Abstract
Since rhinosinusitis is an inflammatory disease, cytokines as key regulators of inflammation play a central role in its pathophysiology. In acute rhinosinusitis, several proinflammatory cytokines of different types have been identified. Initial information about the involvement of the inflammasome in rhinosinusitis has been gained, but this area remains open for more detailed research. Although it has been accepted now that chronic rhinosinusitis (CRS) needs to be differentiated into CRS with and without nasal polyps, it has become clear that this distinction is insufficient to clearly define subgroups with uniform pathophysiology and cytokine patterns. While Th1-cytokines are mostly found in CRSsNP and Th2 cytokines in CRSwNP, there is a substantial overlap, and several other cytokines have also been detected. Attempts to identify CRS endotypes based on cytokines are ongoing but not yet generally accepted. Despite the central role of cytokines in rhinosinusitis, no specific cytokine-targeted therapies are currently available, and only very few studies have specifically addressed the effects of such biologicals in rhinosinusitis.
Keywords: Cytokines, Sinusitis, Biologicals, T helper cells, Nasal polyps, Acute sinusitis, Chronic sinusitis
Introduction
Rhinosinusitis is a widespread and compromising disease and its individual, social, and economic relevance tend to be underestimated. According to the EPOS diagnostic criteria, rhinosinusitis is defined as an inflammation of the nose and paranasal sinuses clinically characterized by the presence of two or more than the following symptoms; one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip), ± facial pain/pressure, ± reduction or loss of smell and either - endoscopic signs of (nasal polyps, and/or mucopurulent discharge primarily from middle meatus, and/or edema/mucosal obstruction primarily in middle meatus and/or - CT changes (mucosal changes within the ostiomeatal complex and/or sinuses). If symptoms last more than 12 weeks without complete resolution, rhinosinusitis becomes a chronic disease (chronic rhinosinusitis (CRS)); otherwise, it should still be termed acute [1••].
Therapy of rhinosinusitis includes intranasal and systemic medication and—in the case of insufficient improvement with drugs alone—surgery. Especially, CRS with nasal polyps (CRSwNP) tends to relapse despite consequent use of all available therapeutic options [1••]. Therefore, new therapies to control or ideally heal this chronic disease are desperately needed. The most promising direction seems to be more targeted drugs aimed toward specific molecular mediators of inflammation. Deep insights into the cellular and molecular processes of rhinosinusitis are a prerequisite to identify such novel therapeutic targets.
It has become obvious in the last years that the relatively simple clinical differentiation of CRS solely dependent on the presence or absence of nasal polyps does not suffice to identify distinct entities with similar clinical and inflammatory patterns. Therefore, attempts to define endotypes in addition to phenotypes of rhinosinusitis are undertaken. Biological endotypes represent subgroups that differ in their pathophysiological mechanisms and the corresponding biomarkers [2••]. The identification of such endotypes is not easy since the pathophysiology of CRS includes endogenous and exogenous factors that come together in each individual in a different extent and form a highly complex network of interaction. Thus, the task is to clarify these mechanisms and categorize them while taking into account the individual variation of endogenous and exogenous factors. Despite these difficulties, the definition of endotypes could be instrumental for the implementation of personalized therapy. Cytokines not only play a key role in orchestrating the cellular interactions but are also key biomarkers for the diverse endotypes and thus very appealing drug targets.
Cytokines in Acute Rhinosinusitis
Pathophysiology and subgrouping in acute rhinosinusitis (ARS) is relatively straightforward compared to CRS. Its etiology can be either viral (mostly rhinoviruses or coronaviruses) or bacterial (mostly Streptococcus pneumonia, Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus). The latter is often preceded by a viral ARS [1••].
For immune defense, both the innate and adaptive immune systems are activated in a stimulus-dependent way. The innate immune system is activated by pathogen-associated molecular patterns (PAMPs), expressed by specific pathogen classes that can activate PAMP receptors on cells of the immune system and other cell types. One type of PAMP receptors are pattern-recognition receptors (PRR) called toll-like receptors (TLR). In response to infection with viruses or bacteria, certain TLRs are addressed. Ten different TLRs have been identified in humans, each one able to bind to specific ligands. Binding initiates intracellular signal cascades with a broad variety of effects on processes like hemostasis, inflammation, apoptosis, and also activation of the adaptive immune system. Activation of the adaptive immune system is induced by regulation of cytokines, chemokines, and other co-stimulatory mediators. Dependent on the pattern of the kind of activated CD4-positive T cells and the upregulated and downregulated mediator, immune answers are initiated. Here, Th1- and Th2-like immune answers are classical. Furthermore, during the last years, Th17-, Th21-, and Treg T cell phenotypes and the corresponding immune responses were defined (Fig. 1). In older studies, these are not considered. Compared to CRS, the role of cytokines in ARS is not explored in detail.
Fig. 1.
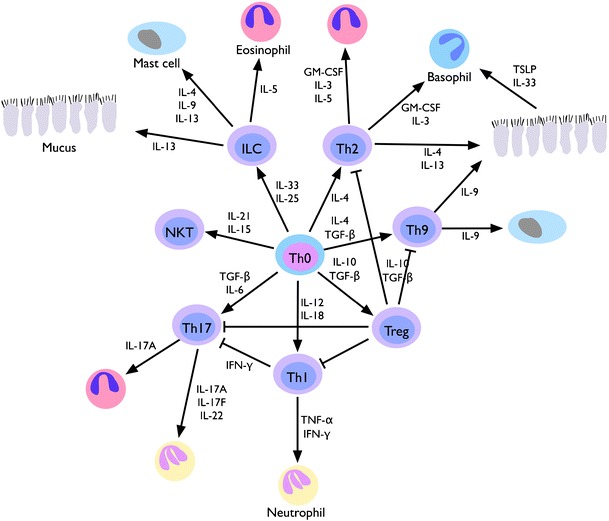
Differentiation of T cell phenotypes relevant in chronic rhinosinusitis and its different endotypes. Under the influence of cytokine patterns and additional signals, Th0 cells develop into phenotypes (in the center of the figure) that influence effector cell types via cytokine release such as epithelial cells, mast cells, eosinophils, basophils, and neutrophils in the nasal tissue and thereby relate to specific aspects of the pathophysiology of CRS. Modified from Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–83
Especially during the last years, cytokine-related investigations in patients with acute rhinosinusitis have rarely been performed and therefore particularly the role of recently defined immune answers and novel cytokines remains largely unclear.
Acute viral infections of the upper aerodigestive tract stimulate a variety of proinflammatory cytokines like IFN-α, IFN-γ, IL-1β, IL-6, IL-8, IL-10, and TNF-α [1••]. Klemens et al. detected upregulated IL-1β, IL-6, IL-7, IL-17, IFN-γ, TNF-α, IL-8, G-CSF, GM-CSF, and elastase in nasal secretion of patients with viral rhinitis [3]. Cytokines in these studies include proinflammatory (e.g., IL1β, IL-6, IL-8), anti-inflammatory (e.g., IL-10), and chemo-attractant (e.g., IL-6) factors belonging to Th1 (TNF-α, IFN-γ), Th2-like (IL-10), Th17-like (IL-17), and Treg (IL-10) immune answers.
Bacterial unmethylated CpG motifs evoke a Th1-immune response after binding to the TLR-9 receptor. CpG administration into the human nose leads to accumulation of Th1-related cytokines IL-1β, IL-6, and IL-8 [4]. Presence of mRNA in nasal epithelium was documented for TLR-1 to TLR-10. PAMPs of other Gram-negative bacteria or S. pneumonia, microbes, often detected in ARS, are known to induce TLR-2 or TLR-4 related immune answers. The former generally initiate Th1-immune responses, and one may assume that this mechanism is also present in ARS. Nevertheless, van Rossum was not able to show upregulated IL-12 (typical for a Th1-response) nor upregulated IL-4 (typical for a Th2-response) in a murine model for nasopharyngeal S. pneumoniae colonization [5]. In contrast to this, Riechelmann et al. detected increased values for IL-2, IL-4, IL-10, IL-12, IL-13, TNF-α, and IFN in nasal fluids from ARS patients compared to CRS patients using a multiplex cytokine assay [6]. An older study from our own laboratory showed elevated IL-8 and IL-3 in mucosal specimen of ARS patients using ELISA [7]. These results suggest a mixed Th1- and Th2-immune response in ARS but also points out that depending on the materials and methods, the results and their interpretation may differ.
The fact that it is quite difficult to clinically specify the transition from acute rhinitis to acute rhinosinusitis [8], it might seem acceptable to deduct results from studies in acute respiratory infection and rhinovirus infection to the pathophysiology of acute rhinosinusitis. A recent study in a large group of patients with community acquired acute respiratory infection demonstrated a close correlation between IL-8 (and neutrophils) in nasal secretions and the severity of nasal symptoms. This underlines both the relevance of neutrophilic inflammation in acute rhinosinusitis and the relevance of IL-8 for neutrophil recruitment into the nasal tissue [9]. In a mouse model of rhinovirus infection, the importance of CCL7 (identical with monocyte chemotactic protein-3) and IFN regulatory factor 7 (IRF-7) to induce and regulate inflammatory and antiviral responses was demonstrated using anti-CCL7 antibodies and an IRF-7-targeting small interfering RNA in vivo [10].
Further research focusing on newer cytokines and patterns of immune responses seems to be warranted in acute rhinosinusitis.
Inflammasome and Rhinosinusitis
In the recent years, a novel concept of activation and regulation of inflammatory reactions has been described. Inflammasomes are complex intracellular molecular arrangements that are often described as a platform. They activate caspase-1 which leads to the activation of the proinflammatory cytokines IL-1β and IL-18 by proteolytic cleavage of their precursors (pro-IL-1β and pro-IL-18). Inflammasomes are pattern-recognition receptors (PRR) that respond not only to microbial components but also to endogenous damage associated molecular patterns. Apart from the initially described Nod-like receptor (NLR) protein 1 (NLPR1), NLRP3, NLRC4, NLRP6, Naip5, and the DNA-sensing AIM2 have been identified [11•].
In the context of rhinosinusitis, especially NLPR3 that can be activated by a large variety of stimuli (microbes, host-derived molecules, and inorganic substances) and AIM2 that is capable of reacting to double-stranded DNA from bacteria, viruses, or human tissue might seem to be interesting areas of research. So far, only few studies have examined inflammasomes in rhinosinusitis.
In a mouse model of acute rhinosinusitis induced by S. aureus, the increasing amount of inflammation after induction of the bacterial inflammation could be correlated to increased expression of NLPR3 proteins and mRNA and elevated levels of IL-1β [12].
The expression of several NOD-like receptors, which also belong to the PRRs, have been investigated in human tonsils, adenoids, nasal polyps, and nasal biopsies from healthy volunteers by Mansson et al. They detected NOD1, NOD2, and NALP3 (the protein encoded by the NLPR3 gene) mRNA, and protein in all samples. CRSwNP patients that were treated with intranasal steroids had lower NLR expression than the tissue from untreated patients [13].
In a study including samples from both CRSsNP and CRSwNP, the involvement of AIM2 in CRSwNP with S. aureus biofilms was demonstrated using human inflammasome PCR arrays. Samples from CRS patients without polyps and without evidence for S. aureus biofilms did not show elevated expression of AIM2-related mRNA [14•].
Given the fact that our understanding of the complexities surrounding inflammasome signaling and regulation is still very limited, these findings have to be regarded as interesting but preliminary.
Cytokines in Chronic Rhinosinusitis
During the last years, it became more and more evident that the clinical picture of CRS only scratches the surface of a disease that involves many different pathomechanisms. Triggers of CRS include genetics, racial differences, anatomic variations, systemic and local allergic inflammation, pathogens like bacteria or fungi, bacterial biofilms, humoral or innate immune deficiencies, gastroesophageal reflux, as well as external noxae like cigarette smoking or air pollution [15].
Clinically, the most evident subdivision is into CRS with and without nasal polyps (CRSwNP, CRSsNP). Histologically, CRSsNP tends toward fibrosis, whereas CRSwNP shows albumin-rich edema and pseudocysts. CRSwNP is furthermore associated with “late onset” asthma and more severe courses of illness. When the inflammatory reaction in the tissue is taken into account, these (clinical) phenotypes of CRS can be subdivided into different molecular endotypes.
Cytokines play a major role in the endotype subclassification of CRS since they relate to the adaptive immune response to exogenous factors (microorganisms, aspirin intolerance, allergy, environmental factors) and influence the local tissue environment by attracting specific cell types and lead to changes in tissue architecture (e.g., remodeling mechanisms, regulation of the epithelial barrier function [16, 17] (Fig. 1). Genetic variations in the genes affecting the cytokine response may furthermore influence the susceptibility to CRS and the course of illness.
Research in cytokines in CRS started detecting that CRSwNP, compared to CRSsNP, seemed to be characterized by a Th2-profile with upregulated IL-4, IL-5, IL-13, and the transcription factor GATA-3 [18, 19]. Eosinophils and total IgE are also elevated in the tissue [20, 21]. High levels of ECP as a marker for eosinophil activation are detected in polyps [21].
In CRSsNP on the other hand, more Th1 cytokines and upregulated TGF-ß and its receptor [22] as well as increased levels of IFN-γ and low ECP are expressed [21]. But ongoing research also demonstrated that a clear-cut subdivision in Th1-CRSsNP and Th2-CRSwNP patients is not possible since CRSwNP and CRSsNP patients also showed mixed Th2 and Th1 patterns [23–25] even in the same individual. Thus, it is obvious that the situation is more complex, especially when considering the advent of multiple additional T helper phenotypes such as Th17-, Th21-, and Treg in this context.
Characteristic for Th17 cells is the production of IL-17A, IL-17F, and IL-22 as well as IL-21. IL-17A induces IL-6 and IL-8 release in bronchial fibroblasts and airway smooth muscle cells as well as GRO-α in the latter, leading to the attraction and activation of neutrophils [26, 27]. In CRSwNP, IL-17A is expressed in a subgroup of individuals [28••, 29]. Especially in Asian patients, IL-17A expression is associated with the development of polyps. If IL-17A expression is associated with eosinophilic accumulation is discussed controversially [29, 30]. Together with IL-17A, serum amyloid A (SSA) and MPO are coincidentally upregulated in Chinese patients [31].
IL-21 seems predominantly upregulated in tissue of CRSwNP and associated with great polyp size, eosinophilia, and asthma development [32]. In serum, IL-21 was found to be elevated both in CRS patients with and without polyps [33].
Although IL-17E (IL-25) has a high sequence homology to members of the IL-17 family, it antagonizes Th17 cell differentiation and triggers the expression of Th2 cytokines. It is expressed in Th2-cells, epithelial cells, mast cells, and eosinophils. Recently, several groups identified IL-25 in CRS tissue, predominantly with nasal polyps and often in the context of eosinophilic inflammation [34, 35]. IL-25 might also have the potential to become a therapeutic target in future, since in a mouse model, IL-25 antibody treatment diminished CRS successfully [36•].
IL-33 is another recently described cytokine that induces the production of Th2-cytokines like IL-4, IL-5, and IL-13. IL-33 is derived from Th2-cells, mast cells, basophils, eosinophils, NKt, and NK cells. Since it also seems to contribute to eosinophilic Th2-mediated inflammation, it could also be another interesting therapeutic target in CRSwNP. ST2, the receptor of IL-33, is elevated in the mucosa of CRSwNP eosinophilic CRS compared to CRSsNP non-eosinophilic CRS [37•, 38]. In allergic rhinitis, it has also been demonstrated that the antagonistic soluble form of the IL-33 receptor, sST2, is inversely correlated to nasal symptoms [39].
IL-32 is a proinflammatory cytokine secreted by T cells, monocytes, NK cell, endothelial and epithelial cells. In these cells, it can activate the production of several other cytokines including TNF, IL-8, Il-1β, and IFN-γ. IL-32 contributes to host defense against viruses and mycobacteria and has been linked to Th2-associated diseases like atopic dermatitis, rhinitis, and asthma [40]. IL-32 is elevated in CRSwNP [41, 42•]. In sinus epithelial cells, it is upregulated by TNF-α and IFN-γ. IL-32 itself is able to block angiogenic factors like VEGF or PDGF in vitro [43].
A human asthma gene array showed an increased IL-19 level in CRSwNP and to a lesser extent in CRSsNP compared to controls [44]. Relatively little information is known to date on the biological functions of IL-19 and its relevance in inflammation. IL-19 was stimulated by LPS, highly expressed in metaplastic epithelium and recombinant IL-19 increased epithelial cell proliferation.
Cytokines and Cellular Interaction in CRS
A heterogeneous T cell population can be found in healthy and inflamed nasal mucosa. It is composed of Th1, Th2, Th17, Th22, Tfh (follicular helper), and Tregs. Their distribution (and activation) is regulated by modifiers—mainly cytokines. Epithelial cells are an important source of regulating cytokines and can release them as a reaction to exogenous stimuli after activation of the innate immune system via pathogen-associated molecular pattern (PAMP) and damage-associated molecular patterns (DAMP) receptors. These epithelial cytokines include IL-1, TNF-α, interferons, eotaxin, RANTES (regulated upon activation normal T cell expressed and secreted), GM-CSF, monodansylcadaverin (MDC), stem cell factor (SCF), monocyte-chemotaxis-protein 4 (MCP-4), B cell activating factor (BAFF), and many more. Epithelial cytokines IL-25, IL-32, and TSLP can polarize dendritic cells and trigger a Th2 response in the tissue of CRSwNP [45•]. On the other hand, both IL-4 and IFN-γ have been demonstrated to influence the epithelial integrity in CRS by acting on tight junctions [46].
Myeloid (mDCs) and plasmacytoid (pDCs) dendritic subpopulations are accumulated in Th2-inflamed tissue. Macrophages, found in CRSwNP with high IL-5, are mostly M2-polarized and may further support the inflammation by release of chemokine ligand 18 (CCL18) that is able to attract DCs, T cells, and Th2 cells [47, 48]. In Th1-polarized inflammation like in CRSsNP M1-macrophages are more dominant [49]. They release IL1ß, IL-12, IL-23, and TNF.
The typical accumulation of eosinophils in CRSwNP is substantially driven by high IL-5 levels that are produced by T cells and support priming and survival of eosinophils [18, 50]. Additionally, eosinophils are attracted by epithelial cells via the chemokines RANTES, MCP 1/2/3/4, and eotaxin [51–53]. Patients with eosinophilic, IL-5- and Th2-dominated inflammation with nasal polyps are clinically prone to “late onset” asthma development. They also show high TSLP levels in the local tissue as well as expression of CRTH2 and ST2 (IL33 receptor) [54•, 55]. T cell activation marker soluble IL-2 receptor and suppressor of cytokine signaling 3 (SOCS3) are elevated in nasal polyps while TGF-ß1 and forkhead box protein 3 are downregulated. This leads to a reduction of Treg cells and their activity in CRSwNP that cannot be observed in CRSsNP [19, 56].
Cytokines and Remodeling
Remodeling is an irreversible process driven by chronic inflammation leading to substantial tissue rearrangements of the lower and upper airways including collagen deposition and fibrosis as key features. Remodeling is also controlled by cytokines, the TGF-ß-family (TGF-ß1 and TGF-ß2), their receptors (matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPS) are relevant in this context. Collagen deposition and an upregulation of the members of the TGF-ß-family and their receptors are shown in CRSsNP, whereas in CRSwNP, these cytokines and receptors as well as collagen deposition are downregulated [22]. Thus, it seems that this type of remodeling is more relevant in CRSsNP.
Cytokine Patterns in Asian Patients
Investigations in Chinese patients with nasal polyps brought another aspect into the discussion. Interestingly, these patients mostly failed to express high levels of Th2-cytokines. Instead, the majority of them expressed low ECP and high levels of IFN-γ, Th17-, and neutrophil-related cytokines (IL-1β, IL-6, and IL-17A) in conjunction with a primarily neutrophilic inflammation [56]. TGF-β is downregulated in both Chinese and white patients with CRSwNP [19, 57]. Whether these differences are related to extrinsic factors like life-style or to genetic factors remains unclear.
The Relevance of Staphylococcus aureus in CRS
A number of studies point to the relevance of S. aureus colonization in CRS. Specific IgE to S. aureus components has been demonstrated in nasal tissue and secretions and correlated to several aspects of disease severity, the degree and type of inflammation [58], and also to the development of asthma [59]. It was shown that S. aureus colonization is associated with eosinophilic inflammation, nasal polyps, and Th2-response though it is nor clear whether S. aureus colonization is facilitated by Th2 milieu or whether the bacterium generates it through exotoxins or other specific proteins [60••, 61]. At least S. aureus superantigens seem to play an important role by generating a cytokine response from CD4+ and CD8+ T cells that intensify the Th2-immune response [62] and eosinophilic inflammation. Treg-associated cytokines IL-10 and TGF-β1 are downregulated in the presence of S. aureus [63].
Therapy and Conclusions
CRS—like most chronic diseases—is difficult to heal and commonly requires therapy for decades. This is especially true for CRSwNP, where repeated surgical interventions for recurring nasal polyps despite the use of intranasal steroids and other drugs over the course of years are quite common. Thus, new forms of therapy would be very desirable. One possible avenue could obviously be targeting cytokines. Despite the introduction of several cytokine-antagonizing drugs in the last years for different diseases, only a very small minority of them have been investigated in chronic rhinosinusitis, and none has been approved for clinical use in CRS.
Two studies have been performed with two different anti-IL-5 antibodies with effects that were quite remarkable [64••, 65]. In a double-blind, placebo-controlled study with mepolizumab in severe CRSwNP, polyp size was reduced for more than 2 months after the last dose in most patients [64••]. Nevertheless, some patients did not demonstrate a significant response. This underlines the relevance of further research aimed at better classifying patients with CRS—possibly via endotyping—and also the need for further investigations into possible molecular targets.
Recently, the results of a controlled study with the anti-IL-4-receptor alpha antibody dupilumab in CRSwNP have been presented as an abstract [66]. In patients treated with intranasal mometasone, the addition of dupilumab led to significant reductions in polyp size, SNOT-22 scores, and CT scores.
Although some studies with new cytokine-antibodies in CRSwNP are on the way, the clinical need for such innovative treatments remains largely underestimated in the medical and pharmaceutical community.
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Rhinosinusitis
Contributor Information
Kathrin Scheckenbach, Phone: +49-211-8117570, Email: Kathrin.Scheckenbach@med.uni-duesseldorf.de.
Martin Wagenmann, Phone: +49-211-8117570, Email: Martin.Wagenmann@uni-duesseldorf.de.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody FM, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;3:1–298. [PubMed] [Google Scholar]
- 2.••.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the european academy of allergy and clinical immunology and the american academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemens C, Rasp G, Jund F, Hilgert E, Devens C, Pfrogner E, et al. Mediators and cytokines in allergic and viral-triggered rhinitis. Allergy Asthma Proc. 2007;28:434–41. doi: 10.2500/aap.2007.28.3017. [DOI] [PubMed] [Google Scholar]
- 4.Månsson A, Bachar O, Adner M, Cardell LO. Nasal CpG oligodeoxynucleotide administration induces a local inflammatory response in nonallergic individuals. Allergy. 2009;64:1292–300. doi: 10.1111/j.1398-9995.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 5.van Rossum AMC, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Bürner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186–91. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, Wagenmann M, Rudack C, Höpken K, Hillebrandt M, Wang D, et al. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy. 1998;53:2–13. doi: 10.1111/j.1398-9995.1998.tb03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwaltney JM, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 9.Henriquez KM, Hayney MS, Xie Y, Zhang Z, Barrett B. Association of interleukin-8 and neutrophils with nasal symptom severity during acute respiratory infection. J Med Virol. 2015;87:330–7. doi: 10.1002/jmv.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girkin J, Hatchwell L, Foster P, Johnston SL, Bartlett N, Collison A, et al. CCL7 and IRF-7 mediate hallmark inflammatory and IFN responses following rhinovirus 1B infection. J Immunol. 2015;194:4924–30. doi: 10.4049/jimmunol.1401362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13:321–4. doi: 10.1038/ni.2257. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y-J, Gong G-Q, Chen S, Xiong L-Y, Zhou X-X, Huang X, et al. NLRP3 inflammasome sequential changes in Staphylococcus aureus-induced mouse model of acute rhinosinusitis. Int J Mol Sci. 2014;15:15806–20. doi: 10.3390/ijms150915806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Månsson A, Bogefors J, Cervin A, Uddman R, Cardell LO. NOD-like receptors in the human upper airways: a potential role in nasal polyposis. Allergy. 2011;66:621–8. doi: 10.1111/j.1398-9995.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 14.•.Jardeleza C, Miljkovic D, Baker L, Boase S, Tan NCW, Koblar SA, et al. Inflammasome gene expression alterations in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Rhinology. 2013;51:315–22. Relation between inflammasome activation andS. aureusbiofilms in CRS. [DOI] [PubMed]
- 15.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kast JI, Wanke K, Soyka MB, Wawrzyniak P, Akdis D, Kingo K, et al. The broad spectrum of interepithelial junctions in skin and lung. J Allergy Clin Immunol. 2012;130:544–4. doi: 10.1016/j.jaci.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, et al. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:74–80. doi: 10.1016/j.jaci.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42. doi: 10.1016/S0091-6749(97)80019-X. [DOI] [PubMed] [Google Scholar]
- 19.Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele TPJ, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 21.Van Zele TPJ, Claeys S, Gevaert P, Van Maele G, Holtappels G, van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Bruaene N, Derycke L, Pérez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–9. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Wagenmann M, Schubert K, Helmig P, Gärtner-Akerboom M, Chaker A, Scheckenbach K. Der ELISPOT-assay, eine hochsensitive methode zur untersuchung der zytokinproduktion der nasenschleimhaut. Allergologie. 2005;28:401–11. doi: 10.5414/ALP28401. [DOI] [Google Scholar]
- 24.Wagenmann M, Gaertner-Akerboom M, Helmig P. Increased production of type-2 and type-1 cytokines in nasal polyps. J Allergy Clin Immunol. 2000;105:S210. doi: 10.1016/S0091-6749(00)91050-9. [DOI] [Google Scholar]
- 25.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–32. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Molet S, Hamid QA, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–8. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 27.Henness S, Johnson CK, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A augments TNF-alpha-induced IL-6 expression in airway smooth muscle by enhancing mRNA stability. J Allergy Clin Immunol. 2004;114:958–64. doi: 10.1016/j.jaci.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 28.••.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–8. Demonstration of the relevance of the immune response toS. aureusfor nasal polyps and asthma and differences between chinese and european nasal polyps. [DOI] [PubMed]
- 29.Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010;151:8–16. doi: 10.1159/000232566. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X-D, Li G-Y, Li L, Dong Z, Zhu D-D. The characterization of IL-17A expression in patients with chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2011;25:171–5. doi: 10.2500/ajra.2011.25.3645. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Bai J, Ding M, Liu W, Xu R, Zhang J, et al. Interleukin-17A contributes to the expression of serum amyloid A in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol. 2013;270:1867–72. doi: 10.1007/s00405-012-2295-x. [DOI] [PubMed] [Google Scholar]
- 32.Xiao L, Wei Y, Zhang YN, Luo X, Yang BY, Yu SF, et al. Increased IL-21 expression in chronic rhinosinusitis with nasalpolyps. Clin Exp Allergy. 2015;45:404–13. doi: 10.1111/cea.12475. [DOI] [PubMed] [Google Scholar]
- 33.Chao P-Z, Hsieh M-S, Lee F-P, Chen S-Y, Cheng C-W, Chang H-W, et al. Serum level of interleukin-21 is elevated in chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29:e1–6. doi: 10.2500/ajra.2015.29.4117. [DOI] [PubMed] [Google Scholar]
- 34.Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2015;114:289–98. doi: 10.1016/j.anai.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Lam M, Hull L, Imrie A, Snidvongs K, Chin D, Pratt E, et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29:175–81. doi: 10.2500/ajra.2015.29.4176. [DOI] [PubMed] [Google Scholar]
- 36.•.Shin H-W, Kim D-K, Park M-H, Eun KM, Lee M, So D, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;135:1476–7. doi: 10.1016/j.jaci.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 37.•.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2013;124:E115–22. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 39.Baumann R, Rabaszowski M, Stenin I, Tilgner L, Gaertner-Akerboom M, Scheckenbach K, et al. Nasal levels of soluble IL-33R ST2 and IL-16 in allergic rhinitis: inverse correlation trends with disease severity. Clin Exp Allergy. 2013;43:1134–43. doi: 10.1111/cea.12148. [DOI] [PubMed] [Google Scholar]
- 40.Keswani A, Kern RC, Schleimer RP, Kato A. Role of interleukin-32 in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2013;13:13–8. doi: 10.1097/ACI.0b013e32835b35d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soyka MB, Treis A, Eiwegger T, Menz G, Zhang S, Holzmann D, et al. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy. 2012;67:790–8. doi: 10.1111/j.1398-9995.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 42.•.Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin-32 in chronic rhinosinusitis with and without nasal polyps. Allergy. 2012;67:25–32. doi: 10.1111/j.1398-9995.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer N, Christoph J, Makrinioti H, Indermitte P, Rhyner C, Soyka M, et al. Inhibition of angiogenesis by IL-32: possible role in asthma. J Allergy Clin Immunol. 2012;129:964–7. doi: 10.1016/j.jaci.2011.12.1002. [DOI] [PubMed] [Google Scholar]
- 44.Pace E, Scafidi V, Di Bona D, Siena L, Chiappara G, Ferraro M, et al. Increased expression of IL-19 in the epithelium of patients with chronic rhinosinusitis and nasal polyps. Allergy. 2012;67:878–86. doi: 10.1111/j.1398-9995.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- 45.•.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134:499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 46.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130:1087–96. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 47.Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L. Alternatively activated macrophages and impaired phagocytosis of S aureus in chronic rhinosinusitis. Allergy. 2011;66:396–403. doi: 10.1111/j.1398-9995.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 48.Poposki JA, Uzzaman A, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–4. doi: 10.1016/j.jaci.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobol SE, Christodoulopoulos P, Manoukian JJ, Hauber H-P, Frenkiel S, Desrosiers M, et al. Cytokine profile of chronic sinusitis in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2002;128:1295–8. doi: 10.1001/archotol.128.11.1295. [DOI] [PubMed] [Google Scholar]
- 50.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–8. [PubMed] [Google Scholar]
- 51.Meyer JE, Bartels J, Görögh T, Sticherling M, Rudack C, Ross DA, et al. The role of RANTES in nasal polyposis. Am J Rhinol. 2005;19:15–20. [PubMed] [Google Scholar]
- 52.Bartels J, Maune S, Meyer JE, Kulke R, Schlüter C, Röwert J, et al. Increased eotaxin-mRNA expression in non-atopic and atopic nasal polyps: comparison to RANTES and MCP-3 expression. Rhinology. 1997;35:171–4. [PubMed] [Google Scholar]
- 53.Jahnsen FL, Haye R, Gran E, Brandtzaeg P, Johansen FE. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J Immunol. 1999;163:1545–51. [PubMed] [Google Scholar]
- 54.•.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 55.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–11. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 56.Zhang N, Van Zele TPJ, Pérez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:1061–8. doi: 10.1016/j.jaci.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE ‘above atopy’. J Intern Med. 2012;272:133–43. doi: 10.1111/j.1365-2796.2012.02559.x. [DOI] [PubMed] [Google Scholar]
- 59.Song W-J, Chang Y-S, Lim M-K, Yun E-H, Kim S-H, Kang H-R, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014;44:553–62. doi: 10.1111/cea.12239. [DOI] [PubMed] [Google Scholar]
- 60.••.Ba L, Zhang N, Meng J, Zhang J, Lin P, Zhou P, et al. The association between bacterial colonization and inflammatory pattern in Chinese chronic rhinosinusitis patients with nasal polyps. Allergy. 2011;66:1296–303. doi: 10.1111/j.1398-9995.2011.02637.x. [DOI] [PubMed] [Google Scholar]
- 61.Corriveau M-N, Zhang N, Holtappels G, Van Roy N, Bachert C. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23:461–5. doi: 10.2500/ajra.2009.23.3367. [DOI] [PubMed] [Google Scholar]
- 62.Akdis M, Simon HU, Weigl L, Kreyden O, Blaser K, Akdis CA. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–75. [PubMed] [Google Scholar]
- 63.Patou J, Gevaert P, Van Zele TPJ, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008;121:110–5. doi: 10.1016/j.jaci.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 64.••.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele TPJ, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 65.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele TPJ, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 66.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Jiao L, et al. Dupilumab in chronic sinusitis with nasal polyposis, with and without asthma. Allergy. 2015;70:107. [Google Scholar]


