Abstract
Immunoengineering is a rapidly growing and interdisciplinary field focused on developing tools to study and understand the immune system, then employing that knowledge to modulate immune response for the treatment of disease. Because of its roles in housing a substantial fraction of the body’s lymphocytes, in facilitating immune cell trafficking, and direct immune modulatory functions, among others, the lymphatic system plays multifaceted roles in immune regulation. In this review, the potential for biomaterials to be applied to regulate the lymphatic system and its functions to achieve immunomodulation and the treatment of disease are described. Three related processes–lymphangiogenesis, lymphatic vessel contraction, and lymph node remodeling–are specifically explored. The molecular regulation of each process and their roles in pathologies are briefly outlined, with putative therapeutic targets and the lymphatic remodeling that can result from disease highlighted. Applications of biomaterials that harness these pathways for the treatment of disease via immunomodulation are discussed.
Keywords: lymphangiogenesis, lymph node, biomaterials
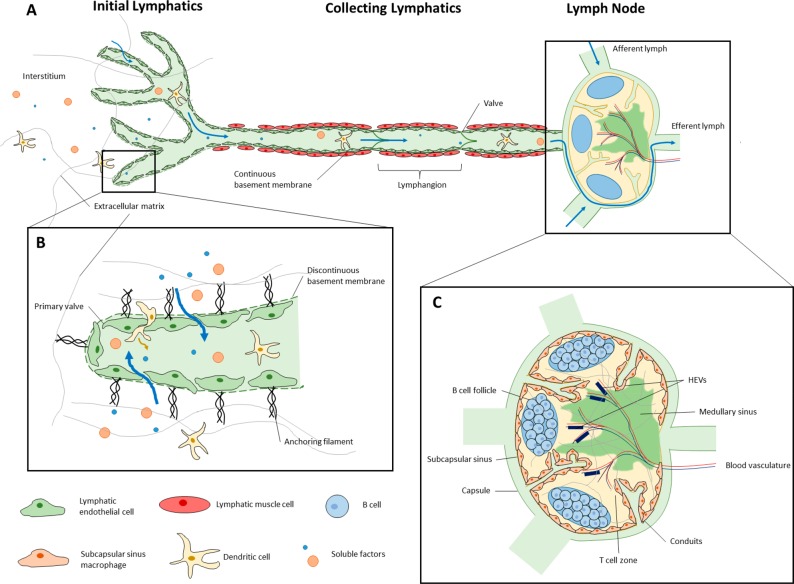
The lymphatic system (Figure 1) is a network of vasculature and immune organs that plays critical roles in fluid balance, lipid transport, and in immune cell trafficking and the immune response. While lymph drainage is critical in removing excess fluid from the interstitial space, relieving edema, and maintaining fluid homeostasis, that fluid also contains migratory immune cells1 and soluble antigen that are transported to downstream lymph nodes (LNs), enabling immune cell interactions and the adaptive immune response. This drainage is critical in a normal immune response, and inhibition of lymphatic function can disrupt both healthy immune responses2 and the very structure of LNs.3,4 In addition to enabling lymph transport and subsequent immune responses, lymphatic vessels have direct, multifaceted immunomodulatory roles5−7 through recruitment of leukocytes and facilitation of cell migration,8 regulation of T cell homeostasis9,10 or activation,11 and dendritic cell modulation.12 These many functions make the lymphatic system a key component of the immune response and a clear target for immunomodulation.
Figure 1.
Structure and function of the lymphatic system. (A) Fluid leaves the tissue interstitium and enters initial lymphatic vessels, flowing through larger collecting vessels and the lymph node. Readers are referred to refs (20−23) for an in depth discussion of lymphatic structure and function. (B) Initial lymphatic vessels are composed of overlapping LECs on a discontinuous basement membrane24 that allow fluid, migratory immune cells, and soluble factors, including nanoparticles in the 20–100 nm size range, to enter the vessels.25 Lymph is moved away from the periphery through larger collecting lymphatic vessels that are surrounded by a layer of specialized lymphatic muscle cells that produce coordinated contractions to propel lymph downstream. (C) Fluid subsequently flows through lymph nodes (LN), secondary lymphoid organs that house cells of the adaptive immune system and are a critical site of antigen presentation. As lymph flows through the LN to eventually exit via an efferent lymphatic vessel,22 soluble antigen can be processed by lymph-sampling sinus-lining macrophages. The conduit system allows molecules smaller than 70 kDa to access deeper regions of the LN,26,27 and LEC-mediated transcytosis facilitates diffusion of antibodies into the LN parenchyma.28 Antigen-presenting cells (APCs) can carry antigen from the periphery29 and traffic into the LN to present their antigen to B and T lymphocytes residing in distinct locations within the LN,22 which then drive the resulting immune response. Cell migration, fluid movement, and antigen transport are all supported by a system of LN stromal cells that provide a scaffold for all critical LN functions to occur.22,28 The structure of LNs is critical to their function and changes with the immune response.
As the lymphatic system is increasingly implicated in playing important immunomodulatory roles and in regulating disease progression and resolution, interest in therapeutic lymphatic modulation increases; just as disease states modulate lymphatic vessels and LN for their benefit, the lymphatic system can be directed to reverse pathologies and promote therapeutic fluid transport and immune responses. Biomaterials have great potential for such in vivo lymphatic modulation. As examples, biomaterials possess surface and mechanical properties that regulate lymphangiogenesis in vitro(13,14) and in vivo;15 are used to tightly control the delivery and presentation of pro-lymphangiogenic factors in vivo;16,17 and enable lymphatic-targeted delivery of immunomodulatory agents for the induction of desired immune responses.18,19 Biomaterials are uniquely valuable for modulating lymphatics in that they enable the delivery of instructive signals to specific lymphatic components, such as vessels or lymph nodes, and can control not only the location of agent release, but also the concentration and context of delivery to precisely regulate the desired response by lymphatic vessels and immune cells alike. This versatile and precise control of the lymphatic response is thus a valuable tool in immunoengineering. Herein, the application of biomaterials to the modulation of lymphangiogenesis, the collecting lymphatic vessel function, and lymph node remodeling for immunomodulation applications are considered.
The Lymphatic System in Immunomodulation
Lymphangiogenesis
Molecular Regulators as Therapeutic Targets
Lymphangiogenesis, or the growth of new lymphatic vessels from preexisting lymphatic vessels, is a tightly regulated process, and an understanding of its regulatory factors is critical to enable directed lymphatic vessel growth. After the generation of the lymphatic system during embryonic development, adult lymphatic vessels are typically quiescent and lymphangiogenesis is primarily observed in disease states such as chronic inflammation,30 LN remodeling in response to infection or disease, tumor growth and metastasis,31,32 and wound healing or tissue regeneration.17 Lymphangiogenesis is primarily regulated by vascular endothelial growth factor receptors and their ligands that also play a critical role in angiogenesis, highlighting the close relationship between the lymphatic and blood vasculature. While angiogenesis, or the growth of new blood vessels, is primarily regulated by VEGFR-1 and VEGFR-2 and their ligand VEGF-A, lymphangiogenesis is primarily regulated by the binding of VEGF-C33,34 or VEGF-D35 to VEGFR-3 expressed on LECs. Lymphangiogenesis is also regulated by the epidermal growth factor (EGF) pathway. Epidermal growth factor receptor (EGFR) has been found to be expressed on healthy LECs, where EGF treatment induces lymphatic vessel growth,36 and on lymphatic vessels in tumor microenvironments where EGF induces lymphangiogenesis and subsequent lymphatic metastasis,37 making EGF modulation a potentially promising therapeutic for regulating lymphangiogenesis. Other growth factors such as fibroblast growth factors (FGFs),38 platelet-derived growth factors (PDGFs),39 and angiopoietin-140 have been shown to regulate lymphangiogenesis, and nongrowth factor regulators such as nitric oxide41 and directional interstitial flow42 are also important in modulating lymphangiogenesis.
Immunomodulatory Effects of Lymphangiogenesis in Disease
Because of the lymphatic system’s critical role in fluid balance, soluble factor transport, and immune cell migration, lymphangiogenesis within peripheral tissues can have significant effects to either help or hinder disease progression depending on context. This makes the growth of lymphatic vessels an enticing target for the treatment of a variety of diseases. See Table 1 for examples of therapeutic modulation of lymphangiogenesis.43
Table 1. Therapeutic Modulation of Lymphangiogenesisa.
| disease model | mechanism of manipulation | major findingsb | refs |
|---|---|---|---|
| Inhibition of Pathological Lymphangiogenesis | |||
| corneal transplantation rejection or suture-induced corneal inflammation | VEGFC/D trap | improves graft survival | (44−46) |
| heart transplantation rejection | VEGFC/D trap and antibody blockade | inhibits LEC-derived chemokine production and immune cell trafficking; improves allograft survival | (47) |
| obliterative bronchiolitis | VEGFC/D trapping | inhibits T cell responses and obliterative bronchiolitis development | (48) |
| chemical carcinogenesis in the skin | VEGFC/D trap | fewer tumors and delayed onset; reduced macrophage number and inflammation | (49) |
| melanoma and lung, prostate, and bladder cancer xenografts | VEGFC/D trap | suppresses LN and distal metastasis | (50−53) |
| tumor xenografts | VEGFC and NRP2 blocking antibody | inhibits distal lymphatic dilation. SMC remodeling, and postsentinel LN metastasis | (54) |
| neuroblastoma xenograft | Anti-VEGFD | inhibits lymphatic metastasis | (55) |
| breast and gastric cancer | Anti-VEGFR3 | suppresses LN and distal metastasis | (56−58) |
| orthotopic breast, spontaneous pancreatic cancer | VEGFR3 TK inhibitor | suppresses tumor growth and LN metastasis | (59) |
| lung cancer xenograft | TK inhibitor of VEGFR2/3 | suppresses tumor growth | (60) |
| heterotopic brain cancer and othotopic breast cancer | Anti-NRP2 | suppresses metastasis to LNs and lungs | (61) |
| breast cancer | Blocking of NRP2-VEGFR3 complex formation | a somatotropin peptide binds to NRP2 and attenuates VEGFR3 signaling | (62) |
| pancreatic cancer xenograft | Anti-ephrinB2 | suppresses angiogenesis, tumor growth | (63) |
| lung cancer xenograft | Anti-ANG2 | inhibits tumor growth and LN metastasis | (64) |
| breast cancer | SphK1 inhibitor | inhibits tumor growth and LN metastasis | (65) |
| breast cancer | Anti-CXCL12 | synergistically inhibits metastasis with anti-VEGFC treatment | (66) |
| suture-induced inflammatory lymphangiogenesis | recombinant thrombospondin-1 | suppresses inflammatory lymphangiogenesis | (67) |
| airway inflammation | anti-TNF-α | reduced leukocyte influx, LV remodeling, and LN hypertrophy | (68) |
| HNSCC | mTOR inhibition | Suppresses tumor lymphangiogenesis and LN metastasis; improves survival | (69) |
| pancreatic cancer | suppresses tumor-derived VEGFC | mTOR inhibitor reduces metastasis | (70) |
| breast cancer xenograft | NSAIDs | inhibits VEGFD-induced prostaglandin synthesis, and thereby collecting lymphatic vessel dilation and metastasis | (71) |
| melanoma | photodynamic laser therapy | destroys tumor-bearing lymphatic vessels and inhibits metastasis | (72) |
| Induction of Therapeutic Lymphangiogenesis | |||
| primary lymphedema | VEGFC application | reduces primary lymphedema in Chy mice | (73) |
| secondary lymphedema | VEGFC application | reduces surgery-induced lymphedema in rabbits | (74) |
| secondary lymphedema | LN transfer and VEGFC application | recovery of lymphatic vessel structure and function | (75−78) |
| hypercholesterolemia | VEGFC application | improves RCT | (79) |
Table taken from ref (43) with permission. Copyright 2014 American Society for Clinical Investigation.
In addition to inhibition of lymphangiogenesis. HNSCC, head and neck squamous cell carcinoma, TK, tyrosine kinase.
Inflammation
Peripheral lymphatic vessels are known to proliferate in chronic inflammatory conditions in a variety of tissues,30,80 a process called inflammation-associated lymphangiogenesis, and lymphangiogenesis plays a complicated role in inflammatory diseases. Depending on the disease context and timing, lymphangiogenesis can either be critical for the resolution of inflammation or a component of the pathology, as reviewed in-depth elsewhere.80,81 Along these lines, in some inflammatory disease contexts, lymphangiogenesis enables beneficial immune responses and fluid clearance. In a Mycoplasma pulmonis lung epithelium infection model of chronic inflammation, as one example, lymphangiogenesis and lymphatic vessel remodeling at the site of infection are observed alongside LN hypertrophy. Inhibition of tracheal lymphangiogenesis worsened local lymphedema and reduced LN hypertrophy, indicative of an impaired response to the infection, suggesting the therapeutic importance of lymphangiogenesis in this context as it provides a drain for excess fluid and supports increased migration of immune cells to LN in response to the infection.82 It is important to note that in this model of airway inflammation, LN hypertrophy reduced with antibiotic treatment and inflammation resolution, but newly generated tracheal lymphatic vessels persisted even after the inflammation was resolved.82 This study thus suggests not only a potential role for lymphangiogenesis enhancement to promote fluid balance and a healthy immune response, but also highlights the importance of treatment timing–in a situation where peripheral lymphangiogenesis is undesirable, preemptive antilymphangiogenic therapy may prevent the growth of new vessels, but those vessels may persist once produced.
Organ transplantation is an entirely different context in which chronic inflammation-associated lymphangiogenesis is associated with reduced graft survival. In kidney transplantation, early renal lymphangiogenesis has been associated with avoiding acute rejection as it provides early clearance of fluid and immune cells.83 Chronic lymphangiogenesis, however, appears to worsen outcomes and contribute to the maintenance of inflammation. In studies of organ rejection, lymphangiogenesis has been observed to negatively affect graft survival,84 primarily by enhancing drainage from the transplant to the draining LN and enabling an immune response; inhibiting lymphangiogenesis has improved outcomes in islet85 and cornea transplantation86 and reduced immune cell infiltration in heart transplants,47 and lymphatic vessels exacerbate inflammation in kidney transplantation.87 Organ transplantation thus represents a context in which chronic lymphangiogenesis contributes to pathology, and highlights a potential application for antilymphangiogenic therapy.
Cancer and Metastasis
Lymphatic vessels are critical in moving fluid, antigen, and immune cells, but are also employed by cancer cells metastasizing from primary tumors. To take advantage of lymphatic vessels for rapid transport to distant tissues in a low fluid shear stress environment, tumors can invade existing lymphatic vessels or express lymphangiogenic factors, such as VEGF-C88 or EGF,37 themselves to promote the formation of tumor-associated lymphatic vessels and increase metastasis.31 These vessels promote cancer cell transport out of the tumor; interstitial flow can drive cancer cell migration toward lymphatic vessels,89 and both lymphatic vessels90 and tumor cells91 express chemokines that can drive cancer cell chemotaxis into lymphatic vessels and enhance drainage to sentinel LNs, priming them for metastasis and modulating the immune response to tumor growth. This mechanism makes lymphangiogenic pathways a clear target for the prevention of LN metastasis, and interventions have been successfully employed; inhibiting lymphangiogenic molecules, particularly VEGF-C, has been shown to reduce lymphangiogenesis and subsequent LN metastasis by many groups.51,92
Though lymphangiogenesis inhibition shows promise in preventing metastasis, the relationship between tumors and their lymphatic vasculature is highly complex. Lymphatic vessels are not merely a route for cancer cell metastasis; in addition, they are responsible for modulation of the tumor immune environment93 and for the interplay between the tumor and immune system that tumors hijack to bypass the normal immune response. Because this tight relationship between tumors and the immune system is mediated by lymphatic vessels, tumor lymphangiogenesis is actually critical in the efficacy of immunotherapies94 and inhibiting lymphangiogenesis has been shown to abrogate the tumor response to photodynamic therapy and checkpoint blockade.95 When targeting tumor lymphangiogenesis, a careful balance between limiting LN metastasis and enabling immunotherapy must be struck, and care taken that lymphatic vasculature alterations induced by treatment do not worsen prognosis by increasing metastasis as has been observed with some chemotherapeutic treatment modalities.96
Cardiovascular Disease
The lymphatic system plays a critical role in maintaining the health of the cardiovascular system.97,98 For example, lymphatic vessel obstruction negatively affects cardiac function, inducing edema and causing altered electrical signaling, ventricular fibrillation, and myofibrillar degeneration.99−101 Chronic edema can also induce fibrosis in the cardiac interstitium and cause reduced cardiac output,102 contributing to the risk of heart failure.97 Modulating lymphangiogenesis to regulate lymph flow and immune cell migration is thus is a potential approach to treat a variety of cardiovascular diseases. For example, lymphatic drainage is critical in mediating reverse cholesterol transport (RCT)103 in the prevention of atherosclerosis. Enhancing lymphangiogenesis with VEGF-C can improve RCT and reduce cholesterol accumulation,79 while inhibiting transport via lymphatic disruption impedes clearance of cholesterol-loaded macrophages from tissue.79,104
Functional cardiac lymphatic vessels are also critical in recovery from myocardial infarction. Enhanced lymphangiogenesis and remodeling of cardiac lymphatic vasculature has been observed after myocardial infarction,105 and blocking the lymphangiogenic response through VEGFR3 inhibition impedes cardiac lymphatic transport, resulting in increased inflammation and edema in a postinfarct heart.16 Enhancing lymphangiogenesis reduces inflammation and fibrosis,105 and enhancing lymph drainage in the heart after ischemia can reduce tissue damage and prevent infarction development.106
Application of Biomaterials for Modulating Lymphangiogenesis
As discussed above, there are a wide variety of approaches to modulating lymphangiogenesis for therapeutic applications (Figure 2). One of the most common approaches is the regulation of the VEGF-C/VEGFR3 pathway. Lymphangiogenesis can be promoted by the addition of VEGF-C protein itself79 or by the implantation of autologous, growth factor-producing LNs.107 Inhibition of this pathway can be achieved through VEGF-C/D trapping through the expression of soluble VEGFR-3 by either adenoviral gene delivery47 or through delivery of anti-VEGFR-3,94 VEGF-C neutralizing antibodies, or small molecule pathway inhibitors.16 Other growth factor pathways can be modulated using similar approaches.36 Though these options are feasible for research purposes, they have translated poorly to the clinic. Viral gene delivery poses a variety of challenges in patients,108 and the use of protein or small molecule regulators raises issues with maintaining spatial and temporal control over delivery. Off-target delivery of therapeutics can negatively impact nontarget tissues or related pathways, resulting in side effects,109,110 and their nontargeted delivery and short half-life can necessitate repeated treatment or increased dosage.
Figure 2.
Modulation of lymphangiogenesis. A wide variety of approaches to modulating lymphangiogenesis are in use (blue background) and biomaterials can, in many cases, be used to expand upon and improve those approaches (orange background).
As interest in modulation of lymphangiogenesis grows, the role of biomaterials in overcoming challenges of traditional therapeutic approaches is becoming increasingly evident. There has been significant research into the use of biomaterials to induce lymphangiogenesis in peripheral tissue for the treatment of a wide variety of pathologies that could benefit from improved lymph flow or a resolution of inflammation. While biomaterial surface and mechanical properties alone have been shown to regulate lymphangiogenesis, biomaterials are often combined with cells or pro-lymphangiogenic growth factors to enhance vessel growth in vivo. These approaches have been shown to enhance lymphangiogenesis and can be used to treat a variety of pathologies, including lymphedema and chronic wounds.
There is great interest in how surface and physical properties of biomaterials directly (e.g., in the absence of coformulated/delivered growth factors) influence LEC proliferation, sprouting, and orientation. BECs and LECs have been observed to proliferate and form networks with architecture similar to the in vivo condition preferentially on different substrates; in hydrogel matrices with cleavable matrix-bound VEGF-C, BECs form vessels preferentially in collagen-containing matrices, while LECs form capillary networks with nearly physiological structure preferentially when implanted in a flexible fibrin matrix.13 However, LECs can be cultured on a wide variety of hydrogel substrates, including gelatin,111 matrigel,112 fibrin,13,113,114 collagen,13,15,114 and hyaluronic acid.115 Hydrogels are not a requirement for LEC growth, however. Rigid polyglycolic acid (PGA) tube scaffolds have been employed for in vitro LEC culture, and generated a tubular structure that obtained a lymphatic-like phenotype when transplanted in vivo.116 The surface properties and fiber alignment of electrospun biomaterial scaffolds have also been observed to regulate the migration and alignment of LECs, driving their orientation in the direction of fiber alignment in vitro and maximizing migration on fibers of a particular size and density.14
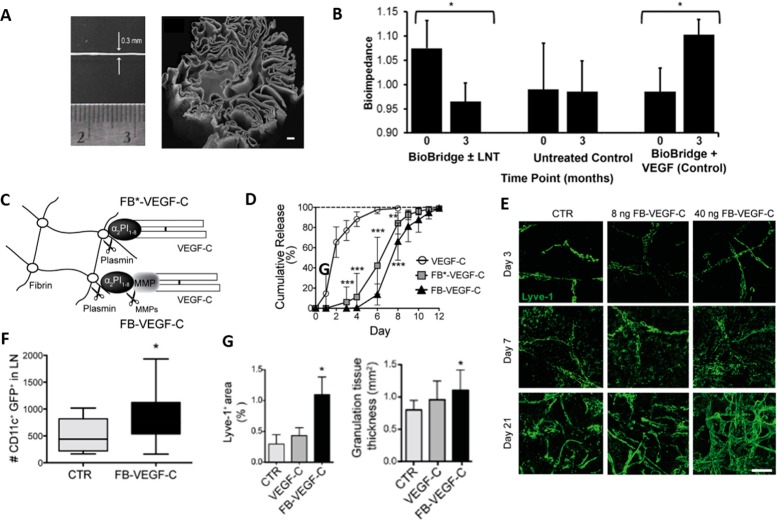
These principles have been put into practice in the clinic for the treatment of secondary lymphedema. BioBridge by Fibralign Corporation is a nanofibrillar collagen scaffold, a thread-like structure with aligned layers that provide a large surface area for cell attachment and migration (Figure 3A. These scaffolds were found to guide the cytoskeletal organization of endothelial cells117 and LECs15 in the direction of the collagen fibers. When implanted in a porcine lymphedema model, the BioBridge scaffold enhanced lymphangiogenesis and the generation of lymphatic collecting vessels, improving lymphatic function and fluid clearance even without the addition of exogenous mediator VEGF-C (Figure 3B).15 BioBridge is currently in clinical testing, highlighting the potential for modulating LEC migration, proliferation, and lymphangiogenesis by careful selection of optimal biomaterials, even without the presence of molecular mediators of lymphangiogenesis.
Figure 3.
Biomaterials for promoting lymphangiogenesis. (A) Hadamitzky et al. implanted nanofibrillar collagen scaffolds into a porcine model of lymphedema, (B) and observed that only when the scaffolds were delivered with or without exogenous LN transfer was there any measurable reduction in interstitial fluid volume, as measured by bioimpedance. Adding exogenous VEGF-C to the implanted scaffold negated any benefit of the scaffold. (C) Guc et al. developed plasmin-cleavable VEGF-C-releasing hydrogels that (D) release their VEGF-C payload in a controlled fashion, compared to rapid release of free protein, in vivo. (E) The hydrogel induces significant expansion of LYVE1+ vessels (green) in a dose-dependent fashion and (F) increases leukocyte trafficking to LNs. (G) In a diabetic wound model, the FB-VEGF-C hydrogels enhanced lymphangiogenesis and improved wound healing, as measured by increased granulation tissue. Panels A and B adapted with permission from ref (15). Copyright 2016 Elsevier. Panels C–G adapted with permission from ref (17), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Copyright 2017 Elsevier.
In addition to the promotion of lymphangiogenesis by material selection alone, significant work has been done to combine biomaterials with additional pro-lymphangiogenic factors, such as cells, growth factors, and small molecule drugs, to further enhance lymphatic vessel growth, and these systems can be applied to a wide variety of diseases. Significant work, for example, has been done in using implanted hydrogels delivering exogenous growth factors, particularly VEGF-C, for the enhancement of lymphatic vessel growth. Such hydrogels provide unique advantages for enhancing peripheral lymphangiogenesis: they are easily modifiable; can contain free or tethered growth factor at controllable concentrations,118 in controllable locations,16 and in combination with other factors; have controllable stiffness; are often biodegradable; and can be injectable for ease of delivery.118
It has been shown that LEC proliferation and sprouting in vitro are most improved when cells are exposed to a constant release of VEGF-C; accordingly, alginate hydrogels that gradually release VEGF-C significantly improve LEC sprouting in a chick chorioallantoic membrane (CAM) assay.118 Similar VEGF-C-releasing hydrogel systems have been employed to treat a wide variety of pathologies in vivo. For example, implantation of a VEGF-C-releasing gel at the site of mouse hindlimb lymphatic injury has been shown to induce lymphangiogenesis and reduce hind paw edema in combination with extracorporeal shock wave therapy.111 When combined with human adipose-derived stem cells, the gel reduced edema and increased density of LYVE-1 positive lymphatic vessels,119 making this system a promising approach for the treatment of lymphedema. Similar systems have also been used to enhance wound healing. A fibrin/collagen hydrogel containing fibrin-binding VEGF-C improved wound healing in a diabetic mouse model after subcutaneous implantation; the VEGF-C variant released from the hydrogel by fibrin cleavage by infiltrating immune cells (Figure 3C,D) induced local stimulation of lymphangiogenesis (Figure 3E) and enhanced leukocyte trafficking (Figure 3F that was not observed with free VEGF-C, resulting in increased lymphangiogenesis and granulation tissue production when implanted in diabetic animals (Figure 3G).17 VEGF-C-releasing hydrogels have also been applied to the heart after myocardial infarction, and found to reduce inflammation, edema, and fibrosis while improving postinfarct function.16 VEGF-C released from such hydrogels may not only act directly on lymphatic vessels through activation of VEGFR-3 on LECS, but may also promote lymphangiogenesis by acting on myeloid cells, which have been shown to express VEGFR-3 in the context of cancer and inflammation120 and to incorporate into lymphatic vessels121 during inflammation-associated lymphangiogenesis.
Biomaterials have also been employed to induce antilymphangiogenic effects in the context of graft rejection and cancer, primarily through the use of nanoparticle (NP) gene delivery systems. The delivery of plasmids expressing a VEGF-binding recombinant construct enhanced survival of corneal grafts by reducing angiogenesis and lymphangiogenesis,122 and the delivery of anti-VEGFR-3 siRNA in polyethylenimine-alginate nanoparticles123 or anti-VEGF-C siRNA in calcium carbonate nanoparticles124 has been shown to inhibit lymphangiogenesis and reduce tumor growth and metastasis, respectively. While biomaterials-based approaches to directly delivering molecular regulators of tumor vascularization are currently mostly limited to antiangiogenic regulator delivery, additional nanoparticle systems specifically targeting lymphangiogenesis may be employed since the role of lymphangiogenesis in cancer progression is continually clarified and antilymphangiogenic therapeutics are approved for clinical use.
Lymphatic Vessel Contraction
Molecular and Cellular Regulation
The contraction of collecting lymphatic vessels is critical to the function of the lymphatic system, and its natural regulatory mechanisms provide many targets for engineering vessel function. The intrinsic contraction of lymphatic vessels is granted by lymphatic muscle cells (LMCs), which share features with both striated cardiac muscle and vascular smooth muscle.125 LMC contractions are regulated by the flux of ions across many ion channels126−128 and by a variety of inflammatory mediators (Table 2), a critical point due to the lymphatic system’s important role in the inflammatory response. Histamine, secreted by immune cells during inflammation, drives lymph formation and flow both by increasing capillary permeability and subsequent interstitial pressure129 and through direct effects on lymphatic muscle cells.130 Arachidonic acid metabolites are also critically involved in lymphatic pumping regulation; prostaglandins have been observed to induce lymphatic contractions, thromboxane synthesis inhibition suppresses contraction,131 and leukotriene B4 antagonism improves lymphatic function and inflammation in a mouse tail lymphedema model.132
Table 2. Regulators of Lymphatic Vessel Contraction.
| regulator | system | effect | refs |
|---|---|---|---|
| NO (iNOS) | mouse hindlimb lymphatics | overwhelms NO gradient, reducing lymphatic contraction strength | (133) |
| NO(eNOS) | mouse hindlimb lymphatics | eNOS inhibition or knockout reduces contraction strength and increases frequency | (133) |
| mouse tail lymphatics | inhibition or knockout reduces lymph velocity through effects on collecting lymphatics | (136) | |
| NO (exogenous) | rat tail lymphatics | addition of GTNO reduces lymph flow through reduced contraction frequency and effective contraction length | (134) |
| prostaglandins | rat iliac lymph node afferent vessel | vessel dilation (PGE2) or vessel constriction | (137) |
| bovine mesenteric vessel | increased contraction frequency and amplitude (PGA2 and PGB2) or decreased contraction amplitude (PGE2; and PGI2) | (138) | |
| bradykinin | rat mesentery | increased contraction frequency and pump flow index | (139) |
| histamine | guinea pig mesentery | increased contraction frequency and decreased contraction amplitude | (130) |
| guinea pig mesentery | increased contraction frequency and decreased contraction amplitude | (140) |
Perhaps the most well-studied regulator of lymphatic contractions is nitric oxide (NO), a reactive small molecule known for its inflammatory roles and its vasoactive effects in the blood vasculature that plays complex roles in lymphatic pumping. LECs express endothelial nitric oxide synthase (eNOS), producing a basal level of nitric oxide required for normal lymphatic pumping; when eNOS is inhibited133 or exogenous NO is added,134 normal pumping function is disrupted. NO production is thought to be tightly regulated both spatially and temporally within lymphatic vessels, with the pulsatile flow of lymph in vessels driving transient, cyclical production of NO, especially in the region of secondary valves, that supports coordinated contraction.135
Immune Regulation of Vessel Contraction in Disease
Inflammation and Cancer
Lymphatic vessel contraction is regulated not only by lymphatic vessels themselves, but also by immune cells in the context of inflammation, further complicating the relationship between lymphatic function and inflammation resolution. Histamine secreted by mast cells causes increased contraction frequency and decreased amplitude, altering lymphatic pumping.140 Nitric oxide is also a critical regulator of lymphatic pumping during the inflammatory response, when inducible nitric oxide synthase (iNOS) expressed by CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) in inflamed tissue overwhelms the natural NO gradient produced by LEC eNOS, resulting in inhibited lymphatic contraction and reduced antigen delivery to draining LNs from the periphery.133 Tumors can also regulate collecting lymphatic vessels to their advantage, producing VEGF-D that regulates prostaglandin production and causes dilation of tumor-associated collecting lymphatic vessels, resulting in increased LN metastasis.71 Normal collecting vessel function is thus hugely important in the immune response, and altered vessel function in the context of inflammation or tumorigenesis can have significant effects.
Lymphedema
Lymphedema occurs when lymphatic drainage is insufficient to maintain physiological interstitial fluid volumes, leading to edema and structural changes in the affected tissue.141 In primary lymphedema, lymphatic vessels do not properly develop to transport homeostatic levels of fluid, often as a result of mutations in genes critical for lymphatic development,142 such as VEGFR-3143 or FOXC2.144 Secondary lymphedema, the more common manifestation of the disease, occurs due to some postnatal insult, such as surgical LN removal in breast cancer treatment145 or filariasis.146 The contraction of lymphatic vessels in patients with lymphedema is often impaired. Lymphedematous legs show irregular contractions too weak to propel lymph,147 and lymphatic congestion lymphoscintigraphy measurements reveal lymphatic pump failure in patients with arm lymphedema after breast cancer surgery.148 Inflammation can also disrupt lymphatic vessel contractility, particularly through the production of iNOS by immune cells surrounding vessels,133 and inflammatory disease is often associated with lymphedema.
Metabolic Disorders
Obesity is a significant risk factor for secondary lymphedema,149,150 and studies have shown that lymphatic function is impaired in obesity.151,152 These effects are mediated in part by iNOS-producing inflammatory cells surrounding lymphatic vessels, and lymphatic vessel function can be restored by iNOS inhibition or the suppression of perilymphatic T cells.153
The barrier integrity of collecting lymphatic vessels also plays a significant role in the immune response, and abnormal vessel permeability can contribute to disease. Though collecting lymphatic vessels are typically not considered permeable to migrating immune cells, lymphatic vessel permeability allows the leakage of lymph and its components into adipose tissue surrounding lymphatic vessels and enables antigen exposure to the dendritic cells associated with lymphatic collecting vessels.154 Collecting vessel permeability is also altered in several pathologies, particularly in metabolic diseases. In diabetic mice, for example, collecting vessels are leakier than in wild type vessels, and can be rescued by increasing NO signaling.155 Similar functional deficiencies have also been implicated in hypercholesterolemia and atherosclerosis, making the barrier function of collecting vessels critical in maintaining normal physiology.
Biomaterials Regulating Collecting Lymphatic Function—Opportunities and Potential
In spite of the clear importance of physiological collecting vessel function in the movement of lymph, avoidance of disease development, and the immune response, the employment of biomaterials to specifically enhance collecting vessel function in vivo has remained largely unexplored. This may be significantly due to the growing state of the field—the contractile physiology and regulators of vessel barrier function are poorly understood, and more is learned every day about the tight relationship between lymphatic vessels and cells of the immune system. Because of this, many studies on improving vessel function currently involve molecular mediators delivered through topical application, local injection, or systemic delivery. For example, when NO is used therapeutically to modulate lymphatic vessel function it is often applied directly to the site of action through topical application134,156 or inhalation;157 these local delivery approaches, however, may be insufficient for the modulation of vessels that are not superficial and accessible. Additionally, many of the regulators of lymphatic vessel pumping and permeability are small molecules (NO, prostaglandins, histamine, etc.), the biodistribution of which can be very challenging to control due to their blood permeability. These molecules are also often involved in other critical physiological functions,158,159 especially in the blood vasculature due to physiological similarities between lymphatic and blood vessels, and systemic delivery could result in side effects due to action in off-target tissues. If small molecule mediators of lymphatic vessel contractility are to be employed therapeutically, sufficient concentrations must be achieved in target tissues while off-target delivery should be avoided to limit side effects, a balancing act that is challenging to manage.
To this end, biomaterials could be valuable in solving this problem as more lymphatic-targeted, controlled drug delivery systems are developed. This could be achieved through conjugation to a nanoparticle, or even in vivo association with a native protein, such as albumin,160 that has access to lymph. This approach is fairly common for targeting drugs to LNs, and has recently been explored for the delivery of NO to lymphatic tissues.161 When NO-loaded nanoparticles are injected in the skin, they create a depot that slowly drains into lymphatic vessels while releasing their drug payload in a lymphatic-targeted fashion that enhances the delivery of NO to lymphatic tissues. While NO accumulation was primarily measured in the LN, nanoparticles are presumably also releasing NO in collecting lymphatic vessels en route to the LN that could modulate lymphatic vessel pumping and lymph flow. This may be true for many drugs delivered in this fashion.
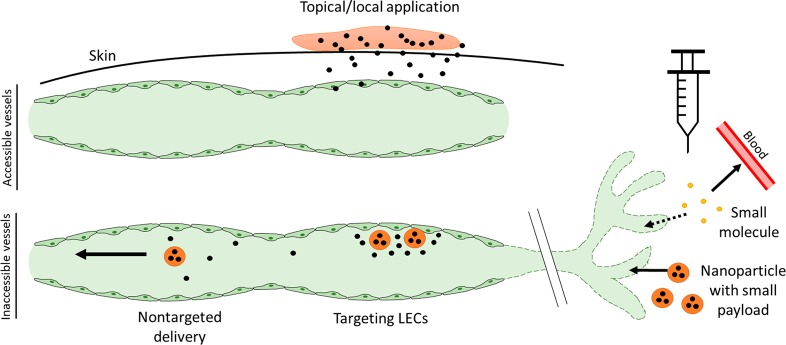
While NP-based systems enable the uptake of a small molecule payload into lymphatic vessels, additional challenges with vessel targeting may arise due to low retention time of NPs within lymphatic vessels. Once a nanoparticle leaves the interstitium and enters the lymph, it transits through the lymphatic vessel over the time scale of minutes,162 to pass through draining LNs and eventually be returned in efferent vessels to the blood circulation at the thoracic duct. During this brief time the particle may only release a fraction of its payload, and any payload retained within the drug delivery system can no longer target the collecting vessels. To enhance the retention time of drug delivery systems within collecting lymphatic vessels and create a drug depot within lymphatic vessels, drug delivery systems with LEC targeting moieties could be employed. While this approach is mostly unexplored in a therapeutic context, it has been used for lymphatic imaging. Metallic nanoparticles have been conjugated to antipodoplanin antibody for imaging of breast cancer lymphatic vessels,163 and lectins have been used to visualize blood and lymphatic vasculature.164 Using similar targeting approaches for more localized and controlled drug release in lymphatic collectors is a promising tool for the treatment of disorders of lymphatic pumping, and could be broadly applied in inflammation and lymphedema (Figure 4).
Figure 4.
Limitations and future directions for modulating lymphatic collecting vessel function. While local administration of small molecule therapeutics is useful in situations where lymphatic vessels are readily accessible, injection of small molecules can result in poor lymphatic uptake and systemic distribution. Employing lymphatic-draining nanoparticles that release a small molecule payload within lymphatic vessels could overcome this limitation, and targeting the nanoparticles to LECs could help ensure local release of drug and a higher concentration within the collecting lymphatic vessel.
Lymph Node Remodeling
Regulation and Impact on Immune Response
LNs are complex secondary lymphoid organs that are critical in the immune response; they have particular structure defined by precise distribution of immune cells, extracellular matrix (ECM), blood and lymphatic vessels, and supportive stromal cells.22 LN biophysical remodeling, or changes in the organization of the LN, occurs in a variety of situations, both healthy and pathological, and includes physical changes in LN structure, such as LN size, stiffness, and matrix composition;165,166 cellular changes as cells migrate and proliferate; and chemical changes as chemokine and cytokine distributions are altered. The organization of these components regulates lymph flow, antigen, and chemokine distribution within the LN, and adhesion, migration, and activation of immune cells. Because the structure of the LN is so tightly tied to the activation, behavior, and location of the cells within it, it follows that changes at the level of the LN can have drastic effects on the subsequent immune response.
Physical features of the LN, such as stiffness, pressure, and size, are regulated by the cells of the LN, both stromal and immune.167 Of particular interest in this context are fibroblastic reticular cells (FRCs), supportive cells that produce extracellular matrix components such as collagen168 and are critical in enabling LN elasticity during expansion.169 LNs can also remodel with respect to the organization of resident cells. Antigen-presenting cells (APCs) must migrate to interact with B and T cells for the induction of adaptive and humoral responses; B cells migrate and proliferate during the formation of germinal centers;170 FRCs remodel and migrate to enable LN expansion;171 and lymphocytes must migrate extensively through the LN during circulation. All of these migratory processes and the maintenance of distinct LN regions are governed by complicated chemokine gradients produced by LN stromal cells, and disruption of these pathways in disease can have predictably significant consequences on immune cell organization and the immune response.
Lymph Node Remodeling in Disease
Cancer
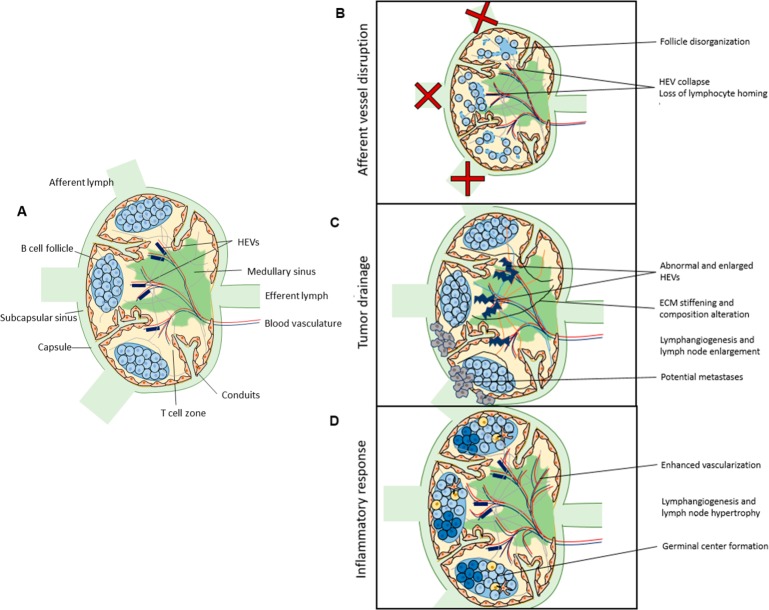
Significant biophysical remodeling is also observed in malignancies (Figure 5); tumor-draining LNs (tdLN) that receive lymph and thus also lymph-borne soluble antigen and tumor-associated factors from the tumor interstitium are a striking example (Figure 5C). Stromal cells play an important role in tdLN remodeling.172,173 For example, BECs, LECs, and FRCs in LNs draining melanoma tumors proliferate as tumors develop,173 and altered chemokine production by FRCs contributes to disorganized immune cell distribution and altered immune cell profiles.173 tdLN show enhanced expression of genes regulating ECM deposition,174 and LNs draining melanomas are enlarged and stiff compared to naïve LNs with high intranodal pressure and altered ECM composition, including increased collagen and hyaluronic acid content,165 changes that are simultaneously observed in the tumor microenvironment during tumor development. These changes in stiffness and ECM density and composition may limit immune cell mobility and recruitment, and limit the immune response to the upstream tumor.
Figure 5.
Lymph node remodeling in disease. (A) The biophysical organization of the LN remodels in the context of disease, including (B) afferent vessel disruption, (C) tumor drainage, and (D) inflammatory response.
Vascular remodeling is also observed in tdLN; high endothelial venules (HEVs) are observed to remodel in LNs draining tumors, enlarging and becoming abnormal particularly in patients with LN metastases,175 and tdLN displayed enhanced BEC proliferation and functional vascularization, measurably enhancing blood flow within the LN.176 LN lymphangiogenesis is also enhanced in the context of nasopharyngeal carcinoma, where an increase in the number of LYVE1+ vessels and dilation of those vessels is observed.176 Together, these changes are postulated to prime the LN for metastasis. Tumor drainage to LNs thus significantly alters LN organization, ECM, and vascularization, potentially altering the LN’s ability to mount an immune response to the growing tumor or priming the lymph node for subsequent metastasis.
Inflammation/Lymph Node Expansion
LNs must expand significantly in a normal adaptive immune response, increasing in size in a reversible fashion without permanent damage to LN structure (Figure 5D). During this expansion, FRCs, BECs, and LECs all proliferate177 and lymphocytes are recruited to the LN,178 resulting in notably enhanced LN size and cellularity. During infection, LEC expansion results in an increase in the number of LYVE1+ vessels within the LN,177 and LN sinus expansion is induced by VEGF-A distributed by interstitial flow alongside FRC expansion and remodeling.178 B cells of inflamed LNs have also been shown to induce lymphangiogenesis to enhance the influx of antigen-presenting DCs from the periphery.179 FRC network remodeling is critical in enabling LN size change, as FRC interaction with C-type lectin receptor 2 (CLEC-2) on migratory dendritic cells relaxes the FRC cytoskeleton and enables its stretching.169 This FRC remodeling maintains the size restriction of molecular access to conduits but enhances conduit permeability, allowing soluble molecules in the conduits improved access to antigen presenting cells and advancing the immune response.180 LN vasculature also undergoes remodeling—BECs proliferate177 and the LN feed arteriole expands to recruit additional lymphocytes from circulation.181 These observed changes in physical structure and cellular properties and localization are critical to the normal immune response.
In disease and chronic inflammation, these normal processes can be interrupted. In HIV infection, for example, CD8+ T cells express high amounts of TGF-β1, inducing the deposition of collagen and subsequent LN fibrosis;182 this fibrosis is associated with damage to the LN T cell zone and limitation of CD4+ T cell populations.183 Enhanced collagen deposition and LN stiffening is observed in LNs draining melanoma tumors,165 and LN fibrosis associated with poor lymph flow through nodes has been observed in lymphedema patients.3,184,185 Fibrosis associated with such chronic inflammation and pathogen exposure can inhibit future immune responses and response to vaccination.166
Impaired Lymph Flow
Lymph flow through LNs from afferent lymphatic vessels is critical for the maintenance of normal LN structure (Figure 5B). Disruption of the afferent lymphatic vessels destroys the physical structure of the LN, causes the collapse of HEVs, and eliminates lymphocyte homing to LNs from the vasculature.4 In mice lacking dermal lymphatic vessels, and thus normal drainage to dermal LNs, LNs show similarly minimized HEVs, along with disorganized FRCs and scattered B cells, perhaps due to altered distribution of CXCL13,2 a chemokine that guides B cell organization. In lymphedema, LNs have been observed to show increased collagen and hyaluronic acid deposition, fibrosis, and lymphocyte depletion.3 These observations together highlight the importance of fluid flow through the LN in regulating the structure, and thus subsequent immune response, of the LN.
Biomaterials for Therapeutic Modulation and Remodeling of Lymph Nodes
Biomaterials enable targeted drug delivery to LN for the induction of therapeutic or investigative remodeling (Figure 6A). By taking advantage of size-based access of molecules to lymphatic vessels, and thus the LNs, biomaterials can be used to enhance the delivery of small molecules, antigen, cytokines, and other modulatory agents to the LN to regulate remodeling and the subsequent immune response. NP carriers from 20 to 100 nm in diameter drain primarily to lymphatic vessels after injection in the interstitial space as they are too large to enter the pores of the blood vasculature, with molecules on the smaller end of the range showing improved lymphatic uptake compared to larger molecules.186 Once in the LN, NPs can modulate the immune response by delivery of antigen, immunogenic agents, or tolerogenic agents. NPs are well-suited for delivery to the APCs that are critical in generating both immunity and tolerance, as APCs readily capture nanoscale particulates. Co-delivery of antigen and drugs by NPs thus promotes the simultaneous exposure of APCs to antigen and immunity- or tolerance-promoting agents to elicit the desired immune response.
Figure 6.
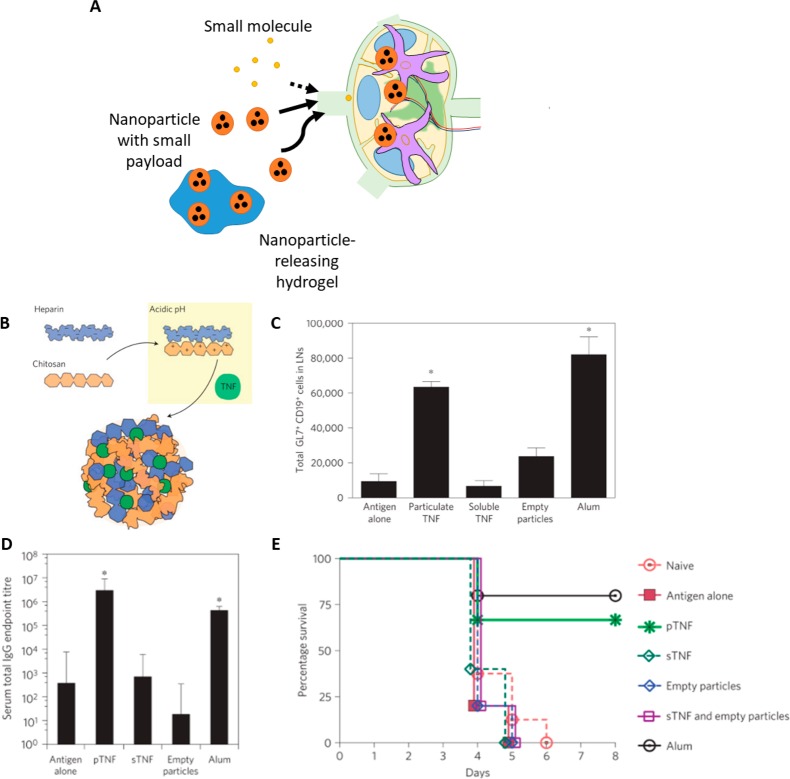
Biomaterials enable LN-targeted drug delivery to regulate LN remodeling and immune response. (A) While small molecules and proteins may poorly drain to lymph and yield low concentrations at target immune cells, nanoparticle formulations can enhance lymphatic drainage, enhance uptake by target immune cells, and precisely deliver combinations of payloads. (B) St. John et al. developed artificial mast cell granules composed of heparin that could be loaded with TNF-α and other drugs. (C) These synthetic granules increased germinal center formation, as measured by the total number of germinal center B cells, compared to controls. (D) After vaccination with hemeagglutinin, granule delivery resulted in improved antibody titers and (E) protected mice from lethal flu challenge. Panels B–E adapted with permission from ref (18). Copyright 2012 Springer Nature.
Particle systems have thus been employed for LN-targeted delivery of adjuvants and antigen to generate immunity. For example, synthetic particles designed to mimic mast cell granules have been developed.18 Mast cell granules are TNF-containing particles secreted by activated mast cells in peripheral inflammation. These particles easily enter lymphatic capillaries and carry their TNF payload to the draining lymph node, where they induce significant lymph node hypertrophy.187 Synthetic mast cell granules can recapitulate the properties of native granules; these heparin-based, TNF-loaded particles (Figure 6B) drained to LNs and induced LN remodeling and germinal center formation with increased numbers of activated B cells (Figure 6C) and improved antibody titers (Figure 6D), and showed improved adjuvant activity compared to free TNF, protecting treated mice against lethal flu challenge after vaccination (Figure 6E). Not only were these particles effective adjuvants, but they could also be loaded with IL-12 to polarize the resulting immune response toward a Th1 phenotype,18 highlighting the potential for simultaneous delivery of multiple cytokines to elicit LN remodeling and a desired immune response. Particle systems can also be used to codeliver an antigen along with stimulatory cytokines for the enhancement of the immune response. Multilamellar lipid vesicles have been used to codeliver VMP001 malaria antigen and monophosphoryl lipid A (MPLA) to LN, and induced improved LN remodeling in response to vaccination. These particles promoted germinal center formation where they accumulated in LNs, while soluble antigen delivery did not, and simultaneously increased proliferation of follicular helper T cells. They also generated a broader humoral response at a lower dose compared to soluble antigen.188
Other biomaterials can be used for the induction of tolerance. In one group, polymer microparticles that are retained in the LN upon intra-LN injection were loaded with myelin oligodendrocyte glycoprotein (MOG), a myelin peptide, and rapamycin, a common immunosuppressant, and delivered to LN in a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE).189 While particles loaded with rapamycin alone did not improve disease symptoms, codelivering both MOG and rapamycin resulted in systemic tolerance and reduced disease severity. The delivery of the particles to LN was critical in the improved outcomes; when MOG/rapamycin-loaded particles were injected intramuscularly rather than intra-LN, no clinical changes were observed, highlighting the importance of LN localization in the generated response.189 A polymeric NP system has also shown promise for tolerance induction when used to codeliver rapamycin and disease-specific antigen.19 Upon subcutaneous injection, the NP drain to LN and promote tolerance that alleviates symptoms in a variety of diseases, including hypersensitivity and EAE. Importantly, tolerance was only induced in the presence of encapsulated rapamycin; when antigen-loaded NPs were delivered with free rapamycin, the tolerogenic effects were lost, highlighting the importance of context and codelivery.19 Other biomaterials systems besides polymeric NPs have been used to deliver antigen alongside other tolerogenic drugs to suppress immune responses; for example, liposomes loaded with antigen and NF-κB inhibitors have also been delivered to LNs, where they are taken up by antigen-presenting cells and induce antigen-specific regulatory T cells to reduce symptoms of arthritis in mice.190
Similar approaches can be employed to deliver LN-targeted therapeutics for the activation of an anticancer immune response. One group employed a silica NP to codeliver CpG DNA and OVA antigen to LNs and observed significantly improved generation of antigen-specific T cells compared to soluble administration, in addition to improved humoral response. The NP formulation induced a protective response against an EG.7-OVA lymphoma, and reduced systemic CpG effects as evidenced by a reduction in spleen size.191 Polymeric NPs made of Pluronic F127 and poly(propylene sulfide) have also been employed for this purpose; when loaded with adjuvanting Toll-like receptor 4 and 9 ligands and injected in the limb ipsilateral to a B16–F10 melanoma (hence resulting in payload accumulation within LNs codraining the melanoma), they promoted dendritic cell maturation and the generation of antigen-specific T cells that reduced tumor size compared to free adjuvant.192
Particle systems have also been developed for the delivery of chemokines to modulate immune cell migration. Because cell migration is based on chemokine gradients, bulk release of chemokine may be insufficient to induce the desired trafficking. Particle delivery can overcome this by providing sustained release and producing necessary gradients of chemokines to mediate cell attraction in vitro(193) and in vivo.194 This approach has been used to induce neutrophil recruitment to LN.195 Hydrogel NPs were synthesized that codelivered interleukin 8 and macrophage inflammatory protein 1α. Upon subcutaneous injection, the particles were taken up into lymph and rapidly accumulated in draining LNs where they induced remodeling, causing subcapsular sinus expansion and an influx of neutrophils to the site, while chemokines delivered without the particle carrier showed a much reduced effect,195 highlighting the potential advantages in targeted, sustained release of chemokines that biomaterials can provide.
Peripherally implanted hydrogels can also be employed in drug delivery for LN remodeling in several contexts. Hydrogels are an excellent tool for providing sustained, local release of drug. Hydrogels can thus be employed as depots for sustained release of lymphatic-draining nanoparticles or molecules, providing spatially controlled delivery of drug-loaded nanoparticles for draining LN remodeling over longer time periods than a single injection of nanoparticles could provide. In one example, pH-sensitive polymeric nanogels containing 50 nm particles loaded with Toll-like receptor 7/8 agonist IMDQ were injected in the mouse footpad.196 Following, 50 nm particles were slowly released from the nanogel and delivered to the draining LN, where they improved the immune response to tuberculosis antigen. Filomicelle scaffolds can also be employed for this purpose,197 converting slowly to lymphatic-draining micellar delivery vehicles during gradual oxidation. The released micelles accumulate in LNs draining the site of injection and are taken up by LN-resident APCs. The use of technologies like these could further enhance current LN-targeted drug delivery systems by enabling the controlled release of NP drug carriers, removing the need for repeated bolus injections.
In addition to biomaterials designed to remodel existing LNs through LN-targeted drug delivery, biomaterials may also be implanted to generate new LN-like tissue. While the generation of LNs in vitro has not yet resulted in implantable lymphoid structures, lymphoid tissues have been generated in vivo using biomaterials approaches. The implantation of collagen scaffolds seeded with dendritic cells and thymic stromal cells expressing lymphotoxin alpha (LTα), a critical regulator of lymphoid tissue development, resulted in the formation of lymphoid-like organoids that showed distinct T and B cell zones, follicular DC networks, germinal centers, high endothelial venule-like structures, and were capable of mounting both humoral and cellular immune responses.198 Further studies revealed that these artificial LNs were capable of supporting antigen-specific secondary antibody responses, and that cells from the generated organoid migrated to the spleen and proliferated at the new location.199 Biomaterials approaches to generating lymphoid tissues in vivo is a young field, and there is much room for further investigation in this area.
While these examples are not exhaustive, they reveal the usefulness of a range of biomaterials including hydrogels and polymeric, silica, lipid, and protein NPs for delivering cargo to the LN, inducing LN remodeling, and regulating the subsequent immune response with improved outcomes compared to the delivery of soluble components, and highlight the potential of biomaterials for the in vivo generation of lymphoid tissue.
Conclusions
The lymphatic system is potently immunomodulatory, with critical roles including antigen transport, leukocyte trafficking, and direct immune cell regulation that make it a valuable target for regulating immune responses. Biomaterials are a powerful tool for lymphatic function regulation, enabling the carefully controlled delivery of cues to modulate lymphatic vessel growth and remodeling, fluid transport via vessel pumping, and LN structure, subsequently regulating the immune response for the treatment of a variety of pathologies. Though the myriad applications of biomaterials in lymphatic modulation and immunoengineering are beginning to be appreciated and experimentally explored, significant work remains to be done to translate these discoveries into the clinic. Applications of engineered biomaterials in immunoengineering via regulation of lymphatic structure and function will continue to expand as the role of the lymphatic system in immunomodulation is further clarified, regulatory molecular targets are identified, and novel biomaterials are developed.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant (R01CA207619), a (CCR15330478) grant from Susan G. Komen, US Department of Defense grant (CA150523), a grant from Winship Cancer Institute of Emory University, a Predoctoral Fellowship from the American Heart Association, and the GT BioMAT T32 grant (NIH T32EB006343).
The authors declare no competing financial interest.
References
- Pflicke H.; Sixt M. (2009) Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925–35. 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. N.; Rutkowski J. M.; Pasquier M.; Kuan E. L.; Alitalo K.; Randolph G. J.; Swartz M. A. (2012) Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 189, 2181–90. 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamatti S. S.; Narasimha A.; Janardhan J. V.; Chowdappa V. (2016) Lymph Node Fibrosis in a Case of Primary Lymphoedema- A Report of Two Cases. J. Clin. Diagnostic Res. 10, ED08–ED09. 10.7860/JCDR/2016/19528.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R. E.; Streeter P. R.; Brevé J.; Duijvestijn A. M.; Kraal G. (1991) The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 115, 85–95. 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel K.; Sasso M. S.; Potin L.; Swartz M. A. (2017) Exploiting lymphatic vessels for immunomodulation: Rationale, opportunities, and challenges. Adv. Drug Delivery Rev. 114, 43–59. 10.1016/j.addr.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo K. P.; Angeli V. (2017) Bidirectional crosstalk between lymphatic endothelial cell and T cell and its implications in tumor immunity. Front. Immunol. 8, 83. 10.3389/fimmu.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card C. M.; Yu S. S.; Swartz M. A. (2014) Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest. 124, 943–52. 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. A.; Jackson D. G. (2008) Cell Traffic and the Lymphatic Endothelium. Ann. N. Y. Acad. Sci. 1131, 119–133. 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- Onder L.; Narang P.; Scandella E.; Chai Q.; Iolyeva M.; Hoorweg K.; Halin C.; Richie E.; Kaye P.; Westermann J.; Cupedo T.; Coles M.; Ludewig B. (2012) IL-7-producing stromal cells are critical for lymph node remodeling. Blood 120, 4675–83. 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K. S.; Kieper W. C.; Jameson S. C.; Lefrançois L. (2000) Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–32. 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Lund A. W.; Duraes F. V.; Hirosue S.; Raghavan V. R.; Nembrini C.; Thomas S. N.; Issa A.; Hugues S.; Swartz M. A. (2012) VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell Rep. 1, 191–199. 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Shimaoka M.; Snoeck H.; Randolph G. J.; Podgrabinska S.; Kamalu O.; Mayer L. (2009) Mac-1/ICAM-1-Dependent Mechanism Dendritic Cell Maturation and Function via Inflamed Lymphatic Endothelium Suppresses and Mihaela Skobe. J. Immunol. 183, 1767–1779. 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm C.-L. E.; Zisch A.; Swartz M. A. (2007) Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol. Bioeng. 96, 167–176. 10.1002/bit.21185. [DOI] [PubMed] [Google Scholar]
- Bouta E. M.; McCarthy C. W.; Keim A.; Wang H. B.; Gilbert R. J.; Goldman J. (2011) Biomaterial guides for lymphatic endothelial cell alignment and migration. Acta Biomater. 7, 1104–13. 10.1016/j.actbio.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky C.; Zaitseva T. S.; Bazalova-Carter M.; Paukshto M. V.; Hou L.; Strassberg Z.; Ferguson J.; Matsuura Y.; Dash R.; Yang P. C.; Kretchetov S.; Vogt P. M.; Rockson S. G.; Cooke J. P.; Huang N. F. (2016) Aligned nanofibrillar collagen scaffolds-Guiding lymphangiogenesis for treatment of acquired lymphedema. Biomaterials 102, 259–267. 10.1016/j.biomaterials.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y.; Polavarapu R.; Eskla K.-L.; Pantner Y.; Nicholson C. K.; Ishii M.; Brunnhoelzl D.; Mauria R.; Husain A.; Naqvi N.; Murohara T.; Calvert J. W. (2018) Impact of Lymphangiogenesis on Cardiac Remodeling After Ischemia and Reperfusion Injury. J. Am. Heart Assoc. 7, e009565 10.1161/JAHA.118.009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güç E.; Briquez P. S.; Foretay D.; Fankhauser M. A.; Hubbell J. A.; Kilarski W. W.; Swartz M. A. (2017) Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 131, 160–175. 10.1016/j.biomaterials.2017.03.033. [DOI] [PubMed] [Google Scholar]
- St John A. L.; Chan C. Y.; Staats H. F.; Leong K. W.; Abraham S. N. (2012) Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 11, 250–7. 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. A.; LaMothe R. A.; Ferrari J. D.; Zhang A. H.; Rossi R. J.; Kolte P. N.; Griset A. P.; O’Neil C.; Altreuter D. H.; Browning E.; et al. (2015) Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 112, E156–E165. 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. (2011) The lymphatic vasculature in disease. Nat. Med. 17, 1371–1380. 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Randolph G. J.; Ivanov S.; Zinselmeyer B. H.; Scallan J. P. (2017) The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 35, 31–52. 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard-Mack C. L. (2006) Normal Structure, Function, and Histology of Lymph Nodes. Toxicol. Pathol. 34, 409–424. 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- Moore J. E.; Bertram C. D. (2018) Lymphatic System Flows. Annu. Rev. Fluid Mech. 50, 459–482. 10.1146/annurev-fluid-122316-045259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak T. C.; Schmid-Schönbein G. W.; Zweifach B. W. (1984) New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc. Res. 28, 95–112. 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Baluk P.; Fuxe J.; Hashizume H.; Romano T.; Lashnits E.; Butz S.; Vestweber D.; Corada M.; Molendini C.; Dejana E.; et al. (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–62. 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R.; Mebius R. E.; Kraal G. (2008) The conduit system of the lymph node. Int. Immunol. 20, 1483–1487. 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- Gretz J. E.; Norbury C. C.; Anderson A. O.; Proudfoot A. E.; Shaw S. (2000) Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–40. 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri L.; Fair-Mäkelä R.; Auvinen K.; Rantakari P.; Jalkanen S.; Ivaska J.; Salmi M. (2019) Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J. Clin. Invest. 129, 3086–3102. 10.1172/JCI125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G. J.; Angeli V.; Swartz M. A. (2005) Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628. 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Pullinger B. D.; Florey H. W. (1937) Proliferation of lymphatics in inflammation. J. Pathol. Bacteriol. 45, 157–170. 10.1002/path.1700450115. [DOI] [Google Scholar]
- Hirakawa S.; Brown L. F.; Kodama S.; Paavonen K.; Alitalo K.; Detmar M. (2006) VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–7. 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadras S. S.; Paul T.; Bertoncini J.; Brown L. F.; Muzikansky A.; Jackson D. G.; Ellwanger U.; Garbe C.; Mihm M. C.; Detmar M. (2003) Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 162, 1951–60. 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V.; Pajusola K.; Kaipainen A.; Chilov D.; Lahtinen I.; Kukk E.; Saksela O.; Kalkkinen N.; Alitalo K. (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290–298. 10.1002/j.1460-2075.1996.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M.; Kaipainen A.; Joukov V.; Meng X.; Lakso M.; Rauvala H.; Swartz M.; Fukumura D.; Jain R. K.; Alitalo K. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–5. 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Veikkola T.; Jussila L.; Makinen T.; Karpanen T.; Jeltsch M.; Petrova T. V.; Kubo H.; Thurston G.; McDonald D. M.; Achen M. G.; et al. (2001) Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20, 1223–31. 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D.; Angehrn Y.; Klein S.; Riccardi S.; Baenziger-Tobler N.; Otto V. I.; Pittelkow M.; Detmar M. (2013) Activation of the epidermal growth factor receptor promotes lymphangiogenesis in the skin. J. Dermatol. Sci. 71, 184–194. 10.1016/j.jdermsci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A.; Cardona A. S.; Tauber S.; Fink A. M.; Steiner A.; Pehamberger H.; Niederleithner H.; Petzelbauer P.; Gröger M.; Loewe R. (2013) Epidermal Growth Factor Facilitates Melanoma Lymph Node Metastasis by Influencing Tumor Lymphangiogenesis. J. Invest. Dermatol. 133, 230–238. 10.1038/jid.2012.272. [DOI] [PubMed] [Google Scholar]
- Chang L. K.; Garcia-Cardeña G.; Farnebo F.; Fannon M.; Chen E. J.; Butterfield C.; Moses M. A.; Mulligan R. C.; Folkman J.; Kaipainen A. (2004) Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl. Acad. Sci. U. S. A. 101, 11658–63. 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R.; Björndahl M. A.; Religa P.; Clasper S.; Garvin S.; Galter D.; Meister B.; Ikomi F.; Tritsaris K.; Dissing S.; et al. (2004) PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333–345. 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Tammela T.; Saaristo A.; Lohela M.; Morisada T.; Tornberg J.; Norrmén C.; Oike Y.; Pajusola K.; Thurston G.; Suda T.; et al. (2005) Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 105, 4642–8. 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J.; Hagendoorn J.; Padera T. P.; Hoshida T.; Nelson G.; Kashiwagi S.; Jain R. K.; Fukumura D. (2009) Endothelial Nitric Oxide Synthase Mediates Lymphangiogenesis and Lymphatic Metastasis. Cancer Res. 69, 2801–2809. 10.1158/0008-5472.CAN-08-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski J. M.; Boardman K. C.; Swartz M. A. (2006) Characterization of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Heart Circ. Physiol. 291, H1402. 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.; Aspelund A.; Alitalo K. (2014) Lymphangiogenic factors, mechanisms, and applications. J. Clin. Invest. 124, 878–87. 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque R. J. C.; Hayashi T.; Cho W. G.; Kleinman M. E.; Dridi S.; Takeda A.; Baffi J. Z.; Yamada K.; Kaneko H.; Green M. G.; et al. (2009) Alternatively spliced VEGF receptor-2 is an essential endogenous inhibitor of lymphatic vessels. Nat. Med. 15, 1023–1030. 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.; Tiem M.; Watkins R.; Cho Y. K.; Wang Y.; Olsen T.; Uehara H.; Mamalis C.; Luo L.; Oakey Z.; et al. (2013) Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 121, 4242–9. 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H.; Cho Y.; Simonis J.; Cahoon J.; Archer B.; Luo L.; Das S. K.; Singh N.; Ambati J.; Ambati B. K. (2013) Dual suppression of hemangiogenesis and lymphangiogenesis by splice-shifting morpholinos targeting vascular endothelial growth factor receptor 2 (KDR). FASEB J. 27, 76–85. 10.1096/fj.12-213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykänen A. I.; Sandelin H.; Krebs R.; Keränen M. A. I.; Tuuminen R.; Kärpänen T.; Wu Y.; Pytowski B.; Koskinen P. K.; Ylä-Herttuala S.; et al. (2010) Targeting Lymphatic Vessel Activation and CCL21 Production by Vascular Endothelial Growth Factor Receptor-3 Inhibition Has Novel Immunomodulatory and Antiarteriosclerotic Effects in Cardiac Allografts. Circulation 121, 1413–1422. 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- Krebs R.; Tikkanen J. M.; Ropponen J. O.; Jeltsch M.; Jokinen J. J.; Ylä-Herttuala S.; Nykänen A. I.; Lemström K. B. (2012) Critical role of VEGF-C/VEGFR-3 signaling in innate and adaptive immune responses in experimental obliterative bronchiolitis. Am. J. Pathol. 181, 1607–20. 10.1016/j.ajpath.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Alitalo A. K.; Proulx S. T.; Karaman S.; Aebischer D.; Martino S.; Jost M.; Schneider N.; Bry M.; Detmar M. (2013) VEGF-C and VEGF-D Blockade Inhibits Inflammatory Skin Carcinogenesis. Cancer Res. 73, 4212–4221. 10.1158/0008-5472.CAN-12-4539. [DOI] [PubMed] [Google Scholar]
- He Y.; Rajantie I.; Pajusola K.; Jeltsch M.; Holopainen T.; Yla-Herttuala S.; Harding T.; Jooss K.; Takahashi T.; et al. (2005) Vascular Endothelial Cell Growth Factor Receptor 3–Mediated Activation of Lymphatic Endothelium Is Crucial for Tumor Cell Entry and Spread via Lymphatic Vessels. Cancer Res. 65, 4739–4746. 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- He Y.; Kozaki K. -i.; Karpanen T.; Koshikawa K.; Yla-Herttuala S.; Takahashi T.; Alitalo K. (2002) Suppression of Tumor Lymphangiogenesis and Lymph Node Metastasis by Blocking Vascular Endothelial Growth Factor Receptor 3 Signaling. JNCI J. Natl. Cancer Inst. 94, 819–825. 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- Yang H.; Kim C.; Kim M.-J.; Schwendener R. A.; Alitalo K.; Heston W.; Kim I.; Kim W.-J.; Koh G. (2011) Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer 10, 36. 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Lalani A. S.; Harding T. C.; Gonzalez M.; Wu W.-W.; Luan B.; Tu G. H.; Koprivnikar K.; VanRoey M. J.; He Y.; et al. (2005) Inhibition of Lymphogenous Metastasis Using Adeno-Associated Virus-Mediated Gene Transfer of a Soluble VEGFR-3 Decoy Receptor. Cancer Res. 65, 6901–6909. 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- Gogineni A.; Caunt M.; Crow A.; Lee C. V.; Fuh G.; van Bruggen N.; Ye W.; Weimer R. M. (2013) Inhibition of VEGF-C Modulates Distal Lymphatic Remodeling and Secondary Metastasis. PLoS One 8, e68755. 10.1371/journal.pone.0068755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima K.; Watanabe M.; Satoh Y.; Hata J.; Ishii N.; Aoki Y. (2012) Inhibition of lymphatic metastasis in neuroblastoma by a novel neutralizing antibody to vascular endothelial growth factor-D. Cancer Sci 103, 2144–2152. 10.1111/cas.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N.; Kloos B.; Cassella M.; Podgrabinska S.; Persaud K.; Wu Y.; Pytowski B.; Skobe M. (2006) Inhibition of VEGFR-3 Activation with the Antagonistic Antibody More Potently Suppresses Lymph Node and Distant Metastases than Inactivation of VEGFR-2. Cancer Res. 66, 2650–2657. 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Pytowski B.; Goldman J.; Persaud K.; Wu Y.; Witte L.; Hicklin D. J.; Skobe M.; Boardman K. C.; Swartz M. A. (2005) Complete and Specific Inhibition of Adult Lymphatic Regeneration by a Novel VEGFR-3 Neutralizing Antibody. J. Natl. Cancer Inst. 97, 14–21. 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Kubo H.; Yamaguchi K.; Kawashima K.; Ueda Y.; Matsuo K.; Awane M.; Shimahara Y.; Takabayashi A.; Yamaoka Y.; et al. (2004) Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 95, 328–333. 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A.; Blanc I.; Gueguen-Dorbes G.; Duclos O.; Bonnin J.; Barron P.; Laplace M.-C.; Morin G.; Gaujarengues F.; Dol F.; et al. (2012) SAR131675, a Potent and Selective VEGFR-3-TK Inhibitor with Antilymphangiogenic, Antitumoral, and Antimetastatic Activities. Mol. Cancer Ther. 11, 1637–1649. 10.1158/1535-7163.MCT-11-0866-T. [DOI] [PubMed] [Google Scholar]
- Heckman C. A.; Holopainen T.; Wirzenius M.; Keskitalo S.; Jeltsch M.; Yla-Herttuala S.; Wedge S. R.; Jurgensmeier J. M.; Alitalo K. (2008) The Tyrosine Kinase Inhibitor Cediranib Blocks Ligand-Induced Vascular Endothelial Growth Factor Receptor-3 Activity and Lymphangiogenesis. Cancer Res. 68, 4754–4762. 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- Caunt M.; Mak J.; Liang W.-C.; Stawicki S.; Pan Q.; Tong R. K.; Kowalski J.; Ho C.; Reslan H. B.; Ross J.; et al. (2008) Blocking Neuropilin-2 Function Inhibits Tumor Cell Metastasis. Cancer Cell 13, 331–342. 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Lee E.; Koskimaki J. E.; Pandey N. B.; Popel A. S. (2013) Inhibition of Lymphangiogenesis and Angiogenesis in Breast Tumor Xenografts and Lymph Nodes by a Peptide Derived from Transmembrane Protein 45A. Neoplasia 15, 112–IN6. 10.1593/neo.121638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abengozar M. A.; de Frutos S.; Ferreiro S.; Soriano J.; Perez-Martinez M.; Olmeda D.; Marenchino M.; Canamero M.; Ortega S.; Megias D.; et al. (2012) Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 119, 4565–4576. 10.1182/blood-2011-09-380006. [DOI] [PubMed] [Google Scholar]
- Holopainen T.; Saharinen P.; D’Amico G.; Lampinen A.; Eklund L.; Sormunen R.; Anisimov A.; Zarkada G.; Lohela M.; Heloterä H.; et al. (2012) Effects of Angiopoietin-2-Blocking Antibody on Endothelial Cell–Cell Junctions and Lung Metastasis. J. Natl. Cancer Inst. 104, 461–475. 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M.; Ramachandran S.; Kim E. Y.; Allegood J. C.; Rashid O. M.; Yamada A.; Zhao R.; Milstien S.; Zhou H.; Spiegel S.; et al. (2012) Sphingosine-1-Phosphate Produced by Sphingosine Kinase 1 Promotes Breast Cancer Progression by Stimulating Angiogenesis and Lymphangiogenesis. Cancer Res. 72, 726–735. 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo W.; Jia L.; Song N.; Lu X. -a.; Ding Y.; Wang X.; Song X.; Fu Y.; Luo Y. (2012) The CXCL12-CXCR4 Chemokine Pathway: A Novel Axis Regulates Lymphangiogenesis. Clin. Cancer Res. 18, 5387–5398. 10.1158/1078-0432.CCR-12-0708. [DOI] [PubMed] [Google Scholar]
- Cursiefen C.; Maruyama K.; Bock F.; Saban D.; Sadrai Z.; Lawler J.; Dana R.; Masli S. (2011) Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 208, 1083–1092. 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P.; Yao L. C.; Feng J.; Romano T.; Jung S. S.; Schreiter J. L.; Yan L.; Shealy D. J.; McDonald D. M. (2009) TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Invest. 119, 2954–2964. 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.; Marsh C. A.; Dorsam R. T.; Mikelis C. M.; Masedunskas A.; Amornphimoltham P.; Nathan C. A.; Singh B.; Weigert R.; Molinolo A. A.; et al. (2011) Decreased Lymphangiogenesis and Lymph Node Metastasis by mTOR Inhibition in Head and Neck Cancer. Cancer Res. 71, 7103–7112. 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S.; Kishimoto T.; Kamata S.; Otsuka M.; Miyazaki M.; Ishikura H. (2007) Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 98, 726–733. 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T.; Shayan R.; Caesar C.; Roufail S.; Harris N. C.; Ardipradja K.; Zhang Y. F.; Williams S. P.; Farnsworth R. H.; Chai M. G.; et al. (2012) VEGF-D Promotes Tumor Metastasis by Regulating Prostaglandins Produced by the Collecting Lymphatic Endothelium. Cancer Cell 21, 181–195. 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Tammela T.; Saaristo A.; Holopainen T.; Ylä-Herttuala S.; Andersson L. C.; Virolainen S.; Immonen I.; Alitalo K. (2011) Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 3, 69ra11. 10.1126/scitranslmed.3001699. [DOI] [PubMed] [Google Scholar]
- Karkkainen M. J.; Saaristo A.; Jussila L.; Karila K. A.; Lawrence E. C.; Pajusola K.; Bueler H.; Eichmann A.; Kauppinen R.; Kettunen M. I.; et al. (2001) A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. U. S. A. 98, 12677–12682. 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.; Murayama T.; Gravereaux E.; Tkebuchava T.; Silver M.; Curry C.; Wecker A.; Kirchmair R.; Hu C. S.; Kearney M.; et al. (2003) VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J. Clin. Invest. 111, 717–725. 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T.; Saaristo A.; Holopainen T.; Lyytikkä J.; Kotronen A.; Pitkonen M.; Abo-Ramadan U.; Ylä-Herttuala S.; Petrova T. V.; Alitalo K. (2007) Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 13, 1458–1466. 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- Hartiala P.; Saaristo A. M. (2010) Growth Factor Therapy and Autologous Lymph Node Transfer in Lymphedema. Trends Cardiovasc. Med. 20, 249–253. 10.1016/j.tcm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Lähteenvuo M.; Honkonen K.; Tervala T.; Tammela T.; Suominen E.; Lähteenvuo J.; Kholová I.; Alitalo K.; Ylä-Herttuala S.; Saaristo A. (2011) Growth Factor Therapy and Autologous Lymph Node Transfer in Lymphedema. Circulation 123, 613–620. 10.1161/CIRCULATIONAHA.110.965384. [DOI] [PubMed] [Google Scholar]
- Honkonen K. M.; Visuri M. T.; Tervala T. V.; Halonen P. J.; Koivisto M.; Lähteenvuo M. T.; Alitalo K. K.; Ylä-Herttuala S.; Saaristo A. M. (2013) Lymph Node Transfer and Perinodal Lymphatic Growth Factor Treatment for Lymphedema. Ann. Surg. 257, 961–967. 10.1097/SLA.0b013e31826ed043. [DOI] [PubMed] [Google Scholar]
- Lim H. Y.; Thiam C. H.; Yeo K. P.; Bisoendial R.; Hii C. S.; McGrath K. C. Y.; Tan K. W.; Heather A.; Alexander J. S. J.; Angeli V. (2013) Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 17, 671–684. 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Kim H.; Kataru R. P.; Koh G. Y. (2014) Inflammation-associated lymphangiogenesis: a double-edged sword?. J. Clin. Invest. 124, 936–42. 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouelkheir G. R.; Upchurch B. D.; Rutkowski J. M. (2017) Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation’s fire?. Exp. Biol. Med. (London, U. K.) 242, 884–895. 10.1177/1535370217697385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P.; Tammela T.; Ator E.; Lyubynska N.; Achen M. G.; Hicklin D. J.; Jeltsch M.; Petrova T. V.; Pytowski B.; Stacker S. A.; et al. (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115, 247–57. 10.1172/JCI200522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuht S.; Gwinner W.; Franz I.; Schwarz A.; Jonigk D.; Kreipe H.; Kerjaschki D.; Haller H.; Mengel M. (2007) Lymphatic neoangiogenesis in human renal allografts: Results from sequential protocol biopsies. Am. J. Transplant. 7, 377–384. 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- Dietrich T.; Bock F.; Yuen D.; Hos D.; Bachmann B. O.; Zahn G.; Wiegand S.; Chen L.; Cursiefen C. (2010) Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol. 184, 535–9. 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin N.; Zhang N.; Xu J.; Shi Q.; Ding Y.; Bromberg J. S. (2011) Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation 92, 25–30. 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C.; Cao J.; Chen L.; Liu Y.; Maruyama K.; Jackson D.; Kruse F. E.; Wiegand S. J.; Dana M. R.; Streilein J. W. (2004) Inhibition of Hemangiogenesis and Lymphangiogenesis after Normal-Risk Corneal Transplantation by Neutralizing VEGF Promotes Graft Survival. Invest. Ophthalmol. Visual Sci. 45, 2666. 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D.; Regele H. M.; Moosberger I.; Nagy-Bojarski K.; Watschinger B.; Soleiman A.; Birner P.; Krieger S.; Hovorka A.; Silberhumer G.; et al. (2004) Lymphatic Neoangiogenesis in Human Kidney Transplants Is Associated with Immunologically Active Lymphocytic Infiltrates. J. Am. Soc. Nephrol. 15, 603–612. 10.1097/01.ASN.0000113316.52371.2E. [DOI] [PubMed] [Google Scholar]
- Salven P.; Lymboussaki A.; Heikkilä P.; Jääskela-Saari H.; Enholm B.; Aase K.; von Euler G.; Eriksson U.; Alitalo K.; Joensuu H. (1998) Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am. J. Pathol. 153, 103–8. 10.1016/S0002-9440(10)65550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haessler U.; Teo J. C. M.; Foretay D.; Renaud P.; Swartz M. A. (2012) Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr. Biol. 4, 401–409. 10.1039/c1ib00128k. [DOI] [PubMed] [Google Scholar]
- Gunn M. D.; Tangemann K.; Tam C.; Cyster J. G.; Rosen S. D.; Williams L. T. (1998) A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 95, 258–63. 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J. D.; Fleury M. E.; Yong C.; Tomei A. A.; Randolph G. J.; Swartz M. A. (2007) Autologous Chemotaxis as a Mechanism of Tumor Cell Homing to Lymphatics via Interstitial Flow and Autocrine CCR7 Signaling. Cancer Cell 11, 526–538. 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Choi J. U.; Chung S. W.; Al-Hilal T. A.; Alam F.; Park J.; Mahmud F.; Jeong J.-H.; Kim S. Y.; Byun Y. (2017) A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials 139, 56–66. 10.1016/j.biomaterials.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Lund A. W.; Wagner M.; Fankhauser M.; Steinskog E. S.; Broggi M. A.; Spranger S.; Gajewski T. F.; Alitalo K.; Eikesdal H. P.; Wiig H.; et al. (2016) Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest. 126, 3389–3402. 10.1172/JCI79434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser M.; Broggi M. A. S.; Potin L.; Bordry N.; Jeanbart L.; Lund A. W.; Da Costa E.; Hauert S.; Rincon-Restrepo M.; Tremblay C.; et al. (2017) Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 9, eaal4712. 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- Muchowicz A.; Wachowska M.; Stachura J.; Tonecka K.; Gabrysiak M.; Wołosz D.; Pilch Z.; Kilarski W. W.; Boon L.; Klaus T. J.; et al. (2017) Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur. J. Cancer 83, 19–27. 10.1016/j.ejca.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Alishekevitz D.; Gingis-Velitski S.; Kaidar-Person O.; Gutter-Kapon L.; Scherer S. D.; Raviv Z.; Merquiol E.; Ben-Nun Y.; Miller V.; Rachman-Tzemah C.; et al. (2016) Macrophage-Induced Lymphangiogenesis and Metastasis following Paclitaxel Chemotherapy Is Regulated by VEGFR3. Cell Rep. 17, 1344–1356. 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K.; Rockson S. G. (2008) The role of the lymphatic circulation in the natural history and expression of cardiovascular disease. Int. J. Cardiol. 129, 309–317. 10.1016/j.ijcard.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Aspelund A.; Robciuc M. R.; Karaman S.; Makinen T.; Alitalo K. (2016) Lymphatic System in Cardiovascular Medicine. Circ. Res. 118, 515–30. 10.1161/CIRCRESAHA.115.306544. [DOI] [PubMed] [Google Scholar]
- Ludwig L. L.; Schertel E. R.; Pratt J. W.; McClure D. E.; Ying A. J.; Heck C. F.; Myerowitz P. D. (1997) Impairment of left ventricular function by acute cardiac lymphatic obstruction. Cardiovasc. Res. 33, 164–171. 10.1016/S0008-6363(96)00177-0. [DOI] [PubMed] [Google Scholar]
- Gloviczki P.; Solti F.; Szlavy L.; Jellinek H. (1983) Ultrastructural and electrophysiologic changes of experimental acute cardiac lymphostasis. Lymphology 16, 185–92. [PubMed] [Google Scholar]
- Sun S. C.; Lie J. T. (1977) Cardiac lymphatic obstruction: ultrastructure of acute-phase myocardial injury in dogs. Mayo Clin. Proc. 52, 785–92. [PubMed] [Google Scholar]
- Laine G. A.; Allen S. J. (1991) Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ. Res. 68, 1713–21. 10.1161/01.RES.68.6.1713. [DOI] [PubMed] [Google Scholar]
- Randolph G. J.; Miller N. E. (2014) Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124, 929–935. 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C.; Li W.; Fulp B.; Platt A. M.; Gautier E. L.; Westerterp M.; Bittman R.; Tall A. R.; Chen S.-H.; Thomas M. J.; et al. (2013) Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123, 1571–9. 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri O.; Pouehe C.; Houssari M.; Galas L.; Nicol L.; Edwards-Lévy F.; Henry J.-P.; Dumesnil A.; Boukhalfa I.; Banquet S.; et al. (2016) Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 133, 1484–1497. 10.1161/CIRCULATIONAHA.115.020143. [DOI] [PubMed] [Google Scholar]
- Taira A.; Uehara K.; Fukuda S.; Takenaka K.; Koga M. (1990) Active Drainage of Cardiac Lymph in Relation to Reduction in Size of Myocardial Infarction: - An Experimental Study. Angiology 41, 1029–1036. 10.1177/000331979004101202. [DOI] [PubMed] [Google Scholar]
- Becker C.; Assouad J.; Riquet M.; Hidden G. (2006) Postmastectomy Lymphedema Long-term Results Following Microsurgical Lymph Node Transplantation. Ann. Surg. 243, 313–315. 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman M. A.; Chalberg T. W.; Schaffer D. V. (2015) Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 17, 63–89. 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- Tsimboukis S.; Merikas I.; Karapanagiotou E. M.; Saif M. W.; Syrigos K. N. (2009) Erlotinib-Induced Skin Rash in Patients with Non–Small-Cell Lung Cancer: Pathogenesis, Clinical Significance, and Management. Clin. Lung Cancer 10, 106–111. 10.3816/CLC.2009.n.013. [DOI] [PubMed] [Google Scholar]
- Roé E.; García Muret M. P.; Marcuello E.; Capdevila J.; Pallarés C.; Alomar A. (2006) Description and management of cutaneous side effects during cetuximab or erlotinib treatments: A prospective study of 30 patients. J. Am. Acad. Dermatol. 55, 429–437. 10.1016/j.jaad.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Kim I. G.; Lee J. Y.; Lee D. S.; Kwon J. Y.; Hwang J. H. (2013) Extracorporeal Shock Wave Therapy Combined with Vascular Endothelial Growth Factor-C Hydrogel for Lymphangiogenesis. J. Vasc. Res. 50, 124–133. 10.1159/000343699. [DOI] [PubMed] [Google Scholar]
- Yan A.; Avraham T.; Zampell J. C.; Haviv Y. S.; Weitman E.; Mehrara B. J. (2011) Adipose-derived stem cells promote lymphangiogenesis in response to VEGF-C stimulation or TGF-β1 inhibition. Future Oncol. 7, 1457–73. 10.2217/fon.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin C.; Overney J.; Shieh A. C.; Dixon J. B.; Swartz M. A. (2010) A multichamber fluidic device for 3D cultures under interstitial flow with live imaging: Development, characterization, and applications. Biotechnol. Bioeng. 105, 982–991. [DOI] [PubMed] [Google Scholar]
- Marino D.; Luginbühl J.; Scola S.; Meuli M.; Reichmann E. (2014) Bioengineering Dermo-Epidermal Skin Grafts with Blood and Lymphatic Capillaries. Sci. Transl. Med. 6, 221ra14. 10.1126/scitranslmed.3006894. [DOI] [PubMed] [Google Scholar]
- Abdalla S.; Makhoul G.; Duong M.; Chiu R. C. J.; Cecere R. (2013) Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction. Interact. Cardiovasc. Thorac. Surg. 17, 767–772. 10.1093/icvts/ivt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T. t.; Jiang Z. h.; Li S. l.; Zhou G. d.; Kretlow J. D.; Cao W. g.; Liu W.; Cao Y. l. (2010) Reconstruction of lymph vessel by lymphatic endothelial cells combined with polyglycolic acid scaffolds: A pilot study. J. Biotechnol. 150, 182–189. 10.1016/j.jbiotec.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Huang N. F.; Okogbaa J.; Lee J. C.; Jha A.; Zaitseva T. S.; Paukshto M. V.; Sun J.; Punjya N.; Fuller G. G.; Cooke J. P. (2013) The Modulation of Endothelial Cell Morphology, Function, and Survival Using Anisotropic Nanofibrillar Collagen Scaffolds. Biomaterials 34, 4038–4047. 10.1016/j.biomaterials.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. T.; Hadley D. J.; Kukis D. L.; Silva E. A. (2017) Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications. PLoS One 12, e0181484. 10.1371/journal.pone.0181484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. H.; Kim I. G.; Lee J. Y.; Piao S.; Lee D. S.; Lee T. S.; Ra J. C.; Lee J. Y. (2011) Therapeutic lymphangiogenesis using stem cell and VEGF-C hydrogel. Biomaterials 32, 4415–4423. 10.1016/j.biomaterials.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D.; Huttary N.; Raab I.; Regele H.; Bojarski-Nagy K.; Bartel G.; Kröber S. M.; Greinix H.; Rosenmaier A.; Karlhofer F.; et al. (2006) Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234. 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- Maruyama K.; Ii M.; Cursiefen C.; Jackson D. G.; Keino H.; Tomita M.; Rooijen N. V.; Takenaka H.; D’amore P. A.; Stein-Streilein J. (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. K.; Uehara H.; Young J. R.; Tyagi P.; Kompella U. B.; Zhang X.; Luo L.; Singh N.; Archer B.; Ambati B. K. (2012) Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest. Ophthalmol. Visual Sci. 53, 2328–36. 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Wang G.; Tan Y.; Wang H. (2014) Inhibition of Lymphangiogenesis of Endothelial Progenitor Cells with VEGFR-3 siRNA Delivered with PEI-alginate Nanoparticles. Int. J. Biol. Sci. 10, 160–170. 10.7150/ijbs.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.-W.; Liu T.; Xiao Y.; Feng Y.-L.; Cheng D.-J.; Tingting G.; Zhang L.; Zhang Y.; Chen Y.-X.; Tingting G.; et al. (2009) Vascular Endothelial Growth Factor-C siRNA Delivered via Calcium Carbonate Nanoparticle Effectively Inhibits Lymphangiogenesis and Growth of Colorectal Cancer In Vivo. Cancer Biother.Radiopharm. 24, 249–259. 10.1089/cbr.2008.0515. [DOI] [PubMed] [Google Scholar]
- von der Weid P.-Y.; Zawieja D. C. (2004) Lymphatic smooth muscle: the motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 36, 1147–1153. 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Telinius N.; Mohanakumar S.; Majgaard J.; Kim S.; Pilegaard H.; Pahle E.; Nielsen J.; de Leval M.; Aalkjaer C.; Hjortdal V.; et al. (2014) Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J. Physiol. 592, 4697–714. 10.1113/jphysiol.2014.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Roizes S.; von der Weid P.-Y. (2014) Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J. Physiol. 592, 5409–27. 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telinius N.; Kim S.; Pilegaard H.; Pahle E.; Nielsen J.; Hjortdal V.; Aalkjaer C.; Boedtkjer D. B. (2014) The contribution of K + channels to human thoracic duct contractility. Am. J. Physiol. Circ. Physiol. 307, H33–H43. 10.1152/ajpheart.00921.2013. [DOI] [PubMed] [Google Scholar]
- Haddy F.; Scott J.; Grega G. (1972) Effects of histamine on lymph protein concentration and flow in the dog forelimb. Am. J. Physiol. 223, 1172–1177. 10.1152/ajplegacy.1972.223.5.1172. [DOI] [PubMed] [Google Scholar]
- Fox J. L. R.; von der Weid P.-Y. (2002) Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br. J. Pharmacol. 136, 1210–8. 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. G.; Gordon J. L. (1981) Regulation of lymphatic contractility by arachidonate metabolites. Nature 293, 294. 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- Tian W.; Rockson S. G.; Jiang X.; Kim J.; Begaye A.; Shuffle E. M.; Tu A. B.; Cribb M.; Nepiyushchikh Z.; Feroze A. H.; et al. (2017) Leukotriene B4antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 9, eaal3920. 10.1126/scitranslmed.aal3920. [DOI] [PubMed] [Google Scholar]
- Liao S.; Cheng G.; Conner D. A.; Huang Y.; Kucherlapati R. S.; Munn L. L.; Ruddle N. H.; Jain R. K.; Fukumura D.; Padera T. P. (2011) Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. U. S. A. 108, 18784–9. 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler M.; Kassis T.; Dixon J. B. (2012) Sensitivity analysis of near-infrared functional lymphatic imaging. J. Biomed. Opt. 17, 066019. 10.1117/1.JBO.17.6.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen H. G.; Gasheva O. Y.; Zawieja D. C. (2011) Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am. J. Physiol. Heart Circ. Physiol. 301, H1897–906. 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendoorn J.; Padera T. P.; Kashiwagi S.; Isaka N.; Noda F.; Lin M. I.; Huang P. L.; Sessa W. C.; Fukumura D.; Jain R. K. (2004) Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 95, 204–209. 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- Mizuno R.; Koller A.; Kaley G. (1998) Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins R790. 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Ohhashi T.; Azuma T. (1984) Variegated effects of prostaglandins on spontaneous activity in bovine mesenteric lymphatics. Microvasc. Res. 27, 71–80. 10.1016/0026-2862(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama S. N.; Benoit J. N. (1996) Effects of bradykinin on lymphatic pumping in rat mesentery. Am. J. Physiol. G752. [DOI] [PubMed] [Google Scholar]
- Plaku K. J.; Von Der Weid P.-Y. (2006) Mast Cell Degranulation Alters Lymphatic Contractile Activity Through Action of Histamine. Microcirculation 13, 219–227. 10.1080/10739680600556902. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R.; Cheung L.; Han J.; Swanson J.; Beilhack A.; An A.; Dadras S. S.; Rockson N.; Joshi S.; Wagner R. (2006) Inflammatory Manifestations of Experimental Lymphatic Insufficiency Methods and Findings. PLoS Med. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S.; Sabine A.; Petrova T. V. (2011) Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–18. 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen M. J.; Ferrell R. E.; Lawrence E. C.; Kimak M. A.; Levinson K. L.; Mctigue M. A.; Alitalo K.; Finegold D. N. (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153. 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Fang J.; Dagenais S. L.; Erickson R. P.; Arlt M. F.; Glynn M. W.; Gorski J. L.; Seaver L. H.; Glover T. W. (2000) Mutations in FOXC2 (MFH-1), a Forkhead Family Transcription Factor, Are Responsible for the Hereditary Lymphedema-Distichiasis Syndrome. Am. J. Hum. Genet. 67, 1382–1388. 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armer J. M. (2005) The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 23, 76–83. 10.1081/CNV-48707. [DOI] [PubMed] [Google Scholar]
- Nutman T. B. (2013) Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphatic Res. Biol. 11, 144–8. 10.1089/lrb.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski W. L. (2002) Contractility Patterns of Normal and Pathologically Changed Human Lymphatics. Ann. N. Y. Acad. Sci. 979, 52–63. 10.1111/j.1749-6632.2002.tb04867.x. [DOI] [PubMed] [Google Scholar]
- Modi S.; Stanton A. W. B.; Svensson W. E.; Peters A. M.; Mortimer P. S.; Levick J. R. (2007) Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J. Physiol. 583, 271–285. 10.1113/jphysiol.2007.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyer L. K.; Varnic M.; Le L. W.; Leong W.; McCready D. (2010) Obesity is a Risk Factor for Developing Postoperative Lymphedema in Breast Cancer Patients. Breast J. 16, 48–54. 10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Greene A. K.; Grant F. D.; Slavin S. A. (2012) Lower-Extremity Lymphedema and Elevated Body-Mass Index. N. Engl. J. Med. 366, 2136–2137. 10.1056/NEJMc1201684. [DOI] [PubMed] [Google Scholar]
- Blum K. S.; Karaman S.; Proulx S. T.; Ochsenbein A. M.; Luciani P.; Leroux J.-C.; Wolfrum C.; Detmar M. (2014) Chronic High-Fat Diet Impairs Collecting Lymphatic Vessel Function in Mice. PLoS One 9, e94713. 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman E. S.; Aschen S. Z.; Farias-Eisner G.; Albano N.; Cuzzone D. A.; Ghanta S.; Zampell J. C.; Thorek D.; Mehrara B. J. (2013) Obesity Impairs Lymphatic Fluid Transport and Dendritic Cell Migration to Lymph Nodes. PLoS One 8, e70703. 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi J. S.; Hespe G. E.; Cuzzone D. A.; Savetsky I. L.; Nitti M. D.; Gardenier J. C.; Nores G. D. G.; Jowhar D.; Kataru R. P.; Mehrara B. J. (2016) Inhibition of Inflammation and iNOS Improves Lymphatic Function in Obesity. Sci. Rep. 6, 1–12. 10.1038/srep19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan E. L.; Ivanov S.; Bridenbaugh E. A.; Victora G.; Wang W.; Childs E. W.; Platt A. M.; Jakubzick C. V.; Mason R. J.; Gashev A. A.; et al. (2015) Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J. Immunol. 194, 5200–10. 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan J. P.; Hill M. A.; Davis M. J. (2015) Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc. Res. 107, 89–97. 10.1093/cvr/cvv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul M. E.; Thomas P. A.; Dosen P. J.; Isbister G. K.; O’Leary M. A.; Whyte I. M.; McFadden S. A.; van Helden D. F. (2011) A pharmacological approach to first aid treatment for snakebite. Nat. Med. 17, 809–811. 10.1038/nm.2382. [DOI] [PubMed] [Google Scholar]
- Datar S. A.; Oishi P. E.; Gong W.; Bennett S. H.; Sun C. E.; Johengen M.; Maki J.; Johnson R. C.; Raff G. W.; Fineman J. R. (2014) Altered reactivity and nitric oxide signaling in the isolated thoracic duct from an ovine model of congenital heart disease with increased pulmonary blood flow. Am. J. Physiol. Circ. Physiol. 306, H954–H962. 10.1152/ajpheart.00841.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashina K.; Tsubosaka Y.; Nakamura T.; Omori K.; Kobayashi K.; Hori M.; Ozaki H.; Murata T. (2015) Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS One 10, e0132367. 10.1371/journal.pone.0132367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. (2005) Nitric oxide as a regulator of inflammatory processes. Mem Inst Oswaldo Cruz 100, 5–9. 10.1590/S0074-02762005000900002. [DOI] [PubMed] [Google Scholar]
- Liu H.; Moynihan K. D.; Zheng Y.; Szeto G. L.; Li A. V.; Huang B.; Van Egeren D. S.; Park C.; Irvine D. J. (2014) Structure-based Programming of Lymph Node Targeting in Molecular Vaccines. Nature 507, 519–522. 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schudel A.; Sestito L. F.; Thomas S. N. (2018) S-nitrosated poly(propylene sulfide) nanoparticles for enhanced nitric oxide delivery to lymphatic tiss. J. Biomed. Mater. Res., Part A 106, 1463–1475. 10.1002/jbm.a.36348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S.; Vannberg F. O.; Dixon J. B. (2016) Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 6, 24436 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Zou L. G.; Zhang S.; Gong M. F.; Zhang D.; Qi Y. Y.; Zhou S. W.; Diao X. W. (2013) Feasibility of MR imaging in evaluating breast cancer lymphangiogenesis using Polyethylene glycol-GoldMag nanoparticles. Clin. Radiol. 68, 1233–1240. 10.1016/j.crad.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Robichaux J. L.; Tanno E.; Rappleye J. W.; Ceballos M.; Stallcup W. B.; Schmid-Schönbein G. W.; Murfee W. L. (2010) Lymphatic/Blood Endothelial Cell Connections at the Capillary Level in Adult Rat Mesentery. Anat. Rec. 293, 1629–1638. 10.1002/ar.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N. A.; McClain J.; Tuell S. L.; Warner A.; Smith B.; Yun Y.; Mohan A.; Sushnitha M.; Thomas S. N. (2015) Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 29, 4512–4522. 10.1096/fj.15-274761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kityo C.; Makamdop K. N.; Rothenberger M.; Chipman J. G.; Hoskuldsson T.; Beilman G. J.; Grzywacz B.; Mugyenyi P.; Ssali F.; Akondy R. S.; et al. (2018) Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Invest. 128, 2763–2773. 10.1172/JCI97377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel I. I.; Nasser R.; Garcia-Sabaté A.; Sapudom J.; Ma C.; Chen W.; Teo J. C. M. (2018) Deconstructing Immune Microenvironments of Lymphoid Tissues for Reverse Engineering. Adv. Healthcare Mater. 8, 1801126. 10.1002/adhm.201801126. [DOI] [PubMed] [Google Scholar]
- Malhotra D.; Fletcher A. L.; Astarita J.; Lukacs-Kornek V.; Tayalia P.; Gonzalez S. F.; Elpek K. G.; Chang S. K.; Knoblich K.; et al. (2012) Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13, 499–510. 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton S. E.; Reis e Sousa C. (2016) Dendritic cells in remodeling of lymph nodes during immune responses. Immunol. Rev. 271, 221–229. 10.1111/imr.12414. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. M. (1994) Germinal Centers. Annu. Rev. Immunol. 12, 117–139. 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Acton S. E.; Farrugia A. J.; Astarita J. L.; Mourão-Sá D.; Jenkins R. P.; Nye E.; Hooper S.; Van Blijswijk J.; Rogers N. C.; Snelgrove K. J.; Rosewell I.; Moita L. F.; Stamp G.; Turley S. J.; Sahai E.; Reis E Sousa C. (2014) Dendritic Cells Control Fibroblastic Reticular Network Tension and Lymph Node Expansion. Nature 514, 498–502. 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford C. D.; Dieterich L. C.; He Y.; Hell T.; Montoya-Zegarra J. A.; Noerrelykke S. F.; Russo E.; Röcken M.; Detmar M. (2018) Mechanisms of Tumor-Induced Lymphovascular Niche Formation in Draining Lymph Nodes. Cell Rep. 25, 3554–3563. 10.1016/j.celrep.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel A.; Shorthouse D.; Haas L.; Hall B. A.; Shields J. (2016) Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 17, 1118–1127. 10.1038/ni.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J. L.; Roman S. S.; Wickline S. A. (2011) Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 71, 3792–3801. 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- Lee S. Y.; Qian C.-N.; Ooi A. S.; Chen P.; Wong H. M. B.; Myint S. S.; Wong J. C.; Hwang S. G. J.; Soo K. C. (2012) Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J. Transl. Med. 10, 206. 10.1186/1479-5876-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C.-N.; Berghuis B.; Tsarfaty G.; Bruch M.; Kort E. J.; Ditlev J.; Tsarfaty I.; Hudson E.; Jackson D. G.; Petillo D.; Chen J.; Resau J. H.; Teh B. T. (2006) Preparing the “‘Soil’”: The Primary Tumor Induces Vasculature Reorganization in the Sentinel Lymph Node before the Arrival of Metastatic Cancer Cells. Cancer Res. 66, 10365–76. 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- Gregory J. L.; Walter A.; Alexandre Y. O.; Hor J. L.; Liu R.; Ma J. Z.; Devi S.; Tokuda N.; Owada Y.; Mackay L. K.; et al. (2017) Infection Programs Sustained Lymphoid Stromal Cell Responses and Shapes Lymph Node Remodeling upon Secondary Challenge. Cell Rep. 18, 406–418. 10.1016/j.celrep.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Tan K. W.; Yeo K. P.; Wong F. H. S.; Lim H. Y.; Khoo K. L.; Abastado J.-P.; Angeli V. (2019) Prolonged Inflammation Restrains Lymph Node Hypertrophy during Expansion of Cortical and Medullary Sinuses. J. Immunol. 188, 4065–4080. 10.4049/jimmunol.1101854. [DOI] [PubMed] [Google Scholar]
- Angeli V.; Ginhoux F.; Llodrà J.; Quemeneur L.; Frenette P. S.; Skobe M.; Jessberger R.; Merad M.; Randolph G. J. (2006) B Cell-Driven Lymphangiogenesis in Inflamed Lymph Nodes Enhances Dendritic Cell Mobilization. Immunity 24, 203–215. 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Martinez V. G.; Pankova V.; Krasny L.; Singh T.; White I. J.; Dertschnig S.; Horsnell H. L.; Kriston-Vizi J.; Burden J. J.; Tape C. J.; et al. (2019) Conduit integrity is compromised during acute lymph node expansion. SSRN Journal 33349 10.2139/ssrn.3334979. [DOI] [Google Scholar]
- Soderberg K. A.; Payne G. W.; Sato A.; Medzhitov R.; Segal S. S.; Iwasaki A. (2005) Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. U. S. A. 102, 16315–20. 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.; Deng J.; Xu W.; Wang H.; Shi L.; Wu F.; Wu D.; Nei W.; Zhao M.; Panyong M.; et al. (2018) CD8+ T cells with high TGF-β1 expression cause lymph node fibrosis following HIV infection. Mol. Med. Rep. 18, 77–86. 10.3892/mmr.2018.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacker T. W.; Nguyen P. L.; Beilman G. J.; Wolinsky S.; Larson M.; Reilly C.; Haase A. T. (2002) Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110, 1133–9. 10.1172/JCI0216413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinmonth J. B.; Wolfe J. H. (1980) Fibrosis in the lymph nodes in primary lymphoedema. Histological and clinical studies in 74 patients with lower-limb oedema. Ann. R. Coll. Surg. Engl. 62, 344–54. [PMC free article] [PubMed] [Google Scholar]
- Rada I. O.; Tudose N.; Bibescu Roxin R. (1983) Lympho-nodal fibrosclerosis in primary lymphedema. Part one: Considerations on lympho-nodal fibrosclerosis in primary lymphedema. Lymphology 16, 217–22. [PubMed] [Google Scholar]
- Reddy S. T.; Rehor A.; Schmoekel H. G.; Hubbell J. A.; Swartz M. A. (2006) In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Controlled Release 112, 26–34. 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Kunder C.; St. John A. L.; Li G.; Leong K. W.; Berwin B.; Staats H. F.; Abraham S. N. (2009) Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 206, 2455–2467. 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J. J.; Suh H.; Li A. V.; Ockenhouse C. F.; Yadava A.; Irvine D. J. (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. U. S. A. 109, 1080–5. 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostanoski L. H.; Chiu Y.-C.; Gammon J. M.; Simon T.; Andorko J. I.; Bromberg J. S.; Jewell C. M. (2016) Reprogramming the Local Lymph Node Microenvironment Promotes Tolerance that Is Systemic and Antigen Specific. Cell Rep. 16, 2940–2952. 10.1016/j.celrep.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capini C.; Jaturanpinyo M.; Chang H.-I.; Mutalik S.; McNally A.; Street S.; Steptoe R.; O’Sullivan B.; Davies N.; Thomas R. (2009) Antigen-specific suppression of inflammatory arthritis using liposomes. J. Immunol. 182, 3556–65. 10.4049/jimmunol.0802972. [DOI] [PubMed] [Google Scholar]
- An M.; Li M.; Xi J.; Liu H. (2017) Silica Nanoparticle as a Lymph Node Targeting Platform for Vaccine Delivery. ACS Appl. Mater. Interfaces 9, 23466–23475. 10.1021/acsami.7b06024. [DOI] [PubMed] [Google Scholar]
- Thomas S. N.; Vokali E.; Lund A. W.; Hubbell J. A.; Swartz M. A. (2014) Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824. 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Jain S.; Larman H. B.; Gonzalez S.; Irvine D. (2005) Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials 26, 5048–5063. 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Irvine D. J. (2011) Engineering Chemoattractant Gradients Using Chemokine-Releasing Polysaccharide Microspheres. Biomaterials 32, 4903. 10.1016/j.biomaterials.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova T.; Teunis A.; Magni R.; Luchini A.; Espina V.; Liotta L.; Popov S. (2015) Chemokine-Releasing Nanoparticles for Manipulation of the Lymph Node Microenvironment. Nanomaterials 5, 298–320. 10.3390/nano5010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhn L.; Vanparijs N.; De Beuckelaer A.; Lybaert L.; Verstraete G.; Deswarte K.; Lienenklaus S.; Shukla N. M.; Salyer A. C. D.; Lambrecht B. N.; et al. (2016) pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. U. S. A. 113, 8098–8103. 10.1073/pnas.1600816113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabin N. B.; Allen S.; Kwon H.-K.; Bobbala S.; Firlar E.; Shokuhfar T.; Shull K. R.; Scott E. A. (2018) Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat. Commun. 03001-9. 10.1038/s41467-018-03001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu S.; Watanabe T. (2004) Generation of a synthetic lymphoid tissue–like organoid in mice. Nat. Biotechnol. 22, 1539–1545. 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- Okamoto N.; Chihara R.; Shimizu C.; Nishimoto S.; Watanabe T. (2007) Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. J. Clin. Invest. 117, 997. 10.1172/JCI30379. [DOI] [PMC free article] [PubMed] [Google Scholar]