Abstract
Aberrant expression, function, and mutation of G protein-coupled receptors (GPCRs) and their signaling partners, G proteins, have been well documented in many forms of cancer. These cell surface receptors and their endogenous ligands are implicated in all aspects of cancer including proliferation, angiogenesis, invasion, and metastasis. Adhesion GPCRs (aGPCRs) form the second largest family of GPCRs, most of which are orphan receptors with unknown physiological functions. This is mainly due to our limited insight into their structure, natural ligands, signaling pathways, and tissue expression profiles. Nevertheless, recent studies show that aGPCRs play important roles in cell adhesion to the extracellular matrix and cell–cell communication, processes that are dysregulated in cancer. Emerging evidence suggests that aGPCRs are implicated in migration, proliferation, and survival of tumor cells. We here review the role of aGPCRs in the five most common types of cancer (lung, breast, colorectal, prostate, and gastric) and emphasize the importance of further translational studies in this field.
Keywords: adhesion GPCRs, metastasis, cancer, extracellular matrix, proliferation
G Protein-Coupled Receptors and Their Role in Cancer
G protein-coupled receptors (GPCRs) are the largest superfamily of cell surface receptors in the human genome and are implicated in various biological processes including sensory perception (vision, olfaction, taste), cellular adhesion, angiogenesis, development, and hormonal regulation, among others.1 Structurally, GPCRs are defined by seven-transmembrane domains with alternating extracellular and intracellular loops2−4 and are grouped into five classes: rhodopsin-like, secretin, glutamate, adhesion, and frizzled (GRAFS classification).5 The diverse stimuli of GPCR signaling and the consequent physiological events make these receptors one of the most intriguing pharmacological targets. To date, approximately 34% of drugs in the global market target 108 unique GPCRs6 and about 56% of the nonolfactory GPCRs have yet to be studied in a clinical trial.6 This underscores the high potential of GPCRs as novel targets in various therapeutic areas.
Compelling evidence suggests that GPCRs play major roles in cancer including growth, migration, metastasis, invasion, and survival.7−10 GPCRs and their cognate heterotrimeric G proteins and signaling circuits are implicated in breast, lung, colorectal, prostate, and brain tumors, among many others.9,11−13 Despite the recent advancements in our structural and functional knowledge of GPCRs,14−16 only eight FDA-approved drugs target GPCRs for cancer therapy.17 This underscores the need to explore the function of both deorphanized and orphan GPCRs in tumorigenesis, with a mission to reveal novel therapeutic targets. Adhesion GPCRs (aGPCRs) form the second-largest (33 members) family of GPCRs, of which only 11 members have been deorphanized.18 The role of some members of the aGPCR family as modulators of proliferation, metastasis, and cancer cell communication is gradually being appreciated.19
Structure and Mechanisms of Activation of aGPCRs
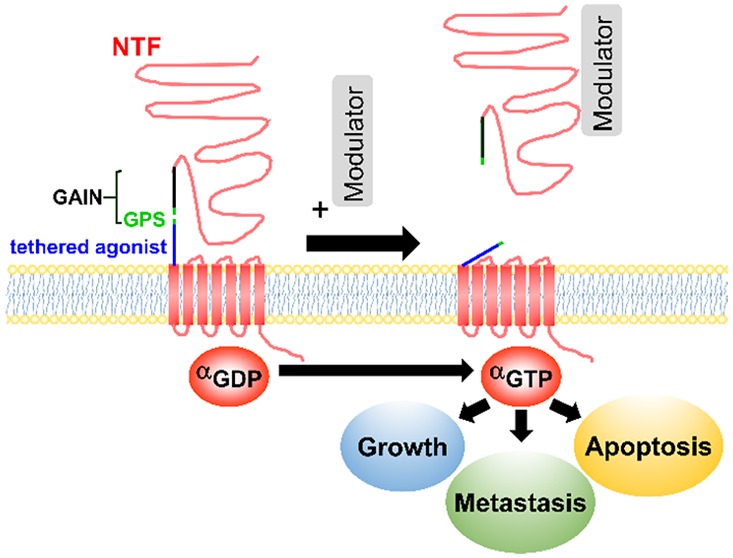
aGPCRs maintain the seven-transmembrane structure but are uniquely identified by a large N-terminal fragment (NTF) (Figure 1). In some aGPCRs, NTF contains several domains including EGF-like, cadherin, pentraxin, and leucine-rich repeats, which enable cells to interact with adhesion molecules (e.g., ADGRE5 with integrins20) or extracellular matrices (ECM) (e.g., ADGRG1 with collagen III;21 ADGRG6 with collagen IV22). aGPCRs, except ADGRA1, include a GPCR Autoproteolysis-Inducing domain (GAIN), which is located N-terminally to the first transmembrane domain.18 Within the GAIN domain is a highly conserved GPCR proteolytic site (GPS) and a stretch of residues that connects GPS to the first transmembrane domain.23 Proteolytic cleavage at the GPS during protein translation divides aGPCRs into NTF and C-terminal fragments (CTF), which remain associated via noncovalent interactions.
Figure 1.
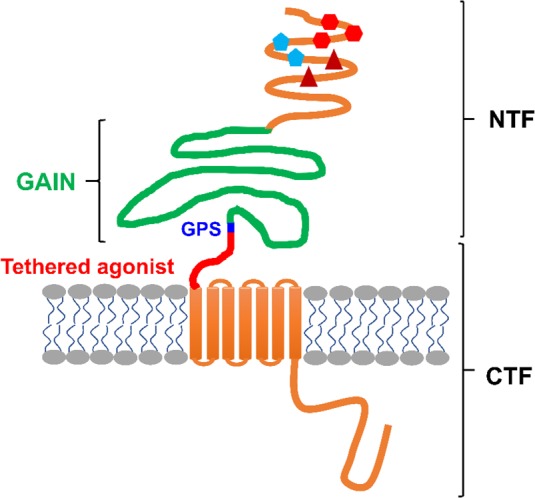
General structure of an aGPCR. All aGPCRs, except ADGRA1, contain a GPCR Autoproteolysis-Inducing domain (GAIN)23 that includes the GPCR proteolysis site (GPS) and a tethered agonist sequence.31 The cleavage at GPS results in a two-subunit molecule, including an N-terminal fragment (NTF) and a C-terminal fragment (CTF) that remain associated via noncovalent interactions. In some aGPCRs, NTF includes additional domains such as EGF-like, cadherin, pentraxin, and leucine-rich repeats. These domains interact with other cell adhesion molecules and extracellular matrices, by which they orchestrate the intracellular signaling.
Several modes of activation of aGPCRs have been proposed, some of which are unique to specific aGPCRs and cellular contexts (reviewed in detail in refs (24 and 25)). Dissociation of NTF from CTF by an extracellular molecular partner unmasks a 15–25 amino acid tethered agonist that remains extracellularly on the N-terminus of CTF. Several studies have reported that NTF-truncated mutants of ADGRB2,26 ADGRG1,27 ADGRG2,29,30,143 ADGRF1,31 and ADGRE532 show constitutive activation of downstream signaling pathways. Further studies proved that this activity is due to the interaction of the tethered agonist with the cognate receptors. For instance, the deletion of tethered agonists in NTF-truncated ADGRD1,33 ADGRG2,143 and ADGRG633 abolished certain signaling pathways and exogenous addition of synthetic peptides, that are identical to tethered agonists, stimulated receptor signaling.30,31,34,143 Alternative mechanisms of activation of aGPCRs exist. Unlike the aforementioned receptors, the interaction of NTF and CTF is required for proper signaling and function of ADGRC2 in the brain.35 Moreover, circulating NTF of several aGPCRs have been reported26,36,37 and the secreted NTF of ADGRB1 (Vasculostatin, a.k.a. Vstat120) showed antiangiogenic and antitumorigenic functions in glioma xenograft models,38 pointing to CTF-independent roles that NTF may play in distant or neighboring cells. To add to the complexity of the aGPCR activation and signaling, recent studies have shown that while NTF and tethered agonist are required for certain signaling pathways of ADGRG1,39 ADGRB1,39 and ADGRG2,143 they can be dispensable for interaction of these receptors with β-arrestins. Together, these studies suggest that GPS, NTF, CTF, tethered agonist, and other domains of aGPCRs play various functional roles.
aGPCRs in Cancer
Multidomain NTF of aGPCRs enables cell–cell communication and cell-extracellular matrix interaction, processes that are dysregulated in cancer. Current evidence suggests that some aGPCRs regulate the cell cycle, proliferation, survival, and dissemination of cancer cells (Table 1).
Table 1. Comprehensive List of aGPCRs, Their Endogenous Ligands, Signaling Pathways, Altered Expression, and Mutation in Various Types of Cancera.
| receptor | ligand/extracellular partner | signaling pathways | cancer | expression/localization | mutation | disease significance | |
|---|---|---|---|---|---|---|---|
| Subfamily I | ADGRL1 (LPHN1) | α-latrotoxin40, Teneurin-2 (Lasso)41,42, FLRT1, 2, 342,43, Neurexin-1α, -1β, -2β, -3β44 | Gαq, Gαo46 | AML47, Lung, Liver, Endometrial carcinoma158 | decreased47 | - | Overexpression of ABCB1 drug transporter47 |
| ADGRL2 (LPHN2) | FLRT343 | - | Breast48, Squamous Cell Lung Carcinoma49 | increased48,49 | Q693H49 | Escape immune surveillance by activating the Tim-3-Galectin-9 pathway48 | |
| ADGRL3 (LPHN3) | FLRT1, 2, 343,50, Teneurin-343 | - | Breast51 | increased51 | - | Correlated with axillary lymph node metastasis51 | |
| ADGRL4 (ELTD1) | - | - | Renal52, Colorectal52, HNSCC52, Ovarian52, Glioblastoma155,156 | increased52 | - | Correlated with a positive prognosis in various cancers by regulating angiogenesis and vessel maturation52 | |
| Subfamily II | ADGRE1 (EMR1) | - | - | CRC54, AML55 | increased54,55 | - | Azoxymethane mouse models of CRC show increased expression of ADGRE154 |
| ADGRE2 (EMR2) | Chondroitin Sulfate B56 | Gα1657 | Lung58,59 AML55, Glioblastoma60, Breast61 | increased55,58,61, localization20 | - | Decreases adhesion and increases proliferation in lung cancer58 Correlated with EMT markers59 Associated with a more invasive phenotype60,61 Cytoplasmic expression correlated with worse prognosis61 | |
| ADGRE3 (EMR3) | - | - | Relapsed CRC62,63, HCC64, Glioblastoma65 | increased62-65 | - | Hypomethylated at CpG islands64 Increase in invasive phenotype65 | |
| ADGRE4 (EMR4) | - | - | - | - | - | ||
| ADGRE5 (CD97) | CD5566, α5β1 integrin (20), Chondroitin Sulfate B56, CD9067 | Gα12/1332 | CRC69-72, Rectal, Adenocarcinoma73, Pancreatic74, IHC75, Gastric76, Breast77, HCC (78), Esophageal (79), Glioblastoma80, Ovarian81, Gallbladder82, AML83,84, OSCC85, Thyroid86, Prostate32 | increased69,73-75,77,78,80-86, localization71 | - | Poor clinical outcome and increased invasion70,72-74,77,80-87 Cytoplasmic ADGRE5 correlates with more malignant phenotype71 Expressed only on the invasive front of rectal tumor cells73 Biliary soluble ADGRE5 is a negative prognostic biomarker in IHC75 Exosome-mediated proliferation (75) Correlated with tumor metastasis78 Hypomethylated promoter79 | |
| Subfamily III | ADGRA1 (GPR123) | - | - | - | - | - | |
| ADGRA2 (GPR124) | Glycosaminoglycans88, WNT789 | β-catenin89 | Glioblastoma90, Osteosarcoma91, Urothelial Carcinoma92, Lung170 | increased and decreased90, increased92 | - | Alteration in ADGRA2 expression leads to altered microtubule dynamics during mitosis leading to chromosomal instability90 Contributes to tumor angiogenesis91 | |
| ADGRA3 (GPR125) | - | - | CRC93 | increased93 | - | Correlates with increased metastasis and worse prognosis93 | |
| Subfamily IV | ADGRC1 (CELSR1) | - | Rho Kinase94 | - | - | - | |
| ADGRC2 (CELSR2) | - | Ca2+95 | Breast96 | localization96 | - | Cytoplasmic localization is a negative prognostic marker in HER2+ breast carcinoma96 | |
| ADGRC3 (CELSR3) | - | Ca2+95 | OSCC97, AML55, HCC98, HNSCC99 | decreased55,97, increased98,99 | - | Highly methylated in oral carcinomas97 Increased expression correlated with poor prognosis in HCC98 Decreased expression is correlated with better survival in HNSCC99 | |
| Subfamily V | ADGRD1 (GPR133) | - | Gαs100 | Glioblastoma101, AML102, Gastric103 | increased101,102, decreased103 | - | Protumorigenic role in hypoxic glioblastoma tumors101 Associated with poor clinical outcome102 Downregulated by lncRNA and miRNAs in gastric cancer103 |
| ADGRD2 (GPR144) | - | - | Lung104, HNSCC99 | increased99 | - | Frequently mutated due to benzo(a)pyrene exposure104 Increased expression is correlated with better survival in HNSCC99 | |
| Subfamily VI | ADGRF1 (GPR110) | Synaptamide105 | Gαs105, Gαq31 | Lung106-108, Prostate106, Breast109, ALL110, Osteosarcoma111, HCC112, Thyroid113 | increased106,108-111 | - | Various polymorphisms associated with NSCLC susceptibility107 Knockdown decreased anchorage-independent growth in BT747 breast cancer cells109 Prostate cancer lines express splice-variants of ADGRF1 (106) Knockdown decreased proliferation and its expression served as a prognostic marker for osteosarcoma111 Genomic deletion of ADGRF1 decreased the hepatocellular carcinogenesis112 Expression can be used to distinguish papillary thyroid cancer from other thyroid malignancies113 |
| ADGRF2 (GPR111) | - | - | - | - | - | - | |
| ADGRF3 (GPR113) | - | - | SBNET114,115 | increased114,115 | - | Increased expression in the primary SBNET and secondary lesions114,115 | |
| ADGRF4 (GPR115) | - | Gα15116 | Lung117 | increased117 | - | Associated with TRIMP58 methylation in lung squamous cell carcinoma, may be a prognostic marker (117) | |
| ADGRF5 (GPR116) | Surfactant Protein D118 | Gαq119 | CRC120, Breast119, Gastric121 | increased119,120 | G2731C121, G2276A121 | Patients with high levels of ADGRF5 showed distant metastasis and poor tumor differentiation120 Expression promotes migration, invasion and metastasis119 | |
| Subfamily VII | ADGRB1 (BAI1) | Phosphatidylserine122, Matrix metalloprotease 14123 | Gα12/1339, β-Arrestin39 | Lung124, Glioblastoma125, CRC126,127, Astrocytoma128, Breast129 | decreased88,129 | - | Functions as an inhibitor of angiogenesis124,125,127,128 Decrease in expression correlates with a favorable prognosis129 Ectopic expression of NTF decreased metastasis to the brain129 |
| ADGRB2 (BAI2) | Glutaminase Interacting Protein (GIP)130, Furin26 | Gα1626 | CRC131 | increased131 | - | Correlates with tumorigenesis and tumor growth (131) | |
| ADGRB3 (BAI3) | C1q-like proteins132 | - | Lung133, Glioma134 | increased133, decreased134 | - | Expression is increased in SCLC and can be used to differentiate from LCNEC133 | |
| Subfamily VIII | ADGRG1 (GPR56) | Collagen III (100),Transglutaminase II21,135 | Gαq/11136, Gα12/13137, β-Arrestin39 | CRC138, Melanoma139,140, Prostate141, AML142 | increased138,139,141,142 | - | Promotes metastasis by inducing EMT138,140,141 Suppresses the expression of VEGF139 Increased expression can be used to differentiate leukemic stem cells142 |
| ADGRG2 (GPR64) | - | Gαq143,144, Gαs29, Gα13143, β-Arrestin143,144 | Breast145,146, ES146, Parathyroid29, Endometrial (147) | increased29,145 decreased147 | - | Knockdown of ADGRG2 decreased cell migration145 Promotes invasiveness and metastasis146 Regulates PTH release by parathyroid adenoma29 Functions as a tumor suppressor in endometrial cancer147 | |
| ADGRG3 (GPR97) | Beclomethasone Dipropionate116 | Gαo116 | - | - | - | - | |
| ADGRG4 (GPR112) | - | NEC148 | increased148 | - | A novel marker for NEC cells148 | ||
| ADGRG5 (GPR114) | - | Gαs116 | AML55 | increased55 | - | Consistently upregulated in AML55 | |
| ADGRG6 (GPR126) | Collagen IV22, Laminin-211149 | Gαs33 | Bladder150 | intronic mutations150 | multiple intronic mutations150 | Mutational burden can be a prognostic marker in bladder cancer150 | |
| ADGRG7 (GPR128) | - | - | Lymphoma151 | gene fusion151 | - | ADGRG7 fused with TRK fused gene (TFG) in lymphoma tumors151 | |
| Subfamily IX | ADGRV1 (GPR98) | - | Gαi152, Gαs153, Gαq153 | CRC154 | increased154 | - | Inhibition by miR-145 sensitizes cells to Oxaliplatin154 |
Notation: ALL, acute lymphoblastic leukemia; AML, acute myelocytic leukemia; CRC, colorectal carcinoma; ES, Ewing’s sarcoma; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; IHC, intrahepatic cholangiocarcinoma; NEC, neuroendocrine carcinoma; OSCC, oral squamous cell carcinoma; SBNET, small bowel neuroendocrine tumors.
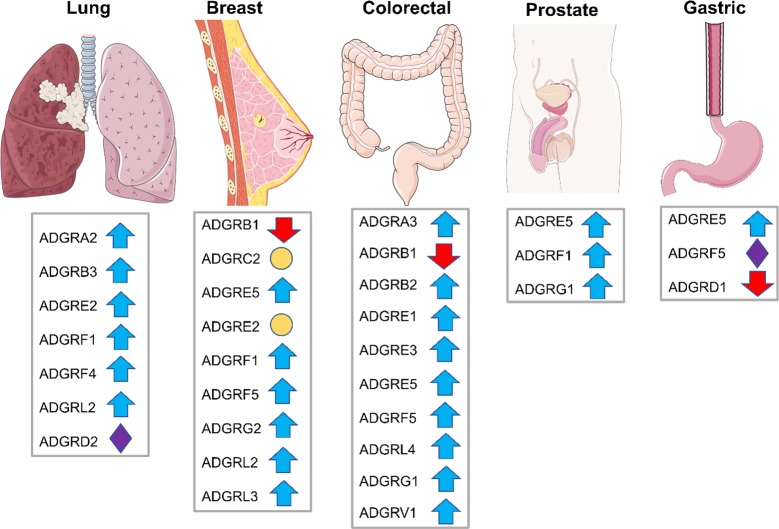
For example, knockdown of ADGRL4 reduced the proliferation of glioblastoma cells in vitro.155,156 ADGRG1 is upregulated in colorectal cancer tissues and cell lines and promotes tumor growth and metastasis via induction of epithelial to mesenchymal transition (EMT).138 However, in melanoma cell lines, ADGRG1 suppressed the production of vascular endothelial growth factor, a known stimulator of tumor angiogenesis, and inversely correlated with melanoma progression in mouse tissues and xenograft models of human melanoma.139 Antigrowth157 and pro-metastasis140 roles have also been reported for ADGRG1 in melanoma studies. ADGRB1 functions as an inhibitor of angiogenesis in pulmonary adenocarcinoma,124 glioblastoma,125 colorectal cancer,127 and astrocytoma.128 ADGRG2 showed functional roles in both benign (parathyroid adenoma29) and highly metastatic (Ewing sarcoma146) tumors. Various carcinoma-associated mutations (endometrial, lung, liver) in ADGRL1 revealed altered surface expression and exaggerated basal activity of the receptor.158 In the era of -omics, there is now evidence of aberrant expression and mutational profile of aGPCRs in different malignancies that warrant future translational studies. Here, we review the current body of knowledge regarding the expression and function of aGPCRs in the five most common types of cancer (Figure 2).159
Figure 2.
List of aGPCRs with altered expression (blue and red arrows), mutation (purple diamonds), or localization (orange circles) in the five most common cancers globally (organ images are taken from https://smart.servier.com).
Lung Cancer
Lung cancer is the leading cause of death and the most common cancer globally, totaling approximately 12% of new cancer cases in 2018.160 ADGRB3 was shown to be one of the most significantly mutated genes in 13% of lung squamous and 5% of lung adenocarcinoma tumors.161 These mutations span NTF, 7TM, and C-terminus of ADGRB3, and authors suggested that this protein might act as a putative tumor suppressor. This is in line with the reported antiangiogenic and antineurogenic activity of other members of this subfamily, ADGRB1 and ADGRB2.162,163 Currently, small cell lung cancer (SCLC) and large cell neuroendocrine lung carcinomas (LCNEC) are differentiated based on morphological analysis, which tends to be a poor determinant of cancer subtype. Immunohistochemical (IHC) analysis of human lung tumors showed that ADGRB3 is expressed in the nucleus of a majority of SCLC samples but is either absent or expressed at low levels in the cytoplasm of LCNEC tissues.133 The ability to use ADGRB3 staining to differentiate between SCLC and LCNEC will be of significant clinical importance. Nuclear localization and signaling of some GPCRs164,165 and β-arrestins166,167 have been reported in HEK293 cells and tumor cells. Interestingly, the NTF-truncated ADGRB3 was shown to interact with β-arrestin2.168 Whether the nuclear ADGRB3 in SCLC cells is the activated form of the receptor that is transported to the nucleus by β-arrestin2 requires further studies.
MicroRNAs (miRNAs) are a family of noncoding small RNAs that regulate gene expression, are dysregulated in various cancers and are either tumor suppressors or oncogenes.169 Down-regulation of miR-138-5p increased the expression of ADGRA2 in nonsmall-cell lung carcinoma (NSCLC) cell lines and patient-derived cells.170 NSCLC patients who are treated with gefitinib, a common tyrosine kinase inhibitor, often become resistant to this drug.171 Interestingly, introducing miR-138-5p to resistant NSCLC cells down-regulated ADGRA2 and resensitized cells to gefitinib.170 Although the endogenous ligand of ADGRA2 is unknown, this receptor has been implicated in tumor angiogenesis, a known mechanism of gefitinib resistance in lung cancer patients.170−173 Whether inhibition of ADGRA2 by a small molecule or biologic can resensitize patients to gefitinib is yet to be explored.
Expression profiling revealed that 97 miRNAs were differentially expressed in NSCLC patients’ lungs compared with normal lung tissues, of which miR-099a was one of the most down-regulated miRNAs in NSCLC tissues. Expression of miR-099a in NCI-H1650, NCI-H1975, and NCI-H1299 lung adenocarcinoma cell lines reduced expression of ADGRE2 and increased cell cycle arrest and apoptosis.58 Rescue experiments suggested that ADGRE2, a target of miR-099a, mediates NSCLC cell migration and its knockdown increases adhesion and decreases proliferation. ADGRE2 expression also correlated with β-catenin expression,58 a known marker for EMT and metastasis in lung adenocarcinoma.59 IHC analysis of 119 lung cancer patient biopsies revealed that ADGRE2 is upregulated in approximately 12% of cases.58 ADGRE2 binds to chondroitin sulfate,56 a proteoglycan that is involved in lung growth174 and is present at elevated levels in lung tumors.175 This evidence, combined with the fact that ADGRE2 couples to Gα15,116 a promiscuous Gα protein that activates phospholipase Cβ, provides strong grounds for the screening of small molecules that interfere with ADGRE2-mediated signaling and migration of NSCLC cells.
Insertional mutagenesis experiments, either by retroviruses or lentiviruses, have been exploited as a tool to identify genes that potentially regulate cell growth and culminate in tumorigenesis.176 Genomic localization of proviral sequences after a retroviral screen in mice suggested that ADGRF1 is a proto-oncogene in mouse leukemia,106 which was corroborated by additional reported insertion sites.177,178 Lum et al. followed this proto-oncogenic indication by mRNA and protein expression analysis and found that, whereas ADGRF1 expression was low in lung cancer cell lines, lung adenocarcinoma tumor samples showed upregulated ADGRF1 compared to either normal lung, squamous, or small lung tumor samples.106
Breast Cancer
Breast cancer was the second most commonly diagnosed cancer in 2018 with over 2 000 000 newly diagnosed cases.160 Several aGPCRs show altered expression or mutation in breast malignancies (Table 1).77,96,109,179 ADGRC2 was initially shown to be down-regulated in human epidermal growth factor receptor 2 (HER2)-positive breast carcinomas.180 Further immunohistochemical studies by the same group did not identify a significant correlation between ADGRC2 expression and either HER2 or estrogen receptor (ER) status of breast tumor tissues or cell lines.179 However, they identified a small group of cell lines and tumors that show striking down-regulation of ADGRC2, pointing to a potential impact of this receptor in a subset of breast cancers. ADGRC2 is a member of the nonclassical cadherin family of proteins due to the presence of several cadherin domains in its NTF. Interestingly, cadherins are involved in cell–cell communication and cell adhesion, and E-cadherin promotes metastasis in diverse models of invasive ductal carcinomas.181−183 Therefore, it would be interesting to know whether the deletion of cadherin domains in ADGRC2 changes the metastatic potential of breast cancer cells.
Localization and expression of ADGRE2 correlated with breast cancer patient prognosis.61 While ADGRE2 is not expressed in normal breast epithelial cells, invasive breast carcinomas and ductal carcinoma in situ (DCIS) showed upregulation of ADGRE2.61 Nuclear expression of ADGRE2 was correlated with lower tumor grades and a longer disease-free survival.61 Since inactive GPCRs do not reside in the nucleus, it is possible that ADGRE2 is either activated on the cell surface and endocytosed to the nucleus or it is not shuttled to the plasma membrane after translation. Further research is necessary to define the mechanism by which ADRGE2 regulates breast cancer cell function and to confirm nuclear localization as a prognostic biomarker.
Data from the Cancer Genome Atlas (TCGA) show that 52% of patients with invasive ductal carcinoma have reduced levels of ADGRB1, which correlates inversely with patient survival.129 This is consistent with the down-regulation of ADGRB1 in several other tumors including glioblastoma,125 colorectal,127 and lung cancer.124 The secreted N-terminal fragment of ADGRB1 (Vasculostatin, a.k.a. Vstat120) was shown to suppress growth in xenograft models of glial tumors.38 Vstat120 contains an arginine–glycine–aspartate domain and five thrombospondin type-1 repeats, motifs that are known modulators of angiogenesis.184,185 Overexpression and consequent secretion of Vstat120 reduced the viability of various subtypes of breast cancer cell lines.129 Injection of Vstat120-expressing virus into the brain of breast cancer-derived brain metastases (BCBM) mouse models significantly decreased the tumor size and disease burden and increased survival.129 This experiment is of significant clinical importance because BCBM is a feature of treatment-resistant HER2-positive and triple-negative breast cancer, for which the standard of care is systemic chemotherapy and radiation with poor prognosis and low survival rates.186 Given the antiangiogenic effects of Vstat120 and its motifs, it would be important to investigate their stability and bioavailability in mouse models of cancer. Also, the therapeutic efficacy of these molecules in combination with other current therapies warrants future research.
ADGRE5 is upregulated in MDA-MB231, MDA-468, MCF-7, and T47D breast cancer cell lines and its knockdown decreased cell growth, proliferation, and migration.77 However, the mechanisms by which ADGRE5 regulates these cellular functions in breast cancer cell lines are mainly unknown. Independent studies have provided contradictory results whether the expression of the endogenous ligand of ADGRE5, CD55, correlates with breast cancer prognosis positively or negatively.187,188 This might be due to the different methods used by authors to define “high and low expression”. It is noteworthy that ADGRE577 and CD55188 are coexpressed on the surface of MCF-7 cells. Further studies may reveal whether this receptor–ligand pair are colocalized in breast tumor tissues as well and if deletion of either or both proteins alters the in vivo manifestation of breast tumor. As elaborated in more detail later in this minireview, the expression of ADGRE5 in various epithelial carcinomas correlates with the stage and progression of the tumor.
As great strides are made in cancer treatments, there are still many patients who develop resistance to targeted therapies. Bhat et al. recently showed that several aGPCRs are expressed in cancer stem cells and anti-HER2 therapy-resistant cells. Using Aldefluor, a nonimmunological fluorescent marker for stemness, Baht et al. found that ADGRB3, ADGRE2, ADGRA2, ADGRF5, and ADGRF1 are all overexpressed in cancer stem cells.109 The only aGPCR found to be expressed in both cancer stem cells and anti-HER2 therapy-resistant cell lines was ADGRF1.109 Knockdown of ADGRF1 in BT747 cells decreased anchorage-independent growth, a common feature of metastatic cell lines and reduced the mammosphere formation, suggesting a role for ADGRF1 in cancer stemness.109 These data warrant further investigation into the downstream effects and potential targeting of ADGRF5 in HER2+ breast cancer.
Knockdown of ADGRF5 in highly metastatic breast cancer cell line, MDA-MB-231 reduced the cell migration in vitro and metastasis in mammary tumor mouse models in vivo.119 The potential role of ADGRF5 in cell invasion was further confirmed by the ectopic expression of the receptor in less-metastatic breast cancer lines (MCF-7 and Hs578T).119 Activation of a well-known cytoskeletal remodeling signaling cascade, Gαq, p63RhoGEF and small GTPases, RhoA and Rac1 was confirmed as the potential mechanism of cell motility by ADGRF5 in breast cancer cells.119 The increased expression of ADGRF5 in human breast cancer tissues correlated with cancer metastasis and poor prognosis,119 further suggesting this receptor as a potential candidate for breast cancer therapy.
The expression of ADGRG2 in breast cancer cell lines has been debated. Richter et al. showed low to no expression of ADGRG2 transcripts in MDA-MB-231 and Hs578T breast cancer cell lines.146 We have also not been able to show the expression of ADGRG2 in MDA-MB231 cells at either mRNA or protein level (data not shown). However, Peeters et al. revealed the effect of ADGRG2 knockdown on migration and adhesion of these cell lines, presumably via its effect on RelB, a member of the NF-κB family.145 Surprisingly, Peeters et al. did not provide expression data for ADGRG2 at either mRNA or protein level in either cell lines. An impedance-based assay (xCELLigence) showed that ADGRG2 knockdown delays breast cancer cell adhesion but does not modulate cell proliferation.145 Constitutive activation of the serum response element (SRE) transcription factor was dependent on the autoproteolysis of ADGRG2 at its GPS site when the receptor was overexpressed in HEK293 cells.145 Unlike reports on the inhibitory function of NTF in ADGRG2 signaling,29,30,143 Peeters et al. showed that NTF plays a crucial role in the activation of both NF-κB and SRE pathways by ADGRG2.145 Further studies to profile the expression and localization of ADGRG2 in breast cancer cell lines and patient breast tumor-derived cells are necessary to provide a thorough understanding of its function in this disease.
Colorectal Cancer
The current standard of care for colorectal cancer (CRC), the third most commonly diagnosed cancer,159 is surgical resection, radiotherapy, and chemotherapy.189 In recent years targeted therapies such as inhibitors of angiogenesis, immune checkpoint, and epidermal growth factor receptor (EGFR) have turned CRC into a highly treatable disease. However, resistance due to tumor mutation and recurrence following surgery warrant further studies.190
The expression of several aGPCRs is changed in CRC. While ADGRB1 is down-regulated in the colon mucosa of CRC patients,126 ADGRB2 is upregulated in advanced CRC131 and ADGRE3 is upregulated in CRC biopsies of relapsed patients compared with patients who are disease-free.54,62,63 In addition, expression of ADGRE1 is decreased in colon tissue biopsies of mouse models of colorectal carcinoma compared with control mice.54 Unfortunately, these aGPCRs have no prognostic indications and no further work has been done to elucidate their mechanistic roles in CRC.
Other aGPCRs have shown more promise in the laboratory and clinical settings. Analysis of three Gene Expression Omnibus (GEO) data sets and one data set from TCGA (151 cases of CRC) revealed that ADGRA3 is down-regulated in 78% of CRC specimens and its elevated expression in 22% of the samples is associated with prolonged recurrence-free survival.93 CRC patients with upregulated ADGRA3 showed reduced KRAS mutation, smaller tumors, and less metastasis.93 Interestingly, gain-of-function mutations in KRAS contribute to the transition from adenoma to CRC191 and are good predictors of resistance to EGFR therapy.190 Overexpression of ADGRA3 in a CRC cell line (HCT116) suppressed the Wnt/β-catenin signaling pathway, a known driver of CRC93,192 and down-regulated c-Myc and cyclin-D1. There is compelling evidence that KRAS mutations can cause aberrant Wnt/β-catenin signaling, which will cause an oncogenic transformation in intestinal epithelial cells.193 Yet, it is unclear whether there is a crosstalk between ADGRA3 and KRAS at the levels of Wnt/β-catenin signaling.
High ADGRE5-expressing CRC cells show greater localization of β-catenin in the nucleus, indicating activation of the Wnt/β-catenin signaling pathway.69,72 Wobus et al. found that CRC cells with increased expression of ADGRE5 showed an elevated invasion and a poor clinical outcome.69,72,194 Given that ADGRE5 has an observable effect on tumor cell invasion, migration, and secondary metastasis in various cancers,32,78,195,196 its interactions with junctional proteins were studied. Proximity ligation and coimmunoprecipitation assays in various human CRC cell lines showed a strong interaction among ADGRE5, β-catenin, and E-cadherin when compared to normal tissues and cell lines.71 Malignant samples had reduced membrane-bound and increased cytoplasmic ADGRE5.71 These results suggest that ADGRE5 plays an important role in the regulation of cell junctions in normal colon tissue. Whether the cytoplasmic ADGRE5 is an indication of its prior activation and endocytosis in CRC cells awaits further investigation. IHC staining of various rectal adenocarcinoma tissue samples showed a strong coexpression of ADGRE5 and CD55 in the invasive front of the tumor.73 However, cells in the center of the tumor showed little to no expression of either ADGRE5 or CD55. Patients with high ADGRE5 expression showed a less favorable prognosis, more metastatic burden, and a higher rate of clinical recurrence.73 These data indicate that ADGRE5 and its ligand, CD55, may have a prognostic role as a biomarker in CRC.
Protein and mRNA analysis of 48 colorectal carcinoma cases showed a significant increase in ADGRF5 levels when compared with normal tissues.120 These results were confirmed in three microarrays from the Oncomine database and IHC staining of over 90 CRC samples.120 Patients expressing high levels of ADGRF5 showed an increase in distant metastasis and histological differentiation.120 Univariate analysis of these results, along with several other in-silico studies, show that high levels of ADGRF5 could act as an unfavorable prognostic indicator in CRC patients. miR-511–5p is known to be down-regulated in a variety of CRC cell lines, and patients who expressed elevated levels of miR-511-5p show higher survival rates.197In vitro overexpression of miR-511-5p mimetics in CRC cells reduced proliferation and colony formation and increased cell apoptosis.197 Interestingly, miR-511-5p binds the 3′UTR of the ADGRF5 gene to repress its transcription, and overexpression of ADGRF5 reverses the antitumor features of miR-511-5p.197 Together, these data support tumorigenic roles for ADGRF5.
mRNA, IHC, and in situ hybridization analyses showed that ADGRG1 is highly expressed in CRC specimens198 and colonic crypt cells199 compared with normal gastrointestinal tissues and cells. This overexpression is intensified in mice that express progastrin, a peptide that is upregulated in CRC and other cancer cell lines.199,200 Jin et al. found that ADGRG1 directly interacts with progastrin to increase the proliferation rate of colonic cells and the genomic deletion of ADGRG1 increases apoptosis in the colonic mucosa and decreases proliferation in mice.199 The increased expression of ADGRG1 predicted a worse prognosis for patients suffering from CRC138 and knockdown of ADGRG1 down-regulated mesenchymal markers, N-cadherin, and vimentin via the PI3K/AKT pathway.138 The current screening method for CRC is an optical colonoscopy that does not detect the early stages of cancer development. It remains to be investigated whether ADGRG1 can potentially be a less invasive diagnostic biomarker for CRC.
Prostate Cancer
There were 1 276 106 newly diagnosed cases of prostate cancer in 2018, globally. Because of the indolent nature and slow progression of prostate cancer many cases remain undiagnosed until later stages of the disease. Examination of prostate cancer screenings such as prostate-specific antigen and digital rectal exam lack internal validity and have shown inconsistent results and false-positives.201
Histological analysis of a prostate tissue array derived from 36 adenocarcinoma cases revealed that ADGRE5 is upregulated in these tumors compared with normal adjacent tissues.32 This was corroborated by the high expression of ADGRE5 in some prostate cancer cell lines (PC3 and DU145) and the low expression in nontransformed prostate cells. The depletion of ADGRE5 in DU145 cells reduced the serum-induced activation of RhoA small GTPase in vitro and cell migration and invasion in Matrigel.32 The described mechanism of ADGRE5-mediated migration of prostate cells is consistent with a previous study, in which ADGRE5 regulated the migration of neural progenitor cells through Gα12/13 G proteins and RhoA small GTPase.137 Mice injected with ADGRE5-depleted PC3 cells showed a significant reduction in bone metastasis but no change in tumor growth when compared with mice that were injected with parental PC3 cells.32 In addition to ADGRE5, the increased expression of lysophosphatidic acid receptor 1 (LPAR1) has been reported in prostate cancer cells.202 Interestingly, the ectopic coexpression of ADGRE5 and LPAR1 in LNCaP cells (an androgen-sensitive prostate adenocarcinoma) revealed that these GPCRs heteromerize and ADGRE5 potentiates the LPA-induced RhoA activation.32 Heteromerization and crosstalk of various GPCRs and the consequent regulation of tumorigenesis and metastasis of prostate,203,204 breast,203,204 and glioblastoma205 cells have been previously reported. Histological examination indicated an association between expression of LPAR1 and ADGRE5 in prostate cancer biopsies.32 Together, these data suggest crosstalk between LPAR1 and ADGRE5 in prostate tumor cells. Considering that LPAR1 antagonists have not yet been approved to mitigate tumor burdens and metastasis, it would be interesting to examine whether inhibition of ADGRE5 by small molecules or specific antibodies suppresses the LPAR1-mediated metastasis.
Prostate biopsies from old subjects, which are prone to benign hyperplasia or have undiagnosed cancer, showed higher expression of ADGRF1.106 There is a spectrum of ADGRF1 expression among main prostate cancer cell lines; high in PC3, low in LNCaP, and negative in DU145. Histological analysis with antibodies raised against two distinct peptides from the NTF of ADGRF1 showed differential staining in prostate adenocarcinoma tissues, suggesting potential expression of different splice variants.106 Such differential staining precluded a comparative analysis of ADGRF1 expression between benign prostate hyperplasia and prostate adenocarcinomas and emphasized the importance of relative quantification of splice variants of aGPCRs in tumors.
Gastric Cancer
Gastric cancers are the fifth most commonly diagnosed cancer in the world.160 Aust et al. found that ADGRE5 was expressed in 44 of 50 gastric cancer biopsies.206 Alternative splicing generates three isoforms of ADGRE5 that contain three, four, or five repeats of EGF domains on their NTF. Overexpression of the smaller isoform, ADGRE5/EGF1,2,5 in BGC-823 stomach adenocarcinoma cells increased their invasive behavior in vitro.207 In line with these findings, orthotopic mouse models of gastric carcinoma that lacked the ADGRE5/EGF1,2,5 showed reduced metastatic spread and tumor volume.196 However, the full-length isoform, ADGRE5/EGF1,2,3,4,5 suppressed the invasion and increased proliferation. These studies indicate that the characteristics of gastric tumor cells may be regulated by the balance of ADGRE5 splice variants.207
Recently, Chao et al. found that the exosomes isolated from the stomach adenocarcinoma cell lines that express wild-type ADGRE5 stimulated migration of other cells in a transwell assay.76 This was accompanied by phosphorylation of the major signaling molecules of the MAPK pathway. Consistent with these findings, exosomes released from ADGRE5-expressing tumors increased metastasis of gastric adenocarcinomas.208 In a footpad mouse model of aggressive gastric adenocarcinoma, Liu et al. found that tumors lacking ADGRE5 show a diminished metastasis and metastatic niche formation.208 Exosomes isolated from SGC-L, an SGC-7901 cell-derived highly metastatic gastric cancer cell line expressing ADGRE5, were also able to increase the metastatic phenotype of tumor cells.208 Taken together, these studies suggest that ADGRE5 increases cell proliferation and metastasis in gastric cancer via vesicle-mediated tumor cell communication and activation of the MAPK pathway.
A recent study showed that ADGRF1 mRNA and protein are significantly upregulated in tumor biopsies compared with paired adjacent normal tissues resected from 117 gastric cancer patients.209 Patients with high ADGRF1 protein levels had shorter survival and increased recurrence after surgery compared with gastric cancer patients with low ADGRF1 expression. These data suggest that ADGRF1 may be a candidate biomarker for diagnostic purposes in gastric cancer patients. N-Docosahexaenoylethanolamine (synaptamide, a.k.a. DHEA), a stimulant of neurite growth, was recently shown to induce cyclic AMP production via ADGRF1.105 It would be interesting to know (a) whether the level of synaptamide, an endogenous metabolite ligand of ADGRF1, is altered in the gastric tumor microenvironment, (b) what the pathologic impact of synaptamide-ADGRF1 interaction is, and (c) what molecular mechanism(s) are used by ADGRF1 in gastric cancer cells.
Conclusions and Potential Therapeutic Approaches
Although the field of aGPCR research has seen constant growth in terms of engaged signaling pathways in the past decade, the physiological functions of these receptors are yet to be explored further. In particular, their role in all aspects of cancer, from tumor initiation to metastasis is incompletely understood. aGPCRs are implicated in diverse diseases from diabetes to various neoplasms. However, there are currently no approved drugs targeting any aGPCRs.
The difficulty in obtaining structural information from aGPCRs has hampered the process of developing small molecules or biologics to target them. On the other hand, the large NTF and its multiple domains provide potential sites to target therapeutically. The recent discovery of an ADGRG1 antagonist210 gives hope for the development of future small molecules with proper pharmacology to regulate the function of these understudied receptors in cancer. In addition to small molecules, antibodies against domains of the NTF can be potentially interesting modulators. Salzman et al. showed that monobodies designed for certain domains on the NTF of ADGRG1 can act as activators or inhibitors of G protein signaling.211 It remains to be explored whether antibodies against either tethered agonist or its binding site(s) will act as antagonists.
Another hypothetical approach that may modulate aGPCR function is the design of cell-permeable inhibitors of autoproteolysis, as this cleavage is required for activation of certain signaling pathways by ADGRG2 and ADGRG1.145,212
aGPCRs interact strongly with β-arrestins, particularly in the absence of NTF.39,143 The signaling bias of classical GPCRs toward either G protein or β-arrestin pathways and their physiological effects have challenged the drug development.213−215 This phenomenon should be taken into account in the development of aGPCR modulators too.
Fibrosis in the tumor microenvironment, a side effect of protease actions and metastasis, alters the composition of ECM dramatically.216 Given the ECM-binding domains on the NTF of some aGPCRs, it would be interesting to examine whether these changes modulate aGPCR activity and thereby proliferation and migration of tumor cells. Also, targeting the large ECM ligands of aGPCRs to interfere with the protein–protein interaction can be a potential approach to regulate aGPCR functions in cancer.
On average, aGPCRs have 19 transcript variants with tissue-dependent expression patterns, leading to functional differences.217 As mentioned above for ADGRF1 in prostate cancer and for ADGRE5 in gastric tumors, splice variants of aGPCRs show a differential impact on tumorigenesis. Therefore, it is crucial to use the genomics/bioinformatics tools such as RNA-Seq to quantify various transcripts of aGPCRs of interest in tumor specimens at the first stages of cancer studies.
The body of evidence provided here points to fundamental roles that aGPCRs may play in promotion or prevention of cancer, and we hope this would trigger future translational studies to explore their potential as therapeutic targets.
Acknowledgments
We thank the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center for continuous support. We are grateful to Michele Vitolo for careful review of the manuscript. This work was supported by NIH Grant R01GM130617 (to N.B.). Student support was provided by NIH-NIGMS Initiative for Maximizing Student Development Grant R25-GM55036 (to A.A.G.).
The authors declare no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
References
- Hall R. A.; Premont R. T.; Lefkowitz R. J. (1999) Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol. 145, 927–932. 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D. M.; Rasmussen S. G.; Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363. 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim Y. Y.; Zurawski Z.; Hamm H. (2018) GPCR regulation of secretion. Pharmacol. Ther. 192, 124–140. 10.1016/j.pharmthera.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco E. M.; Sotgia F.; Clarke R. B.; Lisanti M. P.; Maggiolini M. (2017) G Protein-Coupled Receptors at the Crossroad between Physiologic and Pathologic Angiogenesis: Old Paradigms and Emerging Concepts. Int. J. Mol. Sci. 18, 2713. 10.3390/ijms18122713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R.; Lagerstrom M. C.; Lundin L. G.; Schioth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272. 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Hauser A. S.; Attwood M. M.; Rask-Andersen M.; Schioth H. B.; Gloriam D. E. (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discovery 16, 829–842. 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. L.; Chien A. J.; Moon R. T. (2009) Wnt/Fz signaling and the cytoskeleton: potential roles in tumorigenesis. Cell Res. 19, 532–545. 10.1038/cr.2009.41. [DOI] [PubMed] [Google Scholar]
- Yu M.; He P.; Liu Y.; He Y.; Du Y.; Wu M.; Zhang G.; Yang C.; Gao F. (2015) Hyaluroan-regulated lymphatic permeability through S1P receptors is crucial for cancer metastasis. Med. Oncol. 32, 381. 10.1007/s12032-014-0381-1. [DOI] [PubMed] [Google Scholar]
- Liu Y.; et al. (2016) G protein-coupled receptors as promising cancer targets. Cancer Lett. 376, 226–239. 10.1016/j.canlet.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Dorsam R. T.; Gutkind J. S. (2007) G-protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94. 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Xu X.; Prestwich G. D. (2010) Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer 116, 1739–1750. 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. B.; et al. (2001) The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene 20, 1953–1963. 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- Hu L.; Roth J. M.; Brooks P.; Luty J.; Karpatkin S. (2008) Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 68, 4666–4673. 10.1158/0008-5472.CAN-07-6276. [DOI] [PubMed] [Google Scholar]
- Garcia-Nafria J.; Tate C. G. (2020) Cryo-Electron Microscopy: Moving Beyond X-Ray Crystal Structures for Drug Receptors and Drug Development. Annu. Rev. Pharmacol. Toxicol. 60, 51. 10.1146/annurev-pharmtox-010919-023545. [DOI] [PubMed] [Google Scholar]
- Du Y.; et al. (2019) Assembly of a GPCR-G Protein Complex. Cell 177, 1232–1242. 10.1016/j.cell.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; e1211.
- Insel P. A.; et al. (2019) GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol. Sci. 40, 378–387. 10.1016/j.tips.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V.; et al. (2019) Illuminating the Onco-GPCRome: Novel G protein-coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J. Biol. Chem. 294, 11062–11086. 10.1074/jbc.REV119.005601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J.; et al. (2015) International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol. Rev. 67, 338–367. 10.1124/pr.114.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust G.; Zhu D.; Van Meir E. G.; Xu L. (2016) Adhesion GPCRs in Tumorigenesis. Handb. Exp. Pharmacol. 234, 369–396. 10.1007/978-3-319-41523-9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; et al. (2005) CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood 105, 2836–2844. 10.1182/blood-2004-07-2878. [DOI] [PubMed] [Google Scholar]
- Luo R.; et al. (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc. Natl. Acad. Sci. U. S. A. 108, 12925–12930. 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola K. J.; Sidik H.; Zuchero J. B.; Eckart M.; Talbot W. S. (2014) Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signaling 7, ra76. 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arac D.; et al. (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 31, 1364–1378. 10.1038/emboj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T.; Aust G.; Hamann J. (2013) Sticky signaling--adhesion class G protein-coupled receptors take the stage. Sci. Signaling 6, re3. 10.1126/scisignal.2003825. [DOI] [PubMed] [Google Scholar]
- Purcell R. H.; Hall R. A. (2018) Adhesion G Protein-Coupled Receptors as Drug Targets. Annu. Rev. Pharmacol. Toxicol. 58, 429–449. 10.1146/annurev-pharmtox-010617-052933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima D.; Kudo G.; Yokota H. (2010) Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J. Recept. Signal Transduction Res. 30, 143–153. 10.3109/10799891003671139. [DOI] [PubMed] [Google Scholar]
- Paavola K. J.; Stephenson J. R.; Ritter S. L.; Alter S. P.; Hall R. A. (2011) The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J. Biol. Chem. 286, 28914–28921. 10.1074/jbc.M111.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balenga N.; et al. (2017) Orphan Adhesion GPCR GPR64/ADGRG2 Is Overexpressed in Parathyroid Tumors and Attenuates Calcium-Sensing Receptor-Mediated Signaling. J. Bone Miner. Res. 32, 654–666. 10.1002/jbmr.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demberg L. M.; Rothemund S.; Schoneberg T.; Liebscher I. (2015) Identification of the tethered peptide agonist of the adhesion G protein-coupled receptor GPR64/ADGRG2. Biochem. Biophys. Res. Commun. 464, 743–747. 10.1016/j.bbrc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Stoveken H. M.; Hajduczok A. G.; Xu L.; Tall G. G. (2015) Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc. Natl. Acad. Sci. U. S. A. 112, 6194–6199. 10.1073/pnas.1421785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y.; et al. (2011) LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 71, 7301–7311. 10.1158/0008-5472.CAN-11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher I.; et al. (2014) A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 9, 2018–2026. 10.1016/j.celrep.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C.; et al. (2016) The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J. 30, 666–673. 10.1096/fj.15-276220. [DOI] [PubMed] [Google Scholar]
- Shima Y.; Kengaku M.; Hirano T.; Takeichi M.; Uemura T. (2004) Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev. Cell 7, 205–216. 10.1016/j.devcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Gray J. X.; et al. (1996) CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J. Immunol. 157, 5438–5447. [PubMed] [Google Scholar]
- Krasnoperov V.; et al. (2009) Dissociation of the subunits of the calcium-independent receptor of alpha-latrotoxin as a result of two-step proteolysis. Biochemistry 48, 3230–3238. 10.1021/bi802163p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B.; Brat D. J.; Devi N. S.; Van Meir E. G. (2005) Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 24, 3632–3642. 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- Kishore A.; Purcell R. H.; Nassiri-Toosi Z.; Hall R. A. (2016) Stalk-dependent and Stalk-independent Signaling by the Adhesion G Protein-coupled Receptors GPR56 (ADGRG1) and BAI1 (ADGRB1). J. Biol. Chem. 291, 3385–3394. 10.1074/jbc.M115.689349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S.; Ichtchenko K.; Khvotchev M.; Sudhof T. C. (1998) alpha-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J. Biol. Chem. 273, 32715–32724. 10.1074/jbc.273.49.32715. [DOI] [PubMed] [Google Scholar]
- Silva J. P.; et al. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. U. S. A. 108, 12113–12118. 10.1073/pnas.1019434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard A. A.; Maxeiner S.; Sudhof T. C. (2014) Latrophilins function as heterophilic cell-adhesion molecules by binding to teneurins: regulation by alternative splicing. J. Biol. Chem. 289, 387–402. 10.1074/jbc.M113.504779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M. L.; et al. (2012) FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 73, 903–910. 10.1016/j.neuron.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard A. A.; Ko J.; Sudhof T. C. (2012) High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J. Biol. Chem. 287, 9399–9413. 10.1074/jbc.M111.318659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelianova V. G.; et al. (1997) Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J. Biol. Chem. 272, 21504–21508. 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Kocibalova Z.; Guzyova M.; Imrichova D.; Sulova Z.; Breier A. (2018) Overexpression of the ABCB1 drug transporter in acute myeloid leukemia cells is associated with downregulation of latrophilin-1. Gen Physiol Biophys 37, 353–357. 10.4149/gpb_2018008. [DOI] [PubMed] [Google Scholar]
- Yasinska I. M.; Sakhnevych S. S.; Pavlova L.; Teo Hansen Selnø A.; Teuscher Abeleira A. M.; Benlaouer O.; Goncalves Silva I.; Mosimann M.; Varani L.; Bardelli M.; Hussain R.; Siligardi G.; Cholewa D.; Berger S. M.; Gibbs B. F.; Ushkaryov Y. A.; Fasler-Kan E.; Klenova E.; Sumbayev V. V. (2019) The Tim-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front. Immunol. 10, 1594. 10.3389/fimmu.2019.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. X.; et al. (2013) Whole-exome sequencing to identify novel somatic mutations in squamous cell lung cancers. Int. J. Oncol. 43, 755–764. 10.3892/ijo.2013.1991. [DOI] [PubMed] [Google Scholar]
- Lu Y. C.; et al. (2015) Structural Basis of Latrophilin-FLRT-UNC5 Interaction in Cell Adhesion. Structure 23, 1678–1691. 10.1016/j.str.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotepui M.; Thawornkuno C.; Chavalitshewinkoon-Petmitr P.; Punyarit P.; Petmitr S. (2012) Quantitative real-time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G, KLB and MMP13 mRNA expression in breast cancer. Asian Pac J. Cancer Prev 13, 5879–5882. 10.7314/APJCP.2012.13.11.5879. [DOI] [PubMed] [Google Scholar]
- Favara D. M.; Banham A. H.; Harris A. L. (2014) A review of ELTD1, a pro-angiogenic adhesion GPCR. Biochem. Soc. Trans. 42, 1658–1664. 10.1042/BST20140216. [DOI] [PubMed] [Google Scholar]
- Bader J. E.; et al. (2018) Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. American journal of physiology. Gastrointestinal and liver physiology 314, G22–G31. 10.1152/ajpgi.00229.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiga A.; et al. (2016) Transcriptome analysis of G protein-coupled receptors in distinct genetic subgroups of acute myeloid leukemia: identification of potential disease-specific targets. Blood Cancer J. 6, e431. 10.1038/bcj.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M.; et al. (2003) The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 102, 2916–2924. 10.1182/blood-2002-11-3540. [DOI] [PubMed] [Google Scholar]
- I K.-Y.; Huang Y.-S.; Hu C.-H.; Tseng W.-Y.; Cheng C.-H.; Stacey M.; Gordon S.; Chang G.-W.; Lin H.-H. (2017) Activation of Adhesion GPCR EMR2/ADGRE2 Induces Macrophage Differentiation and Inflammatory Responses via Galpha16/Akt/MAPK/NF-kappaB Signaling Pathways. Front. Immunol. 8, 373. 10.3389/fimmu.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano A.; et al. (2017) miR-99a reveals two novel oncogenic proteins E2F2 and EMR2 and represses stemness in lung cancer. Cell Death Dis. 8, e3141. 10.1038/cddis.2017.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. X.; et al. (2009) WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62. 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M. E.; et al. (2015) Epidermal growth factor-like module containing mucin-like hormone receptor 2 expression in gliomas. J. Neuro-Oncol. 121, 53–61. 10.1007/s11060-014-1606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. Q.; et al. (2011) Leukocyte adhesion-GPCR EMR2 is aberrantly expressed in human breast carcinomas and is associated with patient survival. Oncol. Rep. 25, 619–627. [DOI] [PubMed] [Google Scholar]
- Grone J.; et al. (2011) Molecular profiles and clinical outcome of stage UICC II colon cancer patients. International journal of colorectal disease 26, 847–858. 10.1007/s00384-011-1176-x. [DOI] [PubMed] [Google Scholar]
- Wang Y.; et al. (2004) Gene expression profiles and molecular markers to predict recurrence of Dukes’ B colon cancer. J. Clin. Oncol. 22, 1564–1571. 10.1200/JCO.2004.08.186. [DOI] [PubMed] [Google Scholar]
- Archer K. J.; Mas V. R.; Maluf D. G.; Fisher R. A. (2010) High-throughput assessment of CpG site methylation for distinguishing between HCV-cirrhosis and HCV-associated hepatocellular carcinoma. Mol. Genet. Genomics 283, 341–349. 10.1007/s00438-010-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane A. J.; Sughrue M. E.; Rutkowski M. J.; Phillips J. J.; Parsa A. T. (2010) EMR-3: a potential mediator of invasive phenotypic variation in glioblastoma and novel therapeutic target. NeuroReport 21, 1018–1022. 10.1097/WNR.0b013e32833f19f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J.; Vogel B.; van Schijndel G. M.; van Lier R. A. (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184, 1185–1189. 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandel E.; Saalbach A.; Sittig D.; Gebhardt C.; Aust G. (2012) Thy-1 (CD90) is an interacting partner for CD97 on activated endothelial cells. J. Immunol. 188, 1442–1450. 10.4049/jimmunol.1003944. [DOI] [PubMed] [Google Scholar]
- Wobus M.; Huber O.; Hamann J.; Aust G. (2006) CD97 overexpression in tumor cells at the invasion front in colorectal cancer (CC) is independently regulated of the canonical Wnt pathway. Mol. Carcinog. 45, 881–886. 10.1002/mc.20262. [DOI] [PubMed] [Google Scholar]
- Eichler W.; Hamann J.; Aust G. (1997) Expression characteristics of the human CD97 antigen. Tissue Antigens 50, 429–438. 10.1111/j.1399-0039.1997.tb02897.x. [DOI] [PubMed] [Google Scholar]
- Hilbig D.; Dietrich N.; Wandel E.; Gonsior S.; Sittig D.; Hamann J.; Aust G. (2018) The Interaction of CD97/ADGRE5 With beta-Catenin in Adherens Junctions Is Lost During Colorectal Carcinogenesis. Front. Oncol. 8, 182. 10.3389/fonc.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M.; et al. (2002) Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am. J. Pathol. 161, 1657–1667. 10.1016/S0002-9440(10)64443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. L.; et al. (2010) The impact of expressions of CD97 and its ligand CD55 at the invasion front on prognosis of rectal adenocarcinoma. International journal of colorectal disease 25, 695–702. 10.1007/s00384-010-0926-5. [DOI] [PubMed] [Google Scholar]
- He Z.; Wu H.; Jiao Y.; Zheng J. (2015) Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol. Lett. 9, 793–797. 10.3892/ol.2014.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z. W.; Liu M. C.; Hong H. J.; Du Q.; Chen Y. L. (2017) Expression and prognostic value of soluble CD97 and its ligand CD55 in intrahepatic cholangiocarcinoma. Tumor Biol. 39, 4319. 10.1177/1010428317694319. [DOI] [PubMed] [Google Scholar]
- Li C.; et al. (2015) CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J. Gastroenterol 21, 6215–6228. 10.3748/wjg.v21.i20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.; et al. (2017) Effects of targeted CD97 immune epitopes small interference RNA on cellular biological behaviors in MDA-MB231 malignant breast cancer cell line. Am. J. Transl Res. 9, 4640–4651. [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; et al. (2018) CD97 Promotes Tumor Aggressiveness Through the Traditional G Protein-Coupled Receptor-Mediated Signaling in Hepatocellular Carcinoma. Hepatology 68, 1865–1878. 10.1002/hep.30068. [DOI] [PubMed] [Google Scholar]
- Singh V.; et al. (2015) Esophageal Cancer Epigenomics and Integrome Analysis of Genome-Wide Methylation and Expression in High Risk Northeast Indian Population. OMICS 19, 688–699. 10.1089/omi.2015.0121. [DOI] [PubMed] [Google Scholar]
- Safaee M.; et al. (2015) Proportional upregulation of CD97 isoforms in glioblastoma and glioblastoma-derived brain tumor initiating cells. PLoS One 10, e0111532. 10.1371/journal.pone.0111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G. B.; Kim D. (2019) MicroRNA-503–5p Inhibits the CD97-Mediated JAK2/STAT3 Pathway in Metastatic or Paclitaxel-Resistant Ovarian Cancer Cells. Neoplasia 21, 206–215. 10.1016/j.neo.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Lei L.; Wang S.; Gu D.; Zhang J. (2012) Immunohistochemical expression and prognostic value of CD97 and its ligand CD55 in primary gallbladder carcinoma. J. Biomed. Biotechnol. 2012, 587672. 10.1155/2012/587672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus M.; Bornhauser M.; Jacobi A.; Krater M.; Otto O.; Ortlepp C.; Guck J.; Ehninger G.; Thiede C.; Oelschlagel U. (2015) Association of the EGF-TM7 receptor CD97 expression with FLT3-ITD in acute myeloid leukemia. Oncotarget 6, 38804–38815. 10.18632/oncotarget.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikari V. P.; Yang J.; Wu S.; Alachkar H. (2019) CD97 expression is associated with poor overall survival in acute myeloid leukemia. Exp. Hematol. 75, 64–73. 10.1016/j.exphem.2019.06.474. [DOI] [PMC free article] [PubMed] [Google Scholar]; e64.
- Mustafa T.; et al. (2005) Expression of the epidermal growth factor seven-transmembrane member CD97 correlates with grading and staging in human oral squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev 14, 108–119. [PubMed] [Google Scholar]
- Hoang-Vu C.; Bull K.; Schwarz I.; Krause G.; Schmutzler C.; Aust G.; Kohrle J.; Dralle H. (1999) Regulation of CD97 protein in thyroid carcinoma. J. Clin. Endocrinol. Metab. 84, 1104–1109. 10.1210/jcem.84.3.5557. [DOI] [PubMed] [Google Scholar]
- Aust G.; Hamann J.; Schilling N.; Wobus M. (2003) Detection of alternatively spliced EMR2 mRNAs in colorectal tumor cell lines but rare expression of the molecule in colorectal adenocarcinomas. Virchows Arch. 443, 32–37. 10.1007/s00428-003-0812-4. [DOI] [PubMed] [Google Scholar]
- Vallon M.; Essler M. (2006) Proteolytically processed soluble tumor endothelial marker (TEM) 5 mediates endothelial cell survival during angiogenesis by linking integrin alpha(v)beta3 to glycosaminoglycans. J. Biol. Chem. 281, 34179–34188. 10.1074/jbc.M605291200. [DOI] [PubMed] [Google Scholar]
- Posokhova E.; et al. (2015) GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 10, 123–130. 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry A. E.; Vicente J. J.; Xu C.; Morrison R. S.; Ong S.-E.; Wordeman L.; Stella N. (2019) GPR124 regulates microtubule assembly, mitotic progression, and glioblastoma cell proliferation. Glia 67, 1558–1570. 10.1002/glia.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Li Y.; Zhou F.; Piao Z.; Hao J. (2018) beta-elemene enhances anticancer bone neoplasms efficacy of paclitaxel through regulation of GPR124 in bone neoplasms cells. Oncol. Lett. 16, 2143–2150. 10.3892/ol.2018.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. V.; Platt F. M.; Hurst C. D.; Aveyard J. S.; Taylor C. F.; Pole J. C.M.; Garcia M. J.; Knowles M. A. (2010) High-resolution analysis of genomic alteration on chromosome arm 8p in urothelial carcinoma. Genes, Chromosomes Cancer 49, 642–659. 10.1002/gcc.20775. [DOI] [PubMed] [Google Scholar]
- Wu Y.; et al. (2018) Elevated G-Protein Receptor 125 (GPR125) Expression Predicts Good Outcomes in Colorectal Cancer and Inhibits Wnt/beta-Catenin Signaling Pathway. Med. Sci. Monit. 24, 6608–6616. 10.12659/MSM.910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. L.; et al. (2010) The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum. Mol. Genet. 19, 2251–2267. 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y.; et al. (2007) Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat. Neurosci. 10, 963–969. 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- Jiang L.; et al. (2018) Differential cellular localization of CELSR2 and ING4 and correlations with hormone receptor status in breast cancer. Histology and histopathology 33, 835–842. [DOI] [PubMed] [Google Scholar]
- Khor G. H.; Froemming G. R.; Zain R. B.; Abraham T. M.; Lin T. K. (2016) Involvement of CELSR3 Hypermethylation in Primary Oral Squamous Cell Carcinoma. Asian Pac J. Cancer Prev 17, 219–223. 10.7314/APJCP.2016.17.1.219. [DOI] [PubMed] [Google Scholar]
- Gu X.; et al. (2019) CELSR3 mRNA expression is increased in hepatocellular carcinoma and indicates poor prognosis. PeerJ 7, e7816. 10.7717/peerj.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Liu G.; Wang D.; Li Y. (2019) Analysis of lncRNA-Mediated ceRNA Crosstalk and Identification of Prognostic Signature in Head and Neck Squamous Cell Carcinoma. Front. Pharmacol. 10, 150. 10.3389/fphar.2019.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnekamp J.; Schoneberg T. (2011) Cell adhesion receptor GPR133 couples to Gs protein. J. Biol. Chem. 286, 41912–41916. 10.1074/jbc.C111.265934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayin N. S.; et al. (2016) GPR133 (ADGRD1), an adhesion G-protein-coupled receptor, is necessary for glioblastoma growth. Oncogenesis 5, e263. 10.1038/oncsis.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Wu S.; Alachkar H. (2019) Characterization of upregulated adhesion GPCRs in acute myeloid leukemia. Transl Res. 212, 26–35. 10.1016/j.trsl.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. L.; et al. (2019) Differentially expressed long noncoding RNAs and regulatory mechanism of LINC02407 in human gastric adenocarcinoma. World journal of gastroenterology 25, 5973–5990. 10.3748/wjg.v25.i39.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. J.; et al. (2015) Characterization of Somatic Mutations in Air Pollution-Related Lung Cancer. EBioMedicine 2, 583–590. 10.1016/j.ebiom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-W.; Huang B. X.; Kwon H.; Rashid M. A.; Kharebava G.; Desai A.; Patnaik S.; Marugan J.; Kim H.-Y. (2016) Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 7, 13123. 10.1038/ncomms13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum A. M; Wang B. B; Beck-Engeser G. B; Li L.; Channa N.; Wabl M. (2010) Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer. BMC Cancer 10, 40. 10.1186/1471-2407-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K.; et al. (2017) Polymorphisms in genes related to epithelial-mesenchymal transition and risk of non-small cell lung cancer. Carcinogenesis 38, 1029–1035. 10.1093/carcin/bgx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A. N.; Ahmad M. W.; Madar I. H.; Grace B. L.; Hasan T. N. (2015) An in silico analytical study of lung cancer and smokers datasets from gene expression omnibus (GEO) for prediction of differentially expressed genes. Bioinformation 11, 229–235. 10.6026/97320630011229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. R.; et al. (2018) GPCRs profiling and identification of GPR110 as a potential new target in HER2+ breast cancer. Breast Cancer Res. Treat. 170, 279–292. 10.1007/s10549-018-4751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras T.; et al. (2017) Differential expression of MUC4, GPR110 and IL2RA defines two groups of CRLF2-rearranged acute lymphoblastic leukemia patients with distinct secondary lesions. Cancer Lett. 408, 92–101. 10.1016/j.canlet.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhang G.; Zhao C.; Li J. (2018) Clinical Significance of G Protein-Coupled Receptor 110 (GPR110) as a Novel Prognostic Biomarker in Osteosarcoma. Med. Sci. Monit. 24, 5216–5224. 10.12659/MSM.909555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.; et al. (2017) Gpr110 deficiency decelerates carcinogen-induced hepatocarcinogenesis via activation of the IL-6/STAT3 pathway. Am. J. Cancer Res. 7, 433–447. [PMC free article] [PubMed] [Google Scholar]
- Espinal-Enriquez J.; Munoz-Montero S.; Imaz-Rosshandler I.; Huerta-Verde A.; Mejia C.; Hernandez-Lemus E. (2015) Genome-wide expression analysis suggests a crucial role of dysregulation of matrix metalloproteinases pathway in undifferentiated thyroid carcinoma. BMC Genomics 16, 207. 10.1186/s12864-015-1372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. C.; Sherman S. K.; Wang D.; Dahdaleh F. S.; Bellizzi A. M.; O’Dorisio M. S.; O’Dorisio T. M.; Howe J. R.; et al. (2013) Overexpression of membrane proteins in primary and metastatic gastrointestinal neuroendocrine tumors. Ann. Surg Oncol 20, S739–S746. 10.1245/s10434-013-3318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. K.; et al. (2014) GIPR expression in gastric and duodenal neuroendocrine tumors. J. Surg. Res. 190, 587–593. 10.1016/j.jss.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte J.; et al. (2012) Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett. 586, 1214–1219. 10.1016/j.febslet.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Cui Q.; Qu W.; Ding X.; Jiang D.; Liu H. (2018) TRIM58/cg26157385 methylation is associated with eight prognostic genes in lung squamous cell carcinoma. Oncol. Rep. 40, 206–216. 10.3892/or.2018.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa T.; et al. (2013) Lung surfactant levels are regulated by Ig-Hepta/GPR116 by monitoring surfactant protein D. PLoS One 8, e69451. 10.1371/journal.pone.0069451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.; et al. (2013) GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Galphaq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 73, 6206–6218. 10.1158/0008-5472.CAN-13-1049. [DOI] [PubMed] [Google Scholar]
- Yang L.; Lin X.-L.; Liang W.; Fu S.-W.; Lin W.-F.; Tian X.-Q.; Gao Y.-J.; Chen H.-Y.; Dai J.; Ge Z.-Z. (2017) High expression of GPR116 indicates poor survival outcome and promotes tumor progression in colorectal carcinoma. Oncotarget 8, 47943–47956. 10.18632/oncotarget.18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G.; et al. (2013) Exome sequencing identifies early gastric carcinoma as an early stage of advanced gastric cancer. PLoS One 8, e82770. 10.1371/journal.pone.0082770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.; et al. (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434. 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- Cork S. M.; et al. (2012) A proprotein convertase/MMP-14 proteolytic cascade releases a novel 40 kDa vasculostatin from tumor suppressor BAI1. Oncogene 31, 5144–5152. 10.1038/onc.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H; Oshika Y; Abe Y; Yoshida Y; Hashimoto T; Handa A; Kijima H; Yamazaki H; Inoue H; Ueyama Y; Nakamura M (2000) Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int. J. Mol. Med. 5, 181–183. 10.3892/ijmm.5.2.181. [DOI] [PubMed] [Google Scholar]
- Kaur B.; Brat D. J.; Calkins C. C.; Van Meir E. G. (2003) Brain angiogenesis inhibitor 1 is differentially expressed in normal brain and glioblastoma independently of p53 expression. Am. J. Pathol. 162, 19–27. 10.1016/S0002-9440(10)63794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y; Oshika Y; Fukushima Y; Tokunaga T; Hatanaka H; Kijima H; Yamazaki H; Ueyama Y; Tamaoki N; Miura S; Nakamura M (1999) Expression of angiostatic factors in colorectal cancer. Int. J. Oncol. 15, 1221–1225. 10.3892/ijo.15.6.1221. [DOI] [PubMed] [Google Scholar]
- Fukushima Y; Oshika Y; Tsuchida T; Tokunaga T; Hatanaka H; Kijima H; Yamazaki H; Ueyama Y; Tamaoki N; Nakamura M (1998) Brain-specific angiogenesis inhibitor 1 expression is inversely correlated with vascularity and distant metastasis of colorectal cancer. Int. J. Oncol. 13, 967–970. 10.3892/ijo.13.5.967. [DOI] [PubMed] [Google Scholar]
- Wang W.; et al. (2013) Expression of brain-specific angiogenesis inhibitor 1 is inversely correlated with pathological grade, angiogenesis and peritumoral brain edema in human astrocytomas. Oncol. Lett. 5, 1513–1518. 10.3892/ol.2013.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisen W. H.; et al. (2015) Changes in BAI1 and nestin expression are prognostic indicators for survival and metastases in breast cancer and provide opportunities for dual targeted therapies. Mol. Cancer Ther. 14, 307–314. 10.1158/1535-7163.MCT-14-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zencir S.; et al. (2011) Identification of brain-specific angiogenesis inhibitor 2 as an interaction partner of glutaminase interacting protein. Biochem. Biophys. Res. Commun. 411, 792–797. 10.1016/j.bbrc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. C.; et al. (2008) Gene expression profiling: canonical molecular changes and clinicopathological features in sporadic colorectal cancers. World journal of gastroenterology 14, 6662–6672. 10.3748/wjg.14.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger M. F.; Martinelli D. C.; Sudhof T. C. (2011) The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc. Natl. Acad. Sci. U. S. A. 108, 2534–2539. 10.1073/pnas.1019577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M. F.; et al. (2014) BAI3, CDX2 and VIL1: a panel of three antibodies to distinguish small cell from large cell neuroendocrine lung carcinomas. Histopathology 64, 547–556. 10.1111/his.12278. [DOI] [PubMed] [Google Scholar]
- Kee H. J.; et al. (2004) Expression of brain-specific angiogenesis inhibitor 3 (BAI3) in normal brain and implications for BAI3 in ischemia-induced brain angiogenesis and malignant glioma. FEBS Lett. 569, 307–316. 10.1016/j.febslet.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Xu L.; Begum S.; Hearn J. D.; Hynes R. O. (2006) GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A. 103, 9023–9028. 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little K. D.; Hemler M. E.; Stipp C. S. (2004) Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Galpha q/11 association. Mol. Biol. Cell 15, 2375–2387. 10.1091/mbc.e03-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T.; et al. (2008) Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J. Biol. Chem. 283, 14469–14478. 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- Ji B.; Feng Y.; Sun Y.; Ji D.; Qian W.; Zhang Z.; Wang Q.; Zhang Y.; Zhang C.; Sun Y. (2018) GPR56 promotes proliferation of colorectal cancer cells and enhances metastasis via epithelialmesenchymal transition through PI3K/AKT signaling activation. Oncol. Rep. 40, 1885–1896. 10.3892/or.2018.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; et al. (2011) GPR56 Regulates VEGF production and angiogenesis during melanoma progression. Cancer Res. 71, 5558–5568. 10.1158/0008-5472.CAN-10-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N. Y.; et al. (2017) GPR56/ADGRG1 Activation Promotes Melanoma Cell Migration via NTF Dissociation and CTF-Mediated Galpha12/13/RhoA Signaling. J. Invest. Dermatol. 137, 727–736. 10.1016/j.jid.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Xu L.; et al. (2010) GPR56 plays varying roles in endogenous cancer progression. Clin. Exp. Metastasis 27, 241–249. 10.1007/s10585-010-9322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga S.; et al. (2019) High GPR56 surface expression correlates with a leukemic stem cell gene signature in CD34-positive AML. Cancer Med. 8, 1771–1778. 10.1002/cam4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh P.; Talamantez-Lyburn S. C.; Chang K. T.; Inoue A.; Balenga N. (2019) Spatial regulation of GPR64/ADGRG2 signaling by beta-arrestins and GPCR kinases. Ann. N. Y. Acad. Sci. 1456, 26–43. 10.1111/nyas.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.-L.; Sun Y.-J.; Ma M.-L.; Wang Y.-j.; Lin H.; Li R.-R.; Liang Z.-L.; Gao Y.; Yang Z.; He D.-F.; Lin A.; Mo H.; Lu Y.-J.; Li M.-J.; Kong W.; Chung K. Y.; Yi F.; Li J.-Y.; Qin Y.-Y.; Li J.; Thomsen A. R B; Kahsai A. W; Chen Z.-J.; Xu Z.-G.; Liu M.; Li D.; Yu X.; Sun J.-P. (2018) Gq activity- and beta-arrestin-1 scaffolding-mediated ADGRG2/CFTR coupling are required for male fertility. eLife 7, 33432. 10.7554/eLife.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M. C.; et al. (2015) The adhesion G protein-coupled receptor G2 (ADGRG2/GPR64) constitutively activates SRE and NFkappaB and is involved in cell adhesion and migration. Cell. Signalling 27, 2579–2588. 10.1016/j.cellsig.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Richter G. H.; et al. (2013) G-Protein coupled receptor 64 promotes invasiveness and metastasis in Ewing sarcomas through PGF and MMP1. J. Pathol 230, 70–81. 10.1002/path.4170. [DOI] [PubMed] [Google Scholar]
- Ahn J. I.; Yoo J.-Y.; Kim T. H.; Kim Y. I.; Broaddus R. R.; Ahn J. Y.; Lim J. M.; Jeong J.-W. (2019) G-protein coupled receptor 64 (GPR64) acts as a tumor suppressor in endometrial cancer. BMC Cancer 19, 810. 10.1186/s12885-019-5998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leja J.; et al. (2009) Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod. Pathol. 22, 261–272. 10.1038/modpathol.2008.174. [DOI] [PubMed] [Google Scholar]
- Petersen S. C.; et al. (2015) The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 85, 755–769. 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinet S.; et al. (2019) High Prevalence of a Hotspot of Noncoding Somatic Mutations in Intron 6 of GPR126 in Bladder Cancer. Mol. Cancer Res. 17, 469–475. 10.1158/1541-7786.MCR-18-0363. [DOI] [PubMed] [Google Scholar]
- Chase A.; et al. (2010) TFG, a target of chromosome translocations in lymphoma and soft tissue tumors, fuses to GPR128 in healthy individuals. Haematologica 95, 20–26. 10.3324/haematol.2009.011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. X.; et al. (2014) Constitutive Galphai coupling activity of very large G protein-coupled receptor 1 (VLGR1) and its regulation by PDZD7 protein. J. Biol. Chem. 289, 24215–24225. 10.1074/jbc.M114.549816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.; Lin S. T.; Fu Y. H.; Ptacek L. J. (2013) Very large G protein-coupled receptor 1 regulates myelin-associated glycoprotein via Galphas/Galphaq-mediated protein kinases A/C. Proc. Natl. Acad. Sci. U. S. A. 110, 19101–19106. 10.1073/pnas.1318501110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q.; et al. (2017) [MiR-145 inhibits drug resistance to Oxaliplatin in colorectal cancer cells through regulating G protein coupled receptor 98]. Zhonghua wei chang wai ke za zhi = Chinese journal of gastrointestinal surgery 20, 566–570. [PubMed] [Google Scholar]
- Dai S.; Wang X.; Li X.; Cao Y. (2015) MicroRNA-139–5p acts as a tumor suppressor by targeting ELTD1 and regulating cell cycle in glioblastoma multiforme. Biochem. Biophys. Res. Commun. 467, 204–210. 10.1016/j.bbrc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Serban F.; et al. (2017) Silencing of epidermal growth factor, latrophilin and seven transmembrane domain-containing protein 1 (ELTD1) via siRNA-induced cell death in glioblastoma. J. Immunoassay Immunochem. 38, 21–33. 10.1080/15321819.2016.1209217. [DOI] [PubMed] [Google Scholar]
- Millar M. W.; Corson N.; Xu L. (2018) The Adhesion G-Protein-Coupled Receptor, GPR56/ADGRG1, Inhibits Cell-Extracellular Matrix Signaling to Prevent Metastatic Melanoma Growth. Front. Oncol. 8, 8. 10.3389/fonc.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarko O.; et al. (2018) A Comprehensive Mutagenesis Screen of the Adhesion GPCR Latrophilin-1/ADGRL1. iScience 3, 264–278. 10.1016/j.isci.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Institute for Cancer Research (2018) Global cancer statistics for the most common cancers, Vol. 2019.
- Bray F.; et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 68, 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Kan Z.; et al. (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873. 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Kang X.; et al. (2006) Antiangiogenic activity of BAI1 in vivo: implications for gene therapy of human glioblastomas. Cancer Gene Ther. 13, 385–392. 10.1038/sj.cgt.7700898. [DOI] [PubMed] [Google Scholar]
- Jeong B. C.; et al. (2006) Brain-specific angiogenesis inhibitor 2 regulates VEGF through GABP that acts as a transcriptional repressor. FEBS Lett. 580, 669–676. 10.1016/j.febslet.2005.12.086. [DOI] [PubMed] [Google Scholar]
- Lee D. K.; et al. (2004) Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J. Biol. Chem. 279, 7901–7908. 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Jong Y. J.; O’Malley K. L. (2008) Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J. Biol. Chem. 283, 14072–14083. 10.1074/jbc.M708551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. K.; et al. (2012) beta-arrestin1 mediates metastatic growth of breast cancer cells by facilitating HIF-1-dependent VEGF expression. Oncogene 31, 282–292. 10.1038/onc.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfrocca R.; Tocci P.; Rosano L.; Caprara V.; Sestito R.; Di Castro V.; Bagnato A. (2016) Nuclear beta-arrestin1 is a critical cofactor of hypoxia-inducible factor-1alpha signaling in endothelin-1-induced ovarian tumor progression. Oncotarget 7, 17790–17804. 10.18632/oncotarget.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoud N.; Tran V.; Aimi T.; Kakegawa W.; Lahaie S.; Thibault M.-P.; Pelletier A.; Wong G. W.; Kim I.-S.; Kania A.; Yuzaki M.; Bouvier M.; Cote J.-F. (2018) Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion. Nat. Commun. 9, 4470. 10.1038/s41467-018-06897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Croce C. M. (2016) The role of MicroRNAs in human cancer. Signal Transduct Target Ther 1, 15004. 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; et al. (2014) miR-138–5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem. Biophys. Res. Commun. 446, 179–186. 10.1016/j.bbrc.2014.02.073. [DOI] [PubMed] [Google Scholar]
- Wheeler D. L.; Dunn E. F.; Harari P. M. (2010) Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 7, 493–507. 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. D.; et al. (2011) Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. U. S. A. 108, 2807–2812. 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C.; Smallwood P. M.; Nathans J. (2017) Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron 95, 1056–1073. 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; e1055.
- Shannon J. M.; et al. (2003) Chondroitin sulfate proteoglycans are required for lung growth and morphogenesis in vitro. American journal of physiology. Lung cellular and molecular physiology 285, L1323–1336. 10.1152/ajplung.00226.2003. [DOI] [PubMed] [Google Scholar]
- Li G.; et al. (2017) Glycosaminoglycans and glycolipids as potential biomarkers in lung cancer. Glycoconjugate J. 34, 661–669. 10.1007/s10719-017-9790-7. [DOI] [PubMed] [Google Scholar]
- Ranzani M.; Annunziato S.; Adams D. J.; Montini E. (2013) Cancer gene discovery: exploiting insertional mutagenesis. Mol. Cancer Res. 11, 1141–1158. 10.1158/1541-7786.MCR-13-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K.; Suzuki T.; Stephens R. M.; Jenkins N. A.; Copeland N. G. (2004) RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 32, D523–527. 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren A. G.; et al. (2008) Large-scale mutagenesis in p19(ARF)- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell 133, 727–741. 10.1016/j.cell.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; et al. (2005) Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin. Cancer Res. 11, 4357–4364. 10.1158/1078-0432.CCR-04-2107. [DOI] [PubMed] [Google Scholar]
- Wilson K. S.; Roberts H.; Leek R.; Harris A. L.; Geradts J. (2002) Differential gene expression patterns in HER2/neu-positive and -negative breast cancer cell lines and tissues. Am. J. Pathol. 161, 1171–1185. 10.1016/S0002-9440(10)64394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashaie M. A.; Chowdhury E. H. (2016) Cadherins: The Superfamily Critically Involved in Breast Cancer. Curr. Pharm. Des. 22, 616–638. 10.2174/138161282205160127095338. [DOI] [PubMed] [Google Scholar]
- Usui T.; et al. (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585–595. 10.1016/S0092-8674(00)80046-X. [DOI] [PubMed] [Google Scholar]
- Katoh Y.; Katoh M. (2006) Comparative integromics on FAT1, FAT2, FAT3 and FAT4. Int. J. Mol. Med. 18, 523–528. 10.3892/ijmm.18.3.523. [DOI] [PubMed] [Google Scholar]
- Volpert O. V.; et al. (2002) Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 8, 349–357. 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Maubant S.; et al. (2006) Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood 108, 3035–3044. 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- Kotecki N.; Lefranc F.; Devriendt D.; Awada A. (2018) Therapy of breast cancer brain metastases: challenges, emerging treatments and perspectives. Ther Adv. Med. Oncol 10, 80312. 10.1177/1758835918780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjd Z.; et al. (2004) Loss of CD55 is associated with aggressive breast tumors. Clin. Cancer Res. 10, 2797–2803. 10.1158/1078-0432.CCR-1073-03. [DOI] [PubMed] [Google Scholar]
- Ikeda J.; et al. (2008) Prognostic significance of CD55 expression in breast cancer. Clin. Cancer Res. 14, 4780–4786. 10.1158/1078-0432.CCR-07-1844. [DOI] [PubMed] [Google Scholar]
- Holch J.; Stintzing S.; Heinemann V. (2016) Treatment of Metastatic Colorectal Cancer: Standard of Care and Future Perspectives. Visc Med. 32, 178–183. 10.1159/000446052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkel M.; Riemer P.; Blaker H.; Sers C. (2015) Similar but different: distinct roles for KRAS and BRAF oncogenes in colorectal cancer development and therapy resistance. Oncotarget 6, 20785–20800. 10.18632/oncotarget.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R.; Vogelstein B. (1990) A genetic model for colorectal tumorigenesis. Cell 61, 759–767. 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- Zhan T.; Rindtorff N.; Boutros M. (2017) Wnt signaling in cancer. Oncogene 36, 1461–1473. 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux E.; Cagnol S.; Beaudry K.; Carrier J.; Rivard N. (2015) Oncogenic KRAS signalling promotes the Wnt/beta-catenin pathway through LRP6 in colorectal cancer. Oncogene 34, 4914–4927. 10.1038/onc.2014.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler W. (2000) CD97 isoform expression in leukocytes. J. Leukoc Biol. 68, 561–567. [PubMed] [Google Scholar]
- Huang L. C.; Hueng D. Y. (2014) CD97 and glioma invasion. J. Neurosurg. 120, 579–580. 10.3171/2012.11.JNS12437. [DOI] [PubMed] [Google Scholar]
- Liu D.; et al. (2012) The invasion and metastasis promotion role of CD97 small isoform in gastric carcinoma. PLoS One 7, e39989. 10.1371/journal.pone.0039989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Fan H. Q.; Zhang Y. W. (2019) MiR-511–5p functions as a tumor suppressor and a predictive of prognosis in colorectal cancer by directly targeting GPR116. European review for medical and pharmacological sciences 23, 6119–6130. [DOI] [PubMed] [Google Scholar]
- Sewda K.; Coppola D.; Enkemann S.; Yue B.; Kim J.; Lopez A. S.; Wojtkowiak J. W.; Stark V. E.; Morse B.; Shibata D.; Vignesh S.; Morse D. L. (2016) Cell-surface markers for colon adenoma and adenocarcinoma. Oncotarget 7, 17773–17789. 10.18632/oncotarget.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G.; Sakitani K.; Wang H.; Jin Y.; Dubeykovskiy A.; Worthley D. L.; Tailor Y.; Wang T. C. (2017) The G-protein coupled receptor 56, expressed in colonic stem and cancer cells, binds progastrin to promote proliferation and carcinogenesis. Oncotarget 8, 40606–40619. 10.18632/oncotarget.16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J.; Varro A.; Dimaline R.; Wang T. (2001) The gastrins: their production and biological activities. Annu. Rev. Physiol. 63, 119–139. 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- Chou R.; et al. (2011) Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 155, 762–771. 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- Guo R.; et al. (2006) Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology 147, 4883–4892. 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- Scarlett K. A.; et al. (2018) Agonist-induced CXCR4 and CB2 Heterodimerization Inhibits Galpha13/RhoA-mediated Migration. Mol. Cancer Res. 16, 728–739. 10.1158/1541-7786.MCR-16-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coke C. J.; et al. (2016) Simultaneous Activation of Induced Heterodimerization between CXCR4 Chemokine Receptor and Cannabinoid Receptor 2 (CB2) Reveals a Mechanism for Regulation of Tumor Progression. J. Biol. Chem. 291, 9991–10005. 10.1074/jbc.M115.712661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E.; et al. (2014) Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J. Biol. Chem. 289, 21960–21972. 10.1074/jbc.M114.561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust G.; et al. (2002) CD97, but not its closely related EGF-TM7 family member EMR2, is expressed on gastric, pancreatic, and esophageal carcinomas. Am. J. Clin. Pathol. 118, 699–707. 10.1309/A6AB-VF3F-7M88-C0EJ. [DOI] [PubMed] [Google Scholar]
- O’Seaghdha C. M.; et al. (2010) Common variants in the calcium-sensing receptor gene are associated with total serum calcium levels. Hum. Mol. Genet. 19, 4296–4303. 10.1093/hmg/ddq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; et al. (2016) CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer 19, 754–766. 10.1007/s10120-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Huang G.; Jin P. (2019) Clinicopathological and prognostic significance of aberrant G protein-couple receptor 110 (GPR110) expression in gastric cancer. Pathol., Res. Pract. 215, 539–545. 10.1016/j.prp.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Stoveken H. M.; et al. (2016) Dihydromunduletone Is a Small-Molecule Selective Adhesion G Protein-Coupled Receptor Antagonist. Mol. Pharmacol. 90, 214–224. 10.1124/mol.116.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman G. S.; et al. (2017) Stachel-independent modulation of GPR56/ADGRG1 signaling by synthetic ligands directed to its extracellular region. Proc. Natl. Acad. Sci. U. S. A. 114, 10095–10100. 10.1073/pnas.1708810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.; Luo R.; Jin P.; Li T.; Oak H. C.; Giera S.; Monk K. R.; Lak P.; Shoichet B. K.; Piao X. (2019) GAIN domain-mediated cleavage is required for activation of G protein-coupled receptor 56 (GPR56) by its natural ligands and a small-molecule agonist. J. Biol. Chem. 294, 19246. 10.1074/jbc.RA119.008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. C. S.; McCarthy D.; Li J.; Palczewski K.; Yuan S. (2017) Designing Safer Analgesics via mu-Opioid Receptor Pathways. Trends Pharmacol. Sci. 38, 1016. 10.1016/j.tips.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire S. M.; et al. (2013) A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 344, 708–717. 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Rajagopal S.; Rajagopal K.; Lefkowitz R. J. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discovery 9, 373–386. 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C.; Liu T.; Buckanovich R.; Coffman L. G. (2019) The double edge sword of fibrosis in cancer. Transl Res. 209, 55–67. 10.1016/j.trsl.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim A. B.; Rothe J.; Cakir M. V.; Lede V.; Wilde C.; Liebscher I.; Thor D.; Schoneberg T. (2019) Genetic basis of functional variability in adhesion G protein-coupled receptors. Sci. Rep. 9, 11036. 10.1038/s41598-019-46265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]