Abstract
Lactococcus lactis NZ9000 was selected as an antigen delivery vehicle for mucosal immunization against porcine transmissible gastroenteritis virus (TGEV) infection. An approximately 70 kDa fragment of the N-terminal globular domain of the spike (S) protein (SN protein) from the coronavirus TGEV was used as the transmissible gastroenteritis virus antigen model. Recombinant L. lactis, expressing the SN protein, was constructed with the pNZ8112 plasmid. Expression and localization of the transcribed SN protein from the recombinant LNZ9000-rTGEV-SN were detected via SDS-PAGE, Western blot, and immunofluorescence. BALB/c mice, orally immunized with LNZ9000-rTGEV-SN, produced local mucosal immune responses against TGEV. The induced antibodies demonstrated neutralizing effects on TGEV infection. These data indicated that the recombinant L. lactis could be a valuable tool in the development of future vaccines against TGEV.
Keywords: TGEV S glycoprotein, Lactococcus lactis, Oral immunization
Introduction
Research carried out in recent years has led to the conviction that certain strains of lactic acid bacteria (LAB), in particular strains from the genera Lactobacillus and Bifidobactrium, may promote health in man and animals. LAB possesses a number of properties that make them attractive candidates for oral vaccination purposes. These bacteria are considered to be ‘safe’ organisms with a GRAS (Generally Recognized As Safe) status. In addition, Lactobacilli are able to survive passage through the upper gastrointestinal tract (GIT), and certain strains are capable of colonizing the intestinal tract [1–3]. These properties are particularly relevant when vaccines are to be applied in less industrialized countries. Furthermore, many bacterial, viral, and parasitic pathogens enter the body via the mucosal surfaces of the body. The first line of defense for humans and animals is found, not surprisingly, in the intestines, the largest immunological organ of the human body. More than 60% of antibodies produced daily are secreted into the GIT. Oral immunization frequently evokes both local and systemic immune responses, resulting in an effective elimination of foreign invaders [4–7]. These characteristics make them attractive candidates for delivering pharmaceutical compounds of interest to the mucosa, particularly vaccines and immunomodulators.
Transmissible gastroenteritis virus (TGEV) is a coronavirus that causes a highly contagious enteric disease in pigs, resulting in neonatal mortality and severe economical loses to the affected farms. The viral genome consists of a single-stranded, positive-sense 28.5 kb RNA and is constructed of three major structural proteins: S, M, and N, and a fourth structural protein, the small membrane (sM) protein [8, 9]. Protein S is the major inducer of TGEV neutralizing antibodies. The S protein has been used mainly for the induction of protective immunity against TGEV, and relevant neutralization epitopes have been mapped to the N-terminal domain of this protein. Four major antigenic sites have been described in glycoprotein S, of which site A is immunodominant [10–12]. The protection of suckling piglets against TGEV infection is based on the uptake of specific lactogenic antibodies, mainly of the IgA class, from the milk of the TGEV-immune sows [13, 14].
In the present article, we used Lactococcus lactis NZ9000 as a vector to express the N-terminal globular domain of the spike (S) protein and to act as an antigenic carrier in oral vaccination. Our data indicated that intragastric inoculation with the recombinant bacteria LNZ9000-rTGEV-SN could induce specific immune responses against TGEV.
Materials and methods
Bacterial strain and growth conditions
Lactococcus lactis were grown at 30°C in M17 medium containing 0.5% glucose (GM17). When required, antibiotics (chloramphenicol) were added at 10 μg/ml for L. lactis, unless otherwise stated. Plating of bacteria was performed on GM17 medium with 1.5% agar. The antibiotic concentration used for the selection of Lactococcus transformants was 10 μg/ml of Chloromycetin (Cm, Sigma, St. Louis, MO).
Labeling of Lactococcus with cFDA-SE
Lactococcus lactis NZ9000 was labeled with 5′-(and 6′)-carboxyfluorescein diacetate succinimidyl ester, (cFDA-SE; Molecular Probes-Invitrogen, Carlsbad, CA; Sigma), a non-fluorescent, membrane-permeable ester, which is converted to a fluorescent derivative by a non-specific prokaryotic/eukaryotic intracellular esterase. The derivative is then covalently linked to an intracellular protein via the probe’s succinimidyl group [15]. Briefly, a 100 mM stock solution of cFDA-SE was dissolved in dimethyl sulfoxide and then diluted in ethanol (1 ml, reagent grade). This solution was filter-sterilized before being aliquoted and stored at −20°C. L. lactis NZ9000 was grown overnight at 30°C in M17 medium containing 0.5% glucose. The bacterial cultures were centrifuged at 4,000×g for 10 min, and the pellets were washed twice in sterile PBS and adjusted to a concentration of 1010 CFU/ml before being labeled with cFDA-SE at 37°C for 20 min. Fluorescent labeling was terminated by pelleting the bacteria. The labeled bacteria were washed twice in sterile PBS to remove excess cFDA-SE and resuspended in sterile PBS. The flow cytometry profile (FACS Calibur TM: BD Biosciences, Cell QuestTM Pro) showed that about 99.5% of cells were labeled.
Animal adhesion study
Seven-week-old BALB/c mice were obtained from the Laboratory Animal Center, Harbin Medical University of China. A group of 15 mice were dosed with approximately 109 cFDA-SE-labeled bacteria by oral administration. Another group of 15 mice was orally fed with sterile PBS as the control. Groups of three mice each were killed on days 1, 2, 4, 6, and 7, and then the duodenum, jejunum, ileum, and colon were extracted from each mouse. Individual sections were cut longitudinally, and residual food particles or fecal material was removed from the intestine before being examined for the presence of adhering cFDA-SE-labeled bacteria. This was performed by adding 150 μl of PBS to every 1.0 cm of tissue and dislodging microbes from the mucosal surface of the tissues. Cell extracts were fixed with formaldehyde (0.75%, v/v), prior to flow cytometry analysis [3].
Construction of the TGEV SN gene expression plasmid
TGEV strain TH-98 was isolated and stored in our laboratory [16]. All DNA manipulations were performed according to standard procedures [17]. The pNZ8112 plasmid used in the present study is a cell-surface expression vector containing the ssUSP45 secretion signal peptide and anchor domain structure, a gift from NIZO, The Netherlands. A 2,037-bp fragment of the gene encoding TGEV antigen S that encompasses the four major antigenic neutralization epitopes was obtained by RT-PCR from TGEV genome. The oligonucleotides were: 5′-CTTGCATGCATGAAAAAACTGTTCGTTGTTCTGGTTGTAAT-3′ (forward) containing an SphI site and 5′-CGTCTAGACACACCACTATTATCAGACGGTACACCCACTATGTTG-3′ (reverse) containing an XbaI site. PCR amplification conditions were as follows: 95°C for 5 min; 30 cycles of 94°C for 1 min, 57.9°C for 1.0 min and 72°C for 1.5 min; and 72°C for 10 min of final extension. The PCR product was digested by SphI and XbaI, and inserted into the corresponding sites of pNZ8112.
Generation of recombinant L. lactis NZ9000 with the TGEV SN protein
Electroporation was carried out according to the method described by Josson et al. [18] and Holo and Nes [19], with some modifications. Briefly, 10 μl of recombinant plasmid DNA was added to 150 μl of L. lactis NZ9000, gently mixed and placed at 4°C for 5 min. The mixer was subjected to a single electric pulse (25 μF of 2500 V/cm), resuspended in GM17 medium without antibiotics and incubated under anaerobic conditions at 30°C for 2 h. Recombinant strains were selected on GM17-agar medium containing l0 μg/ml of Cm. The presence and integrity of the constructions were checked by extraction of recombinant plasmid DNA [20], restriction enzyme analysis, and sequencing.
Segregation and structural stability of pNZ8112-SN
To assess the segregation stability of NZ8112-SN in L. lactis NZ9000, the recombinant strain was grown in GM17 medium at 30°C without Cm. Serial subcultures were performed by diluting the culture in fresh broth (1:100) without antibiotics and growing until the mid-exponential phase was reached (OD600 at 1.0). After 80 generations, the percentage of Cm-resistant (Cmr) colonies was determined by plating 100 μl of culture dilutions onto GM17 plates without Cm or plates supplemented with 10 μg/ml Cm. To determine the structural stability of the recombinant plasmid after 80 generations, several colonies were randomly picked from the GM17 plate and analyzed by colony PCR, using forward and reverse primers of the S gene.
Protein expression and localization analysis
To analyze the expression and localization of the SN fusion protein, overnight cultures of strains LNZ9000-rTGEV-SN, NZ9000 (with plasmid pNZ8112), and NZ9000 were introduced into fresh medium at a ratio of 1:250. For the induction of the nisA promoter, strains were grown to an optical density of 0.5 at 600 nm, and nisin (Sigma) was added to a final concentration of 10 ng/ml. Growth was continued for 3 h. For NZ9000, growth was continued without addition of nisin until an optical density of 1.0 at 600 nm was reached. To prepare extracts, cells were pelleted by centrifugation at 5,000×g for 15 min at 4°C. The bacterial pellets were washed twice with sterile, 50 mM Tris-Cl (pH 8.0) and lysed in a Bead-Beater (Biospec, Bartlesville, USA) by vigorous shaking. The lysates were centrifuged at 15,000×g for l0 min, and the supernatants were stored at −20°C until further analysis. The bacterial protein extracts and supernatant were examined using 10% SDS-PAGE. Proteins were electrotransferred onto a nitrocellulose membrane, and the immunoblots were developed using mouse anti-TGEV serum (the antiserum obtained from BALB/c mice immunized with of TGEV). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma, 1:2000) was used, and visualization of the immunolabeled bands was carried out using the Chemiluminescent Substrate reagent (Pierce) according to the manufacturer’s instruction.
Immunofluorescence was also used to analyze the cell-surface display of SN protein. Induced bacterial cultures of LNZ9000-rTGEV-SN (1.0 ml) were pelleted, washed twice with sterile PBS, resuspended in PBS containing mouse anti-S serum, and incubated at 37°C for 2 h. The cells were centrifuged and washed twice with sterile PBS to remove unbound antibodies. The pellets were incubated with FITC-conjugated goat anti-mouse IgG (Sigma) containing 1% Evans blue dye (Pierce) at 37°C for 2 h. The cells were washed twice with sterile PBS, resuspended in sterile PBS and laid on a glass slide. Confocal microscopy was used to visualize the fluorescence on the cell surface of LNZ9000-rTGEV-SN. The strain of Lactococcus lactis NZ9000 harboring the pNZ8112 plasmid and the wide-type L. lactis NZ9000 were used as controls.
Immunization
LNZ9000-rTGEV-SN was cultured, induced with nisin, and centrifuged. Cell pellets were washed once with sterile PBS and resuspended in PBS to a concentration of 1010 CFU/ml. The treatment group of mice received doses of 109 cells of LNZ9000-rTGEV-SN. The control mice were immunized with equivalent doses of L. lactis NZ9000 with pNZ8112. The sham control group received 0.1 ml doses of PBS. All the mice were immunized via oral administration. The immune protocol was administered on three consecutive days at days 0, 1, and 2. A booster immunization was given at days 14, 15, and 16, and a second booster was given at days 28, 29, and 30 [21].
ELISA analysis of serum and intestine mucus
Sera were prepared and stored at −20°C until required. The intestinal mucus of mice was extracted as described previously [22, 23]. Polystyrene microtiter plates were coated with the TGEV propagated on swine testicular (ST) cells overnight at 4°C, and the cultures of swine testicular (ST) cells were used as the negative control antigen. The ELISA plates were washed twice in PBS containing 1% Tween-20 and then saturated with PBS containing 5% skimmed milk at 37°C for 2 h. Serum or intestinal lavage was diluted tenfold in PBS containing 1% BSA and primary antibodies. After incubation at 37°C for 1 h, the plates were washed twice with PBS containing 1% Tween-20. Bound antibodies were detected using HRP-conjugated goat anti-mouse IgA or IgG (Sigma), followed by color development using o-phenylene diamine dihydrochloride (Sigma) as the substrate. The absorbance was measured at 490 nm, and the endpoint titers and concentrations of antibody (IgA/IgG) from the optical density (OD) taken from ELISA data were calculated according to Raghava et al. [24].
Neutralization ability of the induced antibodies
Intestinal fluids and serum samples obtained from the mice immunized with LNZ9000-rTGEV-SN were evaluated using the plaque reduction assay to determine the neutralization ability. Lavages and serum obtained from the mice fed with the non-expressing strain or PBS were used as negative control, and 50 μl of samples in twofold serial dilutions (from 1:2 to 256) were prepared in 96-well plates. TGEV, adjusted to 100 TCID50 in 50 μl of virus diluent (10% concentrated Hank’s balanced salt solution, 0.1% bovine serum albumin, pH 7.4), was added to the cell plate containing serially diluted intestinal lavage and serum. The antibody and virus mixture was mixed and incubated at 37°C for 1 h, and then 100 μl of ST cells (used for virus infection) were added to the antibody–virus mixture and incubated at 5% CO2 and 37°C for 5 days. The overlay medium was then discarded, after which the wells were washed twice with sterile PBS (pH 7.4) and stained with 1% crystal violet solution. Differences in the number of plaques formed between treatments were examined, and the level of significance was determined by analysis of variance (ANOVA) followed by the Student’s t-test.
Results
Adhesion of L. lactis NZ9000
The persistence of L. lactis NZ9000 in the intestinal tract was determined via oral administration of cFDA-SE-labeled Lactococcus to mice and sampling the different regions of the intestine, post-orogastric intubation. Flow cytometric analysis of cell extracts (Table 1) indicated that Lactococcus was able to survive and adhere to different regions of the intestinal tract. Adhesion was most prominent in the ileum, with 8.45 ± 0.67 × 104 cells being detected on the first day. By the seventh day, the amounts of L. lactis NZ9000 that remained adhered to the intestinal mucosa were 22.17, 33.29, 36.33, and 35.76% of that observed on the first day in duodenum, jejunum, ileum, and colon, respectively.
Table 1.
Average number of cFDA-SE labeled L. lactis isolated from different sections of murine intestinal tract and the percentage of the initial population (day 1) that remained attached various days post-oral intubation
| Days post-oral feeding | Duodenum | Jejunum | Ileum | Colon |
|---|---|---|---|---|
| 1 | 8.93 ± 0.64E + 03 | 1.55 ± 0.86E + 04 | 8.45 ± 0.72E + 04 | 6.74 ± 0.69E + 04 |
| 2 | 6.82 ± 0.38E + 03 | 6.36 ± 0.57E + 03 | 6.03 ± 0.54E + 04 | 4.33 ± 0.36E + 04 |
| 4 | 3.89 ± 0.52E + 03 | 5.23 ± 0.48E + 03 | 4.35 ± 0.61E + 04 | 2.42 ± 0.28E + 04 |
| 6 | 2.93 ± 0.68E + 03 | 5.99 ± 0.81E + 03 | 3.35 ± 0.27E + 04 | 2.63 ± 0.61E + 04 |
| 7 | 1.98 ± 0.71E + 03 | 5.16 ± 0.29E + 03 | 3.07 ± 0.38E + 04 | 2.41 ± 0.56E + 04 |
| Percentage of cells remaining as of day 1(%) | ||||
| 2 | 76.37 | 41.03 | 71.36 | 64.24 |
| 4 | 43.56 | 33.74 | 51.48 | 35.90 |
| 6 | 32.81 | 38.65 | 39.64 | 39.02 |
| 7 | 22.17 | 33.29 | 36.33 | 35.76 |
Cell-surface expression of the TGEV SN antigen
The gene encoding the SN protein was amplified and cloned as an SphI–XbaI fragment into the corresponding sites of the expression plasmid, generating pNZ8112-SN (Fig. 1). The recombinant plasmid was electroporated into L. lactis NZ9000, producing the recombinant strain LNZ9000-rTGEV-SN.
Fig. 1.
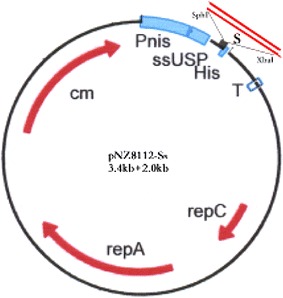
Map of pNZ8112-SN. Cmr resistance determinant; repA and repC replication elements; nisA-promoter and multiple cloning sites with terminator sequence behind the mcs are shown. The usp45 signal sequence ssUSP is translationally fused to the nisA-promoter. Possibility for C-terminal His tag is present. This is a broad-range host vector with the cat-gene and an NcoI-site for translational fusions. The SN gene was obtained from TGEV genome RNA by RT-PCR
LNZ9000-rTGEV-SN colonies were grown overnight in basal GM17 medium and then induced with nisin. The cell lysates were analyzed by SDS-PAGE and Western blot using mouse anti-TGEV serum. Coomassie blue gel-staining showed an approximately 70 kDa fusion protein band in LNZ9000-rTGEV-SN (Fig. 2a lane 2), but not in L. lactis NZ9000 with pNZ8112 (Fig. 2a lane 3) or wild-type L. lactis NZ9000 (data not shown). Similarly, an immune-reactive band was detected (Fig. 2b lane 1) via Western blot in a similar position, as observed in the SDS-PAGE in LNZ9000-rTGEV-SN (Fig. 2a lane 2). As the negative control, L. lactis NZ9000 with pNZ8112 did not display the corresponding immunoreactive band (Fig. 2b lane 2). These results show that the nisin promoter from pNZ8112 could efficiently induce the expression of the TGEV heterologous protein.
Fig. 2.
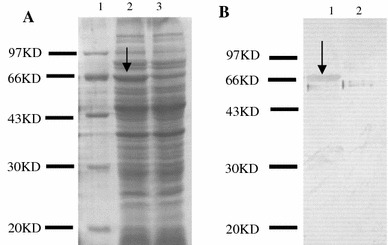
The SDS-PAGE (a) and Western blot (b) analyses of the cell lysates from the expressed product. Lane a1: MW, molecular mass marker; Lane a2: proteins from LNZ9000-rTGEV-SN induced by nisin; Lane a3: proteins from Lactococcus lactis NZ9000 harboring pNZ8112, induced by nisin; Laneb1: western blot analysis of the product of LNZ9000-rTGEV-SN; Lane b2: western blot analysis of the product of Lactococcus lactis NZ9000 harboring pNZ8112. Arrow indicates the expressed protein
The immunofluorescence assay was developed with the mouse anti-TGEV serum and FITC fluorescence-conjugated goat anti-mouse IgG to confirm the cell-surface display of the SN fusion protein. The results indicated that there was green–yellow fluorescence on the cell surface of LNZ9000-rTGEV-SN (Fig. 3a), and the control bacteria were red, as determined with the Evans blue dye (Fig. 3b). The localization analysis results suggested that the SN protein could be exposed externally on the cell wall. The S protein could not be detected in the supernatant from induced cultures of LNZ9000-rTGEV-SN, even after concentrating the supernatant by 50-fold in a Millipore Ultrafree-15 column (data not shown).
Fig. 3.
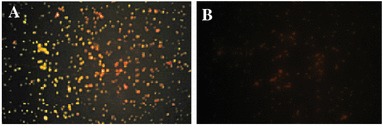
Detection of the expressed product by indirect immunofluorescence (40×). a The recombinant strain of LNZ9000-rTGEV-SN. The bacteria exhibit strong yellow and green fluorescence; b The Lactococcus lactis NZ9000 strain with pNZ8112. There is no fluorescence. The cells were stained red. (Color figure online)
Next, the segregation and structural stability of pNZ8112-SN in L. lactis NZ9000 were analyzed after 80 generations of subcultures in GM17 broth without Cm. The results indicated that the recombinant plasmid was stable and maintained its Cmr phenotype and structural arrangement. Thus, the recombinant Lactococcus could still express the protein of interest (Fig. 4).
Fig. 4.
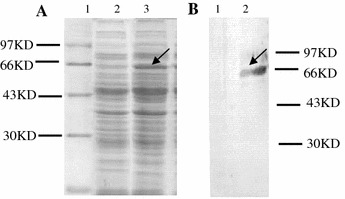
Expression and identification of the SN protein in LNZ9000-rTGEV-SN subcultured for 80 generations without selection for Cmr. Lane a1: MW, molecular mass marker; lanes a2 and a3: Coomassie blue gel staining shows an approximately 70 kDa fusion protein expressed from LNZ9000-rTGEV-SN, induced by nisin or uninduced (lane a2); lanes b1 and b2: immunoreactive band of protein expressed by LNZ9000-rTGEV-SN. No immunoreactive bands were observed in the cell lysates from the recombinant cells without induction by nisin (lane B1). Arrow indicates the expressed protein
Immune responses of the recombinant L. lactis
The mucosal immune response was investigated by measuring the anti-TGEV-SN IgA level in intestinal lavages following intragastric immunization. The concentration of mucosal IgA against TGEV-SN was determined by ELISA, using the TGEV cultures as the coating antigen. The results showed that there was no substantial difference in mucosal IgA levels between experimental and control groups, prior to intragastric immunization. However, oral immunization with recombinant LNZ9000-rTGEV-SN elicited an antigen-specific, mucosal IgA response. After the second booster, a high level of anti-TGEV-SN IgA was observed in intestinal lavages of mice immunized with LNZ9000-rTGEV-SN (Fig. 5), whereas no anti-SN-specific IgA was detected in controls. When the ST cell culture was used as the negative control antigen, no anti-SN-specific IgA was detected (data not shown). Likewise, a similar result was observed with the detection of anti-TGEV-SN-specific IgG (Fig. 6).
Fig. 5.
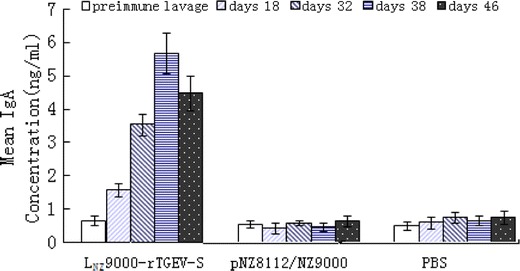
Anti-TGEV-SN-specific IgA level in intestinal lavage after intragastric immunization. Mice received three consecutive doses of 109 CFU/ml LNZ9000-rTGEV-SN, 3 times within a 2-week period. Control mice received 109 CFU/ml pNZ8112/NZ9000, while the negative group received PBS. Intestinal lavages were collected on days 18, 32, 38, and 46 after the first immunization, and were analyzed via ELISA using TGEV as the coating antigen. Bars represent the IgA titer ± S.E.M in each group
Fig. 6.
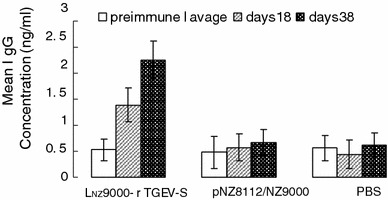
Anti-TGEV-SN serum IgG level after intragastric immunization. Sera from groups of mice immunized orally with 109 CFU/ml LNZ9000-rTGEV-SN and equivalent doses of pNZ8112/NZ9000 and PBS were analyzed for the presence of anti-TGEV-SN-specific IgG by ELISA, using TGEV as the coating antigen. Bars represent the IgG titer ± S.E.M in each group
Plaque reduction assay
Plaque reduction assays were performed to detect whether the antibody responses were specific to the TGEV antigen. Results demonstrated that the presence of anti-TGEV-SN IgA or IgG in the culture medium conferred statistically significant neutralizing effects (P < 0.05) on TGEV infection (Fig. 7). A consistent reduction of 22.3 ± 3.28% in the number of plaques was observed when plaque reduction assays were carried out using two- to eightfold dilutions of sera, and a reduction of 35.6 ± 3.79% was seen with two- to eightfold dilutions of intestinal lavages, which were obtained from the mice immunized with LNZ9000-rTGEV-SN.
Fig. 7.
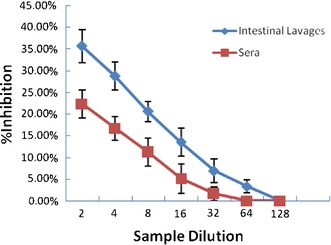
Inhibition of viral plaque formation in intestinal lavages and sera after intragastric immunization. Maximum reduction in the number of plaques is expressed as a percentage of plaques obtained for the negative control samples. Intestinal lavages or sera values collected from mice fed with LNZ9000-rTGEV-SN were 35.6 ± 3.79 and 22.3 ± 3.28%, respectively. Results are mean values and standard errors of triplicates
The inhibitory effect decreased gradually with further dilution and reached a level similar to that of the buffer control or the non-expressing control strain at dilutions 1:64 and 1:128 of intestinal lavage and sera, respectively.
Discussion
In this report, we found that the globular part (N-terminal domain) of the TGEV spike protein (glycoprotein S) expressed in L. lactis NZ9000 retained its antigenic properties and elicited neutralizing antibodies, when used to immunize animals. For many pathogens, the initial infection mainly occurs at the mucosa tissues of intestines. Therefore, it is important to develop vaccines that elicit protective immune responses against infection and replication of the pathogens at the mucosal level. Vaccines administered by parenteral routes generally fail to stimulate mucosal immune responses. Mucosal immunization is a proven, effective approach against colonization of pathogens and their further spread to the systemic circulation [4]. Therefore, it is necessary to develop efficient and safe antigen vectors that could trigger mucosal and systemic immune responses. One promising approach relies on the use of live bacterial vehicles. The potential of recombinant LAB to deliver heterologous antigens to the mucosal immune system has been under investigation [25–27]. This approach offers a number of advantages over the traditional parenteral vaccination, such as non-invasiveness and the possibility to elicit both mucosal and systemic immune responses. A great deal of research, currently being explored for oral application, focused on the development of adequate mucosal vaccines with various vaccine delivery systems. Among available approaches that efficiently stimulate mucosal responses, the use of Lactococcus carriers to deliver vaccine antigens probably constitutes one of the most successful strategies.
The use of LAB as a live vehicle for the delivery of antigens or other therapeutic molecules for mucosal immunization has been proposed. However, only a few of systems have been described using Lactobacillus casei strains as a carrier of heterologous viral antigens in a form that can be presented to and processed by the mammalian host [28]. The TGEV S protein was expressed by Lactobacillus casei [29]. Lactobacillus is the resident strain present in the intestinal tract of mammals. It may produce immunological tolerance, and a practical application for its use should be identified. IgA is the predominant antibody at the mucosal surface, as its local production is greater than other immunoglobulins. Therefore, an efficient TGEV oral vaccine should be able to induce a specific mucosal IgA response. We evaluated the immunogenicity of LNZ9000-rTGEV-SN using BALB/c mice as the animal model. The LNZ9000-rTGEV-SN produced both mucosal and systemic immune responses after intragastric administration. The oral administration regimen consisted of three sets of three successive daily doses of LNZ9000-rTGEV-SN by vaccination. This protocol was adapted from Challacombe’s procedure [21], which was consistently effective when particulate oral vaccines were used to immunize mice. Three successive daily doses of recombinant bacteria were required in order to ensure that a systemic antibody response to TGEV could be elicited in all mice that received the recombinant strain, intragastrically.
In order to confirm the efficiency of the induced antibodies to inhibit the virus, we tested whether intestinal lavages and sera could inhibit the infection of ST cells in a plaque reduction assay. Serum and intestinal samples collected from mice immunized with LNZ9000-rTGEV-SN demonstrated statistically significant inhibition. An important feature of a delivery system is the ability to adhere to and persist in the intestinal tract. Lactobacilli could survive passage through the upper GIT and colonize the intestinal tracts [30, 31]. In addition, Lactobacilli have intrinsic adjuvant activity [32, 33]. In the present study, we investigated the potential adhesion and persistence of NZ9000 in the mouse intestine by using cFDA-SE. It was shown that 8.93 × 103 CFU/ml to 8.45 × 104 CFU/ml of L. lactis NZ9000, orally fed to mice, adhered to the different sections of intestinal tract. The percentage of L. lactis NZ9000 that remained in the different sections of the intestinal tract after initial attachment on the first day varied from 41.03 to 76.37%.
In the present study, we demonstrated that L. lactis NZ9000 was able to survive in the upper GIT and express and secrete the heterologous TGEV SN protein that induced specific immune responses after oral administration. Currently, we are in the process of confirming the immune protection of the recombinant Lactococcus expressing TGEV SN protein in pigs. We are also investigating the mechanisms by which the antigen enters intravascular compartments and induces systemic immune responses. The probiotic effects and the harmless nature of Lactococcus lactis NZ9000 would make it an ideal candidate for delivering heterologous antigens in an oral vaccine.
Conclusions
The present study describes a novel approach for preventing the spread of transmissible gastroenteritis in pigs. We described the capability of L. lactis NZ9000 to adhere to the GIT. We believe that our data could be valuable to intensive host-pathogen interaction studies and preventing TGEV outbreaks. The recombinant Lactococcus expressing SN protein induced both local mucosal and systematical immune responses against TGEV. The induced antibodies demonstrated neutralizing effects on TGEV infection. These data indicated that the recombinant Lactococcus could be a useful tool in the development of future vaccines against TGEV.
Acknowledgments
This work was supported by Grant 706019 from the National Natural Sciences Funds of China and Grant CX2008 from the supported program for innovative research team of Northeast Agricultural University (NEAU). The authors wish to thank NIZO Food Research for providing the pNZ8112 plasmid and the bacterial strain, Lactococcus lactis NZ9000.
References
- 1.Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Appl. Environ. Microbiol. 1999;65:351. doi: 10.1128/aem.65.1.351-354.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesena C, Morelli L, Alander M, Siljander T, Tuomola E, Salminen S, Mattila-Sandholm T, von Wright A. J. Dairy Sci. 2001;84:1001. doi: 10.3168/jds.S0022-0302(01)74559-6. [DOI] [PubMed] [Google Scholar]
- 3.Lee YK, Ho PS, Low CS, Arvilommi H, Salminen S. Appl. Environ. Microbiol. 2004;70:670. doi: 10.1128/AEM.70.2.670-674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermúdez-Humarán LG, Langella P, Cortes-Perez NG, Gruss A, Tamez-Guerra RS, Oliveira SC, Saucedo-Cardenas O, de Oca-Luna RM, Loir YL. Infect. Immun. 2003;71:1887. doi: 10.1128/IAI.71.4.1887-1896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Underdown BJ, Mestecky J, et al. Mucosal Immunoglobulin. In: Ogra P, Mesteck J, Lamm ME, Strober W, McGhee JR, Bienestock J, et al., editors. Handbook of Mucosal Immunology. Boston: Academic Press; 1994. pp. 79–98. [Google Scholar]
- 6.Staats HF, Jackson RJ, Marinaro M, Takahashi I, Kiyono H, McGhee JR. Curr. Opin. Immunol. 1994;6:572. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Pouwels PH, Leer RJ, Boersma WJA. The potential of Lactobacillus as a carrier for oral immunization. J. Biotechnol. 1996;44:183. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 8.Saif LJ, Wedley R. In: Disease of Swine. 8. Straw BE, Allaire S, Mengeling WL, Tayler DJ, editors. Ames: Iowa State University Press; 1982. p. 295. [Google Scholar]
- 9.Paul S. Adv. Virus Res. 2006;66:193. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanagh D. Arch. Virol. 1997;142:629. [PubMed] [Google Scholar]
- 11.Delmas B, Gelfi J, Laude H. J. Gen. Virol. 1986;67:1405. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez G, Correa I, Melgosa MP, Bullido MT, Enjuanes L. J. Virol. 1986;60:131. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez N, Carrillo C, Salinas J, Parra F, Borca MV, Escribano JM. Virology. 1998;249:352. doi: 10.1006/viro.1998.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S, Bruszewski J, Smalling R, Browne JK. Adv. Exp. Med. Biol. 1985;185:63. doi: 10.1007/978-1-4684-7974-4_4. [DOI] [PubMed] [Google Scholar]
- 15.Weston SA, Parish CR. J. Immunol. Methods. 1990;133:87. doi: 10.1016/0022-1759(90)90322-M. [DOI] [PubMed] [Google Scholar]
- 16.Xiao-feng REN, Yi-jing LI, Guang-xing LI, Bao-quan LIU. Chin. J. Vet. Med. 2002;7:3. [Google Scholar]
- 17.Sambrook J, Russel W. Molecular Cloning: A Laboratory Manual. 3. New York: Cold Spring Harbor Laboratory Press; 2002. pp. 597–628. [Google Scholar]
- 18.Josson K, Scheirlinck T, Michiels F, Platteeuw C, Stanssens P, Joos H. Plasmid. 1989;21:9. doi: 10.1016/0147-619X(89)90082-6. [DOI] [PubMed] [Google Scholar]
- 19.Holo H, Nes IF. Appl. Environ. Microbiol. 1989;55:3119. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson DG, McKay LL. Appl. Environ. Microbiol. 1983;46:549. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challacombe SJ. Ann. NY Acad. Sci. 1983;409:177. doi: 10.1111/j.1749-6632.1983.tb26868.x. [DOI] [PubMed] [Google Scholar]
- 22.Chatel JM, Langella P, Adel-Patient K, Wal JM, Corthier G. Clin. Diagn. Lab. Immunol. 2001;8:545. doi: 10.1128/CDLI.8.3.545-551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corthésy B, Boris S, Isler P, Grangette C, Mercenier A. J. Infect. Dis. 2005;192:1441. doi: 10.1086/444425. [DOI] [PubMed] [Google Scholar]
- 24.Raghava GPS, Joshi AK, Agrewala JN. J. Immunol. Methods. 1992;153:263. doi: 10.1016/0022-1759(92)90330-V. [DOI] [PubMed] [Google Scholar]
- 25.Chen H. J. Control Release. 2000;67:117. doi: 10.1016/S0168-3659(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 26.Grangette C, Muller-Alouf H, Goudercourt D, Geoffroy MC, Turneer M, Mercenier A. Infect. Immun. 2001;69:1547. doi: 10.1128/IAI.69.3.1547-1553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira ML, Areas AP, Campos IB, Monedero V, Perez-Martinez G, Miyaji EN, Leite LCC, Aires KA, Lee Ho P. Microbes Infect. 2006;8:1016. doi: 10.1016/j.micinf.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M.Oddone G, Lan QQ, Rawsthorne H, Mills DA, Block DE. Biotechnol. Bioeng. 2006;96:1127. doi: 10.1002/bit.21192. [DOI] [PubMed] [Google Scholar]
- 29.Ho PS, Kwang J, Lee YK. Vaccine. 2005;23:1335. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheppler L, Vogel M, Zuercher AW, Zuercher M, Germond JE, Miescher SM, Stadler M. Vaccine. 2002;26:2913. doi: 10.1016/S0264-410X(02)00229-3. [DOI] [PubMed] [Google Scholar]
- 31.Russell-Jones GJ. J. Control Release. 2000;65:49. doi: 10.1016/S0168-3659(99)00231-X. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Mills DA. Plasmid. 2007;58:275. doi: 10.1016/j.plasmid.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Perdigon G, Fuller R, Raya R. Curr. Issues Intest. Microbiol. 2001;2:27. [PubMed] [Google Scholar]


