Abstract
Advances in structural biology have yielded exponential growth in G protein-coupled receptor (GPCR) structure solution. Nonetheless, the instability of fully active GPCR complexes with cognate heterotrimeric G proteins has made them elusive. Existing structures have been limited to nanobody-stabilized GPCR:Gs complexes. Here we present methods for enhanced GPCR:G protein complex stabilization via engineering G proteins with reduced nucleotide affinity, limiting Gα:Gβγ dissociation. We illustrate the application of dominant negative G proteins of Gαs and Gαi2 to the purification of stable complexes where this was not possible with wild-type G protein. Active state complexes of adenosine:A1 receptor:Gαi2βγ and calcitonin gene-related peptide (CGRP):CLR:RAMP1:Gαsβγ:Nb35 were purified to homogeneity and were stable in negative stain electron microscopy. These were suitable for structure determination by cryo-electron microscopy at 3.6 and 3.3 Å resolution, respectively. The dominant negative Gα-proteins are thus high value tools for structure determination of agonist:GPCR:G protein complexes that are critical for informed translational drug discovery.
GPCRs are premier drug targets and there is substantial interest in structural understanding of these proteins to determine mechanisms of drug interaction and activation. Recent methodological developments in GPCR engineering, including introduction of thermo-stabilizing mutations, replacement of flexible loops with small stable fusion proteins, and specialized binding partners, have enabled the determination of structures of 53 unique receptors.1 Of these structures only five receptors are in a fully active, transducer-complexed, state. This state is only achieved when both agonist and trimeric G protein (or other transducer) are present, commonly referred to as the ternary complex.2 Thus, understanding the structural basis of this state is critical to understanding mechanisms of GPCR signal transduction and for utilizing this information for structure-based drug design. G protein bound active state receptor structures have been achieved using several approaches; of the five published Gαs containing structures, four (three unique receptors) utilized a camilid nanobody, Nb35, to stabilize the Gα–Gβγ interface,3−6 while the structure of Adenosine A2A receptor utilized a highly engineered mini-Gs partner.7 The active state of opsin was determined using a c-terminal peptide fragment of Gt.8
The agonist-bound GPCR acts as a guanine–nucleotide exchange factor (GEF) for the Gα subunit, promoting GDP release and subsequent GTP binding. This occurs while the Gα subunit is bound to the Gβγ heterodimer. Physiologically, where GDP and GTP concentrations are relatively high, the ternary complex is unstable, the G protein heterotrimer dissociates from the receptor, and into its component parts, Gα and Gβγ, that engage downstream signaling effectors.9 This inherent instability makes it extremely challenging to trap and purify complexes of GPCRs bound to heterotrimeric G proteins for structural studies.
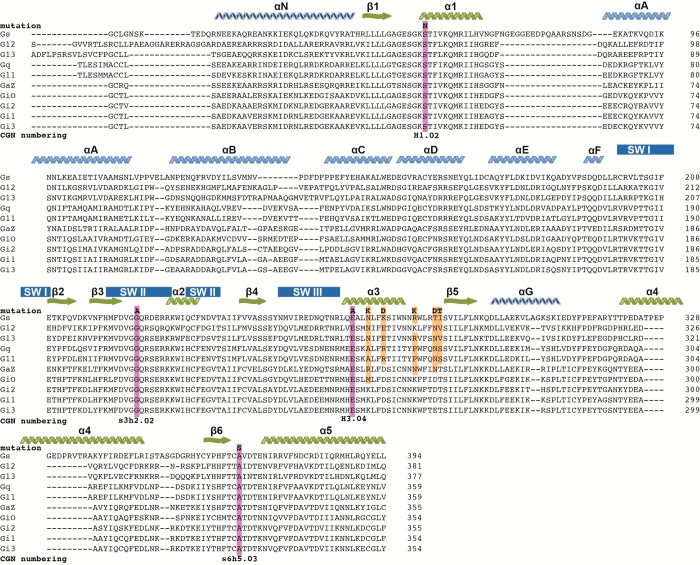
Using the extensive literature on G protein mutagenesis, mutations within Gαi2 and Gαs (highlighted in Figure 1) predicted to stabilize the ternary complex state were selected. These reduce nucleotide-binding affinities and enhance the stability of agonist:GPCR:G protein heterotrimer complexes; achieving the latter is critical for structural studies. Mutations included four residues conserved across all G protein subclasses. SerH1.02 (CGN numbering system10), involved in coordinating Mg2+ and contacting GTP’s β-phosphate, whose mutation to Asn in Gαs11 and Cys in Gαi2,12 Gαo,12 and Gαt13 generates a dominant negative G protein. This inhibits signaling by formation of a stabilized (nondissociating) ternary complex. Glys3h2.02 of Gα forms a backbone amide hydrogen bond with the γ-phosphate of GTP14 with Ala substitution increasing the GDP dissociation rate and blocking GTP induced dissociation from the β2 receptor.15 A combination of Glys3h2.02Ala with GluH3.04Ala generates a dominant negative16 presumably through disruption of the conserved salt-bridge between residues at s3h2.04 and H3.04,14 which is also seen during the conformational rearrangement in the nucleotide free state.3 Alas6h5.03 to Ser substitution increases GDP dissociation rate. Although, with these mutations, the free Gαβγ heterotrimer is thermolabile on its own; in combination with Glys3h2.02Ala, GluH3.04Ala, and Alas6h5.03Ser, it appears more stable, presumably because of constitutive interactions with GPCRs.17 For the Gαs mutant, five additional mutations substituting residues of Gαi2 into Gαs within the α3 helix or α3 helix−β5 strand linker were introduced, improving the dominant negative effect of Gαs.17
Figure 1.
Alignment of human Gα isoforms. Clustalw omega alignment of reference sequences for human Gα isoforms manually adjusted to take into account secondary elements from deposited PDB structures: 1SVK, 3FFB, 1ZCA, 1ZCB, 2BCJ, 1AZT, and 3SN6. α-Helices (zig-zags) from the α-helical domain are indicated in light blue, in dark blue are those outside either core domain and in green are those from the Ras-like domain. β-Strands are indicated in the same color scheme with wavy arrows. Secondary structure elements from the α-helical domain are indicated with letters and the Ras-like domain with numbers. The position and substitution for common DN-substitutions are highlighted in purple with the CGN numbering10 shown below. Highlighted in yellow are Gαi residues that are substituted into Gαs, which improve the dominant negative effect.
Here we present data showing the utility of these engineered dominant negative (DN) Gα subunits to enhance formation of stable complexes for structural studies by cryo-electron microscopy (cryo-EM). These Gαs and Gαi2 constructs have enabled solution of fully active structures of glucagon-like peptide 1 receptor (GLP-1R),6 adenosine A1 receptor (A1-AR),18 and calcitonin gene-related peptide receptor (CGRP-R)19 at resolutions that allow reliable placement of side-chain rotomers for most amino acids in the receptor core, supporting their broad applicability for purification of stable GPCR:heterotrimeric G protein complexes.
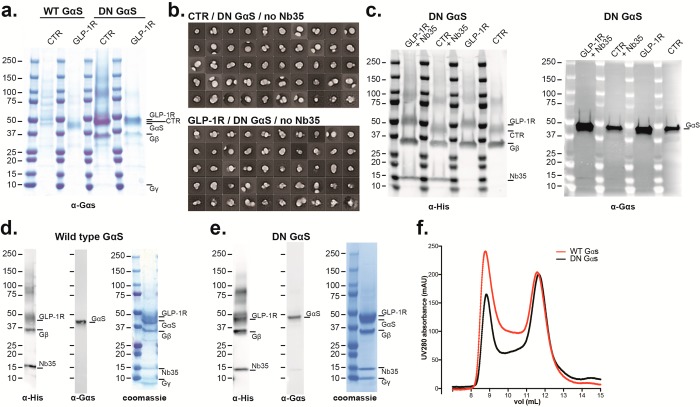
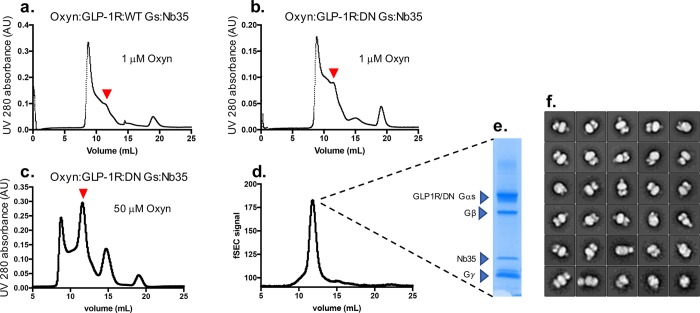
In the case of GLP-1R, purification of GLP-1R:Gs complexes were attempted using exendin-P5 (ExP5), a biased agonist with lower cAMP efficacy than the native GLP-1 that had been used by others to determine a 4.1 Å structure in complex with wild type (WT) Gs.5 The ExP5 complexes were initially formed with ExP5:GLP-1R:WT-Gαs:βγ in the absence of Nb35. These were benchmarked against the same complex containing the DN-Gαs as well as against sCT:CTR containing either WT or DN-Gαs as shown in Figure 2a. The ExP5:GLP-1R:WT-Gαs:βγ could not be purified to homogeneity; however, the inclusion of the DN-Gαs allowed both GLP-1R and CTR containing complexes to be purified to homogeneity (Figure 2a), yielding around 200 μg L–1. These Nb35 free complexes were subjected to negative stain EM and yielded ∼28% full complex out of the total unfiltered particles (Figure 2b). Inclusion of Nb35 with either WT or DN-Gαs generated a complex with similar yield and stoichiometry to Nb35 free complexes as assessed by Western blot (Figure 2c), slightly improved purity as assessed by coomassie stain (compare Figure 2a with 2d, 2e), and a monodispersed peak by size exclusion chromatography (SEC). When either was subjected to negative stain EM, the full complex represented ∼70% of total unfiltered particles (data not shown) and we chose to image the DN complex. This allowed solution of the structure at 3.3 Å.6 We further assessed the utility of DN Gs for formation and purification of complexes with low efficacy agonists, using the endogenous GLP-1R agonist, oxyntomodulin, that has 100-fold lower cAMP potency than GLP-1. All preparations included Nb35 to maximize complex stability and yields. The initial formation of complexes was attempted using 1 μM peptide, as per previous studies. Complexes were weakly formed with both WT (Figure 3a) and DN Gs (Figure 3b), with the latter providing higher yields. Increasing the agonist concentration to 50 μM during initial formation of the complex substantially improved yields with the DN Gs preparation (Figure 3c), enabling recovery of a monodisperse peak following a second round of purification by SEC (Figure 3d). This peak exhibited apparent stoichiometric levels of component proteins by coomassie stain (Figure 3e) and large numbers of 2D classes of the full complex by negative stain EM (Figure 3f).
Figure 2.
Comparison of purification of WT-Gs and DN-Gs containing heterotrimers with CTR and GLP-1R. (a) Coomassie stained gel showing relative abundance of various components of CTR (with salmon calcitonin) and GLP-1R (with exendin-P5)–G protein complexes following FLAG purification in the presence of either WT or DN-Gs but not Nb35. (b) 2D class averages from negative stained EM micrographs of the CTR:DN Gs and GLP-1R:DN Gs complexes in the absence of Nb35. (c) Western blot against His-tag (Gβ, Nb35, and CTR:GLP-1R) and Gαs from purified active complex with DN-Gs in the presence or absence of Nb35. (d,e), Western blot and coomassie stained gel of WT- (d) and DN- (e) containing GLP-1R:Gs complexes in the presence of Nb35 and corresponding SEC traces (f), illustrating that both complexes can be purified but that the proportion of complex in the void/aggregate is slightly higher with the WT-Gαs (c).
Figure 3.
Formation and purification of WT-Gs and DN-Gs containing heterotrimers by oxyntomodulin with GLP-1R. (a,b) Size exclusion chromatography of complexes initiated with 1 μM oxyntomodulin revealed higher yield of complexes with the DN-Gs (b) relative to that seen with WT-Gs (a), although both had relatively low yields for complex formation (the elution position of the complexes is illustrated by the red arrow). (b) Increasing the concentration of oxyntomodulin to 50 μM in the Oxyn:GLP-1R:DN-Gs:Nb35 preparation during the initiation step markedly improved the yield of complexes, which could be purified to homogeneity by an additional size exclusion chromatography step (d). (e) Coomassie stained gel from the peak in panel d, illustrating high purity and recovery of each of the component proteins. (f) 2D class averages from negative stain EM demonstrate the presence of complexes suitable for single-particle cryo-EM structure determination.
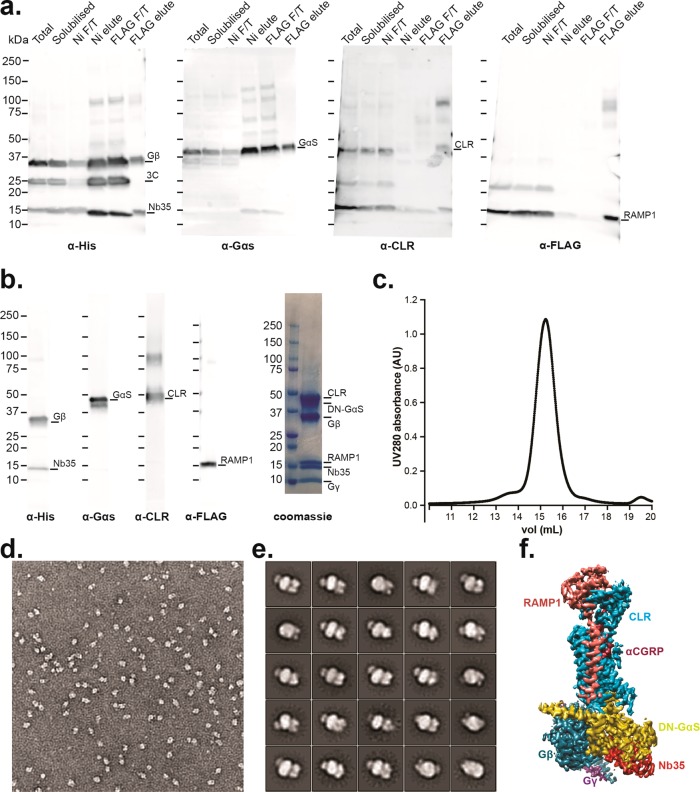
Subsequently, we applied this methodology to structure determination of the CGRP receptor. This receptor is a heterodimeric complex containing the calcitonin receptor-like receptor (CLR) and the single pass transmembrane protein, receptor activity-modifying protein 1 (RAMP1), requiring a more complex purification protocol (see Methods). Formation of this complex using the agonist αCGRP, WT-Gαs, Gβγ, and Nb35 enabled its purification, as shown in Figure 4a; however, the yield was extremely poor (<25 μg L–1) such that insufficient material was available for SEC. In contrast, the use of the DN-Gαs enabled complex purification with a yield similar to that of our GLP-1R complex (∼200 μg L–1) that ran as a monodisperse peak by SEC and contained apparent stoichiometric amounts of all components (CGRP:CLR:RAMP1:DN-Gαs:βγ:Nb35; Figure 4b,c). This was confirmed when complexes were subjected to negative stain EM and 2D class averaging, for which particles were homogeneous and stable (Figure 4d,e). Single particle cryo-EM imaging enabled solution of the fully active CGRP receptor to a resolution of 3.3 Å19 (Figure 4f). Our data, using GLP-1R and CGRP-R as examples, indicate that, while the use of WT Gαs and Nb35 may be sufficient for purification of some GPCR:Gαs complexes, the use of a DN Gαs provides additional advantages for solving active structures by single particle cryo-EM, particularly for agonist:GPCR:Gs protein combinations that may be less stable.
Figure 4.
DN-Gαs allows purification and structure determination of the fully active heteromeric CGRP receptor. (a) Western blots of purification fractions of the CGRP active complex using WT-Gαs showing purification is possible but yield is poor (see text): (left) anti-His antibody against His tags on Gβ1 and Nb-35; (middle left) anti-Gαs antibody for Gαs detection; (middle right) anti-CLR antibody for CLR detection; (right) anti-FLAG antibody against the FLAG tag on RAMP1. (b) (Left) Western blot of the final purified fraction of the CGPR complex formed using DN-Gαs showing that all components are present; (right) coomassie stained gel showing stoichiometric recovery of proteins. (c) Monodisperse peak of the purified complex following size exclusion chromatography. (d) Negative stain EM micrograph. (e) 2D class averages from the negative stain EM data. (f) 3D cryo-EM map from single particle cryo-EM determination of structure, colored according to each protein subunit (the 3D map was adapted from Liang et al.19).
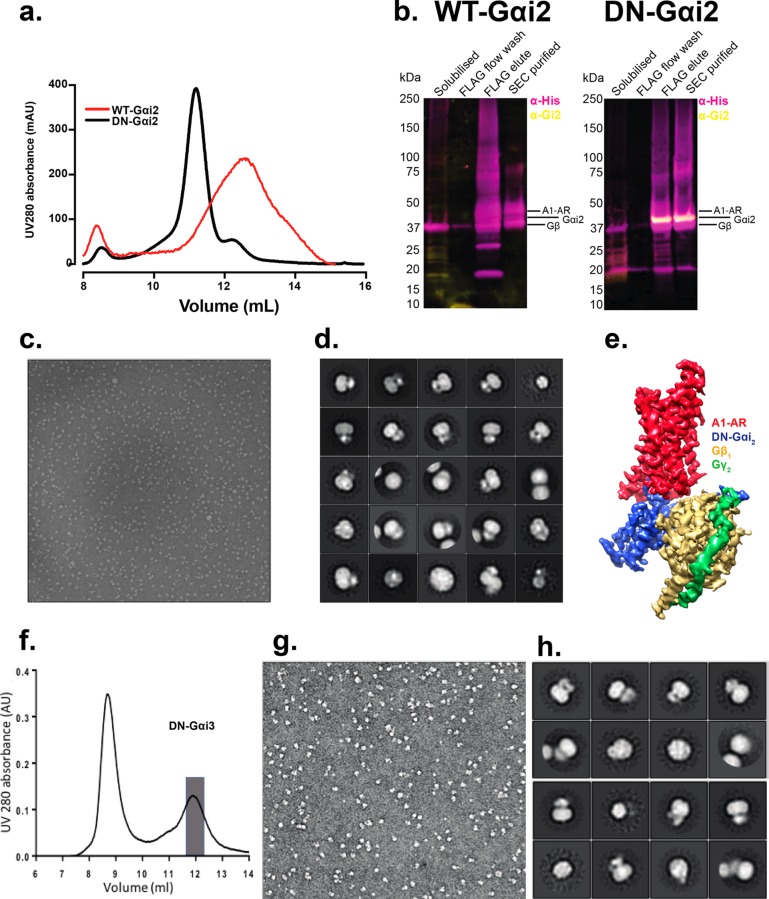
To assess the applicability of using DN G proteins for structural studies on GPCRs with non-Gαs partners, we selected the A1-AR as an exemplar. The A1-AR preferentially couples to Gαi/o proteins. We selected Gαi2 as the G protein partner, because of high expression in both brain and heart; both major organs for therapeutic targeting of the A1-AR.20 Unlike with ExP5:GLP-1R:WT-Gs, we could form an active ADO:A1-AR:WT-Gαi2:βγ complex using the endogenous agonist adenosine (ADO) that could be purified with a yield of approximately 150 μg L–1. When this complex was subjected to SEC it gave a very broad peak, characteristic of a low affinity complex with rapid exchange kinetics (Figure 5a). Consistent with this, the SEC fraction collected from the 11 mL peak contained substoichiometric quantities of G protein components relative to receptor. In contrast, complex formation and purification in the presence of DN-Gαi2 yielded a monodispersed peak by SEC (Figure 5a) and apparent stoichiometric amounts of receptor and G protein when assessed by Western blot (Figure 5b), with a marginally improved yield of 200 μg L–1. Negative stain EM data and 2D class averaging revealed uniformity and stable complex particles (Figure 5c,d) suitable for cryo-EM. This lead to the solution of a high resolution map (3.6 Å) of this small (∼120 kDa) complex (Figure 5e)18· Although the A1-AR exhibits physiologically important coupling to Gi2 proteins, it can couple to other members of the Gi/o subfamily. Stable complexes could also be formed and purified using adenosine:A1-AR:DN-Gi3 (Figure 5f–h), yielding a monodisperse SEC peak (Figure 5f), well behaved particles in negative stain EM (Figure 5g), and a similar distribution of 2D class averages (Figure 5h), to that seen with DN-Gi2 complexes.
Figure 5.
DN-Gαi2 allows purification and structure determination of the fully active A1-AR. (a) Size exclusion chromatography trace showing a broad peak for ADO:A1-AR:G protein complex in the presence of WT Gi2, and a monodisperse peak in the presence of DN Gi2. (b) Western blot of final purified fraction showing (left) limited Gαi2 recovery in the ADO:A1-AR:WT G protein complex and (right) stoichiometric recovery of G protein in the ADO:A1-AR:DN G protein complex (modified from Ext. Data Figure 1c18). (c) Negative stain EM micrograph of the ADO:A1-AR:DN Gi2 protein complex. (d) 2D class averages from the negative stain EM micrographs; (e) 3D cryo-EM map, A1-AR is in red, heterotrimeric DN-Gi2 is in blue, gold, and green for α, β, and γ, respectively. The DN-Gi2 data were adapted from Draper-Joyce et al. 2018.18 (f) Size exclusion chromatography illustrating purification of the ADO:A1-AR:DN-Gi3 complex; the peak fractions (gray box) were pooled and assessed by negative stain EM (g), with 2D class averages shown in panel h.
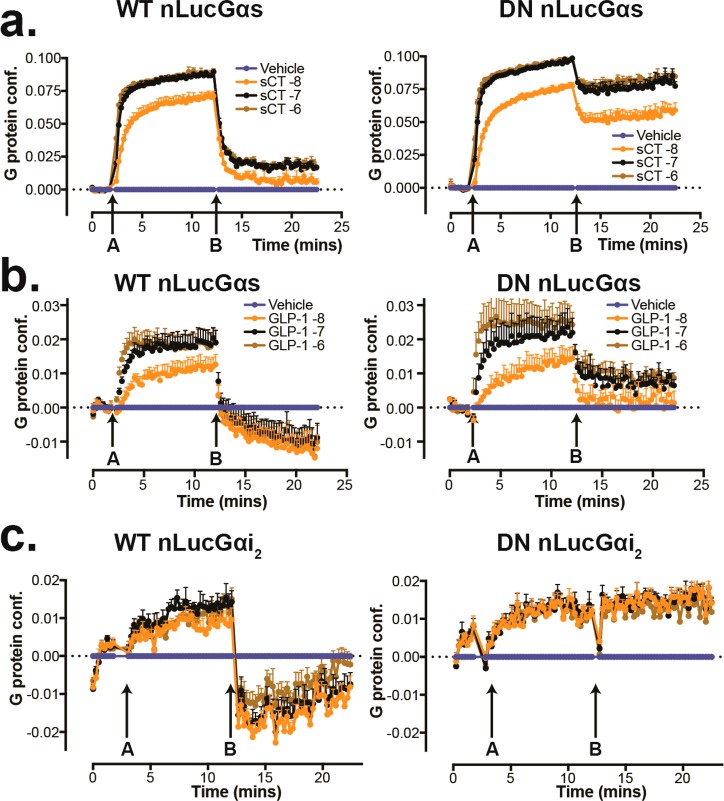
Our data reveal that DN Gα subunits are invaluable structure determination tools. We wanted to confirm that the agonist:GPCR mediated conformational transitions induced within the DN Gα:βγ heterotrimers were similar to those of the WT Gα:βγ. The ability of either DN-Gαs or DN-Gαi2 to promote the high-affinity state of the A1-AR was not different to that of the equivalent WT-Gα subunits.18 Moreover, in membrane-based bioluminescence resonance energy transfer (BRET) assays with CTR (Figure 6a) or GLP-1R (Figure 6b), there was no difference in the rate (Figure 6a,b) or extent of conformational rearrangement of WT- and DN-Gαs (Figure 6a,b) in response to agonist. Similarly, the rate and extent of ligand-induced conformational change mediated by GLP-1R activation at WT- and DN-Gαi2 was not different (Figure 6c). For DN-Gαs, there was reduced sensitivity, whereas Gαi was completely resistant to GTP induced conformational change, supporting the designed reduction in affinity/sensitivity of the G protein to GTP from the introduced mutations (Figure 6a–c).
Figure 6.
Functional analysis of DN-G proteins. (a) Time-course for ligand-induced changes in BRET of WT-Gαs or DN-Gαs (nanoLuc tagged) and Gγ (Venus) at increasing concentrations of salmon calcitonin (sCT) (arrow, A) followed by the addition of 30 μM GTP (arrow, B) on membranes from HEK293 cells that lack endogenous Gαs and were stably transfected with the human CTR; the rate and magnitude of agonist-induced structural rearrangement are similar, but the DN-Gαs is resistant to GTP-induced conformational rearrangement. (b) Comparison of time-courses for agonist-induced changes in BRET of WT-Gαs/Gγ and DN-Gαs/Gγ are shown for GLP-1(7–36)NH2 activated GLP-1R; both WT- and DN-Gαs report the same difference in rate and extent of ligand-induced G protein conformational change (A); however, after the addition of 30 μM GTP (B), the DN-Gαs is less susceptible to conformational change. (c) time-course for GLP-1(7–36)NH2-induced changes in BRET of WT-Gαi2 or DN-Gαi2 (nanoLuc tagged) and Gγ (Venus) (A), followed by the addition of 30 μM GTP (B), on membranes from HEK293 cells that lack endogenous Gαs/Gαq/11/Gα12/13 and were stably transfected with the human GLP-1 receptor. The rate and magnitude of structural rearrangement are similar but the DN-Gαi2 is resistant to GTP-induced conformational rearrangement. All data are N = 3 + SEM.
In conclusion, we show that incorporation of DN mutations in Gαi and Gαs subunits enhances the stability of the G proteins in complex with an agonist-bound receptor. Four of these are absolutely conserved residues across G proteins, and mutation of these would be predicted to have similar effects. Additional substitutions from the Gαi/o family members into Gαs provides further stability to the GPCR:G protein ternary complex, facilitating structural studies. Despite decreased nucleotide affinity, modified Gα-proteins maintain similar allosteric and conformational function to WT. These DN Gα-proteins are extremely valuable tools for high-resolution structure determination of agonist:GPCR:G protein complexes and provide an important advance for translational drug discovery.
Methods
Constructs
The human CTR,4 GLP-1R,6 CLR,19 and A1-AR18 were all modified at the N-terminus with replacement of the signal peptide with that of hemagluttinin (HA) to enhance expression, followed by an N-terminal FLAG epitope and 3C protease cleavage site. At the C-terminus all receptors were appended with a 3C protease site followed by an 8× histidine tag. Human RAMP1 was modified at the N-terminus with a HA signal peptide and N-terminal FLAG tag.
The DN-Gαs construct was generated, as previously described,6 by site-directed mutagenesis to incorporate the following mutations: S(H1.02)N (S54N), G(s3h2.02)A (G226A), E(H3.04)A (E268A), N(H3.07)K (N271K), K(H3.10)D (K274D), R(H3.16)K (R280K), T(h3s5.01)D (T284D), I(h3s5.02)T (I285T), and A(s6h5.03)S (A366S). The format for these mutations is natural residue (CGN number10) mutated residue (natural residue reference sequence number mutated residue)
The DN-Gαi2 construct was generated, as previously described,18 by site directed mutagenesis to incorporate the following mutations: S(H1.02)N (S47N), G(s3h2.02)A (G204A), E(H3.04)A (E246A), and A(s6h5.03)S (A327S). The format for these mutations is natural residue (CGN number) mutated residue (natural residue reference sequence number mutated residue).
The N-terminally tagged Venus-Gγ2 has been previously described.21 The nanoluc tagged Gαs and Gαi2 had the engineered version of the shrimp luciferase from Oplophorus gracilirostris flanked by SGGGGS linkers and was inserted after G(h1ha10) in Gαs or E(HA.03) in Gαi2.
Insect Cell Expression
For CTR, GLP-1R, and CLR:RAMP1, the receptors, together with human WT- or DN-Gαs, His6-tagged human Gβ1 and Gγ2, were coexpressed in Tni insect cells (Expression Systems) using baculovirus. Cell cultures were grown in ESF 921 serum-free media (Expression Systems) to a density of 4 million cells mL–1 and then infected with three separate baculoviruses at a ratio of 1:2:2 for CTR, WT or DN-Gαs, and Gβ1γ2; 2:2:1 for GLP-1R, WT or DN-Gαs, and Gβ1γ2; and 5:1:2:1 for RAMP1, CLR, WT or DN-Gαs, and Gβ1γ2. Cultures were harvested by centrifugation 60 h postinfection and the cell pellet was stored at −80 °C. For the A1-AR, the receptor was expressed separately to the G proteins. The A1-AR was expressed in Tni insect cells using baculovirus. The human WT or DN-Gαi2/Gαi3, His6-tagged human Gβ1 and γ2 were coexpressed in Tni insect cells using baculovirus at a 1:1 ratio. Cell cultures were grown in ESF 921 serum-free media (Expression System) to a density of 4 million cells mL–1 and then infected with baculovirus, at the ratios detailed above. Cultures were grown at 27 °C and harvested by centrifugation 60 h postinfection. Cells were snap frozen and stored at −80 °C for later use.
Complex Purification
All cell pellets, except for A1-AR and heterotrimeric Gi protein, were thawed in 20 mM HEPES pH 7.4, 50 mM NaCl, and 2 mM MgCl2 supplemented with cOmplete Protease Inhibitor Cocktail tablets (Roche). GLP-1R and CLR/RAMP1 were coexpressed with heterotrimeric Gs protein, whereas A1-AR and heterotrimeric Gi protein were expressed separately. Complex formation for GLP-1R and CLR/RAMP1 were initiated by addition of either 1 μM exendin-P5, 50 μM oxyntomodulin (Chinapeptide), or 10 μM αCGRP, Nb35–His (10 μg mL–1), apyrase (25 mU mL–1, NEB) and, in the case of CLR:RAMP1, 3C protease (10 μg mL–1); the suspension was incubated for 1 h at room temperature. Membranes were collected by centrifugation at 30 000g for 30 min. The complex from the membrane fraction was solubilized with 0.5% (w/v) lauryl maltose neopentyl glycol (LMNG, Anatrace) supplemented with 0.03% (w/v) cholesteryl hemisuccinate (CHS, Anatrace) for 2 h at 4 °C in the presence of 1 μM exendin-P5, oxyntomodulin, or αCGRP and apyrase (25 mU mL–1, NEB). For A1-AR, cells from either A1-AR or heterotrimeric Gi protein expression were solubilized separately in 20 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 0.5% (w/v) LMNG, 0.01% (w/v) CHS, supplemented with cOmplete Protease Inhibitor Cocktail tablets (Roche). Complex formation was initiated by combining solubilized A1-AR and heterotrimeric Gi and by addition of 1 mM adenosine (ADO) (Sigma) and apyrase (25 mU ml–1, NEB), followed by 2 h incubation at 4 °C. Insoluble material was removed by centrifugation at 30 000g for 30 min. For the CLR:RAMP1 complex only the solubilized complex was immobilized by batch binding to NiNTA resin. The resin was packed into a glass column and washed with 20 column volumes of 20 mM HEPES pH 7.4, 100 mM NaCl, 2 mM MgCl2, 0.01% (w/v) LMNG, and 0.006% (w/v) CHS, 1 μM CGRP, before bound material was eluted in buffer containing 250 mM imidazole. The solubilized GLP-1R or A1-AR complex, or NiNTA purified CLR:RAMP1 complex was immobilized by batch binding to M1 anti-FLAG affinity resin in the presence of 3 mM CaCl2. The resin was packed into a glass column and washed with 20 column volumes of 20 mM HEPES pH 7.4, 100 mM NaCl, 2 mM MgCl2, 3 mM CaCl2, 1 μM exendin-P5, 0.01% (w/v) LMNG and 0.006% (w/v) CHS before bound material was eluted in buffer containing 5 mM EGTA and 0.1 mg/mL FLAG peptide. The complex was then concentrated using an Amicon Ultra Centrifugal Filter (MWCO 100 kDa) and subjected to size-exclusion chromatography on a Superdex 200 Increase 10/300 column (GE Healthcare) that was pre-equilibrated with 20 mM HEPES pH 7.4, 100 mM NaCl, 2 mM MgCl2, 1 μM exendin-P5 or 1 μM oxyntomodulin, 0.01% (w/v) LMNG and 0.006% (w/v) CHS to separate complex from contaminants. Eluted fractions consisting of receptor and G protein complex were pooled and concentrated.
SDS-PAGE and Western Blot Analysis
Samples collected from each purification step were analyzed by SDS-PAGE and Western blot. For SDS-PAGE, precast gradient TGX gels (Bio-Rad) were used. Gels were either stained by Instant Blue (Expedeon) or immediately transferred to PVDF membrane (Bio-Rad) at 100 V for 1 h. The proteins on the PVDF membrane were probed first with the primary mouse anti-His antibody (cat. no. 34660, QIAGEN), and, for membranes with Gαs coincubated, with rabbit anti-Gs C-18 antibody (cat. no. sc-383, Santa Cruz), followed by washing and incubation with secondary antimouse antibody (680RD). For blots with Gαs 800CW, a goat anti-rabbit antibody (Li-Cor) was used. For blots with Gαi2, membranes were again washed and then incubated with an AlexaFluor488 conjugated anti-Gαi2 mouse monoclonal antibody (cat. no. sc13534, Santa Cruz). Bands were imaged using a Typhoon multimode imager (GE Healthcare life sciences) or Li-Cor Odyssey.
Mammalian Cell Membrane Preparations for G Protein BRET Aassays
HEK293AΔGαs-GLP-1R, HEK293AΔGαs/Gαq/11/Gα12/13-GLP-1R or HEK293AΔGαs-CTR cells were transfected WT or DN Gαs-Nanoluc, WT or DN Gαi2-Nanoluc, and Gγ2-Venus at 1:1:1: ratio using PEI. Cell membranes were prepared as described previously and stored at −80 °C.19 At 24 h after transfection, cells were harvested with membrane preparation buffer (20 mM BisTris [pH7.4], 50 mM NaCl, 1 mM MgCl2, 1 × P8340 (protease inhibitor cocktail, Sigma), 1 mM DTT and 0.1 mM PMSF). For Gαi2 membrane preparation, 50 μM GDP was included in the buffer to improve protein stability. Cells were then homogenized, applied to a stepped sucrose gradient (60%, 40%, homogenate) and centrifuged at 100 000g for 2.5 h at 4 °C. The layers between 40% and homogenate were collected, diluted in membrane preparation buffer, and centrifuged at 160 000g for 30 min at 4 °C. The final pellet was resuspended in membrane preparation buffer and stored at −80 °C.
Agonist-Induced G Protein Conformational Changes
Receptor mediated BRET signals between Gαs and Gγ were measured at 30 °C using a PHERAstar (BMG LabTech, Offenburg, Germany). 5 μg/well cell of membrane was incubated with 1 × Furimazine in assay buffer (1 × HBSS, 10 mM HEPES, 0.1% BSA, 1 × P8340, 1 mM DTT, and 0.1 mM PMSF, pH 7.4) for 5 min in a white 96-well plate before initiation of the assay BRET was measured at 15 s intervals before and after ligand and nucleotide addition at the indicated concentrations.
Negative Stain TEM
SEC eluted fractions were used to prepare specimens for negative stain EM using a conventional negative staining protocol.22 Negative-stained samples were imaged at room temperature with a Tecnai T12 (FEI) electron microscope. Images were collected at a magnification of 52 000× and a defocus value of −1 μm on a FEI Eagle 4K camera with a pixel size of 2.06 Å.
Acknowledgments
This work was supported by the Monash University Ramaciotti Centre for Cryo-Electron Microscopy, National Health and Medical Research Council of Australia (NHMRC) project Grants APP1145420, APP1120919, and NHMRC program Grant (APP1055134). A.C., P.M.S., and D.W. are NHMRC Senior Principal Research, Principal Research, and Career Development Fellows, respectively. AG is an Australian Research Council Discovery Early Career Research Fellows. L.T.M. is an Australian Heart Foundation Future Leaders Fellow. The work was supported by the Monash University Ramaciotti Centre for Cryo-Electron Microscopy.
Author Contributions
# These authors contributed equally.
The authors declare no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
References
- Thal D. M.; Vuckovic Z.; Draper-Joyce C. J.; Liang Y. L.; Glukhova A.; Christopoulos A.; Sexton P. M. (2018) Recent advances in the determination of G protein-coupled receptor structures. Curr. Opin. Struct. Biol. 51, 28–34. 10.1016/j.sbi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2017) Theoretical aspects of GPCR-ligand complex pharmacology. Chem. Rev. 117, 4–20. 10.1021/acs.chemrev.5b00561. [DOI] [PubMed] [Google Scholar]
- Rasmussen S. G.; DeVree B. T.; Zou Y.; Kruse A. C.; Chung K. Y.; Kobilka T. S.; Thian F. S.; Chae P. S.; Pardon E.; Calinski D.; Mathiesen J. M.; Shah S. T.; Lyons J. A.; Caffrey M.; Gellman S. H.; Steyaert J.; Skiniotis G.; Weis W. I.; Sunahara R. K.; Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555. 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. L.; Khoshouei M.; Radjainia M.; Zhang Y.; Glukhova A.; Tarrasch J.; Thal D. M.; Furness S. G. B.; Christopoulos G.; Coudrat T.; Danev R.; Baumeister W.; Miller L. J.; Christopoulos A.; Kobilka B. K.; Wootten D.; Skiniotis G.; Sexton P. M. (2017) Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123. 10.1038/nature22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Sun B.; Feng D.; Hu H.; Chu M.; Qu Q.; Tarrasch J. T.; Li S.; Kobilka T. S.; Kobilka B. K.; Skiniotis G. (2017) Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253. 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. L.; Khoshouei M.; Glukhova A.; Furness S. G. B.; Zhao P.; Clydesdale L.; Koole C.; Truong T. T.; Thal D. M.; Lei S.; Radjainia M.; Danev R.; Baumeister W.; Wang M. W.; Miller L. J.; Christopoulos A.; Sexton P. M.; Wootten D. (2018) Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125. 10.1038/nature25773. [DOI] [PubMed] [Google Scholar]
- Carpenter B.; Nehmé R.; Warne T.; Leslie A. G. W.; Tate C. G. (2016) Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536, 104–107. 10.1038/nature18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P.; Park J. H.; Hildebrand P. W.; Kim Y. J.; Krauss N.; Choe H. W.; Hofmann K. P.; Ernst O. P. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502. 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Clapham D. E.; Neer E. J. (1997) G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 37, 167–203. 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Flock T.; Ravarani C. N. J.; Sun D.; Venkatakrishnan A. J.; Kayikci M.; Tate C. G.; Veprintsev D. B.; Babu M. M. (2015) Universal allosteric mechanism for Gα activation by GPCRs. Nature 524, 173–179. 10.1038/nature14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleator J. H.; Mehta N. D.; Kurtz D. T.; Hildebrandt J. D. (1999) The N54 mutant of Galphas has a conditional dominant negative phenotype which suppresses hormone-stimulated but not basal cAMP levels. FEBS Lett. 443, 205–208. 10.1016/S0014-5793(98)01704-9. [DOI] [PubMed] [Google Scholar]
- Slepak V. Z.; Katz A.; Simon M. I. (1995) Functional analysis of a dominant negative mutant of G alpha i2. J. Biol. Chem. 270, 4037–4041. 10.1074/jbc.270.8.4037. [DOI] [PubMed] [Google Scholar]
- Ramachandran S.; Cerione R. A. (2011) A dominant-negative Galpha mutant that traps a stable rhodopsin-Galpha-GTP-betagamma complex. J. Biol. Chem. 286, 12702–12711. 10.1074/jbc.M110.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara R. K.; Tesmer J. J.; Gilman A. G.; Sprang S. R. (1997) Crystal structure of the adenylyl cyclase activator Gsalpha. Science 278, 1943–1947. 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- Lee E.; Taussig R.; Gilman A. G. (1992) The G226A mutant of Gs alpha highlights the requirement for dissociation of G protein subunits. J. Biol. Chem. 267, 1212–1218. [PubMed] [Google Scholar]
- Iiri T.; Bell S. M.; Baranski T. J.; Fujita T.; Bourne H. R. A. (1999) Gsalpha mutant designed to inhibit receptor signaling through Gs. Proc. Natl. Acad. Sci. U. S. A. 96, 499–504. 10.1073/pnas.96.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlot C. H. (2002) A highly effective dominant negative alpha s construct containing mutations that affect distinct functions inhibits multiple Gs-coupled receptor signaling pathways. J. Biol. Chem. 277, 21080–21085. 10.1074/jbc.M201330200. [DOI] [PubMed] [Google Scholar]
- Draper-Joyce C. J.; Khoshouei M.; Thal D. M.; Liang Y. L.; Nguyen A. T. A.; Furness S. G. B.; Venugopal H.; Baltos J. A.; Plitzko J. M.; Danev R.; Baumeister W.; May L. T.; Wootten D.; Sexton P. M.; Glukhova A.; Christopoulos A. (2018) Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature 558, 559–563. 10.1038/s41586-018-0236-6. [DOI] [PubMed] [Google Scholar]
- Liang Y. L., Khoshouei M., Deganutti G., Glukhova A., Koole C., Peat T. S., Radjainia M., Plitzko J. M., Baumeister W., Miller L. J., Hay D. L., Christopoulos A., Reynolds C. A., Wootten D., and Sexton P. M.. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A.; Gao Z.-G. (2006) Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discovery 5, 247–264. 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness S. G. B.; Liang Y. L.; Nowell C. J.; Halls M. L.; Wookey P. J.; Dal Maso E.; Inoue A.; Christopoulos A.; Wootten D.; Sexton P. M. (2016) Ligand-dependent modulation of G protein conformation alters drug efficacy. Cell 167, 739–749. 10.1016/j.cell.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Peisley A.; Skiniotis G. (2015) 2D Projection Analysis of GPCR complexes by negative stain electron microscopy. Methods Mol. Biol. 1335, 29–38. 10.1007/978-1-4939-2914-6_3. [DOI] [PubMed] [Google Scholar]