Abstract
Hepatitis A virus (HAV) is a wide spread pathogenic agent and is the common cause of acute Hepatitis A worldwide. Passive immunization of HAV plays an extremely important role in post-exposure prophylaxis with clinical applications often requiring large amounts of antibody. As an alternative to the in vitro production of recombinant proteins, expression of monoclonal antibodies (mAbs) in the milk of transgenic animals is currently used being associated with low production costs and high activity. In this paper, eight founder lines of transgenic mice were generated by co-microinjection of the two cassettes encoding the heavy- and light-chains of a neutralizing anti-HAV antibody, respectively. The expressed heavy- and light-chains of the mAb were correctly assembled and modified in the mammary gland as detected by western blotting. High expression levels of the antibody were achieved during the lactation period and found to be independent of the copy numbers of integrated transgenes. The highest level was up to 32.2 mg/ml. The binding specificity and neutralizing activity of the expressed mAb were assayed by ELISA and neutralizing test, showing that it is capable to neutralize the JN strain of Hepatitis A virus efficiently. Therefore, our results suggest that a large-scale and efficient production of the anti-HAV mAb in the milk of transgenic farm animals would be feasible in the future.
Keywords: Hepatitis A virus, Monoclonal antibody, Transgenic mice, Milk
Introduction
Hepatitis A was a term first introduced by Krugman et al. in 1967 (Krugman et al. 1967), and there is still a moderate-to-high risk for hepatitis A in the majority of the world’s population (Victor et al. 2007). Approximately 1.4 million cases of hepatitis A occur every year worldwide, predominantly in developing countries (Di Giammarino and Dienstag 2005). Consequently, it is responsible for a substantial economic burden to society in terms of direct treatment cost and indirect productivity loses (Keystone and Hershey 2008). This disease is caused by Hepatitis A virus (HAV) which belongs to the genus Hepatovirus of the Picornaviridae family that primarily infects hepatocytes. It can cause effects that range from the slight symptoms such as fever, anorexia, nausea to death from fulminant hepatitis (Martin and Lemon 2006). Transmission of HAV is mainly through the faecal–oral route by contaminated water and food in global perspective (Hadler et al. 1980). However, in some areas, e.g., the United States, the most common reported source of infection is household or other close contact with an infected person (Victor et al. 2007).
Hepatitis A vaccines have been licensed since 1992 and currently provide pre-exposure protection from HAV infection (CDC 1999). They are long-term protective and highly immunogenic in both children and adults (Van Damme and Van Herck 2005). The administration of Immunoglobulins (Igs) provides short-term effective protection (Larralde et al. 2007). It is mainly for people exposed to the high risk of infection, such as people traveling to places where the virus is endemic or who have close personal contact with a Hepatitis A patient (Kim et al. 2004). Furthermore, Igs are the only product recommended for post-exposure prophylaxis in the United States and Igs administered intramuscularly is the product used for the prevention of HAV infection (CDC 2006). However, Igs against HAV is mainly derived from human plasma, which led to limited supply and possible healthy risks due to its impurity. Therefore, the application of human monoclonal antibodies (mAbs) has been considered as an alternative approach against HAV infection, because mAbs have proven to be the well-characterized and highly effective products with better activity than their polyclonal counterparts (Lonberg 2005; Marasco and Sui 2007).
Through nearly 30 years of development, mAbs have progressively played an important role in therapeutic strategies (Reichert et al. 2005). Accordingly, the rapidly growing market demand has driven a wide range of mAbs applications. Even with conservative prediction, mAbs will probably have global sales of $20 billion by the year 2010 (Gottschalk 2005). The platforms for the mAbs production are variable but critical owing to the significant impact on the level and quality of the products (Keefer 2004). Most of the mAbs approved for commercialization are currently produced by mammalian cell culture; however, this system faces a shortage of production capacity and scale-up production will be inevitably associated with extremely high costs. Besides, in different culture conditions, there are pitfalls in complicated purification steps as well as variable mAbs levels and post-translational modifications (Castillo 1999; Farid 2007; Kozlowski and Swann 2006; Winokur and Stapleton 1992). These limitations have motivated researches to develop alternative production technologies with higher efficiency.
Technology advances in genetic engineering opened up a way for the cost-effective, large-scale production of mAbs using the mammary gland of transgenic animals. This method is capable of high level expression at low cost, associated with the required complex post-translational modifications, constant secretion and stable bioactivity of these proteins. Thus this production system can avoid the pitfalls associated with other expression systems and makes transgenic production of mAbs attractive (Dove 2002; Dyck et al. 2003; Houdebine 2002). In recent years, there have been many successful examples of mAbs expression in the milk of transgenic mice with production levels of up to several mg per ml; such as mAbs against Coronavirus, Hantavirus, CD20 and CD69 (Castilla et al. 1998; Molina et al. 2003; Sola et al. 1998; Tang et al. 2008; Yu et al. 2006). Moreover, it is noteworthy that recombinant human antithrombin III produced in the mammary gland of transgenic goats has been approved as a drug by the European Medicines Evaluation Agency (EMEA) in 2006, which launched as ATryn in the treatment of blood clotting. This demonstrates the commercial reality of protein mass-production by transgenic animals. Therefore in this paper, we expressed a human mAb in the milk of transgenic mice, and explored its potential effect on immunoprophylaxis of HAV infection. This study aimed to demonstrate the feasibility for a large-scale production of the mAbs against HAV in the milk of transgenic farm animals in the future.
Materials and methods
Construction of transgenes
The coding regions of H chain (HC) and L chain (LC) genes of a human IgG1 mAb against HAV were amplified by PCR from plasmids pHAVH3 and pHAVL3 (Wei et al. 2004). The HC was amplified using primers HC-F (5′-GTCTCGAGCCATGGGATGGAGCTGTA-3′) and HC-R (5′-GTCTCGAGAGATTATTTTCCCGGACT-3′), both containing a XhoI site. Primers LC-F (5′-GTGTCGACCCATGGGATGGAGCTGTA-3′) and LC-R (5′-GTGTCGACACTAGTCCCTC TAACACT-3′), both with an added SalI site were used to amplify the LC. Then the two amplified fragments were separately cloned into the expression plasmid pBC1 digested with XhoI. The resulting plasmids designated pBC-hGHC and pBC-hGLC were under the control of the goat β-casein regulatory sequences to direct gene expression specifically in the mammary gland duration lactation.
Generation of transgenic mice
The hGHC and hGLC transgenes were, respectively, released from the plasmids with NotI/NruI or NotI/SalI digestion, leading to a 18.1 kb or a 16.5 kb DNA fragment, containing of chicken β-globin insulator, goat β-casein promoter, Ig secretory leader, either the human VH-Cγ1 gene (with introns) or VL-Cκ gene, and β-casein 3′ genomic DNA, respectively (Fig. 1). These two fragments were purified after agarose gel electrophoresis (Omega, USA) and subsequently co-microinjected at a 1:1 molar ratio into pronuclei of fertilized Kunming White eggs according to standard protocols. Genomic DNA was extracted from tail biopsies of mice as described previously (Hogan et al. 1994). The presence of the transgenes was identified by PCR with primers pBC-F (5′-GATTGACAAGTAATACGCTGTTTCCTC-3′) and pBC-R (5′-CATCAGAAGTTAAACAGCACAGTTAG-3′). The primers were specific to the β-casein intron 1 and intron 7 sequences and could therefore detect both heavy and light chain genes simultaneously. Genomic DNA (10 μg) was digested with EcoRI and analyzed by Southern blotting (Sambrook and Russell 2001) with the hybridization probes labelled by [α-32P] dCTP. The probes were the amplified products of 2.4 kb HC and 1.2 kb LC genes. Transgene copy numbers were determined by signal quantification via a phosphorimager (Storm 820; Molecular Dynamics, Sunnyvale, CA).
Fig. 1.
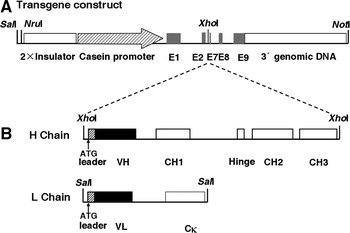
Schematic presentation of the hGHC/hGLC transgene constructs used for microinjection. a Structure of the transgene construct released from pBC1 vector with NotI/NruI for HC and NotI/SalI for LC. The transgene backbone contains 2 × chicken β-globin insulator, goat β-casein promoter, untranslated exons (E)1, parts of E2 and 7, E8, E9 and β-casein 3′ genomic DNA. The dotted lines indicate insertion of the HC and LC sequences to the unique XhoI restriction site, respectively. b Hatched boxes and solid boxes represent Ig secretory leader and the variable regions (VH and VL), respectively. Exons encoding the constant regions as open boxes are indicated in CH1, Hinge, CH2 and CH3 for HC or Cκ for LC. Translational start codons are indicated in the H chain and the L chain of Ig, respectively. Relevant restriction enzymes sites are shown
RT-PCR analysis
Total RNA was isolated using Trizol (Tiangen, CN) from mammary glands tissues of three female transgenic mice during lactation periods. One microgram of total RNA was used for first-strand cDNA synthesis by using M-MLV Reverse Transcriptase (Progema, USA). The reaction was carried out for 1 h at 37°C for oligodT in a total volume of 25 μl. Primers used to detect the chimeric mRNA expression were Exon1-F (5′-TCCATTCAGCTTCTCCTTCA-3′) and VH-R (5′-CCTCGGCTCTCAGATTGTTC-3′), Exon1-F (5′-TCCATTCAGCTTCTCCTTCA-3′) and VL-R (5′-GACTTGGAGCCAGAGAATCG-3′), which were complementary to the exon 1 of the goat β-casein gene and V-region of the HC and LC, amplifying 408 bp and 322 bp fragments, respectively. The mouse β-actin was used as RT-PCR internal control. The primers of mouse β-actin were β-actin F (5′-TTCTACAATGAGCTGCGTGTGG-3′) and β-actin R (5′-GGTGTTGAAGGTCTCAAACATGAT-3′), amplifying a 142 bp fragment.
SDS-PAGE
Milk from transgenic mice and non-transgenic mice was collected at day 7 and 20 of lactation, diluted with distilled water to 500 μl, and defatted by centrifugation at 4°C or 15 min at 10,000×g. The skim milk obtained was mixed with sample buffer and then separated on 10% SDS-PAGE gels under both reducing and non-reducing conditions, respectively. Proteins were visualized by staining with Coomassie brilliant blue.
Western blotting
Separated proteins were electrophoretically transferred to nitrocellulose membranes (Amersham Pharmacia, UK), and then incubated overnight in blocking buffer (3% BSA in PBS-T) at 4°C. Immunodetection was carried out with the HRP-conjugated goat anti-human Fab specific antibody (Sigma, US) and ECL western blotting reagents (Amersham Biosciences, UK) according to the manufacturer’s instructions. Purified human IgG (Sigma, US) was used as the positive control.
ELISA
A 96-well microtitre plate was coated with goat anti-human Fab (Sigma, US) and incubated at 4°C overnight. After being washed with PBS containing 0.05% Tween-20 (PBS-T), the wells were blocked with 0.5% BSA in PBS-T for 2 h at room temperature. One hundred microliters of milk samples were added per well at twofold serial dilutions from 1/1,000 to 1/512,000, respectively. After the incubation and washing, plates were incubated with 100 μl of HRP-conjugated goat anti-human IgG Fc antibody (Sigma, US) at a dilution of 1/2,000 per well. A standard curve was generated using twofold serial dilutions of purified human IgG. The colorimetric reaction was developed with the substrate TMB (Pierce, US) and the absorbance value was measured at 450 nm. The plate was washed six times in PBS-T between each step and all incubations were carried out at room temperature for 1 h. The specific binding activity of the milk samples to HAV antigen was determined by ELISA using purified Hepatitis A antigen (Cao et al. 2004) coated microtitre plates. HRP-conjugated anti-human Fc specific antibody at a 1/2,000 dilution was used. The HA78 antibody (2.5 mg/ml), which was expressed by CHO cells, was used as the positive control.
In vitro neutralization activity test
Neutralizing activity against HAV was determined in a modified cell culture assay, based on MacGregor et al. (1983). First, HAV JN strain was diluted in 10-fold steps from 10−1 to 10−5 to determine the 50% tissue culture infective dose (TCID50) in fetal rhesus monkey kidney (FRhK-4) cells; then the titre of infectious virus was determined based on the TCID50 endpoint. 100TCID50 of HAV were used for the following experiment. The milk samples were initially diluted to the concentration close to 2.5 mg/ml. Then, both the milk samples and recombinant purified antibody (positive control, 2.5 mg/ml) were diluted using twofold dilutions from 1/20 to 1/640, and were incubated with 0.1 ml of virus at 37°C for 2 h separately. Four replicates of each sample were inoculated onto the monolayers of FRhK-4 cells growing in 24 wells plates. Mock infected cells were included as HAV negative control. In addition, HAV virus titre was also measured by ELISA. After all the plates were incubated at 33°C for 1.5 h and the medium removed, 1.0 ml of fresh culture medium was added to the wells of each plate. Monolayers were further incubated for 21 days and the culture medium was replaced every 6–7 days. The cells were harvested after 21 days, with 0.2 ml of PBS plus 0.05% of Tween-20 and disrupted by three cycles of freeze–thawing. The presence of HAV was monitored by ELISA with the condition that polyclonal human anti-HAV antibody (CDC, CN) was used as coating antibody and HRP-conjugated polyclonal human anti-HAV antibody (CDC, CN) used as detecting antibody. The 50% neutralization endpoint was calculated (Reed and Muench 1938).
Results
Generation of the transgenic mice
To generate transgenic mice expressing the anti-HAV mAb at high levels in milk, two separate cassettes were constructed encoding the IgG HC and LC genes, each under control of the goat β-casein promoter (Fig. 1). These two cassettes were co-injected into the fertilized mouse eggs. Four males (No. 1, 2, 14, 15) and 4 females (No. 7, 8, 9, 35) transgenic founders were obtained from 36 mice analyzed by PCR (Fig. 2a); all contained both the HC and LC transgenes. In addition, there were another five founders containing either HC or LC transgenes; these were not analyzed further.
Fig. 2.
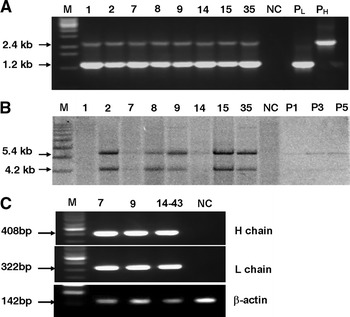
Molecular characterization of transgenic mice. a PCR detection of transgenic founders. M, 1 kb DNA ladder; P: positive plasmid control; NC, negative control; transgenic founders numbered 1, 2, 7, 8, 14, 15 and 35. The amplified products are 2.4 kb for HC and 1.2 kb for LC. b Southern blot analysis of the transgenes. The digested genomic DNA was hybridized with the mixture probes of amplified HC and LC fragments and hybridization signals for HC and LC are indicated. NC, genomic DNA of non-transgenic mice as a negative control; P1, P3, P5, are positive plasmid DNA equivalent to 1, 3 and 5 gene copies, respectively. The transgene copy numbers are presented in Table 1. c RT-PCR analysis of the transgenes expression in the mammary gland tissues of transgenic mice. RT-PCR was performed to demonstrate the presence of the chimeric mRNA. M, 100 bp ladder; Transgenic mice are 7, 9 and 14–43; NC, the non-transgenic mouse. The mouse β-actin gene was used as the internal control
Southern blotting was further used to confirm that both transgenes were integrated into the genome of all eight transgenic mice (Fig. 2b) as well as to determine the transgene copy number. The probes specific for 2.4 kb HC and 1.2 kb LC fragments were mixed together allowing both transgenes to be detected simultaneously, enabling comparison of the relative copy numbers. The copy numbers of the transgenes in eight founders were different, ranging from 3 to 20 for both HC and LC by comparing with the band intensity with those of known amount of control plasmids (Table 1). Six transgenic founders (8 and 35 died) were mated with wild type mice and all of them transmitted the transgenes to their offspring. A total of 26 F1 transgenic mice were identified among 54 offspring, 15 of them contained double transgenes, while the remaining contained either the HC or LC transgene. This probably reflects multiple integration loci in some founder animals.
Table 1.
Correlation of the transgene copy number and mAb concentration in different transgenic lines
| Line F0 (Sex) | Offspring F1a | Copy No.b | Expression level (mg/ml)c | |
|---|---|---|---|---|
| HC | LC | |||
| 7 (F) | 5 | 5 | 22.3 | |
| 9 (F) | 15 | 10 | 17.6 | |
| 9–49 (F) | 32.2 | |||
| 9–50 (F) | 6.7 | |||
| 14 (M) | 3 | 3 | ||
| 14–43 (F) | 16.5 | |||
| 15 (M) | 20 | 20 | ||
| 15–24 (F) | 75.3 × 10−3 | |||
aOffspring F1 refers to the F1 progeny of founders
bTransgene copy number was determined as described in “Material and methods”
cThe expression levels are presented based on the ELISA assay
Expression of the anti-HAV mAb in the mammary glands of transgenic mice
The HC and LC transgenes mRNA expression in the mammary gland of lactating transgenic mice were analyzed by RT-PCR (Fig. 2c). We found that both the HC and LC transgenes were expressed in the females of three detected transgenic lines (mice 7, 9 and 14–43). This result confirmed that the recombinant mAb was expressed from the chimeric mRNA, which contains untranslated exons 1 and 2 of the goat β-casein gene.
Milk samples were collected from five transgenic founder mice or F1 mice that carried both transgenes. The structure and expression of the anti-HAV mAb in the milk was analyzed by western blotting. A single band was detected for both the HC and LC under reducing condition with the expected molecular weights (MW) of 50 kDa (HC) and 25 kDa (LC), respectively (Fig. 3a). The results indicated the presence of the HC and LC expression in all the detected lines. Transgenic milk samples were also analyzed under non-reducing conditions which showed strong bands with the MW of 150 kDa (Fig. 3b). The relative molecular mass of the major detected bands were identical to the purified human IgG, indicating that the recombinant mAb was assembled and modified correctly in these transgenic mice. However, some additional weak bands with lower MW were also observed, probably reflecting the presence of incompletely assembled IgG molecules.
Fig. 3.
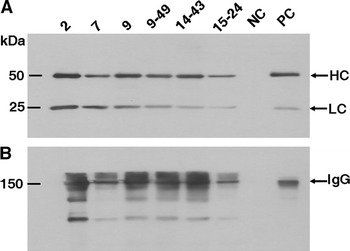
Western blot analysis of transgenic lines expressing the recombinant mAb against HAV. The milk samples were separated by SDS-PAGE under reducing (a) and non- reducing conditions (b). Following transferred to the nitrocellulose membranes, samples were detected with HRP-conjugated goat anti-human Fab specific antibody. Molecular weight markers were indicated in the left lane. 2, 7, 9, 9–49, 14–43, 15–24, different transgenic mice; NC, negative control (milk of non-transgenic mice); PC, positive control (purified human IgG)
The stability of the mAb production in the milk was also detected by analyzing eight mice (F2 generation) in four transgenic lines. Within each line, the amount of mAb was constant (data not shown). This result may be due to the co-integration of both transgenes in the same chromosome for most of the founders.
To assess the expression levels of the recombinant mAb, the skim milk obtained at day 7 and 20 of lactation periods from four different transgenic lines (Line 7, 9, 14 and 15) was measured using an ELISA approach specific to human IgG Fab fragment. Four lines were shown to express the anti-HAV mAb higher than 1 mg/ml with the highest level was up to 32.2 mg/ml. Line 15 showed a low expression level (Table 1). No apparent differences in expression of the mAb were observed between the milk samples collected on day 7 and those collected on day 20 in each line (data not shown).
Characterization of the mAb binding specificity to HAV antigen
The binding specificity of the milk samples to HAV antigen was examined by ELISA. As expected, the milk samples could bind to HAV antigen in a dose-dependent manner and their affinities for HAV appeared to be equivalent (except line 15) which had nearly the same binding specificity as CHO-derived IgG (HAIgG78) (Fig. 4). The highest antibody titer could reach 106 with a concentration of 2.5 ng/ml.
Fig. 4.
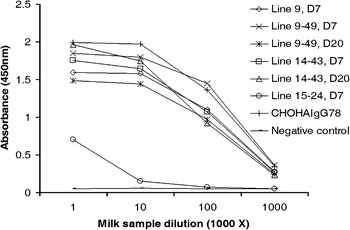
ELISA detection of the expressed mAb binding activity to HAV antigen. Dilutions of the milk samples from three transgenic lines 9, 14 and 15 (mice 9, 9–49, 14–43 and 15–24), and a female non-transgenic mouse (negative control) were added to microtitre wells coated with HAV. The specificity was detected with HRP-conjugated anti-human Fc specific antibody. Those legends with D7 and D20 denotes the corresponding milk samples collected at 7th and 20th day of lactation, respectively. The CHO-derived HAIgG78 antibody (2.5 mg/ml), which recognize HAV antigen, was used as the positive control. Values are presented as OD value at 450 nm
To further evaluate the neutralizing activity of mAb, milk from two high expressing transgenic lines, line 9 and 14, was assayed by neutralizing test based on their ability to block HAV infection in FRhK-4 cells. Dilutions of transgenic skim milk were considered to have virus-neutralizing activity if less than 50% of the FRhK-4 cells were infected by HAV. It was shown that all the three transgenic milk samples exhibited neutralizing activity against HAV in a dose-dependent manner (Fig. 5). In particular, the milk sample of mouse 9 reduced HAV infectivity by 50% at a dilution of 1/280, while the milk samples of mice 9–49 and 14–43 reduced HAV infectivity by 50% at a dilution of 1/320. For the milk sample of mouse 9–49, the concentration of the recombinant mAb required for 50% protection was estimated to be 0.3 μg/ml at a dilution of 1/320.
Fig. 5.
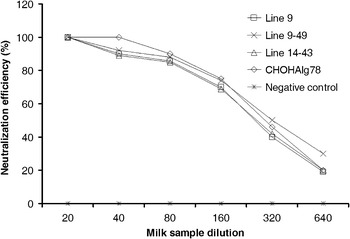
Comparison of the specific activity of the recombinant mAbs in HAV neutralization. Serial dilutions from 1/20 to 1/640 of the milk samples (initially diluted to the concentration of 2.5 mg/ml) from two transgenic lines 9 and 14 (mice 9, 9–49, 14–43), and a female non-transgenic mouse (negative control) were assayed with HAV JN strain. The CHO-derived HAIgG78 (2.5 mg/ml) antibody, was used as the positive control
No obvious differences were observed in the neutralizing activity among the detected milk samples. In addition, protection levels were similar to HAIgG78. In contrast, milk samples from non-transgenic mice had no virus neutralizing capacity at any of the tested dilutions.
Discussion
Towards engineering passive immunization in order to protect HAV infection in human beings, a transgenic mice model expressing the human anti-HAV mAb has been constructed. In this paper, we demonstrate that both the HC and LC of the anti-HAV antibody can be expressed in the milk of transgenic mice under control of the goat β-casein promoter, as well as be properly assembled into functional IgG molecule against HAV. We incorporated two strategies into vector design to enhance transgene expression. First, the chicken β-globin insulator is predicted to reduce the influence of neighboring regulatory elements, thereby it modulating the positive effects caused by the random integration of the transgenes in the host chromosome (Chung et al. 1997). Secondly, genomic rather than cDNA fragments of IgG constant regions were used to benefit from potential enhancer-like effects of its introns (Whitelaw et al. 1991). The high levels of mAb expression that we observed were consistent with the results using these two strategies, though position effects, e.g., line 15 with extremely low expression level, were still observed. Furthermore, most of the mice carried both transgenes, however, when mating the founder transgenic mice with the non-transgenic mice, the resulting single chain of the transgenic offspring might be due to the segregation of the transgenes from different chromosomal sites for some founders. The majority of the transgenic founders transmitted both transgenes to their offspring, which agrees with most of previous studies reported that co-microinjection normally led to both transgenes co-integrated into a single chromosomal site (Clark et al. 1992; Tang et al. 2008).
The amount of the expressed mAb estimated by ELISA in the milk revealed the expression level was higher than any other mammalian expression systems, with the highest expression level up to 32.2 mg/ml. This expression level is considerably higher than those previously reported in the milk of transgenic mice; such as 0.4 mg/ml (Limonta et al. 1995), 0.7 mg/ml (Kolb et al. 2001), 0.8 mg/ml (Newton et al. 1999), 5 mg/ml (Castilla et al. 1998), and 6 mg/ml (Sola et al. 1998). The expression level was maintained over the lactating periods, which validated the fact that goat β-casein promoter could stably drive high level expression of the recombinant proteins to the mammary gland at the lactating stage (Young et al. 1997). No direct relationship between the transgene copy number and the amount of mAb was observed in the detected transgenic mice, implying that the integration site of the transgene had a greater effect on the expression level than that of transgene copy number (Table 1) which is consistent with the previous finding (Sola et al. 1998).
Like other therapeutic recombinant proteins, we have to characterize mAb well in order to ensure their structural and functional activity. Specifically, in our experiment, presence of additional weak bands in the western blotting under non-reducing condition indicated that there were small portion of the incompletely folded IgG molecules. Several explanations have been proposed to interpret this phenomenon; we suggest the most likely explanation relates to the presence of free sulfhydryl in the mAb (Zhang and Czupryn 2002). Each IgG molecule consists of two heavy and two light chains covalently linked by interchain disulfide bonds, with in addition, each domain of the heavy or light chain containing one additional disulfide bond. Some mAb species containing free sulfhydryl are likely to be formed during the protein folding process in the endoplasmic reticulum. Consequently, in our experiment, minor proportion of the formed recombinant mAb in the epithelial cells in the mammary gland of mice may lack interchain disulfide bonds but remain noncovalently associated under non-reducing conditions presenting as low MW fragments in our analysis.
Noteworthily, our data exhibits significant advantages in producing the neutralizing mAb against HAV in the transgenic milk with high-affinity and high-specificity in protein synthesis, as compared with the previously study proposed production of neutralizing mAbs to HAV using phage-displayed antibody library (Kim et al. 2004). The antigen-binding activity of mAb was demonstrated in the specific ELISA, which represented a nearly 40-fold increase in the antibody affinity. Through a virus neutralization assay with previously tested CHO-derived HAIgG78 as the control, we verified that transgenic milk produced mAb was as effective as the HAIgG78 in neutralizing HAV and preventing infection of FRhK4 cells (Fig. 5).
In conclusion, we have generated transgenic mice expressing high levels of HAV neutralizing mAb in milk. This work solidifies the concept of using larger transgenic animals for the production of a potentially therapeutic anti-HAV antibody. With the ever-growing market need for large quantities of mAbs, transgenic large animals will provide a cost-competitive large-scale production alternative for these complex proteins. Furthermore, this method will also facilitate the commercial production of mAbs using transgenic bioreactors for immunoprophylaxis.
Acknowledgements
We would like to thank Dr. Bruce Whitelaw (The Roslin Institute, UK) for revising the manuscript. This work was supported by “863” High-Tech Research Development Project (Project Grant No. 2002AA206111, 2001AA213091) and National Natural Scientific Foundation of Beijing (Project Grant No. 5030001).
Contributor Information
Mifang Liang, Phone: +86-10-63550268, FAX: +86-10-63550268, Email: mifangl@vip.sina.com.
Ning Li, Phone: +86-10-62731146, FAX: +86-10-62733904, Email: ninglcau@cau.edu.cn.
References
- Cao JY, Liang MF, Meng QL, Wang XF, Xu YG, Guo KQ, Zhan MY, Bi SL, Li DX. Baculovirus expression of two human recombinant neutralizing IgG monoclonal antibodies to hepatitis A virus. Chin J Exp Clin Virol. 2004;18:20–23. [PubMed] [Google Scholar]
- Castilla J, Pintado B, Sola I, Sanchez-Morgado JM, Enjuanes L. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat Biotechnol. 1998;16:349–354. doi: 10.1038/nbt0498-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo FJ (1999) Production of clinical grade monoclonal antibodies. In: Presentation at international business communications fifth annual antibody production & downstream processing conference, pp 17–19
- CDC Prevention of Hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1999;48(RR12):1–37. [PubMed] [Google Scholar]
- CDC Prevention of Hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 2006;55(RR07):1–23. [PubMed] [Google Scholar]
- Chung JH, Bell AC, Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc Natl Acad Sci USA. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Cowper A, Wallace R, Wright G, Simons JP. Rescuing transgene expression by co-integration. Biotechnology (NY) 1992;10:1450–1454. doi: 10.1038/nbt1192-1450. [DOI] [PubMed] [Google Scholar]
- Di Giammarino L, Dienstag JL. Hepatitis A—the price of progress. N Engl J Med. 2005;353:944–946. doi: 10.1056/NEJMe058152. [DOI] [PubMed] [Google Scholar]
- Dove A. Uncorking the biomanufacturing bottleneck. Nat Biotechnol. 2002;20:777–779. doi: 10.1038/nbt0802-777. [DOI] [PubMed] [Google Scholar]
- Dyck MK, Lacroix D, Pothier F, Sirard MA. Making recombinant proteins in animals—different systems, different applications. Trends Biotechnol. 2003;21:394–399. doi: 10.1016/S0167-7799(03)00190-2. [DOI] [PubMed] [Google Scholar]
- Farid SS. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:8–18. doi: 10.1016/j.jchromb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Gottschalk U. Downstream processing of monoclonal antibodies: from high dilution to high purity. BioPharm Int. 2005;18:42–52. [Google Scholar]
- Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302:1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Houdebine LM. Antibody manufacture in transgenic animals and comparisons with other systems. Curr Opin Biotechnol. 2002;13:625–629. doi: 10.1016/S0958-1669(02)00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer CL. Production of bioproducts through the use of transgenic animal models. Anim Reprod Sci. 2004;82–83:5–12. doi: 10.1016/j.anireprosci.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Keystone JS, Hershey JH. The underestimated risk of hepatitis A and hepatitis B: benefits of an accelerated vaccination schedule. Int J Infect Dis. 2008;12:3–11. doi: 10.1016/j.ijid.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jang MH, Stapleton JT, Yoon SO, Kim KS, Jeon ES, Hong HJ. Neutralizing human monoclonal antibodies to hepatitis A virus recovered by phage display. Virology. 2004;318:598–607. doi: 10.1016/j.virol.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Kolb AF, Pewe L, Webster J, Perlman S, Whitelaw CB, Siddell SG. Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis. J Virol. 2001;75:2803–2809. doi: 10.1128/JVI.75.6.2803-2809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S, Swann P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev. 2006;58:707–722. doi: 10.1016/j.addr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200:365–373. doi: 10.1001/jama.200.5.365. [DOI] [PubMed] [Google Scholar]
- Larralde OG, Martinez R, Camacho F, Amin N, Aguilar A, Talavera A, Stott DI, Perez EM. Identification of hepatitis A virus mimotopes by phage display, antigenicity and immunogenicity. J Virol Methods. 2007;140:49–58. doi: 10.1016/j.jviromet.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Limonta J, Pedraza A, Rodriguez A, Freyre FM, Barral AM, Castro FO, Lleonart R, Gracia CA, Gavilondo JV, de la Fuente J. Production of active anti-CD6 mouse/human chimeric antibodies in the milk of transgenic mice. Immunotechnology. 1995;1:107–113. doi: 10.1016/1380-2933(95)00010-0. [DOI] [PubMed] [Google Scholar]
- Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- MacGregor A, Kornitschuk M, Hurrell JG, Lehmann NI, Coulepis AG, Locarnini SA, Gust ID. Monoclonal antibodies against hepatitis A virus. J Clin Microbiol. 1983;18:1237–1243. doi: 10.1128/jcm.18.5.1237-1243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43:S164–S172. doi: 10.1002/hep.21052. [DOI] [PubMed] [Google Scholar]
- Molina A, Valladares M, Magadan S, Sancho D, Viedma F, Sanjuan I, Gambon F, Sanchez-Madrid F, Gonzalez-Fernandez A. The use of transgenic mice for the production of a human monoclonal antibody specific for human CD69 antigen. J Immunol Methods. 2003;282:147–158. doi: 10.1016/j.jim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Newton DL, Pollock D, DiTullio P, Echelard Y, Harvey M, Wilburn B, Williams J, Hoogenboom HR, Raus JC, Meade HM, Rybak SM. Antitransferrin receptor antibody-RNase fusion protein expressed in the mammary gland of transgenic mice. J Immunol Methods. 1999;231:159–167. doi: 10.1016/S0022-1759(99)00154-4. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench IL. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sola I, Castilla J, Pintado B, Sanchez-Morgado JM, Whitelaw CB, Clark AJ, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Yu S, Zheng M, Ding F, Zhao R, Zhao J, Dai Y, Li N (2008) High level expression of a functional human/mouse chimeric anti-CD20 monoclonal antibody in milk of transgenic mice. Transgenic Res 727–732. doi:10.1007/s11248-007-9162-3 [DOI] [PubMed]
- Van Damme P, Van Herck K. Effect of hepatitis A vaccination programs. JAMA. 2005;294:246–248. doi: 10.1001/jama.294.2.246. [DOI] [PubMed] [Google Scholar]
- Victor JC, Monto AS, Surdina TY, Suleimenova SZ, Vaughan G, Nainan OV, Favorov MO, Margolis HS, Bell BP. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694. doi: 10.1056/NEJMoa070546. [DOI] [PubMed] [Google Scholar]
- Wei JS, Tao R, Sun WW, Jia Q, Li C, Liang MF. Purification and characterization of recombinant human anti-HAV monoclonal antibody. Chin J Biotechnol. 2004;20:257–261. [PubMed] [Google Scholar]
- Whitelaw CB, Archibald AL, Harris S, McClenaghan M, Simons JP, Clark AJ. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991;1:3–13. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- Winokur PL, Stapleton JT. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992;14:580–586. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]
- Young MW, Okita WB, Brown EM, Curling JM. Production of biopharmaceutical proteins in the milk of transgenic dairy animals. Biopharm Int. 1997;10:34–38. [Google Scholar]
- Yu S, Liang M, Fan B, Xu H, Li C, Zhang Q, Li D, Tang B, Li S, Dai Y, Wang M, Zheng M, Yan B, Zhu Q, Li N. Maternally derived recombinant human anti-hantavirus monoclonal antibodies are transferred to mouse offspring during lactation and neutralize virus in vitro. J Virol. 2006;80:4183–4186. doi: 10.1128/JVI.80.8.4183-4186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Czupryn MJ. Free sulfhydryl in recombinant monoclonal antibodies. Biotechnol Prog. 2002;18:509–513. doi: 10.1021/bp025511z. [DOI] [PubMed] [Google Scholar]


