Abstract
Rhinosinusitis (RS) is a heterogeneous group of diseases. It is a significant and increasing health problem that affects about 15% of the population in Western countries. It has a substantial impact on patients’ health-related quality of life and daily functioning and represents a huge financial burden to society and the health care system as a result of the direct and indirect costs. In addition, RS is not well-understood, and little is known about the etiology and pathophysiology. In the past decade, many papers have been published that have changed our understanding of RS. RS is commonly classified into acute and chronic RS based on symptom duration. In acute RS, an inflammatory reaction initiated by a viral infection characterizes most uncomplicated, mild to moderate cases. Therefore, the first line of treatment for these cases are intranasal steroids and not antibiotics. In severe and complicated cases, antibiotics combined with topical steroids remain the treatment of choice. On the other hand, chronic RS is actually subdivided into two distinct entities (chronic rhinosinusitis with and without polyps), as growing evidence indicates that these entities have specific inflammatory pathways and cytokine profiles. The authors review recent data regarding the clinical presentations, cytokine profiles, tissue remodeling, and modalities of treatment for each form of RS.
Keywords: Rhinosinusitis, ARS, CRS with and without polyps, Cytokine profiles, Fungi, Staphylococcus aureus, Enterotoxins, Tissue remodeling, Treatment
Introduction
Rhinosinusitis is a large and heterogeneous group of diseases. Despite the fact that many papers have been published on this topic in the past decade, little is known about the etiology and pathophysiology. Nevertheless, the amount of knowledge available in the worldwide literature has changed our understanding of this entity.
Rhinosinusitis is commonly classified into acute (ARS) and chronic rhinosinusitis (CRS) based on symptom duration. In ARS, an inflammatory reaction characterizes most uncomplicated, mild to moderate cases. Therefore, there is no longer an argument to prescribe an antibiotic as the first line of treatment but rather to privilege an anti-inflammatory treatment such as intranasal steroids. In severe and complicated cases, antibiotics combined with topical steroids should remain the treatment of choice. On the other hand, CRS is actually subdivided into two distinct entities (CRS with and without polyps), as growing evidence indicates that these entities have specific inflammatory pathways and cytokine profiles.
This paper is a review of the recent data on rhinosinusitis. The authors hope that this review helps clinicians gain a better understanding of how to manage such conditions.
Definition of Rhinosinusitis
Rhinosinusitis is a heterogeneous group of diseases, a significant and increasing health problem that affects about 15% of the population in Western countries [1, 2]. It has a substantial impact on patients’ health-related quality of life and daily functioning. It represents a huge financial burden for society and the health care system as a result of direct and indirect costs. This establishes rhinosinusitis as one of the most expensive disorders experienced by, for example, the US population [2–4].
On the basis of the International Consensus on Rhinosinusitis, rhinosinusitis is subdivided into acute, subacute, recurrent, and chronic rhinosinusitis. Table 1 summarizes the different forms of rhinosinusitis.
Table 1.
Classification of rhinosinusitis
| Temporal designation | Duration of symptoms |
|---|---|
| Acute | ≤4 wk |
| Subacute | 4–12 wk of unresolved acute symptoms |
| Chronic | ≥12 wk (symptoms of varying severity but similar to those seen during the acute episode; CT or MRI typically abnormal) |
| Recurrent | ≥3 episodes of acute rhinosinusitis/y |
Acute Rhinosinusitis
ARS is a common upper respiratory tract disorder that involves inflammation of the nasal and paranasal sinus mucosa. It can be mild, moderate, or severe. Unlike a common cold, which typically resolves in less than 5 days, the symptomatology of ARS worsens after 5 days or persists more than 10 days, but in all cases, it resolves in less than 4 weeks [5, 6]. In the European Position Paper on Rhinosinusitis and Nasal Polyps document, the symptoms can persist up to 12 weeks and be intermittent or persistent [1]. Primary care physicians diagnose and treat a large proportion of these infections. With appropriate therapy, signs and symptoms of ARS resolve completely. The diagnosis of ARS is based on patient history and clinical examination [6]. The symptomatology includes nasal congestion, purulent discharge, fever, headache, facial pain/pressure, dental pain, postnasal drip, cough, and tenderness around the sinus area [5, 6]. Table 2 summarizes the symptomatology and classifies it into major and minor symptoms and those found in the cases of complications. The symptoms typically worsen in the recumbent position and may interfere with sleep.
Table 2.
Signs and symptoms of acute rhinosinusitis
| Major criteria (very important, frequent) | Minor criteria (not as relevant) | In case of complication |
|---|---|---|
| Nasal congestion | Fever | Local extension, palpable frontal or malar masses and deformity, frontal swelling |
| Purulent nasal discharge | Facial pain and tenderness | Orbital pain |
| Facial pressure | Fatigue | Periorbital edema |
| Hyposmia/anosmia | Intractability, prolonged course | Proptosis |
| Headache | Ophthalmoplegia | |
| Halitosis | Intracranial infection: meningitis or brain abscess | |
| Dental pain, toothache | ||
| Cough |
(Adapted from Report of the Rhinosinusitis Task Force Committee Meeting [157])
Nasal endoscopy reveals the mucosa and turbinates to be red and swollen with clear secretions that turn thicker, colored, and opaque within 5–7 days and then become clear again before symptom resolution. Symptoms that require immediate referral to a specialist are the following: periorbital edema, displacement of the eyeball, diplopia, ophthalmoplegia, impaired extraocular movements, reduced visual acuity, severe headache or unilateral facial or orbital pain, frontal lump, and neurological signs and deficits. ARS is typically preceeded by a viral infection or an allergic reaction.
Viruses account for at least 80% to 90% of the causative agents of ARS. Among them, rhinovirus, coronavirus, influenza, respiratory syncitial virus, and parainfluenza virus play a major role [7–9]. ARS becomes a bacterial infection in about 0.5% to 2% of the cases. This is the case when the RS is severe or complicated. In these particular conditions, the “infernal trio” (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarhalis infections) must be considered. Anaerobes have been reported in up to 30% of cases.
The pathophysiology of ARS involves interaction between a predisposing condition (allergic rhinitis, septal deformity, immune deficiency, environmental factors), a viral infection, and a consequent inflammatory response in the mucosal lining of the nose and paranasal sinuses. Table 3 summarizes different predisposing factors that can play a role in the development of ARS. The inflammatory process leads to the development of edema, engorgement, fluid extravasation, mucus production, and obstruction of the sinus ostium. This ostial obstruction impedes the normal ventilation and drainage of the sinus. There is then a decrease in the partial pressure in oxygen, a decrease in the ciliary clearance, a stasis of secretion, and a secundary bacterial infection. Thus, ARS is first regarded as an infectious process initiated by a viral infection.
Table 3.
Predisposing factors for acute rhinosinusitis
| Infection |
| •Viral upper respiratory tract infection (most common) |
| Allergic rhinitis |
| •Perennial/seasonal |
| •Persistent/intermittent (ARIA classification) |
| Nonallergic rhinitis |
| •Vasomotor rhinitis |
| •Aspirin intolerance (AERD) |
| •Nonallergic, noninfectious perennial rhinitis |
| Medication related (rhinitis medicamentosa) |
| •Topical decongestants |
| •β-Blockers |
| •Oral contraceptives |
| •Antihypertensives |
| Coexisting medical conditions |
| •Pregnancy |
| •Hypothyroidism |
| •Horner syndrome |
| •Wegener’s granulomatosis |
| •Cystic fibrosis |
| •Vascular headache |
| •Cerebrospinal fluid rhinorrhea |
| Anatomic variants |
| •Deviated septum |
| •Concha bullosa |
| •Nasal polyps |
| •Foreign body |
| •Tumor |
AERD aspirin-exacerbated respiratory disease, ARIA allergic rhinitis and its impact on asthma
(Adapted from Derosiers [6])
When we consider the cytokine profile, ARS results from a T-helper type 1 (Th1) cytokine polarization associated with a high level of tumor necrosis factor-β and interferon-γ. There is also release of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-8. These cytokines are considered very potent chemoattractive agents for neutrophils. Figure 1 is a schematic of the inflammatory cascade in the case of a rhinovirus infection.
Fig. 1.
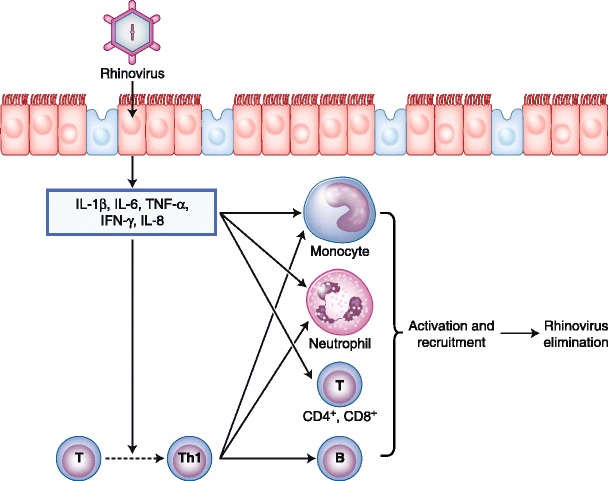
Schematic of the inflammatory cascade in the case of a rhinovirus infection. IFN—interferon; IL—interleukin; Th1—T-helper type 1; TNF—tumor necrosis factor
The goals of treatment of ARS are to alleviate or minimize symptoms, eradicate pathogens and the underlying cause with therapies that halt inflammation, and promote sinus drainage. It is interesting to point out that 65% of ARS cases resolve spontaneously within 2 weeks [6]. Table 4 summarizes the different treatment options in cases of ARS. In the early phase of ARS, symptomatic treatment is sufficient. It consists of nasal douches, decongestants, exspectorants, mucolytics, and painkillers. In moderate ARS, monotherapy with an intranasal glucosteroid is recommended [5, 6, 10].
Table 4.
Evidence for treatment of acute rhinosinusitis
| Therapy | Level of evidence | Recommendation | Relevance |
|---|---|---|---|
| Antibiotic | Ia | A | Yes, after 5–10 d, or in severe cases |
| Topical corticosteroid | Ib | A | Yes |
| Addition of topical steroid to antibiotic | Ib | A | Yes |
| Oral steroid | Ib | A | Yes, reduce pain in severe disease |
| Addition of oral antihistamine | Ib | B | Yes, in allergic patients only |
| Nasal douche | Ib | D | No |
| Decongestant | Ib | D | Yes, as symptomatic relief |
| Mucolytics | No evidence | None | No |
Glucocorticoids act on the glucocorticoid receptor to inhibit transcription of proinflammatory mediators, which are upregulated during the inflammatory response. As a consequence, they reduce the mucosal inflammation, edema, cellular infiltration, and nasal congestion; improve the permeability of the sinus ostium; and thus facilitate the ventilation and drainage of the sinus. By reducing the eosinophilia, they can also be of great help in cases of comorbid allergic rhinitis, which occurs frequently in young patients and is a possible predisposing factor for the development of ARS. Treatment with intranasal steroids (INS) has proven to be safe and well-tolerated, with an incidence of adverse events no greater than those observed with placebo. In moderate to severe cases, topical steroids must be used in association with a broad spectrum antibiotic [11–13].
Many studies including randomized, placebo-controlled trials have demonstrated the clinical effectiveness of such a combination. Actually, it provides greater symptomatic improvement than antibiotic therapy alone, shorter time to recovery, and greater regression of imaging abnormalities [12, 13].
The antibiotic is chosen acccording to the local bacterial resistance patterns, effectiveness, and safety. It must be effective against S. pneumoniae and H. influenzae, which are the most common bacteria implicated in uncomplicated, community-acquired acute bacterial rhinosinusitis. That is why in the United States, the first choice is amoxycillin [9, 14, 15]. It is also clear that the use of antibiotics is warranted in patients with severe or rapidly worsening symptoms regardless of the duration of illness, and that referral to an ear, nose, and throat specialist is mandatory in such cases.
Chronic Rhinosinusitis
CRS is characterized by chronic inflammation of the nasal and paranasal sinus mucosa, cytokine release and tissue remodeling that includes changes in the extracellular matrix (ECM), protein deposition, and tissue structure [16].
According to the European Position Paper on Rhinosinusitis and Nasal Polyps document, CRS is defined by two or more symptoms associated with signs via nasal endoscopic or CT scan [1]. A CT scan is therefore not required to diagnose CRS. CRS is also defined by the persistence of symptoms for more than 12 weeks without clinical improvement despite attempts of medical treatments. Table 5 lists the criteria to define CRS according to the European Position Paper on Rhinosinusitis and Nasal Polyps document. Unlike ARS, which is usually infectious, CRS is considered an inflammatory condition.
Table 5.
Rhinosinusitis: signs and symptoms
| Major symptoms (2 of the following): |
| •Nasal congestion or obstruction |
| •Nasal discharge (anterior or posterior) ± |
| •Facial pain or pressure |
| •Olfactory disturbance: reduction or loss of smell |
| and/or |
| Endoscopic signs (1 or more of the following): |
| •Polyps |
| •Mucopurulent discharge from the middle meatus |
| •Edema/obstruction at the middle meatus |
| or |
| CT signs (e.g., mucosal changes at ostiomeatal complex and/or in the sinus) |
(From Fokkens et al. [1]; with permission)
Based on nasal endoscopy, CRS can be subdivided into two categories: CRS with and without nasal polyps. In the past, these two entities were considered as the expressions of one single disease, nasal polyposis being considered the end point of the evolution of CRS without nasal polyps. Nowadays, despite clinical similarities, there is growing evidence that these entities are completely disparate based on distinct inflammatory pathways, cytokine profiles, and different tissue remodeling [16–23].
CRS without polyps accounts for approximately 60% of CRS cases [16]. Table 6 summarizes the criteria to define CRS without polyps. It is a heterogeneous condition in which structural abnormalities, host factors, environmental factors, allergic factors, viruses, bacteria or fungi, biofilms, and superantigens variably contribute to the disease.
Table 6.
Definition of chronic rhinosinusitis without polyps
| A. Symptomatology present for >12 wk |
| B. Requires more than 2 of the following symptoms: |
| •Anterior or posterior mucopurulent discharge |
| •Nasal congestion |
| •Facial pain/pressure |
| •Decreased sense of smell |
| C. Objective documentation |
| •Nasal endoscopy: purulence, edema, crust |
| •CT: diffuse opacity of the ethmoidal cells |
(From Fokkens et al. [1]; with permission)
The symptomatology consists of nasal congestion, anterior and posterior rhinorrhea, reduction of the sense of smell, and recurrent upper respiratory tract infections (URTIs). Facial pain, pressure and/or fullness are very common in this group in contrast to CRS with polyps.
The role of the bacteria and fungi in CRS is a very tricky issue. They can infect the sinuses or simply colonize them, leading to a complex and aggressive inflammatory reaction. Bacterial organisms can infect the paranasal sinuses. They are involved in acute exacerbations of CRS. The bacteria encountered are often one of the “infernal trio,” but other bacteria have been identified (coagulase-negative staphylococci, Staphylococcus aureus, enterobacteriaceae, Corynebacterium spp, Pseudomonas aeruginosa, and less commonly the gram-negative enteric bacteria). Anaerobes can also be present (e.g., Propionobacterium spp, bacteroides, or peptococci). When a dental origin is suspected, microaerophilia streptococci must be encountered. These bacteria are usually not present in ARS [7, 8, 14, 15, 24, 25]. In CRS without polyps, medical treatment is usually not as effective. This can be explained by some “bacteriological” factors.
The bacteria are more prone to be resistant to the commonly prescribed antibiotics, such as gram-negative bacteria or methicillin-resistant S. aureus [7, 8, 26]. Bacteria can also form biofilms on the sinonasal mucosa of patients with CRS. Sequestration of bacteria within biofilms allows the bacteria to resist antibiotic treatment and persist as a low-grade infection within the sinus mucosa. The exact role of biofilms in the pathogenesis of CRS is still unclear, but they can explain the persistence of rhinorrhea and crusting despite an in vitro active antibiotic [27, 28].
Local osteitis of the underlying bone can also play a critical role in the elaboration of CRS by inducing persistent inflammatory changes in the surrounding mucosa [29, 30]. Concurrent osteitis can be found in 36% to 53% of patients with CRS using radiographic and pathological criteria, respectively. Although a causal relationship between osteitis and CRS cannot be inferred from these data, these clinical findings correlate well with previous evidence of bone involvement in CRS found in animal models, further reaffirming the association between underlying osteitis and the pathogenesis of CRS.
Bacterial colonization with enterotoxin-producing S. aureus is found with increased prevalence in patients with nasal polyps. In contrast, patients with CRS without polyps do not have an increased prevalence of enterotoxin-specific IgE antibodies [31].
The role of fungi in CRS has been debated since the first publication by Ponikau et al. [32] advocating that fungi that normally colonize the nose and sinuses could elicit an inflammatory response characterized by an intense eosinophilic infiltration into the nose and sinuses, leading to the development of CRS. Figure 2 depicts the role of fungi in CRS. With a particular method of collecting surgical samples and a specific method of identification, those investigators demonstrated that fungal cultures of nasal secretions were positive in 202 (96%) of 210 consecutive CRS patients [33, 34]. Allergic mucin was found in 97 (96%) of 101 consecutive surgical cases of CRS. Allergic fungal sinusitis (AFS) was diagnosed in 94 (93%) of 101 consecutive surgical cases with CRS based on histopathologic findings and culture results.
Fig. 2.
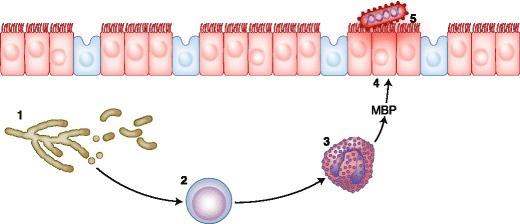
Role of fungi in chronic rhinosinusitis. Fungi (1) elicit an inflammatory response by lymphocytes (2). The lymphocytes then trigger the release of major basic protein (MBP, 4) by eosinophils (3). The MBP is normally synthesized to destroy foreign agents such as viruses or parasites. In this case, the MBP causes ulcers in the mucus membrane (5) of the nose and sinuses, giving rise to bacterial sinusitis. (Adapted from Ponikau et al. [32])
The group also demonstrated that 1) CRS patients react to certain fungal stimuli by producing significantly (P < 0.05) higher amounts of IL-5 and IL-13 compared with controls; 2) CRS patients have an enhanced humoral response (significantly elevated IgG levels to Alternaria spp); and 3) CRS patients react independently to an IgE-mediated allergy, as evidenced by that fact that nonallergic CRS patients also produced IL-5 in response to fungal stimuli [35, 36]. Finally, they demonstrated the benefits of treatment with topical amphotericin B administrated in nasal lavages for patients suffering from CRS [37, 38].
This theory was discussed during the past decade in many international congresses, meetings, and roundtables. Unfortunately, some skepticism still exists, as these results have not been confirmed by other studies, particularly the efficacy of amphotericin B [39–41]. Moreover, with an adequate method of identification, fungi are detected in patients with CRS and controls [32–34, 42]. Finally, the classic inspissated allergic mucin that is grossly and histopathologically identical to that found in patients with AFS can also be seen in some patients without the presence or involvement of fungi [43, 44].
Nevertheless, AFS is a true universally recognized fungal disease even if the underlying immune process is still under discussion [45–50]. AFS is defined as CRS accompanied by the presence of a peanut-buttery extramucosal sinus inspissate called allergic mucin that histopathologically contains masses of pyknotic eosinophils (eosinophil concretions), Charcot-Leyden crystals (lysophospholipase), and sparse numbers of fungal hyphae highlighted by fungal silver staining. Most AFS cases involve dematiaceous fungi, such as Bipolaris spicifera, Curvularia spp, and Drechslera spp. AFS is a noninvasive form of fungal rhinosinusitis that represents an allergic hypersensitivity (IgE mediated in some cases) disorder analogous to allergic bronchopulmonary aspergillosis. AFS is a very common disease in countries with warm and humid climates (e.g., United States and India) but is very rare in Europe.
Besides this entity, CRS is sometimes an eosinophilic mucin rhinosinusitis and sometimes is associated with a neutrophilic infiltration. The eosinophilic infiltration seems to be more frequent in cases of CRS with nasal polyps, whereas a neutrophilic infiltration is associated with CRS without polyps. Thus, the role of fungi in these two entities is still controversial and needs further investigation. CRS is characterized by a chronic inflammation with release of proinflammatory cytokines and subsequent tissue remodeling. Remodeling is defined as a process leading to transient or permanent changes in tissue architecture that involves the breakdown of tissue structures such as basement membrane and interstitial stroma, as well as repair [51, 52]. CRS without nasal polyps is characterized by a predominant neutrophilic inflammation in the stroma with a lesser concentration of eosinophils.
The mucosal lining in CRS without polyps is characterized by basement membrane thickening [20, 23, 53, 54]; goblet cell hyperplasia; glandular hyperplasia; limited subepithelial edema; prominent subepithelial fibrosis; paucity in glands; and chronic monocellular cell infiltration with neutrophils, lymphocytes, mast cells, and plasma cells [55]. Mast cells and lymphocytes are sometimes organized in lymphatic follicles.
The ECM plays an essential role in tissue integrity, and matrix metalloproteinases (MMPs) are the major proteolytic enzymes involved in ECM damage or repair. In CRS without polyps, it has been demonstrated that there is an increased concentration of MMP-9 and its natural inhibitor, T-cell immunoglobulin mucin 1. The ratio seems to be counterbalanced. On the other hand, in CRS with nasal polyps, there is an increased concentration of MMP-9 and MMP-7, but their inhibitors are not upregulated. This could contribute to the development of pseudocysts seen in nasal polyposis [56]. Figure 3 shows the inflammation mediators and tissue remodeling in CRS without polyps.
Fig. 3.
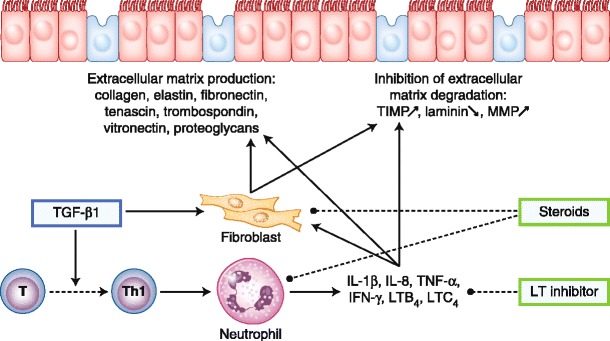
Inflammation mediators and tissue remodeling in chronic rhinosinusitis (CRS) without polyps. Transforming growth factor (TGF)-β1 is thought to play a critical role in the development of CRS without polyps. TGF-β1 stimulates fibroblast proliferation and collagen deposition and inhibits matrix metalloproteinases (MMPs) by enhancing tissue inhibitor of metalloproteinases (TIMP). IFN—interferon; IL—interleukin; LT—leukotriene; Th1—T-helper type 1; TNF—tumor necrosis factor
The cytokine profile of CRS without polyps is mainly a Th1 cytokine polarization with CD3, CD25, and CD68 T lymphocytes. The inflammatory infiltrate is made up of mostly neutrophils, with a low percentage of eosinophils, mast cells, and plasma cells. There is an upregulation of proinflammatory cytokines such as interferon-γ, transforming growth factor (TGF)-β1, TGF-β2, and IL-1. Unlike in nasal polyposis, forkhead box P3 (FoxP3), T-box transcription factor (Tbet), and GATA-binding protein 3 are not significantly elevated compared with controls. There is also a polymorphism within the IL receptors and α1 antitrypsin [17, 18, 20, 23, 31, 57, 58]. Table 7 reviews the different cytokines, adhesion molecules, and growth factors implicated in the different forms of CRS.
Table 7.
Inflammatory pathways, cytokine profiles, biomarkers, and tissue remodeling in CRS
| CRS without polyps | CRS with polyps | CRS in Chinese patients | |
|---|---|---|---|
| T-cell profile | Th1 | Th2 | Th17 |
| Inflammatory cells | Prominent neutrophils (low percentage of eosinophils, mast cells), T cells | Prominent eosinophils, B cells, T cells | Neutrophils (MPOs), T cells |
| Cluster of differentiation | CD3, CD25, CD68 | CD3, CD25, CD138, CD68 | CD4, CD8 |
| Cytokines and chemokines | IFN-γ↑, TGF-β1↑, IL-1↑, IL-3↑, IL-6↑, IL-8↑; TNF-α, IL-5 not increased | IL-4↑, IL-5↑, IL-13↑, ECP↑; overproduction of IL-8, RANTES, eotaxin (from epithelial cells) | IL-17, IL-6, IFN-γ, IL-4, IL-5, IL-10, TGF-β |
| Immunoglobulins | IgE↑ in cases of allergic rhinitis; IgA unknown | Local production of polyclonal IgE (Staphylococcus aureus, enterotoxin); IgA, IgG, IgM elevated | – |
| Growth factors | GM-CSF increased | GM-CSF (epithelial cells), VEGF | – |
| Adhesion molecules | VCAM-1 and IL-5 not increased | Upregulated ICAM-1 and VCAM-1, E-selectin, P-selectin | – |
| Transcription factors | FoxP3 upregulated; Tbet and GATA-3 are similar to controls | Tbet and GATA-3 upregulated; FoxP3 downregulated. | Tbet, GATA-3 |
| Matrix remodeling proteins | Collagen, MMP-9 counterbalanced by natural inhibitor TIMP-1, fibrosis | MMP-1, MMP-2, MMP-9, and MMP-7 upregulated; epithelial shedding pseudocyst formation containing albumin | – |
| Subsequent tissue remodeling | Basement membrane thickening, goblet cell hyperplasia, limited subepithelial edema, prominent fibrosis and mononuclear cells | Epithelial damage, epithelial shedding, pseudocyst formation containing albumin thickened basement membrane, reduced number of blood vessels and glands, no neuronal structures | – |
| Genes | Polymorphisms within IL receptors, α1-antitrypsin | Polymorphisms within COX1/COX2 pathways, leukotriene pathways, and receptors related to AA metabolites | – |
| Comorbidities | Recurrent URTIs; allergic rhinitis can be associated | Asthma, AERDs | – |
AA arachidonic acid, AERD aspirin-exacerbated respiratory disease, COX cyclooxygenase, CRS chronic rhinosinusitis, ECP eosinophil cationic protein, FoxP3 forkhead box P3, GATA-3 GATA-binding protein 3, GM-CSF granulocyte-macrophage colony-stimulating factor, ICAM intercellular adhesion molecule, IFN interferon, IL interleukin, MMP matrix metalloproteinase, MPO myeloperoxidase, RANTES regulated on activation, normal T-cell expressed and secreted, Tbet T-box transcription factor, TGF transforming growth factor, Th T-helper type cell, TIMP tissue inhibitor of metalloproteinases, TNF tumor necrosis factor, URTI upper respiratory tract infection, VCAM vascular cell adhesion molecule, VEGF vascular endothelial cell growth factor
Treatment of CRS without polyps is medical and surgical [59–61]. The first step should be management of the underlying cause and contributing factors [62, 63]. Antibiotics are frequently prescribed by general practitioners for CRS. The antibiotics are clearly indicated for the treatment of acute bacterial exacerbations of CRS. Amoxicillin and amoxicillin plus clavulanic acid remain the first choices [64, 65]. The use of fluoroquinolones for URTIs has been evoked as acting against gram-negative bacteria. Nevertheless, these should not be the first line of treatment of URTIs, and we must keep in mind that CRS is more a complex, multifactorial inflammatory disease rather than solely an infectious problem. One clear indication to prescribe fluoroquinolone is to treat infection by P. aeruginosa.
Prolonged use of low-dose macrolides has been proposed [61, 66, 67] due to their anti-inflammatory effects. The duration of the treatment varies from 6 to 12 weeks.
Nasal douches with intranasal saline was shown to decrease nasal symptoms in chronic sinusitis [68]. They improve quality of life among patients with CRS and reduce the amount of secretions, postnasal drip, and load of mediators in the secretions.
Glucocorticoids administered topically, intranasally, or systemically are the foundation of the treatment of CRS in association with antibiotics. Several randomized, double-blind studies have shown glucocorticoids to be effective in the treatment of CRS with or without polyps [69–75]. However, these studies demonstrate only minor improvement without concomitant surgery. This was partially explained by a lack of penetration into the sinuses by topically applied drugs. In CRS with eosinophilic inflammation, topical steroids are routinely prescribed for at least 3–6 months, with few reports of systemic or local adverse events. Table 8 reviews the different options for the medical treatment of CRS with and without polyps.
Table 8.
Medical treatment of chronic rhinosinusitis with and without nasal polyps
| Treatment | Grade of recommendation | Clinically relevant? |
|---|---|---|
| Nasal saline douche | A | Yes (for additional therapy) |
| Topical corticosteroids | A | Yes |
| Systemic corticosteroids | A | Yes |
| Addition of oral antihistamine | A | Yes (in cases of allergic rhinitis, itching, sneezing) |
| Long-term oral antibiotics (macrolides) | A | Yes (>12 wk) |
| Short-term oral antibiotics | C | Acute/severe exacerbations only |
| Allergen avoidance in allergic patients | D | Yes |
| Mucolytics | C | No |
| Bacterial lysates | C | No |
| Topical antibiotics | D | No |
| Proton pump inhibitor | D | No |
| Oral steroids | D | No |
| Decongestants | D | No |
| Antimycotics (systemic/topical) | D | No |
| Immunotherapy | D | No |
Surgery should be considered when there is persistence or partial relief of the symptomatology despite a maximal medical treatment. Surgery may also be required to improve sinus aeration and nasal access for topical therapy. Surgery should be functional. The concept is to restore the aeration and drainage of the paranasal cavities while preserving healthy mucosa on the ethmoid roof and lamina papyracea. Postoperatively, most patients experience significant reductions in headache, nasal obstruction, and postnasal drip, and have improved quality-of-life scores [76–78]. In patients with asthma, functional endoscopic sinus surgery improves peak expiratory flow and reduces the use of inhaled and systemic glucocorticosteroids [79–83]. Some publications suggest the use of topical steroids in the postoperative period to improve wound healing [84].
Chronic Rhinosinusitis with Nasal Polyps
Nasal polyposis is a subgroup of CRS. It remains one of the most difficult challenges in otolaryngology, as its etiology and pathophysiology are still unclear, medical treatment is unsatisfactory, and because of frequent recurrences, repeated surgical procedures are often necessary.
Nasal polyps present as edematous, semitranslucent masses originating from the mucosal linings of the sinuses and prolapsing into the nasal cavities. Nasal endoscopy shows them to be bilateral. They are lateral to the middle turbinates in the middle meatus and are frequently present in the superior meatus. When the polyposis is unilateral, another diagnosis must be considered (e.g., a Schneiderian papilloma or a tumor in adults, or a dermoid cyst, meningoencephalocele, or nasal glioma in children).
The symptomatology is similar to that of CRS without polyps (Table 5), with frequent nasal congestion, anterior and posterior nasal discharge, and recurrent episodes of respiratory tract infection. Reduction or loss of the sense of smell is a very important symptom in patients with nasal polyps. Headache and facial pain/pressure are less common than in CRS without polyps.
Concerning the bacteriology, all the organisms described in the section “Chronic Rhinosinusitis Without Polyps” can infect a nasal polyposis. The “infernal trio” can be associated with recurrence of the polyposis during an acute bacterial exacerbation of sinusitis. Antibiotic-resistant organisms, biofilms, and osteitis also can be associated with the polyposis. A specific item to discuss in case of nasal polyposis is the role of bacterial colonization by S. aureus–producing enterotoxins [31, 85–87].
Enterotoxins from S. aureus act locally as superantigens on T lymphocytes and induce a multiclonal B-cell activation. Release of cytokines (IL-5) from Th2 cells results in an eosinophilic activation with release of eosinophilic cationic protein (ECP). ECP causes tissue damage, edema formation, and albumin accumulation. B-cell activation will result in the production of multiclonal IgE by plasma. In contrast, in patients with CRS without polyps, there is no evidence of increased prevalence of enterotoxin-specific IgE antibodies. Figure 4 shows the possible role of enterotoxins producing S. aureus in the pathophysiology of nasal polyps.
Fig. 4.
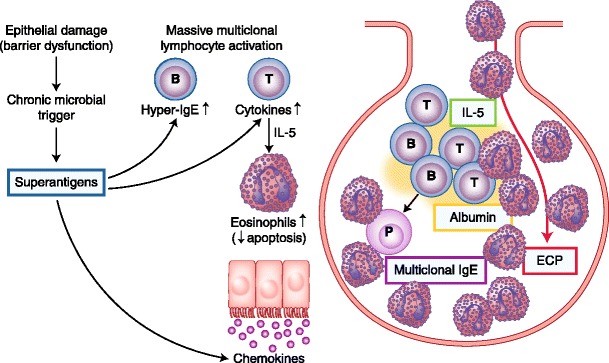
Possible role of enterotoxins producing Staphylococcus aureus in the pathophysiology of nasal polyps. Enterotoxins from S. aureus act locally as superantigens on T lymphocytes and induce a multiclonal B-cell activation. Release of cytokines (interleukin [IL]-5) from T-helper type 2 cells results in an eosinophilic activation with release of eosinophilic cationic protein (ECP). ECP causes tissue damage, edema formation, and albumin accumulation. B-cell activation will result in the production of multiclonal IgE by plasma. In contrast, in patients with chronic rhinosinusitis without polyps, there is no evidence of increased prevalence of enterotoxin-specific IgE antibodies. (Adapted from Van Zele et al. [31], Verbruggen et al. [85], Bachert et al. [86], Gevaert et al. [87], and Perez-Novo et al. [91])
Nasal polyposis constitutes a heterogeneous group of diseases. Based on the type of inflammatory infiltrate, we can subdivide the nasal polyps into two groups: eosinophilic and neutrophilic. Table 9 lists the different subpopulations of nasal polyps.
Table 9.
Different subpopulations of nasal polyps and clinical entities
| Type of infiltrate | Eosinophils predominant | Neutrophils predominant |
|---|---|---|
| “Etiology” | Idiopathic in Caucasian patients (most common) | Idiopathic in Asian patients (Chinese polyp) |
| Aspirin intolerance (Samter’s triad): 8%–30% of patients with nasal polyps | Cystic fibrosis: polyps in 6%–48% of patients | |
| Allergic fungal sinusitis: 85% of patients with nasal polyps | Primary ciliary dyskinesia | |
| Churg-Strauss syndrome: 50% of patients with nasal polyposis | ||
| Remodeling | Epithelial damage | – |
| Epithelial shedding | ||
| Pseudocyst formation containing albumin | ||
| Edema | ||
| Thickened basement membrane | ||
| Reduced number of blood vessels and glands, no neuronal structures | ||
| Laboratory findings | Eosinophils increased | See Table 7 |
| Eosinophil cationic protein increased | ||
| Local interleukin-5 production | ||
| Multiclonal production of IgE |
Nasal polyposis affects about 4% of the general population in Western countries. It is not more common in atopic individuals than in healthy controls. It is interesting to note that 29% of the Belgian population is sensitized to at least one aeroallergen [88]. More than 40% of nasal polyposis is associated with nonallergic asthma.
About 25% of nasal polyposis is associated with aspirin sensitivity (Samter’s triad). Aspirin-exacerbated respiratory disease represents a subset of patients with severe recalcitrant nasal polyposis and asthma in the setting of aspirin sensitivity. The underlying pathophysiology appears to be dysregulation in the eicosanoid metabolism pathway, leading to increased production of cysteinyl leukotrienes and decreased levels of prostaglandins.
Churg-Strauss syndrome, or allergic granulomatous angiitis is characterized by inflammation of the small arteries and veins in individuals with a history of asthma or allergy. Besides inflammation of the blood vessels, there is also an increase in the number of eosinophils and inflammatory nodular lesions. Nasal polyps are present in about 50% of patients. AFS is associated with nasal polyposis in more than 80% of cases.
Histologically, polyps are covered by respiratory pseudostratified columnar epithelium with some areas of squamous metaplasia. There is frequent epithelial damage (epithelium shedding) and variable stages of thickened basement membrane. Pseudocyst formation and edema are two major characteristics of nasal polyps. These pseudocysts contain albumin and other plasma proteins. The number of vessels and glands is reduced, and there is virtually no neuronal structure. Fibroblasts and infiltrating inflammatory cells are localized around pseudocyst formations. EG2+ (activated) eosinophils are usually located around vessels and glands and are predominant in about 80% of patients with nasal polyps. The number of degranulating epithelial mast cells is high [51, 53, 55, 89].
In nasal polyps, the ECM is severely damaged. This can be explained by the fact that the balance between MMPs and tissue inhibitors of MMPs has been shown to be displaced in favour of MMPs, especially MMP-9 and MMP-7 [56]. It also has been demonstrated that there are modifications in the angiogenesis in nasal polyps via the vascular endothelial growth factor [90].
The vascular endothelial growth factor plays a role in inducing edema, angiogenesis, and fibrosis. It was demonstrated that it is intensively expressed in nasal polyps, mainly in inflammatory cells but also in epithelial cells. TGF-β1 upregulates its secretion.
Nasal polyposis in Caucasian patients has a predominantly Th2-biased eosinophilic inflammation (Table 7) [17, 18, 20, 23, 57, 58, 91–93]. This is associated with high levels of IL-5, RANTES (regulated on activation, normal T-cell expressed and secreted), granulocyte-macrophage colony-stimulating factor, and eotaxin that play a role in the recruitment and activation of eosinophils and decrease their apoptosis [94–96].
Concentrations in ECPs are high. There is an upregulation of cysteinyl-leukotriene synthesis, but no significant differences in polyps were found between patients with and without Samter’s triad and asthma. This can explain some benefit observed with a treatment with anti-leukotriene modifiers in nasal polyposis [97].
There is a decrease in FoxP3 expression accompanied by upregulation of Tbet and GATA-binding protein 3 and a downregulation of TGF-β1 [57]. After treatment with INS, FoxP3 expression is increased [58].
Elevated levels of total and specific IgE in the polyp tissue are another major characteristic in nasal polyposis. Their concentrations may be very high (>1,000 kU/L) but are often unrelated to serum IgE levels and to skin prick test positivity, which is in contrast to allergic rhinitis. The production is very heterogeneous. Bachert et al. [31, 91, 98, 99] demonstrated three different categories of patients: nasal polyposis group 1 demonstrated no measurable specific IgE; nasal polyposis group 2 selected specific IgE; and the third group demonstrated a multiclonal specific IgE, including IgE to S. aureus enterotoxins, a high total IgE level, and a high prevalence of asthma. In this latter group, patients are usually sensitized to aspirin [31, 91, 98, 99].
Before considering the treatment, it is interesting to note that Chinese patients have neutrophilic and not eosinophilic polyps. Such polyps are also seen in inherited diseases such as primary ciliary diskinesia and cystic fibrosis. Chinese patients with polyps are characterized by B- and T-cell activation, a minor eosinophilic inflammation compared with polyps from white individuals, and a decrease in TGF-β1 in comparison with controls. One third of patients with polyps showed an IgE response to S. aureus enterotoxins [100]. Figure 5 shows the putative mechanisms, cells, and mediators implied in CRS with polyps and Chinese polyps.
Fig. 5.
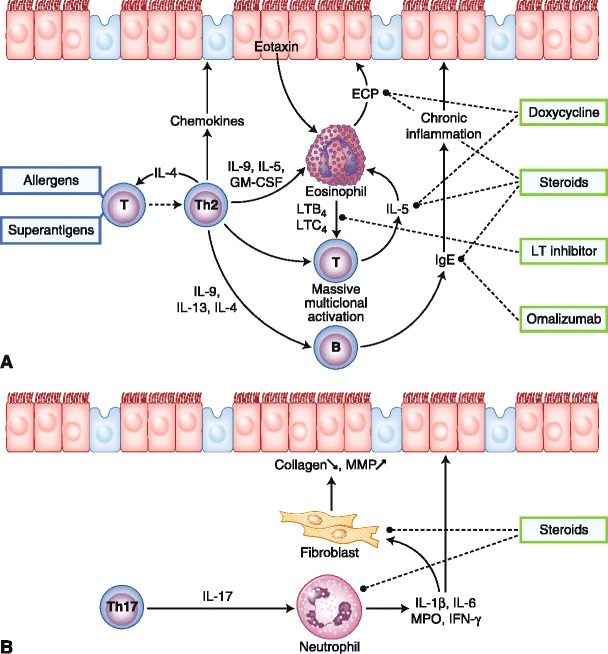
Putative mechanisms, cells, and mediators implied in white patients with chronic rhinosinusitis (CRS) with polyps (a) and polyps of Chinese patients (b). Treatments are indicated in the frames. In white patients, nasal polyposis is thought to be orchestrated by T-helper type 2 (Th2) cells, with interleukin (IL)-5 as the major cytokine. IL-5 has a critical role in the activation of eosinophils and the production of IgE. Steroids, anti-leukotrienes (LTs), antihistamines, and anti-IgE (omalizumab) may inhibit polyps disease at different levels. Downregulation of transforming growth factor (TGF)-β is observed in Asian and white individuals with nasal polyps. The predominant T-effector cell in Asian patients is the Th17 cell, which secretes IL-17, resulting in a predominance of neutrophils. Inflammation mediators and tissue remodeling in CRS without polyps are shown. TGF-β is thought to play a critical role in the development of CRS without polyps. TGF-β stimulates fibroblast proliferation and collagen deposition and inhibits matrix metalloproteinases (MMPs) by enhancing tissue inhibitor of metalloproteinases. ECP—eosinophil cationic protein; GM-CSF—granulocyte-macrophage colony-stimulating factor; IFN—interferon; MPO—myeloperoxidase
The management of nasal polyps is extremely individual and must be discussed case by case, as the expectations of one patient are not necessary the same as those of others. The treatment can be medical and surgical [101, 102].
The goals of treatment are to re-establish the nasal airway and nasal breathing, minimize symptoms, improve the sense of smell, treat co-existing diseases such as asthma, improve quality of life, and prevent complications [103]. Before starting any therapy, it is important to inform patients that nasal polyposis is a chronic sinus disease that is impossible to cure, but that we can potentially stabilize it with different medications. Recurrence is the rule, but observance of (compliance with) the treatment can postpone for as long as possible the relapse of the disease. In Samter’s triad, it is important to avoid definitively any form of acetylsalicylic acid and NSAIDs.
Corticosteroids are the first-choice treatment approach for an eosinophilic polyposis, as they can suppress many phases of the inflammatory process. For example, they inhibit the liberation of vasoactive mediators, reducing vasodilatation, fluid extravasation, edema, and local deposit of mediators. They also reduce recruitment of inflammatory cells, fibroblast proliferation, and synthesis of extracellular matrix proteins.
Systemic application affects all polyp tissue within the nose and sinuses but has the disadvantage of systemic adverse effects when used for long-term treatment. Hissaria et al. [104] demonstrated with a randomized, placebo-controlled trial the efficacy of a 14-day course of 50 mg of prednisolone on patients’ symptoms, quality of life, and endoscopic score. Nevertheless, from a clinical point of view, some patients may experience a “rebound effect” after a course of systemic corticosteroids [104–106].
Topical application of corticosteroids dramatically reduces the risk of systemic side effects [107]. Many studies have demonstrated the safety and efficacy of INS in nasal polyposis even if the intrasinus portion of the polyps is not reachable with a nasal spray [108–112]. Among all the specialities, mometasone furoate and fluticasone furoate are the newest and safest molecules with the lowest bioavailability.
In nasal polyposis, antibiotics at full dose are recommended to treat acute bacterial exacerbation of sinusitis, but in recent years, considerable evidence has emerged to suggest that macrolide antibiotics at low dose could play a role in the treatment of nasal polyposis [113–120]. Macrolides have immunomodulatory properties in addition to their well-established antimicrobial activity. Indeed, macrolide antibiotics inhibit synthesis and/or secretion of proinflammatory cytkines (IL-5, IL-8, granulocyte-macrophage colony-stimulating factor, TGF-β, IL-6, IL-8, tumor necrosis factor). They have many effects on neutrophils: they decrease the neutrophil oxidative burst, inhibit neutrophil migration to inflammatory sites, increase the apoptosis of the neutrophils, and inhibit the neutrophil adhesion. They also decrease the eosinophilic inflammation, reduce the goblet cell secretion, and decrease bronchoconstriction.
All the studies confirm clinical efficacy with a significant reduction in the size of the polyps and reduction in symptoms (nasal obstruction, nasal discharge, purulence, headache), and the relapse of the polyps seems to be delayed. However, all the studies demonstrated the necessity of long-term use (3–12 months) and the slow onset. After 2 weeks of treatment, only 5% of patients indicated improvement, while after 4 weeks, 48% were improved, after 8 weeks, 63% were improved, and after 12 weeks, 71% were improved.
When we consider such a long-term treatment, gastrointestinal intolerance can be a problem for the observance of the treatment. Moreover at low dose, emergence of antibiotic-resistant bacteria should be taken into consideration.
Antihistamines are typically one of the medications of choice to treat allergic rhinitis. Despite several theoretical pharmacologic properties applicable for the treatment of nasal polyposis (Consensus Group on New-Generation Antihistmaines [CONGA] [121]), little has been published about such an indication [122–127]. Anti-leukotrienes have shown some effects on nasal polyposis [128, 129].
Changes in the arachidonic acid metabolism have been suggested to be involved in the pathogenesis of nasal polyposis, especially in aspirin-sensitive individuals, and cysteinyl leukotrienes have been found in increased levels in nasal tissue from those patients, whereas concentrations of prostaglandin E2 were decreased. Furthermore, the leukotriene C4 synthase was found to be upregulated in patients with nasal polyps, as were as the number of leukocytes expressing the cysteinyl leukotriene 1 receptor as compared with their non–aspirin-sensitive counterparts. Thus, the use of leukotrienes antagonists, especially in aspirin-sensitive nasal polyp patients, seems appropriate. However, large-scale, controlled trials in clearly characterized patients—with or without aspirin sensitivity—are lacking thus far. In patients with aspirin sensitivity, aspirin desensitization can be recommended [130, 131].
Aspirin desensitization consists of administering incremental oral doses to reach a maintenance dose of greater than 650 mg/d, then inducing a refractory period of a few days. Continuous treatment over years may lead to a significant reduction in number of sinus infections per year, improvement in olfaction, and a reduction in use of systemic corticosteroids. Treatment with daily aspirin may be a therapeutic option for patients who do not respond to topical and systemic corticosteroids. As new treatment options, we must consider doxycycline, anti–IL-5, and anti-IgE.
Doxycycline is an antibiotic that also inhibits the synthesis of MMPs and MMP-9 activity in vitro. However, doxycycline does not affect the MMP-9 inhibitors (tissue inhibitor of metalloproteinases 1 and 2) [132]. A double-blind, placebo-controlled study demonstrated in 32 patients a significant decrease in the endoscopic nasal polyp score 4–12 weeks after the start of the doxycline treatment compared with placebo. The concentrations of IgE, myeloperoxidase, MMP-9, and ECP decreased significantly in nasal secretions of doxycycline-treated patients. In a clinical setting, the use of doxycycline offers an additional advantage of providing antimicrobial and anti-inflammatory effects, resulting in a decrease in nasal polyp size [133].
As IL-5 is increased in polyp tissue and related to eosinophilic inflammation, and anti–IL-5 monoclonal antibody treatment in vitro induced eosinophil apoptosis, this cytokine was a reasonable target for antibody therapy in nasal polyps. Different humanized anti–IL-5 monoclonal antibodies have been developed and studied in asthma, and a pilot study in nasal polyposis has provided promising results [134, 135]. Considering the marked local production of IgE antibodies in nasal polyps and their relation to severity of disease, it appears that local IgE is functional and involved in the regulation of chronic inflammation. Thus, strategies to antagonize IgE could be relevant. Treatment of allergic asthma and rhinitis with omalizumab, a humanized anti-IgE monoclonal antibody, causes a marked reduction in circulating free IgE levels. No studies in nasal polyposis have been published. However, at least two teams have already thought about possible clinical applications of anti-IgE in severe nasal polyposis regardless of whether it is associated with corticodependent asthma [85, 136].
The management of nasal polyposis usually involves medical treatment and frequently surgery as an adjunct to the medical treatment. Surgical removal of nasal polyps is indicated for patients not responding adequately to medical management, those with continued or recurrent infections, as well as patients who are developing mucoceles or other complications of sinusitis. Patients with polyps and asthma may benefit from surgery by reduction of one trigger of asthma.
The type of surgery to perform is the subject of debate in Europe. What is the respective place of endoscopic-guided polypectomy versus functional endoscopic sinus surgery versus nasalization as recommended by Dr. Jankowski from Nancy? Is functional endoscopic sinus surgery still the gold standard? Must we open all the ethmoid compartments? Must we clear the frontal recess completely? Must we do a large sphenoidotomy in all cases? All these questions remain unanswered.
As long as a complete ethmoidectomy is recommended, powered instrumentation with microdebrider appears particularly helpful in extensive polyposis [137–140]. It allows a precise removal of the polyps under a good visual control with preservation of the anatomy. It minimizes mucosal trauma and stripping and guarantees minimal crust formation, a rapid mucosal healing, and a low incidence of synechiae formation.
In revision surgery, because the anatomy can be highly distorted, but even in huge polyposis, the surgery can be conducted with a navigation system [141–143]. This gives the surgeon more confidence, particularly for the frontal sinus area and the sphenoid sinus, and seems to reduce the rate of complication.
Endoscopic sinus surgery has been shown to lead to significant improvement in total nasal resistance as measured with rhinomanometry, in nasal volume as measured by acoustic rhinometry, in ciliary beat frequency, in mucociliary clearance as measured by saccharin test, and in olfaction as measured by University of Pennsylvania Smell Identification Test. Increasing evidence also indicates that management of polyposis has a benefit for the lower airway and for the patient’s health-related quality of life [80, 82, 83, 144–153]. However, surgery, as complete as it can be, is not the final and definitive step to treat a nasal polyposis that remains as long as we know a chronic inflammatory disease whose etiology and pathophysiology are still poorly understood. Long-term treatment with topical steroids after surgery remains a rule for eosinophilic polyps to reduce the incidence of polyp recurrences or prolong the symptom-free time interval [154–156].
Conclusions
Rhinosinusitis represents a very large and heterogeneous group of diseases. ARS is mainly an inflammatory disorder initiated by a viral infection. INS seem to be the best first option for treatment of mild to moderate cases.
CRS without polyps is a mainly a neutrophilic inflammation. Antibiotics combined with INS is the first choice of treatment, but functional sinus surgery is usually necessary to improve the patient. CRS with polyps is the most frustrating disease for clinicians, as recurrences are the rule. Asthma and aspirin intolerance are the most common comorbidities. In Caucasian patients, it is associated with an eosinophilic infiltration. Fungi or S. aureus enterotoxins could play a role in the pathophysiology. Glucocorticoids associated with large sinus surgery were for a long time the only treatment, but new treatment options seem promising, specifically doxycycline, anti–IL-5, or anti-IgE.
Acknowledgments
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on nasal polyps. Rhinology. 2007;45(suppl. 20):1–139. [PubMed] [Google Scholar]
- 2.Vancauwenberge P, Watelet JB. Epidemiology of chronic rhinosinusitis. Thorax. 2000;55(Suppl 2):S20–1. doi: 10.1136/thorax.55.suppl_2.S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadley J, Sharp BS, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999–2002. Arch Otolaryngol Head Neck Surg. 2007;133(3):260–5. doi: 10.1001/archotol.133.3.260. [DOI] [PubMed] [Google Scholar]
- 4.Benson V, Marano MA. Current estimates from the National Health Interview Survey, 1992. Vital Health Stat. 1994;10(189):1–269. [PubMed] [Google Scholar]
- 5.Bachert C, Meltzer EO. Effect of mometasone furoate nasal spray on quality of life of patients with acute rhinosinusitis. Rhinology. 2007;45:190–6. [PubMed] [Google Scholar]
- 6.Desrosiers M. Diagnosis and management of acute rhinosinusitis. Postgrad Med. 2009;121(3):83–9. doi: 10.3810/pgm.2009.05.2006. [DOI] [PubMed] [Google Scholar]
- 7.Brook I. Bacteriology of acute and chronic ethmoid sinusitis. J Clin Microbiol. 2005;3(7):3479–80. doi: 10.1128/JCM.43.7.3479-3480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.PB Van Cauwenberge, Vander Mijnsbrugge AM, Ingels KJAO. The microbiology of acute and chronic sinusits and otitis media: a review. Eur Arch Otorhinolaryngol. 1993;250:S3–6. doi: 10.1007/BF02540108. [DOI] [PubMed] [Google Scholar]
- 9.Anon JB, Jacobs MR, Poole MD, Ambrose PG, Benninger MS, Hadley JA, Craig WA. Sinus and Allergy Health Partnership. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130(1 Suppl):1–45. doi: 10.1016/j.otohns.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol. 2005;116:1289–95. doi: 10.1016/j.jaci.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer EO, Orgel HA, Backhaus JW, Busse WW, Druce HM, Metzger WJ, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812–23. doi: 10.1016/0091-6749(93)90058-N. [DOI] [PubMed] [Google Scholar]
- 12.Barlan IB, Erkan E, Bakir M, Berrak S, Basaran MM. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Ann Allergy Asthma Immunol. 1997;78:598–601. doi: 10.1016/S1081-1206(10)63223-1. [DOI] [PubMed] [Google Scholar]
- 13.Dolor RJ, Witsell DL, Hellkamp AS, Williams JW, Jr, Califf RM, Simel DL. Comparison of cefuroxime with or without intranasal fluticasone for the treatment of rhinosinusitis. The CAFFS Trial: a randomized controlled trial. JAMA. 2001;286:3097–105. doi: 10.1001/jama.286.24.3097. [DOI] [PubMed] [Google Scholar]
- 14.Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 pt 2):S41–9. doi: 10.1016/S0194-5998(97)70006-8. [DOI] [PubMed] [Google Scholar]
- 15.Benninger MS. Antimicrobial update in rhinology: perspectives from the sinus & allergy health partnership. Symposium held during the 2003 American Rhinologic Society Scientific Meeting, September 20, 2003, Orlando, Florida.
- 16.Dykewicz ML, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 17.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 18.Bachert C, Gevaert P, Holtappels G, et al. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–90. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 19.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20(6):625–8. doi: 10.2500/ajr.2006.20.2907. [DOI] [PubMed] [Google Scholar]
- 20.Pawankar R. Nasal polyposis: an update. Curr Opin Allergy Clin Immunol. 2003;3:1–6. doi: 10.1097/00130832-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Bateman ND, Fahy C, Woolford TJ. Nasal polyps: still more questions than answers. J Laryngol Otol. 2003;117:1–9. doi: 10.1258/002221503321046577. [DOI] [PubMed] [Google Scholar]
- 22.Fokkens W, Lund V, Bachert C, EAACI et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy. 2005;60:583–601. doi: 10.1111/j.1398-9995.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 23.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006;61:1275–9. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 24.Niederfuhr A, Kirsche H, Riechelmann H, Wellinghausen N. The bacteriology of chronic rhinosinusitis, with and without nasal polyps. Arch Otolaryngol Head Neck Surg. 2009;135(2):131–6. doi: 10.1001/archoto.2008.531. [DOI] [PubMed] [Google Scholar]
- 25.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. doi: 10.1016/S0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi M, Mir Mohammad Sadeghi M, Omrani MR, Torabi MA. Microbiology and antimicrobial resistance in chronic resistant rhino sinusitis with or without polyp after functional endoscopic sinus surgery. J Res Med Sci (JRMS) 2005;10(3):167–71. [Google Scholar]
- 27.Kilty SJ, Desrosiers MY. The role of bacterial biofilms and the pathophysiology of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2008;8(3):227–33. doi: 10.1007/s11882-008-0038-2. [DOI] [PubMed] [Google Scholar]
- 28.Harvey RJ, Lund VJ. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology. 2007;45(1):3–13. [PubMed] [Google Scholar]
- 29.Lee JT, Kennedy DW, Palmer JN, Feldman MC, Chiu AG. The incidence of concurrent osteitis in patients with chronic rhinosinusitis: a clinicopathological study. Am J Rhinol. 2006;20(3):278–82. doi: 10.2500/ajr.2006.20.2857. [DOI] [PubMed] [Google Scholar]
- 30.Perloff JR, Gannon FH, Bolger WE, Montone KT, Orlandi R, Kennedy DW. Bone involvement in sinusitis: an apparent pathway for the spread of disease. Laryngoscope. 2000;110(12):2095–9. doi: 10.1097/00005537-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Van Zele T, Gevaert P, Watelet JB, Claeys G, Haoltappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Ponikau JU, Sherris DA, Kern EB, Homburger HA, et al. The diagnosis and incidence of Allergic Fungal Sinusitis. Mayo Clin Proc. 1999;74:877–84. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 33.Taylor MJ, Ponikau JU, Sherris DA, Kern EB, Gaffey TA, Kephart G, et al. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol Head Neck Surg. 2002;127(5):377–83. doi: 10.1067/mhn.2002.128896. [DOI] [PubMed] [Google Scholar]
- 34.Sherris DA, Ponikau JU, Kern EB. Eosinophilic Mucin rhinosinusitis. Laryngoscope. 2001;111:1670–1. doi: 10.1097/00005537-200109000-00034. [DOI] [PubMed] [Google Scholar]
- 35.Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA. Chronic rhinosinusitis : an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Ponikau JU, Sherris DA, Kephart GM, Adolphson C, Kita H. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2005;5:472–6. doi: 10.1007/s11882-005-0028-6. [DOI] [PubMed] [Google Scholar]
- 37.Ponikau JU, Sherris DA, Kita H, Kern EB. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2002;110(6):862–6. doi: 10.1067/mai.2002.130051. [DOI] [PubMed] [Google Scholar]
- 38.Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a randomized, placebo-controlled, double –blind pilot trial. J Allergy Clin Immunol. 2005;115(1):125–31. doi: 10.1016/j.jaci.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Weschta M, Riechelmann H. Effects of nasal antifungal therapy on nasal cell activation markers in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2006;132(7):743–7. doi: 10.1001/archotol.132.7.743. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy DW, Kuhn FA, Hamilos DL, Zeinreich SJ, Buffler D, Warsi G, et al. Treatment of chronic rhinosinusitis with high dose oral terbinafine: a double blind, placebo controlled study. Laryngoscope. 2005;115(10):1793–9. doi: 10.1097/01.mlg.0000175683.81260.26. [DOI] [PubMed] [Google Scholar]
- 41.Liang KL, Su MC, Shiao JY, Tseng HC, Hsin CH, Lin JF, et al. Amphotericin B irrigation for the treatment of chronic rhinosinusitis without polyps: a randomized, placebo-controlled, double blind study. Am J Rhinol. 2008;22(1):52–8. doi: 10.2500/ajr.2008.22.3115. [DOI] [PubMed] [Google Scholar]
- 42.Ebbens FA, Georgalas C, Rinia AB, van Drunen CM, Lund VJ, Fokkens WJ. The fungal debate: where do we stand today? Rhinology. 2007;45:178–89. [PubMed] [Google Scholar]
- 43.Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110:799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Ramadan HH, Quraishi HA. Allergic mucin sinusitis without fungus. Am J Rhinol. 1997;11:145–7. doi: 10.2500/105065897782537269. [DOI] [PubMed] [Google Scholar]
- 45.Katzenstein AA, Sale SR, Greenburger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72:89–93. doi: 10.1016/0091-6749(83)90057-X. [DOI] [PubMed] [Google Scholar]
- 46.Gourley DS, Whisman BA, Jorgensen NL, Martin ME, Reid MJ. Allergic Bipolaris sinusitis: clinical and immunopathologic characteristics. J Allergy Clin Immunol. 1990;85:583–91. doi: 10.1016/0091-6749(90)90097-N. [DOI] [PubMed] [Google Scholar]
- 47.Corey JP. Allergic fungal sinusitis. Otolaryngol Clin North Am. 1992;25:225–30. [PubMed] [Google Scholar]
- 48.Manning SC, Mabry RL, Schaefer SD. Evidence of IgE-mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope. 1993;103:717–21. doi: 10.1288/00005537-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 49.deShazo RD, Swain RE. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995;96:24–35. doi: 10.1016/S0091-6749(95)70029-3. [DOI] [PubMed] [Google Scholar]
- 50.Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol. 1998;102:387–94. doi: 10.1016/S0091-6749(98)70125-3. [DOI] [PubMed] [Google Scholar]
- 51.Watelet JB, Van Zele T, Gjormarkaj M, Canonica GW, Dahlen SE, Fokkens W, et al. Tissue remodelling in upper airways: where is the link with lower airway remodelling? Allergy. 2006;61(11):1249–58. doi: 10.1111/j.1398-9995.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 52.Watelet JB, Eloy P, Vancauwenberge P. Drug management in chronic rhinosinusitis: identification of the needs. Ther Clin Risk Manag. 2007;3(1):47–57. doi: 10.2147/tcrm.2007.3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Gaffrey TA, Tarara BA, et al. Features of airway remodeling and eosinophilic inflammation in chronic rhinosinusitis: is the histopathology similar to asthma? J Allergy Clin Immunol. 2003;112:877–82. doi: 10.1016/j.jaci.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Pawankar R, Nonaka M, Masuno S, Kimura S. Current concepts on the pathomechanisms of chronic rhinosinusitis and nasal polyps. In: Onerci TM, Ferguson BJ, editors. Nasal polyposis: pathogenesis, medical and surgical treatment. New York: Springer; 2010. pp. 185–90. [Google Scholar]
- 55.Meltzer EO, Hamilos DL, Hadley JA, Lanza DJ, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:S155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watelet JB, Bachert C, Claeys C, Vancauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 57.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121(6):1435–41. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Li HB, Cai KM, Liu Z, Xia JH, Zhang Y, Xua R, et al. Foxp3+ T regulatory cells (Tregs) are increased in nasal polyps (NP) after treatment with intranasal steroid. Clin Immunol. 2008;129:394–400. doi: 10.1016/j.clim.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Eloy P, Watelet JB, Rombaux P, Daele J, Bertrand B. Management of chronic rhinosinusitis without polyps in adults. B-ENT. 2005;(Suppl. 1):65–74, quiz 75–76. [PubMed]
- 60.Watelet JB, Eloy P, Van Cauwenberg P. Drug management in chronic rhinosinusitis: identification of the needs. Ther Clin Risk Manag. 2007;3(1):47–57. doi: 10.2147/tcrm.2007.3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gillespie MB, Osguthorpe JD. Pharmacologic management of chronic rhinosinusitis, alone or with nasal polyposis. Curr Allergy Asthma Rep. 2004;4(6):478–85. doi: 10.1007/s11882-004-0015-3. [DOI] [PubMed] [Google Scholar]
- 62.Ulualp SO, Toohill RJ, Hoffmann R, et al. Possible relationship of gastroesophagopharyngeal acid reflux with pathogenesis of chronic sinusitis. Am J Rhinol. 1999;13:197–202. doi: 10.2500/105065899781389777. [DOI] [PubMed] [Google Scholar]
- 63.DiBaise JK, Olusola BF, Huerter JV, et al. Role of GERD in chronic resistant sinusitis: a prospective, open label, pilot trial. Am J Gastroenterol. 2002;97:843–50. doi: 10.1111/j.1572-0241.2002.05598.x. [DOI] [PubMed] [Google Scholar]
- 64.Dinis PB, Monteiro MC, Martins ML, et al. Sinus tissue pharmacokinetics after oral administration of amocicillin/clavulanic acid. Laryngoscope. 2000;110:1050–5. doi: 10.1097/00005537-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 65.Passali D, Mazzei T, Novelli A, et al. Amoxicillin/clavulanate in chronic rhinosinusitis: tissue and serum distribution. Acta Otorhinolaryngol Belg. 2001;55:259–64. [PubMed] [Google Scholar]
- 66.Suzuki H, Ikeda K. Mode of action of long-term low-dose macrolide therapy for chronic sinusitis in the light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy. 2002;1:117–26. doi: 10.2174/1568010023344832. [DOI] [PubMed] [Google Scholar]
- 67.Wallwork B, Coman W. Chronic rhinosinusitis and eosinophils: do macrolides have an effect? Curr Opin Otolaryngol Head Neck Surg. 2004;12:14–7. doi: 10.1097/00020840-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Taccariello M, Parikh P, Darby Y, et al. Nasal douching in chronic rhinosinusitis: a randomised, single blind study comparing alkaline nasal douche and sterile sea water. Rhinology. 1999;37:29–32. [PubMed] [Google Scholar]
- 69.Keith P, Nieminen J, Hollingworth K, et al. Efficacy and tolerability of fluticasone proprionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy. 2000;30:1460–8. doi: 10.1046/j.1365-2222.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 70.Parikh A, Scadding GK, Darby Y, et al. Topical corticosteroids in chronic rhinosinusitis: a randomized double-blind, placebo-controlled trial using fluticasone proprionate aqueous nasal spray. Rhinology. 2001;39:75–9. [PubMed] [Google Scholar]
- 71.Giger R, Pasche P, Cheseaux C, et al. Comparison of once-versus twice-daily use of beclomethasone diproprionate aqueous nasal spray in the treatment of allergic and non-allergic chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2003;260:135–40. doi: 10.1007/s00405-002-0543-1. [DOI] [PubMed] [Google Scholar]
- 72.Dijkstra MD, Ebbens FA, Poublon RM, et al. Fluticasone proprionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clin Exp Allergy. 2004;34:1395–400. doi: 10.1111/j.1365-2222.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- 73.Lund VJ, Black JH, Szabo LZ, et al. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42:57–62. [PubMed] [Google Scholar]
- 74.Patel RS, Shaw RS, Wallace AM, et al. Efficacy and systemic tolerability of mometasone furoate and betamethasone sodium phosphate. J Laryngol Otol. 2004;118:866–71. doi: 10.1258/0022215042703769. [DOI] [PubMed] [Google Scholar]
- 75.Aukema AA, Mulder PG, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone proprionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol. 2005;115:1017–23. doi: 10.1016/j.jaci.2004.12.1144. [DOI] [PubMed] [Google Scholar]
- 76.Damm M, Quante G, Jungehueslsing M, et al. Impact of functional endoscopic sinus surgery on symptoms and quality of life in chronic rhinosinusitis. Laryngoscope. 2002;112:310–5. doi: 10.1097/00005537-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 77.Chiu AG, Kennedy DW. Surgical management of chronic rhinosinusitis and nasal polyposis: a review of evidence. Curr Allergy Asthma Rep. 2004;4:486–9. doi: 10.1007/s11882-004-0016-2. [DOI] [PubMed] [Google Scholar]
- 78.Iro H, Mayr S, Wallisch C, et al. Endoscopic sinus surgery: its subjective medium-term outcome in chronic rhinosinusitis. Rhinology. 2004;42:200–6. [PubMed] [Google Scholar]
- 79.Dhong HJ, Jung YS, Chung SK, et al. Effect of endoscopic sinus surgery on asthmatic patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124:99–104. doi: 10.1067/mhn.2001.111596. [DOI] [PubMed] [Google Scholar]
- 80.Dunlop G, Scadding GK, Lund VJ. The effect of endoscopic sinus surgery on asthma: management of patients with chronic rhinosinusitis, nasal polyposis and asthma. Am J Rhinol. 1999;13:261–5. doi: 10.2500/105065899782102809. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein MF, Grundfast SK, Dunsky EH, et al. Effect of functional endoscopic sinus surgery on bronchial asthma outcomes. Arch Otolaryngol Head Neck Surg. 1999;125:314–9. doi: 10.1001/archotol.125.3.314. [DOI] [PubMed] [Google Scholar]
- 82.Ikeda K, Tanno N, Tamura G, et al. Endoscopic sinus surgery improves pulmonary function in patients with asthma associated with chronic sinusitis. Ann Otol Rhinol Laryngol. 1999;108:355–9. doi: 10.1177/000348949910800407. [DOI] [PubMed] [Google Scholar]
- 83.Senior BA, Kennedy DW, Tanabodee J, et al. Long-term impact of functional endoscopic sinus surgery on asthma. Otolaryngol Head Neck Surg. 1999;121:66–8. doi: 10.1016/S0194-5998(99)70127-0. [DOI] [PubMed] [Google Scholar]
- 84.Jorrissen M, Bachert C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology. 2009;47(3):280–6. doi: 10.4193/Rhin08.227. [DOI] [PubMed] [Google Scholar]
- 85.Verbruggen K, Van Cauwenberge P, Bachert C. Anti-IgE for the treatment of allergic rhinitis—and eventually nasal polyps? Int Arch Immunol. 2009;148(2):87–98. doi: 10.1159/000155739. [DOI] [PubMed] [Google Scholar]
- 86.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8(1):34–8. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 87.Gevaert P, Johannson S, Bachert C. Aspirin sensitivity and IgE antibodies to Staphylococcus aureus enterotoxins in nasal polyposis: studies on the relationship. Int Arch Allergy Immunol. 2004;133:255–60. doi: 10.1159/000076832. [DOI] [PubMed] [Google Scholar]
- 88.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–64. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 89.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest. 2003;123:417–22. doi: 10.1378/chest.123.3_suppl.417S-a. [DOI] [PubMed] [Google Scholar]
- 90.Coste A, Brugel L, MaöÄtre B, Boussat S, Papon JF, Wingerstmann L, et al. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J. 2000;15:367–72. doi: 10.1034/j.1399-3003.2000.15b24.x. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Novo CA, Kowalski ML, Kuna P, Ptasinska A, Holtappels G, Van Cauwenberge P, et al. Aspirin sensitivity and IgE antibodies to Staphylococcus aureus enterotoxins in nasal polyposis: studies on the relationship. Int Arch Allergy Immunol. 2004;133:255–60. doi: 10.1159/000076832. [DOI] [PubMed] [Google Scholar]
- 92.Bachert C, Wagenmann M, Rudack C, Höpken K, Hillebrandt M, Wang D, et al. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy. 1998;53:2–13. doi: 10.1111/j.1398-9995.1998.tb03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bachert C, Gevaert P, Holtappels G, van Cauwenberge P. Mediators in nasal polyposis. Curr Allergy Asthma Rep. 2002;2:481–7. doi: 10.1007/s11882-002-0088-9. [DOI] [PubMed] [Google Scholar]
- 94.Nonaka M, Pawankar R, Saji F, et al. Eotaxin synthesis by nasal polyp fibroblasts. Acta Otolaryngol. 1999;119:816–20. doi: 10.1080/00016489950180478. [DOI] [PubMed] [Google Scholar]
- 95.Nonaka M, Pawankar R, Saji F, et al. Distinct expression of RANTES and GM-CSF by lipopolysaccharide in human nasal fibroblasts but not in other airway fibroblasts. Int Arch Allergy Immunol. 1999;119:314–21. doi: 10.1159/000024209. [DOI] [PubMed] [Google Scholar]
- 96.Hans-Uwe S, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–8. [PubMed] [Google Scholar]
- 97.Chao SS, Graham SM, Brown CL, Kline JN, Hussain I. Cysteinyl leukotriene 1 receptor expression in nasal polyps. Ann Otol Rhinol Laryngol. 2006;115(5):394–7. doi: 10.1177/000348940611500513. [DOI] [PubMed] [Google Scholar]
- 98.Bachert C, Gevaert P, Holtappels G, Johansson SGO, Van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 99.Bachert C, Gevaert P, Howarth P, Holtappels G, van Cauwenberge P, Johansson SGO. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol. 2003;111:1131–2. doi: 10.1067/mai.2003.1389. [DOI] [PubMed] [Google Scholar]
- 100.Zhang N, Holtappels G, Claeys C, Huang G, Vancauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006;20(4):445–50. doi: 10.2500/ajr.2006.20.2887. [DOI] [PubMed] [Google Scholar]
- 101.Bachert C, Watelet JB, Gevaert P, Van Cauwenberge P. Pharmacological management of nasal polyposis. Drugs. 2005;65(11):1537–52. doi: 10.2165/00003495-200565110-00006. [DOI] [PubMed] [Google Scholar]
- 102.Assanasen P, Naclerio RM. Medical and surgical management of nasal polyps. Curr Opin Otolaryngol Head Neck Surg. 2001;9:27–36. doi: 10.1097/00020840-200102000-00007. [DOI] [Google Scholar]
- 103.Scadding GK. Comparison of medical and surgical treatment of nasal polyposis. Curr Allergy Asthma Rep. 2002;2:494–9. doi: 10.1007/s11882-002-0090-2. [DOI] [PubMed] [Google Scholar]
- 104.Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118(1):128–33. doi: 10.1016/j.jaci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Van Camp P, Clement PAR. Results of oral steroid treatment in nasal polyposis. Rhinology. 1994;32:5–9. [PubMed] [Google Scholar]
- 106.Lildholdt T, Rundccrantz H, Bende M, et al. Glucocorticoid treatment for nasal polyps: a study of budesonide powder and depot-steroid injection. Arch Otolaryngol Head Neck Surg. 1997;123:595–600. doi: 10.1001/archotol.1997.01900060037006. [DOI] [PubMed] [Google Scholar]
- 107.Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008;63(10):1292–300. doi: 10.1111/j.1398-9995.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 108.Holmberg K, Juliusson S, Balder B, et al. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Ann Allergy Asthma Immunol. 1997;78:270–6. doi: 10.1016/S1081-1206(10)63180-8. [DOI] [PubMed] [Google Scholar]
- 109.Lund VJ, Flood J, Sykes AP, et al. Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg. 1998;124:513–8. doi: 10.1001/archotol.124.5.513. [DOI] [PubMed] [Google Scholar]
- 110.Lildholt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonide. Clin Otolaryngol. 1995;20:26–30. doi: 10.1111/j.1365-2273.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 111.Lildholdt T, Fogstrup J, Gammelgaard N, et al. Management of nasal polyps by steroid nose drops. Am J Rhinol. 1991;5:1–3. doi: 10.2500/105065891781976818. [DOI] [Google Scholar]
- 112.Penttila M, Poulsen P, Hollingworth K, et al. Dose-related efficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily and twice daily in the treatment of bilateral nasal polyposis: a placebocontrolled randomized study in adult patients. Clin Exp Allergy. 2000;30:94–102. doi: 10.1046/j.1365-2222.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- 113.Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma and COPD. Chest. 2004;125:52S–61. doi: 10.1378/chest.125.2_suppl.52S. [DOI] [PubMed] [Google Scholar]
- 114.Cervin A. The anti-inflammatory effect of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 2001;121(1):83–92. doi: 10.1080/000164801300006326. [DOI] [PubMed] [Google Scholar]
- 115.Matsune S, Sun D, Ohori J, Nishimoto K, Fukuiwa T, Ushikai M, et al. Inhibition of vascular endothelial growth factor by macrolides in cultured fibroblasts from nasal polyps. Laryngoscope. 2005;115(11):1953–6. doi: 10.1097/01.mlg.0000177031.06112.50. [DOI] [PubMed] [Google Scholar]
- 116.Yamada T, Fujieda S, Mori S, et al. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am J Rhinol. 2000;14(3):143–8. doi: 10.2500/105065800782102717. [DOI] [PubMed] [Google Scholar]
- 117.Nonaka M, Pawankar R, Tomiyama S, et al. A macrolide antibiotic, roxithromycin, inhibits the growth of nasal polyp fibroblasts. Am J Rhinol. 1999;13(4):267–72. doi: 10.2500/105065899782102791. [DOI] [PubMed] [Google Scholar]
- 118.Iino Y, Sasaki Y, Kojima C, et al. Effect of macrolides on the expression of HLA-DR and costimulatory molecules on antigen-presenting cells in nasal polyps. Ann Otol Rhinol Laryngol. 2001;110(5):457–63. doi: 10.1177/000348940111000512. [DOI] [PubMed] [Google Scholar]
- 119.Ichimura K, Shimazaki Y, Ishibashi T, et al. Effect of new macrolide roxithromycin upon nasal polyps associated with chronic sinusitis. Auris Nasus Larynx. 1996;23:48–56. doi: 10.1016/s0385-8146(96)80008-3. [DOI] [PubMed] [Google Scholar]
- 120.Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116:189–93. doi: 10.1097/01.mlg.0000191560.53555.08. [DOI] [PubMed] [Google Scholar]
- 121.Holgate ST, Canonica GW, Simons FER, Taglialatela M, Tharp M, Timmermans H, et al. Consensus group on new-generation antihistamines (CONGA): present status and recommendations. Clin Exp Allergy. 2003;33:1305–24. doi: 10.1046/j.1365-2222.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 122.Keith PK, Conway M, Evans S, et al. Nasal polyps: effects of seasonal allergen exposure. J Allergy Clin Immunol. 1994;93(3):567–74. doi: 10.1016/S0091-6749(94)70068-0. [DOI] [PubMed] [Google Scholar]
- 123.Crampette L, Mainprice B, Bloom M, et al. Inhibition of mediator and cytokine release from dispersed nasal polyp cells by terfenadine. Allergy. 1996;51(5):346–9. doi: 10.1111/j.1398-9995.1996.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 124.Campbell A, Michel FB, Bremard-Oury C, et al. Overview of allergic mechanisms: ebastine has more than an antihistamine effect. Drugs. 1996;52(Suppl. 1):15–9. doi: 10.2165/00003495-199600521-00005. [DOI] [PubMed] [Google Scholar]
- 125.Carayol N, Crampette L, Mainprice B, et al. Inhibition of mediator and cytokine release from dispersed nasal polyp cells, by mizolastine. Allergy. 2002;57(11):1067–70. doi: 10.1034/j.1398-9995.2002.23452.x. [DOI] [PubMed] [Google Scholar]
- 126.Vignola AM, Crampette L, Mondain M, et al. Inhibitory activity of loratadine and descarboethoxyloratadine on expression of ICAM-1 and HLA-DR by nasal epithelial cells. Allergy. 1995;50(3):200–3. doi: 10.1111/j.1398-9995.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 127.Haye R, Aanesen JP, Burtin B, et al. The effect of cetirizine on symptoms and signs of nasal polyposis. J Laryngol Otol. 1998;112(11):1042–6. doi: 10.1017/S0022215100142422. [DOI] [PubMed] [Google Scholar]
- 128.Alobid I, Cardelus S, Picado C, Mullol J. Antileukotrienes in rhinosinusitis and nasal polyposis. Expert Rev Clin Immunol. 2008;4(3):331–7. doi: 10.1586/1744666X.4.3.331. [DOI] [PubMed] [Google Scholar]
- 129.Di Rienzo L, Artuso A, Cerqua N. Antileukotrienes in the prevention of postoperative recurrence of nasal polyposis in ASA syndrome. Acta Otolaryngol Ital. 2000;20(5):336–42. [PubMed] [Google Scholar]
- 130.Stevenson DD, Hankammer MA, Mathison DA, et al. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol. 1996;98(4):751–8. doi: 10.1016/S0091-6749(96)70123-9. [DOI] [PubMed] [Google Scholar]
- 131.Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol. 2003;24(2):159–68. doi: 10.1385/CRIAI:24:2:159. [DOI] [PubMed] [Google Scholar]
- 132.Fiotta N, Altamura N, Moretti M, Wasserman ST, Zacchigna S, Farra R, et al. Short term effects of doxycycline on Matrix Metalloproteinases 2 and 9. Cardiovasc Drugs Ther. 2009;23(2):153–9. doi: 10.1007/s10557-008-6150-7. [DOI] [PubMed] [Google Scholar]
- 133.Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125(5):1069–1076.e4. doi: 10.1016/j.jaci.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 134.Bachert C, Wagenmann M, Hauser U, et al. IL-5 is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99(6):837–42. doi: 10.1016/S0091-6749(97)80019-X. [DOI] [PubMed] [Google Scholar]
- 135.Gevaert P, Bachert C, Holtappels G, et al. Enhanced soluble interleukin 5 receptor alpha expression in nasal polyposis. J Allergy Clin Immunol Allergy. 2003;58(5):371–9. doi: 10.1034/j.1398-9995.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 136.Penn R, Mikula S. The role of anti-IgE immunoglobulin therapy in nasal polyposis: a pilot study. Am J Rhinol. 2007;21(4):428–32. doi: 10.2500/ajr.2007.21.3060. [DOI] [PubMed] [Google Scholar]
- 137.Mendelson MG, Gross CW. Soft-tissue shavers in pediatric sinus surgery. Otolaryngol Clin North Am. 1997;30:443–9. [PubMed] [Google Scholar]
- 138.Setliff R, Parsons D. The “hummer”: new instrumentation for functional endoscopic sinus surgery. Am J Rhinol. 1994;8:275–8. doi: 10.2500/105065894781874232. [DOI] [Google Scholar]
- 139.Zweig JL, Schaitkin BM, Fan CY, et al. Histopathology of tissue samples removed using the microdebrider technique: implications for endoscopic sinus surgery. Am J Rhinol. 2000;14:27–32. doi: 10.2500/105065800781602902. [DOI] [PubMed] [Google Scholar]
- 140.Bernstein JM, Lebowitz RA, Jacobs JB. Initial report on postoperative healing after endoscopic sinus surgery with the microdebrider. Otolaryngol Head Neck Surg. 1998;118:800–3. doi: 10.1016/S0194-5998(98)70272-4. [DOI] [PubMed] [Google Scholar]
- 141.Anon JB. Computer-aided endoscopic sinus surgery. Laryngoscope. 1998;108:949–61. doi: 10.1097/00005537-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 142.Kacker A, TabaEE A, Anand V. Computer-assisted surgical navigation in revision endoscopic sinus surgery. Otolaryngol Clin North Am. 2005;38(3):473–82. doi: 10.1016/j.otc.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 143.Caversaccio M, Zheng G, Nolte LP. Computer-aided surgery of the paranasal sinuses and the anterior skull base. HNO. 2008;56(4):376–8. doi: 10.1007/s00106-008-1705-2. [DOI] [PubMed] [Google Scholar]
- 144.Metson RB, Gliklich RE. Clinical outcomes in patients with chronic sinusitis. Laryngoscope. 2000;110(suppl 94):24–8. doi: 10.1097/00005537-200003002-00007. [DOI] [PubMed] [Google Scholar]
- 145.Keles N, Ilicali OC, Deger K. Objective and subjective assessment of nasal obstruction in patients undergoing endoscopic sinus surgery. Am J Rhinol. 1998;12:307–9. doi: 10.2500/105065898780182408. [DOI] [PubMed] [Google Scholar]
- 146.Rowe-Jones JM, Mackay IS. A prospective study of olfaction following endoscopic sinus surgery with adjuvant medical treatment. Clin Otolaryngol. 1997;22:377–81. doi: 10.1046/j.1365-2273.1997.00004.x. [DOI] [PubMed] [Google Scholar]
- 147.Hafner B, Davris S, Riechelmann H, et al. Endonasal sinus surgery improves mucociliary transport in severe chronic sinusitis. Am J Rhinol. 1997;11:271–4. doi: 10.2500/105065897781446612. [DOI] [PubMed] [Google Scholar]
- 148.Kaluskar SK. Pre- and postoperative mucociliary clearance in functional endoscopic sinus surgery. Ear Nose Throat J. 1997;76:884–6. [PubMed] [Google Scholar]
- 149.Elwany S, Hisham M, Gamaee R. The effect of endoscopic sinus surgery on mucociliary clearance in patients with chronic sinusitis. Eur Arch Otorhinolaryngol. 1998;255:511–4. doi: 10.1007/s004050050109. [DOI] [PubMed] [Google Scholar]
- 150.Delak KW, Stoll W. Olfactory function after functional endoscopic sinus surgery for chronic sinusitis. Rhinology. 1998;36:15–9. [PubMed] [Google Scholar]
- 151.Shin SH, Park JY, Sohn JH. Clinical value of olfactory function tests after endoscopic sinus surgery: a short-term result. Am J Rhinol. 1999;13:63–6. doi: 10.2500/105065899781389894. [DOI] [PubMed] [Google Scholar]
- 152.Amar YG, Frenkiel S, Sobol SE. Outcome analysis of endoscopic sinus surgery for chronic sinusitis in patients having Samter’s triad. J Otolaryngol. 2000;29:7–12. [PubMed] [Google Scholar]
- 153.Nakamura H, Kawasaki M, Higuchi Y, et al. Effects of sinus surgery on asthma in aspirin triad patients. Acta Otolaryngol. 1999;119:592–8. doi: 10.1080/00016489950180856. [DOI] [PubMed] [Google Scholar]
- 154.Hartwig S, Linden M, Laurent C, et al. Budesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial) J Laryngol Otol. 1988;102(2):148–51. doi: 10.1017/S0022215100104372. [DOI] [PubMed] [Google Scholar]
- 155.Virolainen E, Puhakka H. The effect of intranasal beclomethasone dipropionate on the recurrence of nasal polyps after ethmoidectomy. Rhinology. 1980;18(1):9–18. [PubMed] [Google Scholar]
- 156.Stjärne P, Olsson P, Alenius M. Use of mometasone furoate to prevent polyp relapse after endoscopic sinus surgery. Arch Otolaryngol Head Neck Surg. 2009;135(3):296–302. doi: 10.1001/archoto.2009.2. [DOI] [PubMed] [Google Scholar]
- 157.Rhinosinusitis Task Force Committee Report of the Rhinosinusitis Task Force Committee Meeting, Alexandria, VA, August 17, 1996. Otolaryngol Head Neck Surg. 1997;117:S1–68. doi: 10.1016/S0194-5998(97)70001-9. [DOI] [PubMed] [Google Scholar]


