Abstract
We developed and evaluated a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for detecting Japanese encephalitis virus (JEV). The sensitivity of the JEV RT-LAMP assay was in concordance with that of real-time RT-PCR and 10-fold more sensitive than that of conventional RT-PCR, which the detection limit was 24 copies/μl. The JEV RT-LAMP was highly specific, which no cross-reactivity was found with dengue-2 virus, rabies virus, norovirus, astrovirus and human enterovirus 71. The JEV RT-LAMP assay was more simple and less time-consuming compared to the conventional RT-PCR and real-time RT-PCR, which the amplification could be completed in a single tube within 1 h under isothermal conditions at temperature of 63°C. The results suggest that the RT-LAMP assay can be applied as a practical molecular diagnostic tool for JEV infection and surveillance.
Keywords: Japanese encephalitis virus, Reverse transcription loop-mediated isothermal amplification (RT-LAMP), Real-time RT-PCR, RT-PCR
Introduction
Japanese encephalitis virus (JEV) is a member of the genus flavivirus of the family flaviviridae. JEV exists in a zoonotic cycle among mosquitoes, pigs and some birds, and human being as incidental and dead-end hosts. In humans, JEV infection can cause Japanese encephalitis (JE) with severe central nervous system disorders. JE is the leading cause of viral encephalitis in China, India, Japan, Southeast Asia and Western Pacific regions, where 30,000–50,000 cases are reported annually, among these cases, about 30% are fatal. 30% of those who survive will remain irreversible neurologic damage [1]. JE can be controlled by vaccination. The implementation of control program is dependent on accurate surveillance data, which include laboratory confirmation of JEV infection.
Diagnosis of JEV infection is mainly based on serological, viral culture and molecular approaches. Serological methods are simple and feasible. However, antibody test results may be confusing due to considerable cross-reactivity with other flaviviruses [2]. Virus isolation is a definitive diagnosis for JEV infection, however, this assay is usually unavailable owing to less sensitive and more time consuming. RT-PCR assays for detection of specific genomic sequence of JEV have been showed high sensitivity and specificity [3–5]. However, these assays compared with serologic tests are more time-consuming and require expensive and sophisticated equipments. Most of cases of JE occur in rural areas, where routine RT-PCR diagnostic facilities are limited. Therefore, it is necessary to develop a rapid, simple, sensitive, and specific diagnostic method for JEV infection and surveillance.
Loop-mediated isothermal amplification (LAMP) is a novel PCR method developed by Notomi et al. [6]. The most important advantage of the LAMP is amplification of nucleic acids under isothermal conditions at a temperature range of 60–65°C for 1 h or less. Another advantage of the method is that the amplification from stem-loop structures leads to the accumulation of large amounts of products of various lengths, making detection of amplified nucleic acids much easier [7]. Recently, LAMP technology has been successfully applied for rapid detection of various pathogens, such as West Nile virus (WNV), SARS-CoV, Mycobacterium tuberculosis, Paracoccidioides brasiliensis, Plasmodium [8–12]. A reverse transcription LAMP (RT-LAMP) assay for detecting JEV was firstly reported by Toriniwa and Komiya and the sensitivity similarly to conventional RT-PCR [13]. Later, Parida et al. [14] reported the application of RT-LAMP assay to detect JEV in the cerebrospinal fluid samples from patients with clinical diagnosis of acute encephalitis, which the sensitivity of JEV detection was comparable to conventional RT-PCR. Yet they have not compared the sensitivity and specificity of RT-LAMP with real-time RT-PCR assay. Today, real-time PCR assays have a trend to replace conventional PCR or viral culture method in clinical diagnosis for a lot of viral infections because of their high sensitivity and specificity [15]. Here, we report a comparative study of sensitivity and specificity of RT-LAMP, conventional RT-PCR and real-time RT-PCR assays for detection of JEV.
Materials and methods
Viruses
Nakayama strain of JEV was used as positive standard in the present study. The live-attenuated Japanese encephalitis vaccine (derived from SA14-14-2 viral strain, Chengdu Institute of Biological Products, China) also used as positive control. Negative control included five species of viruses, dengue-2 virus (New Guinea C strain), rabies virus (Flury strain, low egg passage), norovirus, astrovirus and human enterovirus 71. Working stocks of Nakayama strain of JEV and the rabies virus were propagated by inoculation of suckling mouse brains, the dengue virus was propagated by in C6/36 cells. The stool specimens containing norovirus, astrovius or enterovirus 71 were obtained from laboratory confirmed patients. A 10% (wt/vol) suspension of stool was made then kept at −80°C until used.
Samples
Two JEV-positive and ten JEV-negative specimens of bat brain tissues that had been confirmed by real-time PCR (data not shown).
Five clinical samples of cerebrospinal fluid (CSF) and two serum samples were collected from patients with non-JE at Nanfang Hospital, Guangzhou city. Simulated JEV-positive samples were prepared as follows. Nakayama strain of JEV propagated in BHK-21 cells. After cultivation and harvest, 10 μl of cultivated viral suspensions were added into sterile tubes containing 300 μl of CSF or serum samples.
Designing of primers for RT-LAMP of JEV
The oligonucleotide primers used for the RT-LAMP of JEV were designed from the envelope (E) gene region of the published sequence of strain JaOArS982 (GenBank accession no. M18370). We designed a set of six primers comprising two outer primers (F3 and B3), two inner primers (FIP and BIP) and two loop primers (Loop F and Loop B) that recognizing eight distinct regions on the target sequence by the LAMP primer designing software PrimerExplorer V3 (http://primerexplorer.jp/elamp3.0.0/index.html). Based on the criteria described by Notomi et al. [6], the primers were selected. The details of the selected primers with regard to their positions in the genomic sequences are shown in Table 1.
Table 1.
The primers used in the RT-LAMP amplification of E gene of JEV
| Primer name | Nucleotide positiona | Sequence (5′–3′) |
|---|---|---|
| F3 | 1036–1054 | AATGACATGACCCCCGTTG |
| B3 | 1239–1256 | GCTGCCAGTCTTTGAGCT |
| FIP |
F1c: 1113–1132 F2: 1066–1084 |
AGGGGGGTTCCATCTCGACC–ACAGTGAACCCTTTCGTCG |
| BIP |
B1c: 1152–1171 B2: 1205–1222 |
GGTTGGGAGGGGAGACAAGC–AAAAGGCCTTGCCTAGCG |
| Loop F | 1085–1104 | TGAGTTGGCACTGGAAGTCG |
| Loop B | 1172–1193 | AGATCAACCACCATTGGCACAA |
aNucleotide position according to the sequence of E gene region of JEV strain JaOArS982 (GeneBank accession no. M18370)
RNA extraction
Extraction of RNA from viral working stocks, suspension of stools, bat brain tissues and clinical samples was performed using QIAamp viral RNA mini kit (Qiagen, Germany), according to the manufacturer’s protocols. The viral RNA was eluted from the QIAamp spin columns in a volume of 60 μl of elution buffer and stored at −80°C until used.
In order to evaluate the detection limit of the assays, the amount of JEV RNA was determined by spectrophotometric reading and converted to molecular copies by using the following formula [16].
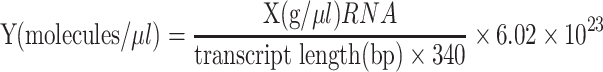 |
Then, 10-fold serial dilutions of the JEV RNA from 10−1–10−8 were used for detection of sensitivity of the assays.
RT-LAMP reaction and detection
The RT-LAMP reaction was carried out in a total 25 μl reaction volume containing 2.5 μl of 10 × ThermoPol Reaction Buffer, 8U of Bst DNA polymerase (8 U/μl, New England Biolabs), 200 U of SuperScript III Reverse Transcriptase (200 U/μl, Invitrogen), 2.5 μl of dNTP Mix (10 mM, Invitrogen), 4.0 μl of Betaine (5 M, Sigma), 3.0 μl of MgCl2 (50 mM, Invitrogen), 1.0 μl of each of the F3 and B3 primers (5 μM), 1.0 μl of each of the BIP and FIP primers (40 μM), 1.0 μl of each of the Loop F and Loop B primers (20 μM), and 5.0 μl of target RNA. The mixture was incubated at 63°C for 1 h and heated at 80°C for 5 min to terminate the reaction.
After reaction, the amplified products of RT-LAMP were observed by electrophoresis on a 1.5% agarose gel electrophoresis (Biowest Agarose, Spain) following ethidium bromide staining under ultraviolet light. Besides, the products also were observed directly with the naked eye by adding SYBR Green I. 2 μl of original SYBR Green I (Invitrogen) was added to the reaction mixture tube. The solution turned green in the presence of LAMP amplification products, while it retained orange in the absence of the amplicon.
RT-CR and real-time RT-PCR
First-strand cDNA was generated using Superscript III first-strand synthesis system (Invitrogen). The mixture was consisted of 50 ng of random hexamers (50 ng/μl), 1.0 μl of dNTP mix (10 mM) and 8.0 μl of total RNA and incubated at 65°C for 5 min, then on ice for at least 1 min. Afterwards, following reagents were added to each of reactions: 2.0 μl of 10 × RT buffer, 4.0 μl of 25 mM MgCl2, 2.0 μl of 0.1 MDTT, 40U of RNase OUT (40 U/μl, Invitrogen) and 200 U of Superscript III reverse transcriptase (200 U/μl). The reaction mixture was sequentially incubated at 25°C for 10 min, 50°C for 50 min and 85°C for 5 min. The synthesized cDNA was stored at −20°C.
RT-PCR was performed with the JEV specific primers targeting E gene of viral genome (GenBank accession no. L48961), JE1 (5′-ACCGTGGGGTCAAAGTCA-3′) (position no. 1590–1607) and JE2 (5′-TGCATTTTAGGTGGCCTGAT-3′) (position no. 1820–1839). The reaction was carried out in a total 50 μl mixture volume using Ex Taq™ system (TaKaRa, Japan) which contained 0.25 μl of TaKaRa Ex Taq polymerase (5 U/μl), 4.0 μl of dNTP mix (2.5 mM), 1.0 μl of each primer (10 μM), 5.0 μl of Ex Taq buffer, 5.0 μl of cDNA and 33.75 μl of nuclease-free water. The reaction was at 94°C for 5 min, followed by 35 cycles (94°C for 45 s, 55°C for 45 s, 72°C for 1 min) and a final extension cycle at 72°C for 10 min. The size of amplified product was 250 bp.
SYBR Green I-based real-time PCR amplification was performed with the detection of NS3 gene of JEV in the Mx3005P quantitative PCR system (Stratagene, USA) [17], JEF (5′-AGA GCG GGG AAA AAG GTC AT-3′, position no. 5739–5758) and JER (5′-TTT CAC GCT CTT TCT ACA GT-3′, position no. 5900–5881) (GenBank accession no. M18370). The amplification was carried out in a 25 μl total reaction volume using SYBR® Premix Ex Taq™ (Perfect Real Time) kit (Takara, Japan). The reaction mixture contained 12.5 μl of 2 × SYBR® Premix Ex Taq™, 0.5 μl of 50 × ROX Reference Dye II, 0.5 μl of each of forward and reverse primers (10μΜ), 2.0 μl of cDNA template and 9.0 μl of nuclease free water. The thermal profile for Real-time PCR was 95°C for 30 s, followed by 40 cycles (95°C for 5 s, 60°C for 10 s, 72°C for 10 s). After amplification, a melting curve analysis was performed to verify the authenticity of the amplified product by its specific melting temperature (Tm).
Results
Amplification and specificity of the RT-LAMP assay
The reaction mixture containing JEV genome was incubated at 63°C for 1 h, positive amplification could be detected by the presence of a ladder-like pattern on a 1.5% agarose gel electrophoresis under ultraviolet light. No positive reactions were found in dengue-2 virus, rabies virus, norovirus, astrovirus and human enterovirus 71 (Fig. 1).
Fig. 1.
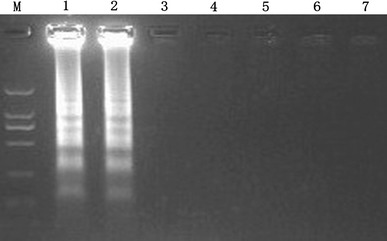
Specificity of the RT-LAMP assay for JEV. Positive amplifications show a ladder-like pattern on a 1.5% agarose gel electrophoresis under UV (lane 1 and lane 2). From lane 3 to lane 7, there is no cross reactivity with the control tested viruses. Lane M, DL2000 DNA Marker; lane 1–2, positive standard (JEV); lane 3, DEN-2 virus; lane 4, rabies virus; lane 5, norovirus; lane 6, astrovirus; lane 7, human enterovirus 71
Detection limit of the RT-LAMP assay
The transcript copy number of the initial concentration was deduced from the RNA concentration that was 1.49 μg/ml by using a SmartSpec 3000 spectrophotometer (Bio-Rad, USA). Depending on the formula by Krieg [16], the JEV RNA initial concentration was converted to 2.4 × 108 copies/μl. As observed on 1.5% agarose gel electrophoresis, the detection limit of the RT-LAMP assay was estimated to be 24 copies (Fig. 2). And the sensitivity of naked-eye inspection with the addition of 2 μl of SYBR GreenI dye was 240 copies (Fig. 3).
Fig. 2.
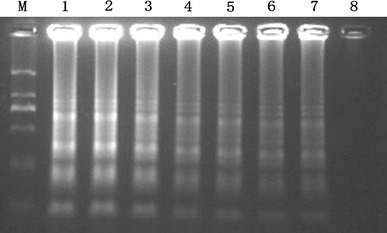
Sensitivity of the RT-LAMP assay for E gene of JEV, the products were electrophoresed on a 1.5% agarose gels. This figure shows that the detection limit of JEV RT-LAMP is 24 copies/μl. The concentrations of JEV RNA are from 2.4 × 107 to 2.4 copies/μl in 10-fold serial dilutions (Lanes 1–8). Lane M is DL2000 DNA Marker
Fig. 3.
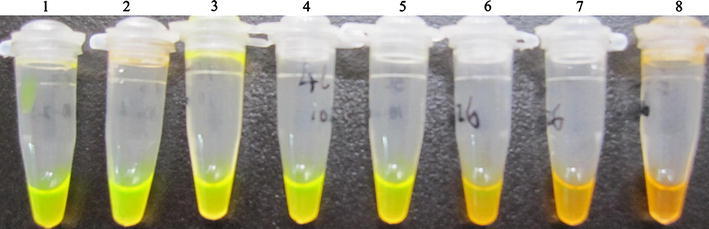
Naked-eye inspection of the RT-LAMP reaction stained with SYBR GreenI under normal light. Tubes 1–8, the concentrations of JEV RNA are ranged from 2.4 × 107 to 2.4 copies/μl in serial 10-fold dilutions. Tubes 1–6 are positive amplifications (the original orange color of SYBR GreenI turn to be green in the online version). Tubes 7–8 are negative reactions (the tubes remain orange color in the online version). It demonstrates the detection limit of JEV RT-LAMP is 2.4 × 102 copies/μl by naked-eye inspection
Comparison of the RT-LAMP, RT-PCR and real-time RT-PCR
Serial 10-fold dilutions of JEV RNA were parallelly tested by RT-PCR and real-time RT-PCR. The detection limits of conventional RT-PCR and real-time RT-PCR were 240 copies and 24 copies, respectively (Figs. 4, 5). Figure 6 shows JEV special standard curve constructed using the known concentrations of 10-fold serial dilutions of JEV RNA. Melting curve analysis showed that specific amplification melting temperature was 84.7°C (Fig. 7). According to the amplification and dissociation plots, the amplified product could be assigned as specific genomic sequence.
Fig. 4.
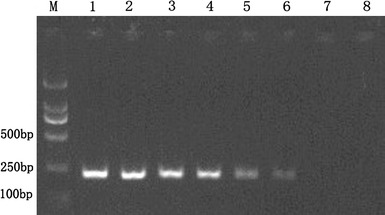
Sensitivity of RT-PCR assay for E gene of JEV. Amplified products of the RT-PCR assay are observed on a 1.5% agarose gel electrophoresis under UV. The amplicon size is 250 bp. This figure shows that the detection limit is 2.4 × 102 copies/μl. Lanes 1–8, the concentrations of JEV RNA are from 2.4 × 107 to 2.4 copies/μl in 10-fold serial dilutions. Lane M is DL2000 DNA Marker
Fig. 5.
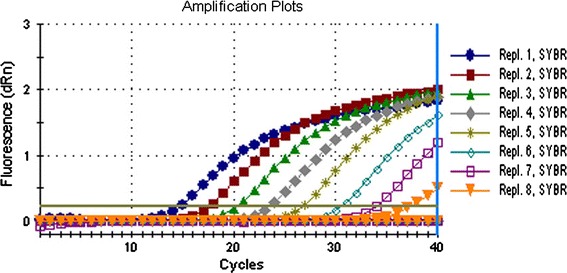
The detection sensitivity of real-time RT-PCR assay for JEV RNA. The amplification plots for detecting JEV show that the detection limit of the assay is 24 copies/μl. Repl.1-Repl.8 represent the concentrations of JEV RNA ranging from 2.4 × 107 to 2.4 copies/μl in serial 10-fold dilutions
Fig. 6.
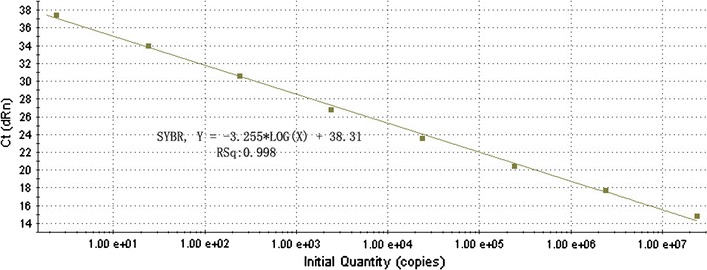
Standard curve of real-time PCR for detecting JEV. The assay standard curve was generated by the known concentration of virus RNA in serial 10-fold dilutions
Fig. 7.
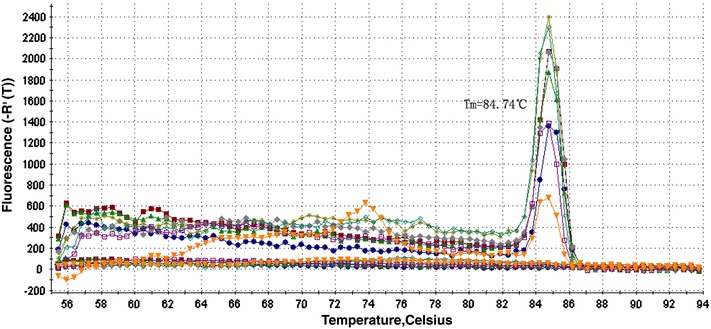
Dissociation curve of real-time PCR for detecting JEV, with specific melting temperature of 84.7°C. The amplified product with a melting temperature of 84.74°C can usually be assigned as specific DNA
The JEV RT-LAMP on agarose gel electrophoresis analysis demonstrated an equivalent sensitivity with the real-time RT-PCR, 10-fold more sensitive than conventional RT-PCR. While the sensitivity of RT-LAMP by visual inspection using SYBR GreenI stain was found to be similar to the RT-PCR, which was inferior to the electrophoresis analysis. The details are summarized in Table 2.
Table 2.
Comparisons of RT-PCR, real-time PCR and RT-LAMP methods for detecting sensitivity of JEV RNA
| Methods | Concentrations of JEV RNA (copies/μl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.4 × 107 | 2.4 × 106 | 2.4 × 105 | 2.4 × 104 | 2.4 × 103 | 2.4 × 102 | 2.4 × 101 | 2.4 × 100 | ||
| Conventional RT-PCR | + | + | + | + | + | + | − | − | |
| Real-time RT-PCR | + | + | + | + | + | + | + | − | |
| RT-LAMP | |||||||||
| Naked-eye inspection | + | + | + | + | + | + | − | − | |
| Agarose gel analysis | + | + | + | + | + | + | + | − | |
+ Positive reaction; − Negative reaction
The RT-LAMP amplification was completed within 1 h which was less than that of conventional RT-PCR (3.5 h) and real-time RT-PCR (2.5 h).
Application of JEV RT-LAMP in the samples
Two known JEV positive samples of bat train tissues, seven simulated clinical samples of JEV infection and the SA14-14-2 vaccine were detected for JEV by RT-LAMP assay. All 10 samples were positive by the assay (Fig. 8). By contrast, no positive amplification was observed in clinical samples from non-JE patients and 10 bat brain tissues that known JEV-negative.
Fig. 8.

Detection of the samples by the RT-LAMP. Lane M is DL2000 marker. Positive reactions (colored green in the online version) present in the CSF samples (from lane 1 to lane 5), blood samples (lane 6 and lane 7), SA14-14-2 Japanese encephalitis vaccine (lane 8), and bat brain tissues known JEV-positive (lane 9 and 10). Negative reaction is seen (colored orange in the online version) in known JEV-negative CSF sample from non-Japanese encephalitis patients (lane 11)
Discussion
In the past decades, several nucleic acid-based amplification methods for detection of JEV infection have been established, which including conventional RT-PCR, nested RT-PCR, probe real-time RT-PCR, dye real-time RT-PCR and LAMP RT-PCR [3, 4, 18–20] Compared to conventional RT-PCR and nested RT-PCR, real-time RT-PCR has engendered wider acceptance due to its improved rapidity, sensitivity and the reduced risk of laboratory contamination. However, real-time RT-PCR assays have several disadvantages, such as requiring expensive instruments, high cost of consumables and reagents. Hence, real time RT-PCR assays are often unavailable to routine clinical diagnosis, especially in remote rural areas where are usually at high risk of JEV infection.
The LAMP method does not rely on expensive and sophisticated facilities such as thermal cyclers. The amplified products of the reaction are shown as a ladder-like pattern by agarose gel electrophoresis staining with ethidium bromide under UV light. The products are also observed through naked eye inspection under UV lamp or normal light by adding fluorescent dye such as SYBR Green I. A by-product of the LAMP reaction, magnesium pyrophosphate, can be detected by real-time turbidimeter, or directly can be inspected by naked eye when the accumulation of magnesium pyrophosphate forming visual white turbidity [6, 7, 21].
To date, two reports have described the LAMP assay for detection of JEV. The report by Toriniwa and Komiya have showed that the sensitivity of detection of JEV RNA was 1PFU and 10PFU, with or without loop primers, respectively. The detection limit with loop primer was the same as that of one step RT-PCR [13]. Parida et al. reported a RT-LAMP assay with the added loop primers to detection of JEV in CSF specimens from acute phase patients clinically diagnosed as encephalitis. They compared the RT-LAMP with conventional RT-PCR. Concordance was 85%, sensitivity was 100%, specificity was 86%, none of the samples positive by RT-PCR were missed by RT-LAMP [14].
Here, we described the establishment of one step, single tube RT-LAMP assay for rapid detection of the envelope gene of JEV genome and compared sensitivity and specificity with conventional RT-PCR and real-time RT-PCR. The JEV RT-LAMP assay showed good specificity due to none of positive results found in negative control samples, including dengue-2 virus, rabies virus, norovirus, astrovirus and human enterovirus 71 under the same experimental conditions. Notwithstanding, it was regret that only two strains of flaviviruses were involved for evaluation of specificity of JEV RT-LAMP in this study.
The results showed that the detection limit of the JEV RT-LAMP assay was equivalent to that of SYBR Green real-time RT-PCR when LAMP amplified products by electrophoretic analysis, 10-fold more sensitive than conventional RT-PCR. The detection limit was 24 copies/μl. However, the sensitivity of detection by the naked-eye inspection based on color change was slightly lower than that by electrophoresis. The detection limit of was 240 copies/μl, which was equivalent to conventional RT-PCR assay. Nevertheless, considering the time-consuming of the RT-LAMP obviously less than that of conventional RT-PCR (2.5 h vs. 1 h), the RT-LAMP by the naked-eye inspection is superior to conventional RT-PCR.
In conclusion, this study presented a simple, sensitive and specific RT-LAMP assay for detection of specific nucleic acid sequence of JEV E gene. Considering the advantages of high sensitivity, rapid amplification, simple equipment and operation, the RT-LAMP assay can be applied as a practical molecular diagnostic tool for JEV infection and surveillance.
Acknowledgments
This study was funded by National Natural Science Foundation of China (Grant no. 30972525) and Guangdong Province “211 Project” (Grant no. GW201004).
References:
- 1.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15(1):1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holbrook MR, Shope RE, Barrett AD. Use of recombinant E protein domain III-based enzyme-linked immunosorbent assays for differentiation of tick-borne encephalitis serocomplex flaviviruses from mosquito-borne flaviviruses. J Clin Microbiol. 2004;42(9):4101–4110. doi: 10.1128/JCM.42.9.4101-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang JL, Lin HT, Wang YM, Weng MH, Ji DD, Kuo MD, Liu HW, Lin CS. Sensitive and specific detection of strains of Japanese encephalitis virus using a one-step TaqMan RT-PCR technique. J Med Virol. 2004;74(4):589–596. doi: 10.1002/jmv.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang DK, Kweon CH, Kim BH, Lim SI, Kim SH, Kwon JH, Han HR. TaqMan reverse transcription polymerase chain reaction for the detection of Japanese encephalitis virus. J Vet Sci. 2004;5(4):345–351. [PubMed] [Google Scholar]
- 5.Swami R, Ratho RK, Mishra B, Singh MP. Usefulness of RT-PCR for the diagnosis of Japanese encephalitis in clinical samples. Scand J Infect Dis. 2008;40(10):815–820. doi: 10.1080/00365540802227102. [DOI] [PubMed] [Google Scholar]
- 6.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16(3):223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 8.Endo S, Komori T, Ricci G, Sano A, Yokoyama K, Ohori A, Kamei K, Franco M, Miyaji M, Nishimura K. Detection of gp43 of Paracoccidioides brasiliensis by the loop-mediated isothermal amplification (LAMP) method. FEMS Microbiol Lett. 2004;234(1):93–97. doi: 10.1111/j.1574-6968.2004.tb09518.x. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto T, Sonobe T, Hayashi K. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J Clin Microbiol. 2003;41(6):2616–2622. doi: 10.1128/JCM.41.6.2616-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon LL, Leung CS, Tashiro M, Chan KH, Wong BW, Yuen KY, Guan Y, Peiris JS. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin Chem. 2004;50(6):1050–1052. doi: 10.1373/clinchem.2004.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aonuma H, Suzuki M, Iseki H, Perera N, Nelson B, Igarashi I, Yagi T, Kanuka H, Fukumoto S. Rapid identification of Plasmodium-carrying mosquitoes using loop-mediated isothermal amplification. Biochem Biophys Res Commun. 2008;376(4):671–676. doi: 10.1016/j.bbrc.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 12.Parida M, Posadas G, Inoue S, Hasebe F, Morita K. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol. 2004;42(1):257–263. doi: 10.1128/JCM.42.1.257-263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toriniwa H, Komiya T. Rapid detection and quantification of Japanese encephalitis virus by real-time reverse transcription loop-mediated isothermal amplification. Microbiol Immunol. 2006;50(5):379–387. doi: 10.1111/j.1348-0421.2006.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 14.Parida MM, Santhosh SR, Dash PK, Tripathi NK, Saxena P, Ambuj S, Sahni AK, Lakshmana RP, Morita K. Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J Clin Microbiol. 2006;44(11):4172–4178. doi: 10.1128/JCM.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30(6):1292–1305. doi: 10.1093/nar/30.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg PA. Improved synthesis of full-length RNA probe at reduced incubation temperatures. Nucleic Acids Res. 1990;18(21):6463. doi: 10.1093/nar/18.21.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santhosh SR, Parida MM, Dash PK, Pateriya A, Pattnaik B, Pradhan HK, Tripathi NK, Ambuj S, Gupta N, Saxena P, Lakshmana RP. Development and evaluation of SYBR Green I-based one-step real-time RT-PCR assay for detection and quantitation of Japanese encephalitis virus. J Virol Methods. 2007;143(1):73–80. doi: 10.1016/j.jviromet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Tso S, Chen B. Detection of Japanese encephalitis virus in samples of JE patients by RT-PCR. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2000;14(2):184–187. [PubMed] [Google Scholar]
- 19.Paranjpe S, Banerjee K. Detection of Japanese encephalitis virus by reverse transcription polymerase chain reaction. Acta Virol. 1998;42(1):5–11. [PubMed] [Google Scholar]
- 20.Saxena V, Mishra VK, Dhole TN. Evaluation of reverse-transcriptase PCR as a diagnostic tool to confirm Japanese encephalitis virus infection. Trans R Soc Trop Med Hyg. 2009;103(4):403–406. doi: 10.1016/j.trstmh.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289(1):150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]


