Abstract
Objective:
A companion article reports the trajectory of long-term mortality and significant health-related quality of life (HRQL) disability among children encountering septic shock. In this article, the investigators examine critical illness factors associated with these adverse outcomes.
Design:
Prospective, cohort-outcome study, conducted 2013–2017.
Setting:
Twelve United States academic pediatric intensive care units (PICUs).
Patients:
Critically ill children, 1 month-18 years, with community-acquired septic shock requiring vasoactive-inotropic support.
Interventions:
Illness severity, organ dysfunction, and resource utilization data were collected during PICU admission. Change from Baseline HRQL at the Month 3 follow-up was assessed by parent proxy-report employing the Pediatric Quality of Life Inventory or the Stein-Jessop Functional Status Scale.
Measurements and Main Results:
In univariable modeling, critical illness variables associated with death and/or persistent, serious HRQL deterioration were candidates for multivariable modeling using Bayesian Information Criterion. The most clinically relevant multivariable models were selected among models with near optimal statistical fit.
Results:
Three months following septic shock, 346/389 (88.9%) subjects were alive and 43/389 (11.1%) had died; 203/389 (52.2%) had completed paired HRQL surveys. Pediatric Risk of Mortality, cumulative Pediatric Logistic Organ Dysfunction (PELOD) scores, PICU and hospital durations of stay, maximum and cumulative vasoactive-inotropic scores, duration of mechanical ventilation, need for renal replacement therapy, extracorporeal life support or cardiopulmonary resuscitation, and appearance of pathologic neurological signs were associated with adverse outcomes in univariable models. In multivariable regression analysis (OR [95%CI]), summation of daily PELOD scores, 1.01/per point [1.01–1.02], p<0.001; highest vasoactive-inotropic score, 1.02/per point) [1.00–1.04], p=0.003; and any acute pathological neurologic sign/event, 5.04 [2.15–12.01], p<0.001 were independently associated with death or persistent, serious deterioration of HRQL at Month 3.
Conclusions and Relevance:
Biologically plausible factors related to sepsis-associated critical illness organ dysfunction and its treatment were associated with poor outcomes at Month 3 follow-up among children encountering septic shock.
Keywords: septic shock, mortality, health-related quality of life morbidity, critical illness variables, children, organ dysfunction
BACKGROUND
In resource rich settings, in-hospital mortality associated with pediatric sepsis is less than 10% [1–3]. However, a companion article reports that children surviving sepsis remain at significant, enduring risk for mortality as well as significant health-related quality of life (HRQL) morbidity at least one year following hospitalization for septic shock [4]. Accordingly mortality alone no longer adequately describes the overall impact of sepsis on children [5]. Contemporary sepsis definitions stress the importance of life-threatening organ dysfunction [6]. Moreover, critical care provided for children with septic shock typically focuses on avoiding, treating, and hastening resolution of organ dysfunction. In a recent survey investigation, both families of critically ill children and critical care providers chose, after survival, HRQL/functional status and duration of organ dysfunction, as the most personally important outcome measures for a hypothetical interventional trial enrolling critically ill children [7]. Multiple publications have reported the relationship between individual and composite organ dysfunctions and risk for mortality among critically ill children including those with sepsis [8–17].
Recently the Surviving Sepsis Campaign identified, “What are the predictors of sepsis long-term morbidity and mortality?”, as one of the top six clinical sepsis research priorities [18]. Accordingly, Specific Aim 2 of the Life After Pediatric Sepsis Evaluation (LAPSE) prospective, cohort-outcome investigation (R01HD073362) was conducted to determine the association between risk of long-term mortality and persistent, serious HRQL disability with critical illness variables related to treatment of septic shock. LAPSE investigators hypothesized that intensity and duration of critical care for septic shock organ dysfunction would be associated with risk of death or persistent, serious HRQL morbidity 3 months following hospitalization for sepsis.
METHODS
Performance Sites; Study Participants
Details of LAPSE performance sites, study participants including study inclusion and exclusion criteria, and serial assessment of patient functional status and HRQL are provided in the LAPSE companion article [4].
Critical Illness Factors Potentially Associated with Poor Outcomes
Critical illness exposures examined for their association with long term mortality and HRQL morbidity following pediatric septic shock included: chronic comorbid condition classification (Pediatric Medical Complexity Algorithm) [19], immunodeficiency status, initial illness severity per Pediatric Risk of Mortality (PRISM), version IV [20], composite organ dysfunction per the Pediatric Logistic Organ Dysfunction score (PELOD), version 2 [21], mechanical ventilation duration (invasive or non-invasive positive pressure support, excluding high flow nasal cannula oxygen) [22], vasoactive-inotropic support per Vasoactive-Inotropic Score (VIS) [23], receipt of packed erythrocyte transfusion [24], renal replacement therapy (RRT), extracorporeal life support (ECLS), cardiopulmonary resuscitation (CPR), magnitude of acute deterioration of Pediatric Cerebral Performance Category (PCPC) [25], Pediatric Overall Performance Category (POPC) [25], and Functional Status Scale [26] comparing Baseline and Day 7, PICU and hospital durations of stay, and any acute, pathological neurologic sign or event (anisocoria, pathologic breathing pattern, stereotypic or flaccid posture, new seizure activity documented clinically or by electroencephalography, new anoxic-ischemic-reperfusion injury noted on brain imaging, treatment for increased intracranial pressure, and autonomic storming).
Primary Outcome Measure
A secondary aim of the LAPSE investigation was to ascertain the feasibility of a novel primary outcome measure for future pediatric sepsis interventional trials. LAPSE investigators focused on the patient-centered, clinically meaningful, composite outcome of death or persistent, serious deterioration of HRQL as compared to baseline (PSD-HRQL) [27]. A priori, PSD-HRQL was defined as HRQL persisting > 25% below baseline HRQL (before the sepsis event) as assessed 3 months following PICU admission for treatment of septic shock. Percent deterioration, instead of absolute change (number of points) from baseline HRQL was chosen, because children encountering sepsis exhibit a spectrum of baseline HRQL [4].
Participating families completed serial parent-proxy assessments of their child’s HRQL utilizing the Pediatric Quality of Life Inventory (PedsQL™) [28, 29] or the Stein-Jessop Functional Status Scale (FSII-R) [30], as some families of children with severe developmental disabilities reported that the FSII-R instrument better quantified their child’s status. These tools have been compared side-by-side [31]. Both are reliable, valid instruments with internal consistency. PedsQL™ addresses the dimensions of physical, emotional, social and school functioning, while FSII-R addresses eating, sleeping, play behavior and emotional. Scores from either instrument correlate highly with concurrently measured POPC scores [32, 33]. Although the name, FSII-R, suggests this instrument is primarily a functional status measure, in fact, it is generally regarded as a validated measure of general health status for children of all ages (15). Both instruments employ a 0–100 point scale.
Persistent 25% deterioration below baseline can be envisioned for the PedsQL™ instrument with an established minimal clinically important difference (MCID) of 4.5 points [34]: For generally healthy children (about 50% of LAPSE patients) with normative PedsQL™ scores of 82.5±14.9 (mean±SD), a 25% decrease from baseline would be 20.6 points or 1.4 SD or 4.6 MCID. For children with chronic comorbid conditions (about 50% of LAPSE patients) with normative PedsQL™ scores of 71.8±18.4, a 25% decrease from baseline would be 18.0 points or 1.0 SD or 4.0 MCID. For the FSII-R instrument, MCID has not been reported. However, for generally healthy children with normative FSII-R scores of 96.1±8.2, a 25% decrease from baseline would be 24.0 points or 2.9 SD. For children with chronic comorbid conditions and normative FSII-R scores of 86.8±15.7, a 25% decrease from baseline would be 21.7 points or 1.4 SD. For each scenario, a persistent 25% deterioration below baseline HRQL would be significant and serious.
Again with reference to potential future sepsis interventional trials, this report focuses on Month 3, but also provides information related to Month 1 follow-up. While the latter has been a traditional time point for (mortality) outcome assessment in clinical trials, the former represents a more realistic time for long-term assessment of the effect of a septic shock insult, without undue influence of post-discharge external factors that might also impact long-term mortality or HRQL disability. This report provides associations of critical illness variables with the outcomes of mortality, PSD-HRQL and the composite of these two measures as assessed at Month 3.
Data Analysis and Reporting
Descriptive statistics are presented using counts and percentages for categorical variables, and the median and interquartile range for continuous variables. Differences in long-term outcomes were measured using standard statistical tests such as the Wilcoxon rank-sum test, Fisher’s exact test, the Cochran-Armitage test for trend, and the Spearman correlation coefficient with 95% confidence intervals (CI).
Univariable and multivariable logistic regression modeling were used to examine associations with clinical risk factors and adverse long-term outcomes. Variables assessed in >90% of the cohort and at least marginally associated with outcome (p < 0.20) were chosen as candidate predictors in multivariable modeling (eTables 4, 5, and 6). Multivariable models using every possible combination of candidate predictors were systematically constructed for each of the three outcomes as assessed at Month 3. The Bayesian Information Criteria (BIC) was used for model comparisons. Multivariable models of near optimal statistical fit were presented to LAPSE investigators (eTables 7, 8, and 9), from which one model for each outcome was chosen based on clinical relevance for emphasis in text discussions (Table 3). All analyses were performed using SAS 9.4 (SAS Institute; Cary, NC). P-values are based on a two-sided alternative with values of <0.05 considered significant. Results are reported according to STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) Guidelines for cohort studies [35].
TABLE 3.
Selected Multivariable Models for Mortality, PSD-HRQL, and Mortality or PSD-HRQL at Month 3
| Mortality Odds Ratio (95% CI) [P-Value] | PSD-HRQL Odds Ratio (95% CI) [P-Value] | Mortality or PSD-HRQL Odds Ratio (95% CI) [P-Value] | |
|---|---|---|---|
| Δ BIC = 0.6 | Δ BIC = 0.0 | Δ BIC = 0.0 | |
| Variable | Modeling Based on 341 Complete Records | Modeling Based on 201 Complete Records | Modeling Based on 229 Complete Records |
| ∑ Daily PICU PELOD Scores | -------- | 1.01 (1.01, 1.02) [<0.001] | 1.01 (1.01, 1.02) [<0.001] |
| Highest VIS in PICU | -------- | -------- | 1.02 (1.00, 1.04) [0.003] |
| Pathologic Neurologic Signs/Events | |||
| No | -------- | -------- | Reference |
| Yes | -------- | -------- | 5.04 (2.15,12.01) [<0.001] |
| Age (years) | -------- | 1.13 (1.04, 1.23) [0.003] | -------- |
| Sum of VIS in PICU | 1.01 (1.00, 1.01) [<0.001] | -------- | -------- |
| Anisocoria or absence of pupillary response | |||
| No | Reference | -------- | -------- |
| Yes | 5.26 (2.06, 13.20) [<0.001] | -------- | -------- |
Abbreviations: PSD-HRQL, persistent, severe health-related quality of life deterioration, defined as > 25% below baseline health-related quality of life, as assessed at Month 3; BIC, Bayesian information criterion; PICU, pediatric intensive care unit; PELOD, Pediatric Logistic Organ Dysfunction, version 2; VIS, Vasoactive-Inotropic Score; PICU, pediatric intensive care unit
RESULTS
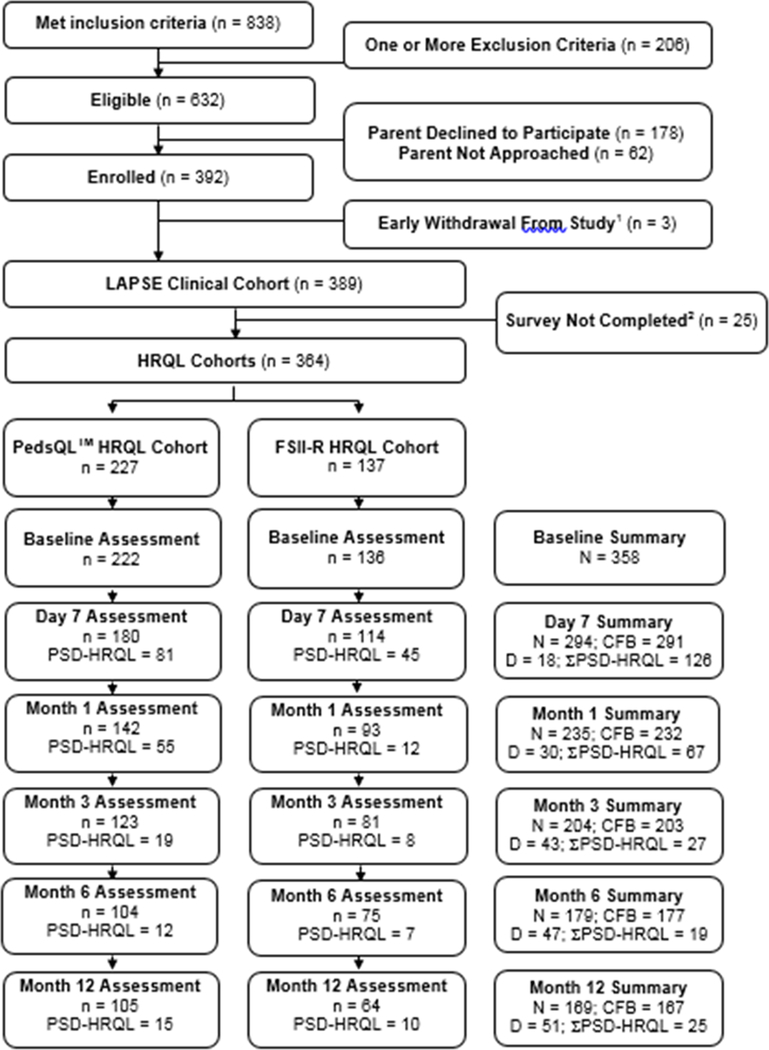
A detailed flow diagram for the LAPSE cohort is presented in Figure 1. At Month 1 following PICU admission for the septic shock event, 259/389 (67%) of the LAPSE participants could be assessed for outcomes. Among these, 30/389 (8%) had died, 232 were alive with complete change-from-baseline HRQL information, and 67/232 (29%) had PSD-HRQL; accordingly death or PSD-HRQL occurred in 95/259 (37%) of evaluable patients at Month 1. At Month 3, 246/389 (63%) of the LAPSE participants could be assessed for outcomes. Among these, 43/389 (11%) had died, 203 were alive with complete change-from-baseline HRQL information, and 27/203 (13%) had PSD-HRQL; accordingly death or PSD-HRQL occurred in 28% of evaluable patients at Month 3. In the interval between Month 1 and Month 3, 13 additional children died, but among evaluable patients, the absolute percent with PSD-HRQL decreased by 16%.
Figure 1.
Detailed flow diagram for the cohort.
Abbreviations: HRQL, health-related quality of life; PedsQLTM, Pediatric Quality of Life Inventory; FSII-R, Stein-Jessop Functional Status Scale; N, total number of patients with available, adequate, change from baseline survey data at a particular time point; CFB, change from baseline; PSD-HRQL, persistent, severe deterioration of HRQL below baseline, specifically, HRQL scores (PedsQLTM or FSII-R) persisting > 25% below the baseline HRQL assessment at follow-up; D, cumulative deaths among the entire LAPSE clinical cohort (n=389). Seven of the 35 patients who died in the hospital did so after the Day 28 study time point. Two subjects where discharged from the hospital alive before Day 28, but died later during the Day 28 time point interval; ΣPSD-HRQL, total patients with persistent, severe deterioration of HRQL below baseline from PedsQLTM or FSII-R cohorts.
1 No clinical data was available due to early family-initiated withdrawal from the study or refusal to complete HRQL surveys.
2 Families never initiated a survey, even the baseline survey, or surveys were inadequately completed and could not be used for analysis.
eTables 1-3 summarize Month 3 participant demographics at PICU admission by HRQL survey status (completed or not completed) and vital status; hospital variables reflecting illness severity, organ dysfunction and resource utilization by HRQL survey completion status and vital status; and PICU admission demographics by outcomes respectively. Families of previously healthy children (without chronic conditions) were less likely to have completed the Month 3 survey (eTable 1). Although PRISM was not different between patients with and without a complete Month 3 survey, higher summation of PELOD and VIS, greater duration of mechanical ventilation, increased need for RRT, ECLS, and CPR, and more frequent occurrence of pathological neurologic signs/events, suggested greater illness severity among patients without a Month 3 survey (eTable 2). Surviving subjects with PSD-HRQL tended to be older and previously healthy (eTable 3).
Table 1 summarizes critical illness related variables reflecting illness severity, organ dysfunction and resource utilization by outcomes at Month 3. Magnitude of deterioration of functional status during the first 7 days of PICU admission, intensity and duration of individual and composite organ dysfunctions, durations of stay in the PICU and hospital, and pathological neurologic signs/events were all associated with mortality or PSD-HRQL. Table 2 summarizes Spearman correlations of change in HRQL from baseline to Month 3, for both PedsQL™ and FSII-R HRQL measures, with various critical illness variables.
TABLE 1.
Critical Illness Related Variables and Outcomes at Month 3
| Patient Characteristic | PSD-HRQL |
Mortality or PDS-HRQL |
||||
|---|---|---|---|---|---|---|
| Yes (N = 27) | No (N = 176) | P-value | Yes (N = 70) | No (N = 176) | P-value | |
| ADMISSION DATA | ||||||
| PRISM1 | 11.0 [6.0, 19.0] | 11.0 [6.0, 16.0] | 0.6484 | 12.0 [7.0, 21.0] | 11.0 [6.0, 16.0] | 0.0294 |
| PELOD, Day 0 | 9.0 [7.0, 14.0] | 7.0 [5.0, 10.0] | 0.0094 | 9.0 [7.0, 14.0] | 7.0 [5.0, 10.0] | <.0014 |
| Immunocompromised | 3 (11.1%) | 29 (16.5%) | 0.5835 | 13 (18.6%) | 29 (16.5%) | 0.7095 |
| PELOD, First Day2 | 9.0 [6.0, 14.0] | 8.0 [6.0, 10.0] | 0.1044 | 11.0 [7.0, 14.0] | 8.0 [6.0, 10.0] | <.0014 |
| HOSPITAL SUMMARY | ||||||
| FSS CFB at Day 7 (>0 implies worsening)3 | 7.5 [4.0, 14.0] | 3.0 [0.0, 8.0] | <.0014 | 10.5 [4.0, 16.0] | 3.0 [0.0, 8.0] | <.0014 |
| PCPC CFB at Day 7 (>0 implies worsening)3 | 1.0 [0.0, 2.0] | 0.0 [0.0, 1.0] | 0.0524 | 2.0 [0.0, 3.0] | 0.0 [0.0, 1.0] | <.0014 |
| POPC CFB at Day 7 (>0 implies worsening)3 | 1.0 [0.0, 3.0] | 0.0 [0.0, 1.0] | 0.0034 | 2.0 [1.0, 3.0] | 0.0 [0.0, 1.0] | <.0014 |
| Sum of PELOD in PICU | 72.0 [52.0, 178.0] | 45.5 [27.0, 74.5] | <.0014 | 91.5 [57.0, 178.0] | 45.5 [27.0, 74.5] | <.0014 |
| PRBC first transfer (relative to Day 0) | 1.0 [0.0, 5.0] | 1.0 [0.0, 2.5] | 0.9004 | 1.0 [0.0, 2.0] | 1.0 [0.0, 2.5] | 0.2604 |
| Hospital length of stay (days) | 28.0 [19.8, 57.9] | 15.0 [9.2, 23.8] | <.0014 | 21.3 [9.5, 47.9] | 15.0 [9.2, 23.8] | 0.0154 |
| PICU length of stay (days) | 14.5 [8.7, 25.4] | 8.1 [4.9, 13.8] | <.0014 | 12.7 [7.9, 24.2] | 8.1 [4.9, 13.8] | <.0014 |
| Highest VIS in PICU | 13.0 [8.0, 25.0] | 10.0 [5.0, 20.0] | 0.1164 | 18.8 [8.0, 30.0] | 10.0 [5.0, 20.0] | <.0014 |
| Highest VIS in PICU | 0.1946 | 0.0026 | ||||
| < 5 | 2 (7.4%) | 29 (16.5%) | 4 (5.7%) | 29 (16.5%) | ||
| 5–30 | 21 (77.8%) | 129 (73.3%) | 50 (71.4%) | 129 (73.3%) | ||
| > 30 | 4 (14.8%) | 18 (10.2%) | 16 (22.9%) | 18 (10.2%) | ||
| Sum of VIS in PICU | 43.0 [27.0, 130.0] | 25.0 [8.0, 57.0] | 0.0054 | 85.5 [25.0, 226.5] | 25.0 [8.0, 57.0] | <.0014 |
| Mechanical ventilator days | 10.0 [7.0, 24.0] | 7.0 [4.0, 11.5] | 0.0054 | 10.5 [6.0, 21.0] | 7.0 [4.0, 11.5] | <.0014 |
| Renal replacement therapy in PICU | 4 (14.8%) | 10 (5.7%) | 0.0975 | 18 (25.7%) | 10 (5.7%) | <.0015 |
| ECLS in PICU | 2 (7.4%) | 5 (2.8%) | 0.2355 | 14 (20.0%) | 5 (2.8%) | <.0015 |
| Anisocoria or absence of pupillary response | 5 (18.5%) | 16 (9.1%) | 0.1685 | 29 (41.4%) | 16 (9.1%) | <.0015 |
| Pathologic breathing pattern | 3 (11.1%) | 16 (9.1%) | 0.7245 | 17 (24.3%) | 16 (9.1%) | 0.0035 |
| Stereotypic posturing or flaccid posture | 3 (11.1%) | 17 (9.7%) | 0.7355 | 15 (21.4%) | 17 (9.7%) | 0.0205 |
| Seizure activity and or abnormal EEG | 4 (14.8%) | 37 (21.0%) | 0.6095 | 23 (32.9%) | 37 (21.0%) | 0.0705 |
| New anoxic-ischemic injury on CT/MRI imaging | 3 (11.1%) | 7 (4.0%) | 0.1335 | 14 (20.0%) | 7 (4.0%) | <.0015 |
| Treatment for increased intracranial pressure | 2 (7.4%) | 2 (1.1%) | 0.0865 | 7 (10.0%) | 2 (1.1%) | 0.0035 |
| Neurologic injury suspected by care provider | 6 (22.2%) | 15 (8.5%) | 0.0415 | 27 (38.6%) | 15 (8.5%) | <.0015 |
| Autonomic storming | 2 (7.4%) | 1 (0.6%) | 0.0475 | 5 (7.1%) | 1 (0.6%) | 0.0085 |
| Cardiopulmonary arrest or chest compressions | 2 (7.4%) | 9 (5.1%) | 0.6435 | 21 (30.0%) | 9 (5.1%) | <.0015 |
| Neurologic insult(s) | 13 (48.1%) | 66 (37.5%) | 0.2985 | 48 (68.6%) | 66 (37.5%) | <.0015 |
Abbreviations: HRQL, health-related quality of life; PSD-HRQL, persistent, serious deterioration of HRQL > 25% below baseline at Month 3; PRISM-IV, Pediatric Risk of Mortality, version IV; PELOD-2, Pediatric Logistic Organ Dysfunction score, version 2; FSS, Functional Status Scale; PCPC, Pediatric Cerebral Performance Category; POPC, Pediatric Overall Performance Category; CFB, change from baseline; PRBC, packed red blood cells; VIS, vasoactive-inotropic support; PICU, pediatric intensive care unit; ECLS, extracorporeal life support; EEG, electroencephalogram; CT/MRI, computerized tomography/magnetic resonance imaging
Collected during a modified 6-hour period of 2 hours prior to PICU admission through 4 hours post PICU admission.
First day is defined as day of admission if admission time is before 12:00 pm or following day if admission is after 12:00 pm.
CFB at Day 7 for PCPC, POPC, and FSS reflect days post ICU admission or hospital discharge whichever occured first.
Wilcoxon rank-sum test.
Fisher’s exact test.
TABLE 2.
Spearman Correlations (rs) of Change in HRQL With Critical Illness Variables
| Severity of Illness Measures | # PedsQL™ | # FSII-R | Δ PedsQL™ Month 3 rs (95% CI) | Δ FSII-R Month 3 rs (95% CI) |
|---|---|---|---|---|
| PRISM-IV | 122 | 81 | −0.11 (−0.282, 0.069) | −0.013 (−0.231, 0.205) |
| PELOD, Day 0 | 122 | 81 | −0.176 (−0.343, 0.002) | 0.012 (−0.207, 0.23) |
| PELOD, First Day | 122 | 81 | −0.142 (−0.312, 0.037) | 0.009 (−0.21, 0.227) |
| Sum of PELOD-2 in PICU | 122 | 81 | −0.276 (−0.433, −0.104) | −0.236 (−0.432, −0.019) |
| PRBC first transfusion (relative to Day 0) | 63 | 32 | 0.071 (−0.18, 0.313) | −0.43 (−0.677, −0.096) |
| Highest VIS in PICU | 122 | 81 | −0.14 (−0.31, 0.038) | 0.013 (−0.206, 0.231) |
| Sum of VIS in PICU | 122 | 81 | −0.187 (−0.353, −0.009) | −0.142 (−0.35, 0.079) |
| Mechanical ventilator days | 122 | 81 | −0.194 [−0.359, −0.016] | −0.207 [−0.407, 0.012] |
| PICU length of stay (days) | 122 | 81 | −0.235 (−0.396, −0.06) | −0.26 (−0.452, −0.044) |
| Hospital length of stay (days) | 122 | 81 | −0.254 (−0.413, −0.08) | −0.227 (−0.424, −0.009) |
| PCPC CFB at Day 28 (>0 is worsening) | 122 | 81 | −0.165 (−0.333, 0.014) | 0.01 (−0.209, 0.228) |
| PCPC CFB at Day 7 (>0 is worsening) | 122 | 81 | −0.188 (−0.354, −0.01) | −0.038 (−0.254, 0.182) |
| POPC CFB at Day 28 (>0 is worsening) | 122 | 81 | −0.284 (−0.44, −0.112) | −0.165 (−0.37, 0.055) |
| POPC CFB at Day 7 (>0 is worsening) | 122 | 81 | −0.251 (−0.41, −0.076) | −0.172 (−0.376, 0.048) |
| FSS CFB at Day 28 (>0 is worsening) | 122 | 81 | −0.368 (−0.513, −0.204) | −0.301 (−0.488, −0.089) |
| FSS CFB at Day 7 (>0 is worsening) | 121 | 80 | −0.259 (−0.418, −0.085) | −0.272 (−0.464, −0.055) |
| PedsQL™ Baseline | 122 | . | −0.531 (−0.647, −0.39) | |
| PedsQL™ CFB at Day 7 | 112 | . | 0.393 (0.223, 0.539) | |
| PedsQL™ CFB at Day 28 | 106 | . | 0.692 (0.577, 0.78) | |
| FSII-R Baseline | . | 81 | −0.532 (−0.672, −0.355) | |
| FSII-R CFB at Day 7 | . | 69 | 0.365 (0.14, 0.554) | |
| FSII-R CFB at Day 28 | . | 65 | 0.563 (0.37, 0.709) |
Abbreviations: rs, Spearman correlation; HRQL, health-related quality of life; PedsQL™, Pediatric Quality of Life Inventory; FSII-R, Stein-Jessop Functional Status Scale; PRISM-IV, Pediatric Risk of Mortality score, version IV; PELOD-2, Pediatric Logistic Organ Dysfunction score, version 2; PICU, pediatric intensive care unit; PRBC, packed red blood cells; VIS, Vasoactive-Inotropic Score; PCPC, Pediatric Cerebral Performance Category; CFB, change from baseline; POPC, Pediatric Overall Performance Category; FSS, Functional Status Scale;
eTables 4, 5, and 6 summarize univariable logistic regression modeling for death, PSD-HRQL, and death or PSD-HRQL as outcomes at Month 3. Initial illness severity (PRISM), first day and cumulative PELOD scores, maximal and cumulative VIS, cumulative MV days, need for RRT, ECLS, and CPR, acute deterioration of functional status (comparing baseline and Day 7), and new pathological neurologic signs/events were most strongly associated with risk for mortality (eTable 4). First day and cumulative PELOD scores, maximal and cumulative VIS, cumulative MV days, and acute deterioration of functional status as well as patient age and duration of PICU and hospital stays were most strongly associated with PSD-HRQL at Month 3 (eTable 5). Initial illness severity (PRISM), first day and cumulative PELOD scores, maximal and cumulative VIS, cumulative MV days, need for RRT, ECLS, and CPR, acute deterioration of functional status, new pathological neurologic signs/events, and PICU duration of stay were most strongly associated with risk for mortality or PSD-HRQL at Month 3 (eTable 6). In general, illness severity measures were negatively correlated with the absolute measures of each individual HRQL assessment. Change in FSS or POPC from baseline to Day 7 or to Day 28 were negatively correlated with HRQL measures at Month 3, and the Day 28 correlations were slightly stronger.
Statistically equivalent multivariable models, with mortality, PSD-HRQL, and mortality or PSD-HRQL at Month 3, as the outcome measure, are provided in eTable 7, eTable 8, and eTable 9. These models highlight the association of single and composite organ dysfunctions during septic shock critical illness with long-term adverse outcomes. Table 3 summarizes clinically relevant multivariable models for mortality, PSD-HRQL, and mortality or PSD-HRQL at Month 3 following PICU admission for septic shock. Risk of mortality at Month 3 was independently associated with cumulative VIS scores and new pathological pupillary activity. It is estimated that for every 1-point increase in the sum of VIS scores, the odds of mortality increases between 0–1 percent, and a new pathological pupillary sign is associated with 5.3 fold increased risk of death. Among children surviving septic shock at Month 3, sum of daily PICU PELOD scores and age were independently associated with increased odds of PSD-HRQL. It is estimated that for every 1-point increase in the sum of daily PICU PELOD scores, the odds of a PSD-HRQL outcome increases between 1–2 percent, while every additional year increases the risk by 4–23 percent. Sum of daily PELOD scores while in the PICU, maximum VIS, and new pathological neurologic signs/events were independently associated with mortality or PDS-HRQL. It is estimated that for every 1-point increase in the sum of daily PICU PELOD scores, the odds of a poor outcome increases between 1–2 percent; that for every 1-point increase in the highest VIS score, the odds of a poor outcome increases between 0–4 percent; and that a new pathological neurologic sign/event during critical care for septic shock increases the odds of poor outcome five-fold.
DISCUSSION
Univariable and multivariable anlyses verify that magnitude and duration of organ dysfunction and need for organ failure rescue (RRT, ECLS, CPR) during treatment of pediatric septic shock highlight risk factors consistently associated with death and/or PSD-HRQL three months following admission to the PICU for a septic shock event. Magnitude of acute functional status deterioration during the first week of sepsis; duration of PICU stay, reflecting the interval of support for dysfunctional organs; and duration of hospitalization, reflecting ongoing acute convalescence following critical illness, were also associated with adverse outcomes. Among children surviving septic shock older age was also associated with risk for PSD-HRQL.
As previously noted, multiple investigations have ascertained a dose-response association of number of dysfunctional organs with risk for death among children with sepsis [8–17]. Similarly, in an adult prospective, multicenter, observational, cohort study, a high Sequential Organ Failure Assessment score was significantly associated with increased risk of death 3 months following admission for septic shock [36]. Dose-response hazard ratios for death from pediatric SIRS, sepsis, severe sepsis and septic shock in relation to severity of sepsis-associated organ dysfunction were validated utilizing the PELOD (organ dysfunction) score [15]. Post hoc analysis of the RESTORE (REsearching severe Sepsis and Organ dysfunction in children: A gLobal perspective) [37] database revealed a strong association between both illness severity (PRISM) and number of organ dysfunctions with poor functional outcome (POPC) at 28 days [38]. Similarly in a sub-investigation of the SPROUT (Sepsis Prevalence, Outcomes, and Therapy) international point prevalence study [39], children with a history of new or progressive multiple organ dysfunction syndrome, exhibited a higher mortality, and among survivors, increased frequency of moderate-to-severe disability at hospital discharge [17].
Supporting the association of the magnitude of vasoactive-inotropic support with adverse outcomes, VIS at 48 hours after PICU admission, was independently associated with short-term outcomes including duration of ventilation and PICU stay, as well as the composite outcome of cardiac arrest/need for ECLS/in-hospital mortality, among children with sepsis requiring vasoactive-inotropic support [23]. Not surprisingly new pathologic neurologic signs/symptoms identified during critical illness for septic shock have previously been noted to be highly associated with poor outcomes [40]. A large retrospective cohort of critically ill children, reported that greater illess severity, longer PICU duration of stay, as well as the rescue interventions of invasive MV, CPR, RRT, and ECLS were associated with acquired global dysfunction and cognitive disability [41].
Other investigations have demonstrated older age as a risk factor for poorer HRQL outcomes compared to population norms among children surving critical illness, including sepsis [42, 43]. This finding may indicate greater resilience among younger children, but might also reflect increasing ability of participant survivors to provide self-report input information to their parents conducting HRQL proxy-reporting. In addition it is possible that rapidly developing infants and toddlers may demonstrate the impact of septic shock on subsequent HRQL by lack of developmentally expected improvement rather than actual decline [43].
Other investigators have emphasized the importance of chronic, comorbid conditions as a risk factor for impaired HRQL following pediatric critical illness [44–46]. Similarly children with chronic conditions surviving sepsis, were reported to be at particularly increased risk for hospital readmission and late mortality [47]. However, in the current investigation, utilizing univariable analyses, medical complexity algorithm category was not strongly associated with adverse outcomes. However, it should be stressed that the current investigation employed paired HRQL assessments in relation to baseline status, that likely affected this lack of association.
LAPSE is the first investigation to identify specific variables encountered by critically ill children with septic shock treated in the PICU, and risk for 3 month mortality and/or PSD-HRQL. Clearly a strength of the current study was quantifying participants’ baseline HRQL and assessing change from baseline for subsequent measures. As detailed in the companion manuscript [4], the primary liability of this examination of critical care variables associated with adverse outcomes following pediatric septic shock, relates to significant, non-random loss of subjects for assessment of HRQL at Month 3 follow-up. However, participants without completed surveys exhibited some measures of higher illness severity, and higher illness severity, associated with higher risk for adverse outcomes, was confirmed with imputation of missing data. The current analysis focused on critical illness variables; certainly factors intrinsic to the individual and environment will also influence long term risk for mortality and/or HRQL disability following septic shock and represent the subject of additional scrutiny of the data set [48]. As presently defined, sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [6]. LAPSE did not primarily examine a “dysregulated host response”, although a subanalysis of selected sepsis host-response biomarkers in relation to risk of poor outcome is in progress [49].
Consistent with previous recommendations [27], data from this investigation establishes the biological plausibility and logistical feasibility of the patient-centered, clinically meaningful, composite outcome, death or PSD-HRQL, at one or three months following PICU admission for pediatric septic shock. At these timepoints this adverse outcome measure occurred in 37% and 28% of patients. To assess an intervention with a relative treatment effect of 25%, α 0.05 and power 0.9, would require 568 or 967 patients in each treatment arm respectively.
CONCLUSIONS
This investigation suggests that the early morbidity of septic shock, reflected as organ dysfunction and the need for PICU supportive care, exemplifies biologically plausible antecedents associated with risk of death and/or PSD-HRQL three months following hospitalization for the septic shock event. Although a good save from septic shock requires that a child resolve sepsis-associated organ dysfunction [1], LAPSE establishes that this achievement alone no longer exemplifies complete sepsis treatment. Specifically, intensity and duration of sepsis-associated organ dysfunction portends ongoing risk for long-term mortality and HRQL disability after children are discharged from the PICU and hospital.
Supplementary Material
ACKNOWLEDGMENTS
The LAPSE Investigators thank all subjects and families for participating in the LAPSE prospective, observational cohort investigation.
Following is a summary of LAPSE Performance Sites, Principal Investigators (PI), Co-investigators (CI), Research Coordinators (RC), and Allied Research Personnel.
Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, PI; Sabrina Heidemann, CI; Ann Pawluszka, RC; Melanie Lulic, RC.
Children’s Hospital of Philadelphia, Philadelphia, PA: Robert A Berg, PI; Athena Zuppa, CI; Carolann Twelves, RC; Mary Ann DiLiberto, RC.
Children’s National Medical Center, Washington, DC: Murray Pollack, PI; David Wessel, PI; John Berger, CI; Elyse Tomanio, RC; Diane Hession, RC; Ashley Wolfe, RC.
Children’s Hospital of Colorado, Denver, CO: Peter Mourani, PI; Todd Carpenter, CI; Diane Ladell, RC; Yamila Sierra, RC; Alle Rutebemberwa, RC.
Nationwide Children’s Hospital, Columbus, OH: Mark Hall, PI; Andy Yates, CI; Lisa Steele, RC; Maggie Flowers, RC; Josey Hensley, RC.
Mattel Children’s Hospital, University of California Los Angeles, Los Angeles, CA: Anil Sapru, PI; Rick Harrison, CI, Neda Ashtari, RC; Anna Ratiu, RC.
Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA: Joe Carcillo, PI; Michael Bell, CI; Leighann Koch, RC; Alan Abraham, RC.
Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA: Patrick McQuillen, PI; Anne McKenzie, RC; Yensy Zetino, RC.
Children’s Hospital of Los Angeles, Los Angeles, CA: Christopher Newth, PI; Jeni Kwok, RC; Amy Yamakawa, RC.
CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Michael Quasney, PI; Thomas Shanley, CI; CJ Jayachandran, RC.
Cincinnati Children’s Hospital, Cincinnati, OH: Ranjit Chima PI; Hector Wong, CI; Kelli Krallman, RC; Erin Stoneman, RC; Laura Benken, RC; Toni Yunger, RC.
St Louis Children’s Hospital, Washington University, St Louis, MO: Alan Doctor, PI; Micki Eaton, RC.
Seattle Children’s Hospital, Seattle Children’s Research Institute (LAPSE Follow-up Center), University of Washington, Seattle, WA: Jerry J Zimmerman, PI; Catherine Chen, RC; Erin Sullivan, RC; Courtney Merritt, RC; Deana Rich, RC; Julie McGalliard; Wren Haaland; Kathryn Whitlock, Derek Salud.
University of Utah (LAPSE Data Coordinating Center), Salt Lake City, UT: J Michael Dean, PI; Richard Holubkov, CI; Whit Coleman, RC; Samuel Sorenson, RC; Ron Reeder; Russell Banks; Angie Webster; Jeri Burr; Stephanie Bisping; Teresa Liu; Emily Stock, Kristi Flick.
Texas A&M University, College Station, TX: James Varni
LAPSE was funded by grant R01HD073362 from the Eunice Kennedy Shriver Institute for Child Health and Development, Pediatric Trauma and Critical Illness Branch. Collaborative Pediatric Critical Care Research Network (CPCCRN) Clinical Centers that participated in LAPSE included the Children’s Hospital of Los Angeles, Children’s Hospital of Michigan, Children’s Hospital of Philadelphia, Children’s Hospital of Pittsburgh, Children’s National Medical Center, Phoenix Children’s Hospital, and the University of Michigan, and were supported by Cooperative Agreements U10-HD050012, U10-HD050096, U10-HD063108, U10-HD049983, U10-HD049981, U10-HD063114, and U10-HD063106, respectively, from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD). The CPCCRN Data Coordinating Center at the University of Utah is supported by Cooperative Agreement U01-HD049934 from the National Institute for Child Health and Human Development (NICHD).
This investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362, and was supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934.
Limited aspects of this manuscript have previously been presented in abstract form at the 2019 Society of Critical Care Medicine Annual Congress
Copyright form disclosure: Dr. Zimmerman’s institution received funding from National Institutes of Child Health and Human Development (NICHD) and Immunexpress, and he received funding from Elsevier Publishing (royalties) and the Society of Critical Care Medicine (travel reimbursements). Drs. Zimmerman, Banks, Berg, Zuppa, Newth, Wessel, Pollack, Meert, Hall, Sapru, Carcillo, McQuillen, Mourani, Wong, Chima, Holubkov, Coleman, Sorenson, Varni, Whitlock, Dean, and Reeder received support for article research from the National Institutes of Health. Dr. Banks’s institution received funding from NICHD/CPCCRN, and he disclosed government work. Dr. Berg, Zuppa, Newth, Wessel, Pollack, Meert, Hall, Sapru, Carcillo, Mourani, Wong, Holubkov, Varni, Whitlock, Dean, and Reeder’s institution received funding from the NIH. Dr. Newth received funding from Philips Research North America and Hamilton Medical AG. Dr. McQuillen’s institution received funding from the NICHD UG1 HD083166. Dr. Holubkov received funding as a DSMB member from Pfizer, Medimmune, and Revance, and funding from biostatistical consulting for Physicians Committee for Responsible Medicine and DURECT Corporation. Dr. Coleman’s institution received funding from Seattle Children’s. Dr. Sorenson’s institution received funding from Seattle Children’s Research Institute. Dr. Varni disclosed that he holds the copyright and the trademark for the PedsQL and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory. Dr. McGalliard disclosed work for hire.
Footnotes
No performance site investigators disclose financial interests, activities, relationships, or affiliations that could be construed as real or potential conflicts of interest related to the manuscript or the related investigation. Dr. James W Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™. Dr Varni provided consultation on original study design and final manuscript edits, but played no role in data acquisition or analysis.
No reprints will be ordered for this article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 2.Ruth A, McCracken CE, Fortenberry JD, et al. : Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15:828–838 [DOI] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman JJ, Banks R, Berg RA, et al. : Trajectory of mortality and health related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med 2019; xx:zzz-zzz [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon DW, Clark RS, Watson RR: No pain, no gain in pediatric sepsis?. Pediatr Crit Care Med 2014; 15:264–266 [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merritt C, Menon K, Agus MSD, et al. : Beyond Survival: Pediatric Critical Care Interventional Trial Outcome Measure Preferences of Families and Healthcare Professionals. Pediatr Crit Care Med 2018; 19:e105–e111 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson JD, Pollack MM, Glass NL, et al. : Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr 1987; 111:324–328 [DOI] [PubMed] [Google Scholar]
- 9.Proulx F, Gauthier M, Nadeau D, et al. : Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med 1994; 22:1025–1031 [DOI] [PubMed] [Google Scholar]
- 10.Duke TD, Butt W, South M: Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med 1997; 23:684–692 [DOI] [PubMed] [Google Scholar]
- 11.Doughty L, Clark RS, Kaplan SS, et al. : sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res 2002; 52:922–927 [DOI] [PubMed] [Google Scholar]
- 12.Kutko MC, Calarco MP, Flaherty MB, et al. : Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med 2003; 4:333–337 [DOI] [PubMed] [Google Scholar]
- 13.Leteurtre S, Martinot A, Duhamel A, et al. : Validation of the Paediatric Logistic Organ Dysfunction (PELOD) Score: prospective, observational, multicentre study. Lancet 2003; 362(9379):192–197 [DOI] [PubMed] [Google Scholar]
- 14.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. : The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003; 167:695–701 [DOI] [PubMed] [Google Scholar]
- 15.Leclerc F, Leteurtre S, Duhamel A, et al. : Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med 2005; 171:348–353 [DOI] [PubMed] [Google Scholar]
- 16.Typpo KV, Petersen NJ, Hallman DM, et al. : Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med 2009; 10:562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JC, Spinella PC, Fitzgerald JC, et al. : New or Progressive Multiple Organ Dysfunction Syndrome in Pediatric Severe Sepsis: A Sepsis Phenotype With Higher Morbidity and Mortality. Pediatr Crit Care Med 2017; 18:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coopersmith CM, De Backer D, Deutschman CS, et al. : Surviving Sepsis Campaign: Research priorities for sepsis and septic shock. Crit Care Med 2018; 46:1334–1356 [DOI] [PubMed] [Google Scholar]
- 19.Simon TD, Cawthon ML, Stanford S, et al. : Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014; 133:e1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Funai T, et al. : The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med 2016, 17(1):2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leteurtre S, Duhamel A, Salleron J, et al. : PELOD-2: an update of the Pediatric Logistic Organ Dysfunction Score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 22.Khemani RG, Thomas NJ, Venkatachalam V, et al. : Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 2012; 40:1309–1316 [DOI] [PubMed] [Google Scholar]
- 23.McIntosh AM, Tong S, Deakyne SJ, et al. : Validation of the Vasoactive-Inotropic Score in pediatric sepsis. Pediatr Crit Care Med 2017; 18:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacroix J, Hebert PC, Hutchison JS, et al. : Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007; 356:1609–1619 [DOI] [PubMed] [Google Scholar]
- 25.Fiser DH, Long N, Roberson PK, et al. : Relationship of Pediatric Overall Performance Category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000; 28:2616–2620 [DOI] [PubMed] [Google Scholar]
- 26.Pollack M, Holubkov R, Glass P, et al. : The Functional Status Score (FSS): A new pediatric outcome measure. Pediatrics 2009; 124:e18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson GA: Mathematical morbidity in paediatric intensive care. Lancet 2003; 362(9379):180–181 [DOI] [PubMed] [Google Scholar]
- 28.Varni JW, Limbers CA, Burwinkle TM: Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varni JW, Limbers CA, Neighbors K, et al. : The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011; 20:45–55 [DOI] [PubMed] [Google Scholar]
- 30.Stein RE, Jessop DJ: Functional status II(R). A measure of child health status. Med Care 1990; 28:1041–1055 [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Kurtin PS: Pediatric health-related quality of life measurement technology: A guide for health care decision makers. J Clin Outcomes Manag 1999. 6:33–40 [Google Scholar]
- 32.Abecassis IJ, Nerva JD, Barber J, et al. : Toward a comprehensive assessment of functional outcomes in pediatric patients with brain arteriovenous malformations: the Pediatric Quality of Life Inventory. J Neurosurg Pediatr 2016; 18:611–622 [DOI] [PubMed] [Google Scholar]
- 33.Keenan HT, Runyan DK, Nocera M: Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 2006; 117:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varni JW, Burwinkle TM, Seid M, et al. : The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3:329–341 [DOI] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–349 [DOI] [PubMed] [Google Scholar]
- 36.Pavon A, Binquet C, Kara F, et al. : Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit Care Med 2013; 41:2600–2609 [DOI] [PubMed] [Google Scholar]
- 37.Nadel S, Goldstein B, Williams MD, et al. : Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 2007; 369(9564):836–843 [DOI] [PubMed] [Google Scholar]
- 38.Farris RW, Weiss NS, Zimmerman JJ: Functional outcomes in pediatric severe sepsis: further analysis of the Researching Severe Sepsis and Organ Dysfunction in Children: A Global Perspective trial. Pediatr Crit Care Med 2013; 14:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuler A, Wulf DA, Lu Y, et al. : The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med 2018; 46:843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bone MF, Feinglass JM, Goodman DM: Risk factors for acquiring functional and cognitive disabilities during admission to a PICU. Pediatr Crit Care Med 2014; 15:640–648 [DOI] [PubMed] [Google Scholar]
- 42.Morrison AL, Gillis J, O’Connell AJ, et al. : Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med 2002; 3:1–5 [DOI] [PubMed] [Google Scholar]
- 43.Killien EY, Farris RWD, Watson RS, et al. : Health related quality of life among survivors of pediatric sepsis. A retrospective cohort study. Pediatr Crit Care Med 2019; 20:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choong K, Fraser D, Al-Harbi S, et al. : Functional recovery in critically ill children, the “WeeCover” multicenter study. Pediatr Crit Care Med 2018; 19:145–154 [DOI] [PubMed] [Google Scholar]
- 45.Kyosti E, Ala-Kokko TI, Ohtonen P, et al. : Factors associated with health-related quality of life 6 years after ICU discharge in a Finnish paediatric population: a cohort study. Intensive Care Med 2018, 44:1378–1387 [DOI] [PubMed] [Google Scholar]
- 46.Griffith DM, Salisbury LG, Lee RJ, et al. : Determinants of health-related quality of life after ICU: Importance of patient demographics, previous comorbidity, and severity of illness. Crit Care Med 2018; 46:594–601 [DOI] [PubMed] [Google Scholar]
- 47.Czaja AS, Zimmerman JJ, Nathens AB: Readmission and late mortality after pediatric severe sepsis. Pediatrics 2009; 123:849–857 [DOI] [PubMed] [Google Scholar]
- 48.Wilson IB, Cleary PD: Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995; 273:59–65 [PubMed] [Google Scholar]
- 49.Wong HR, Cvijanovich NZ, Anas N, et al. : Pediatric Sepsis Biomarker Risk Model-II: Redefining the Pediatric Sepsis Biomarker Risk Model with septic shock phenotype. Crit Care Med 2016; 44:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.