Abstract
The aims of the present study were to determine (i) the profiles of phylogroup and (ii) the antimicrobial susceptibility of pathogenic Escherichia coli strains isolated from calves, and of Salmonella spp. strains isolated from calves and pigs in Minas Gerais State, Brazil. Sixty-one pathogenic E. coli strains and Salmonella spp. (n = 24) strains isolated from fecal samples of calves and Salmonella spp. (n = 39) strains previously isolated from fecal samples of growing/finishing pigs were tested. The minimum inhibitory concentration (MIC) using the agar dilution method was determined for nalidixic acid, amikacin, amoxicillin, ampicillin, cefoxitin, norfloxacin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole. All E. coli isolates were susceptible to amikacin. Tetracycline was the antimicrobial that presented the higher frequency of resistance among E. coli strains, followed by ampicillin, trimethoprim-sulfamethoxazole, amoxicillin, nalidixic acid, norfloxacin, gentamicin, and cefoxitin. E. coli (n = 61) strains isolated from calves belonged to different phylogroup namely, phylogroup A (n = 26), phylogroup B1 (n = 31), phylogroup E (n = 3), and phylogroup F (n = 1). Phylogroups B2, C, and D were not identified among the E. coli in the present study. All Salmonella spp. (n = 24) strains isolated from fecal samples of calves were susceptible to amikacin, amoxicillin, ampicillin, norfloxacin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole. Resistance to nalidixic acid and cefoxitin was detected in 16.66 and 8.33 % of the Salmonella spp. strains, respectively. Among the Salmonella spp. (n = 39) strains isolated from fecal samples of pigs, the higher frequency of resistance was observed to tetracycline, followed by amoxicillin, gentamicin, ampicillin, trimethoprim-sulfamethoxazole, nalidixic acid, cefoxitin, and norfloxacin. All strains were susceptible to amikacin. Forty-eight (78.68 %) of the E. coli strains were classified as multidrug-resistant, whereas among Salmonella spp. strains, the percentage of multidrug resistance was 57.14 %, being all multidrug-resistant strains isolated from pigs (92.30 %). The results from the present study indicate a high frequency of antimicrobial resistance among pathogenic E. coli strains isolated from calves and Salmonella spp. strains isolated from pigs and a high rate of susceptibility to most antimicrobials tested among Salmonella spp. strains isolated from calves. Our study highlights the presence of multidrug-resistant strains of E. coli and Salmonella spp. isolated from food-producing animals in Minas Gerais, Brazil.
Electronic supplementary material
The online version of this article (doi:10.1007/s11250-016-1152-0) contains supplementary material, which is available to authorized users.
Keywords: Salmonella, Escherichia coli, Antimicrobial resistance, Calves, Pigs
Introduction
Diarrhea accounts for more than half of all mortality of calves, being one of the most common and probably one of the most important diseases of young cattle (Foster and Smith 2009). In pigs, diarrheal diseases are also responsible for high morbidity and mortality (Laine et al. 2008). For both species, neonatal enteric bacterial infections have a great impact on future performance, besides being often treated with antimicrobials (Laine et al. 2008; Foster and Smith 2009).
The most common pathogens associated with diarrhea in calves are rotavirus, coronavirus, Salmonella spp., and diarrheogenic Escherichia coli (Blanchard 2012). Diarrhea caused by E. coli has been identified as an important disease of young cattle, responsible for great economic losses (Kolenda et al. 2015). E. coli is a component of normal intestinal microbiota of calves; however, its phenotypic and genotypic characteristics allow the identification of pathogenic strains or pathovars (Croxen et al. 2013). Different pathovars cause diarrhea in calves, such as Enterotoxigenic E. coli (ETEC), Enterohaemorrhagic E. coli (EHEC), Shiga toxin-producing E. coli (STEC), Enteropathogenic E. coli (EPEC), and Necrotoxigenic E. coli (NTEC) (Moxley and Smith 2010; Coura et al. 2014, 2015a; Kolenda et al. 2015).
E. coli strains can also be classified in phylogenetic groups (Croxen et al. 2013), which are not randomly dispersed and can be associated with the source of infection (Clermont et al. 2013; Coura et al. 2015c). Phylogenetic characterization is an important tool to improve the understanding of E. coli population and the relation among strains and disease (Tenaillon et al. 2010); however, only few studies have been performed to identify the phylogenetic groups of E. coli isolated from calf feces (Tramuta et al. 2008; Salvarani et al. 2012).
Salmonella sp. is also one of the major pathogens associated with enteric diseases in animal production (Brenner 2000). The different clinical manifestations of salmonellosis include diarrhea, abortion, pneumonia, septic arthritis, meningitis, gangrene of distal extremities, and others, which are associated with the virulence of the serotypes, infectious dose, and host immunity (Mohler et al. 2009; Nielsen 2013; Coura et al. 2015b). Salmonella spp. serotypes can be host adapted, such as bovine S. Dublin and swine S. Choleraesuis, or non-host-adapted, such as S. Typhimurium (Mohler et al. 2009; Nielsen 2013).
Many Salmonella spp. serotypes can infect cattle; Typhimurium and Dublin serotypes are the most common (Mohler et al. 2009). S. Typhimurium is frequently associated with enteric disease in calves less than 2 months of age, and S. Dublin is associated with young and adult cattle and is more invasive than S. Typhimurium (Mohler et al. 2009). Clinical salmonellosis in pigs generally results in septicemia caused by host-restricted serotypes such as S. Choleraesuis and enterocolitis caused by broad host-range serotypes mainly S. Typhimurium. Weaned pigs intensively reared are most frequently affected by Salmonella spp. infections, although animals in other phases may also be affected; however, in pig farms, Salmonella spp. infections without clinical signs are more common than the clinical disease (Barrow et al. 2010).
In Minas Gerais, Brazil, although only a few reports studied pathogenic E. coli, infection frequencies as high as 59.25 % were observed in young calves (Lage et al. 1993; Andrade et al. 2012). Moreover, in the same region, frequency of Salmonella spp. infection was reported to be 16.4 % in calves and 6.52 % in growing and finishing pigs (Viott et al. 2013; Coura et al. 2015a).
Additionally to the importance of E. coli and Salmonella spp. infections in animals, both bacteria are food-borne pathogens. Food-producing animals represent an important source of EHEC in the food chain (Martin and Beutin 2011). Cattle and other ruminants are the natural reservoir of STEC/EHEC, and although not all pathovars of E. coli are of important public health concern, E. coli has a great genetic diversity and the potential to cause disease (Croxen et al. 2013). Furthermore, S. enterica serotypes are one of the most important foodborne pathogens, resulting in enteric disease, hospitalization, and deaths worldwide (Hur et al. 2012). Salmonella sp. has been identified in all links of the pork production chain (Rostagno and Callaway 2012) and in zoonotic outbreaks associated with dairy farms (Mateus et al. 2008). Moreover, the historical and growing emergence of drug resistance among E. coli and Salmonella spp. strains isolates from humans and animals has increased the debate on public health hazard associated with the use of antibiotics in animal production (Tadesse et al. 2012; Hur et al. 2012; Rostagno and Callaway 2012; Keelara et al. 2013).
Thus, due to the importance of E. coli and Salmonella spp. infections to animal production and public health, the aims of the present study were to determine (i) the profiles of phylogroup and (ii) the antimicrobial susceptibility of pathogenic E. coli strains isolated from calves, and the antimicrobial susceptibility of Salmonella spp. strains isolated from calves and pigs in Minas Gerais State, Brazil.
Materials and methods
Escherichia coli and Salmonella spp. strains and culture conditions
Sixty-one pathogenic E. coli strains were tested. These strains were previously isolated (Andrade et al. 2012) from fecal samples of calves up to 60 days old at 12 dairy farms in Minas Gerais, Brazil, in 2010, and the pathotypes of E. coli were identified by means of a multiplex PCR based on Franck et al. (1998) (Andrade et al. 2012). The pathotypes identified were STEC (n = 36), EHEC (n = 12), ETEC (n = 5), EPEC (n = 1), and others (n = 7) (Andrade et al. 2012).
Twenty-four Salmonella spp. strains isolated from fecal samples of calves up to 90 days of age in 2008 (Coura et al. 2015a) and Salmonella spp. (n = 39) strains isolated from fecal samples from growing/finishing pigs between January 2008 and February 2009 (Viott et al. 2013) in the State of Minas Gerais, Brazil, were also tested. Salmonella spp. isolates were serotyped at the Salmonella Reference Laboratory in the Instituto Oswaldo Cruz/Fundação Oswaldo Cruz (Rio de Janeiro, RJ, Brazil). The serotypes identified among the Salmonella strains isolated from calves were Agona (n = 16), Typhimurium (n = 4), Enteritidis (n = 2), and S. enterica subsp. enterica (n = 2) (Coura et al. 2015a). The serotypes identified among strains isolated from pigs were Typhimurium (n = 32), Agona (n = 5), and S. enterica subsp. enterica (n = 2) (Viott et al. 2013).
E. coli and Salmonella spp. isolates were cultured in MacConkey agar (Difco, USA) and incubated for 18–24 h at 37 °C under aerobic conditions (Quinn et al. 1994).
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) was determined using agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) M07-A9 manual (CLSI 2012a, 2012b) for nalidixic acid (Sigma-Aldrich, Saint Louis, USA), amikacin (Sigma-Aldrich, USA), amoxicillin (Sigma-Aldrich, USA), ampicillin (Sigma-Aldrich, USA), cefoxitin (Fluka, USA), norfloxacin (Fluka, USA), gentamicin (Sigma-Aldrich, USA), tetracycline (Sigma-Aldrich, USA), sulfamethoxazole (Sigma-Aldrich, USA), and trimethoprim (Sigma-Aldrich, USA) (19 parts of Sulfamethoxazole to 1 part Trimethoprim) in 14 twofold dilutions from 0.03125 to 256 μg/mL. Briefly, Mueller-Hinton agar (Difco, USA) plates plus the antimicrobial concentrations tested were inoculated with bacterial suspensions adjusted to turbidity equivalent to a 0.5 McFarland standard and incubated for 24 h at 37 °C (M07-A9, CLSI 2012a).
MIC determination was performed in duplicated. All antibiotics were tested with the reference strains: E. coli ATCC 25922, E. coli ATCC 35918, Enterococcus faecalis ATCC 29212, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 29213 to ensure that the results were within acceptable limits of quality control for susceptibility testing according to CLSI document M100-S22 (CLSI 2012b). In all assays, Mueller-Hinton agar plates without antibiotics were used as growth control at the beginning (two plates) of the antibiotic plating sequence and at the end of this sequence (two plates).
MIC50 and MIC90 levels were defined as the lowest concentration of the antibiotic at which 50 and 90 % of the strains were inhibited, respectively. Strains were classified as resistant, intermediate, or sensitive to antimicrobials according to CLSI manual M100-S22 (CLSI 2012b).
Multidrug resistance was defined as resistance to three or more antimicrobial groups (Magiorakos et al. 2012). The antimicrobial groups were as follows: (i) quinolones (nalidixic acid and norfloxacin), (ii) aminoglycosides (amikacin and gentamicin), (iii) β-lactams (amoxicillin), (iv) penicillin (ampicillin), (v) cephalosporin (Cefoxitin), (vi) tetracycline (tetracycline), and (vii) sulfonamides (sulfamethoxazole).
Phylogenetic group determination of E. coli strains
Pathogenic E. coli strains were tested by PCR for characterization of phylogenetic groups A, B1, B2, C, D, E, and F according to Clermont et al. (2013).
Statistical analysis
Correspondence analysis (Greenacre and Blasius 2006) was used to study the relationship between E. coli phylogroups, E. coli pathotypes, Salmonella serovars, and the antimicrobial susceptibility. In the correspondence analyses, the relationship between the categories was represented in a two-dimensional graph and their relatedness was demonstrated by evaluating which variables were plotted closely together.
Results
Antimicrobial susceptibility and phylotyping of pathogenic E. coli strains isolated from diarrheic calves
The MIC range, MIC50, and MIC90 found for the 61 pathogenic E. coli strains studied are shown in Fig. 1. All E. coli isolates were susceptible to amikacin. Tetracycline was the antimicrobial that presented the higher percentage of resistance among E. coli strains, with 91.80 % (56/61) of resistance strains, followed by ampicillin [75.41 % (46/61)], trimethoprim-sulfamethoxazole [67.21 % (41/61)], amoxicillin [63.93 % (39/61)], nalidixic acid [54.09 % (33/61)], norfloxacin [21.31 % (13/61)], gentamicin [16.39 % (10/61)], and cefoxitin [8.19 % (5/61)]. STEC (n = 36) were 100 % susceptible to amikacin but showed higher percentage of resistance to tetracycline [91.06 % (33/36)], followed by ampicillin [75 % (27/36)], trimethoprim-sulfamethoxazole [69.14 % (25/36)], amoxicillin [61.11 % (22/36)], nalidixic acid [38.88 % (14/36)], norfloxacin [22.22 % (8/36)], gentamicin [16.66 % (6/36)], and cefoxitin [2.77 % (1/36)]. EHEC (n = 12) strains were 100 % sensitive to amikacin, cefoxitin, norfloxacin, and gentamicin, while ETEC (n = 5) and EPEC (n = 1) strains were 100 % susceptible to amikacin and gentamicin (Fig. 1).
Fig. 1.

Scatter plot of minimal inhibitory concentrations (MIC) determined by the agar dilution method to nalidixic acid (NA), amikacin (AMK), amoxicillin (AMX), ampicillin (AMP), cefoxitin (CFX), norfloxacin (NRF), gentamicin (GEN), tetracycline (TET), and trimethoprim-sulfamethoxazole (SMX) of pathogenic E. coli strains [STEC (n = 36), EHEC (n = 12), ETEC (n = 5), EPEC (n = 1), and other pathovars (n = 7)] isolated from fecal samples of calves in Minas Gerais State, Brazil. Resistant strains are indicated in red and intermediate susceptibility profile in yellow. Ellipses indicate the MIC50 for each antimicrobial agent, while dotted rectangles indicate the MIC90. ETEC Enterotoxigenic E. coli, EHEC Enterohaemorrhagic E. coli, STEC Shiga toxin-producing E. coli, EPEC Enteropathogenic E. coli, Others other pathovars
E. coli (n = 61) strains isolated from calves belonged to different phylogroup namely phylogroup A [42.63 % (26/61)], phylogroup B1 [50.81 % (31/61))], phylogroup E [4.92 % (3/61)], and phylogroup F [1.64 % (1/61)]. Phylogroups B2, C, and D were not identified. Among phylogroup A strains, the higher percentage of resistance was observed to tetracycline [96.15 % (25/26)], followed by ampicillin [84.61 % (22/26)], amoxicillin [65.36 % (17/26)], trimethoprim-sulfamethoxazole [61.53 % (16/26)], nalidixic acid [30.76 % (8/26)], gentamicin [11.53 % (3/26)], and norfloxacin [3.84 % (1/26)]. All phylogroup A strains were susceptible to amikacin and cefoxitin (Fig. 2).
Fig. 2.
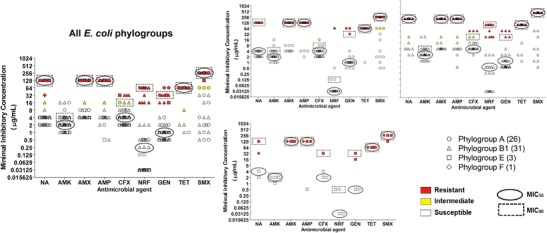
Scatter plot of the minimal inhibitory concentrations (MIC) determined by the agar dilution method to nalidixic acid (NA), amikacin (AMK), amoxicillin (AMX), ampicillin (AMP), cefoxitin (CFX), Norfloxacin (NRF), gentamicin (GEN), tetracycline (TET), and trimethoprim-sulfamethoxazole (SMX) of E. coli strains of phylogroups A (n = 26), B1 (n = 31), E (n = 3), and F (n = 1) isolated from fecal samples of calves in Minas Gerais State, Brazil. Resistant strains are indicated in red and intermediate susceptibility profile in yellow. Ellipses indicate the MIC50 for each antimicrobial agent, while dotted rectangles indicate the MIC90
The antibiotic with the lowest activity against phylogroup B1 was tetracycline with 87.09 % (27/31) of resistant strains, followed by nalidixic acid [74.19 % (23/31)], trimethoprim-sulfamethoxazole [70.96 % (22/31)], ampicillin [67.74 % (21/31)], amoxicillin [58.06 % (18/31)], gentamicin [16.12 % (5/31)], and cefoxitin [9.67 % (3/31)] (Fig. 2).
Only three strains of phylogroup E were detected, all susceptible to amikacin and norfloxacin. Resistance to amoxicillin and tetracycline was detected in 100 % of the strains, while resistance to ampicillin and gentamicin was detected in 66.66 % (2/3), and resistance to nalidixic acid and cefoxitin in 33.33 % (1/3) of the tested strains (Fig. 2).
One phylogroup F E. coli was detected, being classified as susceptible to amikacin, cefoxitin, norfloxacin, and gentamicin, and resistance to nalidixic acid, amoxicillin, ampicillin, tetracycline, and trimethoprim-sulfamethoxazole (Fig. 2).
Correspondence analysis was performed to evaluate the relationship between the antimicrobial susceptibility profile, pathotype, and phylogroup of E. coli. In correspondence analysis, it was not observed association between antibiotic susceptibility and pathovars or phylogroups. The cumulative chi-square was considered low, the three dimensions explains 38.32 % of the total variation, with 16.32 % explained by first dimension, 12.52 % by the second dimension, and 9.48 % by the third dimension (Online Research 1).
Antimicrobial susceptibility and serotyping of Salmonella spp. strains isolated from diarrheic calves and pigs
The serotypes of Salmonella spp. isolated from calves were S. Agona (16/24), S. Enteritidis (4/24), S. Typhimurium (2/24), and S. enterica subsp. enterica (2/24). The serotypes of Salmonella spp. obtained from feces of pigs were S. Typhimurium (32/39), S. Agona (5/39), and S. enterica subsp. enterica (2/39). The MIC range, MIC50, and MIC90 found for the Salmonella strains isolated from fecal samples of calves (n = 24) and for the Salmonella spp. strains isolated from fecal samples of pigs (n = 39) are shown in Fig. 3.
Fig. 3.

Scatter plot of the minimal inhibitory concentrations (MIC) determined by the agar dilution method to nalidixic acid (NA), amikacin (AMK), amoxicillin (AMX), ampicillin (AMP), cefoxitin (CFX), norfloxacin (NRF), gentamicin (GEN), tetracycline (TET), and trimethoprim-sulfamethoxazole (SMX) of Salmonella spp. (n = 24) strains isolated from fecal samples of calves and the Salmonella spp. (n = 39) strains isolated from fecal samples of pigs in Minas Gerais State, Brazil. Resistant strains are showed in red and intermediate susceptibility profile in yellow. Ellipses indicate the MIC50 for each antimicrobial agent, while dotted rectangles indicate the MIC90
All Salmonella spp. (n = 24) strains isolated from fecal samples of calves were susceptible to amikacin, amoxicillin, ampicillin, norfloxacin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole. Resistance to nalidixic acid and cefoxitin were detected in 16.66 % (4/24) and 8.33 % (2/24), respectively, of the strains isolated from calves. Moreover, Salmonella spp. strains isolated from calves also exhibited intermediate susceptibility to amikacin [4.16 % (1/24)], cefoxitin [20.83 % (5/24)], and tetracycline [8.33 % (2/24)].
Among Salmonella spp. (n = 39) strains isolated from fecal samples of pigs, the higher percentage of resistance was observed to tetracycline [97.43 % (38/39)], followed by amoxicillin [89.74 % (35)], gentamicin [87.17 % (34/39)], ampicillin [82.05 % (32/39)], trimethoprim-sulfamethoxazole [53.84 % (21/39)], nalidixic acid [33.33 % (13/39)], cefoxitin [2.56 % (1/39)], and norfloxacin [2.56 % (1/39)]. All strains were susceptible to amikacin and one strain (2.56 %) showed intermediate susceptibility to trimethoprim-sulfamethoxazole.
The correspondence analysis was performed using the antimicrobial susceptibility profile and Salmonella spp. serotype from calves and pigs. Analysis of Salmonella spp. from calves showed that S. enterica subsp. enterica strains were associated to intermediate resistance to amikacin and S. Enteritidis appears to be related to resistant to nalidixic acid, whereas S. Agona was associated to susceptibility to all tested antibiotics. The representation of the two dimensions and expression of the values of the third dimension are shown in Fig. 4. Those three dimensions explain 65.64 % of the total variation, with 27.02 % explained by first dimension, 21.75 % by the second dimension, and 16.87 % by the third dimension. Regarding to Salmonella spp. strains isolated from pigs, the correspondence analysis showed that S. enterica subsp. enterica appears to be related to sensitive to ampicillin, gentamycin, and amoxicillin, whereas S. Typhimurium was associated to resistance to most tested antimicrobials (ampicillin, gentamicin, tetracycline, norfloxacin, and cefoxitin) and sensitivity to nalidixic acid. The representation of the two dimensions and expression of the values of the third dimension are shown in Fig. 4. Those three dimensions explain 58.20 % of the total variation, with 32.12 % explained by first dimension, 13.31 % by the second dimension, and 12.76 % by the third dimension.
Fig. 4.
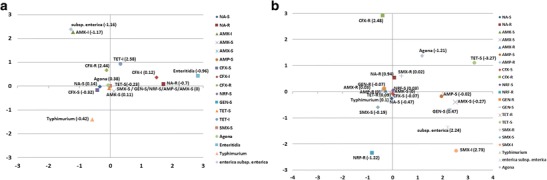
Correspondence analysis of the relationship between the antimicrobial susceptibility profile and Salmonella spp. serotypes: a isolates from calves and b isolates from pigs. The correspondence analysis dimensional representation is interpreted by considering which categories are plotted closely together. N alidixic acid (NA), amikacin (AMK), amoxicillin (AMX), ampicillin (AMP), cefoxitin (CFX), norfloxacin (NRF), gentamicin (GEN), tetracycline (TET), trimethoprim-sulfamethoxazole (SMX), resistant (R), intermediate (I), susceptible (S)
The susceptibility profile of tested E. coli, Salmonella spp. isolated from calves, and Salmonella spp. isolated from pigs to the nine antimicrobials is shown in Fig. 5. Classification into susceptibility profiles was created for grouping strains with similar susceptibilities to antimicrobials and then facilitates the identification of the number of strains with resistant, intermediate, and sensitive profiles. Strains resistant to three or more antimicrobial groups were considered multidrug-resistant (Magiorakos et al. 2012). Forty-eight [78.68 % (48/61)] E. coli strains were classified as multidrug-resistant, whereas among Salmonella spp. strains, the percentage of multidrug-resistant strains was 57.14 % (36/63), being all multidrug-resistant strains isolated from pigs [92.30 % (36/39)].
Fig. 5.

Antimicrobial susceptibility profile of pathogenic E. coli a isolated from calves and Salmonella spp. strains isolated from calves (b) and pigs (c) in Minas Gerais State, Brazil. Resistant (black), intermediate (dark gray), susceptible (light gray). N alidixic acid (NA), amikacin (AMK), amoxicillin (AMX, ampicillin (AMP), cefoxitin (CFX), norfloxacin (NRF), gentamicin (GEN), tetracycline (TET), trimethoprim-sulfamethoxazole (SMX)
Discussion
Antimicrobial agents are indispensable for decreasing mortality and morbidity associated with infectious diseases in animals and humans (Tadesse et al. 2012). In veterinary medicine, they have been used for therapy, metaphylaxis, prophylaxis, and growth promotion (Schwarz et al. 2001), being the enteric diseases one of the main animal infections treated with antibiotics (Teuber 2001). Nonetheless, the extensive use of antimicrobial agents in animals as well as in humans has encouraged the appearance of antimicrobial-resistant bacteria (Hur et al. 2012). Emergence of resistant and multidrug-resistant pathogens among animal isolates is a major public health concern, since some antibiotics used in animal production are also used in treatment of human infections and several animal pathogens are zoonotic (Landers et al. 2012). Therefore, in the present study, we investigated the susceptibility profile of E. coli isolated from feces of calves and Salmonella isolated from feces of calves and pigs to nine antimicrobial agents and observed that tetracycline, ampicillin, and trimethoprim-sulfamethoxazole were the antibiotics with lowest activity against E. coli from calves and Salmonella spp. strains from pigs, whereas a highest rate of susceptible strains were found among Salmonella spp. isolates from calves.
Assessment of the antimicrobial susceptibility profile among the different E. coli pathovars and phylogroups did not show any association among the variables (Online research 1). This could be the result of the absence of relationship among those characteristics or even due to the low representativeness of some of E. coli groups assessed. However, considering all pathogenic E. coli studied, some important conclusions can be drawn. Pathogenic E. coli strains isolated from dairy calves in Minas Gerais, Brazil, in 2010, exhibited high rates of resistance to tetracycline, ampicillin, and trimethoprim-sulfamethoxazole, whereas the highest rate of susceptible strains were found to amikacin, cefoxitin, and gentamicin (Fig. 1). Similarly, high resistance of E. coli isolated from calves to tetracycline, ampicillin, and sulfamethoxazole has been observed in other countries as Australia, Iran, Ireland, Turkey, and USA (Güler et al. 2008; Scaria et al. 2010; Shahrani et al. 2014; Gibbons et al. 2014; Abraham et al. 2014). Likewise, a study conducted in São Paulo State, Brazil, also showed that the most common resistance profile among E. coli isolated from calves was to cefalotin, tetracycline, trimethoprim-sulfadiazine, and ampicillin (Rigobelo et al. 2006). Moreover, in Australia, E. coli isolates from other food animal sources (pork, poultry, and lamb), besides cattle, also exhibited a high resistance rate to tetracycline, ampicillin, trimethoprim/sulfamethoxazole, and streptomycin, whereas none of the isolates were resistant to imipenem or amikacin (Abraham et al. 2014). In fact, in the present study, amikacin was also the antibiotic that showed lower resistance rate among E. coli isolates from cattle, as well as observed in several studies in which it was included (Fig. 5a) (Güler et al. 2008; Scaria et al. 2010; Wani et al. 2013).
The high level of resistance to tetracycline and ampicillin observed in the present study and elsewhere is probably a direct reflex of their intense use in veterinary medicine, especially among cattle. Indeed, albeit statistic on the veterinary antibiotic market in Brazil is not available, data from USA and from all European Union countries showed that tetracycline followed by penicillin are the two classes of antimicrobial most sold (FDA 2010; EMA 2015). Corroborating this hypothesis, sulfonamides, observed in the present study as the third antibiotic with lower activity against E. coli, are also the third most marketed antimicrobial in USA and Europe (FDA 2010; EMA 2015). Resistance to tetracycline, penicillin, and sulfonamides has a great clinical importance, since those groups of antimicrobials are frequently used in the treatment of human infections, especially urinary tract infections caused by E. coli (Gupta et al. 2011). Furthermore, cattle represent an important source of EHEC in the food chain, being considered a potential source of infection to humans (Martin and Beutin 2011).
In contrast to the results obtained for E. coli isolated from calves, Salmonella spp. strains isolated from fecal samples of calves exhibited high susceptibility rates to most of the studied antibiotics (Figs. 3 and 5). Moreover, in the correspondence analysis, the susceptibility to all tested antibiotic was plotted close to S. Agona serotype, suggesting that any of the eight antimicrobials tested could be used to treat infection by this serotype in cattle. In contrast, S. Enteritidis was plotted close to resistance to nalidixic acid, a first-generation quinolone. This result could be explained by the wide use of quinolones in veterinary medicine due its broad spectrum, low toxicity, and excellent concentrations in blood and tissues. However, it is important to note that among Salmonella spp. strains from calves, it was observed a low rate of resistance to the most antibiotics tested, in contrast to studies in Africa (Ahmed et al. 2009), Italy (Bonardi et al. 2013), and USA (Louden et al. 2012), in which Salmonella spp. isolated from cattle exhibited a high frequency of resistant and multidrug-resistant strains. However, in Australia, S. enterica isolated from confirmed cases of salmonellosis in livestock demonstrated to be mostly susceptible to all the studied antimicrobials (Abraham et al. 2014). The wide variation in the observed results for Salmonella spp. strains isolated from calves could be due to differences on the tested antimicrobials, geographical regions, number of isolates, and age of animals. Nonetheless, our results indicate that Salmonella spp. strains isolated from feces of calves in Minas Gerais State are susceptible to many antibiotics commonly used in veterinary medicine, such as amoxicillin, tetracycline, and trimethoprim-sulfamethoxazole (Fig. 5b).
Regarding the large difference in the antimicrobial susceptibility profile observed between E. coli and Salmonella spp. strains from calves in the present study, it is important to consider that although both strains were isolated from calves in Minas Gerais state, the years of isolation of the strains were different and the herds sampled were not also the same. Therefore, the sampled animals possibly were under different management practices, which strongly influence the appearance of antibiotic resistance among enteric bacteria. Furthermore, E. coli is a commensal bacterium, present in high number in the gastrointestinal tract since birth, while Salmonella spp. is a pathogenic bacterium and infection may result or not in colonization for a long period (Gyles et al. 2010). This feature allows E. coli strains to be more exposed to antimicrobials, thus more prone to disseminate genes of resistance.
Contrarily to the observed for Salmonella spp. isolated from calves, Salmonella spp. strains isolated from stool samples of pigs were only susceptible to amikacin and showed high rates of resistance, being the S. Typhimurium strains strongly related to the antimicrobial susceptibility profile resistant to the majority of the drugs tested and sensitivity only to nalidixic acid (Figs. 4 and 5). Similarly to our data, Salmonella spp. strains isolated from pigs in USA presented high resistance to tetracycline, oxytetracycline, and chlortetracycline, and less resistance rates to amikacin and enrofloxacin (Malik et al. 2011). In another study in England and Wales, resistance of Salmonella spp. isolated from pig farms occurred most frequently to tetracycline, sulfonamide compounds, ampicillin, trimethoprim-sulfamethoxazole, streptomycin, and chloramphenicol, whereas resistance to amikacin, amoxicillin/clavulanic acid, ceftazidime, ciprofloxacin, and cefotaxime was not identified (Miller et al. 2011). Collectively, these data indicate high percentages of Salmonella spp. strains isolated from pigs resistant to several antimicrobials (Fig. 5c).
In Brazil, 17 groups of antimicrobials are authorized to be used in animals as growth promoters, mainly in swine and poultry production (Silva et al. 2013). Additionally, it is also important to consider that Brazil is the leading exporter of beef and the fourth exporter of pork in 2010 (BRASIL 2010) and that Salmonella and some E. coli pathotypes are important food-borne pathogens (Martin and Beutin 2011; Gomes et al. 2013). Moreover, in 2010, Brazil was the third largest consumer of antimicrobials in livestock production and the projected increase in antimicrobial consumption will keep the country in this position by the year 2030 (Van Boeckel et al. 2015). The differences on the origin of antimicrobial resistance between Salmonella spp. isolated from pigs and calves are reinforced by the lower antimicrobial consumption in cattle industry worldwide compared to pork production (Van Boeckel et al. 2015).
In our study, besides the high rate of resistance to most of the tested antimicrobials, the majority of the pathogenic E. coli strains and Salmonella spp. strains isolated from pigs also exhibited multidrug resistance. As already discussed, data on use of antibiotics in Brazil and worldwide strongly suggest that the extremely high rate of multidrug resistance observed in the present study could be the result of the indiscriminate use of antibiotics in animal production (FDA 2010; Silva et al. 2013; EMA 2015; Van Boeckel et al. 2015). The fact that no single Salmonella spp. strain isolated from pigs and just one E. coli strain were sensitive to all evaluated antibiotics is of great concern to public health (Fig. 5). The resistance to several antimicrobials previously reported (Hur et al. 2012) and highlighted in our study survival for months in the environment in the presence of organic matter and the occurrence of carrier animals that can shed the bacteria for months (Nielsen 2013) reinforce the importance of our findings concerning the high rates of multidrug-resistant Salmonella spp. strains from pigs. Therefore, the transfer of antibiotic-resistant foodborne pathogens, opportunistic or commensal bacteria to human population, is of great apprehension (Teuber 2001), since these resistant organisms can disseminate to humans via direct contact with animals or via the food chain (Call et al. 2008). As a result of the extensive use or misuse of antimicrobials in livestock and poultry, antimicrobial-resistant bacteria have emerged (Hur et al. 2012), being detected in the environment of farming operations, on food-animal-derived products and also as the cause of clinical infections in humans (Landers et al. 2012).
Our data on E. coli phylogroup showed that B1 was the most common phylogroup of E. coli from calves followed by phylogroup A (Fig. 2). Moreover, phylogroups B2 and D were not detected. Few studies have identified the phylogenetic groups of E. coli obtained from calves (Tramuta et al. 2008; Coura et al. 2015c), but our results are in agreement with those studies, demonstrating that phylogroup B1 is the most frequent phylogroup of E. coli isolated from calves, while phylogroups B2 and D are rare. These results suggest that E. coli isolated from fecal samples of calves are clustered in phylogroups considered as intestinal pathogens, such as B1 and A, while phylogroups B2 and D that mostly cluster with extraintestinal pathogenic E. coli were not identified among E. coli isolated from fecal samples (Escobar-Páramo et al. 2004; Clermont et al. 2011).
Among Salmonella spp. strains isolated from calves, most of Salmonella serotypes identified are not the most pathogenic for calves, namely Salmonella serotypes Dublin and Typhimurium (Gyles et al. 2010). Regarding the Salmonella serotypes isolated from pigs, the serotypes identified are also not considered so pathogenic for swine, except Typhimurium, that was strongly related to multidrug resistance profile (Fig. 4). Salmonella serotypes associated with disease in pigs are mainly the host-restrict serotype S. Cholerasuis and the ubiquitous S. Typhimurium (Gyles et al. 2010).
Overall, the results from the present study indicate a high frequency of antimicrobial resistance among pathogenic E. coli strains isolated from calves and Salmonella spp. strains isolated from pigs and, a low resistance rate to most antimicrobials tested among Salmonella strains isolated from calves. Moreover, our study highlights the presence of high rates of multidrug-resistant strains of E. coli and Salmonella spp. isolated from food-producing animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Correspondence analysis of the relationship between the antimicrobial susceptibility profile and E. coli pathovars (A) or phylogroups (B). The correspondence analysis dimensional representation is interpreted by considering which categories are plotted closely together. Nalidixic acid (NA); amikacin (AMK); amoxicillin (AMX); ampicillin (AMP); cefoxitin (CFX); norfloxacin (NRF); gentamicin (GEN); tetracycline (TET); trimethoprim-sulfamethoxazole (SMX); (R) resistant; (I) intermediate; (S) susceptible; ETEC (Enterotoxigenic E. coli); EHEC (Enterohaemorrhagic E. coli); STEC (Shiga toxin-producing E. coli); EPEC (Enteropathogenic E. coli); OTHERs (others pathovars); Phylogroups (A), (B1), (E) and (F). JPG 471 kb
Acknowledgments
MSMS was supported by scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes. FMC, EMSD, APRS, and APL were supported by fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq. APL is also supported by the Programa Pesquisador Mineiro—PPM (00923–15), from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais—Fapemig. This work was supported by Fapemig and CNPq
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Monalisa S. M. Souto, Fernanda M. Coura and Elaine M. S. Dorneles contributed equally to this work.
References
- Abraham S, Groves MD, Trott DJ, Chapman T a, Turner B, Hornitzky M, Jordan D. Salmonella enterica isolated from infections in Australian livestock remain susceptible to critical antimicrobials. International Journal of Antimicrobial Agents. 2014;43:126–130. doi: 10.1016/j.ijantimicag.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Ahmed, A.M., Younis, E.E. a, Ishida, Y., Shimamoto, T., 2009. Genetic basis of multidrug resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from diarrheic calves in Egypt. Acta Tropica, 111, 144–149. doi:10.1016/j.actatropica.2009.04.004 [DOI] [PubMed]
- Andrade GI, Coura FM, Santos ELS, Ferreira MG, Galinari GCF, Facury Filho EJ, de Carvalho AU, Lage AP, Heinemann MB. Identification of virulence factors by multiplex PCR in Escherichia coli isolated from calves in Minas Gerais, Brazil. Tropical Animal Health Production. 2012;44:1783–90. doi: 10.1007/s11250-012-0139-8. [DOI] [PubMed] [Google Scholar]
- Barrow, P.A., Jones, M.A., Thomson, N., 2010. Salmonella. In: Gyles, C.A., Prescott, J.F., Songer, J.G., Thoen, C.O. Pathogenesis of bacterial infections in animals, (Wiley-Blackwell, Ames)
- Blanchard PC. Diagnostics of dairy and beef cattle diarrhea. Veterinary Clinics of North America: Food Animal Practice. 2012;28:443–64. doi: 10.1016/j.cvfa.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi S, Bassi L, Brindani F, D’Incau M, Barco L, Carra E, Pongolini S. Prevalence, characterization and antimicrobial susceptibility of Salmonella enterica and Yersinia enterocolitica in pigs at slaughter in Italy. International Journal of Food Microbiology. 2013;163:248–257. doi: 10.1016/j.ijfoodmicro.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Brasil, 2010. Brazilian Agribusiness in figures. Retrieved from http://www.agricultura.gov.br
- Brenner FW. Guest Commentary: Salmonella Nomenclature. Journal of Clinical Microbiology. 2000;17:69–74. doi: 10.1128/jcm.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call DR, Davis M, Sawant A. Antimicrobial resistance in beef and dairy cattle production. Animal Health Research Reviews. 2008;9:159–167. doi: 10.1017/S1466252308001515. [DOI] [PubMed] [Google Scholar]
- Clermont O, Olier M, Hoede C, Diancourt L, Brisse S, Keroudean M, Glodt J, Picard B, Oswald E, Denamur E. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infection, Genetic and Evolution. 2011;11:654–62. doi: 10.1016/j.meegid.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environmental Microbiology Reports. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- CLSI, 2012a. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically ; Approved Standard — Ninth Edition, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standar- Ninth Edition. 32 (2), M07-A9.
- CLSI Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Clinical and Laboratory Standards Institute. 2012;32(3):M100–S22. [Google Scholar]
- Coura FM, Lage AP, Heinemann MB. Patotipos de Escherichia coli causadores de diarreia em bezerros: uma atualização. Pesquisa Veterinaria Brasileira. 2014;34:811–818. doi: 10.1590/S0100-736X2014000900001. [DOI] [Google Scholar]
- Coura FM, Freitas MD, Ribeiro J, de Leme RA, de Souza C, Alfieri AA, Facury Filho EJ, de Carvalho AÚ, Silva MX, Lage AP, Heinemann MB. Longitudinal study of Salmonella spp., diarrheagenic Escherichia coli, Rotavirus, and Coronavirus isolated from healthy and diarrheic calves in a Brazilian dairy herd. Tropical Animal Health and Production. 2015;47:3–11. doi: 10.1007/s11250-014-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura FM, Uribe JAZ, Lasmar PVF, Carvalho AÚ, Facury Filho EJ, Silva MVP, Lage AP, Heinemann MB. Systemic and enteric salmonellosis in calves. Semina: Ciências Agrárias. 2015;36:20–41. [Google Scholar]
- Coura FM, Diniz SDA, Silva MX, Maria J, Mussi S, Barbosa SM, Lage AP, Heinemann MB. Phylogenetic Group Determination of Escherichia coli Isolated from Animals Samples. Science World. 2015;2015:1–4. doi: 10.1155/2015/258424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxen M a, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clinical Microbiology Reviews. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Páramo, P., Clermont, O., Blanc-Potard, a. B., Bui, H., Le Bouguénec, C., Denamur, E., 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Molecular Biology and Evolution, 21, 1085–1094. doi:10.1093/molbev/msh118 [DOI] [PubMed]
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption, 2015. ‘Sales of veterinary antimicrobial agents in 26 EU/EEA countries in 2013’. (EMA/387934/2015).
- FDA (2010) Summary Report On Antimicrobials Sold or Distributed for Use in Food-Producing Animals: Food and Drug Administration.
- Foster DM, Smith GW. Pathophysiology of Diarrhea in Calves. Veterinary Clinics of North America: Food Animal Practice. 2009;25:13–36. doi: 10.1016/j.cvfa.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck SM, Bosworth BT, Moon HW. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. Journal of Clinical Microbiology. 1998;36:1795–1797. doi: 10.1128/jcm.36.6.1795-1797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JF, Boland F, Buckley JF, Butler F, Egan J, Fanning S, Markey BK, Leonard FC. Patterns of antimicrobial resistance in pathogenic Escherichia coli isolates from cases of calf enteritis during the spring-calving season. Veterinary Microbiology. 2014;170:73–80. doi: 10.1016/j.vetmic.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Gomes BC, Franco BDGDM, De Martinis ECP. Microbiological Food Safety Issues in Brazil: Bacterial Pathogens. Foodborne Pathogens and Disease. 2013;10:197–205. doi: 10.1089/fpd.2012.1222. [DOI] [PubMed] [Google Scholar]
- Greenacre, M., Blasius, J., 2006. Multiple Correspondence Analysis and Related Methods. CRC Press.
- Güler L, Gündüz K, Ok U. Virulence factors and antimicrobial susceptibility of Escherichia coli isolated from calves in Turkey. Zoonoses Public Health. 2008;55:249–57. doi: 10.1111/j.1863-2378.2008.01121.x. [DOI] [PubMed] [Google Scholar]
- Gupta, K. Thomas M. Hooton,Kurt G. Naber, Bjo¨ rn Wullt,Richard Colgan, Loren G. Miller, Gregory J. Moran, Lindsay E. Nicolle, Raul Raz, Anthony J. Schaeffer, and David E. Soper (2011) Iernational Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical Practice Guidelines d CID 2011:52 [DOI] [PubMed]
- Gyles CL, Prescott JF, Songer JG, Thoen CO. Pathogenesis of Bacterial Infections in Animals. Iowa: Wiley-Blackwell; 2010. [Google Scholar]
- Hur J, Jawale C, Lee JH. Antimicrobial resistance of Salmonella isolated from food animals: A review. Food Research International. 2012;45:819–830. doi: 10.1016/j.foodres.2011.05.014. [DOI] [Google Scholar]
- Keelara S, Scott HM, Morrow WM, Gebreyes WA, Correa M, Nayak R, Stefanova R, Thakur S. Longitudinal study of distributions of similar antimicrobial-resistant Salmonella serovars in pigs and their environment in two distinct swine production systems. Applied and Environmental Microbiology. 2013;79:5167–5178. doi: 10.1128/AEM.01419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenda, R., Burdukiewicz, M., Schierack, P., 2015. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Frontiers in Cellular and Infection Microbiology, 5. doi:10.3389/fcimb.2015.00023 [DOI] [PMC free article] [PubMed]
- Laine TMT, Lyytikäinen T, Yliaho M, Anttila M. Risk factors for post-weaning diarrhoea on piglet producing farms in Finland. Acta Veterinaria Scandinavica. 2008;50:21. doi: 10.1186/1751-0147-50-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage AP, Carvalho ACT, Leite RC, Yano T, Serafim MB. Toxigenic Escherichia coli in calves with diarrhea in Minas Gerais. Brazil, Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 1993;45:352–359. [Google Scholar]
- Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Reports. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louden BC, Haarmann D, Han J, Foley SL, Lynne AM. Characterization of antimicrobial resistance in Salmonella enterica serovar Typhimurium isolates from food animals in the U.S. Food Research International. 2012;45:968–972. doi: 10.1016/j.foodres.2011.03.055. [DOI] [Google Scholar]
- Magiorakos A, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology Infection. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Malik YS, Chander Y, Olsen K, Goyal SM. Antimicrobial resistance in enteric pathogens isolated from Minnesota pigs from 1995 to 2004. Canadian Journal of Veterinary Research. 2011;75:117–121. [PMC free article] [PubMed] [Google Scholar]
- Martin A, Beutin L. Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. International Journal of Food Microbiology. 2011;146:99–104. doi: 10.1016/j.ijfoodmicro.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Mateus, a., Taylor, D.J., Brown, D., Mellor, D.J., Bexiga, R., Ellis, K., 2008. Looking for the unusual suspects: a Salmonella Dublin outbreak investigation. Public Health, 122, 1321–1323. doi:10.1016/j.puhe.2008.02.007 [DOI] [PubMed]
- Miller, a. J., Twomey, D.F., Davies, R.H., Teale, C.J., Williamson, S.M., Reichel, R., Featherstone, C. a., Cook, a. J.C., Snow, L.C., Armstrong, J.D., 2011. Salmonella Serovars and Antimicrobial Resistance Patterns on a Sample of High Seroprevalence Pig Farms in England and Wales (2003–2008). Zoonoses Public Health, 58, 549–559. doi:10.1111/j.1863-2378.2011.01402.x [DOI] [PubMed]
- Mohler VL, Izzo MM, House JK. Salmonella in Calves. Veterinary Clinics of North America: Food Animal Practice. 2009;25:37–54. doi: 10.1016/j.cvfa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Moxley, R. a, Smith, D.R., 2010. Attaching-effacing Escherichia coli infections in cattle. Veterinary Clinics of North America: Food Animal Practice, 26, 29–56, table of contents. doi:10.1016/j.cvfa.2009.10.011 [DOI] [PMC free article] [PubMed]
- Nielsen LR. Review of pathogenesis and diagnostic methods of immediate relevance for epidemiology and control of Salmonella Dublin in cattle. Veterinary Microbiology. 2013;162:1–9. doi: 10.1016/j.vetmic.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Quinn, P.J., Carter, M.E., Markey, B., Carter, G.R., 1994. Enterobacteriaceae. In: Quinn, P.J., Carter, M.E., Markey, B., Carter, G.R. Clinical Veterinary Microbiology. (Wolfe, London)
- Rigobelo HJ, Gamez JM, Marin C, Macedo JA, Ambrosin FAÁ. Virulence factors of Escherichia coli isolated from diarrheic calves. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2006;58:305–310. doi: 10.1590/S0102-09352006000300003. [DOI] [Google Scholar]
- Rostagno MH, Callaway TR. Pre-harvest risk factors for Salmonella enterica in pork production. Food Research International. 2012;45:634–640. doi: 10.1016/j.foodres.2011.04.041. [DOI] [Google Scholar]
- Salvarani S, Tramuta C, Nebbia P, Robino P. Occurrence and functionality of cycle inhibiting factor, cytotoxic necrotising factors and cytolethal distending toxins in Escherichia coli isolated from calves and dogs in Italy. Research in Veterinary Science. 2012;92:372–7. doi: 10.1016/j.rvsc.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Scaria J, Warnick LD, Kaneene JB, May K, Teng CH, Chang YF. Comparison of phenotypic and genotypic antimicrobial profiles in Escherichia coli and Salmonella enterica from the same dairy cattle farms. Molecular and Cellular Probes. 2010;24:325–345. doi: 10.1016/j.mcp.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Kehrenberg C, Walsh TR. Use of antimicrobial agents in veterinary medicine and food animal production. International Journal of Antimicrobial Agents. 2001;17:431–437. doi: 10.1016/S0924-8579(01)00297-7. [DOI] [PubMed] [Google Scholar]
- Shahrani M, Dehkordi F, Momtaz H. Characterization of Escherichia coli virulence genes, pathotypes and antibiotic resistance properties in diarrheic calves in Iran. Biological Research. 2014;47:28. doi: 10.1186/0717-6287-47-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, K.C., Knobl, T., Moreno, A.M., 2013. Antimicrobial resistance in veterinary medicine : mechanisms and bacterial agents with the greatest impact on human health. Brazilian Journal of Veterinary Research and Animal Science, 50, 171–183.
- Tadesse D a, Zhao S, Tong E, Ayers S, Singh A, Bartholomew MJ, McDermott PF. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerging Infectious Diseases. 2012;18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nature Reviews Microbiology. 2010;8:207–17. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- Teuber M. Veterinary use and antibiotic resistance. Current Opinion in Microbiology. 2001;4:493–499. doi: 10.1016/S1369-5274(00)00241-1. [DOI] [PubMed] [Google Scholar]
- Tramuta C, Robino P, Nebbia P. Phylogenetic background of attaching and effacing Escherichia coli isolates from animals. Veterinary Research Communications. 2008;32:433–437. doi: 10.1007/s11259-008-9042-1. [DOI] [PubMed] [Google Scholar]
- Van Boeckel, T.P., Brower, C., Gilbert, M., Grenfell, B.T., Levin, S.A., Robinson, T.P., Teillant, A., Laxminarayan, R., 2015. Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences USA, 112, 5649–5654. doi:10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed]
- Viott, a M., Lage, a P., Cruz, E.C.C., Guedes, R.M.C., 2013. The prevalence of swine enteropathogens in Brazilian grower and finish herds. Brazilian Journal of Microbiology, 44, 145–151. doi:10.1590/S1517-83822013005000033 [DOI] [PMC free article] [PubMed]
- Wani, S. a, Hussain, I., Beg, S. a, Rather, M. a, Kabli, Z. a, Mir, M. a, Nishikawa, Y., 2013. Diarrhoeagenic Escherichia coli and salmonellae in calves and lambs in Kashmir absence, prevalence and antibiogram. Revue Ccientifique et Technique.32, 833–840. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correspondence analysis of the relationship between the antimicrobial susceptibility profile and E. coli pathovars (A) or phylogroups (B). The correspondence analysis dimensional representation is interpreted by considering which categories are plotted closely together. Nalidixic acid (NA); amikacin (AMK); amoxicillin (AMX); ampicillin (AMP); cefoxitin (CFX); norfloxacin (NRF); gentamicin (GEN); tetracycline (TET); trimethoprim-sulfamethoxazole (SMX); (R) resistant; (I) intermediate; (S) susceptible; ETEC (Enterotoxigenic E. coli); EHEC (Enterohaemorrhagic E. coli); STEC (Shiga toxin-producing E. coli); EPEC (Enteropathogenic E. coli); OTHERs (others pathovars); Phylogroups (A), (B1), (E) and (F). JPG 471 kb


