Abstract
Ferns are an important phytogenetic bridge between lower and higher plants. Historically they have been used in many ways by humans, including as ornamental plants, domestic utensils, foods, and in handicrafts. In addition, they have found uses as medicinal herbs. Ferns produce a wide array of secondary metabolites endowed with different bioactivities that could potentially be useful in the treatment of many diseases. However, there is currently relatively little information in the literature on the phytochemicals present in ferns and their pharmacological applications, and the most recent review of the literature on the occurrence, chemotaxonomy and physiological activity of fern secondary metabolites was published over 20 years ago, by Soeder (Bot Rev 51:442–536, 1985). Here, we provide an updated review of this field, covering recent findings concerning the bioactive phytochemicals and pharmacology of fern species.
Keywords: Ferns, Phytochemicals, Pharmacology, Medicine, Food
Introduction
It is well established that chemicals extracted from plants have a wide range of pharmacological applications (Lanzotti 2014; Xiao 2015, 2016a, b, c; Zheng et al. 2016). However, studies on the pharmacology of phytochemicals have mainly focused on angiosperms rather than pteridophytes in general. This may be because angiosperms exhibit greater biodiversity, more varied adaptations, and are more widely distributed, making them accessible to a greater number of research groups.
Although pteridophytes (Fig. 1) are less widely distributed than angiosperms, they are reportedly used for medicinal purposes in places where they do occur, suggesting that they produce secondary metabolites with specialized ecological functions relating to herbivore defence (Morais-Braga et al. 2012a). The pteridophytes are a group of vascular plants that is divided into two monophyletic lineages, the lycophytes and the ferns, which differ phylogenetically: the ferns more closely resemble the seed-bearing plants (Prado and Sylvestre 2010).
Fig. 1.

Different species of ferns identified on the Araripe Plateau, Crato, CE, Brazil. (Courtesy of Flaviana Morais-Braga)
In the botanical kingdom, ferns represent an important phylogenetic bridge between lower and higher plants. Because of their unique evolutionary history and biology, they produce a distinct set of secondary metabolites, many of which are not found in other plants. There are almost 12,000 species of ferns around the world, most of which are native to tropical and subtropical areas. Around 2600 of these species are found in China, over 300 of which are used in traditional Chinese medicine (Ching 1988). Phytochemical studies on ferns have revealed that they contain a wide range of alkaloids (Dong et al. 2012), flavonoids (Xia et al. 2014), polyphenols (Socolsky et al. 2012), terpenoids (Socolsky et al. 2007), and steroids (Ho et al. 2012). The structures of these compounds usually differ from those of related secondary metabolites produced by other higher plants, making them a potentially valuable source of chemical diversity.
Several reviews on the ferns have been published since 2012: Liu et al. (2012b) reviewed ferns eaten in China, Christenhusz and Chase (2014) highlighted recent trends and concepts in fern classification, and Plackett et al. (2015) discussed missing links in shoot evolution and the development of ferns. However, there have been no review articles covering studies on the phytochemistry and pharmacology since 1985, when Soeder (1985) summarized the occurrence, chemotaxonomy and physiological activity of ferns’ chemical constituents. Since then, the number of known phytochemicals from ferns has increased dramatically, as has their range of potential pharmacological applications. This review summarizes current knowledge regarding the phytochemistry and pharmacology of fern species.
Overview of fern species used in medicinal applications
Ferns have historically been used extensively by humans as ornamental plants, in domestic utensils, in handicrafts, as components of cosmetic formulations and foodstuffs, and for medicinal purposes (Morais-Braga et al. 2012a). Reports of therapeutic effectiveness, as well as scientific curiosity and the need for new drugs have prompted several groups to conduct pharmacological research on ferns and related plants. Pharmacological and ethnopharmacological studies have revealed that substances in ferns exhibit diverse pharmacological effects such as cytotoxicity (Radhika et al. 2010), hepatoprotective activity (Wills and Asha 2006), antihyperglycemic activity (Zheng et al. 2011a, b), leishmanicidal activity (Socolsky et al. 2015), trypanocidal activity (Morais-Braga et al. 2013a, b), anti-nociceptive activity, anti-inflammatory activity (Yonathan et al. 2006), immunomodulatory activity (Wu et al. 2005), and chemopreventive effects (Wills and Asha 2009). Because of the need for new medicines with such activities, pteridophytes and their secondary metabolites could potentially be of great medicinal value.
Phytochemicals from fern species
Huperziaceae
The Huperziaceae comprise two genera, Huperzia and Phlegmariurus. The former produces a wide range of secondary metabolites including lycopodium alkaloids, triterpenes, flavones and phenolic acids (Lu et al. 2001; Tong et al. 2003b; Ma and Gang 2004; Zhou et al. 2003a, b; Shi et al. 2005). Huperzia serrata (Thunb.) Trev. is a typical member of the lycophytes, and is synonymous with Lycopodium serratum (Thunb.). Alkaloids from H. serrata have been investigated by Chinese phytochemists since the mid-1980s, resulting in the identification of huperzine A, which has been proposed to have anti-Alzheimer’s activity (Liu et al. 1986a, b). More than 200 alkaloids had been indentified from H. serrata and related genera by 2004 (Ma and Gang 2004). To date, over 100 Lycopodium alkaloids have been identified in H. serrata, around 80 of which were previously unknown (Table 1). Based on their structures and proposed biogenesis, these compounds can be classified as lycodine, lycopodine, fawcettimine, or phlegmarine alkaloids (Fig. 2). Lycopodine and fawcettimine alkaloids are the major lycopodium alkaloids found in H. serrata.
Table 1.
80 New lycopodium alkaloids from H. serrata
| Chemical name | Source (references) |
|---|---|
| I. Lycopodium class | |
| 12-Epilycodoline | H. miyoshiana (Tong et al. 2003b) |
| 12-Epilycodoline N-oxide | H. serrata (Tan and Zhu 2004) |
| 4,6-Dihydroxyserratidine | H. serrata (Tan et al. 2002d) |
| 4-Hydroxyserratidine | H. serrata (Tan et al. 2002d) |
| 4,6-Dihydroxylycopodine | H. serrata (Tan and Zhu 2004) |
| 6-Hydroxylycopodine | H. serrata (Yuan et al. 1995) |
| 6-Hydroxyserratidine | H. serrata (Tan et al. 2002d) |
| 7-Hydroxylycopodine | H. serrata (Tan and Zhu 2004) |
| Clavolonine | H. miyoshiana (Tong et al. 2003b) |
| Flabelliformine | H. miyoshiana (Tong et al. 2003b) |
| Gnidioidine | H. carinata (Thorroad et al. 2014) |
| Huperzine E | H. serrata (Zhu et al. 1996; Wang et al. 2001) |
| Huperzine F | H. serrata (Zhu et al. 1996; Wang et al. 2001) |
| Huperzine G | H. serrata (Wang et al. 1998, 2000) |
| Huperzine O | H. serrata (Wang et al. 2000) |
| Lucidioline | H. serrata (Zhou et al. 1993; Ma et al. 1998) |
| Lycocarinatine A | H. carinata (Thorroad et al. 2014) |
| Lycodoline | H. miyoshiana (Tong et al. 2003b), H. serrata (Yuan et al. 1995), H. carinata (Thorroad et al. 2014) |
| Lycopodine | H. miyoshiana (Tong et al. 2003b), H. serrata (Yuan et al. 1995) |
| Lycoposerramine K | H. carinata (Thorroad et al. 2014) |
| Lycoposerramine U N-oxide | H. squarrosa (Thorroad et al. 2014) |
| Miyoshianine A | H. miyoshiana (Tong et al. 2003b) |
| Miyoshianine B | H. miyoshiana (Tong et al. 2003b) |
| Phlegmariurine B | H. carinata (Thorroad et al. 2014), H. squarrosa (Thorroad et al. 2014) |
| Sauroine | H. saururus (Ortega et al. 2004) |
| Selagoline | H. selago (Staerk et al. 2004) |
| Serratidine | H. serrata (Tan et al. 2002d), H. selago (Staerk et al. 2004) |
| II. Lycodine class | |
| 12-Epilycodine N-oxide | H. squarrosa (Thorroad et al. 2014) |
| 6-Hydroxyhuperzine A | H. serrata (Yuan and Zhao 2000) |
| 8,15-Dihydrohuperzine A | H. carinata (Thorroad et al. 2014) |
| Des-N-methyl–obscurine | H. serrata (Yuan et al. 1995) |
| Huperserine E | H. serrata (Jiang et al. 2014) |
| Huperzine A | H. serrata (Liu et al. 1986a, b), H. selago (Staerk et al. 2004), H. carinata (Thorroad et al. 2014), Huperzia squarrosa (Thorroad et al. 2014) |
| Huperzine B | H. serrata (Liu et al. 1986a, b) |
| Huperzine C | H. serrata (Liu and Huang 1994) |
| Huperzine D | H. serrata (Liu and Huang 1994) |
| Huperzine U | H. serrata (Tan et al. 2003) |
| Huperzinine | H. serrata (Yuan and Wei 1988; Jiang et al. 2014) |
| Lycodine | H. serrata (Yuan et al. 1994) |
| Lycoflexine N-oxide | H. squarrosa (Thorroad et al. 2014) |
| N,N-Dimethylhuperzine A | H. serrata (Hu et al. 1992) |
| N-Demethyl-sauroxine | H. saururus (Vallejo et al. 2013) |
| N-Methyl-huperzine B | H. serrata (Yuan and Wei 1988; Southon and Buckingham 1989) |
| III. Fawcettimine class | |
| 11-Hydroperoxyphlegmariurine B | H. serrata (Tan et al. 2003) |
| 11-Hydroxyphlegmariurine B | H. serrata (Tan et al. 2002e) |
| 11-Oxophlegmariurine B | H. serrata (Tan et al. 2002b) |
| 2-Hydroxyphlegmariurine B | H. serrata (Tan et al. 2002b) |
| 2-Oxophlegmariurine B | H. serrata (Tan et al. 2002b) |
| 7,11-Dihydroxy-phlegmariurine B | H. serrata (Tan et al. 2002e) |
| 7-Hydroxyphlegmariurine B | H. serrata (Tan et al. 2002e) |
| 7-Hydroperoxyphlegmariurine B | H. serrata (Tan et al. 2003) |
| 8-Hydroxyphlegmariurine B | H. serrata (Tan et al. 2000b) |
| 8-Hydroxyphlegmariurine B | H. serrata (Yuan and Zhao 2003) |
| Fawcettimine | H. serrata (Tan et al. 2000a), H. carinata (Thorroad et al. 2014) |
| Huperserines A | H. serrata (Jiang et al. 2014) |
| Huperserines B | H. serrata (Jiang et al. 2014) |
| Huperserines C | H. serrata (Jiang et al. 2014) |
| Huperserines D | H. serrata (Jiang et al. 2014) |
| Huperserratinine | H. serrata (Zhu et al. 1994) |
| Huperzine H | H. serrata (Gao et al. 1999) |
| Huperzine I | H. serrata (Gao et al. 2000b) |
| Huperzine P | H. serrata (Tan et al. 2000a) |
| Huperzine Q | H. serrata (Tan et al. 2002c) |
| Huperzine R | H. serrata (Tan et al. 2002a) |
| Huperzine S | H. serrata (Tan et al. 2003) |
| Huperzine T | H. serrata (Tan et al. 2003) |
| Huperzine W | H. serrata (Tan et al. 2002d) |
| Neohuperzinine | H. serrata (Yuan et al. 2002) |
| N-Oxyhuperzine Q | H. serrata (Tan et al. 2002c) |
| Phlegmariurine A | H. serrata (Tan et al. 2000b) |
| Phlegmariurine B | H. serrata (Yuan et al. 1994; Tan et al. 2000a, b) |
| Serratine | H. serrata (Zhang et al. 1990) |
| Serratinine | H. serrata (Zhou et al. 1993; Ma et al. 1998) |
| IV. Miscellaneous group | |
| Huperzine J | H. serrata (Gao et al. 2000a) |
| Huperzine K | H. serrata (Gao et al. 2000a) |
| Huperzine L | H. serrata (Gao et al. 2000a) |
| Huperzine V | H. serrata (Liu et al. 2004) |
| Huperzinine B | H. serrata (Yuan et al. 2001) |
| Phlegmariurine N | H. serrata (Miao et al. 1989; Yuan and Zhao 2000) |
| Lycobeline A | H. goebelii (Hirasawa et al. 2012) |
| Lycobeline B | H. goebelii (Hirasawa et al. 2012) |
| Lycobeline C | H. goebelii (Hirasawa et al. 2012) |
| Lycotetrastine A | H. tetrasticha (Hirasawa et al. 2011) |
| Huperminone A | H. phlegmaria (Hirasawa et al. 2013) |
| Hupermine A | H. phlegmaria (Hirasawa et al. 2014) |
Fig. 2.
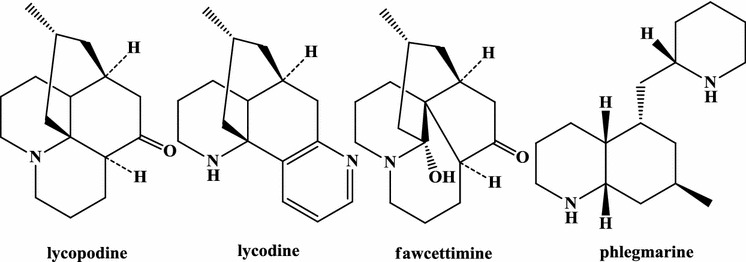
Representative skeletons of Lycopodium alkaloids from Huperzia
Lycopodium alkaloids
Lycopodium alkaloids (Fig. 2) have been isolated and reported from 13 Huperziaceae species and varieties (Table 1): H. serrata, H. serrata (Thunb.) Trev.f. longipetiolata (Spring) Ching, H. selago (L.) Bernh. ex Schrank et Mart., H. lucidula (Michx.) Ching, H. chinensis (Christ) Ching, H. miyoshiana (Makino) Ching, H. saururus (Lam.) Trevis, H. kunmingensis Ching, H. goebelii, H. tetrasticha, H. phlegmaria, H. carinata (Desv. Ex. Poir.) Trevis, and H. squarrosa (G. Forst) Trevis (Hirasawa et al. 2012, 2013). A novel C20N-type Lycopodium alkaloid, lycotetrastine A, with an unprecedented fused-hexacyclic ring system featuring lactone, aza-cycloheptene, aza-cyclohexane, cyclohexane, cyclopentane, and tetrahydrofuran rings, has been isolated from the club moss H. tetrasticha (Hirasawa et al. 2011). Lycobelines A–c alkaloids featuring a decahydroquinoline ring system with an aminohexyl side chain, have been isolated from the club moss H. goebelii (Hirasawa et al. 2011). A novel C16N-type Lycopodium alkaloid known as huperminone A featuring a decahydroquinoline and a cyclohexanone has been isolated from the club moss H. phlegmaria (Hirasawa et al. 2013) along with hupermine A, a new alkaloid with a novel skeleton consisting of a quinolizidine with a 6-dimethylaminohexyl side chain (Hirasawa et al. 2014). Huperminone A has a unique C16N-type skeleton with one fewer nitrogen atom than its closest structural relatives, and may be derived from the C16N2 phlegmarane skeleton. N-demethyl-sauroxine, a novel Lycopodium alkaloid, has been obtained from H. saururus (Lam.) Trevis. Eleven Lycopodium alkaloids including three new alkaloids—8,15-dihydrohuperzine A, lycocarinatine A, and lycoposerramine U N-oxide—have been isolated from whole plants of H. carinata (Desv. Ex. Poir.) Trevis and H. squarrosa (G. Forst) Trevis. Huperzia and Lycopodium species continue to be rich sources of novel heterocyclic alkaloids with C11N, C16N, C16N2, C22N2 and C27N3 skeletons, many of which represent challenging targets for total synthesis. All the Lycopodium alkaloids have complex polycyclic carbon skeletons, albeit with variable levels of oxidation. The most prominent compound in this group is huperzine A, although lycopodine was the first Lycopodium alkaloid to be identified and appears to be the most widely distributed (Ma and Gang 2004).
Lycodine alkaloids
Members of the lycodine alkaloid family found in the Huperziaceae include huperzine A and lycodine derivatives with an opened C-ring such as huperzines B–U and huperserine E (Jiang et al. 2014; Liu et al. 1986a, b; Tan et al. 2003). Lycodine alkaloids typically feature four fused six-membered rings (Jiang et al. 2010; Tan et al. 2002a; Wang et al. 1998, 2000; Wang et al. 2007; Yang et al. 2010; Zhu et al. 1996), and sometimes exhibit N-oxidation (Takayama et al. 2003; Tan and Zhu 2004; Wang et al. 2009a, b; Ying et al. 2014).
Fawcettimine alkaloids
Of the Huperzia species, that which produces the largest number of known fawcettimine alkaloids is H. serrata. The fawcettimine alkaloids differ from the lycopodine alkaloids in that the carbon–carbon single bond between C-4 and C-13 found in the lycopodine alkaloids is replaced by a C-13 to C-12 bond, yielding a fused tetracyclic structure (Ayer et al. 1994; Gao et al. 2000a, b; Jiang et al. 2014; Katakawa et al. 2007, 2011; Takayama et al. 2001). Surprisingly, N-oxidized variants of these alkaloids have been identified, such as N-oxyhuperzine Q (Tan et al. 2002b). The chemical bond between C-12 and C-13 in the fawcettimine alkaloids can be broken, expanding the heterocyclic ring to yield the isoforms known as the phlegmariurine B alkaloids (Tan et al. 2000a, b, c, d, 2003). In addition, the single bond between the N atom and C-13 can be broken to yield derivatives with nine-membered heterocyclic rings such as lycoposerramines A, B and T (Takayama et al. 2001; Katakawa et al. 2005, 2009). A variety of phlegmarine-type alkaloids have been isolated from H. serrata, including N-oxides such as huperzines J–N, lycoposerramines X–Z, and huperserramine A (Gao et al. 2000a, 2008a, b; Katakawa et al. 2006; Ying et al. 2014). Derivatives featuring a single bond between C-4 and C-12 of the phlegmarine skeleton have also been identified; this modification introduces a new five-membered ring and is observed in phlegmarine alkaloids from H. serrata such as lycoposerramine R and huperzimine (Katakawa et al. 2009; Yu et al. 2014). Finally, a simple alkaloid designated huperzine W has been isolated from H. serrata (Tan et al. 2002e).
Triterpenoids
Studies on the non-alkaloidal fraction of Huperzia species have revealed the presence of serratene-type triterpenoids in addition to alkaloids (Shi et al. 2005; Zhou et al. 2003a, b) (Table 2). Serratenes are a group of naturally occurring pentacyclic triterpenoids with seven tertiary methyl groups and a seven-membered C ring (instead of eight methyl groups and a six-membered C ring as found in common pentacyclic triterpenoids), usually with a double bond between C-14 and C-15, and oxygen functionalities at both C-3 and C-21. They have been detected in fern allies and conifers (Pinaceae) (Wittayalai et al. 2012; Tanaka et al. 2004; Zhou et al. 2003a, b).
Table 2.
Serratene-type triterpenoids from Huperzia plants
| Chemical name | Source (references) |
|---|---|
| 14β,15β-Epoxyserratan-3β,21β,29-triol | H. serrata (Zhou et al. 2004) |
| 14β,15β-Epoxy-3β-hydroxyserratan-21α-ol | H. serrata (Zhou et al. 2003b) |
| 14β,15β-epoxy-3β-hydroxyserratan-21α-ol-3β-O-acetate | H. serrata (Zhou et al. 2003b) |
| 14β,15β-epoxy-3β-hydroxyserratan-21β-ol | H. serrata (Zhou et al. 2003b) |
| 16-oxo-21β-hydroxyserrat-14-en-yl acetate | H. serrata (Zhou et al. 2003a) |
| 16-oxo-3α,21β-dihydroxy-serrat-14-en-24-al | H. serrata (Zhou et al. 2013) |
| 16-oxo-3α,21β-dihydroxy-serrat-14-en-24-oic acid | H. serrata (Zhou et al. 2013) |
| 16-oxo-3α-hydroxyserrat-14-en-21β-ol | H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
| 16-oxodiepiserratenediol | H. serrata (Zhou et al. 2013; Li et al. 1988; Zou et al. 2004) |
| 16-oxoserratriol | H. serrata (Zhou et al. 2013) |
| 21-epi-serratenediol | H. serrata (Zhou et al. 2003a), H. kunmingensis (Li et al. 2013), H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
| 21-episerratenediol-3- acetate | H. serrata (Zhou et al. 2003a), H. kunmingensis (Li et al. 2013), H. miyoshiana (Tong et al. 2003b) |
| 21α-hydroxy-serrat-14-en-3β-yl dihydrocoumarate | H. serrata (Zhou et al. 2003a) |
| 21α-hydroxy-serrat-14-en-3β-yl p-dihydrocaffeate | H. serrata (Zhou et al. 2003a) |
| 21α-hydroxy-serrat-14-en-3β-yl propanedioic acid monoester | H. serrata (Zhou et al. 2003a) |
| 21β-hydroxyserrat-14-en-3α-ol | H. serrata (Zhou et al. 2003a), H. phlegmaria (=L. phlegmaria) (Wittayalai et al. 2012) |
| 2lα-hydroxy-serrat-14-en-3β-yl-acetate | H. miyoshiana (Tong et al. 2003b) |
| 2lβ-hydroxy-serrat-14-en-3β-yl-acetate | H. miyoshiana (Tong et al. 2003b), H. phlegmaria (Wittayalai et al. 2012) |
| 3-O-acetyltohogenol | H. miyoshiana (Tong et al. 2003a, b) |
| 3α,21β,24-trihydroxy-serrat-14-en-16-one | H. serrata (Zhou et al. 2003a), H. kunmingensis (Li, et al. 2013), H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
| 3α,2lα-dihydroxy-serrat-14-en-24-oic acid | H. serrata (Zhou et al. 2003a) |
| 3α,2lβ-dihydroxy-serrat-14-en-24,29-diol | H. serrata (Zhou et al. 2003a) |
| 3α,2lβ-dihydroxy-serrat-14-en-24-ol | H. serrata (Zhou et al. 2003a), H. miyoshiana (Tong et al. 2003b) |
| 3β,14β,21α,24-Serratanetetrol (tohogeninol) | H. serrata (Sano et al. 1970) |
| 3β,14β,21α-Serratanetriol (tohogenol) | H. miyoshiana (Tong et al. 2003a) |
| 3β,21β-Dihydroxy-serrat-14-en-16-one | H. serrata (Zhou et al. 2003a), H. miyoshiana (Tong et al. 2003b) |
| 3β,2lα-Dihydroxy-serrat-14-en (serratenediol) | H. serrata (Zhou et al. 2003a, 2004; Li et al. 1988), H. crispate Ching (Pei et al. 2004), H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011), H. kunmingensis (Li et al. 2013) |
| 3β,2lα-Dihydroxy-serrat-14-en-24-ol | H. serrata (Zhou et al. 2003a) |
| 3β,2lβ-Dihydroxy-serrat-14-en-24-ol | H. serrata (Zhou et al. 2003a), H. miyoshiana (Tong et al. 2003b) |
| 3β,2lβ-Dihydroxy-serrat-14-en-29-ol | H. serrata (Zhou et al. 2003a), H. kunmingensis (Li, et al. 2013), H. miyoshiana (Tong et al. 2003b) |
| 3β-Hydroxy-serrat-14-en-21-one | H. serrata (Zhou et al. 2003a) |
| Lycoclavanol | H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
| Lycophlegmarin | H. phlegmaria (Shi et al. 2005) |
| Lycophlegmariol A | H. phlegmaria (Wittayalai et al. 2012) |
| Lycophlegmariol B | H. phlegmaria (Wittayalai et al. 2012) |
| Lycophlegmariol C | H. phlegmaria (Wittayalai et al. 2012) |
| Lycophlegmariol D | H. phlegmaria (Wittayalai et al. 2012) |
| Miyoshianois A | H. miyoshiana (Tong et al. 2003b) |
| Miyoshianois B | H. miyoshiana (Tong et al. 2003b) |
| Miyoshianois C | H. miyoshiana (Tong et al. 2003b) |
| Tohogenin | H. serrata (Zhou et al. 2003a) |
| Miyoshianol A | H. miyoshiana (Tong et al. 2003a) |
| Serrat-14-en-3,21β,24,29-tetraol | H. serrata (Zhou et al. 2004) |
| Serrat-14-en-3α,21β,24,29-tetraol | H. serrata (Zhou et al. 2003a) |
| Serrat-14-en-3β,21α,24-triol | H. serrata (Zhou et al. 2003a) |
| Serrat-14-en-3β,21α-diyl-acetate | H. miyoshiana (Tong et al. 2003b) |
| Serrat-14-en-3β,21β,24-triol | H. serrata (Zhou et al. 2003a) |
| Serrat-14-en-3β,21β,29-triol | H. serrata (Zhou et al. 2004) |
| Serrat-14-en-3β,2lβ,29-triol | H. serrata (Zhou et al. 2003a) |
| Serratenediol (3β-hydroxyserrat-14-en-21α-ol) | H. serrata (Zhou et al. 2004; Li et al. 1988), H. crispate Ching (Pei et al. 2004), H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
| Serratenediol-21-acetate | H. serrata (Zhou et al. 2003a) |
| Serratenediol-3-acetate | H. serrata (Zhou et al. 2003a), H. kunmingensis (Li, et al. 2013), H. crispate Ching (Pei et al. 2004), H. serrata (thunb.) Trev.f. longipetiolata (Spring) Ching (Pei et al. 2011) |
Serratenediol, a representative serratene-type triterpenoid with a seven-membered C ring and seven tertiary methyl groups (Fig. 3), was first isolated from the Japanese club moss Lycopodium serratum (or H. serrata) in 1964 (Tong et al. 2003b). Subsequent studies in this field led to the discovery of several related triterpenoids in Huperzia plants, particularly H. serrata. For example, Zhou and co-workers reported the isolation of many serratane-type triterpenoids from this species (Zhou et al. 2003a, b, 2004). In addition, thirteen triterpenoids, including the previously unknown miyoshianois A–C, were isolated from H. miyoshiana (Tong et al. 2003b). Serratene-type triterpenoids have also been isolated from H. kunmingensis and H. serrata (Thunb.) Trev.f. longipetiolata (Spring) Ching (Li et al. 2013). Finally, five new serratene-type triterpenes, lycophlegmarin (Shi et al. 2005) and lycophlegmariol A–D, along with an abietane-type diterpene have been isolated from the methanol extract of the club moss H. phlegmaria (=L. phlegmaria L.) (Wittayalai et al. 2012).
Fig. 3.

Skeleton of serratene-type triterpenoids from Huperzia
Other secondary metabolites
In addition to alkaloids and triterpenoids, H. serrata also produces phenols and flavonoids (Gao et al. 2000b; Lu et al. 2001; Sano et al. 1970; Zhou et al. 2004). For example, a new flavone glycoside, identified as 5,5′-dihydroxy-2′,4′-dimethoxy-flavone-7-O-β-d-(6″-O-Z-p-coumaroyl)- glucopyranoside was recently isolated from H. serrata (Thunb.) (Yang et al. 2008).
Pteridaceae
Phytochemical studies on the genus Pteris (Pteridaceae) have yielded a variety of secondary metabolites including ent-kaurane diterpenoids and pterosin-sesquiterpenes (Wang et al. 2011a, b, c; Liu et al. 2011a, b, c; Shu et al. 2012; Murakami et al. 1980; Tanaka et al. 1982), flavonoids (Chen et al. 2007; Lu et al. 1999), benzenoids, and benzenoid derivatives (Chen et al. 2007, 2013a, b). Pterosins and ent-kaurane diterpenoids are the characteristic constituents of the fern family Pteridaceae.
Diterpenoids
All of the diterpenoid secondary metabolites isolated from the Pteridaceae to date have either an ent-kaurane or an ent-kaurene skeleton (Table 3) with hydroxyl groups at some or all of the C-2, 6, 7, 9, 11, and 15 positions. In addition, a minority bear hydroxyl groups at C-16, 17, and/or C-18. In some cases, the C-15 hydroxyl is further oxidized to yield 15-oxygenated ent-kaurane or ent-kaurene derivatives. Glycosidic linkages are usually formed via the C-2 or C-4 hydroxyl groups, and the sugar moieties are mainly glucopyranosyl and/or allopyranosyl (Fig. 4) (Chen et al. 2007, 2013a, b).
Table 3.
ent-Kaurane diterpenoids from Pteridaceae species
| Name | Source | References |
|---|---|---|
| 2β,16α-Dihydroxy-ent-kaurane | P. angustipinna, P. cretica, P. dactylina, P. multifida | Murakami et al. (1985a, b), Murakami and Tanaka (1988) |
| 2β,16α-Dihydroxy-ent-kaurane 2-O-β-d-glucoside | P. angustipinna, P. cretica, P. dactylina, P. multifida | Murakami et al. (1985a, b), Murakami and Tanaka (1988) |
| 2β,6β,16α-Trihydroxy-ent-kaurane | P. cretica | Murakami et al. (1985a, b), Murakami and Tanaka (1988) |
| 2β,6β,16α-Trihydroxy-ent-kaurane 2-O-β-d-glucoside | P. cretica | Murakami and Tanaka (1988) |
| 2β,16α,18-Trihydroxy-ent-kaurane | P. ryukyuensis | Tanaka et al. (1978) |
| 2β,15α,16α,17-Tetrahydroxy-ent-kaurane | P. cretica | Murakami and Tanaka (1988) |
| 2β,14β,15α,16α,17-pentahydroxy-ent-kaurane | P. cretica | Murakami and Tanaka (1988) |
| 11β,16β-Epoxy-ent-kauran-19-oic acid | P. longipes | Murakami et al. (1981) |
| (16R)-11β-Hydroxy-15-oxo-ent-kauran-19-oic acid | P. dispar, P. semipinncta | Murakami et al. (1976a, b), Aoyama et al. (1977) |
| (16R)-11β-Hydroxy-15-oxo-ent-kauran-19-oic acid 19-β-d-glucoside | P. dispar, P. semipinncta | Murakami et al. (1976a, b), Aoyama et al. (1977) |
| (16S)-11β-Hydroxy-15-oxo-ent-kauran-19-oic acid | P. dispar, P. semipinncta | Murakami et al. (1976a, b), Aoyama et al. (1977) |
| (16R)-7β,9-Dihydroxy-15-oxo-ent-kauran-19,6β-olide (6F) | P. dispar, P. semipinncta | Murakami et al. (1976a, b), Aoyama et al. (1977) |
| 2β,15α-Dihydroxy-ent-kaur-16-ene | P. angustipinna, P. cretica, P. dactylina, P. multifida | Murakami et al. (1985a, b), Murakami and Tanaka (1988), Liu and Qin (2002) |
| 2β,15α-Dihydmxy-ent-kant-16-ene 2-O-β-d-glucoside | P. angustipinna, P. cretica, P. dactylina, P. multifida, P. plumbaea | Murakami et al. (1985a, b), Murakami and Tanaka (1988) |
| 2β,16β,15α-Trihydroxy-ent-kaur-16-ene | P. cretica, P. multifida | Murakami and Tanaka (1988), Liu and Qin (2002) |
| 2β,6β,15α-trihydroxy-ent-kaur-16-ene 2-O-β-d-glucoside | P. cretica | Murakami and Tanaka (1988) |
| 2β,14β,15α-Trihydroxy-ent-kaur-16-ene | P. plumbaea | Murakami and Tanaka (1988) |
| 2β,14β,15α-Trihydroxy-ent-kaur-16-ene 2-O-β-d-glucoside | P. plumbaea | Murakami and Tanaka (1988) |
| 2β,6β,14β,15α-Tetrahydroxy-ent-kaur-16-ene | P. plumbaea | Murakami and Tanaka (1988) |
| 2β,13,14β,15α-Tetrahydroxy-ent-kaur-16-ene | P. plumbaea | Murakami and Tanaka (1988) |
| 2β,14β,15α,19-Tetrahydroxy-ent-kaur-16-ene | P. plumbaea | Murakami and Tanaka (1988) |
| 9-Hydroxy-ent-kaur-16-en-19-oic acid | P. longipes | Murakami et al. (1981) |
| 15-Oxo-ent-kaur-16-en-19-oic acid | P. longipes | Murakami et al. (1981) |
| 9-Hydroxy-15-oxo-ent-kaur-16-en-19-oic acid | P. livida, P. longipes | Murakami et al. (1981), Tanaka et al. (1981) |
| 9-Hydroxy-15-oxo-ent-kaur-16-en-19-oic acid19-β-d-glueoside | P. altissima, P. livida | Tanaka et al. (1981) |
| 11β-Hydroxy 15-oxo-ent-kaur-16-en-19-oic acid (5F) | P. dispar, P. livida, P. semipinncta | Murakami et al. (1976a, b, 1983), Aoyama et al. (1977), Tanaka et al. (1981) |
| 11β-Hydroxy 15-oxo-ent-kaur-16-en-19-oic acid19-β-d-glueoside | P. altissima, P. dispar, P. livida, P. semipinncta, P. tremula | Murakami et al. (1985a, b), Tanaka et al. (1981) |
| 12β-Hydroxy15-oxo-ent-kaur-16-en-19-oic acid19-β-d-glueoside | P. tremula | Murakami et al. (1985a, b) |
| 9,15β-Dihydroxy-ent-kaur-16-en-19-oic acid | P. longipes | Murakami et al. (1981) |
| 11β,15β-Dihydroxy-ent-kaur-16-en-19-oic acid | P. longipes | Murakami et al. (1981) |
| 12β,15β-Dihydroxy-ent-kaur-16-en-19-oic acid | P. longipes | Murakami et al. (1981) |
| 6β,9-Dihydroxy-15-oxo-ent-kaur-16-en-19-oic acid 19-β-d-glucoside | P. livida | Tanaka et al. (1981) |
| 6β,11β-Dihydroxy-15-oxo-ent-kaur-16-en-19-oic acid 19-β-d-glucoside | P. altissima, P. livida | Tanaka et al. (1981) |
| 7β,9-Dihydroxy-15-oxo-ent-kaur-16-en-19,6β-olide | P. dispar, P. purpureorachis | Murakami et al. (1976a, b), Li et al. (1998) |
| 7β,11β-Dihydroxy-15-oxo-ent-kaur-16-en-19,6β-olid (A) | P. semipinncta | Li et al. (1998) |
| 9,11β-Epoxy-15-oxo-ent-kaur-16-en-19-oic acid | P. purpureorachis | Murakami et al. (1983) |
| 9,11β-Epoxy-15-oxo-ent-kaur-16-en-19-oic acid 19-β-d-glucoside | P. purpureorachis | Murakami et al. (1983) |
| 9,15β-Dihydroxy-ent-kaur-16-en-19-oic acid | P. purpureorachis | Tanaka et al. (1981) |
| 9-Hydroxy-ent-kaur-16-en-19-oic acid | P. purpureorachis | Tanaka et al. (1981) |
| 9-Hydroxy-ent-kaur-16-en-19-oic acid 19-β-d-glucoside | P. purpureorachis | Tanaka et al. (1981) |
| 16-Hydroxy-ent-kaurane-2-β-d-glucoside (creticoside B) | P. multifida | Liu and Qin (2002) |
| Pterokaurane M1 | P. multifida | Ge et al. (2008) |
| Pterokaurane M2 | P. multifida | Ge et al. (2008) |
| Pterokaurane M3 | P. multifida | Ge et al. (2008) |
| 2β,15β-Dihydroxy-ent-kaur-16-ene 2-O-β-d-glucopyranoside | P. cretica Linn. | Harinantenaina et al. (2009) |
| 5,11β,12β-Trihydroxy-15-oxo-ent-kuar-16-en-19-oic acid | P. dispar | Gou et al. 2011 |
| Pterisolic acid A | P. semipinnata | Liu et al. (2011a, b, c) |
| Pterisolic acid B | P. semipinnata | Liu et al. (2011a, b, c) |
| Pterisolic acid C | P. semipinnata | Liu et al. (2011a, b, c) |
| Pterisolic acid D | P. semipinnata | Liu et al. (2011a, b, c) |
| Pterisolic acid E | P. semipinnata | Liu et al. (2011a, b, c) |
| Pterisolic acid F | P. semipinnata | Liu et al. (2011a, b, c) |
Fig. 4.
Ent-kaurane-type diterpenoids from genus Pteris (Chen et al. 2007, 2013a, b)
The novel tetrahydroxylated ent-kaurane pterokaurane M2 (which is hydroxylated at C-2, C-14, C-15, and C-18) was isolated from Pteris multifida (Ge et al. 2008). Although C-15 hydroxylated kaurenes had previously been isolated from P. cretica (Harinantenaina et al. 2009), this 2,15-dihydroxy-ent-kaur-16-ane 2-O-d-glucopyranoside is the first ent-kaurene derivative with an α-oriented hydroxyl group at C-15 to be isolated from any Pteris species (Wang et al. 2011a, b, c). In addition, ent-atisane type diterpenes, which represent a small proportion of the diterpenoids in Pteris, were isolated from the fronds of P. purpureorachis COPEL (Fig. 5) (Tanaka et al. 1981; Murakami et al. 1983).
Fig. 5.
Atisane-type diterpenoids from genus Pteris
P. semipinnata L. is a member of the genus Pteris that grows in northern China. Phytochemical studies on this species have led to the isolation of over thirty terpenoids, including diterpenoids and sesquiterpenoids. Most of the novel diterpenoids from P. semipinnata are ent-kaurane derivatives such as 6β,11α-dihydroxy- 15-oxo-ent-kaur-16-en-19-oic acid and 7α,11α-dihydroxy-15-oxo-ent-kaur-16-en-19-oic acid (Bai et al. 2013), 7 β-hydroxy-11β,16β-epoxy-ent-kauran-19-oic acid (Zhan et al. 2009), and pterisolic acids A–F (Wang et al. 2011a, b, c). In addition, a new ent-kaurane diterpenoid glucoside known as pteriside has been obtained from P. semipinnata (Shi and Bai 2010). In addition to ent-kaurane ditertenoids, a novel labdane diterpenoid glucoside, 15-O-β-d-glucopyranosyl-labda-8(17), 13E-diene-3β,7β-diol has been identified (Jin et al. 2010).
Flavonoids
Many flavonoids, especially flavonols, have been isolated from ferns and characterized (Harborne and Williams 1988; Cao et al. 2013a, b). Flavonoids are abundant in Pteris species, and epidemiological and medical data suggest that they play key roles in preventing and managing diseases (Chen et al. 2017; Xiao and Kai 2012; Xia et al. 2014; Xiao et al. 2016; Chen et al. 2017; Xiao 2016c). Flavonoids from 20 fern species belonging to the genus Pteris have been studied (Gong et al. 2007; Chen et al. 2007). Most flavonoids from this genus are α- or β-glycosides such as flavonoid glucosides, galactosides, rhamnosides, or arabinosides. The most numerous glycosylated flavonoids are flavonol O-glycosides. Most of the flavonoid glycosides are 3- or 7-O-glycosides, but the hydroxyl groups at C-5, 4, and 8 positions are sometimes glycosylated as well (Gong et al. 2007; Imperato 2003, 2006; Cai et al. 2000; Cao et al. 2012). In addition, some C-glycosylflavones have been isolated from Pteris (Imperato 2004, 2006). The basic flavonoid aglycones in this genus are apigenin, kaempferol, quercetin, and luteolin. For example, two known flavonoids, apigenin 7-O-α-d-glucoside and apigenin 7-O-α-d-glucuronide, have been obtained from P. semipinnata (Zhan et al. 2010).
New flavonoids are still being isolated from Pteris. For example, 3,8-di-C-arabinosylluteolin, 3-O-(2,3-di-O-p-coumaroyl)-glucosides, 7-O-rhamnoside, 7-O-p-hydroxybenzoate and three di-C-glycosylflavones have been isolated from P. vittata (Fig. 6) (Imperato 2003, 2004, 2006). The main flavonoids in P. multifida are rutin, luteolin, apigenin, and their glycosides (Lu et al. 1999). The Sword Brake fern (P. ensiformis Burm.) is used extensively in traditional Taiwanese herbal drinks. Chen et al. (2007) reported the isolation of three new phenolic compounds in aqueous extracts of this species: kaempferol 3-O-l-rhamnopyranoside-7-O-[-d-apio-furanosyl-(1-2)-O-d-glucopyranoside], 7-O-caffeoylhydroxymaltol 3-O-d-glucopyranoside and hispidin 4-O-d-glucopyranoside, along with the known compounds kaempferol 3-O-l-rhamnopyranoside-7-O-d-glucopyranoside, caffeic acid, 5-caffeoylquinic acid, 3,5-di-caffeoylquinic acid, and 4,5-di-caffeoylquinic acid.
Fig. 6.

Pteris vittata L. (Courtesy of Jianguo Cao)
A novel bihomoflavanonol with an unprecedented skeleton, designated pteridium III, was recently isolated from P. aquilinum, and glycosides with O-d-xylopyranosyl and O-d-glucopyranoside moieties have been isolated from P. esculentum. This represents the second time that unusual disaccharide analogues of this kind have been found in this family.
The species Pityrogramma calomelanos (Fig. 7), syn. Acrostichum calomelanos, belongs to the family Pteridaceae and is distributed across tropical America, where it is very often found on the edges of paths and roads and in disturbed areas at either high or low altitude (Moran and Davidse 1995; Prado 2005b). Dihydrochalcone was evaluated in India by Sukumaran and Kuttan (1991). The complex flavonoids, calomelanols A-J, were isolated from the farinose exudate of Pityrogramma calomelanos in early 1990s (Asai et al. 1991, 1992a, b).
Fig. 7.

Aerial parts of the fern Pityrogramma calomelanos. (Courtesy of Flaviana Morais-Braga)
Sesquiterpenes
Phytochemical investigations on Pteris have revealed that the C14 and C15 illudane-type sesquiterpenoids known as pterosins (Fig. 8), are key chemotaxonomical constituents of the genus (Wang et al. 2011a, b, c; Liu et al. 2011a, b, c; Shu et al. 2012; Murakami et al. 1980; Tanaka et al. 1982). The pterosins are a large group of naturally occurring sesquiterpenes with an indanone skeleton.
Fig. 8.

Typical pterosins found in genus Pteris
Bracken (Pteridium spp.) is a ubiquitous fern that has been described as one of the five most common plants on earth; it has a long history of poisoning grazing livestock. P. aquilinum is the most widespread species within the family Pteridaceae, with 11 subspecies occurring predominantly in the northern hemisphere (Yamada et al. 2007; Vetter 2009). In the 1970s, several indanone-type sesquiterpenes (Table 4), such as pterosin B, were isolated as characteristic constituents of bracken (Fukuoka et al. 1978; Hikino et al. 1970, 1971, 1972; Kuroyanagi et al. 1974a, 1979). In addition to diterpenoids, several new sesquiterpenoid indanone derivatives including pterisemipol, (2R)-norpterosin B, (2R)-12-O-β-d-glucopyranosylnorpterosin B and semipterosin A (Fig. 9) have been found in P. semipinnata (Zhang and Xuan 2007; Zhan et al. 2010). Pterosin sesquiterpenoids (Table 4) were first isolated from bracken, P. aquilinum var. latiusculum (Pteridaceae) (Hikino et al. 1970), and proved to be the long-sought bracken carcinogens (Hirono 1987). To date, over 60 pterosin sesquiterpenoids, all 2,5,7-trimethyl-indan-1-one derivatives, have been isolated from Pteris (Fig. 9) (Wang et al. 2011a, b, c; Liu et al. 2011a, b, c; Shu et al. 2012; Murakami et al. 1980; Tanaka et al. 1982). Several of them, including pterosin Z and acetyl-Δ2-dehydropterosin B have proven to be cytotoxic (Chen et al. 2008a, b).
Table 4.
1H-indan-1-one sesquiterpenoids from Pteridaceae species
| Chemical name | Type | Source | References |
|---|---|---|---|
| Pterosin A | I | P. aqullinum var. Latiusculum; D. seabra (wall) Moore; P. cretica L; M. substrigosa Tagawa; D. wilfordii (Moore) Christ; | Yoshihira et al. (1972, 1978), Murakami et al. (1976a), Kuraishi et al. (1985), Tanaka et al. (1981) |
| Pterosin B | I | P. aqullinum var. Latiusculum; P. bella Tagawa; J. scammanae Tryon; P. tremula Br; P. dactylina Hook; P. multifida Poir; P. grevilleana Wall; P. cretica L; P. ryukyuensis Tagawa; | Yoshihira et al. (1978), Murakami et al. (1974, 1975, 1976a), Kuroyanagi et al. (1974b), Satake et al. (1984), Kuraishi et al. (1985), Tanaka et al. (1981) |
| Pterosin C | I | P. aqullinum var. Latiusculum; P. wallichiana Agardh; P. bella Tagawa; P. aqullinum subsp wightianum (Wall) Shich; P. ashimensis Hieron; H. incise (Thunb) Smith; P. multifida Poir; P. cretica L; P. ryukyuensis Tagawa; P. livida Mett; P. podophylla | Yoshihira et al. (1978), Murakami et al. (1974, 1975, 1976a), Kuroyanagi et al. (1974a), Hikino et al. (1972), Kuraishi et al. (1985), Tanaka et al. (1981) |
| Pterosin D | I | P. aqullinum var. Latiusculum; P. aqullinum subsp wightianum (Wall) Shich; H. punctata (Thunb) Mett; J. scammanae Tryon; M. speluncae (L.) Moore; M. strigosa (Thunb) Presl; D. wilfordii (Moore) Christ | Yoshihira et al. (1978), Kuroyanagi et al. (1974a, 1979), Murakami et al. (1980), Tanaka et al. (1978, 1981) |
| Pterosin E | I | P. aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Pterosin F | I | P. aqullinum var. Latiusculum; P. aqullinum subsp wightianum (Wall) Shich; P. tremula Br; P. dactylina Hook; P. multifida Poir; Pteris cretica L | Yoshihira et al. (1978), Kuroyanagi et al. (1974a), Murakami et al. (1974, 1976a), Satake et al. (1984), Kuraishi et al. (1985) |
| Pterosin G | I | P. aqullinum var. Latiusculum; P. podophylla Swartz | Yoshihira et al. (1978), Tanaka et al. (1981) |
| Pterosin H | I | P. aqullinum var. Latiusculum; P. aqullinum subsp wightianum (Wall) Shich; M. speluncae (L.) Moore; M. Trepeziformis (Boxb.) Kuhn; M. obtusiloba Hayata; M. substrigosa Tagawa | Yoshihira et al. (1972, 1978), Kuroyanagi et al. (1974a, 1979), Murakami et al. (1980) |
| Pterosin I | I | P. aqullinum var. Latiusculum; P. aqullinum subsp wightianum (Wall) Shich; M. speluncae (L.) Moore; M. obtusiloba Hayata | Yoshihira et al. (1978), Kuroyanagi et al. (1974a, 1979), Murakami et al. (1980) |
| Pterosin J | I | P. aqullinum var. Latiusculum; P. tremula Br; P. dactylina Hook | Yoshihira et al. (1978), Murakami et al. (1976a, b), Tanaka et al. (1981) |
| Pterosin K | I | P. aqullinum var. Latiusculum; | Yoshihira et al. (1978) |
| Pterosin L | I | P. aqullinum var. Latiusculum; H. punctata (Thunb) Mett; J. scammanae Tryon; M. speluncae (L.) Moore; M. strigosa (Thunb) Presl; D. wilfordii (Moore) Christ | Yoshihira et al. (1978), Kuroyanagi et al. (1974a, 1979), Murakami et al. (1980), Tanaka et al. (1978, 1981) |
| Pterosin M | II | O. japonicum | Yoshihira et al. (1978) |
| Pterosin N | I | P. aqullinum var. Latiusculum; P. ashimensis Hieron; H. incise (Thunb) Smith | Yoshihira et al. (1978), Hikino et al. (1972), Murakami et al. (1976a, b), Kuroyanagi et al. (1974a) |
| Pterosin O | I | P. aqullinum var. Latiusculum; P. dactylina Hook; P. multifida Poir | Yoshihira et al. (1978), Satake et al. (1984), Murakami et al. (1974), Kuroyanagi et al. (1974a) |
| Pterosin P | II | P. aquilinum | Kuroyanagi et al. (1974a, 1979) |
| Pterosin Q | II | P. kiuschiuensis Hieron; P. bella Tagawa; P. oshimensis Hieron; H. incise (Thunb) Smith; P. dactylina Hook; P. ryukyuensis Tagawa | Fukuoka et al. 1978, Murakami et al. (1974, 1975, 1976a), Hikino et al. (1972), Satake et al. (1984), Kuroyanagi et al. (1974a) |
| Pterosin R | II | C. barometz | Murakami et al. (1980) |
| Pterosin S | II | P. kiuschiuensis Hieron; J. scammanae Tryon; P. multifida Poir; P. cretica L; P. livida Mett | Fukuoka et al. (1978), Kuroyanagi et al. (1974a), Murakami et al. (1974), Kuraishi et al. (1985), Tanaka et al. (1981) |
| Pterosin T | II | P. kiuschiuensis Hieron; P. bella Tagawa | Fukuoka et al. (1978), Murakami et al. (1975) |
| Pterosin U | II | P. kiuschiuensis Hieron; | Fukuoka et al. (1978) |
| Pterosin V | II | D. seabra (wall) Moore | Murakami et al. (1976b) |
| Pterosin W | II | P. fauriei Hieron; P. inaqualis Baker var. Aequata (Miq) Tagawa; | Tanaka et al. (1982), Hikino et al. (1971) |
| Pterosin X | II | P. fauriei Hieron; P. inaqualis Baker var. Aequata (Miq) Tagawa | Tanaka et al. (1982), Hikino et al. 1971 |
| Pterosin Y | II | C. japonica | Murakami et al. (1980) |
| Pterosin Z | I | P. aqullinum var. Latiusculum; P. aqullinum subsp wightianum (Wall) Shich | Yoshihira et al. (1978), Kuroyanagi et al. (1974a) |
| (2R)-Norpterosin B | I | P. semipinnata | Zhan et al. (2010) |
| (2S, 3S)-Pterosin C | I | P. semipinnata | Zhan et al. (2010) |
| Norpterosin C | I | P. semipinnata | Zhan et al. (2010) |
| (2S)-13-Hydroxypterosin A | I | P. ensiformis | Chen et al. (2013a, b) |
| (2S,3S)-12-Hydroxypterosin Q | I | P. ensiformis | Chen et al. (2013a, b) |
| 1α,3β-Dihydroxylnorpterosin C | I | P. dispar | Gou et al. (2011) |
| 2R,3S-Acetylpterosin C | I | P. multifida Poir | Shu et al. (2012) |
| (2S,3S)-Acetylpterosin C | I | P. multifida Poir | Shu et al. (2012) |
| Acetylpterosin B | I | P. multifida Poir | Wang et al. (2013) |
| Acetylpterosin C | I | P. aqullinum var. Latiusculum; P. ashimensis Hieron; H. incise (Thunb) Smith | Yoshihira et al. (1978), Hikino et al. (1972), Murakami et al. (1976a, b) |
| Benzoylpterosin B | I | P. aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Bimutipterosins A | II | P. multifida Poir | Liu et al. (2011a, b, c) |
| Bimutipterosins B | II | P. multifida Poir | Liu et al. (2011a, b, c) |
| Isocrotonylptersin B | I | P. aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Palmitylpterosin A | I | P. aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Palmitylpterosin B | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Palmitylpterosin C | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Phenylacetylpterosin C | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978), Kuroyanagi et al. (1974a) |
| Pterisemipol | III | Pteris semipinnata L. | Zhang and Xuan 2007 |
| Semipterosin A | I | Pteris semipinnata L. | Zhan et al. (2010) |
| Pteroside A | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Pteroside B | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Pteroside C | I | Pteridium aqullinum var. Latiusculum; Pteris bella Tagawa; Pteris grevilleana Wall | Yoshihira et al. (1978), Murakami et al. (1975, 1985) |
| Pteroside D | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Pteroside K | I | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978) |
| Pteroside M | II | Pteridium aqullinum var. Latiusculum; Onychium japonicum | Kuroyanagi et al. (1974a) |
| Pteroside P | II | Pteridium aqullinum var. Latiusculum | Yoshihira et al. (1978), Kuroyanagi et al. (1974a) |
| Pteroside Q | II | Pteris bella Tagawa; Pteris ashimensis Hieron; Histiopteris incise (Thunb) Smith | Murakami et al. (1974, 1975, 1976a), Hikino et al. (1972) |
| Pteroside S | II | Pteris fauriei Hieron; Pteris inaqualis Baker var. Aequata (Miq) Tagawa; Pteris tremula Br | Tanaka et al. (1982), Hikino et al. (1971), Murakami et al. (1976a, b) |
| Pteroside T | II | Pteris fauriei Hieron; Pteris inaqualis Baker var. Aequata (Miq) Tagawa | Tanaka et al. (1982), Hikino et al. 1971 |
| Pteroside U | II | Pteris fauriei Hieron; Pteris inaqualis Baker var. Aequata (Miq) Tagawa | Tanaka et al. (1982), Hikino et al. (1971) |
| Pteroside W | II | Pteris fauriei Hieron; Pteris inaqualis Baker var. Aequata (Miq) Tagawa | Tanaka et al. (1982), Hikino et al. (1971) |
| Pteroside X | II | Pteris fauriei Hieron; Pteris inaqualis Baker var. Aequata (Miq) Tagawa | Tanaka et al. (1982), Hikino et al. (1971) |
| Pteroside Z | I | Pteridium aqullinum var. Latiusculum; Microlepia speluncae (L.) Moore; Miaolepia Trepeziformis (Boxb.) Kuhn; Microlepia substrigosa Tagawa | Yoshihira et al. (1972, 1978), Murakami et al. (1980) |
| Wallichoside | I | Pteridium aqullinum var. Latiusculum; | Yoshihira et al. (1978) |
| (2R)-12-O-β-d-Glucopyranosylnorpterosin B | I | Pteris semipinnata | Zhan et al. (2010) |
| 2R,3R-13-Hydroxy-pterosin L 3-O-β-d-glucopyranoside | I | Pteris multifida Poir | Shu et al. (2012) |
| Multifidoside A | I | Pteris multifida Poir | Ge et al. (2008) |
| Multifidoside B | I | Pteris multifida Poir | Ge et al. (2008) |
| Multifidoside C | I | Pteris multifida Poir | Ge et al. (2008) |
Fig. 9.
Illudane glycosides and some pterosins found in Bracken (Pteridium spp.)
New chemicals related to the pterosins continue to be discovered. For example, a novel pterisane skeleton sesquiterpenoid, pterisemipol (Fig. 10), was isolated from P. semipinnata L. (Fig. 11). Its skeleton appears to be formed via a rearrangement of protoilludane, which was isolated from the mycelia of Fomitopsis insularis. Interestingly, its biosynthesis does not appear to follow the isoprene rule (Zhang et al. 2007). Recently, two novel isomeric C14 pterosin dimers designated bimutipterosins A and B were isolated from a whole P. multifida plant (Fig. 10). This novel type of pterosin dimer was reported for the first time (Liu et al. 2011a, b, c). From a biogenetic point of view, these compounds, which have a central cyclobutene motif, could be regarded as [2 + 2] dimerization products of dehydropterosin Q, a known compound that has also been isolated from this plant.
Fig. 10.

Skeleton of 1H-indan-1-one sesquiterpenoids from Pteridaceae
Fig. 11.

Pteris semipinnata. (Courtesy of Jianguo Cao)
Terpene glycosides
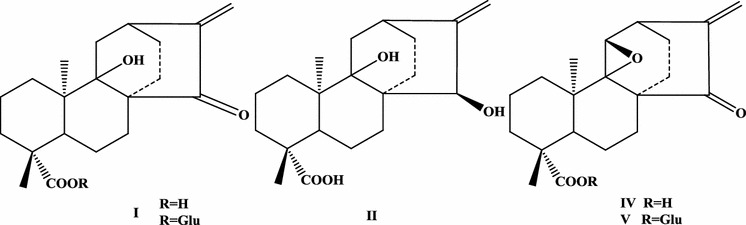
After the discovery of ptaquiloside, more ptaquiloside-related terpene glycosides were isolated. Two new ptaquiloside-related compounds, isoptaquiloside and caudatoside were isolated from fresh fronds of P. aquilinum var. caudatum in 1997 (Castillo et al. 1997). Isoptaquiloside is a C-8 epimer of ptaquiloside. Subsequently, a bioassay-guided separation of an aqueous extract of fresh fronds of the Neotropical bracken P. aquilinum var. caudatum yielded ptaquiloside Z. Studies on the biogenesis of illudane sesquiterpene glucosides such as ptaquiloside resulted in the discovery of a proto-illudane sesquiterpene glucoside, pteridanoside, which was first isolated from the bracken P. aquilinum var. caudatum (Castillo et al. 1999). More recently, a novel norsesquiterpene glucoside ptesculentoside was isolated from the Australian bracken P. esculentum (Fletcher et al. 2010). Finally, two spirocyclic polyketide natural products, pteridic acids A and B, were isolated by Igarashi group from the fermentation broth of Streptomyces hygroscopicus TP-A0451 obtained from the stems of the bracken P. aquilinum, collected in Toyama, Japan (Igarashi et al. 2002).
Triterpenoids
Adiantum is another genus in the family Pteridaceae; it comprises 150–200 species (Brahmachari et al. 2003; Pan et al. 2011). Phytochemical analyses have revealed a variety of chemical compounds derived from various Adiantum species, primarily triterpenoid compounds with a variety of structural motifs (Ibraheim et al. 2011; Reddy et al. 2001; Shiojima et al. 1993). In addition, Adiantum extracts reportedly contain flavonoids, phenylpropanoids, and sterols (Pan et al. 2011). The list of Adiantum-derived phytochemicals continues to expand; the latest additions include 30-normethyl fernen-22-one and hopan-3β-ol, two new triterpenoids isolated from the ethanol extract of A. capillus-veneris L. fronds (Haider et al. 2013).
Sitosterols
β-Sitosterol has been isolated from P. multifida. To date, P. multifida is the only Pteris species shown to contain large amounts of quinic acid derivatives (Harinantenaina et al. 2009). New benzoyl glucosides have been isolated from P. ensiformis Burm. (Chen et al. 2008a, b). In addition, an analysis of the “volatile oils” from P. semipinnata revealed 30 distinct compounds that accounted for 97.35% of their mass, including 3-methoxy-1,2-propanediol, 3-hexen-1-ol, 1-hexanol, 4-hydroxy-2-butanone, and 3-methyl pentanol (Gong et al. 2005).
Dryopteridaceae
The major constituents that have been identified with in Dryopteris plants are flavonoids, polyphenols, and terpenoids (Harborne 1966, 1988; Hiraoka 1978; Gao et al. 2003). Ten flavonol O-glycosides (based on kaempferol and quercetin), two flavanone O-glycosides (based on naringenin and eriodictyol), and three C-glycosylflavones (vitexin, vitexin 7-O-glucoside and orientin) have been identified with in 18 Dryopteris species by Hiraoka (1978). Harborne (1966) found 3-desoxyanthocyanins in D. erythrosora. In addition, kaempferol 7-O-(6″-succinyl-glucoside) was found in four Dryopteris species and an unusual flavan was isolated from D. filix-mas (L.) Schott. (Harborne 1988). Three new kaempferol glycosides, namely kaempferol 3-α-l-(2,4-di-O-acetyl) rhamnopyranoside-7-α-l-rhamnopyranoside, kaempferol 3-α-l-(3,4-di-O-acetyl) rhamnopyranoside-7-α-l-rhamnopyranoside, and kaempferol 3-α-l-(2,3-di-O-acetyl) rhamnopyranosside-7-α-l-rhamnopyranoside were isolated from the rhizome of D. crassirhizoma (Aspidiaceae) (Min et al. 2001). Ten flavonoids (seven flavonol glycosides based on kaempferol and quercetin including a new compound identified as kaempferol 3-O-(acetylrutinoside) and three flavonoid aglycones (apigenin, kaempferol and quercetin) were found in D. villarii (Bell.) Woynar by Imperato (2006). More recently, the same group found five new flavonoids, quercetin 3-O-(X″- acetyl-X″-cinnamoyl-glucoside), quercetin 3-O-(glucosylrhamnoside), kaempferol 3-O-(caffeoylrhamnoside), apigenin 4′-O-(caffeoylglucoside) and 4′-O-(feruloylglucoside) in D. villarii (Imperato 2007a, b).
The Autumn Fern (D. erythrosora) (Fig. 12) is native to China and Japan and is widely known as the Japanese Red Shield Fern because its youngest leaves have a coppery red, bronze, or pink coloration during spring, as shown in the third photo below (as shown in June). Chang et al. (2005) reported that the total flavonoid content of D. erythrosora leaves is around 0.89%, while Cao et al. (2013a) reported the total flavonoid content in whole D. erythrosora plants to be around 14.33%. The main flavonoids in D. erythrosora were identified as gliricidin 7-O-hexoside, apigenin 7-O-glucoside, quercetin 7-O-rutinoside, quercetin 7-O-galactoside, kaempferol 7-O-gentiobioside, kaempferol-3-O-rutinoside, myricetin 3-O-rhamnoside and quercitrin by means of HPLC–DAD–ESI–MS analysis.
Fig. 12.

Dryopteris erythrosora (Courtesy of Jianguo Cao)
Phytochemical investigations have revealed the presence of phloroglucinols (e.g. flavaspidic acids PB and AB), triterpenes (e.g. dryopteric acids A and B), flavonoids and other phenolic analogs in D. crassirhizoma (Chang et al. 2006; Gao et al. 2008b; Min et al. 2001; Noro et al. 1973; Shiojima et al. 1990). Interestingly, such secondary metabolites were not detected by gas chromatography-mass spectrometry (GC–MS) analysis of a methanol extract of the fern (Ban et al. 2012). Rather, GC–MS analysis of the fern extract revealed mainly primary metabolites, including monosaccharides and disaccharides (e.g. fructose, glucose and sucrose), fatty acids (e.g. palmitic, linoleic and oleic acids) and sugar alcohols (e.g. glycerol, xylitol and mannitol) (Ban et al. 2012).
Thelypteridaceae
Macrothelypteris (H. Ito) Ching is a fern species of intermediate size. Protoapigenone, 5,7-dihydroxy-2-(1,2-isopropyldioxy-4-oxo-cyclohex-5-enyl)-chromen-4-one, and 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-cyclohex-4-oxo)-chromen-4-one, were isolated from M. viridifrons (Tagawa) Ching (Wei et al. 2011a). Protoapigenone was also isolated from M. oligophlebia (Wu et al. 2011a).
Extracts of Abacopteris penangiana (Hook.) Ching contain many flavonoids, including novel flavan-4-ol derivatives such as abacopterins A-K, (2S,4R)-4,5,7-trihydroxy-4′-methoxy-6,8-dimethylflavan-5-O-β-d-6-acetylglucopyranoside-7-O-β-d-glucopyranoside, (2S,4R)-5,7-dihydroxy-4,4′-dimethoxy-6,8-dimethylflavan-5-O-β-d-6-acetylglucopyranoside-7-O-β-d-glucopyranoside, (2R,4S)-6,8-dimethyl-7-hydroxy-4′-methoxy-4,2″-oxidoflavan- 5-O-β-d-6″-O-acetyl-glucopyranoside and (2R,4S)-5,7-O-β-d-diglucopyranosyloxy-4′-methoxy-6,8-dimethyl-4,2″-oxidoflavane (Zhao et al. 2006, 2007a, b, 2010a, b, 2011; Lei et al. 2011). In addition, new flavanoids, (2S)-5,2′,5′-trihydroxy-7-methoxyflavanone and abacopterin L together with (7′Z)-3-O-(3,4-dihydroxyphenylethenyl)-caffeic acid were identified from the rhizomes of A. penangiana (Zhao et al. 2011; Wei et al. 2011a, b; Fu et al. 2013).
M. torresiana (Gaud.) Ching is another fern whose phytochemistry and pharmacology have been studied in some detail. This plant grows in southern China and is rich in flavonoids including some that were previously unknown such as protoapigenone, 5,6-dihydroxy-6-methoxyprotoapigenone, protoapigenin, flavotorresin, multiflorin C, (2S)-5,7,2′,5′- tetrahydroxyflavanone 2′-O-β-d-6′′-O-acetylglucopyranoside, (2S)-5,7,2′,5′- tetrahydroxyflavanone 2′-O-β-d-glucopyranoside, 5,7-dihydroxy-2-(1,2-isopropyldioxy- 4-oxocyclohex-5-enyl)-chromen-4-one, 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-4- oxo-cyclohex)-chromen-4-one, 2-(cis-1,2-dihydroxy-4-oxo-cyclohex-5-enyl)-5,7- dihydroxy-chromone, and 2-(trans-1,4-dihydroxy-cyclohexyl)-5,7-dihydroxy-chromone, along with a sesquiterpene, a steroid and two phenols (Lin et al. 2005a, 2007; Fu et al. 2009; Tang et al. 2009, 2010; Lei et al. 2011).
Polypodiaceae
Fernblock® is a polyphenol-enriched hydrophilic extract of the aerial part of Polypodium leucotomos (Choudhry et al. 2014) that is widely used in the formulation of topical gels, creams, sprays, and makeup powder as well as oral dietary supplement capsules marketed for their photoprotective effects. However, the phytochemical composition of P. leucotomos and Fernblock® has not been fully characterised. LC–MS analysis revealed the presence of 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, 4-hydroxycinnamoyl-quinic acid, ferulic acid, and five chlorogenic acid isomers in Fernblock® (García et al. 2006). Studies on the transepithelial transport of caffeic, p-coumaric, ferulic, vanillic and chlorogenic acids across a Caco-2 cell monolayer suggested that such phenolics may be fully absorbed in humans following oral administration (Gombau et al. 2006). This implies that the bioactivity of Fernblock® when taken orally may be attributable at least in part to such phenolics. Oral administration of P. leucotomos extract apparently has no mutagenic or other toxic side effects, enabling repeated use (Choudhry et al. 2014; Gonzalez 2009).
The significance of P. leucotomos extract as a photoprotective nutraceutical as well as the molecular and cellular mechanisms underlying its effects have been discussed in recent reviews (Gonzalez 2009; Gonzalez et al. 2011; Parrado et al. 2014).
The bioactive constituents isolated and identified from P. hastata include flavonol glycosides (e.g. kaempferol 3,7-di-O-α-l-rhamnopyranoside and kaempferol 3-O-α-l- arabinofuranosyl 7-O-α-l-rhamnopyranoside), phenolic acids (e.g. trans-caffeic acid and protocatechuic acid), and their derivatives (e.g. trans-caffeic acid 3-O-d-glucopyranoside) (Duan et al. 2012a, b). Protocatechuic acid and myricetin were detected in both leaf and rhizome aqueous extracts of P. triloba. Sinapic acid was found in the leaf, but not in the rhizome extract (Chai et al. 2013b). Conversely, p-hydroxybenzoic and gallic acids were detected in the rhizome were undetectable in the leaf extract (Chai et al. 2013b).
Selaginellaceae
Selaginella is a genus including more than 700 species that has a wide global distribution (Weng and Noel 2013). It is frequently regarded as one of the oldest lineages of surviving vascular plants (Banks 2009; Weng and Noel 2013) and new Selaginella species are continually being identified and reported, including S. wangpeishanii (Zhang et al. 2014), S. longistrobilina (Zhang et al. 2012a, b, c, d), and S. amasrae (Šimunek and Thomas 2012). Studies on different Selaginella species have resulted in the identification of over 100 natural products, including flavonoids, lignans, selaginellins, phenolics, alkaloids and terpenoids (Weng and Noel 2013). Weng and Noel (2013) have summarized the chemodiversity of Selaginella, which continues to expand as more powerful and advanced analytical techniques are applied.
Biflavonoids
Selaginella-derived biflavonoids are particularly noteworthy because they exhibit a range of interesting pharmacological properties. They are typically dimeric, being linked by a C–O–C or C–C bond. Amentoflavone, hinokiflavone, heveaflavone, neocryptomerin, pulvinatabiflavone and 7″-O-methylamentoflavone were isolated from S. tamariscina (Cheng et al. 2008; Zhang et al. 2012a, b, c, d). New biflavonoids continue to be reported, a recent example being 2,3-dihydrorobustaflavone 7,7″-dimethyl ether, isolated from S. doederleinii Hieron (Han et al. 2013). In addition, an unusual macrocyclic biflavone with an unprecedented methylene bridge, selacyclicbiflavone A, was recently isolated from S. uncinata (Zou et al. 2016a, b).
Involvenflavones
Six new flavonoids, involvenflavones A–F (Fig. 13), were isolated from S. involven. All six are apigenin derivatives with 3′-aryl substituents; this is the first time apigenin derivatives with this substitution pattern have been isolated from a natural source (Long et al. 2015). Two other new flavonoids, uncinataflavones A and B were isolated from S. uncinata (Desv.) Spring. Both are apigenin derivatives with 6-aryl substituents (Zou et al. 2016a, b).
Fig. 13.

Involvenflavones from S. involven (Long et al. 2015)
Alkaloids
Alkaloids were isolated from S. tamariscina (Beauv.) Spring and S. moellendorfii Hieron (Zheng et al. 2004; Wang et al. 2009a, b; Zou et al. 2013). In addition, eight new pyrrolidinoindoline alkaloids (selaginellic acid, 5-hydroxyselaginellic acid, 5-hydroxy-N8,N8-dimethylpseudophrynaminol, N-selaginelloyl-l-phenylalanine, N-(5-hydroxyselaginelloyl)-l-phenylalanine, neoselaginellic acid, N-neoselaginelloyl-l-phenylalanine, and N-(5-hydroxyneoselaginelloyl)-l-phenylalanine) were isolated from whole plants of S. moellendorfii Hieron (Wang et al. 2009a, b). These alkaloids have a 3-carboxybut-2-enyl group at C-3a and two methyl groups at N-8. More recently, another new pyrrole alkaloid was isolated from this plant (Zou et al. 2013).
Selaginellins
The selaginellins are a group of phenols with a unique alkynylphenol carbon skeleton that have only been found in the genus Selaginella to date. The first member of this compound class, selaginellin, was isolated from S. sinensis as a racemic mixture. It features a p-quinone methide unit and an alkynylphenol moiety. Since 2007, several related compounds, selaginellins A–S (Fig. 14) have been isolated from a range of Selaginella species. Thus, selaginellin along with selaginellins A–C and I–Q were found in S. tamariscina, while selaginellins C-H, M, and P-S were found in S. pulvinata. Most recently, selaginellin S was isolated from S. moellendorffii (Zhu et al. 2016). A novel isoquinoline-type selaginellin, selaginisoquinoline A, was isolated from S. pulvinata (Cao et al. 2015a, b), it was isolated as a racemate because of quinone methide-phenol tautomerism. Selariscinins A–D (Fig. 15) from S. tamariscina are also selaginellin derivatives via tautomerism (Nguyen et al. 2015a, b).
Fig. 14.
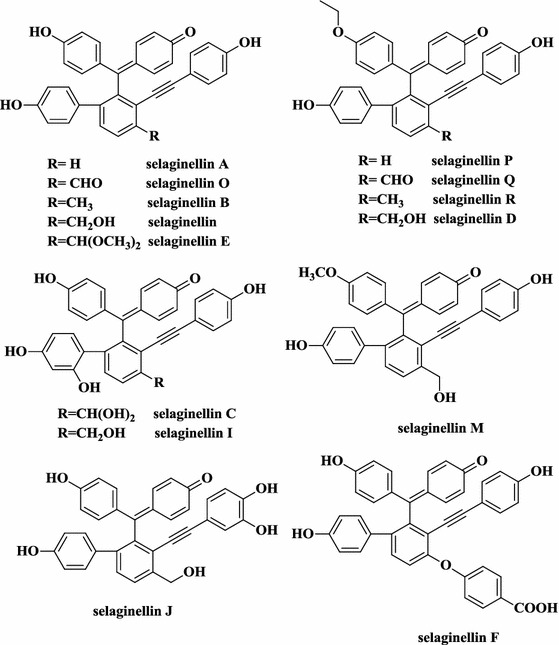
Selaginellins A–S from several Selaginella species
Fig. 15.

Selariscinins A-D and selaginisoquinoline A
Others
A new sesquilignan was isolated from S. sinensis (Desv.) Spring (Wang et al. 2007). Other recently identified natural products from the Selaginellaceae include four new phenols with unprecedented 9H-fluorene skeletons (selaginpulvilins A–D, isolated from S. pulvinata) (Liu et al. 2013b) and a new sesquilignan glycoside, sinensioside A, isolated from S. sinensis (Chen et al. 2014a, b). Additionally, the first abietane diterpenoid from the genus Selaginella (isolated from S. involven Spring) was reported in 2014 (Long et al. 2014). Two novel C-28 spirostene monosides, chrysocauloside A (1β,3β-dihydroxy-20S,22R-spirost-5-ene-1-yl-β-d-glucopyranoside) and chrysocauloside B (1β,3β-dihydroxy-20S,22R-spirost-5-ene-1-yl-β-d-galactopyranoside), were identified from S. ehrysocaulos; both compounds are O-glycosylated at C-1 and bear a methyl group at C-24 and C-25 (Kunert et al. 2015).
Gleicheniaceae
Terpenoids
Terpenoids including labdane-type and clerodane-type diterpenoids, diterpenoid glycosides, and triterpenoids are the major phytochemicals produced by the family Gleicheniaceae (Li et al. 2006, 2008; Hu et al. 2011; Socolsky et al. 2007). Fifteen new diterpenoid glycosides (Fig. 16) were isolated from an Argentine collection of the bitter fern G. quadripartita (Socolsky et al. 2007). Dicranopteris dichotoma Bernb is a very common fern belonging to the genus Dicranopteris that grows in most provinces of northern China and is sometimes known as D. pedata. Phytochemical studies on its fronds led to the identification of eleven clerodane-type diterpenes including nine new ones and two unprecedented phenolic derivatives (Aoki et al. 1997; Li et al. 2006, 2007). In addition, there were four phenolic glycosides, two of which had previously been found by Japanese phytochemists (Kuraishi et al. 1983). Another fern from the family Gleicheniaceae is Hicriopteris glauca (Thunb.) Ching, which belongs to the genus Diplopterygium and is distributed across southern China. Recent phytochemical studies have shown that it produces ent-kaurane diterpenoids as well as flavonoids and phytoecdysones (Fang et al. 2013; Takemoto et al. 1973; Zhang et al. 2009). One of its diterpenoids, ent-2-β-hydroxyl-16-ene-kauran-19-oic acid, was previously unknown.
Fig. 16.
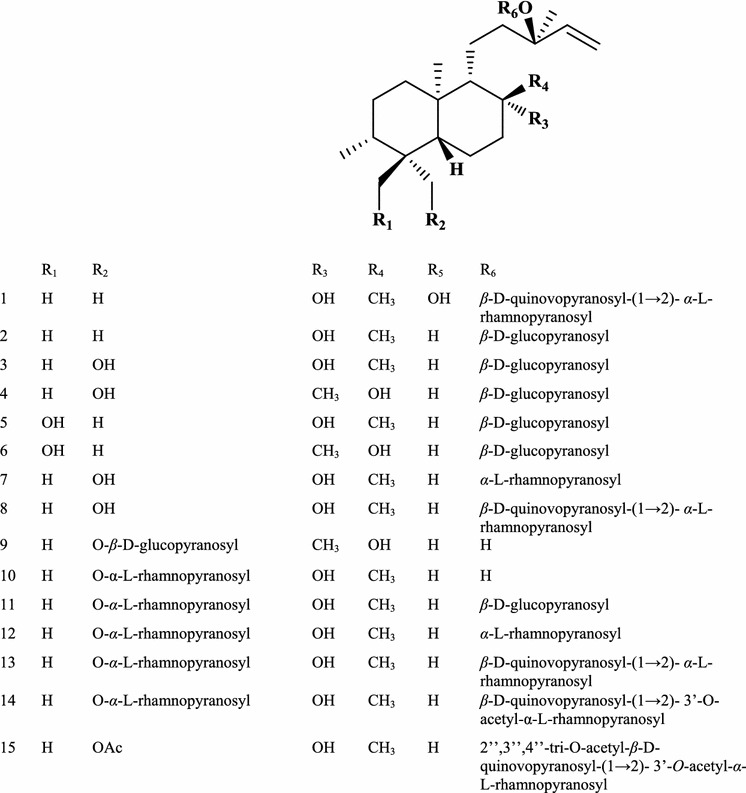
Diterpenoid glycosides from G. quadripartita (Socolsky et al. 2007)
Five ecdysteroids, (22R,24R,25S,26S)-2β,3β,14α,20R-tetrahydroxy-26α-methoxy-6-oxo-stigmast-7-ene-22,26-lactone (1), (22R,24R,25S)-2β,3β,14α,20R,26S-pentahydroxy-6-oxo-stigmast-7-ene-22,26-lactone (2), (22R,25S)-2β,3β,14α,20R,24S-pentahydroxy-6,26-dioxo-stigmast-7-ene-22,26-lactone (3), (22R,25S)-2β,3β,14α,20R,24S,26S-hexahydroxy-6-oxo-stigmast-7-ene-22,26-lactone (4), and capitasterone (5) (Fig. 17) (Hu et al. 2014), as well as two new diterpenoids, (3#,13S)-3-O-[6-O-acetyl-beta-d-glucopyranosyl]-13-O-alpha-l-rhamnopyranosyl-labda-8(17),14-diene and (4R,13S)-18-O-beta-d-glucopyranosyllabda-8(17),14-dien-13-ol (Hu et al. 2011), were detected in a 95% EtOH extract of D. rufopilosum from Yunnan province in China.
Fig. 17.
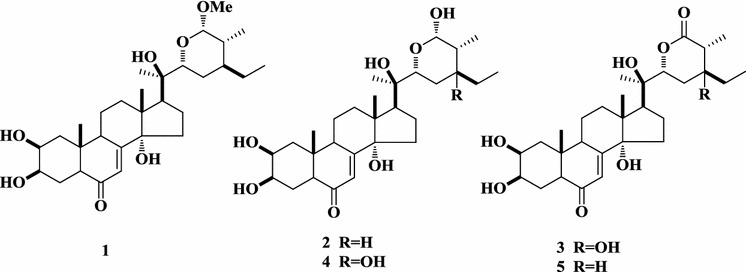
Ecdysteroids from D. rufopilosum of China (Hu et al. 2014)
Flavonoids
Favonol glycosides are also present in the family Gleicheniaceae. G. hirta Bl., G. microphylla R. Br., G. longissima Bl. and G. blotiana C. Chr. produce kaempferol and quercetin, while genkwanin and luteolin are present in G. blotiana C. Chr. and G. hirta Bl, and acacetin in G. microphylla R. Br. (Yusuf et al. 2003). The flavonols in Gleichenia leaves were found to be present as 3-glucosides, 3-rhamnosides, 3-rutinosides, 3,4′-diglucosides, 7-glucosides and 7-arabinoside. Quercetin-3-glucoside was identified as a major flavonoid component of all species studied (Yusuf et al. 2003).
Equisetaceae
The Equisetaceae are bushy perennial herbs native to the northern hemisphere that are commonly known as horsetails. They are represented by a single extant genus, Equisetum, which comprises around 30 species (Fig. 18). The content of inorganic substances (mainly silicic acid and potassium salts) in E. arvense is over 10%. E. arvense is also rich in sterols (β-sitosterol, campesterol, and isofucosterol) (D’Agostino et al. 1984), ascorbic acid, polienic acids, rare dicarboxylic acids (including equisetolic acid), flavonoids (Wichtl 1994; Veit et al. 1990; Oh et al. 2004), styrylpyrones (Veit et al. 1995a), and phenolic acids (cinnamic acids, caffeic acid, di-E-caffeoyl-meso-tartaric acid, and 5-O-caffeoylshikimic acids) (Veit et al. 1995b; Mimica-Dukic et al. 2008). The methanol extract of E. arvense L. was found to contain two phenolic petrosins, onitin and onitin 9-O-glucoside, along with four flavonoids, apigenin, luteolin, kaempferol 3-O-glucoside, and quercetin 3-O-glucoside (Oh et al. 2004).
Fig. 18.

Representative plants of Equisetaceae (a, b) and Ophioglossaceae (c, d). A. E. arvense L., sterile stems. B. E. arvense, fertile stem. C. Botrychium lunaria (L.) Sw. D. Ophioglossum vulgatum L. (Courtesy of Remo Bernardello)
A series of alkaloids have been isolated from E. palustre, namely palustrine, N5-formylpalustrine, N5-acetylpalustrine, palustridiene, and N5-formylpalustridiene, to which the toxicity of the plant is ascribed (Cramer et al. 2015).
Helminthostachyaceae
It was previously reported that four flavonoids, ugonins A–D, were isolated from the rhizomes of Helminthostachys zeylanica (Murakami et al. 1973a, b). Eight flavonoids, ugonins E–L, were isolated from the rhizomes of H. zeylanica (Huang et al. 2003).
Three new cyclized geranyl stilbenes, ugonstilbenes A–C were isolated from the dried rhizomes of H. zeylanica (Chen et al. 2003). Four new prenylated flavonoids, 4″a,5″,6″,7″,8″,8″a-hexahydro-5, 3′, 4′-trihydroxy-5″, 5″, 8″a-trimethyl-4H-chromeno[2″, 3″:7, 6]flavone (1), 4″a,5″,6″,7″,8″,8″a-hexahydro-5,3′,4′,-trihydroxy-5″,5″,8″a-trimethyl-4H-chromeno[2″,3″:7,8]flavone (2), 2-(3,4-dihydroxyphenyl)6-((2,2-dimethyl-6-methylenecyclohexyl)methyl)-5,7-dihydroxy-chroman-4-one (3), and 2-(3,4-dihydroxy-2-[(2,6,6-trimethylcyclohex-2-enyl)methyl] phenyl)-3,5,7-trihydroxy-4H-chromen-4-one (4) (Fig. 19), were isolated from H. zeylanica (Huang et al. 2010a, b, c).
Fig. 19.

Prenylated flavonoids from Helminthostachys zeylanica (Huang et al. 2010a, b, c)
Recently, two novel quercetin glucosides, namely 4′-O-β-d-glucopyranosyl-quercetin 3-O-β-d-glucopyranosyl-(1 → 4)-β-d-glucopyranoside and 4′-O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranosyl-quercetin 3-O-β-d-glucopyranosyl-(1 → 4)-β-d-glucopyranoside were isolated from H. zeylanica roots (Yamauchi et al. 2013).
Ophiolossaceae
Botrychium ternatum is a member of the Ophiolossaceae family that is distributed across China, Korea, and Japan. It is used in folk medicine to treat dizziness, headache, cough, and fevers. Twenty-six kaempferol glycosides, and four quercetin glycosides were identified from its methanol extracts (Warashina et al. 2012), including the new kaempferol glycosides ternatumosides I-XVII (Fig. 20).
Fig. 20.

Ternatumosides I-XVII from B. ternanum (Warashina et al. 2012)
Ophioglossaceae
To date, only a few flavonoids have been identified from Ophioglossum species. However, these species are rich in homoflavonoids. Seven new homoflavonoid glucosides, pedunculosumosides A–G, were isolated from ethanolic extracts of whole O. pedunculosum plants (Wan et al. 2012). Six homoflavonoids, ophioglonin, ophioglonin 7-O-β-d-glucopyranoside, ophioglonol, ophioglonol prenyl ether, ophioglonol 4′-O-β-d-glucopyranoside, and isoophioglonin 7-O-β-d-glucopyranoside, quercetin, luteolin, kaempferol, 3,5,7,3′,4′-pentahydroxy-8-prenylflavone, and quercetin 3-O-methyl ether, were isolated from O. petiolatum (Lin et al. 2005a, b). Homoflavonoids were also found in O. vulgatum and O. thermale (Wan et al. 2013). These compounds can be divided to two groups: type I homoflavonoids, which have an additional carbon atom attached to the C-3 position of ring C, and undergo competing losses of H2O and CH2O from their aglycone ions; and type II homoflavonoids, which bear an additional carbon atom at the C-2′ position of ring B, forming a new ring (Wan et al. 2013; Lin et al. 2005a, b; Wan et al. 2012).
In addition, several flavonoids were isolated from the aerial parts of the fern O. vulgatum L., including 3-O-methylquercetin and its glucosides, 5′-isoprenyl-3-O-methylquercetin 4′,7-di-β-d-glucopyranoside, 3-O-methylquercetin 4′-β-d-glucopyranoside 7-[O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside], and 3-O-methylquercetin 7-[O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside], were isolated from O. pedunculosum (Wan et al. 2012). 3-Methylquercetin, quercetin 3-O-[(6-caffeoyl)-β-glucopyranosyl(1 → 3)α- rhamnopyranoside]-7-O-α-rhamnopyranoside and kaempferol 3-O-[(6-caffeoyl)-β-glucopyranosyl(1 → 3)α-rhamnopyranoside]-7-O-α-rhamnopyranoside were isolated from O. pedunculosum (Clericuzio et al. 2012).
Different prenylated flavonoids have been isolated from Helminthostachys zeylanica (L.) Hook., including the new molecules neougonins A and B, and the previously-described ugonin D, 2-(3,4-dihydroxyphenyl)-6-((2,2-dimethyl-6-methylene cyclohexyl) methyl)-5,7-dihydroxy-chroman-4-one, ugonins E, J, L, and S, 4″a,5″,6″,7″,8″,8″a-hexahydro-5,3′,4′-trihydroxy-5″,5″,8″a-trimethyl-4H-chromeno [2″,3″:7,6]flavone, 4″a,5″,6″,7″,8″,8″a-hexahydro-5,3′,4′-trihydroxy-5″,5″,8″atrimethyl-4H-chromeno[2″,3″:7,8] flavone, and ugonin N (Su et al. 2016). Besides flavonoids, the new peroxy fatty acids thermalic acids A, and B, have been isolated from O. thermal (Dong et al. 2016).
Lygodiaceae
Lygodium venustum (Fig. 21), a cosmopolitan fern belonging to the family Lygodiaceae is widely distributed across Latin America, from Mexico to Paraguay and islands of the Caribbean, where it grows at altitudes of up to 1100 m above sea level (Costa and Pietrobon 2007; Mehltreter 2006; Prado 2005a). L. venustum is rich in flavonoids, including kaempferol 3-O-B-d-glucopyranoside, acacetin, acacetin 7-O-β-d-glucopyranoside, acacetin 7-O-rutinoside, diosmetin 7-O-rutinoside, 7-O-(6″-O-α-l-rhamnopyranosy1)-β-sophoroside, and kaempferol 3-O-rutinoside (Wang et al. 2011a, b, c). L. japonicum (Fig. 22) is another fern species from the same family that is used in traditional Chinese medicine (Chinese name 海金沙). Its main bioactive constituents are phenolic and flavonoid glycosides. Phenylpropanoid glucosides including 4-O-caffeoyl-d-glucopyranose, 3-O-caffeoyl-d-glucopyranose, 2-O-caffeoyl-d-glucopyranose, 6-O-caffeoyl-d-glucopyranose, 4-O-p-coumaroyl-d-glucopyranose, 6-O-p-coumaroyl-d-glucopyranose were isolated from the roots of L. japonicum (Duan et al. 2012a, b). 3,4-Dihydroxybenzoic acid 4-O-(4′-O-methyl)-β-d-glucopyranoside (Ye et al. 2007) and a new ecdysteroside, 2,3,14,20R,22R-pentahydroxy-24R-methyl-5-cholest-7-en-6-one-3-O-d-glucopyranoside were isolated from the roots of L. japonicum (Thunb.) (Fig. 23) (Zhu et al. 2009). 1,4-Naphthoquinone (Chen et al. 2010) and two new tetracyclic triterpenoids, lygodipenoids A and B, with a new 9,19 : 24,32-dicyclopropane skeleton, were also isolated from whole plants of L. japonicum (Han et al. 2012).
Fig. 21.

Aerial parts of the lianescent fern Lygodium venustum. (Courtesy of Flaviana Morais-Braga)
Fig. 22.

Lygodium japonicum (Thunb.) Sw (Courtesy of Jianguo Cao)
Fig. 23.

Lycopodium japonicum Thunb (Courtesy of Jianguo Cao)
Lindsaeaceae
Stenoloma chusanum (L.) Ching (Fig. 24) belongs to the family Lindsaeaceae and is widely distributed in southern China. It has a very high total flavonoid content (up to 30% w/w) and shows strong antioxidant and antibacterial activities (Xia et al. 2014). The total flavonoid content of S. chusanum exhibits clear seasonal dynamics, peaking at 24.63 ± 1.34% in February (Wu et al. 2016). Two new phenolic compounds, 4-O-β-d-(6-O-gentisoylglucopyranosyl) vanillic acid, 2-O-β-d-(6-O-gentisoylglucopyranosyl) gentisic acid, vanillic acid, syringic acid, and gentisic acid, were isolated from whole S. chusanum plants (Ren et al. 2009).
Fig. 24.

Stenoloma chusanum Ching (Courtesy of Jianguo Cao)
Athyriaceae and Aspleniaceae
Umikalsom et al. (1994) compared the flavonoid contents of 18 Athyriaceae species and 15 Aspleniaceae of Malaysian origin. Flavonol 3-O-glycosides (quercetin and kaempferol) were the main flavonoids in the Athyriaceae, with some Diplazium and Deparia species also having appreciable contents of flavone C-glycosides (apigenin C-glycosides). The flavonoid profiles of the Aspleniaceae are much more complex. Kaempferol 3,7-glycosides predominate, but kaempferol 3,4′-diglycosides and 3,7,4′-triglycosides were also found. O-Methylated kaempferol glycosides were found in A. marinum. Luteolin and apigenin C-glycosides, sometimes with O-glycosylated C-sugars were detected in Aspleniaceae species (Umikalsom et al. 1994).
The profiles and bioactivities of flavonoids extracted from Dryoathyrium boryanum (Willd.) Ching were investigated by Cao et al. (2013b). Based on HPLC–DAD–ESI–MS analyses, the main flavonoids in D. boryanum were tentatively identified as 3-hydroxyphloretin 6′-O-hexoside, quercetin-7-hexoside, apigenin7-O-glucoside, luteolin 7-O-glucoside, apigenin 7-O-galactoside, acacetin 7-O-(α-d-apio-furanosyl)(1 → 6)-β-d-glucoside, 3-hydroxy phloretin 6-O-hexoside, and luteolin 6-C-glucoside (Cao et al. 2013b).
Davalliaceae
Some species of the genus Davallia, such as Davallia divaricata, D. mariesii, D. solida, D. formosana, Drynaria fortunei (Kunze) J.Sm., D. cylindrica (Fig. 25) are used in Gusuibu (Chinese name 骨碎补), a famous traditional Chinese Medicine used to treat inflammation, cancers, aging, bone injuries, and osteoporosis (Chang et al. 2007). The main bioactive constituents of Davallia species are flavanones, flavan-3-ols, procyanidins, and proanthocyanidins (Chen et al. 2008a, b; Cui et al. 1990; Ko et al. 2012; Cheng et al. 2012) (Tables 5, 6). The total flavonoid content of D. cylindrica Ching was determined to be around 164.41 mg/g (w/w) (Cao et al. 2014), and flavan-3-ol dimers, trimers and tetramers are particularly abundant in these species. Thus, procyanidin B-2 (dimer), epicatechin-(−(4β → 8)-epicatechin-(4β → 6)-epicatechin (trimer), epiafzelechin-(4β → 6)-epicatechin-(4β → 8)-epicatechin-(4β → 6)-epicatechin (tetramer) have all been isolated from D. mariesii MOORE and D. divaricata Blume (Hwang et al. 1989, 1990; Cui et al. 1990, 1993). Additionally, (−)-epiafzelechin-(4β → 8)-4β-carboxymethyl-(−)-epicatechin methyl ester, (−)-epiafzelechin-(4β → 8)-4α-carboxymethyl-(−)epiafzelechin ethyl ester, (−)-epiafzelechin-(4β → 8)-(−)-epiafzelechin-(4β → 8)-4β-carboxymethyl-(−)-epiafzelechin methyl ester were isolated from the rhizomes of D. fortunei (Liang et al. 2011).
Fig. 25.

Davallia cylindrica (Courtesy of Jianguo Cao)
Table 5.
New kaempferol glycosides from B. ternanum (Warashina et al. 2012)
| Name | Structure |
|---|---|
| Ternatumoside I | Kaempferol 3-O-β-d-quinovopyranosyl-(1 → 2)-α-l-rhamnopyranoside |
| Ternatumoside II | Kaempferol 3-O-β-d-glucopyranosyl-(1 → 3)-α-l-rhamnopyranoside |
| Ternatumosides III | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside |
| Ternatumosides IV | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside |
| Ternatumoside V | kaempferol 3-O-(2,3,4-tri-O-β-d-glucopyranosyl)-α-l-rhamnopyranoside |
| Ternatumoside VI | Kaempferol 3-O-[β-Dglucopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside |
| Ternatumoside VII | Kaempferol 3-O-[β-d-xylopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside |
| Ternatumoside VIII | Kaempferol 3-O-(2,3-di-O-β-d-glucopyranosyl)-α-l-rhamnopyranoside-7-O-α-l-rhamnopyranoside |
| Ternatumoside IX | Kaempferol 3-O-β-d-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside |
| Ternatumoside X | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-α-l-rhamnopyranoside |
| Ternatumoside XI | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-glucopyranosyl-(1 → 2)-α-Lrhamnopyranoside-7-O-β-d-glucopyranoside |
| Ternatumoside XII | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-β-d-glucopyranoside |
| Ternatumoside III | Kaempferol 3-O-[β-Dxylopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy- (E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-glucopyranosyl-(1 → 2)-α-Lrhamnopyranoside-7-O-α-l-rhamnopyranoside |
| Ternatumoside XIV | Kaempferol 3-O-[β-d-xylopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-α-l-rhamnopyranoside |
| Ternatumoside XV | Kaempferol 3-O-[β-d-xylopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-Lrhamnopyranoside-7-O-β-d-glucopyranoside |
| Ternatumoside XVI | Kaempferol 3-O-[β-d-glucopyranosyl-(1 → 4)]-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-β-d-glucopyranoside |
| Ternatumoside XVII | Kaempferol 3-O-[β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 3)]-β-d-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1 → 2)-α-l-rhamnopyranoside-7-O-α-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside |
Table 6.
Flavan-3-ols, procyanidins and phenolic acids in Davallia species
| Source | Polyphenols and phenolic acids | References |
|---|---|---|
| D. formosana | 6,8-Dihydroxychromone 7-C-β-d-glucopyranoside, 6,8,3′,4′-tetrahydroxyflavanone-7-C-β-d-glucopyranoside, 6,8,4′-Trihydroxyflavanone 7-C-β-d-glucopyranoside, 8-(2-pyrrolidinone-5-yl)-catechin 3-O-β-d-allopyranoside, epiphyllocoumarin 3-O-β-d-allopyranoside, (−)-epicatech-3-O-β-d-allopyranoside, (−)-epicatech-3-O-β-d-(2′′-O-vanillyl)-allopyranoside, (−)-epicatech-3-O-β-d-(3′′-O-vanillyl)-allopyranoside, eriodictyol-8-C-β-d-glucopyranoside, davallioside A, davallioside B, caffeic acid-4-O-β-d-glucopyranoside, p-coumaric acid 4-O-β-d-glucopyranoside, protocatehuic acid, 4-hydroxy-3,5-dimethylbenzoic acid, vanillic acid | Chen et al. (2014a, b), Cui et al. (1990, 1992), Hsu and Chen (1993) |
| D. fortunei (Kunze) J.Sm. | 4α-Carboxymethyl-(+)-catechin methyl ester, (+)-afzelechin 3-O-β-allopyranoside, (+)-afzelechin 6-C-β-glucopyranoside, (−)-epiafzelechin-(4β → 8)-4β-carboxymethyl-(−)-epicatechin methyl ester, -(−)-epiafzelechin-(4β → 8)-4α-carboxymethyl-(−)epiafzelechin ethyl ester, (−)-epiafzelechin-(4β → 8)-(−)-epiafzelechin-(4β → 8)-4β-carboxymethyl-(−)-epiafzelechin methyl ester | Liang et al. (2011) |
| D. solida (Forst.) Sw | Mangiferin, 3′-O-p-hydroxybenzoylmangiferin, 4′-O-p-hydroxybenzoylmangiferin, 4β-carboxymethyl-(−)-epicatechin methyl ester, 6′-O-p-hydroxybenzoylmangiferin, icariside E-3, 3-O-p-hydroxybenzoylmangiferin, eriodictyol, eriodictyol 8-C-β-d-glucopyranoside, 2-C-β-d-4β-carboxymethyl-(−)-epicatechin, icariside E-5, xylopyranosyl-1,3,6,7-tetrahydroxyxanthone, 2-C-β-d-xylopyranosyl-1,3,6,7-tetrahydroxyxanthone | Chen et al. (2008a, b), Rancon et al. (1999) |
| D. mariesii MOORE | Davallin, 5-O-beta-d-(6-O-vanilloylglucopyranosyl)gentisic acid, 4-O-beta-d-(6-O-vanilloylglucopyranosyl)vanillic acid, procyanidin B-2 and B-5, epicatechin-(-(4β → 8)-epicatechin-(4β → 6)-epicatechin, epicatechin-(4β → 6)–epicatechin-(4β → 8)-epicatechin-(4β → 6)-epicatechin, protocatechuic acid, 1-naphthol-β-d-glucopyranoside, davallialactone, (±)-eriodictyol 7-O-beta-d-glucuronide, caffeic acid, 4-O-beta-d-glucopyranosylcaffeic acid, 4-O-beta-d-glucopyranosyl-p-coumaric acid | Cui et al. (1990, 1993) |
| D. divaricata Blume | Davallic acid, (+)-catechin 3-O-β-d-allopyranoside, (−)epicatechin 3-O-β-d-allopyranoside, procyanidins B-1 and B-2, trimeric procyanidin, β-carboxymethyl-(−)-epicatechin, and epiafzelechin-(4β → 6)-epicatechin-(4β → 8)-epicatechin-(4β → 6)-epicatechin | Hwang et al. (1989, 1990) |
The genus Elaphoglossum, recently relocated in the family Dryopteridaceae, comprises around 600 species and is widely spread in South America. The acylphloroglucinols (Fig. 26) are found in E. lindbergii (Socolsky et al. 2011a, 2016), E. yungense (Socolsky et al. 2010a, b), E. piloselloides (Socolsky et al. 2009) and E. gayanum (Socolsky et al. 2010b). Specially, the prenylated acylphloroglucinols, elaphogayanin A-B, elaphopilosin C- E, lindbergins A-F and yungensins A-F were identified from E. gayanum (Socolsky et al. 2010b), E. piloselloides (Socolsky et al. 2010b), E. lindbergii (Socolsky et al. 2011a, 2015) and E. yungense (Socolsky et al. 2010a).
Fig. 26.

New prenylated phloroglucinols from genus Elaphoglossum (Socolsky et al. 2010a, b, 2016)
Diphasiastrum
The genus Diphasiastrum includes more than 23 species, 11 of which have been reported to produce lycopodium alkaloids; the distribution of these alkaloids across this genus was recently reviewed by Halldorsdottir et al. (2015). Most lycopodium alkaloids identified in the Diphasiastrum to date belong to the lycopodine, lycodine, or fawcettimine families.
Biological activity of fern species
Pteridaceae
Pteris is one of the largest and most economically important fern genera, comprising some 200–250 species that are distributed across all the world’s continents bar Antarctica. Its species diversity is greatest in the tropical and subtropical regions (Zhang et al. 2014). Many species belonging to this genus have significant value as medicines and spices (Jiang Su New College of Medicine 1985). The most important and intensively studied by far are P. semipinnata L. and P. multifida, both of which are discussed in traditional Chinese medicine texts and used extensively to treat various symptoms (Jiang Su New College of Medicine 1985). P. multifida Poiret, also known as Fong-Wei-Cao, is not only one of the most widely used vegetables and herbs in China but also is a popular component of herbal beverages in Taiwan (Lu et al. 1999; Harinantenaina et al. 2008). P. semipinnata L. is widely distributed in China and is used in folk medicine to treat toothache, diarrhoea, jaundices, and viper bites (Zhang et al. 1999, 2007). Pteris vittata L. is the first reported arsenic-hyperaccumulating fern. Traditionally, it is used to treat abdominal pains, diarrhoea, and flu (Li 2006). There is also preliminary evidence that aqueous extracts of this species are cytotoxic towards K562 leukaemic cells (Chai et al. 2015a). P. multifida is another arsenic hyperaccumulator that is widely distributed in China and other Asian countries. It has been used to treat enteritis, hepatitis, bacterial dysentery, hematemesis, hematuria, tonsillitis, parotitis and eczema (Cao et al. 2004; Srivastava et al. 2005; Rahman 2008; Rahman et al. 2014; Jiang Su New College of Medicine 1985).
There are also reports discussing the use of Pityrogramma calomelanos as a medicinal plant. It appears to be very versatile: its parts are used to treat renal, urinary and circulatory disturbances, digestive problems related to biliary calculi, cough, cold, chills, pains, fever, arterial hypertension, and bleeding, among other things (Barros and Andrade 1997; Cheryl 2006; May 1978).
Adiantum is another important genus within the family Pteridaceae (Brahmachari et al. 2003; Pan et al. 2011), whose members are distributed across tropical, sub-tropical, and temperate regions. Many species in this genus have ethnopharmacological applications. For instance, A. capillus-veneris Linn. is used to treat skin diseases and measles in Northwestern Pakistan (Abbasi et al. 2010), and to treat inflammatory diseases (Haider et al. 2011). In traditional Chinese medicine, members of this genus have been used to treat fevers, promote urination, and relieve swelling (Pan et al. 2011).
Anticancer activity
The genus Pteris is a rich source of bioactive ent-kaurane diterpenoids. Many compounds in this class exhibit very good anticancer activity (Liu et al. 2011a, b, c; Shu et al. 2012; Qin and Zhu 2004), and it has been reported that the Michael-accepting capability of their α,β-unsaturated ketone moieties is essential for this cytotoxicity (Sun et al. 2006; Ge et al. 2008).
Pteris semipinnata L. is used as a medicinal plant for the treatment of venomous snake bites in Chinese folk medicine. In recent years, pharmacological investigations on this species have primarily focused on the ent-kaurane diterpenoid ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid, which was shown to exhibit significant cytotoxicity and anticancer activity by Zhang et al. (1996, 1999, 2007). ent-11α-Hydroxy-15-oxo-kaur-16-en-19-oic-acid has inhibitory effects in various cancer cell lines: it induces apoptosis in human colon cancer HT-29 cells by increasing the abundance of p38 and iNOS. However, this effect can be mitigated by overexpression of Bcl-2 or Bcl-xL, which upregulates NF-κB activity and leads to apoptotic offset (Chen et al. 2004, 2008a, b). Interestingly, ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid exhibited stronger cytotoxicity in the gastric cancer cell line MKN-45, which expresses the wild-type p53 protein, than in the related gastric cancer line MKN-28, which expresses a p53 variant with a missense mutation. Further investigations revealed that ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis in MNK-45 (in the presence of wild-type p53) by causing the translocation of Bax into mitochondria, leading to a reduction in ΔΨm and DNA fragmentation (Liu et al. 2005a). In addition, the release of cytochrome c and apoptosis inducing factor from mitochondria into the cytosol was also observed during the apoptosis of anaplastic thyroid carcinoma cells treated with ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid (Liu et al. 2005b). In vitro experiments showed ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits the proliferation of the human lung cancer cell lines A549, NCI-H23 and CRL-2066, arrested the cell cycle in the G2 phase, and induced apoptosis through a mitochondria-mediated pathway. In keeping with previous results, mechanistic investigations indicated that ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis by triggering the overexpression and translocation of Bax into the mitochondria, leading to the release of cytochrome c into cytosol, activation of caspase-3, and translocation of AIF from mitochondria into the nucleus (Li et al. 2010, 2012). In addition, ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid significantly inhibited the development of NNK-induced mouse lung cancer in vivo by inducing apoptosis and exerting anti-proliferation effects with minimal side effects (Li et al. 2010). In vivo and in vitro investigations confirmed that ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid effectively inhibits hepatocellular carcinoma (HCC), significantly reducing the number of tumor foci and the size of tumors in a diethylnitrosamine-induced mouse HCC model with minimal side effects. It also induced the death of HCC cells by stabilizing IkBα to inhibit NF-κB (Chen et al. 2012a). In tests against CNE-2Z nasopharyngeal carcinoma (NPC) cells, ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid exhibits significant inhibitory effects, causing cell cycle arrest in G2 phase and apoptosis by increasing the Bax/Bcl-2 ratio and the level of cytochrome c in the cytosol while reducing levels of NF-κB-p65 and increasing those of IκB (Wu et al. 2013). Finally, ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid sensitised A549 cells to cisplatin despite reducing cisplatin-induced ROS production (Li et al. 2012).
In addition to ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid, 7,9-dihydroxy-15-oxo-ent-kaur-16-en-19,6-olide and 7,11-dihydroxy-15-oxo-ent-kaur-16-en-19,6-olide from P. semipinnata can inhibit lung adenocarcinoma cells by acting as inhibitors of DNA topoisomerases (TOPO) and tyrosine protein kinase (TPK) (Tomšík 2014). 7,9-Dihydroxy-15-oxo-ent-kaur-16-en-19,6-olide inhibited TOPO II at a concentration of 0.01 mg/L, while 7,11-dihydroxy-15-oxo-ent-kaur-16-en-19,6-olide was a moderately potent inhibitor of membrane TPK and also reduced the expression of the oncogene c-myc (Li et al. 2001).
Bracken (Pteridium spp.) illudane glycosidess are labile biologically active terpenoids that undergo decomposition under mildly acidic or alkaline conditions, under heating, or in the presence of degradative enzymes (Cáceres-Peña et al. 2013). The novel bihomoflavanonol pteridium III, which has an unprecedented skeleton, was isolated from P. aquilinum and exhibits in vitro antitumor activity against NCI-H46 lung cancer cells, A375 melanoma cells, and U-7MG glioma cells with IC50 values of 22.9, 106.7, and 1540.5 μmol/L, respectively (Chen et al. 2013a, b).
The pterosins (Fig. 8) are a large group of naturally occurring sesquiterpenes with indanone skeletons. They are widely distributed among the Pteridophyte and exhibit both cytotoxicity and smooth muscle relaxant activity. Pterosin Z and acetyl-Δ2-dehydropterosin B were found to be particularly cytotoxic (Chen et al. 2008a, b). Pterosin Z was later isolated from Microlepia speluncae (L.) Moore, M. trapeziformis (Roxb. ex Griff.) Kuhn and M. substrigosa Tagawa (Tomšík 2014). In addition, bimutipterosins A and B, isolated from Pteris multifida, exhibited cytotoxicity against the HL60 human leukemia cell line (Liu et al. 2011a, b, c).
Pterosin C and other C14 and C15 sesquiterpenoids, along with C20-monoxygenated ent-kaurane diterpenoids and 4, 5-dicaffeoylquinic acid, were identified as the cytotoxic constituents of P. multifida Poir. (Ge et al. 2008; Harinantenaina et al. 2008; Ouyang et al. 2010). The pterosin sesquiterpenes 2R,3R-13-hydroxy-pterosin L 3-O–D-glucopyranoside, 2R,3S-acetylpterosin C, and 2S,3S-acetylpterosin C also showed cytotoxicity against HL60 cells with IC50 values of 14.6, 48.3 and 35.7 mM, respectively (Shu et al. 2012). The new C14 pterosin-sesquiterpenoids multifidosides A and B showed cytotoxicity against the HepG2 tumor cell line, with IC50 values below 10 µM, and also displayed inhibitory effects on K562 cells with IC50 values of 10.63 and 9.57 µM, respectively (Ge et al. 2008). The new diterpene 5,11,12-trihydroxy-15-oxo-ent-kuar-16-en-19-oic acid and the sesquiterpene 1, 3-dihydroxylnorpterosin C from Pteris dispar showed potent cytotoxicity against KB cells in vitro, with IC50 values of 59.8 and 36.5 mol/L by the MTT method (Gou et al. 2011)..
Pterosin B, one of the main pterosins found in the genus Pteris, exhibits potent cytotoxic activity against HL60 (human leukemia) cells (Qin and Zhu 2004; McMorris et al. 1992). While searching for the carcinogenic constituent of bracken, Yoshihira et al. (1978) monitored the development of cytotoxicity-related morphological changes in HeLa cells upon incubation with bracken components. These studies failed to detect the potent carcinogen ptaquiloside, but did lead to the isolation of several indanone-type sesquiterpenoidspterosins and their glycosides, the pterosides.
The in vitro antitumor activity of P. calomelanos and its isolated dihydrochalcones (DHCs) was evaluated in India by Sukumaran and Kuttan (1991) using the trypan blue exclusion assay with Dalton’s lymphoma ascites tumor cells (DLA cells) and Ehrlich ascites tumor cells (EA cells). The extract concentrations leading to 50% cytotoxicity in these cell lines were 16 and 18 µg/mL, respectively. The isolated DHCs exhibited greater cytotoxicity against these cell lines, with IC50 values of 6.1 and 11.5 µg/mL, respectively. They also exerted cytotoxic effects in human myelogenous leukemia K562 cells and human nasopharyngeal KB cells, with IC50 values of 1.1 and 8 µg/mL, respectively. A liposome preparation of the isolated DHCs was also tested in vivo in female Swiss albino mice to evaluate its effect on their survival after injection with Ehrlich ascites tumor cells. The treatment increased the animals’ lifespan by 52 and 57% when applied at doses of 5 and 25 mg/kg, respectively. Because tumors are characterized by rapid cell division, the capacity of DHC to inhibit DNA synthesis was investigated by monitoring the incorporation of labelled thymidine in tumor cells, yielding an estimated IC50 of 8 µg/mL. These results indicate that P. calomelanos has antitumor activity and is cytotoxic because of its DHC content.
In addition to the promising results discussed above, an ethanol extract of Adiantum venustum Don. was shown to increase the mean survival time in carcinoma-bearing mice relative to a positive control group treated with the established drug vincristine (Viral et al. 2011).
Antiprotozoal activity
As part of an ethnopharmacological screening program seeking medicinal plants with antiprotozoal activity, the fern P. calomelanos was investigated by Valadeau et al. (2009). Tests for antiplasmodial activity were conducted utilizing erythrocytes infected with Plasmodium falciparum FCR3, and an ethanolic extract of this plant exhibited an IC50 of 49.9 μg/mL in the assay. While promising, this result did not satisfy the study’s predefined activity criterion, under which an extract was considered to have good activity if its assayed IC50 was below 10 μg/mL. The investigators also examined the extract’s antileishmanial activity against axenic amastigotes of Leishmania amazonensis (strain MHOM/BR/76/LTB-012), obtaining an IC50 of 88 μg/mL. When studying the antiprotozoal activity of ethanolic extracts of P. calomelanos and extract subfractions against L. braziliensis, Souza et al. (2013a) observed 100% inhibition of the promastigote forms upon treatment with either the ethanolic extract or the ethyl acetate fraction at a concentration of 500 μg/mL. However, these extract concentrations induced significant cytotoxicity in tests using mammalian fibroblasts from the NCTC929 cell line. Consequently, there is a need for further investigations to determine whether the compounds responsible for the extracts’ leishmanicidal activity are also the ones that are harmful to mammalian cells.
Souza et al. (2012a) also conducted a similar screening campaign to identify plant extracts with trypanocidal activity against the epimastigote forms of the Trypanosoma cruzi CL-B5 strain. They observed that the hexane fraction of the ethanolic extract of P. calomelanos achieved 73.57% trypanosomal growth inhibition at a concentration of 50 μg/mL, and that this treatment had no detectable adverse effect on mammalian fibroblasts. However, the ethanolic extract exhibited complete cytotoxicity at a concentration of 500 μg/mL, with only 55.26% inhibition of epimastigotes. It thus appears that a detailed analysis of the hexane fraction may reveal promising trypanocidal compounds.
Antidiabetic activity
Pterosin A is a low-molecular-weight natural product that has been isolated from several different ferns (Qin and Zhu 2004). Its antidiabetic activity has been studied in several diabetic mouse models, in which it effectively alleviates hyperglycemia, glucose intolerance, insulin resistance, dyslipidemia, and islet hypertrophy. Pterosin A can suppress the reduction of body weight in diabetic mice as well as increases in body weight in fed mice and db/db diabetic mice. Moreover, it reverses the diabetes-associated reduction in GLUT-4 translocation from the cytosol to the membrane in the skeletal muscles of diabetic mice, and the increased PEPCK expression in their livers. An AMPK-regulated signalling pathway is involved in the activation of muscle GLUT-4 and inhibition of liver PEPCK expression by pterosin A. Pterosin A also increases GSK3 phosphorylation, further enhancing intracellular glycogen synthesis in liver cells. In addition, pterosin A treatment effectively reversed islet hypertrophy in db/db mic. Its antidiabetic activity is associated with inhibition of gluconeogenesis in the liver and enhanced glucose consumption in peripheral tissues. These findings indicate that pterosin A may be a viable therapeutic option for diabetes (Hsu et al. 2013).
Chai et al. (2015a) reported that an aqueous extract of P. vittata fronds exhibited moderate, dose-dependent antiglucosidase activity (EC50 87 µg/mL) when compared to myricetin (EC50 53 µg/mL). The identity of the alpha-glucosidase inhibitors in the extract is unknown.
Antitubercular activity
P. ensiformis Burm. was shown to exhibit antitubercular activity in vitro when screened against the M. tuberculosis H37Rv strain. (2S)-13-Hydroxypterosin A, (2S, 3S)-12-hydroxypterosin Q, pterosin B, and alpha-tocopheryl quinone from P. ensiformis exhibited antitubercular activity (MIC = 40 g/ml) against M. tuberculosis H37Rv in vitro. (2S)-13-Hydroxypterosin A was the most effective of the tested antitubercular compounds, with an MIC value of 6.25 g/ml against M. tuberculosis H37Rv (Chen et al. 2013a).
Antimicrobial activity
Extracts of many different ferns have been tested for activity against bacteria, fungi and viruses (Zhou and Li 1998; Xu et al. 2005). Water extracts of P. multifida showed remarkable antibacterial activity against Shigella sp., Escherichia coli, Proleus vulgaris, Staphylococcus aureus, and Bacterium pyocyaneum (Zhou and Li 1998; Kubo et al. 1992). In addition, water extracts and alcohol extracts from 20 species of medicinal pteridophytes were tested for bacteriostatic activity against S. aureus, E. coli, S. lutea, P. vulgaris, B. subtilis and S. cerevisiae. Water and alcohol extracts of P. ensiformis Burm. and P. semipinnata exhibited particularly strong activity in these experiments (Cai et al. 2003). The inhibitory effects of polysaccharides from eight species of pteridophytes against eight species of microorganisms (E. coli, P. vulgaris, S. aureus, R. solanacearum, A. flavus, P. sp., S. cerevisiae, M. grisea.) were tested using the disk agar diffusion method, revealing that polysaccharide extracts of P. aquilinum, P. vittata, P. multifida exhibited clear inhibitory effects against bacteria and fungi including E. coli, P. vulgaris, S. aureus, S. cerevisiae, and Penicillium sp. (Xu et al. 2005).
The antibacterial activity of fern extracts has also been studied extensively by Souza et al. (2012b, 2013b). This group determined the MIC values of the ethanolic extract and various fractions of P. calomelanos by the microdilution method but did not detect any clinically relevant activity, obtaining MIC values above 1024 µg/mL for both bacteria and fungi (genus Candida). However, interesting results were obtained in drug-modifying assays where the natural product extracts were combined with conventional drugs. In these studies, the ethanolic extract as well as the hexane and methanolic fractions potentiated the action of antifungals against the yeasts C. albicans, C. krusei and C. tropicalis. Against bacteria, the extract and fractions exhibited drug-modifying potential in conjunction with some aminoglycoside antibiotics when tested against E. coli and S. aureus. Subsequent experiments (Souza et al. 2013c) demonstrated that the interaction between the natural products (i.e. the ethanolic extract and the ethyl acetate fraction) and the tested drugs (amikacin, gentamicin, and neomycin) was additive.
A flavonoid isolated from the ethyl acetate fraction of a P. calomelanos extract was evaluated for antibacterial activity. The isolated compound was 2′, 6′-di-hydroxy-4′-methoxydihydrochalcone, and it was tested against Gram-positive and Gram-negative bacteria by the disk diffusion method. It exhibited an intermediate level of antimicrobial activity, forming inhibition halos of 18.43 mm against S. aureus and 18.70 mm against E. coli. Luciano-Montalvo et al. (2013) conducted a separate ethnopharmacological antimicrobial screening using the disk diffusion method (with some modifications) with extracts from 13 plant species, revealing that a decoction of P. calomelanos leaves inhibited bacterial growth in the order P. aeruginosa > S. aureus > S. saprophyticus, with inhibition percentages above 20% relative to a positive control (streptomycin).
Extracts of several Adiantum species have also demonstrated potent antimicrobial activity. A. venustum is especially noteworthy, as its methanol extract was found to inhibit a broad range of Gram-positive and Gram-negative bacteria. In addition, an extract of A. capillus-veneris L. had a very low MIC value (0.48 µg/mL) against E. coli; for comparative purposes, the corresponding value for the reference antibiotic gantamicin is 7.81 µg/mL (Singh et al. 2008).
Antioxidant activity
Ferns are widely used as traditional medicinal herbs in part because of their content of phenolic compounds. These substances are potent antioxidants that play an important role in human nutrition as protective agents against several diseases. Many flavonoids, especially flavonols, have been isolated and characterized from ferns (Harborne and Williams 1988; Cao et al. 2012), and the DPPH radical scavenging activity of fern extracts has been shown to increase with their total flavonoid concentrations (Xia et al. 2014). An extract of D. boryanum (Willd.) Ching (Cao et al. 2013a, b) with a total flavonoid content of around 145.8 mg/g exhibited very strong superoxide anion radical scavenging potential at a concentration of 0.21 mg/ml, which is higher than that of rutin (0.25 mg/mL). Poudel (2011) reported that the polyphenol content of the ethyl acetate fraction of D. boryanum is 266 μg GAE/mg and that it exhibits potent antioxidative activity based on its DPPH free radical scavenging activity and hydrogen peroxide scavenging activity. The ethyl acetate fraction of D. boryanum also reduced lipid oxidation by 35% when applied to the 3T3-L1 cell line at 100 μg/mL.
Phytochemical investigations on the Pteris genus have identified various phenolic compounds (Lu et al. 1999; Gong et al. 2007). Aqueous extracts of the Sword Brake fern (P. ensiformis Burm.), a common ingredient in traditional Taiwanese herbal drinks, showed strong antioxidant activity in DPPH assay. Moreover, caffeic acid and its derivatives 7-O-caffeoylhydroxymaltol 3-O-β-d-glucopyranoside, 5-O-caffeoyl-quinic acid, 3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid as well as hispidin glucoside (hispidin 4-O-β-D-glucopyranoside) were found to have similar or superior DPPH scavenging activities to the common antioxidant supplement alpha-tocopherol. This suggests that a catechol moiety facilitates DPPH scavenging. Yung-Husan Chen et al. (2007) reported that 3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid (both of which are present in the Sword Brake fern) have strong DPPH scavenging potential, with IC50 values of around 10 µM and a TEAC of around 2 mM. P. multifida Poiret is another fern that is commonly used in Taiwanese herbal beverages, and is also widely eaten as a vegetable in mainland China. An aqueous extract of P. multifida Poiret showed high antioxidant activities in a conjugated diene assay. Moreover, at a concentration of 20 mg/mL, it exhibited high radical scavenging activity towards DPPH, hydroxyl, and ferrous radicals (82.5, 80.1 and 85.4 mg/mL, respectively) as well as a high level of reducing power based on absorbance at 700 nm. Its antioxidant activity is somewhat variable, presumably because of variation in the distribution and relative abundance of its naturally occurring antioxidant components (Lan et al. 2011).
Antioxidant tests in vitro showed that the purified polysaccharide prepared from P. aquilinum is a powerful scavenger of superoxide radicals and the DPPH radical, as well as an effective inhibitor of 1,2,3-phentriol self-oxidation. In addition, the lipopolysaccharides had a high FRAP value (827.6 mol/L) comparable to that of vitamin C. These results suggest that water-soluble polysaccharides warrant further exploration as potential natural antioxidants (Xu et al. 2009).
Lai and Lim (2011) investigated the capacity of methanolic extracts of 15 pteridophyte species, including P. calomelanos, to sequester free radicals. Three radical scavenging assays were used—DPPH assay, FRAP assay, and bleaching assays. The extracts’ overall activity was moderate and correlated with their total phenol content, which was also moderate. Morais-Braga et al. (2012a) utilized the DPPH assay to study the antioxidant capacity of the hexane, chloroform, ethyl acetate and methanolic fractions of P. calomelanos, revealing that the ethanolic extract and methanolic fraction showed the best results, with IC50 values of 43.4 and 123.57 μg/mL, respectively. The activity was attributed to presence of polar compounds in the extracts such as phenolic acids and flavonoids, which have known antioxidant activity.
Toxicity and carcinogenic activity
Ptaquiloside is a norsesquiterpene glucoside of the illudane type that was shown to be responsible for many conditions associated with bracken consumption by livestock, including acute haemorrhagic disease in cattle (bracken poisoning), bright blindness in sheep, bovine enzootic haematuria, and upper alimentary carcinoma. The biological activity of this reactive glycoside has been attributed to the facile elimination of glucose to form an unstable conjugated dieneone intermediate that acts as a powerful alkylating agent of amino acids and DNA (Yamada et al. 2007).
A new toxic unstable sesquiterpene glycoside, ptaquiloside Z, from the neotropical bracken fern P. aquilinum var. caudatum showed toxicity toward brine shrimp (LC50 62.5 g/mL at 24 h and LC50 7.8 g/mL at 48 h) (Castillo et al. 1998).
The only vascular plant known to cause cancer in humans is the bracken fern P. aquilinum (L.) Kuhn. Bracken is currently the most common fern worldwide and one of the most aggressive and most widely distributed weeds of all; the only regions where it is not found are those with polar or desert climates (Vetter 2009). The toxicity of bracken, its mutagenic, carcinogenic and teratogenic effects in animals and humans, and its assumed mechanism of action have been described at length (Yamada et al. 2007; Vetter 2009). Ptaquiloside was discovered in 1983, and its carcinogenicity was clearly demonstrated in 1984: intragastric administration of ptaquiloside to rats induced mammary cancer (100%) and ileal tumours (91%) (Yamada et al. 2007). The unstable norsesquiterpene glucoside ptaquiloside has been conclusively identified as the main mutagenic, clastogenic and carcinogenic constituent of bracken. However, the carcinogenic potential of bracken is enhanced by its co-occurrence with other closely related illudane glycosides (IG) that are less well characterized. In particular, isoptaquiloside and caudatoside from the Venezuelan species P. caudatum (L. Maxon) (Castillo et al. 1997) and ptesculenoside from the Australian species P. esculentum (G. Forst.) (Fletcher et al. 2010) have been observed in quantities comparable to those ptaquiloside, with lesser amounts of ptaquiloside Z also being found in P. caudatumthus.
Insecticidal activity
Many methanol fern extracts were recently reported to possess insecticidal activity against houseflies (Musca domestica) and mosquitoes (Aedes albopictus). For example, an extensive screening study showed that methanolic extracts of whole Onychium japonicum plants exhibit potent insecticidal activity, suggesting that these plants could be used as botanical insecticides. M. domestica adults treated for 48 h with extracts of P. vittata leaves and roots exhibited mortalities above 90% (Huang et al. 2010a, b, c).
Immunomodulatory activity
The Sword Brake fern (P. ensiformis Burm.) is widely used in traditional Chinese herbal medicine. Aqueous extracts of the Sword Brake fern exert immunomodulatory effects by inhibiting the release of TNF-α, IL-1β, IL-6, NO, and PGE2 in LPS-activated RAW264.7 cells (Wu et al. 2005).
Anti-inflammatory activity
The hexane fraction of A. capillus-veneris L. showed anti-inflammatory effects against croton oil-induced and formalin-induced inflammation in mice (Ibraheim et al. 2011). A recent work evaluated the anti-inflammatory effect of an A. capillus-veneris L. ethanol extract at the transcriptional and translational levels using a luciferase gene reporter assay and Western blotting, revealing a potential link between the Adiantum-mediated anti-inflammatory effect and inhibition of the transcription factor (NF-κB) pathway (Yuan et al. 2013a, b).
Other bioactivities
Ali et al. (2013) reported that methanol extracts of A. philippense L. leaves exhibited thrombolytic activity in a blood clot lysis study. Additionally, Ibraheim et al. (2011) reported that A. capillus-veneris extracts possess hypoglycemic activity.
Huperziaceae
Huperzia is a famous source of Lycopodium alkaloids with acetylcholinesterase (AChE) inhibiting activity (Liu et al. 1986a; Ma and Gang 2004), particularly huperzine A (Ma et al. 2007). Huperzine A was isolated from whole plants of H. serrata (Thunb. ex Murray) Trev. (Liu et al. 1986b), which is used in traditional medicine to treat contusions, strain, swelling, and schizophrenia (Jiang et al. 2014). As a potent AChE inhibitor, huperzine A has potential applications in the treatment of mild to moderate Alzheimer’s disease and was examined in a phase II trial to evaluate its safety, tolerability, and efficacy (Zhang et al. 2004; Yang et al. 2003; Rafii et al. 2011). Treatment with this alkaloid can improve learning and reduce memory impairment in patients, and has neuroprotective effects in rats as a result of modulating the oxidative stress, apoptosis, mitochondrial dysfunction and inflammatory responses, and ameliorate Aβ25-35 induced apoptosis of rat cortical neurons via inhibition of reactive oxygen species production and activation of caspase-3 (Xiao et al. 2002; Zhang et al. 2008).
Because of these promising results, huperzine A has become an important lead compound for the development of new anti-Alzheimer’s drugs (Wu et al. 2011a, b). Huperzine A itself has been marketed in China as a new drug for Alzheimer Disease (AD) treatment, and a derivative known as ZT-1 is being developed as a new anti-AD drug candidate in China and Europe (Ma et al. 2007; Zhang 2012).
In addition to the activities described above, huperzine A promotes hippocampal neurogenesis in vitro and in vivo by stimulating the proliferation of neural stem cells, and attenuates hippocampal cognitive deficits in rats exposed to acute hypobaric hypoxia (Ma et al. 2013a, b; Shi et al. 2012). It can also alleviate chronic pain in rats induced by spinal cord compression injury (Yu et al. 2013).
Huperzine A is a more powerful AChE inhibitor with higher oral bioavailability than some approved AChE-inhibiting drugs, is more capable of penetrating the blood–brain barrier, and has a longer half-life (Wang and Yan 2006).
2α,11α-Dihydroxyfawcettidine and lycoposerramine H also inhibit AChE, with IC50 values of 27.9 and 16.7 μM, respectively (Katakawa et al. 2007), and the known compound lycopodine-6α,11α-diol inhibited α-glucosidase more potently (IC50 = 148 ± 5.5 μM) than the approved anti-diabetes drug acarbose (IC50 376.3 ± 2.7 μM) (Ying et al. 2014). Additionally, 12-deoxyhuperzine O antagonizes the N-methyl-D-aspartate receptor with an IC50 of 0.92 μM (Yang et al. 2010). Alcohol extracts of H. serrata and specific triterpenoids within those extracts can inhibit the proliferation of HL-60 human leukemia cells and induce their apoptosis (Ham et al. 2012).
A novel Lycopodium alkaloid was obtained from H. saururus (Lam.) Trevis and its AChE inhibitory activity was evaluated (IC50 = 209.6 ± 1.1 μM) (Vallejo et al. 2013). Lycopodium alkaloids have attracted considerable research interest in recent years, with many in vitro and in vivo studies having been conducted to study their AChE-inhibiting activity. For example, lycotetrastine A isolated from H. tetrasticha inhibited AChE from bovine erythrocytes with an IC50 of 85 μM; for comparative purposes, the IC50 of (−)-huperzine A for this enzyme is 53 μM (Hirasawa et al. 2011). A separate in vitro AChE inhibition assay showed that the new lycodine-type alkaloid huperserine E exhibited moderate anti-AChE activity with an IC50 value of 6.71 μM (Jiang et al. 2014).
H. saururus is a native Argentine species used in ethnomedicine as aphrodisiac and memory improver (Vallejo et al. 2007). In experiments with male Wistar rats, treatment with its main alkaloid component sauroine improved memory retention in the step-down test while significantly increasing hippocampal plasticity as determined by electrophysiological experiments and behavioral tests (Vallejo et al. 2008). A purified alkaloid extract of H. saururus strongly inhibited AChE (IC50 = 0.58 μg/mL) based on a slight modification of Ellman’s method, but the most abundant alkaloids present in the extract had much weaker inhibitory effects when isolated and purified: sauroxine has an IC50 of 8.9 ± 0.4 μg/mL and was present at a concentration of 32.3 μM, 6-hydroxylycopodine has an IC50 78.1 ± 3.5 μg/mL (present at 298.8 ± 13.3 μM), and the new alkaloid N-demethyl-sauroxine has an IC50 of 54.5 μg/mL (209.6 ± 1.1 μM). The inhibitory activities of all three compounds are several orders of magnitude weaker than that of the extract, suggesting a possible synergistic effect between the different alkaloids (Vallejo et al. 2013). As demonstrated by the results presented above, none of the Lycopodium alkaloids identified to date have exhibited comparable in vitro AChE inhibitory activity to huperzine A (Konrath et al. 2013).
The Lycopodium serratene triterpenoids lycophlegmariol B, D and 21β-hydroxyserrat-14-en-3α-ol from H. phlegmaria (=L. phlegmaria L.) showed inhibitory effects against MOLT-3 acute lymphoblastic leukemia (T-lymphoblast) with IC50 values of 14.7, 3.0 and 2.9 μM, respectively (Wittayalai et al. 2012). Lycophlegmarin also showed modest growth-inhibitory activity against BEL 7402 human hepatoma cells (Shi et al. 2005).
Selaginellaceae
Selaginella-based treatments were first documented in the traditional Chinese medicine textbook Shen Nong Ben Cao Jing in 2737 BC (Yang and Flaws 1998). Many of the Selaginella species have been used in traditional ethnomedicines for different purposes, including analgesia and the treatment of depression (S. convoluta, northern Brazil) (de Sá et al. 2012), the treatment of chronic tracheitis (S. sinensis, Traditional Chinese Medicine) (Chen et al. 2014a, b), ‘Sanjeevani’ or resurrection (S. bryopteris, Indian Ayurvedic Medicine) (Mishra et al. 2011), or for treatment of renal disorders (S. lepidophylla, Mexico) (Aguilar et al. 2013).
As a genus, Selaginella is medicinally significant because many of its species exhibit pharmacologically relevant properties including anti-carcinogenic, anti-hyperglycemic, anti-nociceptive, antioxidative and neuroprotective activity (Chai and Wong 2012; de Sá et al. 2012; Girish and Muralidhara 2012; Weng and Noel 2013). Several studies have been conducted to characterize the mechanisms of action of Selaginella-derived natural products and to identify the phytochemicals responsible for specific observed activities.
Anticancer activity
Ethyl acetate extracts of S. doederleinii Hieron exhibit pronounced antitumor activity in vitro and in vivo without obvious toxicity towards normal cells, and efficiently induce apoptosis (Wang et al. 2015). The mechanisms of anti-tumor activity and cell apoptosis induced by S. doederleinii extracts may be associated with reductions in the ratio of mRNAs from tumor suppressor genes, caspase-3 activation, the suppression of survivin, and reduced expression of the COX-2, 5-LOX, FLAP, and 12-LOX mRNAs (Wang et al. 2015). The main active components in S. doederleinii extracts are bioflavonoids, and two biflavonoids (biapigenin and binaringenin derivatives) isolated from S. doederleinii Hieron have been linked to cytotoxicity towards human cancer cell lines (Li et al. 2014). In addition, an extract of S. tamariscina (Beauv.) Spring was shown to exhibit anti-metastatic properties that resulted from the down-regulation of metalloproteinases and reduced activation of an Akt kinase (Yang et al. 2013). In this context, it is interesting that an earlier report had suggested that caspase-3 plays a functional role in S. tamariscina-induced apoptosis in leukemia cells (Ahn et al. 2006).
Antidiabetic activity
S. tamariscina extracts have also been shown to exert anti-hyperglycemic effects in diabetic rats and HepG2 cells (Zheng et al. 2011a). Specifically, total flavonoids of S. tamariscina were found to increase the expression of PPAR-γ and IRS-1, two protein mediators with functional roles in insulin-stimulated signalling and glucose metabolism (Zheng et al. 2011b). A later study using a diabetic mouse model validated the proposed connection between antioxidative and antidiabetic activities by showing that treatment with S. tamariscina total flavonoids reduced levels of nitric oxide and nitric oxide synthase while increasing levels of superoxide dismutase and other antioxidative enzymes (Zheng et al. 2013).
Biflavonoids from S. tamariscina (amentoflavone, robustaflavone, cupressuflavone, taiwaniaflavone, and 3,8″-biapigenin) potently inhibited protein tyrosine phosphatase 1B, with IC50 values ranging from 4.5 ± 0.1 to 13.2 ± 0.8 μM, and also had significant stimulatory effects on the glycose uptake in 3T3-L1 adipocyte cells (Nguyen et al. 2015a). Selaginellins (selaginellin, selariscinin A, selariscinin B, selariscinin C, selariscinin E, selaginellin M) isolated from S. tamariscina strongly stimulated glucose uptake in 3T3-L1 adipocyte cells and also significantly inhibited the PTP1B enzyme, with IC50 values ranging from 4.6 ± 0.1 to 21.6 ± 1.5 μM (Nguyen et al. 2015a, b).
Neuroprotective and phosphodiesterase inhibitory activities
Recent publications have discussed the neuroprotective and phosphodiesterase-inhibiting properties of Selaginella extracts, suggesting that they may have applications in the treatment of Parkinson’s disease and pulmonary conditions (Chandran 2014; Liu et al. 2013b).
Lygodiaceae
Lygodium venustum, which has been used as a medicinal plant by indigenous populations in Mesoamerica, reportedly exhibits antiseptic, fungicidal and trichomonacidal activities, and is indicated for the treatment of dermatoses, mycoses and infections (Duke 2008). It is also used to treat gastrointestinal and gyneco-obstetric disorders, and as a postpartum anti-inflammatory agent (Argueta Villamar et al. 1994). Traditionally, it is used to prepare the hallucinogenic drink ayahuasca by the Sharanahua and indigenous peoples of the upper Purus River in the Peruvian Amazon (Rivier and Lindgren 1972). In Brazil, this fern is utilized by mystical Afro-Brazilian cults in cleansing baths and to treat nervousness and emotional instability (Albuquerque et al. 1997; Rahman 2008).
The pharmacological activities of L. venustum have been investigated in various places, particularly Brazil and Mexico. These studies have mainly adopted an ethno-directed approach, although other approaches have also been utilized. Because L. venustum is rich in flavonoids, much of its bioactivity is attributed to these secondary metabolites. However, further studies will be needed to confirm this assumption and identify the precise compounds responsible for specific effects.
Antimicrobial activity
The first report on the bioactivity of L. venustum was published in 2005: Alanis et al. conducted a screening study in which 26 Mexican medicinal plants were tested for activity against eight bacterial strains using the disk-diffusion method. Methanolic and aqueous extracts of L. venustum achieved less than 50% inhibition in all tested cases, indicating that the plant has only weak inhibitory effects on bacterial growth. Microbiological tests were also performed by Morais-Braga et al. (2012a, b, 2013a, b), who determined the plant’s MIC using the broth microdilution method and showed that extracts of the whole plant and subfractions of L. venustum had no clinically relevant effects, with a MIC ≥ 1024 µg/mL against the bacteria Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus, and the yeasts Candida albicans, Candida tropicalis, and Candida krusei. Tests of these extracts and fractions at subinhibitory concentrations (MIC/8) in combination with conventional drugs revealed no potentiation of antifungal activity. However, the authors did observe an interesting effect whereby the extracts and fractions enhanced the activity of aminoglycoside antibiotics (amikacin, kanamycin, neomycin and gentamicin) against E. coli and S. aureus. This effect was especially pronounced for the ethyl acetate fraction, which potentiated all of the tested aminoglycosides. The interactions between the antibiotics and the extract or the ethyl acetate fraction were evaluated using the checkerboard method, which demonstrated that the natural products had additive or synergistic effects when combined with the conventional drugs, clearly demonstrating their antibiotic-modifying ability (Morais-Braga et al. 2012a).
Antidiarrheal activity
Because of its use in treating gastrointestinal disorders such as diarrhoea, L. venustum was tested for anti-secretory activity by Velázquez et al. (2006) in an in vivo model with induction by the cholera toxin. Methanolic and aqueous extracts of L. venustum were evaluated at a concentration of 300 mg/kg; the aqueous extract had no effect but the methanolic extract achieved 51.6% inhibition, indicating interesting antidiarrheal activity. A methanolic extract of the same concentration was investigated by Calzada et al. (2010) to assess its inhibition of peristalsis of the intestinal tract in rats. The effect observed was moderate (42%) but superior to that of the drug used as the positive control, loperamide, which achieved 34% inhibition at a dose of 10 mg/kg.
Antiprotozoal activity
The antiprotozoal activity of pteridophytes in general and L. venustum in particular was investigated by Calzada et al. (2007) in a study where 22 plants were tested for trichomonacidal effects. L. venustum exhibited moderate activity, with an IC50 of 60.9 μg/mL. The same group conducted an in vitro screening of plants based on ethnomedicinal use for treatment of giardiasis and amebiasis, in which methanolic extracts of 26 plants were tested, including L. venustum. The IC50 of the L. venustum extract against Entamoeba histolytica was 178.4 µg/mL, which was much higher than that found for Giardia lamblia (74.3 µg/mL). The effect against Giardia was classified as being of intermediate strength (Calzada et al. 2006).
Morais-Braga et al. (2013a) examined the antileishmanial activity of an ethanolic extract of L. venustum and its hexane, ethyl acetate, dichloromethane and methanolic fractions against the promastigote forms of protozoa of the family Kinetoplastida. With the exception of the methanolic fraction, all of the tests indicated insignificant activity; the methanolic fraction exhibited a moderate effect, achieving 68% inhibition at a concentration of 500 µg/mL. This fraction also exhibited antiepimastigote activity against Trypanosoma cruzi, achieving 63% inhibition at a concentration of 500 µg/mL. The hexane fraction similarly achieved 63% inhibition of the epimastigote forms, but at twice the concentration.
Antioxidant activity
The antioxidant effect of L. venustum was evaluated by Morais-Braga et al. (2012a) in a study using a photocolorimetric method based on the reduction of the 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical. The ethanolic extract of the fern exhibited antioxidant activity with an IC50 of 67.58 μg/mL. The ethyl acetate fraction exhibited still lower antioxidant activity, with an EC50 of 82.70 μg/mL.
Cytotoxicity
In view of the bioactivities shown by the fern L. venustum, it was tested for cytotoxicity in vitro using mammalian fibroblasts (NCTC-929). The ethanolic extract and the hexane, ethyl acetate and methanolic fractions exhibited varied cytotoxicities at a concentration of 100 µg/mL but the dichloromethane fraction showed no cytotoxicity at this concentration (Morais-Braga et al. 2013a, b). However, an interesting and beneficial cytotoxic effect was observed when the extracts and fractions of L. venustum were combined with a heavy metal such as mercury in prokaryotic cells (E. coli).
Equisetaceae
Extracts of E. arvense, the common horsetail, are widely used for pain relief and to relieve inflammation in treatments for diseases such as tuberculosis, kidney and bladder disorders, dysmenorrhea and hemorrhages, rheumatic diseases, gout, swellings and traumas, problematic wounds, ulcers, and frostbite (Asgarpanah and Roohi 2012; Sandhu et al. 2010). In addition, E. arvense extracts are used in various cosmetics such as anti-aging, moisturizer, anti-wrinkle, anti-acne, and hair conditioning products (Sandhu et al. 2010).
Diverse bioactivities of E. arvense
Investigations on E. arvense have revealed antioxidant, anti-inflammatory, analgesic, antibacterial, antifungal, antitumor and neurotropic effects. Alcoholic extracts of the plant’s stem reportedly induce anticonvulsant, sedative, anxiolytic, and neuroprotective effects in rodents (Santos et al. 2005; Santos-Junior et al. 2005; Samura and Dovzhenok 2002; Singh et al. 2011), while methanolic extracts have shown antidiabetic activity in streptozotocin-induced diabetic rats (Soleimani et al. 2007); and the ethyl acetate extract reportedly exerts antiproliferative effect in tumor cell lines (Četojević-Simin et al. 2010). The only clinical data for such extracts relates to the induction of diuretic effects by a standardized dried extract (Carneiro et al. 2014). It has been suggested that E. arvense extracts could be beneficial silicon supplements, but this has been questioned based on the limited quantities of water-extractable silicon in the plant’s tissues (Bey et al. 2010).
Hepatoprotective activities of E. arvense
Hepatoprotective activity-guided fractionation of the MeOH extract of Equisetum arvense L. resulted in the isolation of two phenolic petrosins, onitin and onitin-9-O-glucoside (Oh et al. 2004). Onitin exhibited hepatoprotective activities against tacrine-induced cytotoxicity in human liver-derived Hep G2 cells, with EC50 value of 85.8 ± 9.3 µM. For comparative purposes, the positive control agent silybin achieved an EC50 value of 69.0 ± 3.3 µM. Onitin also showed superoxide and DPPH free radical scavenging activity. These results support the use of this plant for the treatment of hepatitis in oriental traditional medicine.
Bioactivities of other Equisetum species
Secondary metabolites from various other horsetail species have also been reported to have interesting bioactivity. For example, a kaempferol diglucoside from E. palustre has been proposed as an antiulcerogenic agent (Yesilada and Gurbuz 2010), and ointments prepared from E. pyramidale and E. arvense promoted the healing of cutaneous wounds in diabetic rats (Corrêa et al. 2013; Ozay et al. 2013). An extract from E. hiemale inhibited platelet aggregation and thrombosis in rats, suggesting a potential use in stroke prevention (Qi and Wang 2004). Finally, an alkaloid preparation from E. pratense exerted a sedative effect in rats by reducing the levels of central monoamine neurotransmitters (Ji and Gao 2005). A study on various polar and nonpolar extracts from E. ramosissimum has revealed antimelanoma and anti-melanogenesis effects (Li et al. 2016).
Helminthostachyaceae
The rhizome of Helminthostachys zeylanica is used in traditional medicine in China, India and Sri Lanka as an aperient, analgesic, anti-inflammatory, antipyretic, and hepatoprotective principle, and to treat malaria, jaundice, syphilis, and impotence (Huang et al. 2003; Suja et al. 2003). Its liver-protecting activity was confirmed in a study on CCl4-induced liver damage in Wistar rats (Suja et al. 2004), while acetogenin and prenylated flavonoids from the rhizome have been shown to inhibit superoxide and elastase release by neutrophils (Huang et al. 2010a, b, c). In addition, novel quercetin glucosides from the rhizome were found to stimulate melanin biosynthesis in murine B16 melanoma cells (Yamauchi et al. 2013).
Ugonins E–L (1–8) were evaluated for their antioxidative activity in DPPH assay. Ugonins J, K and L were more active than Trolox, with IC50 values of 5.29, 7.23, and 7.93 mg/mL, respectively (Huang et al. 2003).
Botrychiaceae
Botrychium (=Sceptridium) ternanum is used in China and Japan as a folk remedy for dizziness, headache, cough, asthma, and fever (Wang et al. 2001; Warashina et al. 2012). A study on its anti-asthma activity in a mouse model revealed effects on the regulation of Th1/Th2 balance and leukotriene receptors (Yuan et al. 2013a, b). An extract from B. virginianum induced keratinocyte proliferation and inhibited melanin biosynthesis, suggesting possible cosmetic uses (Koshimizu et al. 2004).
Small, eusporangiate ferns produce a single leaf divided into a sporophore, bearing sporangia, and a trophophore. Ethnobotanical surveys have reported several uses of these ferns as folk remedies, and the therapeutic properties of some species have been explored using experimental approaches (Azaizeh et al. 2006; Kao 1985; Srivastava 2007).
Ophioglossaceae
The fern Ophioglossum vulgatum, known as the adder’s tongue, is frequently used in folk medicine for dermatological, hemostatic, antiparasitic, and alimentary purposes (Kala et al. 2011; Nwosu 2002; Sarker and Hossain 2009). An ointment known in England as ‘‘Green Oil of Charity’’ has long had a reputation as a vulnerary. Accordingly, galactoglycerolipids and flavonol derivatives from aerial parts of O. vulgatum have been shown to promote in vitro keratinocyte scratch-wound healing (Clericuzio et al. 2012, 2014). In addition, the fern’s aqueous extract has been found to counteract bovine viral diarrhoea virus (BVDV) (Herrmann et al. 2011).
Homoflavonoids from O. petiolatum have anti-hepatitis B virus activity (Lin et al. 2005b; Wan et al. 2013). O. pedunculosum contains 3-O-methylquercetin glucosides that inhibit lipopolysaccharide-induced NO production in macrophages (Wan et al. 2012), and a mannan-specific lectin with antifungal activity (He et al. 2011). An alcohol-ethyl acetate fraction from O. thermale has shown anti-inflammatory effects on carrageenan-induced inflammation, which may be related to the extract’s antioxidant properties (Zhang et al. 2012d).
Gleicheniaceae
In Chinese folk medicine, Dicranopteris dichotoma Bernb. is used to treat urinary disease, trauma, and fevers in children, while Hicriopteris glauca (Thunb.) Ching. is used to treat bleeding and trauma. The most abundant phytochemicals in the family Gleicheniaceae are terpenoids, many of which exhibit distinct pharmacologically relevant activities (Kamisan et al. 2014). For example, the diterpene (6S, 13S)-cleroda-3, 14-diene-6, 13-diol exhibits moderate anti-HIV activity (Li et al. 2007). In a study of highland ferns in Malaysia, two species occurring at high altitude (1495 m), Gleichenia truncata and Dicranopteris curranni, were found to be potential sources of natural antiglucosidase, antibacterial, and antioxidant agents (Chai et al. 2013a).
Antiglucosidase activity
Aqueous extracts of G. truncata and D. curranii were found to have alpha-glucosidase inhibitory activity, suggesting their potential as sources of natural antidiabetic or antihyperglycaemic agents (Chai et al. 2013b). The EC50 values of the leaf and rhizome extracts of G. truncata in these assays were 408 and 175 µg/mL, respectively, while the corresponding values for D. curranii were 143 and 179 µg/mL, respectively (Chai et al. 2013a).
Antibacterial activity
MIC assays performed with extracts at concentrations of up to 50 mg/mL revealed that a G. truncata leaf extract inhibited the growth of both Gram-positive (Staphylococcus aureus; Micrococcus luteus) and Gram-negative (Escherichia coli; Pseudomonas aeruginosa) bacteria (Chai et al. 2013a). This suggests that G. truncata may be a potential source of broad-spectrum antibacterial agents that warrants further investigation. Leaf and rhizome extracts of D. curranii and the rhizome extract of G. truncata only inhibited the growth of Gram-positive bacteria (Chai et al. 2013b).
Lindsaeaceae
The activities of the compounds isolated from whole S. chusanum (L.) Ching plants against Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis, Epidermophyton floccosum, and Aspergillus niger were determined, yielding minimal inhibitory concentrations (MIC) of 25–100 μg/mL (Ren et al. 2009). Extracts from this species also exhibited inhibitory activity against tyrosinase and the proliferation and apoptosis of K562 cells (Wu et al. 2016).
In addition to the studies described above, four S. chusanum extracts were tested to determine their antidotal effects in mice suffering from acute poisoning with arsenic and ammonium chloride. Two of the extracts (B and C) dramatically reduced mortality in arsenic-poisoned mice, and extract C increased tolerance of arsenic, raising the LD50 from 31.1 ± 4.3 to 38.2 ± 5.9 mg/kg. In the case of mice poisoned with ammonium chloride, the four extracts also reduced mortality but not in a statistically significant fashion when compared to a control group at the P < 0.05 level (Yang et al. 1989).
Thelypteridaceae
Parathelypteris nipponica (Franch.et Say.) Ching is widely used to treat burns, scale, hematemesis and dysentery in Chinese folk medicine (Editorial Board of China Herba 1998). To explore the origins of its putative beneficial health effects, the antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of its extracts have been studied (Fu et al. 2010).
Another investigation showed that methanolic extracts of Pronephrium megacuspe (Bak.) Holtt. exhibited toxicity towards Musca domestica adults and the 4th instar larva of Aedes albopictus and Myzus persicae, while also having antifeedant effects on 3rd instar larvae of Plutella xylostella, Ostrinia furnacalic and P. rapae (Huang et al. 2012).
Abacopteris penangiana (Hook.) Ching. is commonly used in traditional medicine to treat acute or chronic pharyngitis, dysentery, upper respiratory infections and other conditions (Ding 1982; Zhao et al. 2006). Because of its diverse biological activities, the pharmacological properties of its extracts and phytochemicals have been studied extensively. Compounds isolated from A. penangiana were found to possess cytotoxic activity (Zhao et al. 2006, 2008). Specifically, biological evaluations of flavonoids from this species revealed cytotoxicity towards the HepG2, HeLa and L929 human cell lines (Zhao et al. 2006, 2010b; Fang et al. 2010), antioxidative activity (Zhao et al. 2007a, b), and dopamine-induced neurotoxicity in PC12 cells (Wei et al. 2013).
Further pharmacological investigations on A. penangiana have clarified the diverse activities of its extracts and phytochemicals. Total flavonoids extracted from this species and their hydrolysates can prevent testosterone-induced benign prostatic hyperplasia in rats via their anti-inflammatory, antioxidant and anti-proliferative effects (Wei et al. 2012; Yang et al. 2014a). Such mixtures also exert anti-prostatitis effects on carrageenan-induced rats, again as a result of their antioxidative and anti-inflammatory activities (Yang et al. 2014b).
Metabolic syndrome is usually characterized by hyperglycemia, hyperlipidemia, and vascular injury. Total flavonoids from A. penangiana effectively ameliorate these symptoms in diabetic rats when induced by high fat diet and streptozotocin, and the A. penangiana-derived flavonoid abacopterin A exhibited hypolipidemic and anti-inflammatory activity in C57BL/6J mice fed on a high fat diet. These effects have been linked to the modulation of oxidative stress and the mitigation of inflammatory responses associated with metabolic disorder (Chen et al. 2011; Lei et al. 2011). Many other studies on the bioactivities of phytochemicals from A. penangiana have focused on their neuroprotective effects in vitro and in vivo. Three other flavonoids from this species—5,2′,5′-trihydroxy-7-methoxyflavone (TMF), abacopterin E—significantly attenuated H2O2-induced damage in PC12 cells and neurotoxicity in mice induced by d-galactose (Wei et al. 2011a, b; Lei et al. 2011; Wei et al. 2013; Fu et al. 2013).
The genus Macrothelypteris (H. Ito) Ching consists of around ten species of moderately large terrestrial ferns (Xing 1991), many of which are used in traditional medicine. Ethanolic extracts of M. torresiana, which is distributed across southern China (Chang and Wu 1999), exhibited strong cytotoxicity (An et al. 2005, 2007), and the flavonoid compound protoapigenone isolated from M. torresiana (Gaud.) Ching proved to be highly cytotoxic to various tumor cells, with potential pro-apoptotic activity (Chang et al. 2008a, b). This compound and another flavonoid isolated from M. torresiana (Gaud.) Ching, 2-(cis-1, 2-dihydroxy 4-oxo-cyclohex-5-enyl)-5, 7-dihydroxy-chromone (DEDC), was identified as being potentially useful in cancer chemoprevention (Liu et al. 2011a, b, c). In a separate study, protoapigenone, 5,7-dihydroxy-2-(1,2-isopropyldioxy-4-oxo-cyclohex-5-enyl)-chromen-4-one, and 5,7-dihydroxy-2-(1-hydroxy-2,6-dimethoxy-cyclohex-4-oxo)-chromen-4-one isolated from M. viridifrons (Tagawa) Ching showed strong concentration-dependent anti-proliferative effects on six tumor cell types (Wei et al. 2011a, b). Protoapigenone was also isolated from M. oligophlebia, and exhibited clear antitumor activity in vitro and in vivo (Wu et al. 2011a, b).
Total polyphenols from M. torresiana were shown to have potential in the treatment of chronic nephrotic syndrome in puromycin aminonucleoside-induced hyperlipidemic mice (Chen et al. 2012a, b). In addition to this renal protective effect, extracts and phytochemicals from M. torresiana have various anti-cancer effects. Total flavonoids from M. torresiana showed significant antitumor activity relative to 5-fluorouracil and low acute/subacute oral toxicity in BALB/c mice (Huang et al. 2010a, b, c). In vitro evaluations have shown that protoapigenone possesses significant cytotoxic activity against the cancer cell lines Hep G2, Hep 3B, Tca-8113, MCF-7, A549, MDA-MB-231, M5 and K562, with its lowest IC50 value being 0.23 μg/ml towards M5 cancer cells (Huang et al. 2010a, b, c; Lin et al. 2005a, b). Although DEDC (2-(cis-1,2-dihydroxy-4-oxo-cyclohex-5-enyl)-5,7-dihydroxy-chromone) exhibited only weak cytotoxicity towards HepG2, MCF-7 and K562 cells, it can induce apoptosis of human neuroblastoma SH-SY5Y cells by triggering the production of ROS and activation of NF-κB (Liu et al. 2012a, b). DICO (5,7-Dihydroxy-2-(1,2-isopropyldioxy-4-oxo-cyclohex-5-enyl)-chromen-4-one) is a novel flavonoid with a nonaromatic B-ring that inhibits the growth of Hep G2 cells, causes arrest at the G2/M phase of the cell cycle, and, induces apoptosis via a ROS-mediated mitochondrial pathway (Zhou et al. 2013).
Aqueous extracts of Christella arida, C. dendata and Cyclosorus interruptus were cytotoxic towards the K562 cell line (Chai et al. 2015b), achieving EC50 values of 478.62, 194.50, and 314.52 µg/mL, respectively; for comparative purposes, that of the anticancer drug 5-fluorouracil was 212.86 µg/mL. These results suggest that C. dendata may be a valuable source of potent cytotoxic agents with potential applications in cancer therapy (Chai et al. 2015b). Aqueous extracts of C. arida and C. dendata also exhibit antiglucosidase activity, with EC50 values of 559.87 and 87.48 µg/mL, respectively. However, the aqueous extract of C. interruptus had no detectable antiglucosidase activity (Chai et al. 2015b). Extracts of C. dendata thus contain both cytotoxic and antiglucosidase agents, indicating that further studies on the phytochemistry and pharmacology of this species are warranted.
Dryopteridaceae
The Dryopteridaceae are a family of ferns comprising around 1400 species around the world, many of which have been identified as medicinal herbs (Wu 1991). The rhizomes of Arachniodes exilis (Hance) Ching have been used in folk medicine to treat acute icterus hepatitis, inflammation, dysentery and burn scalds (Chinese Materia Medica Editorial Board 1999). D. filix-mas (L.) Schott had been used to treat parasitic diseases in Europe since 1750; its use has been recorded in pharmacopeias from England, Germany, Switzerland, Japan, and America (Zuo and Chen 2005). D. crassirhizoma Nakai is another fern in this family that occurs in Korea, Japan, and northern China (Han et al. 1998). Its rhizomes are traditionally used as a vermicide (Han et al. 1998), and it has also been used to treat viral diseases including severe acute respiratory syndrome (SARS) (Zhao et al. 2007b).
Anti-tumor activity
A n-hexane extract of D. fragrans was tested in an in vitro short-term assay for anti-tumor promoting agents. Flavonoids extracted from D. erythrosora showed clear cytotoxic effects on A549 cells and exhibited dose-dependent inhibition of acetylcholinesterase (Zhang et al. 2012a, b, c, d). Moreover, the anticancer activity of fern flavonoids was shown to correlate weakly but positively with their antioxidant potential (Cao et al. 2013a, b). The ethanol extract of D. crassirhizoma was reported to have anti-cancer activity and to inhibit the proliferation of human prostate cancer cell lines in a dose- and time-dependent manner (Chang et al. 2010). Flow cytometry and Western blot experiments indicated that its anti-proliferative activity were due to the induction of cell cycle arrest in the G0/G1 phase and apoptosis. The extract’s active anti-proliferative components were not identified, but it exhibited no cytotoxicity towards normal spleen cells, suggesting the potential for development as an anti-cancer agent with minimal side effects (Chang et al. 2010).
Fatty acid synthase (FAS) is an important therapeutic target for anti-cancer drug discovery (Flavin et al. 2010). Ten phloroglucinol derivatives with anti-FAS activity were isolated from a methanol extract of the D. crassirhizoma rhizome. Among these, flavaspidic acid PB and methylene-bis-methylphlorobutyrophenone exhibited the strongest inhibition of FAS (Na et al. 2006). These results highlight the potential value of D. crassirhizoma as a source of lead compounds for anti-cancer drug development.
Antiviral activity
Human immunodeficiency virus (HIV)-1 protease and reverse transcriptase are important therapeutic targets for the development of anti-HIV chemotherapies. Three kaempferol acetylrhamnosides isolated from methanol extracts of D. crassirhizoma (crassirhizomoside A, crassirhizomoside C, and sutchuenoside A) were found to be potent inhibitors of both the RNA-dependent and the DNA-dependent DNA polymerase activities of the HIV-1 reverse transcriptase (Min et al. 2001). In addition, four HIV-1 protease inhibitors were isolated from the fern’s methanol extract: two triterpenes (dryopteric acids A and B), ursolic acid, and 3,4-dihydroxybenzaldehyde. All four compounds had only moderate HIV-1 protease inhibitory activity compared to the positive control agent acetyl-pepstatin. However, acetylation of dryopteric acid A drastically improved its protease inhibitor activity, yielding an IC50 value only 19-fold greater than that of acetyl-pepstatin (Lee et al. 2003).
Antibacterial activity
The antibacterial properties of Polystichum tsus-simense, P. neolobatum, Cyrtomium fortune, C. falcatum, D. uniformis and D. cycadina have been investigated, revealing that species from the genus Polystichum showed more promising antibacterial activity than the others that were tested (Song et al. 2008).
Flavaspidic acids PB and AB from D. crassirhizoma were also found to have activity against Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus, Streptococcus mutans, and Bacillus subtilis (Lee et al. 2009). In keeping with this result, the hexane fraction of a methanol extract of D. crassirhizoma exhibited good antibacterial activity against clinical isolates and the standard ATCC strain of methicillin-resistant S. aureus (Kwon et al. 2007).
The potential of D. crassirhizoma as a source of antimicrobial agents for the prevention of dental caries has been highlighted in the literature. Ban et al. (2012) reported that the methanol extract of the fern had bactericidal and bacteriostatic effects on Streptococcus mutans, a cariogenic dental pathogen. At concentrations below the minimum inhibitory concentration, the extract attenuated the pathogen’s virulence potential by inhibiting its acid production, acid tolerance, water-insoluble glucan formation, and sucrose-dependent adherence in a dose-dependent manner (Ban et al. 2012). A more recent study confirmed the effectiveness of the hexane fraction of a methanol extract of D. crassirhizoma in controlling S. mutans biofilm formation, and identified linoleic acid as a key component of the hexane fraction in this context (Jung et al. 2014). Both the hexane fraction and linoleic acid could thus be used in anti-biofilm agents for topical application (Jung et al. 2014).
Antioxidant activity
An ethanol extract of A. exilis exhibited anti-oxidant activity in vitro and hepatoprotective activity in vivo against CCl4-induced hepatotoxicity (Zhou et al. 2010). Flavaspidic acids PB and AB isolated from the D. crassirhizoma rhizome exhibited lipid peroxidation inhibitory activity comparable to that of alpha-tocopherol and the synthetic antioxidant butylated hydroxyanisole. The two compounds also showed moderate free radical scavenging activity (Lee et al. 2003).
Zhang et al. (2012a, b, c, d) investigated the total flavonoid contents and free radical scavenging activity of extracts from the leaves, stems, rachis and roots of D. erythrosora. Their results indicated that the total flavonoid content of the different plant parts declined in the order stems > roots > rachis > leaves, while the DPPH free radical scavenging abilities (IC50) of 50% ethanol extracts of the different parts declined in the order stems > root > rachis ≫ leaves (Zhang et al. 2012a, b, c, d). The free radical scavenging ability of the different plant parts thus appears to be proportional to their total flavonoid content.
Molluscicidal activity
Socolsky et al. (2011a) evaluated the molluscicidal activity of 12 phloroglucinol derivatives isolated from E. piloselloides, E. gayanum, E. yungense, and E. lindbergii against the schistosomiasis vector snail Biomphalaria peregrina. The prenylated desaspidins elaphopilosins A and B showed the greatest activity in these assays, and a QSAR analysis showed that there is an optimum molecular volume for high activity, which is probably related to the size of the active site of the receptor(s) that these compounds interact with.
Other relevant biological activities
Water and ethanol extracts of D. fragrans both reportedly help relieve the symptoms of psoriasis (Shen et al. 2002). Flavon-3-ol glycosides from D. filix-mas (L.) Schott can be used as histidine decarboxylase inhibitors for the treatment of peptic ulcers and atopic dermatitis (Minoru et al. 1996). In vitro and in vivo results suggest that water extracts of D. crassirhizoma can mitigate bone loss by suppressing osteoclast differentiation and function (Ha et al. 2013). The active components responsible for these effects were not identified, but the results suggest that the fern may be useful in the treatment of bone diseases (Ha et al. 2013). In addition, an ethanol extract of D. crassirhizoma suppressed inflammatory reactions in macrophages and protected against acute inflammatory lesions of the stomach in mice, suggesting that this fern (or compounds derived from it) could be useful as an anti-inflammatory agent. These effects have been attributed to the presence of kaempferol, quercetin and resveratrol in the extract (Yang et al. 2013).
Polypodiaceae
Two Phymatopteris species (Family Polypodiaceae) are used in traditional medicine. P. hastata is used in China as a remedy for various diseases including diarrhoea, bronchitis, and influenza, as well as conditions such as carbuncles, furunculosis, and viper bites (Su et al. 2011). On the other hand, in Nepal, P. quasidivaricata is used as a traditional treatment for musculo-skeletal problems and dermatological infections (Uprety et al. 2010). Such uses imply that the Phymatopteris genus is a promising source of phytochemicals with therapeutically-relevant bioactivities. However, its phytochemistry and pharmacology/bioactivity remain underexplored; the only Phymatopteris that have been studied in this context are P. hastata and P. triloba. A tropical fern from the same family, Polypodium leucotomos, which is native to Central and South America is traditionally used as an antiphlogistic, anti-tumor, and anti-inflammatory agent, as well as a general tonic and psoriasis remedy (Ho et al. 2011; Horvath et al. 1967).
Anticancer activity
Several lines of evidence indicate that fern extracts can protect against the development of UV-induced skin cancer. For instance, fern extracts inhibited skin tumor formation in mice exposed to UVB (Alcaraz et al. 1999). Oral aministration of P. leucotomos extracts also reduced the formation of mutagenic cyclobutane pyrimidine dimers in human subjects (Middelkamp-Hup et al. 2004) and mice (Zattra et al. 2009) following exposure to UV radiation. The antioxidant activity of P. leucotomos extracts is considered central to their ability to mitigate UV-induced DNA damage (Gonzalez et al. 2011). In mice, the fern extract also inhibited UV-induced cyclooxygenase-2 expression and activated tumor suppressor p53 (Zattra et al. 2009), both potentially contributing to the extract’s chemopreventive activity against skin cancer (Parrado et al. 2014).
Anti-inflammatory activity
The beneficial actions of P. leucotomos extracts as an adjuvant treatment for vitiligo, psoriasis, melasma, and atopic dermatitis have recently been reviewed (Choudhry et al. 2014; Nestor et al. 2014). Oral P. leucotomos extract supplementation has been shown to enhance repigmentation in patients with vitiligo undergoing NB-UVB (narrowband ultraviolet B) (Middelkamp-Hup et al. 2007) and PUVA (psoralen plus ultraviolet A) (Reyes et al. 2006) phototherapies. This may be due to the antioxidant properties of the fern extract (Gomes et al. 2001). In keeping with this proposal and the fact that vitiligo is a chronic inflammatory skin disorder (Taïeb 2012), improved repigmentation in vitiligo patients following a combined NB-UVB and oral antioxidant (vitamin E) supplementation therapy has been reported (Elgoweini and Din 2009). In UV-irradiated mice, the fern extract reduced acute inflammation by inhibiting cyclooxygenase-2 (Zattra et al. 2009). Oral administration of a P. leucotomos extract, in combination with PUVA therapy, suppressed the proliferation of peripheral blood mononuclear cells in vitiligo patients (Reyes et al. 2006). In vitro, the fern extract also decreased the levels of pro-inflammatory cytokines (IL-2, IFN-, and TNF-α) and induced the production of the anti-inflammatory cytokine IL-10 in human peripheral blood mononuclear cells (Gonzalez et al. 2000).
Antidiabetic activity
The antidiabetic potential of P. hastata and P. triloba has been studied using cell-based and biochemical assays. (−)-Epiafzelechin 3,5-di-O-β-d-apiofuranoside isolated from P. hastata improved glucose consumption by insulin-resistant HepG2 cells as well as the translocation of glucose transporter 4 (GLUT4) to the surface of skeletal muscle cells (Ma et al. 2013a, b). On the other hand, leaf and rhizome aqueous extracts of P. triloba have demonstrated in vitro glucosidase inhibitory activity (Chai et al. 2013a). Notably, despite the crude nature of the P. triloba leaf extract, its EC50 value was on the same order of magnitude as that of quercetin, indicating that they have similar potencies (56 vs. 22 g/mL). These results, although still preliminary, suggest that the genus Phymatopteris produces bioactive compounds that can affect different therapeutic targets relevant to type 2 diabetes.
The potential of Phymatopteris phytochemicals as antidiabetic agents may be enhanced by their antioxidant activity. Selected flavonol glycosides, phenolic acids and phenolic acid derivatives isolated from P. hastata have shown radical scavenging activity (Duan et al. 2012a), and the antioxidant activity of organic solvent fractions of P. hastata has been correlated with their phenolic contents (Su et al. 2011). A crude aqueous extract of P. triloba leaves exhibited both antiglucosidase activity and stronger superoxide scavenging activity than ascorbic acid (Chai et al. 2013b). The extract also exhibited nitric oxide scavenging and ferric reducing activities (Chai et al. 2013a, b). Phenolics detected in P. triloba leaf extracts, myricetin and sinapic acid (Chai et al. 2013b), are known to have antiglucosidase/anti-hyperglycemic (Cherng et al. 2013; Tadera et al. 2006) and antioxidant activities (Cos et al. 1998; Jalaludeen and Pari 2011). Oxidative stress is a key factor in the pathogenesis of diabetic complications associated with postprandial hyperglycemia (Aryangat and Gerich 2010). Therefore, dual-function anti-hyperglycemic agents with antioxidant activity may provide additional benefits compared to single-function anti-hyperglycemic agents. Taken together, these findings on the bioactivities and chemical constituents of P. hastata and P. triloba justify deeper investigations into the antidiabetic potential of species from the Phymatopteris genus.
Davalliaceae
A flavonoid-rich (0.258 mg/mL) extract of D. cylindrica exhibited a free radical (O2−, DPPH· and ABTS·) scavenging potential slightly greater than that of rutin (0.25 mg/mL), along with cytotoxic effects on A549 cells and dose-dependent inhibition of acetylcholinesterase (Cao et al. 2014).
Athyriaceae
Athyrium multidentatum (Doll) Ching is one of the most common ferns in the north-east of China and exhibits several pharmacologically relevant effects such as tranquilization, blood pressure reduction, and diuresis. Polysaccharide-rich fractions from this fern were found to exhibit strong antioxidant activity (Sheng et al. 2011; Liu et al. 2011a, b, c, 2013a, b; Sheng 2014; Sheng and Sun 2014), and a polysaccharide extract from A. multidentatum (Doll.) Ching showed remarkable anti-aging activity (Liu et al. 2015). Striatisporolide A isolated from rhizomes of A. multidentatum exhibited significant cytoproliferative and minor cytoprotective effects on HUVECs arising from interference with ROS generation and apoptosis (Liu et al. 2016).
Perspectives
Fern species are essential constituents of ecosystems and produce a wide array of bioactive components with diverse activities. Many of them are used in traditional medicines and could potentially be used to treat various diseases. However, wild fern plants around the world are subject to many severe threats due to environmental change, and significant losses of fern species and habitats have occurred, leading to a profound loss of biodiversity. At present, there is comparatively little data in the literature relating to the phytochemistry of ferns and their bioactivities, despite their potential as sources of novel bioactive compounds. Further analysis and testing of wild ferns to determine their biological properties and identify their active constituents could thus enable important improvements in human healthcare and help to valorize natural biodiversity. To this end, a survey will be performed to identify rare, traditional and wild fern species used in foods and medicines. High value products, pharmaceuticals and bioactive food ingredients from ferns will be harvested sustainably for analysis, and their nutritional and phytochemical profiles will be investigated to determine the bioactivity and toxicity of their extracts and components. In addition, the antioxidant and anti-inflammatory activity of selected fractions/purified compounds and ingredients will be examined in selected in vitro and cellular models. The ability of a diet enriched with fern foods and ingredients selected based on in vitro and ex vivo experiments to modulate clinically relevant parameters, oxidative and inflammatory stress, and cardiovascular function in overweight subjects will be assessed.
Acknowledgments
This study was supported by the opening fund of the State Key Laboratory of Quality Research in Chinese Medicine, University of Macau (No. SKL-QRCM-2014-2016-008).
Footnotes
Tsun-Thai Chai and Xin Wang have same contibutuion as the first author.
Contributor Information
Hui Cao, Email: hui_cao0830@yahoo.com.
Tsun-Thai Chai, Email: chaitt@utar.edu.my.
Xin Wang, Email: wangxinishappy@163.com.
Maria Flaviana B. Morais-Braga, Email: flavianamoraisb@yahoo.como.br
Jing-Hua Yang, Email: yangjh@ynu.edu.cn.
Fai-Chu Wong, Email: wongfc@utar.edu.my.
Ruibing Wang, Email: rwang@umac.mo.
Huankai Yao, Email: hkyao@xzhmu.edu.cn.
Jianguo Cao, Email: cao101@shnu.edu.cn.
Laura Cornara, Email: cornara@dipteris.unige.it.
Bruno Burlando, Email: burlando@mfn.unipmn.it.
Yitao Wang, Email: ytwang@umac.mo.
Jianbo Xiao, Phone: +853-88228522, Email: jianboxiao@umac.mo.
Henrique D. M. Coutinho, Email: hdmcoutinho@gmail.com
References
- Abbasi AM, Khan M, Ahmad M, et al. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J Ethnopharmacol. 2010;128:322–335. doi: 10.1016/j.jep.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Aguilar MI, Mejía IA, Menchaca C, et al. Determination of biflavonoids in four Mexican species of Selaginella by HPLC. J AOAC Int. 2013;96:712–716. doi: 10.5740/jaoacint.11-029. [DOI] [PubMed] [Google Scholar]
- Ahn SH, Mun YJ, Lee SW, et al. Selaginella tamariscina induces apoptosis via a caspase-3-mediated mechanism in human promyelocytic leukemia cells. J Med Food. 2006;9:138–144. doi: 10.1089/jmf.2006.9.138. [DOI] [PubMed] [Google Scholar]
- Albuquerque U, Barros I, Chiapetta A. Pteridófitas utilizadas nos cultos afro-brasileiros em Recife–PE: um estudo etnobotânico. Biol Brasílica. 1997;7:23–30. [Google Scholar]
- Alcaraz M, Pathak M, Rius F, et al. An extract of Polypodium leucotomos appears to minimize certain photoaging changes in a hairless albino mouse animal model: a pilot study. Photodermatol Photoimmunol Photomed. 1999;15:120–126. doi: 10.1111/j.1600-0781.1999.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Ali MS, Amin MR, Kamal CMI, et al. In vitro antioxidant, cytotoxic, thrombolytic activities and phytochemical evaluation of methanol extract of the A. philippense L. leaves. Asian Pacific J Trop Biomed. 2013;3:464–469. doi: 10.1016/S2221-1691(13)60097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Ohro T, Hiraga Y, et al. Biologically active clerodane-type diterpene glycosides from the root-stalks of Dicranopteris pedata. Phytochemistry. 1997;46:839–844. doi: 10.1016/S0031-9422(97)00377-4. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Tanaka N, Suzuki N, et al. Neue pterosin-derivate aus Pteris wallichiana Agaroh und P. semipinnata L. Chem Pharm Bull. 1977;25:2461–2464. doi: 10.1248/cpb.25.2461. [DOI] [Google Scholar]
- Argueta Villamar A, Cano Asseleih L, Rodarte M. Atlas de las plantas de las medicina tradicional Mexicana. Mexico: Instituto Nacional Indigenista; 1994. [Google Scholar]
- Aryangat AV, Gerich JE. Type 2 diabetes: postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manag. 2010;6:145–155. doi: 10.2147/vhrm.s8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai F, Iinuma M, Tanaka T, et al. Complex flavonoids in farinose exudate from Pityrogramma calomelanos. Phytochemistry. 1991;30:3091–3093. doi: 10.1016/S0031-9422(00)98259-1. [DOI] [Google Scholar]
- Asai F, Iinuma M, Tanaka T. Complex flavonoids in the farinose exudate of Pityrogramma calomelanos. Phytochemistry. 1992;31:2487–2490. doi: 10.1016/0031-9422(92)83306-J. [DOI] [Google Scholar]
- Asai F, Iinuma M, Tanaka T, et al. Two complex flavonoids in the farinose exudate of Pityrogramma calomelanos. Heterocycles. 1992;33:229–233. doi: 10.3987/COM-91-S21. [DOI] [Google Scholar]
- Asgarpanah J, Roohi E. Phytochemistry and pharmacological properties of Equisetum arvense L. J Med Plants Res. 2012;6:3689–3693. [Google Scholar]
- Ayer WA, Ma Y, Liu J, et al. Macleanine, a unique type of dinitrogenous Lycopodiurn alkaloid. Can J Chem. 1994;72:128–130. doi: 10.1139/v94-020. [DOI] [Google Scholar]
- Azaizeh H, Saad B, Khalil K, et al. The state of the art of traditional Arab herbal medicine in the Eastern region of the Mediterranean: a review. ECAM. 2006;3:229–235. doi: 10.1093/ecam/nel034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai R, Zhou Y, Deng S, et al. Two new ent-kaurane diterpenoids from Pteris semipinnata. J Asian Nat Prod Res. 2013;15:1107–1111. doi: 10.1080/10286020.2013.818661. [DOI] [PubMed] [Google Scholar]
- Ban SH, Kim JE, Pandit S, et al. Influences of Dryopteris crassirhizoma extract on the viability, growth and virulence properties of Streptococcus mutans. Molecules. 2012;17:9231–9244. doi: 10.3390/molecules17089231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JA. Selaginella and 400 million years of separation. Annu Rev Plant Biol. 2009;60:223–238. doi: 10.1146/annurev.arplant.59.032607.092851. [DOI] [PubMed] [Google Scholar]
- Barros I, Andrade L. Pteridófitas medicinais (samambaias, avencas e plantas afins) Recife: Ed UFPE; 1997. [Google Scholar]
- Bey R, Thingstad SF, Paulsen BS. Horsetail (Equisetum spp.) as a source of silicon supplement in human nutritional myth? J Herbs Spices Med Plants. 2010;16:119–125. doi: 10.1080/10496475.2010.504405. [DOI] [Google Scholar]
- Brahmachari G, Mondal S, Chatterjee D, et al. Phytochemicals and biological activities of Adiantum species. J Sci Ind Res. 2003;62:1119–1130. [Google Scholar]
- Cai JX, Wu WS, Wu LY, et al. Total flavonoid contents of 22 kinds of fern plants. J Fujian Teach Univ Nat Sci Ed. 2000;16:63–66. [Google Scholar]
- Cai J, Wu W, Ge Q. Bacteriostatic activities of extracts from 20 medicinal pteridophytes. Subtropi Plant Sci. 2003;33:22–25. [Google Scholar]
- Calzada F, Yépez-Mulia L, Aguilar A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2006;108:367–370. doi: 10.1016/j.jep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Calzada F, Lilian YM, Contreras AT. Effect of Mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalis trophozoites. J Ethnopharmacol. 2007;113:248–251. doi: 10.1016/j.jep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Calzada F, Arista R, Pérez H. Effect of plants used in Mexico to treat gastrointestinal disorders on charcoal–gum acacia-induced hyperperistalsis in rats. J Ethnopharmacol. 2010;128(1):49–51. doi: 10.1016/j.jep.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Cao X, Ma LQ, Tu C. Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.) Environm Poll. 2004;128:317–325. doi: 10.1016/j.envpol.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Cao J, Xia X, Chen X, et al. Flavonoids contents, antioxidant and anticancer activities of 78 species of ferns from China. Biomed Pap. 2012;156:S33. [Google Scholar]
- Cao J, Xia X, Chen X, et al. Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem Toxicol. 2013;51:242–250. doi: 10.1016/j.fct.2012.09.039. [DOI] [PubMed] [Google Scholar]
- Cao J, Xia X, Dai X, et al. Flavonoids profiles, antioxidant, acetylcholinesterase inhibition activities of extract from Dryoathyrium boryanum (Willd.) Ching. Food Chem Toxicol. 2013;55:121–128. doi: 10.1016/j.fct.2012.12.051. [DOI] [PubMed] [Google Scholar]
- Cao J, Xia X, Dai X, et al. Chemical composition and bioactivities of flavonoids-rich extract from Davallia cylindrica Ching. Environ Toxicol Pharmacol. 2014;37:571–579. doi: 10.1016/j.etap.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu Y, Duan J. A new selaginellin derivative from Selaginella pulvinata. Acta Pharma Sin. 2015;50:199–202. [PubMed] [Google Scholar]
- Cao Y, Yao Y, Huang XJ, et al. Four new selaginellin derivatives from Selaginella pulvinata: mechanism of racemization process in selaginellins with quinone methide. Tetrahedron. 2015;71:1581–1587. doi: 10.1016/j.tet.2015.01.017. [DOI] [Google Scholar]
- Carneiro DM, Freire RC, Honório TCD, et al. Randomized, double-blind clinical trial to assess the acute diuretic effect of Equisetum arvense (field horsetail) in healthy volunteers. ECAM. 2014;2014:1–8. doi: 10.1155/2014/760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo UF, Wilkins AL, Lauren DR, et al. Isoptaquiloside and caudatoside, illudane-type sesquiterpene glucosides from Pteridium aquilinum var. caudatum. Phytochemistry. 1997;44:901–906. doi: 10.1016/S0031-9422(96)00593-6. [DOI] [Google Scholar]
- Castillo UF, Ojika M, Alonso-Amelot M, et al. Ptaquiloside Z, a new toxic unstable sesquiterpene glycoside from the neotropical bracken fern Pteridium aquilinum var. caudatum. Bioorg Med Chem. 1998;6:2229–2233. doi: 10.1016/S0968-0896(98)00168-0. [DOI] [PubMed] [Google Scholar]
- Castillo UF, Sakagami Y, Alonso-Amelot M, et al. Pteridanoside, the first protoilludane sesquiterpene glucoside as a toxic component of the neotropical bracken fern Pteridium aquilinum var. caudatum. Tetrahedron. 1999;55:12295–12300. doi: 10.1016/S0040-4020(99)00728-0. [DOI] [Google Scholar]
- Četojević-Simin DD, Čanadanović-Brunet JM, Bogdanović GM, et al. Antioxidative and antiproliferative activities of different horsetail (Equisetum arvense L.) extracts. J Med Food. 2010;13:452–459. doi: 10.1089/jmf.2008.0159. [DOI] [PubMed] [Google Scholar]
- Chai TT, Wong FC. Antioxidant properties of aqueous extracts of Selaginella willdenowii. J Med Plants Res. 2012;6:1289–1296. [Google Scholar]
- Chai TT, Yong AL, Ong HC. Antibacterial, anti-glucosidase, and antioxidant activities of selected highland ferns of Malaysia. Bot Stud. 2013;54:55. doi: 10.1186/1999-3110-54-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai TT, Quah Y, Ooh KF, et al. Anti-proliferative, antioxidant and iron-chelating properties of the tropical highland fern, Phymatopteris triloba (Houtt) Pichi Serm (Family Polypodiaceae) Trop J Pharma Res. 2013;12:747–753. [Google Scholar]
- Chai TT, Yeoh LY, Ismail NIM, et al. Evaluation of glucosidase inhibitory and cytotoxic potential of five selected edible and medicinal ferns. Trop J Pharma Res. 2015;14:449–454. doi: 10.4314/tjpr.v14i3.13. [DOI] [Google Scholar]
- Chai TT, Yeoh LY, Ismail NIM, et al. Cytotoxicity and antiglucosidase potential of six selected edible and medicinal ferns. Acta Poloniae Pharma Drug Res. 2015;72:297–401. [PubMed] [Google Scholar]
- Chandran G. Insights on the neuromodulatory propensity of Selaginella (Sanjeevani) and its potential pharmacological applications. CNS Neur Dis Drug Targets. 2014;13:82–95. doi: 10.2174/18715273113126660188. [DOI] [PubMed] [Google Scholar]
- Chang RC, Wu ZY. Flora of China. Beijing: Science Press; 1999. p. 79. [Google Scholar]
- Chang Y, Hu J, Jiang S, et al. Study on the distribution and the total flavonoids content of medicial pteridophytes in Nanjing Zijin Mountain. J Northeast Agric Univ. 2005;36:320–323. [Google Scholar]
- Chang X, Li W, Koike K, et al. Phenolic constituents from the rhizomes of Dryopteris crassirhizoma. Chem Pharma Bull. 2006;54:748–750. doi: 10.1248/cpb.54.748. [DOI] [PubMed] [Google Scholar]
- Chang HC, Huang GJ, Agrawal DC, et al. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Bot Stud. 2007;48:397–406. [Google Scholar]
- Chang HL, Su JH, Yeh YT, et al. Protoapigenone, a novel flavonoid, inhibits ovarian cancer cell growth in vitro and in vivo. Cancer Lett. 2008;267:85–95. doi: 10.1016/j.canlet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Chang HL, Wu YC, Su JH, et al. Protoapigenone, a novel flavonoid, induces apoptosis in human prostate cancer cells through activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase 1/2. J Pharmacol ExperTher. 2008;325:841–849. doi: 10.1124/jpet.107.135442. [DOI] [PubMed] [Google Scholar]
- Chang SH, Bae JH, Hong DP, et al. Dryopteris crassirhizoma has anti-cancer effects through both extrinsic and intrinsic apoptotic pathways and G0/G1 phase arrest in human prostate cancer cells. J Ethnopharmacol. 2010;130:248–254. doi: 10.1016/j.jep.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Chen CC, Huang YL, Yeh PY, et al. Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica. Planta Med. 2003;69:964–967. doi: 10.1055/s-2003-45112. [DOI] [PubMed] [Google Scholar]
- Chen G, Liang NC, Lee J, et al. Over-expression of Bcl-2 against Pteris semipinnata L-induced apoptosis of human colon cancer cells via a NF-kappa B-related pathway. Apoptosis. 2004;9:619–627. doi: 10.1023/B:APPT.0000038041.57782.84. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chang FR, Lin YJ, et al. Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.) Food Chem. 2007;105:48–56. doi: 10.1016/j.foodchem.2007.03.055. [DOI] [Google Scholar]
- Chen YH, Chang FR, Lin YJ, et al. Identification of antioxidants from rhizome of Davallia solida. Food Chem. 2008;107:684–691. doi: 10.1016/j.foodchem.2007.08.066. [DOI] [Google Scholar]
- Chen YH, Chang FR, Lu MC, et al. New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm. Molecules. 2008;13:255–266. doi: 10.3390/molecules13020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang G, He J, et al. New naphthoquinone from the root of Lygodium japonicum (Thunb.) Sw. J Nat Med. 2010;64:114–116. doi: 10.1007/s11418-009-0376-y. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen X, Lei Y, et al. Vascular protective potential of the total flavanol glycosides from Abacopteris penangiana via modulating nuclear transcription factor-κB signaling pathway and oxidative stress. J Ethnopharmacol. 2011;136:217–223. doi: 10.1016/j.jep.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Chen GG, Leung J, Liang NC, et al. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits hepatocellular carcinoma in vitro and in vivo via stabilizing IkBα. Inv New Drugs. 2012;30:2210–2218. doi: 10.1007/s10637-011-9791-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Lei Y, Wu G, et al. Renoprotective potential of Macrothelypteris torresiana via ameliorating oxidative stress and proinflammatory cytokines. J Ethnopharmacol. 2012;139:207–213. doi: 10.1016/j.jep.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Chen JH, Wang TC, Yang CK, et al. New pterosin sesquiterpenes and antitubercular constituents from Pteris ensiformis. Chem Biodivers. 2013;10:1903–1908. doi: 10.1002/cbdv.201300072. [DOI] [PubMed] [Google Scholar]
- Chen ND, Chen ND, Chen CW, et al. A novel bihomoflavanonol with an unprecedented skeleton from Pteridium aquilinum. Chin Herb Med. 2013;5:96–100. [Google Scholar]
- Chen CY, Huang CC, Tsai KC, et al. Evaluation of the antihyperuricemic activity of phytochemicals from Davallia formosana by enzyme assay and hyperuricemic mice model. ECAM. 2014;2014:1–10. doi: 10.1155/2014/873607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hao ZY, Wang XL, et al. Sinensioside A, a new sesquilignan glycoside from Selaginella sinensis. Chin J Nat Med. 2014;12:148–150. doi: 10.1016/S1875-5364(14)60024-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Teng H, Xie ZL, et al. Modifications of dietary flavonoids towards improved bioactivity: an update on structure–activity relationship. Crit Rev Food Sci Nutr. 2017 doi: 10.1080/10408398.2016.1196334. [DOI] [PubMed] [Google Scholar]
- Cheng XL, Ma SC, Yu JD, et al. Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina. Chem Pharma Bull. 2008;56:982–984. doi: 10.1248/cpb.56.982. [DOI] [PubMed] [Google Scholar]
- Cheng AS, Chang WC, Cheng YH, et al. The effects of Davallic acid from Davallia divaricata Blume on apoptosis induction in A549 lung cancer cells. Molecules. 2012;17:12938–12949. doi: 10.3390/molecules171112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng YG, Tsai CC, Chung HH, et al. Antihyperglycemic action of sinapic acid in diabetic rats. J Agric Food Chem. 2013;61:12053–12059. doi: 10.1021/jf403092b. [DOI] [PubMed] [Google Scholar]
- Cheryl AL. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;13:45–51. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Materia Medica Editorial Board . Chinese materia medica. Shanghai: Shanghai Scientific and Technical Press; 1999. [Google Scholar]
- Ching R. The Chinese fern families and genera: systematic arrangement and historical origin. Selected papers of Ching Ren Chang. Beijing: Science Press; 1988. [Google Scholar]
- Choudhry SZ, Bhatia N, Ceilley R, et al. Role of oral Polypodium leucotomos extract in dermatologic diseases: a review of the literature. J Drugs Dermatol. 2014;13:148–153. [PubMed] [Google Scholar]
- Christenhusz MJ, Chase MW. Trends and concepts in fern classification. Ann Bot. 2014;113:571–594. doi: 10.1093/aob/mct299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clericuzio M, Tinello S, Burlando B, et al. Flavonoid oligoglycosides from Ophioglossum vulgatum L. having wound healing properties. Planta Med. 2012;78:1639–1644. doi: 10.1055/s-0032-1315149. [DOI] [PubMed] [Google Scholar]
- Clericuzio M, Burlando B, Gandini G, et al. Keratinocyte wound healing activity of galactoglycerolipids from the fern Ophioglossum vulgatum L. J Nat Med. 2014;68:31–37. doi: 10.1007/s11418-013-0759-y. [DOI] [PubMed] [Google Scholar]
- Corrêa ACL, Hans Filho GD, Matias R, et al. Healing effect of the ointment made of Equisetum pyramidale in the treatment of cutaneous lesions in diabetic rats. Braz Arch Biol Technol. 2013;56:377–382. doi: 10.1590/S1516-89132013000300005. [DOI] [Google Scholar]
- Cos P, Ying L, Calomme M, et al. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–76. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- Cramer L, Ernst L, Lubienski M, et al. Structural and quantitative analysis of Equisetum alkaloids. Phytochemistry. 2015;116:269–282. doi: 10.1016/j.phytochem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Cui CB, Tezuka Y, Kikuchi T, et al. Constituents of a fern, Davallia mariesii Moore. I. Isolation and structures of davallialactone and a new flavanone glucuronide. Chem Pharma Bull. 1990;38:3218–3225. doi: 10.1248/cpb.38.3218. [DOI] [PubMed] [Google Scholar]
- Cui CB, Tezuka YT, Kikuchi H, et al. Constituents of a fern, Davallia mariesii Moore. IV. Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide, and two epicatechin glycosides. Chem Pharma Bull. 1992;40:2035–2040. doi: 10.1248/cpb.40.2035. [DOI] [Google Scholar]
- Cui CB, Tezuka YT, Yamashita H, et al. Constituents of a Fern, Davallia mariesii MOORE. V. Isolation and structures of Davallin, a new tetrameric proanthocyanidin, and two new phenolic glycosides. Chem Pharma Bull. 1993;41:1491–1497. doi: 10.1248/cpb.41.1491. [DOI] [Google Scholar]
- Cáceres-Peña YC, Naya M, Calcagno-Pissarelli MP, et al. Influence of Bracken Fern (Pteridium caudatum L. Maxon) pre-treatment on extraction yield of illudane glycosides and pterosins. Phytochem Anal. 2013;24:290–295. doi: 10.1002/pca.2409. [DOI] [PubMed] [Google Scholar]
- de Sá PGS, Nunes XP, Lima JT, et al. Antinociceptive effect of ethanolic extract of Selaginella convoluta in mice. BMC CAM. 2012;12:187. doi: 10.1186/1472-6882-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HS. The medicinal spore plants of China. Shanghai: Shanghai Science and Technology Publishers; 1982. [Google Scholar]
- Dong L, Yang J, He J, et al. Lycopalhine A, a novel sterically congested Lycopodium alkaloid with an unprecedented skeleton from Palhinhaea cernua. Chem Commun. 2012;48:9038–9040. doi: 10.1039/c2cc34676a. [DOI] [PubMed] [Google Scholar]
- Dong JW, Cai L, Li XJ, et al. Two new peroxy fatty acids with antibacterial activity from Ophioglossum thermale Kom. Fitoterapia. 2016;109:212–216. doi: 10.1016/j.fitote.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Duan S, Tang S, Qin N, et al. Chemical constituents of Phymatopteris hastate and their antioxidant activity. China J Chin Mater Med. 2012;37:1402–1407. [PubMed] [Google Scholar]
- Duan YH, Dai Y, He RR, et al. A new phenylpropanoid glucoside from the aerial parts of Lygodium japonicum. J Asian Nat Prod Res. 2012;14:286–292. doi: 10.1080/10286020.2011.650690. [DOI] [PubMed] [Google Scholar]
- Duke JA. Duke’s handbook of medicinal plants of Latin America. London: CRC Press; 2008. [Google Scholar]
- D’Agostino M, Dini A, Pizza C, et al. Sterols from Equisetum arvense. Boll Soc Ital Biol Sper. 1984;60:2241. [PubMed] [Google Scholar]
- Editorial Board of China Herba . Editorial Board of China Herba. State Administration of Traditional Chinese Medicine. China. ChinaHerbal. Shanghai: Shanghai Scientific and Technical Publishers; 1998. [Google Scholar]
- Elgoweini M, Din NNE. Response of vitiligo to narrowband ultraviolet B and oral antioxidants. J Clin Pharmacol. 2009;49:852–855. doi: 10.1177/0091270009335769. [DOI] [PubMed] [Google Scholar]
- Fang JB, Chen JC, Duan HQ. Two new flavan-4-ol glycosides from Abacopteris penangiana. J Asian Nat Prod Res. 2010;12:355–359. doi: 10.1080/10286021003764453. [DOI] [PubMed] [Google Scholar]
- Fang X, Lin X, Liang S, Zhang WD, Feng Y, Ruan KF. Phytochemical study of Hicriopteris glauca. Chem Nat Compd. 2013;49:514–515. doi: 10.1007/s10600-013-0655-4. [DOI] [Google Scholar]
- Flavin R, Pelus S, Nguyen PL, et al. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher MT, Hayes PY, Somerville MJ, et al. Ptesculentoside, a novel norsesquiterpene glucoside from the Australian bracken fern Pteridium esculentum. Tetrahedron Lett. 2010;51:1997–1999. doi: 10.1016/j.tetlet.2010.02.032. [DOI] [Google Scholar]
- Fu W, Fang W, Ruan J. Two new flavanone glycosides from Macrothelypteris torresiana (Gaud.) Ching. Chin Chem Lett. 2009;20:579–581. doi: 10.1016/j.cclet.2008.12.025. [DOI] [Google Scholar]
- Fu W, Chen JL, Cai YL, et al. Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) J Ethnopharmacol. 2010;130:521–528. doi: 10.1016/j.jep.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Fu W, Du G, Liu D, et al. Neuroprotective effect of a caffeic acid derivative from Abacopteris penangiana. Pharm Biol. 2013;51:376–382. doi: 10.3109/13880209.2012.732581. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Kuroyanagi M, Yoshihira K, et al. Chemical and toxicological studies on bracken fern Pteridium aquilinum var. latiusculum. IV. Surveys on bracken constituents by mutagen test. J Pharm Biodyn. 1978;1:324–331. doi: 10.1248/cpb.26.2365. [DOI] [PubMed] [Google Scholar]
- Gao WY, Li YM, Wang BD, Zhu DY. Huperzine H, a new Lycopodium alkaloid from Huperzia serrata. Chin Chem Lett. 1999;10:463–466. [Google Scholar]
- Gao W, Li Y, Jiang S, et al. Three Lycopodium alkaloid N-oxides from Huperzia serrata. Planta Med. 2000;66:664–667. doi: 10.1055/s-2000-8630. [DOI] [PubMed] [Google Scholar]
- Gao W, Wang B, Li Y, et al. A new alkaloid and arbutin from the whole plant of Huperzia serrata. Chin J Chem. 2000;18:614–616. [Google Scholar]
- Gao ZP, Li RF, Wang BH, et al. Progress in chemical constituents of genus Dryopteris. Chin J Exp Trad Med Form. 2003;9:50–55. [Google Scholar]
- Gao W, Li Y, Jiang S, et al. Two new nitrone alkaloids from Huperzia serrata. Helv Chim Acta. 2008;91:1031–1035. doi: 10.1002/hlca.200890110. [DOI] [Google Scholar]
- Gao Z, Ali Z, Zhao J, et al. Phytochemical investigation of the rhizomes of Dryopteris crassirhizoma. Phytochem Lett. 2008;1:188–190. doi: 10.1016/j.phytol.2008.09.005. [DOI] [Google Scholar]
- García F, Pivel JP, Guerrero A, et al. Phenolic components and antioxidant activity of Fernblock®, an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find Exp Clin Pharmacol. 2006;28:157–160. doi: 10.1358/mf.2006.28.3.985227. [DOI] [PubMed] [Google Scholar]
- Ge X, Ye G, Li P, et al. Cytotoxic diterpenoids and sesquiterpenoids from Pteris multifida. J Nat Prod. 2008;71:227–231. doi: 10.1021/np0706421. [DOI] [PubMed] [Google Scholar]
- Girish C, Muralidhara Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: implications for Parkinson’s disease. Neurotoxicology. 2012;33:444–456. doi: 10.1016/j.neuro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Gombau L, García F, Lahoz A, et al. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol In Vitro. 2006;20:464–471. doi: 10.1016/j.tiv.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Gomes AJ, Lunardi CN, Gonzalez S, et al. The antioxidant action of Polypodium leucotomos extract and kojic acid: reactions with reactive oxygen species. Braz J Med Biol Res. 2001;34:1487–1494. doi: 10.1590/S0100-879X2001001100018. [DOI] [PubMed] [Google Scholar]
- Gong XL, Chen ZH, Dian LH, et al. Analysis of the chemical constituents of volatile oil from Pteris semipinnata by GC–MS. Lishizhen Med Mat Med Res. 2005;16:697–698. [Google Scholar]
- Gong XL, Chen ZH, Liang NC. Advances in study on chemical constituents and pharmacological activities of plants of genus Pteris. Chin J Chin Mater Med. 2007;32:1382–1387. [PubMed] [Google Scholar]
- Gonzalez S. Polypodium leucotomos extract: a natural antioxidant and photoprotective tool for the management of UV-induced skin damage and phototherapy. Cosmet Dermatol. 2009;22:604–609. [Google Scholar]
- Gonzalez S, Alcaraz MV, Cuevas J, et al. An extract of the fern Polypodium leucotomos (Difur®) modulates Th1/Th2 cytokines balance in vitro and appears to exhibit anti-angiogenic activities in vivo: pathogenic relationships and therapeutic implications. Anticancer Res. 2000;20:1567–1575. [PubMed] [Google Scholar]
- Gonzalez S, Gilaberte Y, Philips N, et al. Fernblock, a nutriceutical with photoprotective properties and potential preventive agent for skin photoaging and photoinduced skin cancers. Int J Mol Sci. 2011;12:8466–8475. doi: 10.3390/ijms12128466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou ZP, Liang NC, Hou J, et al. Two new diterpene and sesquiterpene from Pteris dispar. Chin Chem Lett. 2011;22:1451–1453. doi: 10.1016/j.cclet.2011.09.001. [DOI] [Google Scholar]
- Ha H, Shim KS, Kim T, et al. Water extract of Dryopteris crassirhizoma attenuates bone loss by suppressing osteoclast differentiation and function. ECAM. 2013;2013:1–9. doi: 10.1155/2013/852648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Nazreen S, Alam MM, et al. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from Adiantum capillus veneris Linn. J Ethnopharmacol. 2011;138:741–747. doi: 10.1016/j.jep.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Haider S, Kharbanda C, Alam MS, et al. Anti-inflammatory and anti-nociceptive activities of two new triterpenoids from Adiantum capillus-veneris Linn. Nat Prod Res. 2013;27:2304–2310. doi: 10.1080/14786419.2013.828292. [DOI] [PubMed] [Google Scholar]
- Halldorsdottir ES, Kowal NM, Olafsdottir ES. The Genus Diphasiastrum and Its Lycopodium Alkaloids. Planta Med. 2015;81:995–1002. doi: 10.1055/s-0035-1546182. [DOI] [PubMed] [Google Scholar]
- Ham Y, Yoon W, Park S, et al. Investigation of the component of Lycopodium serratum extract that inhibits proliferation and mediates apoptosis of human HL-60 leukemia cells. Food Chem Toxicol. 2012;50:2629–2634. doi: 10.1016/j.fct.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Han BH, Suh Y, Chi HJ. Medicinal plants in the Republic of Korea. Manila: Natural Products Research Institute, Seoul National University/WHO Regional Office for the Western Pacific; 1998. [Google Scholar]
- Han QH, Liu X, Yao WQ, et al. Unusual 9, 19: 24, 32-dicyclotetracyclic triterpenoids from Lygodium japonicum. Planta Med. 2012;78:1971–1975. doi: 10.1055/s-0032-1320686. [DOI] [PubMed] [Google Scholar]
- Han AR, Lee NY, Nam JW, et al. Identification of a new biflavonoid from Selaginella doederleinii Hieron. Bull Korean Chem Soc. 2013;34:3147–3149. doi: 10.5012/bkcs.2013.34.10.3147. [DOI] [Google Scholar]
- Harborne JB. Comparative biochemistry of flavonoids-II.: 3-desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry. 1966;5:589–600. doi: 10.1016/S0031-9422(00)83637-7. [DOI] [Google Scholar]
- Harborne JB. The flavonoids, advances in research since 1980. London: Chapman and Hall; 1988. pp. 427–468. [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 1988;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Harinantenaina L, Matsunami K, Otsuka H. Chemical and biologically active constituents of Pteris multifida. J Nat Med. 2008;62:452–455. doi: 10.1007/s11418-008-0265-9. [DOI] [PubMed] [Google Scholar]
- Harinantenaina L, Matsunami K, Otsuka H. Chemical constituents of Pteris cretica Linn. (Pteridaceae) Biochem Sys Ecol. 2009;37:133–137. doi: 10.1016/j.bse.2009.01.012. [DOI] [Google Scholar]
- He XM, Ji N, Xiang XC, et al. Purification, characterization, and molecular cloning of a novel antifungal lectin from the roots of Ophioglossum pedunculosum. Appl Biochem Biotechnol. 2011;165:1458–1472. doi: 10.1007/s12010-011-9367-z. [DOI] [PubMed] [Google Scholar]
- Herrmann F, Romero MR, Blazquez AG, et al. Diversity of pharmacological properties in Chinese and European medicinal plants: cytotoxicity, antiviral and anti-trypanosomal screening of 82 herbal drugs. Diversity. 2011;3:547–580. doi: 10.3390/d3040547. [DOI] [Google Scholar]
- Hikino H, Takahashi T, Arihara S, et al. Structure of Pteroside B, Glycoside of Pteridium aquilinum var. latiusculum. Chem Pharm Bull. 1970;18:1488–1491. doi: 10.1248/cpb.18.1488. [DOI] [Google Scholar]
- Hikino H, Takhashi T, Takemoto T, et al. Structure of Pteroside Z and D. Glycosides of Pteridium aqullinum var. latiusculum. Chem Pharm Bull. 1971;19:2424–2425. doi: 10.1248/cpb.19.2424. [DOI] [Google Scholar]
- Hikino H, Takhashi T, Takemoto T, et al. Structure of Pteroside A and C, Glycosides of Pteridium aqullinum var. latlusculum. Chem Pharm Bull. 1972;20:210–212. doi: 10.1248/cpb.20.210. [DOI] [Google Scholar]
- Hiraoka A. Flavonoid patterns in Athyriaceae and Dryopteridaceae. Biochem Syst Ecol. 1978;6:171–175. doi: 10.1016/0305-1978(78)90003-0. [DOI] [Google Scholar]
- Hirasawa Y, Astulla A, Shiro M, et al. Lycotetrastine A, a novel hexacyclic alkaloid from Huperzia tetrasticha. Tetrahedron Lett. 2011;52:4216–4218. doi: 10.1016/j.tetlet.2011.05.133. [DOI] [Google Scholar]
- Hirasawa Y, Matsuya R, Shaari K, et al. Lycobelines A-C, Novel C 16 N 2-type Lycopodium alkaloids from Huperzia goebelii. Tetrahedr Lett. 2012;53:3971–3973. doi: 10.1016/j.tetlet.2012.05.080. [DOI] [Google Scholar]
- Hirasawa Y, Kato Y, Wong CP, et al. Huperminone A, a novel C 16N-type Lycopodium alkaloid from Huperzia phlegmaria. Tetrahedron Lett. 2013;54:1593–1595. doi: 10.1016/j.tetlet.2013.01.048. [DOI] [Google Scholar]
- Hirasawa Y, Kato Y, Wong CP, et al. Hupermine A, a novel C16N2-type Lycopodium alkaloid from Huperzia phlegmaria. Tetrahedron Lett. 2014;55:1902–1904. doi: 10.1016/j.tetlet.2014.01.141. [DOI] [Google Scholar]
- Hirono I. Naturally occurring carcinogens of plant origin. Kodansha: Elsevier Science Pub. Co.; 1987. [Google Scholar]
- Ho R, Teai T, Bianchini JP, Lafont R, Raharivelomanana P. Ferns: from traditional uses to pharmaceutical development, chemical identification of active principles. In: Fernández H, Kumar A, Revilla MA, editors. Working with Ferns. New York: Springer; 2011. pp. 321–346. [Google Scholar]
- Ho R, Girault JP, Raharivelomanana P, et al. E-and Z-isomers of new phytoecdysteroid conjugates from French Polynesian Microsorum membranifolium (Polypodiaceae) Fronds. Molecules. 2012;17:11598–11606. doi: 10.3390/molecules171011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Alvarado F, Szöcs J, et al. Metabolic effects of calagualine, an antitumoral saponine of Polypodium leucotomos. Nature. 1967;214:1256–1258. doi: 10.1038/2141256a0. [DOI] [PubMed] [Google Scholar]
- Hsu FL, Huang CF, Chen YW, et al. Antidiabetic effects of pterosin A, a small-molecular-weight natural product, on diabetic mouse models. Diabetes. 2013;62:628–638. doi: 10.2337/db12-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Gross ML, Yuan SQ, Wei TT, Lu YQ. Mass spectrometric differentiation of huperzinine, N,N-dimethylhuperzine A and N-methylhuperzine B. Org Mass Spectrom. 1992;27:99–104. doi: 10.1002/oms.1210270206. [DOI] [Google Scholar]
- Hu J, Chen XQ, Zhao QS. Diterpenoids from Diplopterygium rufopilosum. Helv Chim Acta. 2011;94:1085–1090. doi: 10.1002/hlca.201000337. [DOI] [Google Scholar]
- Hu J, Shi X, Mao X, et al. Ecdysteroids from the ethanol extract of Diplopterygium rufopilosum. Phytochem Lett. 2014;8:73–76. doi: 10.1016/j.phytol.2014.02.003. [DOI] [Google Scholar]
- Huang YL, Yeh PY, Shen CC, et al. Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica. Phytochemistry. 2003;64:1277–1283. doi: 10.1016/j.phytochem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Zhang ZX, Li YZ, et al. Anti-insect activity of the methanol extracts of fern and gymnosperm. Agric Sci China. 2010;9:249–256. doi: 10.1016/S1671-2927(09)60090-0. [DOI] [Google Scholar]
- Huang XH, Xiong PC, Xiong CM, et al. In vitro and in vivo antitumor activity of Macrothelypteris torresiana and its acute/subacute oral toxicity. Phytomedicine. 2010;17:930–934. doi: 10.1016/j.phymed.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hwang TL, Yang YL, et al. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med. 2010;76:447–453. doi: 10.1055/s-0029-1186221. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Xu HH, Tian YQ, et al. Insecticidal activities and active ingredients of Pronephrium megacuspe. Sci Agric Sin. 2012;1:010. [Google Scholar]
- Hwang TH, Kashiwada Y, Nonaka GI, et al. Flavan-3-ol and proanthocyanidin allosides from Davallia divaricata. Phytochemistry. 1989;28:891–896. doi: 10.1016/0031-9422(89)80138-4. [DOI] [Google Scholar]
- Hwang TH, Kashiwada Y, Nonaka GI, et al. 4-Carboxymethyl flavan-3-ols and procyanidins from Davallia divaricate. Phytochemistry. 1990;29:279–282. doi: 10.1016/0031-9422(90)89050-J. [DOI] [Google Scholar]
- Ibraheim ZZ, Ahmed AS, Gouda YG. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J. 2011;19:65–74. doi: 10.1016/j.jsps.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi Y, Iida T, Yoshida R, et al. Pteridic acids A and B, novel plant growth promoters with auxin-like activity from Streptomyces hygroscopicus TP-A0451. J Antibiot. 2002;55:764–767. doi: 10.7164/antibiotics.55.764. [DOI] [PubMed] [Google Scholar]
- Imperato F. Kaempferol and Quercetin 3-O-(2″, 3″-di-Op-coumaroyl)-glucosides from Pteris vittata. Am Fern J. 2003;93:157–160. doi: 10.1640/0002-8444(2003)093[0157:SN]2.0.CO;2. [DOI] [Google Scholar]
- Imperato F. Vitexin 7-O-rhamnoside, a New Flavonoid from Pteris vittata. Am Fern J. 2004;94:159–162. doi: 10.1640/0002-8444(2004)094[0159:SN]2.0.CO;2. [DOI] [Google Scholar]
- Imperato F. Kaempferol 3-O-(acetylrutinoside), a new flavonoid and two new fern constituents, quercetin 3-O-(acetylglucoside) and 3-O-(acetylrutinoside) From Dryopteris Villarii. Am Fern J. 2006;96:93–96. doi: 10.1640/0002-8444(2006)96[93:SN]2.0.CO;2. [DOI] [Google Scholar]
- Imperato F. A new flavonoid, quercetin 3-O-(X”-acetyl-X”-cinnomoyl-glucoside) and a new fern consituent quescetin 3-O-(glucosylrhamnoside) from Dryopteris villarii. Am Fern J. 2007;97:124–126. doi: 10.1640/0002-8444(2007)97[124:SN]2.0.CO;2. [DOI] [Google Scholar]
- Imperato F. Three new flavonoid glycosides, kaempferol 3-O-(caffeoylrhamnoside), apigenin 4′-O-(caffeoylglucoside) and 4′-O-(feruloylglucoside) from Dryopteris villarii. Am Fern J. 2007;97:233–236. doi: 10.1640/0002-8444(2007)97[233:TNFGKO]2.0.CO;2. [DOI] [Google Scholar]
- Ito H, Muranaka T, Mori K. Ichthyotoxic phloroglucinol derivatives from Dryopteris fragrans and their anti-tumor promoting activity. Chem Pharm Bull. 2000;48:1190–1195. doi: 10.1248/cpb.48.1190. [DOI] [PubMed] [Google Scholar]
- Jalaludeen AM, Pari L. Studies on the antioxidant and free radical-scavenging effect of sinapic acid: an in vivo and in vitro model. J Pharm Sci Res. 2011;3:1447–1455. [Google Scholar]
- Ji YB, Gao SY. Effect of total alkaloid of Equisetum pratense on the contents of monoamine neurotransmitters in brain of rats. Chin J Clin Rehab. 2005;9:185–186. [Google Scholar]
- Jiang Su New College of Medicine . Dictionary of Chinese traditional medicine. Shang Hai: Shang Hai Technology Press; 1985. [Google Scholar]
- Jiang J, Liu Y, Min K, et al. Two new lycopodine alkaloids from Huperzia serrata. Helv Chim Acta. 2010;93:1187–1191. doi: 10.1002/hlca.200900365. [DOI] [Google Scholar]
- Jiang WW, Liu F, Gao X, et al. Huperserines A–E, Lycopodium alkaloids from Huperzia serrata. Fitoterapia. 2014;99:72–77. doi: 10.1016/j.fitote.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Jin ZS, Rao GW, Li CP. A new labdane diterpenoid from Pteris semipinnata. J Chem Res. 2010;34:39–40. doi: 10.3184/030823410X12625315106944. [DOI] [Google Scholar]
- Jung JE, Pandit S, Jeon JG. Identification of linoleic acid, a main component of the n-hexane fraction from Dryopteris crassirhizoma, as an anti-Streptococcus mutans biofilm agent. Biofouling. 2014;30:789–798. doi: 10.1080/08927014.2014.930446. [DOI] [PubMed] [Google Scholar]
- Kala S, Johnson M, Janakiraman N, et al. Pharmacognostic and phytochemical studies on some selected ethnomedicinal plants of Tamilnadu, South India. Int J Med Arom Plants. 2011;1:89–94. [Google Scholar]
- Kamisan FH, Yahya F, Mamat SS, et al. Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liver injury in rats. BMC CAM. 2014;14:1. doi: 10.1186/1472-6882-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MT. Popular herbal remedies of Taiwan. Taipei: SMC Publishing Inc.; 1985. [Google Scholar]
- Katakawa K, Kitajima M, Aimi N, et al. Structure elucidation and synthesis of lycoposerramine-B, a novel oxime-containing Lycopodium alkaloid from Lycopodium serratum Thunb. J Org Chem. 2005;70:658–663. doi: 10.1021/jo0483825. [DOI] [PubMed] [Google Scholar]
- Katakawa K, Kitajima M, Yamaguchi K, et al. Three new phlegmarine-type Lycopodium alkaloids, lycoposerramines-X,-Y and-Z, having a nitrone residue, from Lycopodium serratum. Heterocycles. 2006;69:223–229. doi: 10.3987/COM-06-S(O)16. [DOI] [Google Scholar]
- Katakawa K, Nozoe A, Kogure N, et al. Fawcettimine-related alkaloids from Lycopodium serratum. J Nat Prod. 2007;70:1024–1028. doi: 10.1021/np0700568. [DOI] [PubMed] [Google Scholar]
- Katakawa K, Kogure N, Kitajima M, et al. A new lycopodium alkaloid, lycoposerramine-R, with a novel skeleton and three new fawcettimine-related alkaloids from Lycopodium serratum. Helv Chim Acta. 2009;92:445–452. doi: 10.1002/hlca.200800327. [DOI] [Google Scholar]
- Katakawa K, Mito H, Kogure N, et al. Ten new fawcettimine-related alkaloids from three species of Lycopodium. Tetrahedron. 2011;67:6561–6567. doi: 10.1016/j.tet.2011.05.107. [DOI] [Google Scholar]
- Ko YJ, Wu JB, Ho HY, et al. Antiosteoporotic activity of Davallia formosana. J Ethnopharmacol. 2012;139:558–565. doi: 10.1016/j.jep.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Konrath EL, Passos CDS, Klein-Júnior LC, et al. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J Pharm Pharmacol. 2013;65:1701–1725. doi: 10.1111/jphp.12090. [DOI] [PubMed] [Google Scholar]
- Koshimizu R, Okumura Y, Hanano A (2004) Skin care preparation for external use. JP 2004-043348
- Kubo I, Muroi H, Himejima M. Antibacterial activity of totarol and its potentiation. J Nat Prod. 1992;55:1436–1440. doi: 10.1021/np50088a008. [DOI] [PubMed] [Google Scholar]
- Kunert O, Swamy RC, Kumar BR, et al. Two novel spirostene glycosides from Selaginella chrysocaulos and their chemotaxonomic significance. Nat Prod Commun. 2015;10:887–889. [PubMed] [Google Scholar]
- Kuraishi T, Mitadera Y, Murakami T, et al. Chemical and chemotaxomical studies of filices. XLII. Chemical studies on the constituents of Dicranopteris dichotoma (Thunb.) Bernh and Microlepia obtusiloba Hayata. Yakugaku Zasshi. 1983;103:679–682. doi: 10.1248/yakushi1947.103.6_679. [DOI] [Google Scholar]
- Kuraishi T, Murakami T, Taniguchi T, et al. Chemical and chemotaxonomical studies of ferns. LIV. Pterosin derivatives of the genus Microlepia (Pteridaceae) Chem Pharm Bull. 1985;33:2305–2312. doi: 10.1248/cpb.33.2305. [DOI] [Google Scholar]
- Kuroyanagi M, Fukuoka M, Yoshihira K, Natori S. Pterosin N and O, Phenylacetylpterosin C, and Pteroside P from Bracken, Pteridium aquilinum var. latiusculum. Chem Pharm Bull. 1974;22:2762–2764. doi: 10.1248/cpb.22.2762. [DOI] [Google Scholar]
- Kuroyanagi M, Fukuoka M, Yoshihira K, et al (1974b) The absolute configurations of pterosins, 1-indanone derivatives from Bracken, Pteridium aquilinum var. latiusculum. Chem Pharm Bull 22:723–726 [DOI] [PubMed]
- Kuroyanagi M, Fukuoka M, Yoshishira K, et al. Chemical and toxicological studies on Bracken Fern, Pteridinm aqullinum var. latiusculum. III. Futher characterization of pterosins and pterosides, sesquiterpenes and the glucosides having 1-indanone skeleton, from the rhizomes. Chem Pharm Bull. 1979;27:592–601. doi: 10.1248/cpb.27.592. [DOI] [Google Scholar]
- Kwon DY, Kang OH, Choi JG, et al. Antibacterial effect of Dryopteris crassirhizoma against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2007;78:430–433. doi: 10.1016/j.fitote.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Lai HY, Lim YY (2011) Antioxidant properties of some Malaysian ferns. In: 3rd international conference on chemical, biological and environmental engineering (IPCBEE)
- Lan KP, Shen YP, Lee SH, et al. Antioxidant and free radical-scavenging activities of Pteris multifida Poiret aqueous extract. J Food Qual. 2011;34:252–258. doi: 10.1111/j.1745-4557.2010.00356.x. [DOI] [Google Scholar]
- Lanzotti V. Drugs based on natural compounds: recent achievements and future perspectives. Phytochem Rev. 2014;13:725–726. doi: 10.1007/s11101-014-9385-x. [DOI] [Google Scholar]
- Lee SM, Na MK, An RB, et al. AntioxidaAnt activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol Pharma Bull. 2003;26:1354–1356. doi: 10.1248/bpb.26.1354. [DOI] [PubMed] [Google Scholar]
- Lei YF, Chen JL, Wei H, Xiong CM, Zhang YH, Ruan JL. Hypolipidemic and anti-inflammatory properties of Abacopterin A from Abacopteris penangiana in high-fat diet-induced hyperlipidemia mice. Food Chem Toxicol. 2011;49:3206–3210. doi: 10.1016/j.fct.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Li TS. Taiwanese native medicinal plants: phytopharmacology and therapeutic values. London: CRC Press; 2006. [Google Scholar]
- Li J, Han Y, Liu JS. Studies on triterpenoids of Huperzia serrata Thunb. Yao Xue Xue Bao. 1988;23:549–552. [PubMed] [Google Scholar]
- Li J, Liang N, Mo L, Zhang X, He C. Comparison of the cytotoxicity of five constituents from Pteris semipinnata L. in vitro and the analysis of their structure-activity relationships. Yao Xue Xue Bao. 1998;33:641–644. [PubMed] [Google Scholar]
- Li JH, Liang NC, Mo LE, et al. Effect of active compounds isolated from Pteris semipinnata L on DNA topoisomerases and tyrosine protein kinase and expression of C-MYC in lung adenocarcinoma cells. Chin J Cancer Res. 2001;13:105–109. doi: 10.1007/s11670-001-0025-9. [DOI] [Google Scholar]
- Li XL, Cheng X, Yang LM, et al. Dichotomains A and B: two new highly oxygenated phenolic derivatives from Dicranopteris dichotoma. Org Lett. 2006;8:1937–1940. doi: 10.1021/ol060535i. [DOI] [PubMed] [Google Scholar]
- Li XL, Yang LM, Zhao Y, et al. Tetranorclerodanes and clerodane-type diterpene glycosides from Dicranopteris dichotoma. J Nat Prod. 2007;70:265–268. doi: 10.1021/np0603166. [DOI] [PubMed] [Google Scholar]
- Li XL, Tu L, Zhao Y, et al. Terpenoids from two Dicranopteris species. Helv Chim Acta. 2008;91:856–861. doi: 10.1002/hlca.200890089. [DOI] [Google Scholar]
- Li MY, Leung J, Kong AW, et al. Anticancer efficacy of 5F in NNK-induced lung cancer development of A/J mice and human lung cancer cells. J Mol Med. 2010;88:1265–1276. doi: 10.1007/s00109-010-0676-4. [DOI] [PubMed] [Google Scholar]
- Li L, Chen GG, Lu YN, et al. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits growth of human lung cancer A549 cells by arresting cell cycle and triggering apoptosis. Chin J Cancer Res. 2012;24:109–115. doi: 10.1007/s11670-012-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Zou J, Li JX, et al. Non-alkaloids from Huperzia kunmingensis. Chin Trad Patent Med. 2013;6:031. [Google Scholar]
- Li S, Zhao M, Li Y, et al. Preparative Isolation of six anti-tumour biflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography. Phytochem Anal. 2014;25:127–133. doi: 10.1002/pca.2478. [DOI] [PubMed] [Google Scholar]
- Li PH, Chiu YP, Shih CC, et al. Biofunctional activities of Equisetum ramosissimum extract: protective effects against oxidation, melanoma, and melanogenesis. Oxid Med Cell Longev. 2016;2016:2853543. doi: 10.1155/2016/2853543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YH, Ye M, Yang WZ, et al. Flavan-3-ols from the rhizomes of Drynaria fortunei. Phytochemistry. 2011;72:1876–1882. doi: 10.1016/j.phytochem.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lin AS, Chang FR, Wu CC, et al. New cytotoxic flavonoids from Thelypteris torresiana. Planta Med. 2005;71:867–870. doi: 10.1055/s-2005-871292. [DOI] [PubMed] [Google Scholar]
- Lin YL, Shen CC, Huang YJ, et al. Homoflavonoids from Ophioglossum petiolatum. J Nat Prod. 2005;68:381–384. doi: 10.1021/np0401819. [DOI] [PubMed] [Google Scholar]
- Lin AS, Chang FR, Yen HF, et al. Novel flavonoids of Thelypteris torresiana. Chem Pharma Bull. 2007;55:635–637. doi: 10.1248/cpb.55.635. [DOI] [PubMed] [Google Scholar]
- Liu JS, Huang MF. The alkaloids huperzine-C and huperzine-D and huperzinine from Lycopodiastrum casuarinoides. Phytochemistry. 1994;37:1759–1761. doi: 10.1016/S0031-9422(00)89606-5. [DOI] [Google Scholar]
- Liu QF, Qin MZ. Studies on chemical constituents of rhizomes of Pteris multifida Poir. Chin Trad Herb Drugs. 2002;33:113–114. [Google Scholar]
- Liu JS, Yu CM, Zhou YZ, et al. Study on the chemistry of huperzine-A, huperzine-B. Acta Chim Sin. 1986;44:1035–1040. [Google Scholar]
- Liu JS, Zhu YL, Yu CM, et al. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem. 1986;64:837–839. doi: 10.1139/v86-137. [DOI] [Google Scholar]
- Liu HQ, Tan CH, Jiang SH, Zhu DY. Huperzine V, A new Lycopodium alkaloid from Huperzia serrata. Chin Chem Lett. 2004;15:303–304. [Google Scholar]
- Liu Z, Chen G, Vlantis A, et al. Cell death induced by ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid in anaplastic thyroid carcinoma cells is via a mitochondrial-mediated pathway. Apoptosis. 2005;10:1345–1356. doi: 10.1007/s10495-005-1730-5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ng EK, Liang NC, et al. Cell death induced by Pteris semipinnata L. is associated with p53 and oxidant stress in gastric cancer cells. FEBS Lett. 2005;579:1477–1487. doi: 10.1016/j.febslet.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Liu DM, Sheng JW, Qi HM, et al. Antioxidant activity of polysaccharides extracted from Athyrium multidentatum (Doll) Ching. J Med Plants Res. 2011;5:3061–3066. [Google Scholar]
- Liu H, Xiao Y, Xiong C, et al. Apoptosis induced by a new flavonoid in human hepatoma HepG2 cells involves reactive oxygen species-mediated mitochondrial dysfunction and MAPK activation. Eur J Pharmacol. 2011;654:209–216. doi: 10.1016/j.ejphar.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Liu J, Shu J, Zhang R, et al. Two new pterosin dimers from Pteris mutifida Poir. Fitoterapia. 2011;82:1181–1184. doi: 10.1016/j.fitote.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Liu H, Jiang C, Xiong C, et al. DEDC, a new flavonoid induces apoptosis via a ROS-dependent mechanism in human neuroblastoma SH-SY5Y cells. Toxicol in vitro. 2012;26:16–23. doi: 10.1016/j.tiv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wujisguleng W, Long C. Food uses of ferns in China: a review. Acta Soc Bot Poloniae. 2012;81:1–10. doi: 10.5586/asbp.2012.046. [DOI] [Google Scholar]
- Liu D, Sheng J, Li Z, et al. Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching. Int J Biol Macromol. 2013;56:1–5. doi: 10.1016/j.ijbiomac.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Liu X, Luo HB, Huang YY, et al. Selaginpulvilins A–D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata. Org Lett. 2013;16:282–285. doi: 10.1021/ol403282f. [DOI] [PubMed] [Google Scholar]
- Liu DM, Sheng JW, Qi HM, et al. Anti-aging activities of polysaccharides from Athyrium multidentatum (Doll.) Ching. J Chem Res. 2015;7:386–389. [Google Scholar]
- Liu DM, Sheng JW, Wang SH, et al. Cytoproliferative and cytoprotective effects of striatisporolide A Isolated from rhizomes of Athyrium multidentatum (Doell.) Ching on human umbilical vein endothelial cells. Molecules. 2016;21:1280. doi: 10.3390/molecules21101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HP, Li FS, Xu KP, et al. Bioactive compounds from Selaginella involven Spring that protect PC-12 cells. Chin Chem Lett. 2014;25:805–808. doi: 10.1016/j.cclet.2014.03.024. [DOI] [Google Scholar]
- Long HP, Zou H, Li FS, et al. Involvenflavones A-F, six new flavonoids with 3′-aryl substituent from Selaginella involven. Fitoterapia. 2015;105:254–259. doi: 10.1016/j.fitote.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Lu H, Hu J, Zhang LX, et al. Bioactive constituents from Pteris multifida. Planta Med. 1999;65:586–587. doi: 10.1055/s-2006-960835. [DOI] [PubMed] [Google Scholar]
- Lu C, Mei X, Zhong F. Studies on the flavonoids in the leaves and stems of Huperzia serrata. Nat Prod Res Dev. 2001;14:27–29. [Google Scholar]
- Luciano-Montalvo C, Boulogne I, Gavillán-Suárez J. A screening for antimicrobial activities of Caribbean herbal remedies. BMC CAM. 2013;13:1. doi: 10.1186/1472-6882-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Gang DR. The lycopodium alkaloids. Nat Prod Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- Ma XQ, Liu D, Hu ZB, Zhu DY (1998) Relationship between taxonomic system and DNA fingerprints of Huperzia and its related genera. Zhongguo Yeshen Zhiwu Ziyuan 155(Suppl)
- Ma X, Tan C, Zhu D, et al. Huperzine A from Huperzia species—an ethnopharmacolgical review. J Ethnopharmacol. 2007;113:15–34. doi: 10.1016/j.jep.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Ma S, Duan S, Jin M, et al. A new flavanol glycoside from Phymatopteris hastata with effect on glucose metabolism. China J Chin Mater Med. 2013;38:831–834. [PubMed] [Google Scholar]
- Ma T, Gong K, Yan Y, et al. Huperzine A promotes hippocampal neurogenesis in vitro and in vivo. Brain Res. 2013;1506:35–43. doi: 10.1016/j.brainres.2013.02.026. [DOI] [PubMed] [Google Scholar]
- May LW. The economic uses and associated folklore of ferns and fern allies. Bot Rev. 1978;44:491–528. doi: 10.1007/BF02860848. [DOI] [Google Scholar]
- McMorris TC, Kelner MJ, Wang W, et al. Structure–activity relationships of illudins: analogs with improved therapeutic index. J Org Chem. 1992;57:6876–6883. doi: 10.1021/jo00051a037. [DOI] [Google Scholar]
- Mehltreter K. Leaf phenology of the climbing fern Lygodium venustum in a semideciduous lowland forest on the Gulf of Mexico. Am Fern J. 2006;96:21–30. doi: 10.1640/0002-8444(2006)96[21:LPOTCF]2.0.CO;2. [DOI] [Google Scholar]
- Miao ZC, Yang ZS, Feng R. The structure determination of a new alkaloid phlegmariuine-N by long-range two-dimensional and NOE difference NMR spectroscopy. Yao Xue Xue Bao. 1989;24:114–117. [PubMed] [Google Scholar]
- Middelkamp-Hup M, Bos J, Rius-Diaz F, et al. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21:942–950. doi: 10.1111/j.1468-3083.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51:910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mimica-Dukic N, Simin N, Cvejic J, et al. Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules. 2008;13:1455–1464. doi: 10.3390/molecules13071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BS, Tomiyama M, Ma CM, et al. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem Pharma Bull. 2001;49:546–550. doi: 10.1248/cpb.49.546. [DOI] [PubMed] [Google Scholar]
- Minoru S, Satoru K, Atsushi I (1996) Flavon-3-ol structure-containing glycosides as histidin decarboxlase inhibitors and pharmaceutical compositions containing the inhibitors. JP 082176774A2, 1996-087-27
- Mishra PK, Raghuram GV, Bhargava A, et al. In vitro and in vivo evaluation of the anticarcinogenic and cancer chemopreventive potential of a flavonoid-rich fraction from a traditional Indian herb Selaginella bryopteris. Brit J Nutrit. 2011;106:1154–1168. doi: 10.1017/S0007114511001498. [DOI] [PubMed] [Google Scholar]
- Morais-Braga MF, Souza TM, Santos KK, Guedes GM, Andrade JC, Tintino SR. Phenolic compounds and interaction between aminoglycosides and natural products of Lygodium venustum SW against multiresistant bacteria. Chemotherapy. 2012;58:337–340. doi: 10.1159/000343044. [DOI] [PubMed] [Google Scholar]
- Morais-Braga MFB, Souza TM, Santos KKA, et al. Antimicrobial and modulatory activity of ethanol extract of the leaves from Lygodium venustum SW. Am Fern J. 2012;102:154–160. doi: 10.1640/0002-8444-102.2.154. [DOI] [Google Scholar]
- Morais-Braga MFB, Souza TM, Santos KKA, et al. Citotocixidade e atividade antiparasitária de Lygodium venustum SW. Acta Toxicol Arg. 2013;21:1–12. [Google Scholar]
- Morais-Braga MFB, Souza TM, Santos KKA, et al. Phenol composition, cytotoxic and anti-kinetoplastidae activities of Lygodium venustum SW. (Lygodiaceae) Exp Parasitol. 2013;134:178–182. doi: 10.1016/j.exppara.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Moran R, Davidse G. Pityrogramma Link. Flora Mesoamericana. 1995;1:137–140. [Google Scholar]
- Murakami T, Tanaka N (1988) Occurrence, structure and taxonomic implications of fern constituents. In: Progress in the chemistry of organic natural products, vol 54. Springer, Wien, pp 1–353
- Murakami T, Hagiwara M, Tanaka K, et al. Chemische Untersuchungen uber die Inhaltsstoffe von Helminthostachys zeylanica (L) HOOK. I. Chem Pharma Bull. 1973;21:1849–1851. doi: 10.1248/cpb.21.1849. [DOI] [Google Scholar]
- Murakami T, Hagiwara M, Tanaka K, et al. Chemische Untersuchungen uber die Inhaltsstoffe von Helminthostachys zeylanica (L) HOOK. II. Chem Pharma Bull. 1973;21:1851–1852. doi: 10.1248/cpb.21.1851. [DOI] [Google Scholar]
- Murakami T, Tanaka N, Tanaka K. Pterosin Q and Pterosid Q aus Pteris oshimensis Hieron und Histiopteris incisa (Thunb) J Smith. Chem Pharm Bull. 1974;22:2758–2761. doi: 10.1248/cpb.22.2758. [DOI] [Google Scholar]
- Murakami T, Tanaka N, Chen CM. Weitere lnhaltsstoffe aus Pteris oshimensis Hieron. Chem Pharm Bull. 1975;23:1890–1892. doi: 10.1248/cpb.23.1890. [DOI] [Google Scholar]
- Murakami T, Aoyama K, Tanaka N, et al. Chemische Untersuchungen der lnhaltsstoffe von Pteris wallichiana Agardh. Chem Pharm Bull. 1976;24:173–175. doi: 10.1248/cpb.24.173. [DOI] [Google Scholar]
- Murakami T, Taguchi S, Nomura Y, et al. Weitere lndan-1 -on-Derivate der Gattung Pteris. Chem Pharm Bull. 1976;24(8):1961–1964. doi: 10.1248/cpb.24.1961. [DOI] [Google Scholar]
- Murakami T, Satake T, Ninomiya K, et al. Pterosin-derivate aus der familie pteridaceae. Phytochemistry. 1980;19:1743–1746. doi: 10.1016/S0031-9422(00)83806-6. [DOI] [Google Scholar]
- Murakami T, Lida H, Tanaka N, et al. Chernische and chemotaxonomische untersuchungen von Filices XXXIII Chemische untersuchugefl der Inhaltsstoffe von pterislongipes Don. Chem Pharm Bull. 1981;29:657–662. doi: 10.1248/cpb.29.657. [DOI] [Google Scholar]
- Murakami T, Tanaka N, Komazawa Y, et al. Chemical and chemotaxonomic studies of fronds. XLI. Additional compounds from Pteris purpureorachis Copel. Chem Pharm Bull. 1983;31:1502. doi: 10.1248/cpb.31.1502. [DOI] [Google Scholar]
- Murakami T, Maehashi H, Tanaka N, et al. Chemical and chemotaxonomical studies of Filices. 55. Constituents of several species of Pteris. Yakugaku Zasshi. 1985;105:640–648. doi: 10.1248/yakushi1947.105.7_640. [DOI] [Google Scholar]
- Murakami T, Machashi H, Tanaka N, et al. Chemical and chemotaxonomical studies on Filices. LV. Studies on the constituents of several species of Pteris. J Pharm Soc Jpn. 1985;105:640–648. doi: 10.1248/yakushi1947.105.7_640. [DOI] [Google Scholar]
- Na M, Jang J, Min BS, et al. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg Med Chem Lett. 2006;16:4738–4742. doi: 10.1016/j.bmcl.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Nestor M, Bucay V, Callender V, et al. Polypodium leucotomos as an adjunct treatment of pigmentary disorders. J Clin Aesthetic Dermatol. 2014;7:13. [PMC free article] [PubMed] [Google Scholar]
- Nguyen PH, Ji DJ, Han YR, et al. Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg Med Chem. 2015;23:3730–3737. doi: 10.1016/j.bmc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Zhao BT, Ali MY, et al. Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J Nat Prod. 2015;78:34–42. doi: 10.1021/np5005856. [DOI] [PubMed] [Google Scholar]
- Noro Y, Okuda K, Shimada H, et al. Dryocrassin: a new acylphloroglucinol from Dryopteris crassirhizoma. Phytochemistry. 1973;12:1491–1493. doi: 10.1016/0031-9422(73)80591-6. [DOI] [Google Scholar]
- Nwosu MO. Ethnobotanical studies on some Pteridophytes of southern Nigeria. Econ Bot. 2002;56:255–259. doi: 10.1663/0013-0001(2002)056[0255:ESOSPO]2.0.CO;2. [DOI] [Google Scholar]
- Oh H, Kim DH, Cho JH, et al. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol. 2004;95:421–424. doi: 10.1016/j.jep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Ortega MG, Agnese AM, Cabrera JL. Sauroine-a novel Lycopodium alkaloid from Huperzia saururus. Tetrahedron Lett. 2004;45:7003–7005. doi: 10.1016/j.tetlet.2004.07.149. [DOI] [Google Scholar]
- Ouyang DW, Ni X, Xu HY, et al. Pterosins from Pteris multifida. Planta Med. 2010;76:1896–1900. doi: 10.1055/s-0030-1249934. [DOI] [PubMed] [Google Scholar]
- Ozay Y, Kasim CM, Guzel-Ozay S, et al. Effects of Equisetum arvense ointment on diabetic wound healing in rats. Wounds. 2013;25:234–241. [PubMed] [Google Scholar]
- Pan C, Chen Y, Ma X, et al. Phytochemical constituents and pharmacological activities of plants from the genus Adiantum: a review. Trop J Pharma Res. 2011;10:681–692. [Google Scholar]
- Parrado C, Juarranz A, Gilaberte Y, et al. Fern extract, oxidative stress, and skin cancer. In: Preedy VR, et al., editors. Cancer: oxidative stress and dietary antioxidants. San Diego: Academic Press; 2014. [Google Scholar]
- Pei G, Zhou PH, He GX, Du FL, Jiang DS. Studies on liposoluble constituents of Huperzia crispate ching. Nat Prod Res Dev. 2004;16(3):213–214. [Google Scholar]
- Pei D, Zhang JJ, Li QJ, Qiu MH, Zhao JH, Pan LT. 长柄石杉中Serratene三萜成分的研究. Lishizhen Med Mater Med Res. 2011;22:2233–2234. [Google Scholar]
- Plackett AR, Di Stilio VS, Langdale JA. Ferns: the missing link in shoot evolution and development. Front Plant Sci. 2015;6:1–15. doi: 10.3389/fpls.2015.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel A. Antioxidative and antiobesity activity of Nepalese wild herbs. Nat Prod Sci. 2011;17:123–129. [Google Scholar]
- Prado J. Flora da Reserva Ducke, Amazônia, Brasil: pteridophyta-Schizaeaceae. Rodriguésia. 2005;56:93–97. [Google Scholar]
- Prado J. Flora da Reserva Ducke, Amazonas, Brasil: Pteridophyta Chave para as famílias. Rodriguésia. 2005;56:27–28. [Google Scholar]
- Prado J, Sylvestre LS. As samambaias e licofitas do Brasil. In: Forzza RC, editor. Catalogo de plantas e fungos do Brasil. Rio de Janeiro: Instituto de Pesquisas Jardim Botanico; 2010. [Google Scholar]
- Qi Z-M, Wang Q. Effects of extracts of Equisetum hiemale on platelet aggregation and thrombosis in rats. Chin J Clin Rehab. 2004;8:7738–7739. [Google Scholar]
- Qin B, Zhu DY. Review on the sesquiterpenoids from the spices of Pteridaceae. (II)-Chemical synthesis, transformation and biological activities of 1H-inden-1-one sesquiterpenoids. Huaxue Yanjiu. 2004;15:66–70. [Google Scholar]
- Radhika NK, Sreejith P, Asha V. Cytotoxic and apoptotic activity of Cheilanthes farinosa (Forsk.) Kaulf. against human hepatoma, Hep3B cells. J Ethnopharmacol. 2010;128:166–171. doi: 10.1016/j.jep.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Rafii M, Walsh S, Little J, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76:1389–1394. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AU. Studies in natural products chemistry. London: Elsevier Sci; 2008. [Google Scholar]
- Rahman S, Kim KH, Saha SK, et al. Review of remediation techniques for arsenic (As) contamination: a novel approach utilizing bio-organisms. J Environm Manag. 2014;134:175–185. doi: 10.1016/j.jenvman.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Rancon S, Chaboud A, Darbour N, et al. A new C-glycosyl xanthone isolated from Davallia solida. Phytochemistry. 1999;52:1677–1679. doi: 10.1016/S0031-9422(99)00190-9. [DOI] [Google Scholar]
- Reddy VN, Ravikanth V, Rao TP, et al. A new triterpenoid from the fern Adiantum lunulatum and evaluation of antibacterial activity. Phytochemistry. 2001;56:173–175. doi: 10.1016/S0031-9422(00)00334-4. [DOI] [PubMed] [Google Scholar]
- Ren B, Xia B, Li W, et al. Two novel phenolic compounds from Stenoloma chusanum and their antifungal activity. Chem Nat Comp. 2009;45:182–186. doi: 10.1007/s10600-009-9298-x. [DOI] [Google Scholar]
- Reyes E, Jaén P, de las Heras E, et al. Systemic immunomodulatory effects of Polypodium leucotomos as an adjuvant to PUVA therapy in generalized vitiligo: a pilot study. J Dermatol Sci. 2006;41:213–216. doi: 10.1016/j.jdermsci.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Rivier L, Lindgren JE. “Ayahuasca”, the South American hallucinogenic drink: an ethnobotanical and chemical investigation. Econ Bot. 1972;26:101–129. doi: 10.1007/BF02860772. [DOI] [Google Scholar]
- Samura BA, Dovzhenok IA. Influence of plant collections with horsetail field on nervous system. Fiziologichno Aktivni Rechovini. 2002;2:104. [Google Scholar]
- Sandhu NS, Kaur S, Chopra D. Equisetum arvense: pharmacology and phytochemistry—a review. Asian J Pharm Clin Res. 2010;3:146–150. [Google Scholar]
- Sano T, Tsuda Y, Inubushi Y. Structures of tohogenol and tohogeninol: triterpenoids of Lycopodium serratum. Tetrahedron. 1970;26:2981–2986. doi: 10.1016/S0040-4020(01)92878-9. [DOI] [Google Scholar]
- Santos J, Blanco M, Monte F, et al. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia. 2005;76:508–513. doi: 10.1016/j.fitote.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Santos-Junior JG, Monte FHM, Blanco MM, et al. Cognitive enhancement in aged rats after chronic administration of Equisetum arvense L. with demonstrated antioxidant properties in vitro. Pharmacol Biochem Behavior. 2005;81:593–600. doi: 10.1016/j.pbb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sarker SK, Hossain AE. Pteridophytes of greater Mymensingh district of Bangladesh used as vegetables and medicines. Bangladesh J Plant Taxonomy. 2009;16:47–56. doi: 10.3329/bjpt.v16i1.2746. [DOI] [Google Scholar]
- Satake T, Murakami T, Saiki Y, et al. Chemical and chemotaxonomical studies on filices. LI. Chemical studies on the constituents of Costa Rican ferns. Chem Pharm Bull. 1984;32:4620–4624. doi: 10.1248/cpb.32.4620. [DOI] [Google Scholar]
- Shen ZB, Jin ZX, Zhang DL. Pharmacological study Oil psoriasis of Dryopteris fragrans. Chin Trad Herbal Drugs. 2002;33:844–845. [Google Scholar]
- Sheng J. Preparation and antioxidant activity of Athyrium multidentatum (Doll.) Ching polysaccharide derivatives. J Chem Pharm Res. 2014;6:1129–1135. [Google Scholar]
- Sheng J, Sun Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr Polym. 2014;108:41–45. doi: 10.1016/j.carbpol.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Sheng JW, Liu DM, Li ZJ, et al. Bioactivity-guided fractionation for antioxidant property of Athyrium multidentatum (Doll) Ching. rhizome. J Med Plants Res. 2011;5:7000–7005. [Google Scholar]
- Shi LM, Bai HB. A new diterpenoid glucoside from Pteris semipinnata. J Chem Res. 2010;34:206–207. doi: 10.3184/030823410X12701426376776. [DOI] [Google Scholar]
- Shi H, Li ZY, Guo YW. A new serratane-type triterpene from Lycopodium phlegmaria. Nat Prod Res. 2005;19:777–781. doi: 10.1080/14786410500044906. [DOI] [PubMed] [Google Scholar]
- Shi Q, Fu J, Ge D, et al. Huperzine A ameliorates cognitive deficits and oxidative stress in the hippocampus of rats exposed to acute hypobaric hypoxia. Neurochemi Res. 2012;37:2042–2052. doi: 10.1007/s11064-012-0826-x. [DOI] [PubMed] [Google Scholar]
- Shiojima K, Arai Y, Ageta H. Seasonal fluctuation of triterpenoid constituents from dried leaflets of Dryopteris crassirhizoma. Phytochemistry. 1990;29:1079–1082. doi: 10.1016/0031-9422(90)85406-6. [DOI] [Google Scholar]
- Shiojima K, Sasaki Y, Ageta H. Fern constituents: triterpenoids isolated from the leaves of Adiantum pedatum. 23-Hydroxyfernene, glaucanol A and filicenoic acid. Chem Pharm Bull. 1993;41:268–271. doi: 10.1248/cpb.41.268. [DOI] [Google Scholar]
- Shu J, Liu J, Zhong Y, et al. Two new pterosin sesquiterpenes from Pteris multifida Poir. Phytochem Lett. 2012;5:276–279. doi: 10.1016/j.phytol.2012.01.011. [DOI] [Google Scholar]
- Šimunek Z, Thomas BA. A new species of Selaginella (Selaginellaceae) from the Bolsovian (Carboniferous Period) of the Zonguldak-Amasra Coal Basin, north-western Turkey. Geol Croat. 2012;65:345–350. [Google Scholar]
- Singh M, Singh N, Khare P, et al. Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J Ethnopharmacol. 2008;115:327–329. doi: 10.1016/j.jep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Singh N, Kaur S, Bedi P, et al. Anxiolytic effects of Equisetum arvense Linn. extracts in mice. Indian J Exp Biol. 2011;49:352–356. [PubMed] [Google Scholar]
- Socolsky C, Asakawa Y, Bardón A. Diterpenoid glycosides from the bitter fern Gleichenia quadripartita. J Nat Prod. 2007;70:1837–1845. doi: 10.1021/np070119m. [DOI] [PubMed] [Google Scholar]
- Socolsky C, Borkosky SA, Asakawa Y, et al. Molluscicidal phloroglucinols from the fern Elaphoglossum piloselloides. J Nat Prod. 2009;72:787–790. doi: 10.1021/np800724h. [DOI] [PubMed] [Google Scholar]
- Socolsky C, Arena ME, Asakawa Y, et al. Antibacterial prenylated acylphloroglucinols from the fern Elaphoglossum yungense. J Nat Prod. 2010;73:1751–1755. doi: 10.1021/np1001779. [DOI] [PubMed] [Google Scholar]
- Socolsky C, Borkosky SA, Hernández de Terán M, et al. Phloroglucinols from the Argentine Ferns Elaphoglossum gayanum and E. piloselloides. J Nat Prod. 2010;73:901–904. doi: 10.1021/np1000208. [DOI] [PubMed] [Google Scholar]
- Socolsky C, Borkosky S, Bardon A. Structure-molluscicidal activity relationships of acylphloroglucinols from ferns. Nat Prod Commun. 2011;6:387–391. [PubMed] [Google Scholar]
- Socolsky C, Cartagena E, Asakawa Y, et al. Acylphloroglucinols from the fern Elaphoglossum lindbergii. Arkivoc. 2011;7:450–460. [Google Scholar]
- Socolsky C, Domínguez L, Asakawa Y, et al. Unusual terpenylated acylphloroglucinols from Dryopteris wallichiana. Phytochemistry. 2012;80:115–122. doi: 10.1016/j.phytochem.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Socolsky C, Salamanca E, Giménez A, et al. Prenylated acylphloroglucinols with leishmanicidal activity from the fern Elaphoglossum lindbergii. J Nat Prod. 2015;79:98–105. doi: 10.1021/acs.jnatprod.5b00767. [DOI] [PubMed] [Google Scholar]
- Soeder RW. Fern constituents: including occurrence, chemotaxonomy and physiological activity. Bot Rev. 1985;51:442–536. doi: 10.1007/BF02860970. [DOI] [Google Scholar]
- Soleimani S, Azarbaizani FF, Nejati V. The effect of Equisetum arvense L. (Equisetaceae) in histological changes of pancreatic ß-cells in streptozotocin-induced diabetic in rats. Pak J Biol Sci. 2007;10:4236–4240. doi: 10.3923/pjbs.2007.4236.4240. [DOI] [PubMed] [Google Scholar]
- Song L, Jiang DQ, Li XG, et al. Analysis of antibacterial characteristic of medical pteridophytes of a few species of Dryopteridaceae. J Wuhan Bot Res. 2008;1:019. [Google Scholar]
- Southon IW, Buckingham J. Dictionary of alkaloids. London: Chapman & Hall; 1989. [Google Scholar]
- Souza TM, Morais-Braga MFB, Costa JGM, et al. Additive effects of Pityrogramma calomelanos L. (link.) combinated with aminoglycosides against Staphylococcus aureus. Int J Phytomed. 2012;4:564–566. [Google Scholar]
- Souza TM, Morais-Braga MFB, Saraiva RA, et al. Cytotoxic and tripanocide activities of Pityrogramma calomelanos (L.) Link. Am Fern J. 2012;102:198–207. doi: 10.1640/0002-8444-102.3.198. [DOI] [Google Scholar]
- Souza TM, Morais-Braga MFB, Costa JGM, et al. Herbs in association with drugs: enhancement of the aminoglycoside-antibiotic activity by Pityrogramma calomelanos (L.) Link. J Young Pharma. 2013;5:188–190. doi: 10.1016/j.jyp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza TM, Morais-Braga MFB, Saraiva AÁF, et al. Evaluation of the anti-Leishmania activity of ethanol extract and fractions of the leaves from Pityrogramma calomelanos (L.) link. Nat Prod Res. 2013;27:992–996. doi: 10.1080/14786419.2012.686911. [DOI] [PubMed] [Google Scholar]
- Srivastava K. Ethnobotanical studies of some important ferns. Ethnobot leaflets. 2007;2007:16. [Google Scholar]
- Srivastava M, Ma LQ, Singh N, et al. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot. 2005;56:1335–1342. doi: 10.1093/jxb/eri134. [DOI] [PubMed] [Google Scholar]
- Staerk D, Larsen J, Larsen LA, Olafsdottir ES, Witt M, Jaroszewski JW. Selagoline, a new alkaloid from Huperzia selago. Nat Prod Res. 2004;18:197–203. doi: 10.1080/14786410310001620600. [DOI] [PubMed] [Google Scholar]
- Su W, Li P, Huo L, et al. Phenolic content and antioxidant activity of Phymatopteris hastata. J Serb Chem Soc. 2011;76:1485–1496. doi: 10.2298/JSC101111130S. [DOI] [Google Scholar]
- Su LH, Li YP, Li HM, et al. Anti-inflammatory prenylated flavonoids from Helminthostachys zeylanica. Chem Pharm Bull. 2016;64:497–501. doi: 10.1248/cpb.c15-00661. [DOI] [PubMed] [Google Scholar]
- Suja SR, Latha PG, Pushpangadan P, et al. Aphrodisiac property of Helminthostachys zeylanica in male mice. J Trop Med Plants. 2003;3:191–195. [Google Scholar]
- Suja S, Latha P, Pushpangadan P, et al. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J Ethnopharmacol. 2004;92:61–66. doi: 10.1016/j.jep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Sukumaran K, Kuttan R. Screening of 11 ferns for cytotoxic and antitumor potential with special reference to Pityrogramma calomelanos. J Ethnopharmacol. 1991;34:93–96. doi: 10.1016/0378-8741(91)90194-I. [DOI] [PubMed] [Google Scholar]
- Sun HD, Xu YL, Jiang B. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006;23:673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- Tadera K, Minami Y, Takamatsu K, et al. Inhibition of. ALPHA.-Glucosidase and. ALPHA.-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52:149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- Taïeb A. Vitiligo as an inflammatory skin disorder: a therapeutic perspective. Pigment Cell Melanoma Res. 2012;25:9–13. doi: 10.1111/j.1755-148X.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- Takayama H, Katakawa K, Kitajima M, et al. A new type of Lycopodium alkaloid, Lycoposerramine-A, from Lycopodium serratum Thunb. Org Lett. 2001;3:4165–4167. doi: 10.1021/ol0167762. [DOI] [PubMed] [Google Scholar]
- Takayama H, Katakawa K, Kitajima M, et al. Ten new Lycopodium alkaloids having the lycopodane skeleton isolated from Lycopodium serratum Thunb. Chem Pharm Bull. 2003;51:1163–1169. doi: 10.1248/cpb.51.1163. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Okuyama T, Jin H, et al. Isolation of phytoecdysones from Japanese ferns. I. Chem Pharm Bull. 1973;21:2336–2338. doi: 10.1248/cpb.21.2336. [DOI] [Google Scholar]
- Tan CH, Zhu DY. Lycopodine-type lycopodium alkaloids from Huperzia serrata. Helv Chim Acta. 2004;87:1963–1967. doi: 10.1002/hlca.200490178. [DOI] [Google Scholar]
- Tan CH, Jiang SH, Zhu DY. Huperzine P, a novel Lycopodium alkaloid from Huperzia serrata. Tetrahedron Lett. 2000;41:5733–5736. doi: 10.1016/S0040-4039(00)00893-5. [DOI] [Google Scholar]
- Tan HQ, Wang XJ, et al. Structure assignment of 8α-OH Phlegmariurine B—a combined NMR and density functional theory investigation. Acta Chim Sin. 2000;11:015. [Google Scholar]
- Tan CH, Chen GF, Ma XQ, et al. Huperzine R, a novel 15-carbon Lycopodium alkaloid from Huperzia serrata. J Nat Prod. 2002;65:1021–1022. doi: 10.1021/np0103564. [DOI] [PubMed] [Google Scholar]
- Tan CH, Chen GF, Ma XQ, et al. Three new phlegmariurine B type lycopodium alkaloids from Huperzia serrata. J Asian Nat Prod Res. 2002;4:227–231. doi: 10.1080/10286020290028974. [DOI] [PubMed] [Google Scholar]
- Tan CH, Ma XQ, Jiang SH, et al. Three new hydroxylated serratidine alkaloids from Huperzia serrata. Nat Prod Lett. 2002;16:149–153. doi: 10.1080/10575630290004215. [DOI] [PubMed] [Google Scholar]
- Tan CH, Ma X, Chen GF, et al. Huperzine W, a novel 14 carbons Lycopodium alkaloid from Huperzia serrata. Chin Chem Lett. 2002;13:331–332. doi: 10.1021/np0103564. [DOI] [PubMed] [Google Scholar]
- Tan CH, Ma XQ, Chen GF, et al. Two novel Lycopodium alkaloids from Huperzia serrata. Helv Chim Acta. 2002;85:1058–1061. doi: 10.1002/1522-2675(200204)85:4<1058::AID-HLCA1058>3.0.CO;2-Q. [DOI] [Google Scholar]
- Tan CH, Ma XQ, Chen GF, et al. Huperzines S, T, and U: new Lycopodium alkaloids from Huperzia serrata. Can J Chem. 2003;81:315–318. doi: 10.1139/v03-067. [DOI] [Google Scholar]
- Tanaka N, Kudo M, Taniguchi T, Murakami T, Saiki Y, Chen CM. Chemische Untersuchungen der Inhaltsstoffe von Pteris ryukyuensis Tagawa und Pteris longipinna Hayata. Chem Pharm Bull. 1978;26:1339–1342. doi: 10.1248/cpb.26.1339. [DOI] [Google Scholar]
- Tanaka N, Murakami T, Saiki Y, et al. Chemical and chemotaxonomical studies of ferns. XXXVII. Chemical studies on the constituents of Costa Rican ferns. 2. Chem Pharm Bull. 1981;29:3455–3463. doi: 10.1248/cpb.29.3455. [DOI] [Google Scholar]
- Tanaka N, Satake T, Takahashi A, et al. Chemical and chemotaxonomical studies of ferns. XXXIX. Chemical studies on the constituents of Pteris bella Tagawa and Pteridium aquilinum subsp. wightianum (Wall) Shich. Chem Pharm Bull. 1982;30:3640–3646. doi: 10.1248/cpb.30.3640. [DOI] [Google Scholar]
- Tanaka R, Minami T, Ishikawa Y, et al. Cancer chemopreventive activity of serratane-type triterpenoids from Picea jezoensis. Chem Biodivers. 2004;1:878–885. doi: 10.1002/cbdv.200490070. [DOI] [PubMed] [Google Scholar]
- Tang Y, Fang W, Ma YT, et al. A novel flavonoid from the root of Macrothelypteris torresiana (Gaud.) Ching. Chin Chem Lett. 2009;20:815–816. doi: 10.1016/j.cclet.2009.02.014. [DOI] [Google Scholar]
- Tang Y, Xiong C, Zhou D, et al. A new flavonoid from Macrothelypteris torresiana. Chem Nat Comp. 2010;46:209–211. doi: 10.1007/s10600-010-9570-0. [DOI] [Google Scholar]
- Thorroad S, Worawittayanont P, Khunnawutmanotham N, Chimnoi N, Jumruksa A, Ruchirawat S, Thasana N. Three new Lycopodium alkaloids from Huperzia carinata and Huperzia squarrosa. TETRAHEDRON. 2014;70:8017–8022. doi: 10.1016/j.tet.2014.08.042. [DOI] [Google Scholar]
- Tomšík P. Ferns and lycopods—a potential treasury of anticancer agents but also a carcinogenic hazard. Phytother Res. 2014;28:798–810. doi: 10.1002/ptr.5070. [DOI] [PubMed] [Google Scholar]
- Tong XT, Tan CH, Zhou H, Jiang SH, Ma XQ, Zhu DY. Triterpenoid constituents of Huperzia miyoshiana. Chin J Chem. 2003;21:1364–1368. doi: 10.1002/cjoc.20030211026. [DOI] [Google Scholar]
- Tong XT, Tan CH, Ma XQ, Wang BD, Jiang SH, Zhu DY. Miyoshianines A and B, two new Lycopodium alkaloids from Huperzia miyoshiana. Plant Med. 2003;69:576–579. doi: 10.1055/s-2003-40648. [DOI] [PubMed] [Google Scholar]
- Umikalsom Y, Grayer-Barkmeijer RJ, Harborne JB. A comparison of the flavonoids in Athyriaceae and Aspleniaceae. Biochem Syst Ecol. 1994;22:587–594. doi: 10.1016/0305-1978(94)90071-X. [DOI] [Google Scholar]
- Uprety Y, Asselin H, Boon EK, et al. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J Ethnobiol Ethnomed. 2010;6:1. doi: 10.1186/1746-4269-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadeau C, Pabon A, Deharo E, Albán-Castillo J, Estevez Y, Lores FA, et al. Medicinal plants from the Yanesha (Peru): evaluation of the leishmanicidal and antimalarial activity of selected extracts. J Ethnopharmacol. 2009;123:413–422. doi: 10.1016/j.jep.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Vallejo MG, Ortega M, Cabrera J, et al. Huperzia saururus increases memory retention in rats. J Ethnopharmacol. 2007;111:685–687. doi: 10.1016/j.jep.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Vallejo MG, Ortega MG, Cabrera JL, et al. Sauroine, an alkaloid from Huperzia saururus with activity in Wistar rats in electrophysiological and behavioral assays related to memory retention. J Nat Prod. 2008;72:156–158. doi: 10.1021/np800151v. [DOI] [PubMed] [Google Scholar]
- Vallejo MG, Ortega MG, Cabrera JL, et al. N-Demethyl-sauroxine, a novel Lycodine Group alkaloid from Huperzia saururus. Tetrahedron Lett. 2013;54:5197–5200. doi: 10.1016/j.tetlet.2013.07.068. [DOI] [Google Scholar]
- Veit M, Geiger H, Czygan F-C, et al. Malonylated flavone 5-O-glucosides in the barren sprouts of Equisetum arvense. Phytochemistry. 1990;29:2555–2560. doi: 10.1016/0031-9422(90)85187-K. [DOI] [Google Scholar]
- Veit M, Beckert C, Höhne C, et al. Interspecific and intraspecific variation of phenolics in the genus Equisetum subgenus Equisetum. Phytochemistry. 1995;38:881–891. doi: 10.1016/0031-9422(94)00658-G. [DOI] [Google Scholar]
- Veit M, Geiger H, Kast B, et al. Styrylpyrone glucosides from Equisetum. Phytochemistry. 1995;39:915–917. doi: 10.1016/0031-9422(95)00941-Y. [DOI] [Google Scholar]
- Velázquez C, Calzada F, Torres J, et al. Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J Ethnopharmacol. 2006;103:66–70. doi: 10.1016/j.jep.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Vetter J. A biological hazard of our age: Bracken fern [Pteridium aquilinum (L.) Kuhn]-A review. Acta Vet Hung. 2009;57:183–196. doi: 10.1556/AVet.57.2009.1.18. [DOI] [PubMed] [Google Scholar]
- Viral D, Shivanand P, Jivani N. Anticancer evaluation of Adiantum venustum don. J Young Pharm. 2011;3:48–54. doi: 10.4103/0975-1483.76419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CX, Luo JG, Guo C, et al. 3-O-Methylquercetin glucosides from Ophioglossum pedunculosum and Inhibition of Lipopolysaccharide-induced nitric oxide production in RAW 264.7 Macrophages. Helv Chim Acta. 2012;95:1586–1592. doi: 10.1002/hlca.201100500. [DOI] [Google Scholar]
- Wan CX, Luo JG, Gu YC, et al. Characterisation of homoflavonoids from three Ophioglossum species using liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Phytochem Anal. 2013;24:541–549. doi: 10.1002/pca.2430. [DOI] [PubMed] [Google Scholar]
- Wang R, Yan H. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine1. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- Wang BD, Jiang SH, Gao WY, Zhu DY, Kong XM, Yang YQ. Structural identification of huperzine G. Zhiwu Xuebao. 1998;40:842–845. [Google Scholar]
- Wang BD, Teng NN, Zhu DY. Structural elucidation of huperzine O. Youji Huaxue. 2000;20:812–814. [Google Scholar]
- Wang SM, Ruan JS, Zhuang J. Small Chunhua oral mouse model of chronic bronchitis and pathological forms of influence and expectorant effects. Fujian Chin Med. 2001;32:18–19. [Google Scholar]
- Wang HB, Tan CH, Tan JJ, et al. Lycopodium alkaloids from Huperzia serrata. Helv Chim Acta. 2007;90:153–157. doi: 10.1002/hlca.200790009. [DOI] [Google Scholar]
- Wang HB, Tan CH, Tan JJ, et al. Two new N-oxide Lycopodium alkaloids from Huperzia serrata. Nat Prod Res. 2009;23:1363–1366. doi: 10.1080/14786410802253239. [DOI] [PubMed] [Google Scholar]
- Wang YH, Long CL, Yang FM, et al. Pyrrolidinoindoline alkaloids from Selaginella moellendorfii. J Nat Prod. 2009;72:1151–1154. doi: 10.1021/np9001515. [DOI] [PubMed] [Google Scholar]
- Wang C-X, Zhang P-H, Luo J-G, et al. Homoflavonoid glucosides from Ophioglossum pedunculosum and their anti-HBV activity. J Nat Prod. 2011;74:683–689. doi: 10.1021/np100745z. [DOI] [PubMed] [Google Scholar]
- Wang F, Li YJ, Ren FC, et al. Pterisolic acids A–F, new ent-kaurane diterpenoids from the fern Pteris semipinnata. Chem Pharm Bull. 2011;59:484–487. doi: 10.1248/cpb.59.484. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu J, Xu XR, et al. Research progress in chemical constituents and pharmaceutical activities of Lygodium japonicum (Thunb)Sw. Chin Wild Plants Res. 2011;30:1–4. [Google Scholar]
- Wang YS, Li FY, Huang R, Li Y, Feng XF, Yang JH. Chemical constituents of Pteris multifidi. Chem Nat Compd. 2013;49:629–631. doi: 10.1007/s10600-013-0695-9. [DOI] [Google Scholar]
- Wang JZ, Li J, Zhao P, et al. Antitumor activities of ethyl acetate extracts from Selaginella doederleinii Hieron in vitro and in vivo and its possible mechanism. ECAM. 2015;2015:1–12. doi: 10.1016/j.molcata.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina T, Umehara K, Miyase T. Flavonoid glycosides from Botrychium ternatum. Chem Pharm Bull. 2012;60:1561–1573. doi: 10.1248/cpb.c12-00744. [DOI] [PubMed] [Google Scholar]
- Wei AH, Wu G, Xiong C, et al. Flavonoids with special B-ring from Macrothelypteris viridifrons and their anti-proliferative effects on tumor cell. China J Chin Mater Med. 2011;36:582–584. [PubMed] [Google Scholar]
- Wei H, Wu GH, Lei Y-F, et al. Neuropective constituents from the rhizomes of Abacopteris penangiana. J Asian Nat Prod Res. 2011;13:707–713. doi: 10.1080/10286020.2011.586340. [DOI] [PubMed] [Google Scholar]
- Wei H, Wu G, Shi D, et al. Total flavan glycoside from Abacopteris penangiana rhizomes and its acid hydrolysate: characterisation and anti-benign prostatic hyperplasia potential. Food Chem. 2012;134:1959–1966. doi: 10.1016/j.foodchem.2012.03.128. [DOI] [PubMed] [Google Scholar]
- Wei H, Wu G, Chen J, et al. (2S)-5,2′,5′-Trihydroxy-7-methoxyflavanone, a natural product from Abacopteris penangiana, presents neuroprotective effects in vitro and in vivo. Neurochem Res. 2013;38:1686–1694. doi: 10.1007/s11064-013-1070-8. [DOI] [PubMed] [Google Scholar]
- Weng JK, Noel JP. Chemodiversity in Selaginella: a reference system for parallel and convergent metabolic evolution in terrestrial plants. Front Plant Sci. 2013;4:1–15. doi: 10.3389/fpls.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichtl M. Herbal drug and phytopharmaceuticals. Stuttgart: Medpharm Scientific Publishers; 1994. [Google Scholar]
- Wills P, Asha V. Protective effect of Lygodium flexuosum (L.) Sw. extract against carbon tetrachloride-induced acute liver injury in rats. J Ethnopharmacol. 2006;108:320–326. doi: 10.1016/j.jep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Wills P, Asha V. Chemopreventive action of Lygodium flexuosum extract in human hepatoma PLC/PRF/5 and Hep 3B cells. J Ethnopharmacol. 2009;122:294–303. doi: 10.1016/j.jep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Wittayalai S, Sathalalai S, Thorroad S, et al. Lycophlegmariols A–D: cytotoxic serratene triterpenoids from the club moss Lycopodium phlegmaria L. Phytochemistry. 2012;76:117–123. doi: 10.1016/j.phytochem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Wu ZH. The Catalogue of Fern family and genus in China. Beijing: Science Press; 1991. [Google Scholar]
- Wu MJ, Weng CY, Wang L, et al. Immunomodulatory mechanism of the aqueous extract of sword brake fern (Pteris ensiformis Burm.) J Ethnopharmacol. 2005;98:73–81. doi: 10.1016/j.jep.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Wu GH, Wei AH, Cai YL, et al. Chemical constituents of Macrothelypteris oligophlebia and their antitumor activity in vitro and in vivo. Chin Pharm J. 2011;5:004. [Google Scholar]
- Wu TY, Chen CP, Jinn TR. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol. 2011;50:131–135. doi: 10.1016/j.tjog.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Wu K, Liu Y, Lv Y, et al. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis and cell cycle arrest in CNE-2Z nasopharyngeal carcinoma cells. Oncol Rep. 2013;29:2101–2108. doi: 10.3892/or.2013.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Li J, Wang Q, et al. Seasonal dynamics of the phytochemical constituents and bioactivities of extracts from Stenoloma chusanum (L.) Ching. Food Chem. 2016 doi: 10.1016/j.fct.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Xia X, Cao J, Zheng Y, et al. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind Crops Prod. 2014;58:91–98. doi: 10.1016/j.indcrop.2014.04.005. [DOI] [Google Scholar]
- Xiao JB. Phytochemicals in medicine & food. Phytochem Rev. 2015;14:317–320. doi: 10.1007/s11101-015-9407-3. [DOI] [Google Scholar]
- Xiao JB. Report of the international symposium on phytochemicals in medicine and food (ISPMF 2015) Food Chem. 2016;204:497–498. doi: 10.1016/j.foodchem.2015.09.107. [DOI] [PubMed] [Google Scholar]
- Xiao JB. Phytochemicals in food and nutrition. Crit Rev Food Sci Nut. 2016;56:S1–S3. doi: 10.1080/10408398.2015.1111074. [DOI] [PubMed] [Google Scholar]
- Xiao JB. Dietary flavonoid aglycones and their glycosides: what show better biological benefits? Crit Rev Food Sci Nutr. 2016 doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- Xiao JB, Kai G. A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure–affinity relationship. Crit Rev Food Sci Nut. 2012;52:85–101. doi: 10.1080/10408398.2010.499017. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Zhang HY, Tang XC. Huperzine A attenuates amyloid β-peptide fragment 25–35-induced apoptosis in rat cortical neurons via inhibiting reactive oxygen species formation and caspase-3 activation. J Neurosci Res. 2002;67:30–36. doi: 10.1002/jnr.10075. [DOI] [PubMed] [Google Scholar]
- Xiao JB, Capanoglu E, Jassbi AR, et al. Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr. 2016;56(S1):S29–S45. doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- Xing GX (1991) Flora of China, vol 4, no 1. Science Press, Beijing, pp 72–73
- Xu GY, Zheng Y, Chen XQ. The inhibitory effects of polysaccharide extracts from eightspecies of pteridophytes against bacteria and fungi. J Fujian Normal Univ (Nat Sci) 2005;2:024. [Google Scholar]
- Xu W, Zhang F, Luo Y, et al. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr Res. 2009;344:217–222. doi: 10.1016/j.carres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ojika M, Kigoshi H. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: chemistry, biology and ecology. Nat Prod Rep. 2007;24:798–813. doi: 10.1039/b614160a. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Mitsunaga T, Batubara I. Novel quercetin glucosides from Helminthostachys zeylanica root and acceleratory activity of melanin biosynthesis. J Nat Med. 2013;67:369–374. doi: 10.1007/s11418-012-0672-9. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Flaws B. The Divine Farmer’s material medica: a translation of the Shen Nong Ben Cao Jing. 1. Boulder: Blue Poppy Press; 1998. [Google Scholar]
- Yang JG, Zhou L, Liu MX. Antidotal effect of Stenoloma chusanum (L.) Ching against arsenic and ammonium. China J Chin Mater Med. 1989;14:46–48. doi: 10.1142/S0192415X86000089. [DOI] [PubMed] [Google Scholar]
- Yang C, Lu Z, Zheng C. Efficacy and reliability of huperzine A in mild and moderate Alzheimer’s disease. Chin J Clin Rehabil. 2003;7:4258–4259. [Google Scholar]
- Yang YB, Yang XQ, Xu YQ, et al. A new flavone glycoside from Huperzia serrata. Chin J Nat Med. 2008;6:408–410. doi: 10.3724/SP.J.1009.2008.00408. [DOI] [Google Scholar]
- Yang YF, Qu SJ, Xiao K, et al. Lycopodium alkaloids from Huperzia serrata. J Asian Nat Prod Res. 2010;12:1005–1009. doi: 10.1080/10286020.2010.522180. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee GJ, Yoon DH, et al. ERK1-and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J Ethnopharmacol. 2013;145:499–508. doi: 10.1016/j.jep.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Yang X, Yuan L, Chen J, et al. Multitargeted protective effect of Abacopteris penangiana against carrageenan-induced chronic prostatitis in rats. J Ethnopharmacol. 2014;151:343–351. doi: 10.1016/j.jep.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Yang X, Yuan L, Xiong C, et al. Abacopteris penangiana exerts testosterone-induced benign prostatic hyperplasia protective effect through regulating inflammatory responses, reducing oxidative stress and anti-proliferative. J Ethnopharmacol. 2014;157:105–113. doi: 10.1016/j.jep.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Ye W, Fan C, Zhang L, et al. A new phenolic glycoside from the roots of Lygodium japonicum. Fitoterapia. 2007;78:600–601. doi: 10.1016/j.fitote.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Ying YM, Liu XS, Tong CP, et al. Lycopodium alkaloids from Huperzia serrata. Helv Chim Acta. 2014;97:1433–1439. doi: 10.1002/hlca.201400015. [DOI] [Google Scholar]
- Yonathan M, Asres K, Assefa A, et al. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol. 2006;108:462–470. doi: 10.1016/j.jep.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Yoshihira K, Fukuoka M, Kuroyanagi M, et al. Further characterization of 1-indanone derivatives from bracken, pteridium aquilinum var. latiusculum. Chem Pharm Bull. 1972;20:426–428. doi: 10.1248/cpb.20.426. [DOI] [Google Scholar]
- Yoshihira K, Fukuoka M, Kuroyanagi M, et al. Chemical and toxicological studies on Bracken Fern, Pteridium aquilinum var. latiusculum. I. Introduction, extraction and fractionation of constituents and toxicological studies including carcinogenicity tests. Chem Pharm Bull. 1978;26:2346–2364. doi: 10.1248/cpb.26.2346. [DOI] [PubMed] [Google Scholar]
- Yu D, Thakor DK, Han I, et al. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. PNAS. 2013;110:E746–E755. doi: 10.1073/pnas.1300083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CM, Calhoun LA, Konder RM, et al. Huperzimine, a novel Lycopodium alkaloid from Huperzia serrata. Can J Chem. 2014;92:406–410. doi: 10.1139/cjc-2013-0520. [DOI] [Google Scholar]
- Yuan SQ, Wei TT. Studies on the alkaloids of Huperzia serrata Thunb. Trev. Yao Xue Xue Bao. 1988;23:516–520. [PubMed] [Google Scholar]
- Yuan SQ, Zhao YM. Chemical research on alkaloids from Huperzia serrata IV. Zhongcaoyao. 2000;31:498–499. [Google Scholar]
- Yuan SQ, Zhao YM. A novel phlegmariurine type alkaloid from Huperzia serrata Thunb. Trev. Yao Xue Xue Bao. 2003;38:596–598. [PubMed] [Google Scholar]
- Yuan S, Feng R, Gu G. Alkaloids of Shezushishan (Huperzia serrata) Zhongcaoyao. 1994;25(453–454):473. [Google Scholar]
- Yuan S, Feng R, Gu G. Alkaloids of Shezushishan (Huperzia serrata) Zhongcaoyao. 1995;26:115–117. [Google Scholar]
- Yuan S, Zhao Y, Feng R. Study on alkaloids of Huperzia serrata Thunb. Trev. Junshi Yixue Kexueyuan Yuankan. 2001;25:57–58. [Google Scholar]
- Yuan S, Zhao Y, Feng R. Structural identification of neohuperzinine. Yaoxue Xuebao. 2002;37:946–949. [Google Scholar]
- Yuan Q, Zhang X, Liu Z, et al. Ethanol extract of Adiantum capillus-veneris L. suppresses the production of inflammatory mediators by inhibiting NF-κB activation. J Ethnopharmacol. 2013;147:603–611. doi: 10.1016/j.jep.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Yang B, Ye Z, et al. Sceptridium ternatum extract exerts antiasthmatic effects by regulating Th1/Th2 balance and the expression levels of leukotriene receptors in a mouse asthma model. J Ethnopharmacol. 2013;149:701–706. doi: 10.1016/j.jep.2013.07.032. [DOI] [PubMed] [Google Scholar]
- Yusuf U, Itam K, Abdullah F, et al. Leaf flavonoids in the genus Gleichenia (Gleicheniaceae) Am Fern J. 2003;93:44–46. doi: 10.1640/0002-8444(2003)093[0044:SN]2.0.CO;2. [DOI] [Google Scholar]
- Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175:1952–1961. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan ZJ, Zhang FY, Li CP, et al. A novel ent-kaurane diterpenoid from Pteris semipinnata. J Chem Res. 2009;2009:149–150. doi: 10.3184/030823409X416947. [DOI] [Google Scholar]
- Zhan ZJ, Ying YM, Zhang FY, et al. Three new illudalane sequiterpenoids from Pteris semipinnata. Helv Chim Acta. 2010;93:550–554. doi: 10.1002/hlca.200900281. [DOI] [Google Scholar]
- Zhang HY. New insights into huperzine A for the treatment of Alzheimer’s disease. Acta Pharm Sin. 2012;33:1170–1175. doi: 10.1038/aps.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Xuan LJ. Pterisemipol, a novel sesquiterpenoid from Pteris semipinnata L. Chin Chem Lett. 2007;18:1386–1388. doi: 10.1016/j.cclet.2007.09.028. [DOI] [Google Scholar]
- Zhang X, Cui L, Tanaka N, et al. The active constituents and antitumor action of Pteris semipinnata. Zhongguo yao xue za zhi. 1996;32:37–38. [Google Scholar]
- Zhang X, Li JH, He CW, et al. Study on the diterpenoid constituents and anticancer action of Pteris semipinnata. Chin Pharm J. 1999;34:512–514. [Google Scholar]
- Zhang HY, Yan H, Tang XC. Huperzine A enhances the level of secretory amyloid precursor protein and protein kinase C-α in intracerebroventricular β-amyloid-(1–40) infused rats and human embryonic kidney 293 Swedish mutant cells. Neurosci Lett. 2004;360:21–24. doi: 10.1016/j.neulet.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Zhang LP, Liang YM, Wei XC, et al. A new unusual natural pigment from Selaginella sinensis and its noticeable physicochemical properties. J Org Chem. 2007;72:3921–3924. doi: 10.1021/jo0701177. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Zheng CY, Yan H, et al. Potential therapeutic targets of huperzine A for Alzheimer’s disease and vascular dementia. Chem Biol Interact. 2008;175:396–402. doi: 10.1016/j.cbi.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Zhang PT, He J, Xu G, et al. A new ent-Kauranoid diterpenoid from Hicriopteris glauca (Gleicheniaceae) Yunnan zhiwu yanjiu. 2009;31:183–186. [Google Scholar]
- Zhang GG, Jing Y, Zhang HM, et al. Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina. Planta Med. 2012;78:390–392. doi: 10.1055/s-0031-1298175. [DOI] [PubMed] [Google Scholar]
- Zhang LB, Wang PS, Wang XY. Selaginella longistrobilina (Selaginellaceae), a new species from Guizhou, China, and Selaginella prostrata, a new combination and its lectotypification. Novon. 2012;22:260–263. doi: 10.3417/2012050. [DOI] [Google Scholar]
- Zhang M, Cao J, Dai X, et al. Flavonoid contents and free radical scavenging activity of extracts from leaves, stems, rachis and roots of Dryopteris erythrosora. Iran J Pharm Res. 2012;11:991. [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Kim JH, Lee GS, et al. In vitro antioxidant and in vivo anti-inflammatory activities of Ophioglossum thermale. Am J Chin Med. 2012;40:279–293. doi: 10.1142/S0192415X1250022X. [DOI] [PubMed] [Google Scholar]
- Zhang LB, Sun QW, He H. Selaginella wangpeishanii (Selaginellaceae), a new lycophyte from a limestone cave in Guizhou, China. Phytotaxa. 2014;164:195–199. doi: 10.11646/phytotaxa.164.3.5. [DOI] [Google Scholar]
- Zhao Z, Ruan J, Jin J, et al. Flavan-4-ol glycosides from the rhizomes of Abacopteris penangiana. J Nat Prod. 2006;69:265–268. doi: 10.1021/np050191p. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Jin J, Ruan J, et al. Antioxidant flavonoid glycosides from aerial parts of the fern Abacopteris penangiana. J Nat Prod. 2007;70:1683–1686. doi: 10.1021/np0703850. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Leng C, Wang Z. Identification of Dryopteris crassirhizoma and the adulterant species based on cpDNA rbcL and translated amino acid sequences. Planta Med. 2007;73:1230–1233. doi: 10.1055/s-2007-981588. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Jin J, Ruan J, et al. Two new flavan glycosides from Abacopteris penangiana. Acta Pharm Sin. 2008;43:392–395. [PubMed] [Google Scholar]
- Zhao ZX, Ruan JL, Jin J, et al. Two new acetylated flavan glycosides from rhizomes of the fern Abacopteris penangiana. J Asian Nat Prod Res. 2010;12:1015–1019. doi: 10.1080/10286020.2010.525744. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ruan J, Jin J, et al. A novel anthocyanidin glycoside from the rhizomes of Abacopteris penangiana. Fitoterapia. 2010;81:1171–1175. doi: 10.1016/j.fitote.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ruan J, Jin J, et al. Two new flavonoids from the rhizomes of Abacopteris penangiana. Helv Chim Acta. 2011;94:446–452. doi: 10.1002/hlca.201000223. [DOI] [Google Scholar]
- Zheng X, Bi Y, Feng W, et al. Study on the chemical constituents of Selaginella tamariscina (Beauv.) Spring. Acta Pharm Sin. 2004;39:266–268. [PubMed] [Google Scholar]
- Zheng XK, Li YJ, Zhang L, et al. Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J Ethnopharmacol. 2011;133:531–537. doi: 10.1016/j.jep.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Zheng XK, Zhang L, Wang WW, et al. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol. 2011;137:662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Zheng XK, Wang WW, Zhang L, et al. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) Spring in diabetic mice. J Pharm Pharmacol. 2013;65:757–766. doi: 10.1111/jphp.12035. [DOI] [PubMed] [Google Scholar]
- Zheng YF, Jassbi AR, Xiao JB. Introduction to the 1st international symposium on phytochemicals in medicine and food (ISPMF 2015) J Agric Food Chem. 2016;64:2439–2441. doi: 10.1021/acs.jafc.6b00379. [DOI] [PubMed] [Google Scholar]
- Zhou R, Li S. Study on antibacterial effects of pteridophyta. Nat Prod Res Dev. 1998;11:53–56. [Google Scholar]
- Zhou BN, Zhu DY, Huang MF, Lin LJ, Lin LZ, Han XY, Cordell GA. NMR assignments of huperzine-A, serratinine and lucidioline. Phytochemistry. 1993;34:1425–1428. doi: 10.1016/0031-9422(91)80042-Y. [DOI] [Google Scholar]
- Zhou H, Jiang SH, Tan CH, et al. New epoxyserratanes from Huperzia serrata. Planta Med. 2003;69:91–94. doi: 10.1055/s-2003-37033. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tan CH, Jiang SH, et al. Serratene-type Triterpenoids from Huperzia serrata. J Nat Prod. 2003;66:1328–1332. doi: 10.1021/np0301590. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li YS, Tong XT, Liu HQ, Jiang SH, Zhu DY. Serratane-type triterpenoids from Huperzia serrata. Nat Prod Res. 2004;18:453–459. doi: 10.1080/14786410310001643803. [DOI] [PubMed] [Google Scholar]
- Zhou D, Ruan J, Cai Y, et al. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J Ethnopharmacol. 2010;129:232–237. doi: 10.1016/j.jep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Zhou D, Wei A, Cao C, et al. DICO, a novel nonaromatic B-ring flavonoid, induces G2/M cell cycle arrest and apoptosis in human hepatoma cells. Food Chem Toxicol. 2013;57:322–329. doi: 10.1016/j.fct.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Jiang SH, Huang MF, Lin LZ, Cordell GA. Huperserratinine from Huperzia serrata. Phytochemistry. 1994;36:1069–1072. doi: 10.1016/S0031-9422(00)90493-X. [DOI] [Google Scholar]
- Zhu D, Huang M, Wang B, et al. Study on the structures of huperzines E and F. Chin J Appl Environ Biol. 1996;2:352–355. [Google Scholar]
- Zhu L, Zhang G, Wang S, et al. A new compound from Lygodium japonicum (Thunb.) Sw. Nat Prod Res. 2009;23:1284–1288. doi: 10.1080/14786410802420820. [DOI] [PubMed] [Google Scholar]
- Zhu B, Wang TB, Hou LJ, et al. A new Selaginellin from Selaginella moellendorffii. Chem Nat Comp. 2016;52:624–627. doi: 10.1007/s10600-016-1725-1. [DOI] [Google Scholar]
- Zou ZX, Xu KP, Li FS, et al. A new pyrrole alkaloid from Selaginella moellendorfii Hieron. Chin Chem Lett. 2013;24:114–116. doi: 10.1016/j.cclet.2013.01.028. [DOI] [Google Scholar]
- Zou H, Xu PS, Liu R, et al. Selacyclicbiflavone A, an unusual macrocyclic biflavone from Selaginella uncinata (Desv.) Spring. Tetrahedron Lett. 2016;57:892–894. doi: 10.1016/j.tetlet.2016.01.038. [DOI] [Google Scholar]
- Zou H, Yi ML, Xu KP, et al. Two new flavonoids from Selaginella uncinata. J Asian Nat Prod Res. 2016;18:248–252. doi: 10.1080/10286020.2015.1063617. [DOI] [PubMed] [Google Scholar]
- Zuo L, Chen RY. Advances in studies on chemical constituents and pharmacological activity in plants of Dryopteris Adanson. Chin Trad Herbal Drugs. 2005;36:1426. [Google Scholar]


