Abstract
The current investigation examines the relation between perinatal complications and social anxiety incorporating the potential indirect effect of child temperament. Participants were 149 children 9 to 12 years of age (Mage=9.97, SDage=1.00) screened for behavioral inhibition (BI) and assessed for social anxiety symptoms using parent- and child-report. Participating families also reported on the presence of perinatal complications. Results indicated that children who experienced perinatal complications were higher in BI and social anxiety, compared to children who did not experience complications. Furthermore, there was an indirect effect between perinatal complications and social anxiety via BI. These findings provide further support for the established relation between perinatal complications and anxiety and demonstrate, for the first time, that this relation may be mediated by temperament, setting the stage for longitudinal analyses.
Specific temperamental traits evident early in development predict the emergence of psychopathology later in life. In particular, behavioral inhibition (BI) is a temperament characterized by a tendency to exhibit a fearful disposition and withdrawal in unfamiliar contexts and situations (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984). Children who strongly and consistently exhibit BI early in life are at increased risk for the development of anxiety symptoms and disorders in childhood and in adolescence, especially social anxiety (Pérez-Edgar & Fox, 2005).
For example, a comprehensive meta-analytic review examined seven longitudinal studies investigating the link between BI in children and social anxiety (Clauss & Blackford, 2012). They found that children identified as behaviorally inhibited are at a three- to four-fold increased risk for the development of social anxiety disorder. Additionally, a longitudinal study found that adolescents 14 to 16 years of age were 3.79 times more likely to be diagnosed with lifetime social anxiety disorder if they consistently displayed high levels of BI during childhood, based on maternal report (Chronis-Tuscano et al., 2009). Thus, it is essential to identify early developmental risk factors that may be associated with, and could be used as predictors of, BI as a readily observable phenotype for identifying risk for social anxiety disorder. In the current study, we examined perinatal complications as a potential early developmental risk factor associated with both BI and social anxiety. Moreover, we investigated the potential indirect effect of temperament in the relation between perinatal complications and social anxiety, setting the stage for future studies of mediation.
There are many reasons to believe that perinatal complications may impact children’s temperament and mental health outcomes. Primarily, there is empirical evidence demonstrating that prenatal, perinatal, and postnatal complications are associated with risk for psychopathology, and specifically, anxiety disorders later in life. For example, Cohen & Velez (1989) found that pregnancy problems (e.g., low birth weight, physical trauma, severe illness, complications during pregnancy or delivery, and cesarean sections) were significant risk factors for anxiety in children and adolescents. Another study found that parents of children with anxiety disorders reported higher rates of perinatal complications, including preterm birth, compared to parents of unaffected children (Johnco et al., 2016). Studies that have investigated the relation between perinatal complications and various forms of psychopathology have found that perinatal complications (e.g., poor maternal obstetric history) appear to be a specific risk for anxiety disorders in offspring, as opposed to externalizing disorders and substance use disorders, which tend to be associated with maternal prenatal substance use (Allen, Lewinsohn, & Seeley, 1998; Essau, Sasagawa, Lewinsohn, & Rohde, 2018).
Furthermore, prenatal and perinatal complications (e.g., heavy bleeding, severe illness, hypertension, or excessive fluid retention) predict risk for childhood anxiety disorders, above-and-beyond the familial risk of parental psychopathology (Hirshfeld-Becker et al., 2004). In particular, there appeared to be an additive effect, as children who were exposed to multiple pre- and perinatal complications were at increased risk for anxiety. Finally, a study (Freed et al., 2014) investigated obstetric complications as a mediating factor in the relation between parental lifetime anxiety and child lifetime psychopathology. In this study, children who experienced delivery complications were much more likely to be diagnosed with anxiety disorders. This relation remained significant even after controlling for parental lifetime anxiety disorders.
Associated studies have demonstrated that preterm birth and low birth weight, as specific indicators of perinatal complications, are risk factors for poor socioemotional and personality development. Adults born very preterm (i.e., less than 33 weeks’ gestation) were more likely to self-report lower extraversion scores, higher neuroticism scores, and exhibited a personality profile characterized by greater negative affect and BI and lower positive affect and sensation seeking compared to their term-born counterparts (Allin et al., 2006). In parallel, adults who were born at extremely low birth weight, compared to normal birth weight adults, are more likely to display a profile of significantly higher shyness and BI (Schmidt, Miskovic, Boyle, & Saigal, 2008). Taken together, these findings highlight possible early developmental risk factors associated with the emergence of distinct temperament and personality profiles. These profiles, in turn, may place individuals at risk for social anxiety and other mental health issues, starting very early in development. There is empirical evidence to demonstrate that early neonatal events, such as low birth weight, confer a two-to-four-fold increase in offspring’s risk for anxiety disorders (Nomura et al., 2007).
Finally, associated biological mechanisms further support a relation between perinatal experiences and early temperament. The perinatal period is thought to be a crucial period of development during which the stress-regulation systems of the body are adjusted and finely tuned (Van Den Bergh, 2011). As such experiences during this period may lead to biological changes that produce lasting effects into later development, impacting physical and mental health outcomes. Animal models, for example, suggest that pre- and perinatal stressors are associated with later sensitivity to novelty and BI-like behaviors, potentially mediated by permutations in glucocorticoid functioning (Cavigelli, 2018; Tang, Reeb-Sutherland, Romeo, & McEwen, 2012). Preliminary data in humans also suggest that exposure to prenatal stress may increase infant fearfulness, particularly in females, via epigenetic modification of the glucocorticoid receptor gene (Ostlund et al., 2016)
The studies outlined thus far suggest that perinatal complications are potential early developmental risk factors associated with poor emotional regulation and difficult temperament in infants, children, and adolescents. To our knowledge, there is no research examining the relation between perinatal complications, temperament, and anxiety in childhood. These are important areas to explore, as prior research provides robust evidence demonstrating the importance of early experience and perinatal development and their effects on neurological and behavioral development. Our study aimed to leverage a large-scale study of BI as an initial assessment of the relation between perinatal complications, temperament, and social anxiety. In particular, we evaluated if BI acts as a potential mediating factor between perinatal complications and the presence of social anxiety symptoms in childhood, by first exploring the indirect effects evident in the current sample.
Methods
Participants
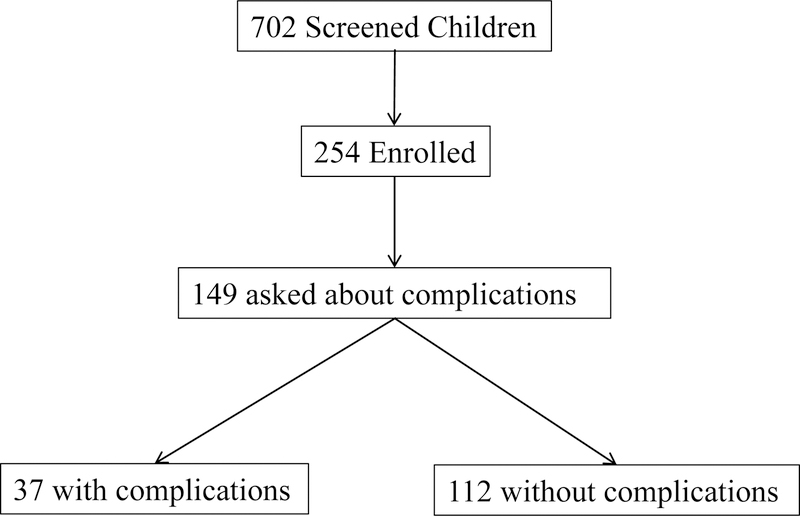
The current analysis drew from a larger laboratory study of temperament, attention, and anxiety. Data were collected from May 2012-December 2016. Figure 1 illustrates the sampling strategy for the current study. We screened 702 9- to 12-year-old children (Mage = 9.97, SDage = 1.00, 365 Male) for participation in the laboratory study using parental report on the Behavioral Inhibition Questionnaire (Bishop, Spence, & McDonald, 2003). Children who met pre-set cutoff scores (≥119 total score or ≥60 social novelty subscale) were identified as BI (N = 130, 18.5%). Cutoff scores were based on a previous study of extreme temperament in children 4 to 15 years of age (Broeren & Muris, 2010). The fully screened sample was 89.9% Caucasian, 2.0% African American, 2.0% Hispanic, 1.3% Asian, 4.0% biracial, and 1.0% declined to respond.
Figure 1.
Diagram illustrating the sampling strategy for the current study.
Over the course of the screening process, we noted verbal reports of birth complications from parents already enrolled in the study. As such, we added questions probing the early medical history of the potential participants, including the presence of perinatal birth complications. Of the full screening sample, 379 families (54.0%) were asked about complications. This subset of families did not differ from the remaining screening sample on core study variables (p’s>0.65).
Screened families were asked two yes/no questions probing if their child was born more than two weeks from the due date and if the child experienced birth complications. An additional open-ended question then allowed parents to describe the nature of the complications. From these responses we removed answers that did not fit the parameters of perinatal complications (e.g., child has a subsequent food allergy). As expected, we found a wide range of concerns and many participants experienced multiple complications (e.g., low birth weight and neonatal intensive care unit (NICU) admission). The most common complications were breech birth (16.2%), NICU admission (16.2%), cardiopulmonary distress (16.2%), maternal distress during or after pregnancy/labor (16.2%), unplanned Cesarean section (13.5%), gastrointestinal complications (8.1%), and perinatal surgery (8.1%). Although this measure relies on self-reported perinatal complications, research has shown that retrospective reports of perinatal events has moderate to high validity, especially among well-educated mothers (Bat-Erdene, Metcalfe, McDonald, & Tough, 2013; Buka, Goldstein, Spartos, & Tsuang, 2004; Coolman et al., 2010; Neiderhiser et al., 2016).
Based on the full sample screen, we invited behaviorally inhibited children to enroll in the larger laboratory study of temperament, attention, and anxiety (e.g., Morales, Taber-Thomas, & Pérez-Edgar, 2017; Thai, Taber-Thomas, & Pérez-Edgar, 2016). Non-inhibited children were also invited to enroll as non-yoked age- and gender-matched controls to the behaviorally inhibited children. In total, 254 children enrolled in the larger study (Figure 1). The enrolled sample was enriched for BI, such that 88 children (34.6%) met the BI criteria, and 166 children were non-BI. Enrolled parents and children were interviewed regarding the child’s social anxiety symptoms using the Diagnostic Interview Schedule for Children version IV (C-DISC 4; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000).
In total, 149 children had available data on BI level, perinatal complications, and both parent- and child-report of social anxiety for the current analysis (Table 1; Figure 1). While the children included in this analysis did not differ from the screened, but excluded, children on demographic variables (p’s>0.43), there was a significant increase in BI level (Mincluded = 95.2 vs. Mexcluded = 89.2, p = 0.04) since we specifically enriched the sample on this variable.
Table 1.
Demographic characteristics and descriptive statistics (mean and standard deviation) of main study variables.
| Full Sample | Birth Complications | ||
|---|---|---|---|
| Yes | No | ||
| N | 149 | 37 | 112 |
| Gender | 77 M/72 F | 18 M/19 F | 59 M/53 F |
| Age | 9.99 (0.93) | 10.14 (0.98) | 9.95 (0.92) |
| Total BIQ | 95.19 (32.90) | 114.00 (31.96)** | 88.98 (30.92)** |
|
Parent-Report Social Anxiety |
1.69 (3.07) | 3.38 (3.79)** | 1.13 (2.58)** |
|
Child-Report Social Anxiety |
3.01 (3.46) | 3.51 (3.29) | 2.84 (3.51) |
p<0.10
p<0.05
p<0.01
Screened families were recruited using the university’s database of families interested in participating in research studies, community outreach, and word-of-mouth throughout the region surrounding State College, PA. All parents and children provided written consent/assent. Participants received monetary compensation for completing the screening questionnaires and additional compensation for participating in the larger study once enrolled. All recruitment and study methods were approved by the institutional review board of The Pennsylvania State University.
Measures
Parents completed the BIQ (Bishop et al., 2003), a 30-item questionnaire consisting of BI-linked behavior in the domains of social and situational novelty assessed on a 7-point Likert scale. The questionnaire has adequate internal consistency and validity in differentiating children with or without BI (Bishop et al., 2003) and parental reports on the BIQ are correlated with laboratory observations of BI (Dyson, Klein, Olino, Dougherty, & Durbin, 2011). In the present study, the BIQ had good internal consistency (α = .86). Continuous total BIQ scores were used for our analyses (Range = 30–165).
To assess social anxiety symptoms, the computer-assisted C-DISC 4 (Shaffer et al., 2000) was administered separately to primary caregivers and the child participants. The C-DISC 4 is a widely used, reliable, and well-validated measure of anxiety symptoms and disorders with strong test-retest reliability (Shaffer et al., 2000; Silverman, Saavedra, & Pina, 2001). A trained research assistant conducted the semi-structured interview, in which participants judged DSM-IV symptoms as either present (“yes”) or absent (“no”). Sample questions from C-DISC 4 Question for Social Anxiety include: “In the last year – that is, since you started fifth grade – was there a time when you felt worried about speaking out loud in class?” and “In the past year have you been very concerned with being liked by others?”. “Yes” responses were tallied to obtain a total symptom score. Total symptom scores ranged from 0 to 12, for both primary caregivers and children. In addition, the C-DISC also asks for symptom frequency, duration, and impairment in order to assess clinical criteria. The series of impairment questions are incorporated at the end of each diagnostic section only if a clinically significant number of symptoms have already been endorsed, usually half or more of those required for the diagnosis. Research assistants conducting the interviews all had bachelor’s or master’s degrees and were trained by a researcher with a master’s degree in clinical psychology.
Model Testing.
We tested a mediation model (Bolin, 2014; Preacher, Rucker, & Hayes, 2007) to assess the relations between perinatal complications, BI, and social anxiety (see Table 3; Figure 2) using SPSS (Version 24; Chicago, IL, USA) PROCESS model 4. Models were run separately for parent-report and child self-report of symptom presentation. Continuous variables were mean centered prior to analysis. Significant conditional indirect effects were determined using 95% bootstrap bias corrected confidence intervals (CIs) based on 10,000 bootstrap samples.
Table 3.
Bivariate correlations for perinatal complications, BIQ score, social anxiety symptoms, and age.
| Complications | BI | Parent-Report | Child-Report | Age | Gender | |
|---|---|---|---|---|---|---|
| Complications | -- | |||||
| BI | 0.330** | -- | ||||
| Parent-Report | 0.318** | 0.514** | -- | |||
| Child-Report | 0.085 | 0.225** | 0.295** | -- | ||
| Age | 0.088 | −0.053 | 0.106 | 0.048 | -- | |
| Gender | 0.035 | 0.102 | 0.178* | 0.197* | 0.223** | -- |
Note: Complications (0=Absent, 1=Present), Gender (0=Male, 1=Female) Total N=149;
p<.10
p<.05
p<.01
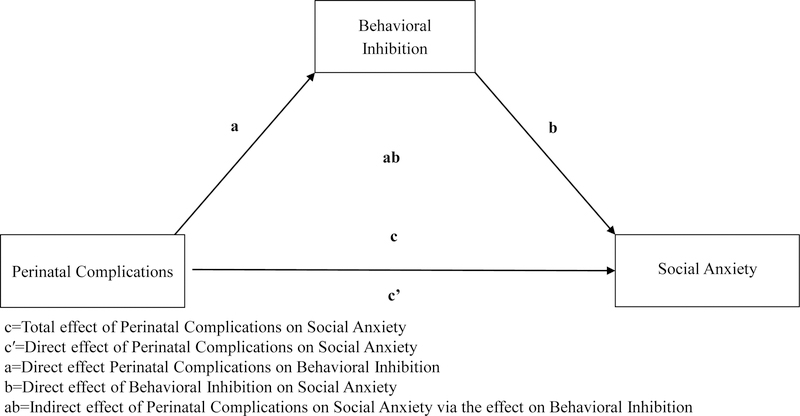
Figure 2.
Conceptual diagram illustrating the mediation model used to test the relations between perinatal complications, BI, and social anxiety symptoms.
The specific models tested here were derived from the available literature in adults (Allin et al., 2006; Schmidt et al., 2008) and our theoretical assumptions regarding the temporal chain between perinatal stress, temperament, and anxiety symptoms. Given that only a relative minority of children experienced birth complications in the final sample, we utilized a model that is robust to differences in group sample size based on the bootstrapping procedure, which provides a representation of the sampling distribution of the indirect effect (Hayes & Rockwood, 2017). Initial analyses included age and sex as covariates in the model. However, we found no systematic effects for either variable and they did not impact the larger indirect effect. They were then removed for parsimony.
Results
In the current analyses, 37 children (24.8%) experienced perinatal complications (Tables 1 and 2). Perinatal complication status did not differ with age or gender (p’s>0.29). Children experiencing perinatal complications were higher in BI, t(147)=−4.23, p<0.001, d=0.70, and had more social anxiety symptoms based on parent-report symptoms, t(147)=−4.06, p<0.001, d=0.67. Although in the same direction, the effect of perinatal complications was not significant for child-report symptoms, t(147)=−1.03, p=0.31, d=0.17. As expected, we found significant intercorrelations between BI scores, parent-report symptoms, and child-report symptoms (Table 3).
Table 2.
Distribution of diagnostic assessments of social anxiety with the DISC for participants as a function of BI status and report of complications.
| Parent-Report | Child-Report | |||||||
|---|---|---|---|---|---|---|---|---|
| Complications | No Complications | Complications | No Complications | |||||
| BI | Non-BI | BI | Non-BI | BI | Non-BI | BI | Non-BI | |
| Low Anxiety | 13 | 10 | 26 | 72 | 14 | 7 | 20 | 58 |
| Subthreshold Anxiety | 10 | 2 | 8 | 3 | 8 | 5 | 14 | 14 |
| Clinical Anxiety | 2 | 0 | 3 | 0 | 3 | 0 | 3 | 3 |
Note: Diagnostic categories are determined by the DISC based on a combination of symptom counts, duration, and frequency, as well as level of impairment. Impairment levels are assessed if the child or parent has endorsed half or more of the queried symptoms. For diagnosis, the symptom disturbance must be evident for six months or longer and have caused significant distress. The distribution of complications and diagnosis by BI-group was significant for both care-givers, X2(2) = 24.11, p < 0.001, and children, X2(2) = 6.93, p = 0.031.
BI and Non-BI categorization for this table was based on the initial parental screening with the BIQ. BI children had cutoff scores ≥119 for the total score or ≥60 for the social novelty subscale. Continuous BIQ scores were used in all of the presented analyses.
Perinatal Complications, Behavioral Inhibition, & Parent-Report Social Anxiety
As shown in Table 4, the model (Figure 2) found that the significant association between perinatal complications and social anxiety (c) is explained through BI. The paths were significant from perinatal complications to BI (a), and from BI to social anxiety symptoms (b). Importantly, the indirect effect of perinatal complications on social anxiety through BI was significant (ab). The direct effect of perinatal complications on social anxiety symptoms (c′) was also significant.
Table 4.
Path results for the mediation models for parent-report and child self-report of child social anxiety.
| Unstandardized Coefficient | Standard Error | t | p | LLCI | ULCI | ||
|---|---|---|---|---|---|---|---|
| Parent-Report of Child Anxiety |
PC→SA c |
2.25 | 0.55 | 4.06 | 0.000 | 1.158 | 3.349 |
|
PC→BI a |
25.02 | 5.91 | 4.23 | 0.000 | 13.341 | 36.697 | |
|
BI→SA b |
0.04 | 0.01 | 6.22 | 0.000 | 0.029 | 0.057 | |
|
PC→SA c′ |
1.18 | 0.52 | 2.25 | 0.026 | 0.143 | 2.214 | |
|
PC→BI→SA ab |
1.07 | 0.27 | -- | -- | 0.560 | 1.629 | |
| Child- Report of Child Anxiety |
PC→SA c |
0.67 | 0.66 | 1.03 | 0.305 | -0.620 | 1.969 |
|
PC→BI a |
25.02 | 5.91 | 4.23 | 0.000 | 13.341 | 36.697 | |
|
BI→SA b |
0.02 | 0.01 | 2.59 | 0.011 | 0.006 | 0.041 | |
|
PC→SA c′ |
0.09 | 0.68 | 0.14 | 0.892 | −1.253 | 1.438 | |
|
PC→BI→SA ab |
0.58 | 0.27 | -- | -- | 0.114 | 1.175 |
PC=Perinatal Complications
BI=Behavioral Inhibition
SA=Social Anxiety
LLCI=Lower Level Confidence Interval
ULCI=Upper Level Confidence Interval
Perinatal Complications, Behavioral Inhibition, and Self-Report Social Anxiety
The model found that the total association between perinatal complications and social anxiety (c) was not significant (Table 4). Here, the direct effect of perinatal complications on social anxiety symptoms (c′) was not significant. However, the paths were again significant from perinatal complications to BI (a), and from BI to social anxiety symptoms (b). As with parent-report, the test of indirect effects was significant for perinatal complications on social anxiety through BI (ab).
Discussion
In the current study, we sought to evaluate perinatal complications as possible early developmental risk factors associated with BI and social anxiety. In addition, we examined BI as an indirect path that could help explain the relation between perinatal complications and the presence of social anxiety symptoms later in life. Our results suggest that perinatal complications may be associated with a broad pattern of development that underscores known risk factors for anxiety. These findings are in line with a broader literature linking the earliest days of life to long-term patterns of psychosocial functioning (Freed et al., 2014; Hirshfeld-Becker et al., 2004; Pollak et al., 2010). While preliminary, the current study provides support for follow-up studies targeting specific perinatal complications and socioemotional function over time in childhood.
Our findings are consistent with prior studies reporting associations between perinatal complications and risk for childhood anxiety disorders (Cohen, Velez, Brook, & Smith, 1989; Freed et al., 2014; Hirshfeld-Becker et al., 2004; Johnco et al., 2016). We extend these findings, highlighting a specific relation between perinatal complications and risk for social anxiety symptoms in children. However, we found the direct relation between perinatal complications and social anxiety symptoms was significant for the parent-report model, but did not reach statistical thresholds for the child self-report model, albeit in the same direction. Finding inconsistent results across informants is not uncommon (De Los Reyes & Kazdin, 2005; De Los Reyes, Thomas, Goodman, & Kundey, 2013).
One possible explanation for this discrepancy could be that there is a stronger relation for the parents because the parent is the informant for both perinatal complications and child social anxiety symptomology. Events surrounding, and then subsequent to, the perinatal complications may color the parent’s view of the child’s functioning. Moreover, parents and children may be assessing and understanding symptoms of anxiety differently. Prior research suggests that parent and child agreement is generally lower for anxiety disorders (Barbosa, Tannock, & Manassis, 2002). In line with this literature, we find a significant but modest correlation between parent and child reports (r = .30), suggesting considerable unique variance for each informant. Given that there is no “gold standard” measure for psychopathology and each informant provides unique information, it is important for future studies to incorporate multiple informants (De Los Reyes & Kazdin, 2005).
Despite the lack of statistical consistency between the parent- and self-report models, the indirect effect of temperament is similar for both models. Specifically, we find that perinatal complications are associated with higher levels of BI and that this temperament trait is related to increased social anxiety. Our findings are unique in that both the parent- and self-report models indicate that BI is an underlying factor linking the relation between perinatal complications and social anxiety symptoms. This result is in line with studies in adults that find poor obstetric outcomes, such as low birth weight and preterm birth, are associated with less adaptive socioemotional and personality development, typically characterized by low extraversion, higher shyness, and BI (Allin et al., 2006; Schmidt et al., 2008). Furthermore, obstetric complications and preterm birth seem to be more strongly associated with anxiety disorders (Essau et al., 2018; Freed et al., 2014), especially when coupled with other risk factors (e.g., maternal hostility; (Neiderhiser et al., 2016). In contrast, other factors, such as maternal substance use during pregnancy (e.g., smoking) are more strongly related to externalizing and attention problems (Wakschlag et al., 1997; Weissman, Warner, Wickramaratne, & Kandel, 1999).
Although our study does make an important contribution, lending support to the proposition that child temperament may be one of the underlying developmental processes linking perinatal complications and social anxiety symptoms, it is difficult to rule out other potential contributing factors.
First, our study did not assess characteristics of the mother. Thus, we were unable to consider how maternal psychological factors may affect the child at different stages of development. Independent studies have found that prenatal maternal stress is a predictor of both perinatal complications (Copper et al., 1996; Dunkel-Schetter, 1998; Lou et al., 1994; Sandman, Davis, & Glynn, 2012) and child temperament (Blair, Glynn, Sandman, & Davis, 2011; Gutteling et al., 2005; Huizink, De Medina, Mulder, Visser, & Buitelaar, 2002; Huttenen, Martin, Noyes, Wisenbaker, & Huttunen, 1999; O’Connor, Heron, Golding, Beveridge, & Glover, 2002). For example, maternal stress during pregnancy has been associated with smaller head circumference at birth, perhaps indicating variations in fetal brain development (Lou et al., 1994). It is also well established that maternal stress during pregnancy is correlated with spontaneous preterm delivery and low birth weight (Copper et al., 1996; Dunkel-Schetter, 1998; Sandman et al., 2012). Beyond obstetric markers, maternal levels of perceived stress during pregnancy are also associated with increased negative affect and emotional and behavioral problems from infancy to childhood (Blair et al., 2011; Gutteling et al., 2005; Huizink et al., 2002; Huttenen et al., 1999; O’Connor et al., 2002).
Thorough assessment of maternal characteristics would also allow future studies to account for the possible influence of the postnatal environment. For instance, both maternal anxiety and perinatal complications may be risk factors for maternal postpartum psychopathology. Prior studies indicate that maternal antenatal anxiety predicts postnatal depression and anxiety, and that the experience of severe obstetric complications is associated with more intense postnatal depressive symptoms, independent of prior anxiety or depression diagnoses (Coelho, Murray, Royal-Lawson, & Cooper, 2011; Heron et al., 2004; Verdoux, Sutter, Glatigny-Dallay, & Minisini, 2002). Maternal postpartum depression and anxiety may indirectly increase the risk for child psychopathology by affecting mother-child interactions. Thus, future studies should carefully consider maternal traits and characteristics both pre- and postpartum in order to understand how these factors may uniquely impact the development of child social anxiety symptoms.
Second, the oversampling of behaviorally inhibited children may limit the generalizability of our findings. Thus, this study should not be utilized as an index for the prevalence of BI and/or perinatal complications. Third, the retrospective self-reporting of perinatal complications is an additional limitation of this study, as mothers may not be able to clearly recall events that occurred during pregnancy and delivery. Future studies should consider examining the relation between perinatal complications, early temperament, and psychopathology using prospective, longitudinal methods. To the best of our knowledge, most studies that have investigated the relation between perinatal complications and anxiety disorders in children do so concurrently and use maternal retrospective report of perinatal complications (for an exception, see Neiderhiser et al., 2016). This allows for many forms of shared method variance. It is difficult to determine the accuracy of the mother’s recollections of the events that occurred during and before birth, and it is possible that mothers with anxiety disorders may be prone to recall more problems during their pregnancies. However, past studies demonstrate that maternal recollection of birth complications is valid particularly for complications like the ones described in this study and among highly educated families (Bat-Erdene et al., 2013; Buka et al., 2004; Coolman et al., 2010; Neiderhiser et al., 2016). Future studies should carefully measure maternal traits and characteristics, incorporate multiple informants, and include behavioral measures for temperament assessment.
Despite these limitations, our study offers important insight as it shows, for the first time in children, that perinatal complications are associated with fearful temperament and may be an early risk factor for social anxiety. Moreover, our findings provide initial evidence that temperament may be one of the underlying developmental processes linking perinatal complications and anxiety. Our findings are in line with the broader literature suggesting that stressors occurring during the perinatal period of development can critically impact neural and behavioral development (Bock, Rether, Gröger, Xie, & Braun, 2014), particularly as expressed in reactive temperamental profiles (Lin, Ostlund, Conradt, Lagasse, & Lester, 2018) Thus, investigating structural and functional abnormalities in brain areas associated with the regulation and mediation of emotionality may provide a more direct link between early life stressors and the later emergence of behavioral problems and psychopathology. Further investigation into the relation between perinatal complications, early temperament, and social anxiety is important in that the findings provide the opportunity to identify and target potential underlying mechanisms, providing new insight into how to more effectively prevent or address the potential consequences of perinatal complications.
Acknowledgments
The authors would like to thank all the families who participated in the study, as well as the students and staff who assisted in data collection. This work was supported by NIH award R01MH094633 to Dr. Pérez-Edgar.
Footnotes
The authors declare no conflict of interest.
References:
- Allen NB, Lewinsohn PM, & Seeley JR (1998). Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Development and Psychopathology, 10(3), 513–529. [DOI] [PubMed] [Google Scholar]
- Allin M, Rooney M, Cuddy M, Wyatt J, Walshe M, Rifkin L, & Murray R (2006). Personality in young adults who are born preterm. Pediatrics, 117(2), 309–316. [DOI] [PubMed] [Google Scholar]
- Barbosa J, Tannock R, & Manassis K (2002). Measuring anxiety: Parent-child reporting differences in clinical samples. Depression and Anxiety, 15(2), 61–65. [DOI] [PubMed] [Google Scholar]
- Bat-Erdene U, Metcalfe A, McDonald SW, & Tough SC (2013). Validation of Canadian mothers’ recall of events in labour and delivery with electronic health records. BMC Pregnancy and Childbirth, 13(1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G, Spence SH, & McDonald C (2003). Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Development, 74(6), 1899–1917. [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Rether K, Gröger N, Xie L, & Braun K (2014). Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Frontiers in Neuroscience, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin JH (2014). Hayes Andrew F. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach New York, NY: The Guilford Press. Journal of Educational Measurement, 51(3), 335–337. [Google Scholar]
- Broeren S, & Muris P (2010). A psychometric evaluation of the behavioral inhibition questionnaire in a non-clinical sample of Dutch children and adolescents. Child Psychiatry & Human Development, 41(2), 214–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Spartos E, & Tsuang MT (2004). The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophrenia Research, 71(2–3), 417–426. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA (2018). Behavioral Inhibition in Rodents: A Model to Study Causes and Health Consequences of Temperament. In Behavioral Inhibition (pp. 35–58). Springer. [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho HF, Murray L, Royal-Lawson M, & Cooper PJ (2011). Antenatal anxiety disorder as a predictor of postnatal depression: a longitudinal study. Journal of Affective Disorders, 129(1–3), 348–353. [DOI] [PubMed] [Google Scholar]
- Cohen P, Velez CN, Brook J, & Smith J (1989). Mechanisms of the relation between perinatal problems, early childhood illness, and psychopathology in late childhood and adolescence. Child Development, 701–709. [DOI] [PubMed]
- Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, & Steegers EA (2010). Medical record validation of maternally reported history of preeclampsia. Journal of Clinical Epidemiology, 63(8), 932–937. [DOI] [PubMed] [Google Scholar]
- Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, … Johnson F (1996). The preterm prediction study: Maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. American Journal of Obstetrics and Gynecology, 175(5), 1286–1292. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, & Kazdin AE (2005). Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Thomas SA, Goodman KL, & Kundey SM (2013). Principles underlying the use of multiple informants’ reports. Annual Review of Clinical Psychology, 9, 123–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel-Schetter C (1998). Maternal stress and preterm delivery. Prenatal and Neonatal Medicine, 3, 39–42. [Google Scholar]
- Dyson MW, Klein DN, Olino TM, Dougherty LR, & Durbin CE (2011). Social and non-social behavioral inhibition in preschool-age children: differential associations with parent-reports of temperament and anxiety. Child Psychiatry & Human Development, 42(4), 390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S, Lewinsohn PM, & Rohde P (2018). The impact of pre-and perinatal factors on psychopathology in adulthood. Journal of Affective Disorders, 236, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed RD, Tompson MC, Otto MW, Nierenberg AA, Hirshfeld-Becker D, Wang CH, & Henin A (2014). Early risk factors for psychopathology in offspring of parents with bipolar disorder: the role of obstetric complications and maternal comorbid anxiety. Depression and Anxiety, 31(7), 583–590. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, & Buitelaar JK (2005). The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child & Adolescent Psychiatry, 14(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Hayes AF, & Rockwood NJ (2017). Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy, 98, 39–57. [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, Glover V, & Team AS (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Faraone SV, Robin JA, Friedman D, Rosenthal JM, & Rosenbaum JF (2004). Pregnancy complications associated with childhood anxiety disorders. Depression and Anxiety, 19(3), 152–162. [DOI] [PubMed] [Google Scholar]
- Huizink AC, De Medina PGR, Mulder EJ, Visser GH, & Buitelaar JK (2002). Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 41(9), 1078–1085. [DOI] [PubMed] [Google Scholar]
- Huttenen MO, Martin RP, Noyes J, Wisenbaker J, & Huttunen MO (1999). Prediction of Early Childhood Negative Emotionality and Inhibition From Maternal Distress During Pregnancy. Merrill-Palmer Quarterly (1982-), 370–391. [Google Scholar]
- Johnco C, Lewin AB, Salloum A, Murphy TK, Crawford EA, Dane BF, … Storch EA (2016). Adverse prenatal, perinatal and neonatal experiences in children with anxiety disorders. Child Psychiatry & Human Development, 47(2), 317–325. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, & Garcia-Coll C (1984). Behavioral inhibition to the unfamiliar. Child Development, 2212–2225.
- Lin B, Ostlund BD, Conradt E, Lagasse LL, & Lester BM (2018). Testing the programming of temperament and psychopathology in two independent samples of children with prenatal substance exposure. Development and Psychopathology, 30(3), 1023–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, & Hetnmingsen R (1994). Prenatal stressors of human life affect fetal brain development. Developmental Medicine & Child Neurology, 36(9), 826–832. [DOI] [PubMed] [Google Scholar]
- Morales S, Taber-Thomas BC, & Pérez-Edgar KE (2017). Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Developmental Science, 20(2), e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Marceau K, Araujo-Greecher MD, Ganiban JM, Mayes LC, Shaw DS, … Leve LD (2016). Estimating the Roles of Genetic Risk, Perinatal Risk, and Marital Hostility on Early Childhood Adjustment: Medical Records and Self-Reports. Behavior Genetics, 46(3), 334–352. 10.1007/s10519-016-9788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Wickramaratne PJ, Pilowsky DJ, Newcorn JH, Bruder-Costello B, Davey C, … Weissman MM (2007). Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression. Comprehensive Psychiatry, 48(5), 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connor TG, Heron J, Golding J, Beveridge M, & Glover V (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry, 180(6), 502–508. [DOI] [PubMed] [Google Scholar]
- Ostlund BD, Conradt E, Crowell SE, Tyrka AR, Marsit CJ, & Lester BM (2016). Prenatal stress, fearfulness, and the epigenome: exploratory analysis of sex differences in DNA methylation of the glucocorticoid receptor gene. Frontiers in Behavioral Neuroscience, 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, & Fox NA (2005). Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics, 14(4), 681–706. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, … Gunnar MR (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81(1), 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, & Hayes AF (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42(1), 185–227. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, & Glynn LM (2012). Psychobiological stress and preterm birth. In Preterm Birth-Mother and Child InTech. [Google Scholar]
- Schmidt LA, Miskovic V, Boyle MH, & Saigal S (2008). Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics, 122(1), e181–e187. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Saavedra LM, & Pina AA (2001). Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. Journal of the American Academy of Child & Adolescent Psychiatry, 40(8), 937–944. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, & McEwen BS (2012). Reducing behavioral inhibition to novelty via systematic neonatal novelty exposure: the influence of maternal hypothalamic-pituitary-adrenal regulation. Biological Psychiatry, 72(2), 150–156. [DOI] [PubMed] [Google Scholar]
- Thai N, Taber-Thomas BC, & Pérez-Edgar KE (2016). Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: An ERP study. Developmental Cognitive Neuroscience, 19, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bergh BR (2011). Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Developmental Medicine & Child Neurology, 53, 19–23. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Sutter AL, Glatigny-Dallay E, & Minisini A (2002). Obstetrical complications and the development of postpartum depressive symptoms: a prospective survey of the MATQUID cohort. Acta Psychiatrica Scandinavica, 106(3), 212–219. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, & Leventhal BL (1997). Maternal smoking during pregnancy and the risk of conduct disorder in boys. Archives of General Psychiatry, 54(7), 670–676. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, & Kandel DB (1999). Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child & Adolescent Psychiatry, 38(7), 892–899. [DOI] [PubMed] [Google Scholar]