Abstract
During fMRI imaging, 12 good and 8 poor writers aged 11 wrote a newly taught pseudoletter and a highly practiced letter. Both letters were formed from the same components, but the pseudoletter had a novel configuration not corresponding to a written English letter form. On the first fMRI contrast between the newly taught pseudoletter and highly practiced letter, based on a group map, good and poor writers significantly activated many common regions; but the poor writers showed spatially more extensive brain activation than did the good writers. The additional regions of significant activation may reflect inefficiency in learning a new letter form. For the second contrast between the highly practiced and newly taught letters, individual brain activation analyses, based on exact clusters, showed that good and poor writers differed significantly in activation only in left fusiform. This individual fusiform activation correlated significantly with behavioral measures of automatic letter writing and expressive orthographic coding. Multiple regression in which both individual fusiform activation and individual orthographic coding were entered explained significant variance in written composition. Results are discussed in reference to the role of the orthographic loop, from internal letter form to external letter writing by hand, in writing letters and composing. The overall results are consistent with prior brain and behavioral studies of writing.
Keywords: Brain and writing development, fMRI writing tasks, Handwriting
Behavioral research on handwriting
The focus of considerable research on handwriting has been on its development and the fine motor processes involved (for a comprehensive review, see Graham & Weintraub, 1996). Other research has focused on the non-motoric, cognitive processes in handwriting that support the translation of internal written language representations (i.e., letter forms) into visual symbols in the external environment. For example, orthographic processes (storing and processing internal representations of written words and their constituent letters and individual letter forms) contributed uniquely over and beyond fine motor processes to handwriting in developing children in grades 1–6 (ages 6–13) (Abbott & Berninger, 1993; Berninger, Cartwright, Yates, Swanson, & Abbott, 1994; Berninger, Yates, & Lester, 1991; Berninger, Yates, Cartwright, Rutberg, Remy, & Abbot, 1992).
The relationship of handwriting to other cognitive processes in writing has also been investigated. Handwriting was related to written spelling in low achieving writers at the end of second grade (Berninger, Abbott, Rogan, Reed, Abbott, Brooks, et al., 1998). Cross-sectional, longitudinal, and experimental research studies have shown that handwriting is related to text generation during the composing process as thought is translated into written language in both developing writers (e.g., Berninger et al., 1991, 1992, 1994 Graham, Berninger, Abbott, Abbott, & Whitaker, 1997; Jones, 2004; Jones & Cristensen, 1999) and skilled writers (Connelly, Campbell, MacLean, & Barnes, 2006; Hayes, 2009; Hayes & Chenoweth, 2006; Hayes & Flower, 1980). Handwriting is also related to the note-taking process while college students listen to lectures (Peverley, 2006).
In addition, results of instructional studies imply causal relationships between handwriting and other written language skills. Teaching automatic letter writing improved compositional fluency: Students wrote texts that were longer (Berninger, Abbott, Whitaker, Sylvester, & Nolen, 1995) and were completed in less time (Berninger, Vaughan, Abbott, Abbott, Brooks, Rogan, et al., 1997; Berninger, Rutberg, Abbott, Garcia, Anderson-Youngstrom, Brooks, et al., 2006; Graham, Harris, & Fink, 2000). Teaching handwriting has also shown transfer to improved word reading (e.g., Berninger, Dunn, Lin, & Shimada, 2004; Berninger et al., 1997, 2006; Dunn & Miller, 2009).
Brain imaging studies of handwriting
Most brain imaging studies of handwriting or handwriting-related motor processes have been done with normal adults or adults who have lost writing function due to strokes, injury, or disease and have been done with brain imaging technologies, such as PET, that are not as susceptible to motor artifact, as is fMRI, which is highly susceptible to motor artifact. Internal codes for letters may form in the left parietal lobe (Basso, Taborelli, & Vignolo, 1978) or fusiform gyrus (Joseph, Gathers, & Piper, 2003), but the graphic-motor code for writing a letter in the external environment is constructed in the left frontal lobe (Brain, 1967) in left BA 6 in right handers (Longcamp, Anton, Roth, & Velay, 2003). Fusiform gyrus is related to orthographic (visual) word-form (e.g., Cohen & Dehaene, 2004; Cohen, Lehéricy, Chhochon, Lemer, Rivaud, & Dehaene, 2002; Dehane, Le Clec’H, Poline, Bihan, & Cohen, Dehane et al., 2002) and letter-form (Joseph et al., 2003) processing.
Letter production (handwriting) enhances letter identification over visual perception of letters alone (James & Gauthier, 2006; Longcamp et al., 2003). Visual perception, which draws greatly on occipital regions, is not specific to visible language and thus is not the same as orthographic coding, which is specific to units of visible language (for review of evidence, see Berninger & Richards, 2002). However, transcription mode may affect letter generation and production. Adults learn novel characters better by writing them by pen than by keyboard (Longcamp et al., 2008). A similar advantage of pen over keyboard was observed in developing writers in grades 2, 4, and 6 who wrote longer essays, wrote words faster, wrote more complete sentences (Berninger, Abbott, Augsburger, & Garcia, 2009a), and expressed more ideas (Hayes & Berninger, 2009) when writing by pen than by keyboard. In addition, handwriting training led to better letter recognition than keyboard training in older preschool children (Longcamp, Zerbato-Poudou, & Velay, 2005). Thus, a brain imaging study of letter writing by pen during middle childhood might advance knowledge of the brain-behavior relationships in handwriting and how these may be related to the composition process.
Advancing knowledge of brain-behavior relationships in developing writing
Participating children were recruited from the larger sample at the end of a 5-year longitudinal study of writing. Annually from first to fifth grade they had completed writing tasks at the university. Although children were generally normal writers displaying a range of abilities from average to well above average, eight children met criteria for writing disability. These eight children and ten good writers participated in the imaging study in the summer after fifth grade. The handwriting tasks during imaging were designed to contrast writing letter forms that were newly taught or highly practiced from kindergarten to fifth grade, but had common components even though the newly taught letter form had a novel configuration.
This contrast between newly taught and highly practiced is best understood in the context of educational practices in letter writing in the United States where the study was conducted. Unlike some European countries such as Italy and France that teach cursive writing from the beginning of formal schooling, in the United States manuscript writing (printing) is taught from kindergarten to second grade, and cursive is not introduced until third grade; and typically cursive writing is not taught after fourth grade. Thereafter, most children use only manuscript printing or a mix of manuscript and cursive (Graham, Berninger, & Weintraub, 1998). Moreover, two of the letters in manuscript form, which are high frequency letters in the language, are not written by hand in the same way they appear in written text in books or computerized word processed text displayed on monitors: a and g. In handwriting, for a, children make a circle (ball) and then a vertical line to the right of the circle that is the same height as the upper most extent of the circle. The most commonly taught style of manuscript is therefore called “ball and stick.” For g, they make a circle and then to the right of it a form that looks like a j without the dot such that its highest point is the same height as the upper most extent of the circle and about half of the j extends below the line on which the circle is resting. Thus, for developing writers in the United States, the “ball and stick” letter production forms for letters named /ay/ and /gee/ are highly familiar and practiced even though children encounter the typed version a and g (letter perception forms) in their written texts.
For purposes of the research study, participating children were taught the letter writing tasks outside the scanner before performing them inside the scanner while their brains were imaged during letter writing. They briefly practiced writing the “ball and stick a” and then were taught to write a pseudoletter composed of the same strokes as the “ball and stick a” but arranged in a novel configuration not previously taught or practiced, that is, a circle with a horizontal line drawn under it rather than at the right side (o). Garner (Garner, 1974; Garner, 1976; Garner, 1981; Garner, Hake, & Eriksen, 1956) developed a paradigm for measuring selective attention to component features versus overall configuration of letter forms and showed that these are separable dimensions of letter forms. Thus, the paradigm kept the motor production requirements for components (strokes) constant, but varied the configural properties of the written letter that was familiar (o with vertical line to right of same height) or novel (pseudoletter o, an o with horizontal line under it). When children wrote this newly taught pseudoletter during the brain scanning, they did not have a long history of practice with this pseudoletter configuration as they did with producing the configuration for “ball and stick a.”
Bidirectional contrasts between these two letter writing tasks were investigated: The first contrast was between the novel, newly taught configuration and the familiar, highly practiced configuration. This contrast identified unique brain activation associated with the newly taught pseudoletter. The second contrast was between the familiar, highly practiced letter form and the novel newly taught letter form. This contrast identified the unique brain activation associated with the more practiced and probably automatic letter form. In addition, because Gabrieli and colleagues (e.g., Hoeft, Ueno, Reiss, Meyler, Whitfield-Gabrieli, Glover, et al., 2009) showed that combining brain and behavioral measures predicted literacy outcomes better than either alone, relationships between individual brain activation and behavioral measures of orthographic coding, automatic letter writing, and written composition were analyzed.
Four hypotheses were tested: The first hypothesis was that good writers show more efficient brain activation than poor writers for the contrast comparing writing a newly taught pseudoletter form (configuration) and writing a highly practiced, familiar letter form. Efficiency was operationalized in reference to the number of brain regions engaged (i.e., showing significant BOLD activation). The second hypothesis was that good writers will show more significant individual brain activation in the fusiform gyrus during the contrast comparing writing a familiar, highly practiced letter form (configuration) and writing a newly taught, not highly practiced pseudoword letter form. The third hypothesis was that individual fusiform activation will correlate with individual behavioral measures of orthographic coding and automatic letter writing. The fourth hypothesis, inspired by Hoeft et al. (2009), was that regression with both brain (fusiform) and behavioral (orthographic coding) predictors will explain significant variance in written composition, thus validating brain-behavior relationships in writing.
The challenge of using tasks requiring overt motor responding in functional magnetic resonance imaging (fMRI) studies is well known. That is probably why relatively few fMRI studies of handwriting have been conducted. In this study we introduced the simple adaptation of using a book at midline on which children wrote with a small wooden stylus. Children had been taught and practiced the handwriting tasks to criterion levels of accuracy and rate of production before being scanned and were able to perform the task in the scanner in a manner that resulted in reliable BOLD activation without motor artifact.
Method
All methods, procedures, and consent forms used by this project were approved by the University of Washington Human Subjects Internal Review Board (Approval no. 96–1872-D12). Parents of participating children gave their informed consent and participating children gave their assent prior to their inclusion in the study. The research was conducted in accordance with ethical and professional guidelines of the American Psychological Association for research with human subjects. All participants were right-handed children who did not wear metal that could not be removed during scanning.
Participants
The children participated in the fMRI study in the summer between fifth and sixth grade. Children were included in the group of good or poor writers based on behavioral measures of handwriting and composing given as part of the longitudinal study. These measures had norms so that children could be compared to their grade or age peers. Automatic letter writing was assessed by number of legible, lowercase, manuscript letters in alphabetic order in the first 15 s on an alphabet writing task (Berninger et al., 1992, 1994). Written composition was assessed on the basis of the Wechsler Individual Achievement Test, Second Edition (WIAT II)Written Expression subtest (Psychological Corporation, 2002). The subtest score is based on extended writing for 10 min on a provided prompt, a sentence combining task (rewrite two provided sentences into one that expresses the same ideas more concisely), and written word fluency (writing example words for provided categories within time limit). An expressive orthographic coding task (adaptation of Berninger, 1987, which required written responses and was used in Berninger, 2001, 2007; Berninger et al., 1994) was also given in the longitudinal study. A written word is briefly presented for 1 s and then removed. The task is to write as the examiner designates, the whole word, a letter in the word (e.g., the last or the 2nd), or a letter group in the word (e.g., first two, last two, 2nd and 3rd).
Children were included in the group of good writers if they were at or near the mean on the measure of automatic letter writing and/or a written composition and were not significantly impaired on either of these writing skills. Children were included in the group of poor writers if they were below the mean in handwriting and/or written expression. Scaling of means was 0 (z) or 100 (ss) and of standard deviations was 3 (z) or 15 (ss). The good writers (n = 12) (handwriting, z = 0.44; written expression ss = 112) and poor writers (n = 8) (handwriting, z = −0.22; written expression ss = 96.3) differed significantly in handwriting, F(1,19) = 5.66, p = 0.029, and written composition, F(1,19) = 15.08, p = 0.001, but not Verbal IQ, F(1,19) = 0.16, p = 0.69 (ss = 119.4 good or 116.7 poor writers on Wechsler Intelligence Scaled for Children, 4th Edition, WISC 4, Wechsler, 2003). The poor writers as a group did not meet criteria for dyslexia, that is, they were not necessarily impaired in accuracy or speed of oral reading.
Of the good writers, 9 were females and 3 were males, and of the poor writers, 7 were males and 1 was female. Both cross-sectional (Berninger & Fuller, 1992, grades 1–6) and longitudinal studies (Martin & Hoover, 1987) showed that typically developing boys are more impaired than typically developing girls in handwriting skills regardless of ethnicity at all developmental levels (Demie, 2001). Thus, it was not surprising that more boys would be in the group of poor writers and more girls in the group of good writers.
Training handwriting tasks before scanning
Children were taught two handwriting tasks outside the scanner until they fully understood them and could perform them with ease and accuracy. Only then were their brains scanned while they performed the handwriting tasks. For the training and practice outside the scanner, children practiced writing the pseudoletter and the real letter with a stylus on the book at midline while they lay on their backs, just as they would do in the scanner. They did not need to look at their hands to write these letters when prompted to write the fake letter or real letter. It is well known in clinical neuropsychology that many finger tasks can be performed with somatosensory or kinesthetic feedback without visual feedback (e.g., Berninger & Rutberg, 1992).
A member of the research team told the children that they were to write the real letter “a” by making a round ball and then writing a stick beside it on the right so that it looked like the letter a they write (without italics), but not the way a looks in written text in books or computers (which has a curved rather than straight line on the right, arches up and over to left, and has a circular-like but not full circle or ball in the interior). The research assistant also modeled writing with a wooden stylus (not a pen or pencil) the round ball and lifting the stylus to form the vertical stroke of the same height on the right of the ball. Likewise, the research assistant modeled for each child how to write the pseudoletter, which was presented as a fake letter, by first writing a circle in a continuous stroke and then lifting the stylus and writing a horizontal stroke under the circle. The children readily grasped that distinction between real and fake letters, seemed to find the contrast interesting, and complied with lifting the stylus to form the line for both real letters and pseudoletters. Children practiced writing the familiar, highly practiced letter and newly taught pseudoletter until the research assistant determined they were writing each as instructed, and doing so with 100% accuracy and at a steady rate. The steady rate criterion, based on the research assistant timing each handwriting production, was one written pseudoletter or letter form each 2 s for 30 s. Children were given feedback as to their accuracy and steady rate for both handwriting tasks.
The two tasks, which were equated in component strokes for letter features but not for overall configuration (Garner, 1974, 1976, 1981; Garner et al., 1956), contrasted in where a straight line of same length was placed—to the right in conventional manner or underneath in novel manner. However, they also differed in the amount of prior practice children had in writing them. Even after they could perform the tasks outside the scanner to criterion levels, children had had at least 5 years of experience in writing the familiar, highly practiced letter form and about 5–10 min prior experience in writing the novel, newly taught pseudoletter.
Performing handwriting tasks during scanning
To minimize motor artifact on handwriting tasks, a book was placed on the child’s chest at midline and the child used a wooden stylus to write on the book either the novel circle with line under it or the familiar, highly practiced circle with a line to the right of it. Note that once scanning began for the tasks, children received no additional instruction for performing the handwriting tasks. However, they were told before entering the scanner that they would be cued with a visual prompt when to switch between writing the fake letter, a circle with line under it, and the real letter, a circle with line to the right of it. In the environment of the scanner, children could not see the letter form they wrote as is typical during sustained writing of text outside the scanner (e.g., Alamargot, Chesnet, Dansac, & Ros, 2006); but they did receive touch sensation and kinesthetic feedback from the motor act of writing novel or familiar letter forms.
The On and Off Tasks were administered by EPRIME using this timing: Fixation for 18 s, 5 cycles of On-30 s followed by Off-30 s, and then fixation for 18 s.
On task
When prompted on visual monitor to write the fake letter, a circle with line under it, the child repeatedly wrote the novel configuration (pseudoletter) with wooden stylus on the touch pad on chest at midline for 30 s at the steady pace practiced outside the scanner.
Off task
When prompted on visual monitor to write the real letter a with ball or circle and stick to right, the child repeatedly wrote the letter with wooden stylus, on the touch pad on chest at midline, for 30 s at the steady pace practiced outside the scanner.
Contrasts were modeled in both directions: (a) the newly taught pseudoletter compared to the highly practiced real letter (On Task and Off Task as just described), and (b) the highly practiced real letter compared to the newly taught pseudoletter (On Task just described becomes the Off Task and the Off Task just described becomes the On Task).
Newly taught versus highly practiced contrast
The first contrast identified unique activation for writing a letter form with novel configuration for placement of component strokes compared to writing a familiar letter form. Children may use a strategy in learning to write a new, novel letter form configuration. If children used a naming strategy, it was likely to be less efficient for the newly taught letter form than for the highly practiced letter form. Each of the two components of the newly taught letter form could be named, but no existing single name could be automatically accessed for the whole novel configuration. In contrast, the highly practiced letter form had a corresponding name and phoneme that could be automatically accessed. Letter naming does facilitate automatic letter production (Berninger et al., 1997).
Highly practiced versus newly taught contrast
The tasks were also compared in the opposite direction on the second contrast. This contrast identified BOLD activation unique to writing highly practiced compared to newly taught letter forms, which are not highly practiced.
Imaging protocol
Structural MR scans and fMRI scans for initial group map analyses were acquired on a Philips Achieva 3-T scanner (version 2.1, Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80 mT/m with a slew rate of 110 mT/m/s or 40 mT/m at a slew rate of 220 mT/m/s) using an 8 channel SENSE Head coil. An MPRAGE localizer was acquired in the sagittal plane for structural analysis and fMRI co-registration of anatomy with parameters: TR/TE 7.0/3.2 ms, SENSE Factor = 1.5 in RL direction, 160 slices, and 3D acquisition resolution matrix 224 × 221 × 160 with reconstructed resolution of 0.94 × 0.94 × 1.0 mm, flip angle = 8 degrees, field of view 240 × 240 × 160 mm, and scan duration 449 s.
Functional MRIs were acquired using the following parameters: gradient echo (single shot) echo-planar pulse sequence (called epi field echo by Philips), TR/TE 3000/30 msecs, FOV 240 mm, slice thickness/gap 4.0/1.0, 32 slices covering the entire brain, 2D matrix 64 × 64, epifactor 63, SENSE factor = 1, number of dynamics 114, and scan duration 349 s. A B0 map (Fast Field Echo, TR/TE 935/20 ms, echo difference time of 4 ms for B0map calculation, scan duration 123 s, 32 slices, 64 × 64 reconstructed matrix, FOV 240 mm) was also acquired at exactly the same slice positions as the fMRI image with a B0 correction using FSL software.
fMRI data analysis
Pre-processing
FSL (FEAT Expert Analysis Tool Version 5.4 in FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) was used for the following pre-statistics processing: motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002); non-brain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 5 mm; mean-based intensity normalization of all volumes by the same factor; and highpass temporal filtering (Gaussian-weighted LSF straight line fitting, with sigma = 50.0 s). FEAT also has a feature for B0 correction, which was used for B0 phase and magnitude maps that Philips automatically produces as part of the B0 map image reconstruction (TE difference 4 ms, dwell time = 0.655 μs, +y polarity, input parameters).
First level
Time-series statistical analysis was carried out using FILM (FMRIB’s Improved Linear Model) (Woolrich, Ripley, Brady, & Smith, 2001) in a block-design with local autocorrelation correction. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.01 (Worsley, Evans, Marrett, & Neelin, 1992). Registration to high resolution and/or standard images was carried out using FLIRT (Jenkinson & Smith, 2001; Jenkinson et al., 2002). ICA-based exploratory data analysis using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) (Beckmann & Smith, 2004) was used to investigate the possible presence of unexpected artifacts or activation.
The individual ICA/MELODIC output components were analyzed by custom software to find out which components had large amounts of activation rimness (greater than 0.65). Rimness is defined as the activation that occurs at boundaries of the brain surface or the ventricular walls. These ICA components were considered to be “artefact” that could arise in part from subject motion. The MELODIC filter option was used to filter out the “artifact” components that were identified in the previous step. The output 4D fMRI data were then re-run through FEAT individual-level analyses to find valid activation. Effects at each voxel were estimated; regionally specific effects were compared using linear contrasts.
Group level
The contrasts for the individual subjects were aggregated for the group in a random effects analysis. Higher-level analysis was carried out using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 only (i.e., without the final MCMC-based stage) (Beckmann, Jenkinson, & Smith, 2003; Woolrich et al. 2001). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.05 (Worsley et al., 1992). Effects at each voxel were estimated; regionally-specific effects were compared using linear contrasts.
Results
Learning to write newly taught letter form
Three kinds of results are reported for the first contrast, which identifies unique BOLD activation for learning to write a newly taught, relatively unfamiliar and unpracticed letter form compared to a familiar, highly practiced letter form. Use of the contrast between a pseudoletter and a real letter, equated on component features but varying in how highly practiced the configuration is, may yield insight into vulnerability in handwriting in older students who have had at least 6 years of handwriting practice of some kind from kindergarten to fifth grade.
To begin with, the results for the good and poor writers are reported separately for each of the regions in the whole brain where BOLD activation significantly exceeded threshold values, indicating neural activity in that region unique to writing the newly taught pseudoletter configuration compared to a highly practiced letter form. Note that all the z-values in Table 1 (good writers) and Table 2 (poor writers) are positive and significant. These analyses support conclusions about which brain regions showed significant BOLD activation in each writing ability group and whether the same brain regions were uniquely activated in the good and poor writers for this handwriting contrast. Table 3 offers a summary of the comparison across writing ability groups regarding common and different brain regions showing significant activation on this contrast. In addition, the group maps are compared for the good and poor writers to determine in which brain regions the mean level of BOLD activation was significantly different between the writing ability groups on this contrast. Both kinds of analyses, which yield qualitatively different kinds of information, can be instructive.
Table 1.
fMRI handwriting results for good writers
| Brain region | Ave. Z | MNI X | MNI Y | MNI Z | Brodmann |
|---|---|---|---|---|---|
| L Precentral | 2.621 | −38 | −24 | 64 | 4 |
| L Calcarine | 2.494 | 2 | −84 | −10 | 17 |
| R Lingual | 2.548 | 4 | −78 | −12 | 17 |
| L Postcentral | 2.569 | −38 | −26 | 54 | 4 |
| L Inf Parietal | 2.452 | −54 | −26 | 50 | 3 |
| R Cerebellum 4_5 | 2.559 | 12 | −52 | −24 | 0 |
| R Cerebellum 6 | 2.531 | 8 | −68 | −28 | 0 |
| L Cerebellum 7b | 2.579 | −6 | −72 | −42 | 0 |
| L Cerebellum 8 | 2.595 | −6 | −70 | −40 | 0 |
| R Cerebellum 8 | 2.775 | 6 | −66 | −34 | 0 |
| L Cerebellum | 2.653 | −8 | −56 | −38 | 0 |
| R Cerebellum 9 | 2.746 | 14 | −52 | −40 | 0 |
| Vermis 6 | 2.777 | 4 | −68 | −26 | 0 |
| Vermis 7 | 3.009 | 4 | −68 | −30 | 0 |
| Vermis 8 | 2.866 | 4 | −68 | −34 | 0 |
| Vermis 9 | 2.657 | 8 | −58 | −38 | 0 |
Brain regions within FSL significant clusters for novel letter > familiar letter contrast for good writers (n = 12)
Table 2.
fMRI handwriting results for poor writers
| Brain region | Ave. Z | MNI X | MNI Y | MNI Z | Brodmann |
|---|---|---|---|---|---|
| L Posterior Cingulum | 2.401 | −12 | −52 | 28 | 0 |
| L Calcarine | 2.577 | −4 | −84 | −14 | 17 |
| R Calcarine | 2.553 | 10 | −94 | 8 | 17 |
| L Cuneus | 2.583 | −4 | −80 | 32 | 18 |
| R Cuneus | 2.614 | 12 | −92 | 12 | 18 |
| L Lingual | 2.77 | −20 | −76 | −14 | 18 |
| R Lingual | 2.576 | 2 | −74 | −6 | 17 |
| L Superior Occipital | 2.763 | −14 | −94 | 0 | 18 |
| L Middle Occipital | 2.809 | −12 | −96 | −2 | 18 |
| L Fusiform | 2.748 | −22 | −76 | −16 | 18 |
| L Superior Parietal | 2.522 | −20 | −62 | 38 | 7 |
| L Precuneus | 2.588 | −2 | −80 | 44 | 7 |
| R Precuneus | 2.522 | 2 | −80 | 44 | 0 |
| L Cerebellum Crus1 | 2.698 | −6 | −84 | −18 | 18 |
| R Cerebellum Crus1 | 2.585 | 16 | −72 | −34 | 0 |
| R Cerebellum Crus2 | 2.611 | 6 | −68 | −32 | 0 |
| R Cerebellum 4_5 | 2.823 | 8 | −58 | −24 | 0 |
| L Cerebellum 6 | 2.864 | −6 | −80 | −16 | 18 |
| R Cerebellum 6 | 2.853 | 28 | −46 | −34 | 37 |
| L Cerebellum 8 | 2.508 | −10 | −62 | −42 | 0 |
| R Cerebellum 8 | 2.664 | 16 | −64 | −60 | 0 |
| R Cerebellum 9 | 2.394 | 16 | −44 | −42 | 0 |
| Vermis 4_5 | 2.754 | 6 | −60 | −20 | 18 |
| Vermis 6 | 3.09 | 4 | −62 | −24 | 0 |
| Vermis 7 | 2.996 | 4 | −66 | −28 | 0 |
| Vermis 8 | 2.862 | 4 | −60 | −28 | 0 |
| Vermis 9 | 2.427 | 6 | −56 | −32 | 0 |
Brain regions within FSL significant clusters for novel letter > familar letter contrast for poor writers (n = 8)
Table 3.
Regions in group maps where good writers activate but poor writers do not, poor writers activate but good writers do not, and both good and poor writers activate on newly taught pseudoletter writing > highly practiced real letter contrast
| Good writers activate but poor writers do not | L Precentral |
| L Postcentral | |
| L Inferior Parietal | |
| L Cerebellum 7b | |
| L Cerebellum 9 | |
| Poor writers activate but good writers do not | L Posterior Cingulum |
| R Calcarine | |
| L and R Cuneus | |
| L Lingual | |
| L Superior Occipital | |
| L Middle Occipital | |
| L Fusiform | |
| L Superior Parietal | |
| L, R Precuneus | |
| L, R Cerebellum Crus 1 | |
| R Cerebellum Crus 2 | |
| L Cerebellum 6 | |
| Vermis 4 and 5 | |
| Both good and poor writers activate | L Calcarine |
| R Lingual | |
| R Cerebellum 4 and 5 | |
| R Cerebellum 6 | |
| L and R Cerebellum 8 | |
| R Cerebellum 9 | |
| Vermis 6, 7, 8, 9 |
Good writers
The significant BOLD activation in good writers associated with the newly taught compared to highly practiced letter form writing contrast occurred in the following regions (see Table 1 for quantitative values and, Figs. 1 and 2 for labeled non-midline and midline regions, respectively): On the left, in precentral (frontal), calcarine (occipital), postcentral (parietal), cerebellar, and inferior parietal regions; and on the right, in lingual (temporal), and cerebellar (including vermis at midline) regions. Of note, some regions of significant activation occurred in each cerebral lobe and bilaterally in reference to the right and left cerebral hemispheres.
Fig. 1.
Group map for fMRI activation for handwriting contrast (novel vs. familiar) for good writers (n = 12) showing left and right surface-rendered views of the brain
Fig. 2.
Group map of fMRI activation for handwriting contrast (novel vs. familiar) for good writers (n = 12) showing midline sagittal section of the brain so that midline structures can be seen (vermis, calcarine, lingual)
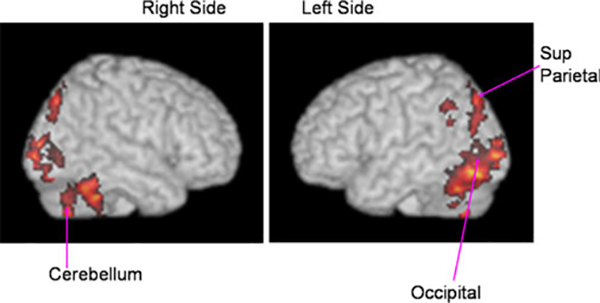
Poor writers
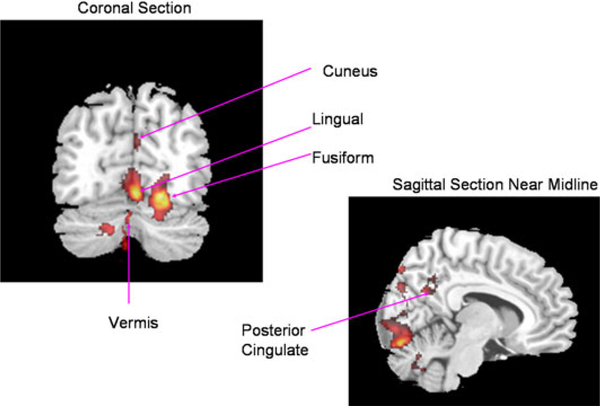
The significant BOLD activation in poor writers associated with the contrast occurred in the following regions (see Table 2 for quantitative values and, Figs. 3 and 4 for midline and non-midline regions, respectively): On the left, in occipital regions including calcarine and cuneus, temporal regions including fusiform and lingual, superior parietal, and posterior cingulate (midline); and on the right, in cuneus and cerebellum including vermis at midline.
Fig. 3.
Group map of fMRI activation for handwriting contrast (novel vs. familiar) for poor writers (n = 8) showing left and right surface-rendered views of the brain
Fig. 4.
Group map of fMRI activation for handwriting task contrast (novel vs. familiar) for poor writers (n = 8) showing coronal and midline sections so that midline structures can be seen (fusiform, lingual, cuneus, posterior cingulate, vermis)
Comparing good and poor writers
Table 3 summarizes the regions where only the good writers activated, regions where only the poor writers activated, and regions where both the good and poor writers activated. Examining which brain regions showed significant activation was instructive. Poor writers activated more brain regions than good writers. Although they activated some of the same brain regions as good writers, differences in which brain regions were activated were also evident. Clearly the good writers and poor writers appear to be orchestrating their distributed neural networks differently on the same contrast for writing a newly taught letter, which probably has not yet been automatized, compared to writing a familiar, highly practiced letter.
Comparison on group maps of the mean levels of BOLD activation across all regions of the brain showed that the writing ability groups did not differ significantly in mean level of BOLD activation in any region. These null results are not reported.
Thus, both kinds of analyses were instructive. Although the good and poor writers did not differ in the mean level of activation in any one brain region, they differed in the number of brain regions activated and which set of brain regions activated. A possible interpretation of the findings is that the poor writers are less efficient than good writers in self-regulation of brain circuitry distributed throughout the brain in practicing the writing of a newly taught letter form: Poor writers may engage more regions and to some extent different regions than do the good writers. If only group maps for the two ability groups had been compared on mean level of BOLD in each brain region, this difference between good and poor writers in which and how many brain regions activated for writing a novel letter would have been missed. At the same time it does not appear that the mean level of BOLD activation differentiates the good and poor writers on this contrast. When the contrast involves differences in strategy application during learning, the brain differences related to neural efficiency may be observed in the spatial extent or number and nature of brain regions activated rather than in the mean level of activation of any one region. Further research is needed on this issue.
Writing highly practiced, familiar real letter forms
Next, we analyzed BOLD activation for the opposite contrast, writing a familiar, highly practiced letter versus a novel pseudoletter. This contrast identifies unique brain activation for writing a familiar, highly practiced, automatic letter compared to writing a novel one that has been hardly practiced. Initially, exact clusters were identified for brain regions that show significant BOLD activation on group maps, which were analyzed separately for good writers and for poor writers and on which good writers and poor writers showed the same and different patterns of BOLD activation (Table 3). In the next step, for these exact clusters, the individual z-scores were averaged for each individual after the brain was co-registered to the standard brain. We have used individual brain analyses in the past to compute correlations for the purpose of identifying and validating brain-behavior relationships in writing (e.g., Richards, Berninger, & Fayol, 2009a; Richards, Berninger, Stock, Altemeier, Trivedi, & Maravilla, 2009b).
After controlling for multiple comparisons on the second contrast for identifying unique brain activation for writing familiar, automatic letters compared to novel letters, based on extent of cluster size as described by Worsley et al. (1992) for corrected cluster significance threshold of p = 0.01, the good and poor writers were compared to find out where they may differ significantly. On this contrast, good and poor writers differed significantly in the individual analyses, based on exact clusters, in left fusiform. Good writers (M = 0.25, SD = 0.49) activated significantly more than the poor writers (M = −1.05, SD = 0.70) in left fusiform, F(1,18) = 23.87, p < 0.00, while writing familiar letters automatically compared to writing novel letters. See Fig. 4, which is based on the first not the second contrast, for location of the fusiform.
Table 4 provides the left fusiform activation values for each individual brain within the groups of good and poor writers. Of the good writers, 9 had positive BOLD and 3 had negative BOLD but of smaller magnitude than 6 of the 8 poor writers. Of the 8 poor writers, 7 had negative BOLD. Thus, the individual brains displayed the same overall pattern as the group means. Negative BOLD activity is not fully understood but one study that combined fMRI and electrophysiology to study primary visual area in monkey brain (V1) found evidence that it may reflect a decrease in neural activity which is influenced by and influences nearby activation in other neural circuits (Shmuel, Augath, Oeltermann, & Logothetis, 2006). Another possible explanation for negative activation is that the control task (writing novel letter) yielded higher activation than the main task (writing familiar letter).
Table 4.
Individual brain BOLD activation within good and poor writers during highly practiced, familiar letter versus newly taught pseudoletter contrast in left fusiform
| Good writers | Poor writers |
|---|---|
| 0.12 | −0.98 |
| 0.01 | −1.4 |
| 0.83 | 0.13 |
| 0.68 | −1.21 |
| −0.41 | −0.87 |
| −0.54 | −0.32 |
| 0.47 | −1.88 |
| 0.70 | −1.86 |
| 0.62 | |
| −0.43 | |
| 0.33 | |
| 0.66 |
Correlations were computed between the individual brain activation values in left fusiform and individual values on the behavioral measures shown to predict writing uniquely in grades 4 to 6 (ages 9 to 12) (Berninger et al., 1994). Left fusiform activation on the contrast for identifying unique activation for the highly practiced automatic letter writing compared to novel letter writing was significantly correlated with two behavioral measures: automatic alphabet letter writing, r = 0.56, p = 0.01; and expressive orthographic coding, r = 0.47, p = 0.04. Prior behavioral research in developing writers had found significant correlations between orthographic coding and automatic alphabet letter writing (Berninger et al., 1992, 1994), indicating that they may be related. The brain-behavioral correlations reported in the current study provide validation for the previous research finding that fusiform gyrus is associated with orthographic coding during middle childhood (e.g., Richards et al., 2009a) and verify that left fusiform activation is also related to handwriting (writing letters), which draws on underlying orthographic representations of letter forms (Abbott & Berninger, 1993).
The multiple regression with the individual left fusiform activation and expressive orthographic coding values accounted for significant variance (32%) in the written composition outcome, F(2,17) = 4.01, p = 0.04. Expressive orthographic coding explained a significant increment in variance, t = 2.11, p = 0.05, over and beyond the fusiform activation in this regression. Prior behavioral research in developing writers had found that orthographic coding explained unique variance in written composition (Berninger et al., 1992, 1994). The earlier finding based only on behavioral measures of writing is replicated even when a brain-related measure is included as a predictor in the regression.
Discussion
Significance of brain activation results for handwriting contrasts
Learning to write new letter forms (configuration of component letter features)
Eleven year-old good and poor writers did not differ in mean level of BOLD activation in specific brain regions on a group map for an fMRI contrast for writing a newly taught letter compared to a familiar, highly practiced one. However, they did differ in the same group map and contrast in spatial extent of activated regions: Poor writers showed significant activation in many more brain regions than did the good writers and showed significant activation in unique regions as well as common ones compared to the good writers. How writers engage their neural circuitry, that is, which brain regions are activated to perform tasks defined by others, is as informative as the level to which they activate discrete regions within the distributed neural networks.
Based on number and nature of the brain regions that were significantly activated (Table 3), the first hypothesis was supported: Good writers were more efficient in that they engaged fewer neural regions to write a newly taught letter than did the poor writers. The component strokes in the novel configuration of the pseudoletter are highly practiced but not in the taught configuration; so the efficiency of production must be related to the overall context or configuration in which those motor strokes are produced. Whereas the highly practiced letter configuration in the familiar letter is more automatic, writing a novel configuration for a pseudoletter may require strategic processing at least initially. See Seitz, Canavan, Yaguez, Herzog, Tellman, Knorr, et al. (1997) for role of automatic and controlled processing in graphomotor functions. Also, different neural regions appear to be specialized for different functions (for review, see Berninger & Richards, 2002). Because activation of brain regions is energy consuming, activating fewer regions may be more efficient in energy requirements than activating more regions. Poor writers may be less efficient in mobilizing their brain resources when they have to apply strategies to learn to write a novel letter. Further research is needed to evaluate whether this finding replicates in other samples that may vary in age and other characteristics and with other tasks to assess the same or similar constructs.
Writing highly practiced letters automatically
Good and poor writers differed significantly in individual BOLD activation in left fusiform, based on an exact cluster of significant BOLD activation, during this second letter writing contrast for identifying unique brain activation associated with writing highly practiced, automatic letters compared to writing novel, not highly practiced letters. One function associated with left fusiform gyrus, which is often referred to as the brain’s Visual Word Form Area (VWFA), is orthographic processing of word-forms (e.g., Cohen et al., 2002; Cohen & Dehaene, 2004; Dehane et al., 2002) and letter-forms (Joseph et al., 2003). Adult good and poor writers have shown differences in left fusiform on handwriting tasks (James & Gauthier, 2006). Richards et al. (2009a) found differences between good and poor child spellers in left fusiform during a long-term spelling contrast. Thus, the second hypothesis was also confirmed: good and poor writers showed significant differences in individual brain activation in left fusiform, a region shown in prior research to be associated with orthographic coding.
Analyzing individual brain activation for the exact clusters allowed testing hypotheses about brain-behavior relationships related to the second contrast. The third hypothesis was also confirmed: Significant correlations occurred between left fusiform and automatic letter writing and between left fusiform and expressive orthographic coding. These findings provide converging validation of brain-behavior relationships between left fusiform and orthographic coding during middle childhood. Both behavioral measures, which have been shown in prior research to explain unique variance in handwriting and composing skills (e.g., Abbott & Berninger, 1993), participate in the orthographic loop that integrates internal written word representations with writing their constituent letters via the hand (and sequential finger movements) in the external environment (see Berninger et al., 2006; Berninger, Raskind, Richards, Abbott, & Stock, 2008; Richards et al., 2009b). Thus, the final hypothesis tested examined whether this brain-behavior relationship between left fusiform and expressive orthographic coding might be relevant for higher-order composing. The fourth hypothesis was also supported in that a set of predictor variables including brain (left fusiform) and behavior (expressive orthographic coding) explained significant variance in written composition. Just as Hoeft et al. (2009) found for reading, both brain and behavior variables contribute to understanding writing.
Significance of the results for writing research
Handwriting
Other research studies have shown that primary motor (precentral gyrus), inferior frontal gyrus, and parietal regions are involved in handwriting. James and Gauthier (2006) identified significant BOLD activation in two left precentral regions on a handwriting task. Hillis, Wityk, Barker and Caramazza (2003) observed that the left posterior inferior frontal gyrus (PIFG) and precentral gyrus (PrG, motor cortex) were essential for handwriting. Sakurai, Onuma, Nakazawa, Ugawa, Momose, Tsuji, et al. (2007) found that the left intraparietal sulcus (IPS) is related to abnormal grapheme formation. Longcamp et al. (2003) reported activation in the left premotor cortex (BA6) during single-letter writing. The current study adds to this research knowledge about the brain basis of handwriting by showing that on a contrast between a pseudoletter writing task and a conventional letter writing task equated in component strokes but not configuration, significant clusters of BOLD activation were found in left precentral gyrus only in good writers and left superior parietal only in poor writers. Left superior parietal region is the only brain region in which gender differences were observed in four brain imaging studies comparing poor and good writers at completion of a longitudinal study of writing: Boys showed less activation than girls on the finger sequencing contrast in this region (Richards et al., 2009b). Lack of differences between good and poor writers in inferior frontal, intraparietal sulcus, and left premotor reported by others may have to do with age of participant (adults), status (normal or acquired writing disorder), or nature of handwriting task; but further research is needed on this issue.
Linguistic recoding of letter forms
In English, letter forms can be verbally recoded into phonology for a single whole word (the name) or word part (e.g., a phoneme that may be in the name or correspond to the letter). For example, the letter a has a name that is associated with the letter form and may be a retrieval cue in letter production but also has sounds associated with it that are used in pronouncing words that contain it. In contrast, each component of a pseudoletter can be named (e.g., circle and line), but the pseudoletter as a whole does not correspond to one name or letter production retrieval cue or one phoneme. Thus, true letters are more likely to have stronger automatic bonds with lexical phonology (a single name for the total configuration) and sublexical phonology (a single phoneme for a word context). These automatic connections to lexical or sublexical phonology of a true alphabet letter may have contributed to the left fusiform activation on the second contrast for highly practiced familiar letters. Fusiform is where orthographic-phonological correspondences begin to be processed (see Berninger & Richards, 2002, for review of the evidence). Alphabetic languages with grapheme-phoneme correspondences may have been invented because writers discovered that written symbols associated with speech sounds require less brain resources and can be produced more efficiently (i.e., requiring activation of fewer brain regions and needing less energy to function).
This link between letter writing and phonological access may explain why children improved in reading real words and orthographic coding when taught word decoding and letter writing in tandem compared to when taught decoding alone without letter writing instruction (Berninger, Dunn, Lin, & Shimada, 2004; Dunn & Miller, 2009). Automatic letter writing, with its automatic access to phonology (lexical names and sublexical phonemes) may also facilitate learning of word spelling. In a sample of low achieving second grade spellers, the poorer handwriters tended to be the poorer spellers before spelling treatment; and handwriting ability was associated with spelling outcome in response to the instructional treatment for spelling (Berninger et al., 1998). Also see Rieben, Ntamakiliro, Gonthier and Fayol (2005), for role of feedback about spelling as children handwrite words. Handwriting (copying) was also related to reading achievement in Chinese (Tan, Spinks, Eden, Perfetti, & Siok, 2005); so the relationship between handwriting and reading (or spelling) may transcend specific orthographies.
Relationship of current study with other brain imaging studies of writing in the same sample
The fMRI study of newly taught and highly practiced letter form writing by hand is the last in a set of four brain imaging studies conducted at the end of a 5-year longitudinal study that focused on the cognitive processes of writing and in which the same sample of well characterized good writers and poor writers participated. Good and poor writers differed during Idea Generation in brain regions associated with working memory as early as idea generation (Berninger, Richards, & Abbott, 2009b; Berninger, Richards, Stock, Abbott, Trivedi, Altemeier, & Hayes, 2009c). Considerable research has documented working memory differences between good and poor child writers (e.g., Olive & Kellogg, 2002; Swanson & Berninger, 1996). Good and poor writers differed in brain activation during storing and processing temporary orthographic representation while learning to spell words; they also differed in long-term memory representations of learned word-specific orthographic representations independent of phonological representations (Richards et al., 2009a). Good and poor writers differed robustly in fMRI activation during a sequential finger tapping contrast in brain regions associated with cognition, executive functions, memory, and language (Richards et al., 2009b). Behavioral measures of handwriting, spelling, and composition reliably predicted individual brain activation on this finger sequencing contrast in five brain regions (right inferior frontal and precuneus, left superior parietal, bilateral inferior temporal) associated with orthographic processing in other imaging studies (Berninger et al., 2009b). As such, these brain regions may be part of the orthographic loop of working memory that supports translation—text generation at the word, sentence, and text levels and transcription at the letter and word levels (Richards et al., 2009a, b).
These brain imaging studies in 11-year-old poor writers cannot be generalized to children with dyslexia. In another series of studies fMRI activation differences were demonstrated between children with and without dyslexia during working memory (Richards, Berninger, Winn, Swanson, Stock, Liang, et al., 2009c) and spelling (Richards, Berninger, Nagy, Parsons, Field, & Richards, 2005; Richards, Aylward, Berninger, Field, Parsons, Richards, & Nagy, 2006). The poor writers who were imaged at the end of the longitudinal study had dysgraphia rather than dyslexia. Dysgraphia is unexpectedly low handwriting and/or spelling. Dyslexia is a disorder in reading (oral reading/decoding and spelling).
Conclusions
Understanding writing development is complex because writing brains are dynamically constructed as brains interact with the environment (Berninger & Richards, 2002; James & Gauthier, 2006). In addition, writing involves many other cognitive processes besides transcription (Alamargot & Chanquoy, 2001; Fayol, 1994, 1999, 2008; Fayol, Jisa, & Mazur-Palandre, 2008; Hayes, 2009; Hayes & Chenoweth, 2006; Hayes & Flower, 1980). However, transcription, including handwriting, is necessary for translating higher-order cognitive processes to spell words and create written text. The brain is a mediating variable during handwriting, spelling, and composing, but other child variables (e.g., interest and motivation) and environmental variables (e.g., instruction) also matter in writing acquisition (Berninger & Richards, 2009). Explicit instruction in letter writing helps developing writers learn to spell words, which are used to communicate ideas and construct the written text to express and elaborate upon ideas (Berninger & Fayol, 2008; Berninger et al., 2009b, c; Hayes, 2009; Hayes & Berninger, 2009). Handwriting is not merely a mechanical, motor skill, but rather a brain-based skill that facilitates meaning-making as writers externalize their cognitions through letter forms, the building blocks of written words and text.
Acknowledgments
The authors thank Jeff Stevenson for his technical help in developing the MR imaging protocols used on the Philips Achieva 3T Scanner. Grants HD 25858 and P50 33812 from the National Institute of Child Health and Human Development (NICHD) supported this research.
Contributor Information
Todd L. Richards, Department of Radiology, University of Washington, Box 357115, Seattle, WA 98195, USA
Virginia W. Berninger, Department of Educational Psychology, University of Washington, Box 353600, Seattle, WA 98195, USA
Pat Stock, Department of Educational Psychology, University of Washington, Box 353600, Seattle, WA 98195, USA.
Leah Altemeier, Department of Educational Psychology, University of Washington, Box 353600, Seattle, WA 98195, USA.
Pamala Trivedi, Department of Educational Psychology, University of Washington, Box 353600, Seattle, WA 98195, USA.
Kenneth R. Maravilla, Department of Radiology, University of Washington, Box 357115, Seattle, WA 98195, USA
References
- Abbott R, & Berninger V (1993). Structural equation modeling of relationships among developmental skills and writing skills in primary and intermediate grade writers. Journal of Educational Psychology, 85(3), 478–508. [Google Scholar]
- Alamargot D, & Chanquoy L (2001). Through the models of writing. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- Alamargot D, Chesnet D, Dansac C, & Ros C (2006). Eye and pen: A new device for studying reading during writing. Behavior Research Methods, 38(2), 287–299. [DOI] [PubMed] [Google Scholar]
- Basso A, Taborelli A, & Vignolo L (1978). Dissociated disorders of speaking and writing in aphasia. Journal of Neurology, Neurosurgery and Psychiatry, 41, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, & Smith S (2003). General multi-level linear modelling for group analysis in FMRI. Neuroimage, 20, 1052–1063. [DOI] [PubMed] [Google Scholar]
- Beckmann C, & Smith S (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–152. [DOI] [PubMed] [Google Scholar]
- Berninger V (1987). Global, component, and serial processing of printed words in beginning readers. Journal of Experimental Child Psychology, 43, 387–418. [Google Scholar]
- Berninger V (2001). Process assessment of the learner (PAL) test battery for reading and writing. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Berninger V (2007). Process assessment of the learner, 2nd edition. Diagnostic for reading and writing (PAL-II RW). San Antonio, TX: The Psychological Corporation/Pearson. [Google Scholar]
- Berninger V, Abbott R, Augsburger A, & Garcia N (2009a). Comparison of pen and keyboard transcription modes in children with and without learning disabilities affecting transcription. Learning Disability Quarterly, 32, 123–141. [Google Scholar]
- Berninger V, Abbott R, Rogan L, Reed E, Abbott S, Brooks A, et al. (1998). Teaching spelling to children with specific learning disabilities: The mind’s ear and eye beat the computer or pencil. Learning Disability Quarterly, 21, 106–122. [Google Scholar]
- Berninger V, Abbott R, Whitaker D, Sylvester L, & Nolen S (1995). Integrating low-level skills and high-level skills in treatment protocols for writing disabilities. Learning Disability Quarterly, 18, 293–309. [Google Scholar]
- Berninger V, Cartwright A, Yates C, Swanson HL, & Abbott R (1994). Developmental skills related to writing and reading acquisition in the intermediate grades: Shared and unique variance. Reading and Writing: An Interdisciplinary Journal, 6, 161–196. [Google Scholar]
- Berninger V, Dunn A, Lin S, & Shimada S (2004). School evolution: Scientist-practitioner educators creating optimal learning environments for ALL students. Journal of Learning Disabilities, 37, 500–508. [DOI] [PubMed] [Google Scholar]
- Berninger V, & Fayol M (2008). Why spelling is important and how to teach it effectively. http://www.literacyencyclopedia.ca/On-Line Encyclopedia of Language and Literacy Development. National Centres for Excellence Canadian Language and Literacy Research Network (CLLRNet). (Published online: 2008–01-22 14:57:52).
- Berninger V, & Fuller F (1992). Gender differences in orthographic, verbal, and compositional fluency: Implications for diagnosis of writing disabilities in primary grade children. Journal of School Psychology, 30, 363–382. [Google Scholar]
- Berninger V, Raskind W, Richards T, Abbott R, & Stock P (2008). A multidisciplinary approach to understanding developmental dyslexia within working-memory architecture: Genotypes, phenotypes, brain, and instruction. Developmental Neuropsychology, 33, 707–744. [DOI] [PubMed] [Google Scholar]
- Berninger V, & Richards T (2002). Brain literacy for educators and psychologists. New York: Academic Press. [Google Scholar]
- Berninger V, & Richards T (2009). Brain and learning In Anderman E & Anderman L (Eds.), Psychology of classroom learning: An encyclopedia (Vol. 1, pp. 15–22). Detroit: Macmillan Reference USA. [Google Scholar]
- Berninger V, Richards T, & Abbott R (2009b). The role of the hand in written idea expression. Proceedings for Writing in all its states. In Alamargot D, Bouchand J, Lambert E, Millogo V, & Beaudet C (Eds.), Proceedings of the international conference « de la France au Québec: l’Ecriture dans tous ses états », University of Poitiers, France, 12–15 November 2008 http://www.poitoucharentes.iufm.fr/spip.php?article1048. [Google Scholar]
- Berninger V, Richards T, Stock P, Abbott R, Trivedi P, Altemeier L, et al. (2009c). fMRI activation related to nature of ideas generated and differences between good and poor writers during idea generation. British Journal of Educational Psychology Monograph Series II, 6, 77–93. [Google Scholar]
- Berninger V, & Rutberg J (1992). Relationship of finger function to beginning writing: Application to diagnosis of writing disabilities. Developmental Medicine and Child Neurology, 34, 198–215. [DOI] [PubMed] [Google Scholar]
- Berninger V, Rutberg J, Abbott R, Garcia N, Anderson-Youngstrom M, Brooks A, et al. (2006). Tier 1 and Tier 2 early intervention for handwriting and composing. Journal of School Psychology, 44, 3–30. [Google Scholar]
- Berninger V, Vaughan K, Abbott R, Abbott S, Brooks A, Rogan L, et al. (1997). Treatment of handwriting fluency problems in beginning writing: Transfer from handwriting to composition. Journal of Educational Psychology, 89, 652–666. [Google Scholar]
- Berninger V, Yates C, Cartwright A, Rutberg J, Remy E, & Abbott R (1992). Lower-level developmental skills in beginning writing. Reading and Writing: An Interdisciplinary Journal, 4, 257–280. [Google Scholar]
- Berninger V, Yates C, & Lester K (1991). Multiple orthographic codes in acquisition of reading and writing skills. Reading and Writing: An Interdisciplinary Journal, 3, 115–149. [Google Scholar]
- Brain L (1967). Speech disorders: Aphasia, apraxia, and agnosia. London: Butterworth. [Google Scholar]
- Cohen L, & Dehaene S (2004). Specialization within the ventral stream: The case for the visual word form area. Neuroimage, 22, 466–476. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chhochon F, Lemer C, Rivaud S, & Dehaene S (2002). Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain, 125, 1054–1069. [DOI] [PubMed] [Google Scholar]
- Connelly V, Campbell S, MacLean M, & Barnes J (2006). Contribution of lower-order skills to the written composition of college students with and without dyslexia. Developmental Neuropsychology, 29, 175–196. [DOI] [PubMed] [Google Scholar]
- Dehane S, Le Clec’H G, Poline J-B, Bihan D, & Cohen L (2002). The visual word form area: A prelexical representation of visual words in the fusiform gyrus. Brain Imaging, 13, 321–325. [DOI] [PubMed] [Google Scholar]
- Demie F (2001). Ethnic and gender differences in educational achievement and implications for school improvement strategies. Educational Research, 43, 91–106. [Google Scholar]
- Dunn A, & Miller D (2009). Who can speak for the children? Implementing research-based practices in an urban school district In Rosenfield S & Berninger V (Eds.), Implementing evidence-based interventions in school settings (pp. 385–414). New York: Oxford University Press. [Google Scholar]
- Fayol M (1994). From declarative and procedural knowledge to the management of declarative and procedural knowledge. European Journal of Psychology of Education, 9, 179–190. [Google Scholar]
- Fayol M (1999). From on-line management problems to strategies in written production In Torrance M & Jefferey G (Eds.), The cognitive demands of writing. Processing capacity and working memory effects in text production (pp. 13–23). Amsterdam: Amsterdam University Press. [Google Scholar]
- Fayol M (2008). Toward a dynamic conception of written production. In Presentation at the Santa Barbara conference on writing research: Writing research across borders Santa Barbara, CA. [Google Scholar]
- Fayol M, Jisa H, & Mazur-Palandre A (2008). Information flow in written and spoken French: A developmental perspective.In Presentation at the Santa Barbara conference on writing research: Writing research across borders Santa Barbara, CA. [Google Scholar]
- Garner WR (1974). The processing of information and structure. Potomac, MD: Erlbaum. [Google Scholar]
- Garner WR (1976). Interaction of stimulus dimensions in concept and choice processes. Cognitive Psychology, 8, 98–123. [Google Scholar]
- Garner WR (1981). The role of configuration in the identification of visually degraded words. Memory & Cognition, 9, 445–452. [DOI] [PubMed] [Google Scholar]
- Garner WR, Hake HW, & Eriksen CW (1956). Operationism and the concept of perception. Psychological Review, 63, 149–159. [DOI] [PubMed] [Google Scholar]
- Graham S, Berninger V, Abbott R, Abbott S, & Whitaker D (1997). The role of mechanics in composing of elementary school students: A new methodological approach. Journal of Educational Psychology, 89, 170–182. [Google Scholar]
- Graham S, Berninger V, & Weintraub N (1998). The relationship of handwriting style and speed and legibility. Journal of Educational Research, 91, 290–296. [Google Scholar]
- Graham S, Harris K, & Fink B (2000). Is handwriting causally related to learning to write? Treatment of handwriting problems in beginning writers. Journal of Educational Psychology, 92, 620–633. [Google Scholar]
- Graham S, & Weintraub N (1996). A review of handwriting research: Progress and prospects from 1980 to 1994. Educational Psychology Review, 8, 7–87. [Google Scholar]
- Hayes JR (2009). From idea to text. In Beard R, Myhill D, Nystrand M, & Riley J (Eds.), SAGE handbook of writing development (in press). [Google Scholar]
- Hayes JR, & Berninger V (2009). Relationships between idea generation and transcription: How act of writing shapes what children write In Braverman C, Krut R, Lunsford K, McLeod S, Null S, Rogers P, & Stansell A (Eds.), Traditions of writing research. New York: Routledge; (in press). [Google Scholar]
- Hayes JR, & Chenoweth N (2006). Is working memory involved in the transcribing and editing of texts? Written Communication, 23, 135–149. [Google Scholar]
- Hayes JR, & Flower LS (1980). Identifying the organization of writing processes In Gregg LW & Steinbert ER (Eds.), Cognitive processes in writing (pp. 3–30). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, & Caramazza A (2003). Neural regions essential for writing verbs. Nature Neuroscience, 6, 19–20. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover G, et al. (2009). Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behavioral Neuroscience (in press). [DOI] [PubMed] [Google Scholar]
- James KH, & Gauthier I (2006). Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia, 44, 2937–2949. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Jones D (2004). Automaticity of the transcription process in the production of written text. Unpublished doctoral dissertation, Graduate School of Education, University of Queensland, Australia. [Google Scholar]
- Jones D, & Cristensen C (1999). The relationship between automaticity in handwriting and students’ ability to generate written text. Journal of Educational Psychology, 91, 44–49. [Google Scholar]
- Joseph J, Gathers A, & Piper G (2003). Shared and dissociated cortical regions for object and letter processing. Cognitive Brain Research, 17, 56–67. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Anton JL, Roth M, & Velay JL (2003). Visual presentation of single letters activates a premotor area involved in writing. Neuroimage, 19, 1492–1500. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Boucard C, Gilhodes J-C, Anton J-L, Roth M, Nazarian B, et al. (2008). Learning through hand- or typewriting influences visual recognition of new graphic shapes: Behavioral and functional imaging evidence. Journal of Cognitive Neuroscience, 20, 802–815. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Zerbato-Puodou M, Velay JL (2005). The influence of writing practice on letter recognition in preschool children: A comparison between handwriting and typing. Acta Psychologia, 119, 67–79. [DOI] [PubMed] [Google Scholar]
- Martin D, & Hoover H (1987). Sex differences in educational achievement: A longitudinal study. Journal of Early Adolescence, 7, 65–83. [Google Scholar]
- Olive T, & Kellogg R (2002). Concurrent activation of high- and low-level production processes in written composition. Memory & Cognition, 30, 594–600. [DOI] [PubMed] [Google Scholar]
- Peverley S (2006). The importance of handwriting speed in adult writing. Implications for automaticity and working memory. Developmental Neuropsychology, 29, 197–216. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. (2002). Wechsler individual achievement test (2nd ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Richards T, Aylward E, Berninger V, Field K, Parsons A, Richards A, et al. (2006). Individual fMRI activation in orthographic mapping and morpheme mapping after orthographic or morphological spelling treatment in child dyslexics. Journal of Neurolinguistics, 19, 56–86. [Google Scholar]
- Richards T, Berninger V, & Fayol M (2009a). FMRI activation differences between 11-year-old good and poor spellers’ access in working memory to temporary and long-term orthographic representations. Journal of Neurolinguistics, 22, 327–353. doi: 10.1016/j.jneuroling.2008.11.002. [DOI] [Google Scholar]
- Richards T, Berninger V, Nagy W, Parsons A, Field K, & Richards A (2005). Brain activation during language task contrasts in children with and without dyslexia: Inferring mapping processes and assessing response to spelling instruction. Educational and Child Psychology, 22(2), 62–80. [Google Scholar]
- Richards T, Berninger V, Stock P, Altemeier L, Trivedi P, & Maravilla K (2009b). fMRI sequential-finger movement activation differentiating good and poor writers. Journal of Clinical and Experimental Neuropsychology, 29, 1–17. doi: 10.1080/13803390902780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Berninger V, Winn W, Swanson HL, Stock P, Liang O, et al. (2009c). Differences in fMRI activation between children with and without spelling disability on 2-back/0-back working memory contrast. Journal of Writing Research, 1(2), 93–123. (Journal of Writing Research is an open access peer reviewed journal available online: Download the articles from the JOWR-website). [Google Scholar]
- Rieben L, Ntamakiliro L, Gonthier B, & Fayol M (2005). Effects of various early writing practices on reading and spelling. Scientific Studies of Reading, 9, 145–166. [Google Scholar]
- Sakurai Y, Onuma Y, Nakazawa G, Ugawa Y, Momose T, Tsuji S, et al. (2007). Parietal dysgraphia: Characterization of abnormal writing stroke sequences, character formation, and character recall. Behavioral Neurology, 18, 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R, Canavan A, Yaguez L, Herzog H, Tellman L, Knorr U, et al. (1997). Representations of graphomotor trajectories in the human parietal cortex: Evidence for controlled processing and automatic performance. European Journal Neuroscience, 9, 378–389. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, & Logothetis N (2006). Negative functional MRI response correlates with decreases in neuronal activity in moneky visual area V1. Nature Neuroscience, 9, 569–577. [DOI] [PubMed] [Google Scholar]
- Smith S (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson HL, & Berninger V (1996). Individual differences in children’s working memory and writing skills. Journal of Experimental Child Psychology, 63, 358–385. [DOI] [PubMed] [Google Scholar]
- Tan L, Spinks J, Eden G, Perfetti C, & Siok W (2005). Reading depends on writing, in Chinese. Proceedings of the National Academy of Sciences, 102, 8781–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler intelligence scale for children, 4th edition (WISC IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage, 14, 1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, & Neelin P (1992). A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism, 12, 900–918. [DOI] [PubMed] [Google Scholar]