Abstract
Left ventricular assist devices (LVADs) improve survival and quality of life in patients with advanced heart failure (HF). Despite these benefits, combined post- and pre-capillary PH (CPC-PH) can be particularly problematic in patients on LVAD support, often exacerbating right ventricular (RV) dysfunction. Both persistently elevated pulmonary vascular resistance (PVR) and RV dysfunction are associated with adverse outcomes including death after LVAD. These observations have led to significant interest in the use of pulmonary vasodilators to treat PH and preserve RV function among LVAD-supported patients. While pulmonary vasodilators are commonly utilized for the treatment of PH and RV dysfunction in LVADs, the benefits of this practice remain unclear. The purpose of this review is to highlight the current challenges in managing pulmonary vascular disease and RV dysfunction in HF patients on LVAD support.
Keywords: Heart Failure, Pulmonary Hypertension, LVAD
Left ventricular assist devices (LVADs) are increasingly being used for the management of select patients with advanced heart failure (HF). Although survival and quality of life are generally improved with this therapy, elevated pulmonary vascular resistance (PVR) and/or right ventricular (RV) dysfunction can limit these benefits. Up to 25% of patients with HF and reduced ejection fraction (HFrEF) will have combined post- and pre-capillary PH (CPC-PH) or “mixed” PH, which can be particularly problematic in patients considered for left sided mechanical support or heart transplant.1–4 Despite the importance of CPC-PH in advanced HF, controversy remains regarding the best treatment approach.
Although limited data support the medical treatment of PH in advanced HF, several case series suggest that pharmacologic or LVAD therapy can improve PVR and facilitate heart transplantation in patients with CPC-PH.5–17 While LVADs may improve secondary PH through sustained lowering of left sided cardiac filling pressures, a subset of patients are left with persistent PH and RV dysfunction, both of which are associated with adverse outcomes.18–24 These observations have led to significant interest in the use of pulmonary vasodilators to treat PH and preserve RV function among patients supported with LVADs.
To date, several small studies have suggested benefit of phosphodiesterase V (PDE-5) inhibitors on hemodynamic and echocardiographic measures of RV function in LVAD-supported patients.20,25–30 While these observations have fueled the use of pulmonary vasodilators for the treatment of PH and RV dysfunction in LVADs, the benefits of this practice remain unclear. The purpose of this review is to highlight the current challenges in managing the dangerous dyad of CPC-PH and RV dysfunction in HF patients on LVAD support. To facilitate this discussion, we will review the pathogenesis of CPC-PH and the clinical data addressing the role of pulmonary vasodilators in HF patients, with a focus on patients receiving LVAD support. The relevant outstanding questions and controversies surrounding the management of CPC-PH in LVAD patients will be highlighted, and future clinical trials of pulmonary vasodilator therapy will be discussed.
Pulmonary Hypertension and Left Heart Disease
Left heart disease is the most common cause of pulmonary hypertension (PH) worldwide. Secondary PH due to left heart disease (WHO Group 2 PH) is defined as the presence of mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg and pulmonary capillary wedge pressure (PCWP) ≥ 15 mmHg in the presence of left heart disease. PH occurs in as many as 80% of patients with advanced HF and is associated with adverse clinical outcomes including diminished exercise capacity, decreased quality of life and increased mortality.31–37 Efforts have been made to distinguish CPC-PH from isolated post-capillary PH (IPC-PH), or passive PH, on the grounds that the former identifies a high-risk subpopulation in which pulmonary vascular remodeling has occurred.36,38 Unlike those with IPC-PH, patients with CPC-PH often are left with persistent PH despite therapeutic lowering of left sided filling pressures, which has implications for clinical outcomes after LVAD or heart transplant. This paradigm implies distinct pathophysiologic mechanisms underlying CPC-PH and suggests novel therapeutic targets.
Although the pathogenesis of CPC-PH is not well understood, it has been suggested that high PVR in these patients results from either structural or functional vascular remodeling. Chronic elevation of left sided filling pressures is thought to trigger pulmonary vascular pathology through mechanical stress in the pulmonary venous system, leading to enhanced endothelin-1 (ET-1) expression, decreased nitric oxide (NO) availability and subsequent arterial remodeling.39–46 Unfortunately, biomarkers, genetic analysis and imaging studies that specifically identify vascular remodeling have not been adapted in practice, leaving clinical determination of CPC-PH to less specific hemodynamic surrogates.
Studies have used a PVR>3 WU and/or TPG>12–15 mmHg to identify patients with pulmonary vascular pathology, as these thresholds are associated with increased mortality.35–38 More specific measures of CPC-PH that are less dependent on cardiac output and filling pressures include diminished PA compliance (PAC) and elevated diastolic pressure gradient (DPG; PAd – PCWP > 7 mmHg), both of which predict mortality among patients with HFrEF and PH.38,47 Low indexed PAC has also been shown to predict RV failure and mortality after LVAD.48 Current consensus defines CPC-PH as Group 2 PH with a DPG>7 mmHg and/or a PVR>3 WU.
Novel approaches are being utilized to better characterize the physiologic and genetic basis of CPC-PH. A recent investigation found that patients with CPC-PH have an increased frequency of gene polymorphisms related to biologic pathways implicated in WHO Group 1 PH (PAH).49 These findings suggest a continuum between PAH and IPC-PH with CPC-PH espousing features of both, and may support a potential role of pulmonary vasodilators in these patients. Moreover, this data argues that adverse pulmonary vascular remodeling in patients with HF may be determined by the interaction between genetic factors and elevated left heart filling pressures. Importantly, CPC-PH may also represent the confluence of IPC-PH and either PAH, intrinsic lung disease/sleep apnea, or chronic thromboembolic disease. Therefore, appropriate screening for alternative causes of elevated PVR in HF patients with CPC-PH is necessary to ensure appropriate therapy.
Treatment of Pulmonary Hypertension in Left Heart Disease
The principle tenet of management for patients with Group 2 PH is to target the passive component of PH through maintenance of a low PCWP. While pulmonary vasodilators may have a role in reversing adverse pulmonary vascular remodeling, there are concerns that these medications may overcome appropriate adaptive changes in the pulmonary vasculature that prevent increased left sided venous return and pulmonary edema.
Despite these concerns, several studies in HFrEF patients have demonstrated favorable short-term hemodynamic effects with pulmonary vasodilators. Sildenafil has been shown to acutely lower mPAP, PVR and SVR while increasing cardiac output in patients with HFrEF and PH, with more robust reductions in PVR and SVR and enhanced cardiac output with co-administration of nitric oxide (NO).25 Short-term epoprostenol has also been shown to reduce PVR, SVR and PCWP in HFrEF while improving cardiac output.50 Also, selective endothelin receptor antagonists (ERAs) have been shown to acutely reduce mPAP and PVR without worsening left sided filling pressures.51,52
Further, longitudinal non-randomized studies have demonstrated beneficial effects of sildenafil on PA pressures, PVR and cardiac output as well as exercise capacity, peak VO2 and patient-reported measures of breathlessness, fatigue and emotional functioning in patients with HFrEF and PH.27–30 Low-dose bosentan has also been associated with improvements in PVR, TPG and survival among patients with CPC-PH on the transplant waiting list, findings which may have implications in patients on LVAD support.53
Large randomized clinical trials have failed to replicate encouraging findings during long-term follow-up (Table 1).50,54–61 Sildenafil did not improve VO2 max or exercise capacity in HFpEF, even among those with PH, and adversely affected renal function, NT-pro-BNP and ET-1 levels.60 Another important investigation of tadalafil among patients with HFrEF and secondary PH was terminated due to enrollment difficulties, limiting the randomized patient experience with PDE-5 inhibitors ( NCT01910389). The soluble guanylate cyclase stimulator riociguat has also been studied in patients with HFrEF and PH, and although the study failed to meet its primary endpoint of reduction in mPAP, improvements in cardiac index and PVR were demonstrated.61 An ongoing investigation of of vericiguat in a similar patient population should shed further light on the role of these medications ( NCT02861534). ERAs have had neutral to harmful effects on clinical outcomes in patients with PH and left heart disease.54–57 Finally, an investigation of long-term epoprostenol use in HFrEF was abandoned due to a trend toward increased mortality (Table 1).50 Lack of clinical benefit with pulmonary vasodilators in HF may be linked to study design limitations including suboptimal target populations (no studies targeting HFrEF and CPC-PH) and inadequate volume status optimization.
Table 1.
Completed Longitudinal Randomized-Controlled Trials of Pulmonary Vasodilators in Patients with Heart Failure
| Study (Year) | Author | Drug | Inclusion | PH (%) | Subjects | Study Duration | Primary Outcome | Results |
|---|---|---|---|---|---|---|---|---|
| FIRST (1996)50 | Califf, et al. | epoprostenol | HFrEF, NYHA IIIB/IV | NR | n=471 | >36 weeks | Survival | Early termination for decreased survival |
| HEAT (2002)54 | Luscher, et al. | darusentan | HFrEF, NYHA III | NR | n=179 | 3 weeks | Hemodynamics (CO, PCWP) | Increased cardiac output, no change in PAP |
| EARTH (2004)55 | Anand, et al. | darusentan | HFrEF, NYHA IIIB/IV | NR | n=642 | 24 weeks | Change in LVESV (MRI) | No benefit |
| ENABLE (2002)56 | Kalra, et al. | bosentan (low-dose)* | HFrEF, NYHA IIIB/IV | NR | n=1613 | 18 months | Mortality, Hospitalization | No benefit, increased early HF |
| REACH-1 (2005)57 | Packer, et al. | bosentan (high-dose)** | HFrEF, NYHA III/IV | NR | n=174 | 26 weeks | Change in clinical state | Early termination for fluid retention, elevated LFTs |
| Guazzi (2007)58 | Guazzi, et al. | sildenafil | HFrEF, NYHA II/III | NR | n=46 | 24 weeks | Peak VO2 | Improved exercise performance, breathlessness |
| Guazzi (2011)59 | Guazzi, et al. | sildenafil | PH due to HFpEF | 100% | n=44 | 12 months | PA pressure/RV function | Improved PAP, RV function |
| RELAX (2013)60 | Redfield, et al. | sildenafil | HFpEF, NYHA II-IV | 53% | n=216 | 24 weeks | Change in peak O2 consumption | No benefit |
| LEPHT (2013)61 | Bonderman, et al. | riociguat | PH due to HFrEF | 100% | n=201 | 16 weeks | Change in mPAP | No change in PAP, decrease in PVR, increase CI |
Data taken from Fang et al, JHLT (2012)32.
Ongoing Trials – Sil-HF HFrEF/PH, BID=twice daily, CI=cardiac index, CO=cardiac output, HF=heart failure, HFpEF=heart failure with preserved ejection fraction, HFrEF=heart failure with reduced ejection fraction, LFTs=liver function tests, LHD=left heart disease, LVESV=left ventricular end systolic volume, NR=not reported, NYHA=New York Heart Association functional class, PAP=pulmonary artery pressure, PCWP=pulmonary capillary wedge pressure, PH=pulmonary hypertension, RV=right ventricle
125 mg BID
500 mg BID
A more specifically targeted study has completed enrollment and aims to assess the safety and tolerability of the ERA macitentan vs. placebo in patients with CPC-PH, paving the way for further studies to assess therapeutic benefit ( NCT02070991). In the meantime, the substantial cost of therapy and potential risks remain important concerns that argue against widespread use of pulmonary vasodilators in the absence of clear clinical benefit. Further, the lack of an efficacy signal in large-scale clinical investigations has implications for the application of these medications to patients on LVAD support.
Reduction in Pulmonary Hypertension with Left Ventricular Assist Devices
LVADs have emerged as an effective modality to improve PH in end-stage HF. Several studies have highlighted substantial improvements in both mPAP and PVR in patients supported with LVADs, allowing for successful heart transplantation in many previously unsuitable candidates with “fixed” PH.6–14 The effects of LVAD support on mPAP and PVR can be seen within days and sustained for several months.6–10,15,16 However, the absence of core laboratory adjudication of pre- and post-LVAD hemodynamics is a major limitation of these studies. Further, use of oral pulmonary vasodilators has often been omitted from reported results, leaving the relative benefits of medications on top of LVAD support unclear.
Persistent Pulmonary Hypertension after LVAD
While LV unloading with LVAD support leads to improvements in the passive components of PH and often decreases PVR owing to enhanced cardiac output and improved PAC, many patients on LVAD support are left with persistent PH.18–20 Tedford et al reported that over 40% of patients had persistent PH with a PVR>3 WU at 1–2 weeks post-LVAD with several patients having a PVR in excess of 5 WU.20 More recently it was shown that among stable outpatients on LVAD support, mPAP was 25.4±8.1 mmHg, (mTPG=11.6 mmHg, mPVR=2.7 WU), despite reasonable LV unloading (mPCWP 13.8 mmHg), indicating persistent PH may occur in a significant subset of patients on LVAD support.19
RV-PA Coupling after LVAD
RV dysfunction and RV failure are important determinants of both short and long-term outcomes after LVAD. Using the INTERMACS definition, RV failure occurs in 20–40% of patients after LVAD.22,24,62,63 Post-LVAD RV failure is associated with post-operative morbidity, short and long-term mortality, increased hospital length-of-stay and lower rates of successful bridging to heart transplant.21–24 Importantly, RV failure is not restricted to the perioperative period with >10% of patients developing late RV failure, which is also associated with poor prognosis.63
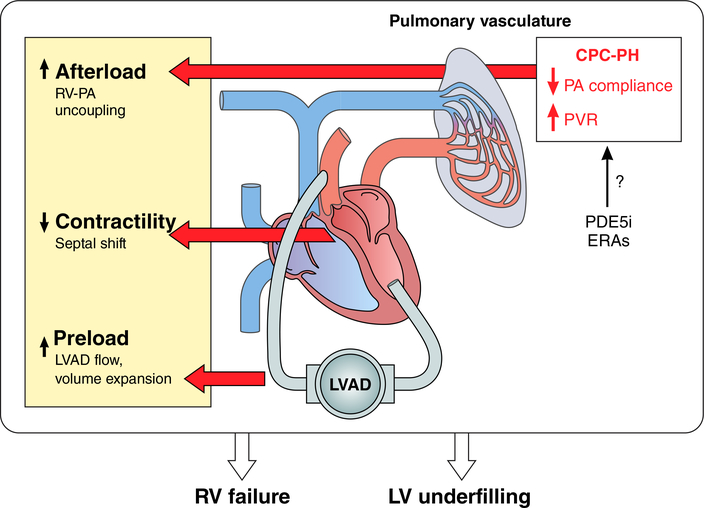
The RV and pulmonary vasculature should be considered as one coupled unit upstream of the LVAD. It is now appreciated that LVAD implantation itself impacts this unit at many levels that are relevant to clinical management. Immediately following LVAD implantation, a marked increase in systemic venous return due to enhanced cardiac output elevates RV preload. RV preload stress may be further exacerbated by perioperative volume loading with intravenous fluids and blood products. As a result, RV work must dramatically increase to maintain cardiac output. LVAD insertion can also produce unfavorable geometric changes that may worsen RV performance. The RV relies heavily on the LV contribution to septal function and contractility, a reliance that is enhanced in the presence of severe RV dysfunction or PH.64–66 During LVAD support, the combination of decreased LV contractility and increased RV free wall to septal distance due to LV unloading reduces RV contractility.66 When RV contractility is impaired and RV preload is elevated, RV work can only be maintained through a reduction in RV afterload. In general, LVAD support will decrease RV afterload owing to a lower PCWP, improved PAC, and often, a fall in PA pressure; however, the presence of CPC-PH can cause persistent RV afterload stress, precipitating RV failure. This is particularly relevant in light of recent data indicating that the RV is more sensitive to afterload stress early after LVAD implant.67 The unique interplay between PH and RV dysfunction in patients on LVAD support highlights the importance of RV afterload reduction as a strategy to improve clinical outcomes (Figure 1).
Figure 1. Interplay between LVAD support, CPC-PH and right ventricular function.
With LVAD unloading, left sided filling pressures decrease and systemic venous return is augmented, which together with the infusion of blood products and crystalloid can increase right ventricular (RV) preload. As the pre-capillary component of PH can persist after normalization of LV filling pressures in those with CPC-PH, the RV also faces a pulmonary vasculature that is less compliant and has a greater resistance. These factors augment RV afterload. LV unloading will also shift septal geometry which can impair RV contractility. The combination of preload stress, increased afterload, and decreased contractility increases the risk of clinical RV failure and the attendant consequences, including persistent heart failure, hepatic congestion, GI bleeding, renal dysfunction, and increased mortality. At the same time, reduced RV-PA coupling leads to LV underfilling which can result in suck down events, ventricular arrhythmias, and pre-syncope/syncope. In patients with CPC-PH, pulmonary vasodilators such as PDE-5 inhibitors or endothelin receptor antagonists (ERAs) may reduce pulmonary vascular resistance and increase PA compliance leading to an improvement in RV-PA coupling. Although unproven, this approach may reduce complications association with impaired RV function in LVAD supported patients.
Use of Pulmonary Vasodilators in the Post-operative Setting After LVAD
In the immediate post-LVAD setting, RV function is optimized by enhancing RV contractility and reducing RV afterload to maintain RV-PA coupling. The former is accomplished through the use of both inotropic support, and if necessary, right ventricular assist device (RVAD) placement. Given the poor outcomes associated with RV failure, additional pharmacologic measures, such as pulmonary vasodilators, are frequently employed to facilitate early weaning of both RVAD and inotropic support.
Use of pulmonary vasodilators in the immediate post-operative setting has gained favor to reduce RV afterload. Milrinone, a PDE-3 inhibitor frequently employed after LVAD for both its positive inotropic effects and vasodilatory properties, has been shown to reduce mPAP after LVAD.68 Inhaled NO has also been demonstrated to reduce mPAP and improve LVAD flow in patients with persistently elevated PVR.69 Sildenafil use in the early post-LVAD period is also associated with rapid decreases in PA pressures and may facilitate weaning from inhaled NO and inotropic support.70,71 Based on several small studies suggesting safety and efficacy and clinical experience supporting use, pulmonary vasodilators are commonly utilized in the short-term management of post-LVAD patients.
Use of Pulmonary Vasodilators During Long-Term Support with LVADs
The beneficial effects seen with pulmonary vasodilators in the short-term have been extrapolated to long-term management of RV dysfunction and persistent PH in clinical practice. ISHLT guidelines recommend treating persistent PH and RV dysfunction after LVAD with PDE-5 inhibitors with the caveat that the benefit of this strategy has not been sufficiently proven.72 As a result, significant heterogeneity exists among LVAD centers in the use of pulmonary vasodilators. The goals of PH treatment in LVAD patients are threefold: 1) Reduction of PVR in patients awaiting heart transplant. 2) Improvement of symptoms and morbidity associated with chronic RV dysfunction. 3) Prevention of late RV failure and its complications.
Reduction of PVR in patients awaiting orthotopic heart transplantation
ISHLT listing criteria suggest PVR > 5 WU or inability to reduce PVR to < 2.5 WU with vasodilator challenge without significant hypotension as relative contraindications to heart transplantation due to risk of early graft dysfunction and mortality related to RV failure.1,4 Numerous studies support the use of LVADs to unload the LV and lower PVR to allow for successful transplantation.5–17 Unfortunately, many of these studies did not report pulmonary vasodilator use, leaving the role of these medications in improving pulmonary hemodynamics uncertain. In small studies of non-LVAD patients with elevated PVR, pulmonary vasodilators have been used to successfully bridge patients to heart transplant.2,3 Based on these proof-of-concept, non-randomized studies, the use of sildenafil has also increased in bridge to transplant patients supported with LVADs.
In the largest clinical study of PDE-5 inhibition in LVAD patients, Tedford et al identified 58/138 patients with a PVR>3 WU 1–2 weeks after LVAD implant and treated a subset with sildenafil.20 Use of sildenafil resulted in marked reductions in PVR (5.87±1.93 WU > 2.96±0.92 WU) and mPAP (36.5±8.6 mmHg > 24.3 ± 3.6 mmHg), while patients who did not receive sildenafil had persistently elevated PVR (>4 WU) at 12–15 weeks.20 Overall, 24 of 26 patients in the treatment group achieved a PVR<3 WU with 19 of these patients becoming eligible for transplant. Although non-randomized, these data suggest a possible role for pulmonary vasodilators among potential heart transplant candidates with persistent elevations of PVR on LVAD support.20
There has also been renewed interest in the use of ERAs for patients with persistent PH after LVAD. In a study of LVAD-supported patients, low-dose bosentan was well tolerated during prolonged therapy and resulted in a reduction in echocardiographically derived PVR from 3.93±1.53 WU in the preoperative setting to 2.58±1.05 WU 3–6 months post-LVAD.18 While the relative benefits of bosentan on top of LVAD support in this study are unclear, the tolerability of this medication was encouraging.
Improvement of symptoms and morbidity associated with chronic RV dysfunction
Many patients who survive post-LVAD RV failure are left with long-term RV dysfunction, and others may develop late RV dysfunction.22–24,62,63,73–75 Among these patients, low cardiac output can result in fatigue and end-organ dysfunction while elevated right-sided pressures can lead to increased HF hospitalizations, increased diuretic requirements, renal failure, cirrhosis, coagulopathy, malnutrition and GI bleeding.22,24,60,73–76 Improving PVR and PAC with vasodilators has the potential to restore RV-PA coupling, which could reduce the effects of RV failure.77 Sildenafil has been associated with improved hemodynamic and echocardiographic measures of RV function in patients with elevated PVR on LVAD support.20 PDE-5 inhibitors are generally well tolerated in LVAD patients, and no major adverse drug effects have been reported.20,78 Bosentan has also been associated with an improvement in RA pressure, RV end-diastolic dimension and RV Tei index in patients on mechanical support.18 Whether these imaging improvements will translate into a reduction in symptoms related to right sided congestion (LE edema, ascites, abdominal pain) and/or LV underfilling (suck down events, syncope, ventricular arrhythmias) will require additional study.
Prevention of late RV failure and its complications
The occurrence of late RV failure is one of the most challenging complications to manage in LVAD supported patients. This is particularly problematic for patients in whom transplant is not a viable bailout option. Among this cohort, reduced RV output often results in refractory HF symptoms and recurrent hospitalizations. Additionally, as RV failure worsens, low LVAD flows, reduced pulsatility and leftward shift of the interventricular septum can occur, resulting in impaired LV filling. This downward spiral is frequently associated with an increased frequency of LVAD alarms, suction events and ventricular arrhythmias.22–24,72–74,79 Use of PDE-5 inhibitors and ERAs is common to prevent late RV failure in high-risk patients; however, the safety, efficacy and relative benefits of these two classes of medications are unknown in this challenging population.
Outstanding Questions and Ongoing Investigation
Who are the target populations to study?
Ongoing clinical trials of Group 2 PH are currently aimed at answering the question of whether pulmonary vasodilators are safe and effective in reducing PVR and improving clinical outcomes, particularly in patients with CPC-PH. The “Clinical Study to Assess the Efficacy and Safety of Macitentan in Patients With Pulmonary Hypertension After Left Ventricular Assist Device Implantation (SOPRANO)” is a multicenter randomized placebo-controlled trial currently enrolling patients with CPC-PH on LVAD support to assess the impact of macitentan on pulmonary hemodynamics with a primary outcome of change in PVR from baseline to 3 months ( NCT02554903). Patients are eligible for enrollment if they are clinically stable with a mPAP>25 mmHg, PVR>3 WU and PCWP<18 mmHg after LVAD implant. The first step is to determine whether pulmonary vasodilators provide hemodynamic benefit in this population. If successful, a focus on clinical outcomes will be critical. Importantly, the placebo group in this study will provide a previously unseen window into the natural history of CPC-PH in LVAD-supported patients. In order for trials like these to succeed, collaboration and engagement are necessary across the community of LVAD centers to overcome enrollment challenges.
It is important to note that SOPRANO will not evaluate patients with RV dysfunction and milder elevations of PVR, a separate but potentially important additional subgroup for future investigation of pulmonary vasodilators. Where clinical equipoise exists, every effort should be made to enroll eligible patients in ongoing clinical trials or to store patient data in a registry format.
What is the most effective strategy to treat PH in left heart disease?
Given the limited data currently available to guide clinical practice, the relative efficacy of different classes of pulmonary vasodilators remains unclear. The cardiovascular and systemic effects of PDE-5 inhibition include mild reductions in systemic vascular resistance and blood pressure due to systemic vasodilation in addition to pulmonary vasodilation.80,81 The effects of ERAs are augmented by the presence of endothelin receptors in the myocardium in addition to the pulmonary and systemic vasculature, which may promote fluid retention. It remains unclear whether these differences favor one strategy in the treatment of PH in patients with HF.82–84 Additionally, combination therapy utilizing both PDE-5 inhibition and ERAs, a favored strategy in the initial management of PAH does not yet have a defined role in the management of CPC-PH. Uncovering the pathways that drive endothelial dysfunction and structural arterial remodeling in patients with CPC-PH will help guide therapy in this patient population. Additionally, more sophisticated phenotyping of patients with CPC-PH may help identify specific subgroups that might benefit more strongly from specific classes of pulmonary vasodilators. The PVDomics study seeks to identify both novel biomarkers and pathways relevant to pulmonary vascular disease and RV function through assessment of genetic, molecular and cellular processes to better focus PH management strategies ( NCT02980887).
How should candidacy and response to therapy be monitored?
The role of invasive hemodynamic assessment in HF patients and LVAD patients remains unclear. Early invasive hemodynamic assessment may identify those patients most likely to benefit from pulmonary vasodilator therapy. If true, routine hemodynamic assessment at prescribed intervals may be warranted to monitor the response to therapy. Of relevance, a recent study found less than 50% of clinically stable LVAD recipients had optimal hemodynamics, further supporting routine hemodynamic assessment for optimization of both RV-PA coupling and LV unloading among this population.19 In the future, ambulatory PA pressure monitoring may also have a role in the management of these patients. This strategy is supported by evidence that in HFrEF patients with PH, treatment directed by ambulatory PA pressure monitoring resulted in a reduction in the composite endpoint of HF hospitalization and mortality.85 Additional study is warranted for patients on LVAD support.
Conclusions
CPC-PH is common in the advanced HF population and persists in a subset of patients on LVAD support. Despite active investigation, the role of pulmonary vasodilator therapy in advanced HF and during LVAD support remains unclear at the present time. Large clinical trials in the HF population have been limited by challenges in enrolling the appropriate patients. Moreover, the use of pulmonary vasodilators for the management of PH and RV dysfunction post-LVAD remains largely unexplored. The substantial burden of RV failure in LVAD-supported patients supports ongoing efforts to determine the effects of pulmonary vasodilators in these patients. Future investigation should begin in patients with CPC-PH and expand to target additional groups, particularly those with RV dysfunction. If successful, such approaches have the potential to reduce the burden of pulmonary vascular disease and RV failure in the advanced HF and LVAD population.
Acknowledgments
Sources of Funding: This study was supported, in part, by research funds from the NIH (NIH grant U10 HL110309, Heart Failure Network).
Footnotes
Disclosures: None.
References
- 1.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates−−2006. J Heart Lung Transplant. 2006;25:1024–42. [DOI] [PubMed] [Google Scholar]
- 2.Jabbour A, Keogh A, Hayward C, Macdonald P. Chronic sildenafil lowers transpulmonary gradient and improves cardiac output allowing successful heart transplantation. Eur J Heart Fail. 2007;9:674–677. [DOI] [PubMed] [Google Scholar]
- 3.Delgado JF, Gómez-Sánchez MA, Sáenz De La Calzada C, Sánchez V, Escribano P, Hernández-Afonso J, Tello R, Gómez De La Cámara A, Rodríguez E, Rufilanchas JJ. Impact of mild pulmonary hypertension on mortality and pulmonary artery pressure profile after heart transplantation. J Hear Lung Transplant. 2001;20:942–948. [DOI] [PubMed] [Google Scholar]
- 4.Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: Testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. [DOI] [PubMed] [Google Scholar]
- 5.Atluri P, Fairman AS, Macarthur JW, Goldstone AB, Cohen JE, Howard JL, Zalewski CM, Shudo Y, Woo YJ. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg. 2013;28:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimpfer D, Zrunek P, Roethy W, Czerny M, Schima H, Huber L, Grimm M, Rajek A, Wolner E, Wieselthaler G. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg. 2007;133:689–695. [DOI] [PubMed] [Google Scholar]
- 7.Mikus E, Stepanenko A, Krabatsch T, Loforte A, Dandel M, Lehmkuhl HB, Hetzer R, Potapov EV. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg. 2011;40:971–7. [DOI] [PubMed] [Google Scholar]
- 8.Torre-Amione G, Southard RE, Loebe MM, Youker KA, Bruckner B, Estep JD, Tierney M, Noon GP. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29:195–200. [DOI] [PubMed] [Google Scholar]
- 9.Kutty RS, Parameshwar J, Lewis C, Catarino PA, Sudarshan CD, Jenkins DP, Dunning JJ, Tsui SS. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg. 2013;43:1237–42. [DOI] [PubMed] [Google Scholar]
- 10.Martin J, Siegenthaler MP, Friesewinkel O, Fader T, van de Loo A, Trummer G, Berchtold-Herz M, Beyersdorf F. Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. Eur J Cardiothorac Surg. 2004;25:971–977. [DOI] [PubMed] [Google Scholar]
- 11.Salzberg SP, Lachat ML, von Harbou K, Zünd G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg. 2005;27:222–5. [DOI] [PubMed] [Google Scholar]
- 12.Liden H, Haraldsson A, Ricksten S-E, Kjellman U, Wiklund L. Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? Eur J Cardiothorac Surg. 2009;35:1029–34–5. [DOI] [PubMed] [Google Scholar]
- 13.Alba AC, Rao V, Ross HJ, Jensen AS, Sander K, Gustafsson F, Delgado DH. Impact of fixed pulmonary hypertension on postheart transplant outcomes in bridge-to-transplant patients. J Hear Lung Transplant. 2010;29:1253–1258. [DOI] [PubMed] [Google Scholar]
- 14.John R, Liao K, Kamdar F, Eckman P, Boyle A, Colvin-Adams M. Effects on pre- and posttransplant pulmonary hemodynamics in patients with continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2010;140:447–452. [DOI] [PubMed] [Google Scholar]
- 15.Etz CD, Welp H a, Tjan TDT, Hoffmeier A, Weigang E, Scheld HH, Schmid C. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg. 2007;83:1697–1705. [DOI] [PubMed] [Google Scholar]
- 16.Pauwaa S, Bhat G, Tatooles AJ, Aggarwal A, Martin M, Kumar A, Modi H, Pappas PS. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J. 2012;19:153–158. [DOI] [PubMed] [Google Scholar]
- 17.Haddad H, Elabbassi W, Moustafa S, Davies R, Mesana T, Hendry P, Masters R, Mussivand T. Left ventricular assist devices as bridge to heart transplantation in congestive heart failure with pulmonary hypertension. Asaio J. 2005;51:456–460. [DOI] [PubMed] [Google Scholar]
- 18.LaRue SJ, Garcia-Cortes R, Nassif ME, Vader JM, Ray S, Ravichandran A, Rasalingham R, Silvestry SC, Ewald GA, Wang I-W, Schilling JD. Treatment of secondary pulmonary hypertension with bosentan after left ventricular assist device implantation. Cardiovasc Ther. 2015;33:50–5. [DOI] [PubMed] [Google Scholar]
- 19.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic Ramp Tests in Patients With Left Ventricular Assist Devices. JACC Hear Fail. 2016;4:208–217. [DOI] [PubMed] [Google Scholar]
- 20.Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB, Shah AS, Yuh D, Conte JV, Champion HC. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail. 2008;1:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaRue SJ, Raymer DS, Pierce BR, Nassif ME, Sparrow CT, Vader JM. Clinical outcomes associated with INTERMACS-defined right heart failure after left ventricular assist device implantation. J Heart Lung Transplant. 2017;36:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–24. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Woldendorp K, Muthiah K, Robson D, Prichard R, Macdonald PS, Keogh AM, Kotlyar E, Jabbour A, Dhital K, Granger E, Spratt P, Jansz P, Hayward CS. Normalisation of haemodynamics in patients with end-stage heart failure with continuous-flow left ventricular assist device therapy. Heart Lung Circ. 2014;23:963–9. [DOI] [PubMed] [Google Scholar]
- 24.Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, Oz MC, Naka Y. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. [DOI] [PubMed] [Google Scholar]
- 25.Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, Semigran MJ. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest. 2005;127:1647–1653. [DOI] [PubMed] [Google Scholar]
- 26.Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–851. [DOI] [PubMed] [Google Scholar]
- 27.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. [DOI] [PubMed] [Google Scholar]
- 28.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–48. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Yang T, Zhou Q, Li S, Huang L. Additional use of a phosphodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: a meta-analysis. Eur J Heart Fail. 2014;16:444–53. [DOI] [PubMed] [Google Scholar]
- 30.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. [DOI] [PubMed] [Google Scholar]
- 31.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–90. [DOI] [PubMed] [Google Scholar]
- 32.Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult--a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31:913–33. [DOI] [PubMed] [Google Scholar]
- 33.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De MT, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. JAmCollCardiol. 2013;62:D100–D108. [DOI] [PubMed] [Google Scholar]
- 34.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. [DOI] [PubMed] [Google Scholar]
- 35.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CSP, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: A community study. J Am Coll Cardiol. 2012;59:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guglin M, Khan H. Pulmonary Hypertension in Heart Failure. J. Card. Fail. 2010;16:461–474. [DOI] [PubMed] [Google Scholar]
- 37.Abramson SV, Burke JF, Kelly JJ, Kitchen JG, Dougherty MJ, Yih DF, McGeehin FC, Shuck JW, Phiambolis TP. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116:888–895. [DOI] [PubMed] [Google Scholar]
- 38.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Hear Fail. 2013;1:290–299. [DOI] [PubMed] [Google Scholar]
- 39.Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, Sotelo T, De La Camara AG, Cortina J, De La Calzada CS. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7:1011–1016. [DOI] [PubMed] [Google Scholar]
- 40.Moraes DL, Colucci WS GMM. Secondary Pulmonary Hypertension in chronic heart failure. The role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–1723. [DOI] [PubMed] [Google Scholar]
- 41.Porter TR, Taylor DO, Cycan a, Fields J, Bagley CW, Pandian NG, Mohanty PK. Endothelium-dependent pulmonary artery responses in chronic heart failure: influence of pulmonary hypertension. J Am Coll Cardiol. 1993;22:1418–1424. [DOI] [PubMed] [Google Scholar]
- 42.Snopek G, Pogorzelska H, Rywik TM, Browarek A, Janas J, Korewicki J. Usefulness of endothelin-1 concentration in capillary blood in patients with mitral stenosis as a predictor of regression of pulmonary hypertension after mitral valve replacement or valvuloplasty. Am J Cardiol. 2002;90:188–9. [DOI] [PubMed] [Google Scholar]
- 43.Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure [published erratum appears in Circulation 1993 Mar;87(3):1064]. Circulation. 1992;85:504–509. [DOI] [PubMed] [Google Scholar]
- 44.Wei CM, Lerman A, Rodeheffer RJ, McGregor CG, Brandt RR, Wright S, Heublein DM, Kao PC, Edwards WD, Burnett JC. Endothelin in human congestive heart failure. Circulation. 1994;89:1580–6. [DOI] [PubMed] [Google Scholar]
- 45.Melenovsky V, Al-Hiti H, Kazdova L, Jabor A, Syrovatka P, Malek I, Kettner J, Kautzner J. Transpulmonary B-Type Natriuretic Peptide Uptake and Cyclic Guanosine Monophosphate Release in Heart Failure and Pulmonary Hypertension. The Effects of Sildenafil. J Am Coll Cardiol. 2009;54:595–600. [DOI] [PubMed] [Google Scholar]
- 46.Chou S-H, Chai C-Y, Wu J-R, Tan M-S, Chiu C-C, Chen I-J, Jeng AY, Chang C-I, Kwan A-L, Dai Z-K. The effects of debanding on the lung expression of ET-1, eNOS, and cGMP in rats with left ventricular pressure overload. Exp Biol Med (Maywood). 2006;231:954–9. [PubMed] [Google Scholar]
- 47.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013;143:758–66. [DOI] [PubMed] [Google Scholar]
- 48.Grandin EW, Zamani P, Mazurek JA, Troutman GS, Birati EY, Vorovich E, Chirinos JA, Tedford RJ, Margulies KB, Atluri P, Rame JE. Right ventricular response to pulsatile load is associated with early right heart failure and mortality after left ventricular assist device. J Hear Lung Transplant. 2016;36:97–105. [DOI] [PubMed] [Google Scholar]
- 49.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, Farber-Eger EH, Sheng Q, Shyr Y, Harrell FE, Newman JH, Brittain EL. Clinical and Biological Insights Into Combined Post- and Pre-Capillary Pulmonary Hypertension. J Am Coll Cardiol. 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FEJ, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134:44–54. [DOI] [PubMed] [Google Scholar]
- 51.Givertz MM, Colucci WS, LeJemtel TH, Gottlieb SS, Hare JM, Slawsky MT, Leier C V, Loh E, Nicklas JM, Lewis BE. Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation. 2000;101:2922–7. [DOI] [PubMed] [Google Scholar]
- 52.Spieker LE, Mitrovic V, Noll G, Pacher R, Schulze MR, Muntwyler J, Schalcher C, Kiowski W, Lüscher TF. Acute hemodynamic and neurohumoral effects of selective ET(A) receptor blockade in patients with congestive heart failure. ET 003 Investigators. J Am Coll Cardiol. 2000;35:1745–1752. [DOI] [PubMed] [Google Scholar]
- 53.Hefke T, Zittermann A, Fuchs U, Schulte-Eistrup S, Gummert JF, Schulz U. Bosentan effects on hemodynamics and clinical outcome in heart failure patients with pulmonary hypertension awaiting cardiac transplantation. Thorac Cardiovasc Surg. 2012;60:26–34. [DOI] [PubMed] [Google Scholar]
- 54.Lüscher TF, Enseleit F, Pacher R, Mitrovic V, Schulze MR, Willenbrock R, Dietz R, Rousson V, Hürlimann D, Philipp S, Notter T, Noll G, Ruschitzka F, Heart Failure ET(A) Receptor Blockade Trial. Hemodynamic and neurohumoral effects of selective endothelin A (ET(A)) receptor blockade in chronic heart failure: the Heart Failure ET(A) Receptor Blockade Trial (HEAT). Circulation. 2002;106:2666–72. [DOI] [PubMed] [Google Scholar]
- 55.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF, EARTH investigators. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 364:347–54. [DOI] [PubMed] [Google Scholar]
- 56.Kalra PR, Moon JCC, Coats AJS. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int. J. Cardiol. 2002;85:195–197. [DOI] [PubMed] [Google Scholar]
- 57.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. [DOI] [PubMed] [Google Scholar]
- 58.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-Term Use of Sildenafil in the Therapeutic Management of Heart Failure. J Am Coll Cardiol. 2007;50:2136–2144. [DOI] [PubMed] [Google Scholar]
- 59.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. [DOI] [PubMed] [Google Scholar]
- 60.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz Elizabeth O Ofili MM, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA - J Am Med Assoc. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: A phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128:502–511. [DOI] [PubMed] [Google Scholar]
- 62.Patel ND, Weiss ES, Schaffer J, Ullrich SL, Rivard DC, Shah AS, Russell SD, Conte JV. Right Heart Dysfunction After Left Ventricular Assist Device Implantation: A Comparison of the Pulsatile HeartMate I and Axial-Flow HeartMate II Devices. Ann Thorac Surg. 2008;86:832–840. [DOI] [PubMed] [Google Scholar]
- 63.Takeda K, Takayama H, Colombo PC, Yuzefpolskaya M, Fukuhara S, Han J, Kurlansky P, Mancini DM, Naka Y. Incidence and clinical significance of late right heart failure during continuous-flow left ventricular assist device support. J Hear Lung Transpl. 2015;34:1024–1032. [DOI] [PubMed] [Google Scholar]
- 64.Santamore WP, Dell’Italia LJ. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog. Cardiovasc. Dis. 1998;40:289–308. [DOI] [PubMed] [Google Scholar]
- 65.Saleh S, Liakopoulos OJ, Buckberg GD. The septal motor of biventricular function. Eur. J. Cardio-thoracic Surg. 2006;29. [DOI] [PubMed] [Google Scholar]
- 66.Klima UP, Lee MY, Guerrero JL, LaRaia PJ, Levine RA, Vlahakes GJ. Determinants of maximal right ventricular function: Role of septal shift. J Thorac Cardiovasc Surg. 2002;123:72–80. [DOI] [PubMed] [Google Scholar]
- 67.Houston BA, Kalathiya RJ, Hsu S, Loungani R, Davis ME, Coffin ST, Haglund N, Maltais S, Keebler ME, Leary PJ, Judge DP, Stevens GR, Rickard J, Sciortino CM, Whitman GJ, Shah AS, Russell SD, Tedford RJ. Right ventricular afterload sensitivity dramatically increases after left ventricular assist device implantation: A multi-center hemodynamic analysis. J Hear Lung Transplant. 2016;35:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haglund NA, Burdorf A, Jones T, Shostrom V, Um J, Ryan T, Shillcutt S, Fischer P, Cox ZL, Raichlin E, Lowes BD, Dumitru I. Inhaled milrinone after left ventricular assist device implantation. J Card Fail. 2015;21:792–797. [DOI] [PubMed] [Google Scholar]
- 69.Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, Smerling AJ, Oz MC. Randomized, Double-Blind Trial of Inhaled Nitric Oxide in LVAD Recipients With Pulmonary Hypertension. Ann Thorac Surg. 1998;65:340–345. [DOI] [PubMed] [Google Scholar]
- 70.Klodell CT Jr., Morey TE, Lobato EB, Aranda JM Jr., Staples ED, Schofield RS, Hess PJ, Martin TD, Beaver TM. Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg. 2007;83:68–71; discussion 71. [DOI] [PubMed] [Google Scholar]
- 71.Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, Staples ED, Beaver TM. Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann. Thorac. Surg. 2005;79:194–197. [DOI] [PubMed] [Google Scholar]
- 72.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore S a, Morgan J a, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi A a, Petty MG, Rogers J. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant. 2013;32:157–87. [DOI] [PubMed] [Google Scholar]
- 73.Patlolla B, Beygui R, Haddad F. Right-ventricular failure following left ventricle assist device implantation. Curr Opin Cardiol. 2013;28:223–33. [DOI] [PubMed] [Google Scholar]
- 74.Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: Prevention and treatment. Best Pract Res Clin Anaesthesiol. 2012;26:217–229. [DOI] [PubMed] [Google Scholar]
- 75.Rich JD. Right Ventricular Failure in Patients with Left Ventricular Assist Devices. Cardiol. Clin. 2012;30:291–302. [DOI] [PubMed] [Google Scholar]
- 76.Sparrow CT, Nassif ME, Raymer DS, Novak E, LaRue SJ, Schilling JD. Pre-Operative Right Ventricular Dysfunction is Associated With Gastrointestinal Bleeding in Patients Supported With Continuous-Flow Left Ventricle Assist Devices. JACC Hear Fail. 2015;3. [DOI] [PubMed] [Google Scholar]
- 77.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J Am Coll Cardiol. 2017;69:236–243. [DOI] [PubMed] [Google Scholar]
- 78.Ravichandran AK, LaRue SJ, Novak E, Joseph SA, Schilling JD. Sildenafil in Left Ventricular Assist Device Is Safe and Well-Tolerated. ASAIO J. 2017 [DOI] [PubMed] [Google Scholar]
- 79.Slaughter MS, Rogers JG, Milano C a, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 80.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. [DOI] [PubMed] [Google Scholar]
- 81.Zusman RM, Morales a, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999;83:35C–44C. [DOI] [PubMed] [Google Scholar]
- 82.Kelso EJ, Geraghty RF, McDermott BJ, Trimble ER, Nicholls DP, Silke B. Mechanical effects of ET-1 in cardiomyocytes isolated from normal and heart-failed rabbits. Mol Cell Biochem. 1996;157:149–155. [DOI] [PubMed] [Google Scholar]
- 83.Thomas PB, Liu EC, Webb ML, Mukherjee R, Hebbar L, Spinale FG. Exogenous effects and endogenous production of endothelin in cardiac myocytes: potential significance in heart failure. Am J Physiol. 1996;271:H2629–37. [DOI] [PubMed] [Google Scholar]
- 84.Onishi K, Ohno M, Little WC, Cheng CP. Endogenous endothelin-1 depresses left ventricular systolic and diastolic performance in congestive heart failure. J Pharmacol Exp Ther. 1999;288:1214–22. [PubMed] [Google Scholar]
- 85.Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–37. [DOI] [PubMed] [Google Scholar]