Abstract
Background and aim
Experience of stigma towards methadone maintenance treatment (MMT) may be a barrier to use of this treatment by people with opioid use disorder. We evaluated the factor structure, internal reliability, construct, and criterion validity of a theory-based stigma measure, the Methadone Maintenance Treatment Stigma Mechanisms Scale (MMT-SMS) and compared this with the Substance Use Stigma Mechanism Scale SU-SMS.
Design
Surveys at the beginning and end of a prospective study together with records of drug use and treatment attendance during that study.
Setting
Community methadone clinic in the Northeastern US.
Participants
Ninety-three participants who were receiving MMT; average daily methadone dose was 84.8mg/day (SD=28.4mg/day).
Measurements
The MMT-SMS uses a self-report questionnaire to assess three dimensions reflecting experiences of anticipated (9 items), enacted (9 items), and internalized stigma (7 items) specifically related to receiving MMT. Anticipated and enacted scales include three stigma source subscales (family, employers, healthcare workers; 3 items each). Responses are recorded on a 5-point Likert-type scale, then averaged to produce the MMT-SMS scale/subscale scores. The SU-SMS is a self-report questionnaire to assess experiences of anticipated, enacted, and internalized stigma regarding substance use history. Both scales were administered at the final parent study visit. Other measures included were assessed in the parent study and used to assess lifetime and recent MMT (e.g. current MMT dose) and drug use experiences (e.g. past 30-day heroin injection).
Findings
The MMT-SMS demonstrated good internal reliability (α=0.806–0.952 for components). Confirmatory factor analysis supported the 7-factor scale structure, distinguishing between experiences of anticipated, enacted, and internalized stigma, and anticipated and enacted stigma source subscales (family, employers, healthcare workers) (RMSEA=0.076, 90%CI=0.061–0.090, p-close=0.003; CFI=0.974; TLI=0.971). Construct validity helped distinguish the MMT-SMS from established substance use stigma constructs. Criterion validity observed associations with substance use experiences while on MMT, likely to predict future MMT success. Internalized MMT stigma was uniquely associated with daily MMT dose. Regarding criterion validity: anticipated MMT and enacted substance use stigma were associated with past 30-day heroin injection, MMT stigma uniquely associated with opioid use behaviors while receiving MMT, and substance use stigma broadly associated with injection-related behaviors.
Conclusions
The Methadone Maintenance Treatment Stigma Mechanisms Scale appears to be a reliable measure of methadone maintenance treatment stigma with robust validity in a sample of persons with opioid use disorders receiving methadone maintenance treatment.
Keywords: stigma, methadone maintenance treatment, medication-assisted treatment, opioid agonist treatment, opioid substitution therapy, opioid use disorder, persons who inject drugs
INTRODUCTION
With an estimated 35 million people who use opioids worldwide,1 there is an urgent need to improve access to pharmacologic treatments for opioid use disorder (OUD). Medication-assisted treatment (MAT) encompasses several types of medications (i.e., methadone, naltrexone, buprenorphine). Methadone is one of the oldest most commonly used treatments, shown to be cost-effective, and improves long-term physical and social outcomes for persons living with an OUD (PLWOUD).2–5 Despite substantial evidence and initiatives to scale up methadone maintenance treatment (MMT) access, methadone remains underutilized.6 Social stigma has been highlighted as a substantial barrier to MMT utilization.7,8
Stigma is a social process involving discrediting and devaluation of individuals based on a characteristic such as drug use,9 resulting in status loss and discrimination when a person is labeled with the stigmatized characteristic (i.e., person who uses drugs).10 Stigma leads to the social and economic exclusion of stigmatized groups, including PLWOUD.11 Substance use stigma is pervasive, contributing to a range of health and social disparities affecting PLWOUD by shaping public attitudes towards public policy interventions and willingness to access or prescribe evidence-based treatment.12–14 Less is known about the stigma PLWOUD experience once they are labeled as someone who has accessed or is accessing MMT. Efforts to understand MMT stigma are needed to better optimize rapid MMT expansion efforts and success.15,16
The literature on stigma and MMT is primarily qualitative and documents perceptions of MMT as an extension of substance use stigma into substance use treatment.17–27 Among persons in substance use treatment and persons receiving MMT, experiencing greater substance use stigma has been found to be associated with having more previous treatment experiences, receiving MMT for longer periods of time, engaging in injection (vs. non-injection) drug use, and more frequent heroin use.22,28 In addition, MMT stigma may hinder persons from seeking or adhering to treatment.17,21,26,27,29 Yet, the individual manifestations of stigma associated with receiving MMT among PLWOUD may be distinct from substance use stigma and remains underexplored.
Specifically, the measurement of MMT stigma has been poorly specified in the existing literature. To date, MMT beliefs, attitudes, and interest in MMT among PLWOUD have been examined without identifying an individual’s experience with MMT stigma.24,30–33 Yet, measuring the impact of MMT stigma on MMT outcomes will likely yield unique points of intervention. Similarly, other studies with PLWOUD have not clearly specified whether stigma was measured in relation to an individual’s substance use or MMT status,22,34 or measured substance use treatment-related stigma more broadly without specifying methadone as distinct from other treatment types.28,35–37 Further, measures have conflated theoretically distinct stigma mechanisms within a single construct (i.e., not able to disentangle the effects of stigma from others vs. oneself) and frequently include items related to how one copes with stigma within the same construct as stigma itself.28,34,38
Theoretical Framework
The Stigma Framework uses stigma theory to parsimoniously articulate three distinct, but related, mechanisms through which persons experience social stigma.39 Specifically, the framework specifies that our understanding of how stigma contributes to poorer health outcomes will be enhanced by measuring the unique effects of anticipated, enacted, and internalized stigma mechanisms. The framework further hypothesizes that our understanding of the relationships between anticipated and enacted stigma on context specific health outcomes (e.g. MMT initiation vs. opioid abstinence) may be improved by distinguishing between stigma sources unique to the target population(s).
Applied to receiving MMT, anticipated stigma reflects future expectations of being stereotyped, encountering prejudice, or being discriminated against due to receiving MMT. Enacted stigma reflects having encountered these experiences firsthand in the past. Anticipated and enacted MMT stigma are perpetuated by others (i.e., stigma sources such as family, employers, or healthcare providers). In contrast, the third stigma mechanism, internalized stigma, reflects the endorsement and application of negative feelings and beliefs about persons receiving MMT towards oneself. Previous research suggests that these stigma mechanisms uniquely influence negative affective, behavioral, and physical health outcomes among persons living with socially devalued characteristics.40,41 To our knowledge, there are no validated measures of anticipated, enacted, and internalized stigma related to receiving MMT among PLWOUD. This study aims to: 1) advance measurement efforts by leveraging the Stigma Framework to develop and evaluate the Methadone Maintenance Treatment Stigma Mechanisms Scale (MMT-SMS), and 2) compare the contributions of MMT and substance use stigma scales in relation to MMT-related outcomes.
METHODS
Scale Development
Informed by the Stigma Framework,39 the MMT-SMS was developed in parallel with the Substance Use Stigma Mechanism Scale (SU-SMS) and the HIV Stigma Mechanisms Scale (HIV-SMS) and implemented among persons receiving MMT, who use substances, and/or persons living with HIV, respectively. As such, the a priori structure of these measures’ primary scales (anticipated, enacted, internalized) and subscales (anticipated and enacted stigma sources) was guided by stigma theory, and an exploratory factor analysis was not conducted. Both the SU-SMS and HIV-SMS are published elsewhere, and demonstrate support for utilizing the Stigma Framework to develop valid, reliable, scales capable of distinguishing between the three stigma mechanisms and distinct sources of anticipated and enacted stigma.40,41
Developing and Piloting Item Content
Within this unified measurement structure; four unique stigma sources were originally drafted for each of the three measures reflecting the types of individuals most proximal to health-related outcomes (i.e. family members, healthcare workers, case managers/social workers, and employers). These stigma sources were identified via qualitative work with substance-involved persons living with HIV (PLWH) in the Bronx, NY, over half of whom had a history of receiving MMT and/or were currently receiving MAT (methadone or buprenorphine).42 Item content for each measure was informed through their respective literatures describing stigmatizing experiences. While the structure of the MMT-SMS and SU-SMS is identical, item content aimed to reflect common stigmatizing experiences related to receiving MMT (e.g. family members will think that I cannot recover) vs. engaging in substance use (e.g. family members will think I cannot be trusted).
Once developed, the SU-SMS and HIV-SMS were piloted with 10 PLWH in the Bronx with a history of substance use. The SU-SMS and MMT-SMS were piloted with 12 clients of the methadone clinic affiliated with this study. Participants completed these measures via in-depth cognitive interviews 43 to assess how individuals process and respond to each item (e.g. relevance of items to personal experiences) and explore factors that may inhibit accurate responses (i.e., social desirability concerns).20 The cognitive interviews produced several changes to the scales. Of note, employers as a stigma source was removed for the HIV-SMS and initial validation of the SU-SMS given high rates of PLWH who had a history of substance use that were on HIV-related disability assistance and unable to seek employment. Employers as a stigma source was retained in the MMT-SMS because participants receiving methadone were largely not on disability and reported significant concerns surrounding stigma from employers (e.g., having to miss work to access methadone). Compared to PLWH only a few female MMT clients with children reported interactions with a case manager/social worker, thus this stigma source was only retained in the HIV-SMS. To best compare the psychometric properties MMT-SMS with the SU-SMS in the methadone clinic sample, the employer items of the SU-SMS have been included in the current analyses. Additionally, item content was slightly modified (e.g. amend adjectives describing a stigmatizing experience).
The three revised measures were subsequently evaluated at these respective sites. Cognitive interview participants were not part of the validation process. Institutional Review Board approval was obtained for the scale development, cognitive interviewing, and scale validation procedures of this sub-study.
Participants and Procedures
Participants for the MMT-SMS validation sub-study were recruited from a group-based HIV prevention trial for individuals receiving outpatient MMT in the New England area (2012–2013).44 Eligible participants were ≥18 years old, HIV-negative, diagnosed with an OUD, and receiving daily MMT. At the parent study’s 12-month assessment, participants were invited to complete supplemental stigma measures (MMT-SMS, SU-SMS) in English via paper and pencil and received an additional $10 remuneration. Participants could either self-administer the measures or elect to have them administered by a trained interviewer. Additional data were obtained from the parent study’s baseline and follow-up assessments at months 3, 6, and 12.
Measures
Sociodemographics
Baseline characteristics that were measured included participants’ age (continuous), gender assessed as male or female. Race/ethnicity was categorized as being Latino (any race), non-Hispanic Black, non-Hispanic White, of another non-Hispanic race (non-Hispanic Other). Primary language spoke at home was coded as English or Spanish; no other languages were reported. Sexual orientation was reported as self-identifying as heterosexual or non-heterosexual (Gay or Bisexual). Indicators of socioeconomic status included baseline education (1=No GED/high school diploma, 0=GED/high school diploma or higher), income was categorized as earning less than $21k (1) or more (0), and housing status (‘what were your usual living arrangements in the past 30 days?’ was coded as unstable (1=controlled environment [jail, inpatient], residential drug/alcohol treatment, or no stable arrangements) or stable (0= living alone or with someone you know in a place that is rented or owned). Employment at baseline (Table 1) and month 12 (post-hoc analysis) was categorized as (1=yes, 0=no) being currently employed (working or a student), unemployed, or on disability/SSI. A single baseline item asked (1=yes, 0=no), ‘Is there someone other than you who uses drugs where you live or stay?’
Table 1.
Participant characteristics (N = 93)
| n (%) | M (SD) | Range | |
|---|---|---|---|
| Age | 38.10 (10.21) | 20–56 | |
| Gender | |||
| Male | 47 (50.5%) | ||
| Female | 46 (49.5%) | ||
| Race/Ethnicity | |||
| Latino | 13 (14.0%) | ||
| Non-Hispanic Black | 13 (14.0%) | ||
| Non-Hispanic White | 63 (67.7%) | ||
| Non-Hispanic Other | 4 (4.3%) | ||
| Primary Language | |||
| English | 88 (94.6%) | ||
| Non-English | 5 (5.4%) | ||
| Sexual Orientation§ | |||
| Heterosexual | 77 (91.7%) | ||
| Gay or Bisexual | 7 ( 8.3%) | ||
| SES Factors | |||
| No GED or HS Diploma | 30 (32.3%) | ||
| Unemployed§ | 62 (73.8%) | ||
| On disability/SSI§ | 17 (20.2%) | ||
| Income < $20K/yr.§ | 74 (88.1%) | ||
| Unstable housing last 30 days§ | 8 (9.5%) | ||
| Drug Use History | |||
| No. Years Opioid Use | 14.10 ( 9.53) | 1.00 – 37.00 | |
| Live with active user(s) | 28 (30.1%) | ||
| Lifetime Injection | 73 (78.5%) | ||
| MMT Status | |||
| Previous MAT History | 82 (88.2%) | ||
| No. Times Previously on MAT | 2.16 (1.84) | 0.00 – 11.00 | |
| Current Methadone Dose (mg/day) | 84.80 (28.39) | 25.00 – 160.00 | |
| Readiness to Change | |||
| Reduce Drug Use (heroin, cocaine) | 4.67 (1.38) | 2.00 – 6.50 | |
| Use New/Clean Needle | 4.05 (1.20) | 1.00 –6.50 | |
| Cumulative Opioid Experiences Last 12 Months | |||
| Mean No. Bags Heroin | 1.90 (4.48) | 0.00 – 19.50 | |
| Mean Opioid Withdrawal | 0.42 (0.63) | 0.00 – 3.00 | |
| Recent Opioid Use | |||
| % Negative UTox Last 6 mo. | 81.75 (23.94) | 0.00 – 100.00 | |
| Injected Heroin Last 6 mo.§ | 18 (21.4%) | ||
| Injected Heroin Last 30 days§ | 9 (9.7%) | ||
9 participants had missing values on this variable. GED= general education diploma. HS= high school. SSI= supplemental security income. K= thousand; Yr.= year. No. = number of. mg = milligrams. Mo.= months. Unstable Housing= reported staying in a controlled environment [jail, inpatient, residential treatment], or no stable arrangement. MMT= methadone maintenance treatment. MAT= medication-assisted treatment (includes methadone, buprenorphine, LAAM). UTox= urine toxicology test for opioids.
Methadone Maintenance Treatment Stigma Mechanisms Scale
The 25-item MMT-SMS (Table 2; Measure S1) measured experiences of anticipated (9-items; “Family members will think that I cannot recover”), enacted (9-items; “Employers have thought that I am still a drug user”), and internalized stigma (7-items; “I feel ashamed of my methadone treatment”) regarding their MMT status. Anticipated and enacted items differentiated between three stigma sources (family, employers, and healthcare workers). Responses were given on a 5-point Likert-type scale; higher scores indicate greater MMT stigma.
Table 2.
Structural Validity: Seven-factor Methadone Maintenance Treatment Stigma Mechanisms Scale model standardized estimates (N=93)
| Construct | Source | Item | Factor Loading (SE) | |
|---|---|---|---|---|
| Anticipated | ||||
| Factor 1 | FAM | 1. Family members will think that I am a drug user. | 0.779 *** | (0.049) |
| FAM | 2. Family members will not support my methadone treatment. † | 0.692 *** | (0.060) | |
| FAM | 3. Family members will think that I cannot recover. | 0.993 *** | (0.033) | |
| Factor 2 | EMP | 4. Employers will think that I’m still a drug user. † | 0.884 *** | (0.026) |
| EMP | 5. Employers will think that I am a bad employee. | 0.909 *** | (0.024) | |
| EMP | 6. Employers will think that managing my methadone treatment schedule will be a problem. | 0.954 *** | (0.023) | |
| Factor 3 | HCW | 7. Healthcare workers will think that I’m still a drug user. † | 0.875 *** | (0.025) |
| HCW | 8. Healthcare workers will give me poor care. | 0.899 *** | (0.026) | |
| HCW | 9. Healthcare workers will not prescribe me medication that I need. | 0.895 *** | (0.024) | |
| Enacted | ||||
| Factor 4 | FAM | 10. Family members have thought that I’m still a drug user. | 0.884 *** | (0.036) |
| FAM | 11. Family members have not supported my methadone treatment. † | 0.879 *** | (0.033) | |
| FAM | 12. Family members have thought that I cannot recover. | 0.935 *** | (0.025) | |
| Factor 5 | EMP | 13. Employers have thought that I’m still a drug user. | 0.949 *** | (0.017) |
| EMP | 14. Employers have thought that I’m a bad employee. | 0.950 *** | (0.019) | |
| EMP | 15. Employers have thought that managing my methadone dosage schedule will be a problem. † | 0.937 *** | (0.018) | |
| Factor 6 | HCW | 16. Healthcare workers have thought that I’m still a drug user. | 0.931 *** | (0.019) |
| HCW | 17. Healthcare workers have given me poor treatment. | 0.947 *** | (0.014) | |
| HCW | 18. Healthcare workers have not prescribed me medication that I need.† | 0.849 *** | (0.032) | |
| Internalized | ||||
| Factor 7 | Self | 19. Receiving methadone makes me feel like I’m a bad person. † | 0.901 *** | (0.022) |
| Self | 20. I feel I’m not as good as others because I receive methadone. | 0.943 *** | (0.015) | |
| Self | 21. I feel ashamed of my methadone treatment. † | 0.912 *** | (0.019) | |
| Self | 22. I think less of myself because I receive methadone. † | 0.913 *** | (0.020) | |
| Self | 23. Receiving methadone makes me feel unclean. | 0.948 *** | (0.016) | |
| Self | 24. Being on methadone treatment is disgusting to me. | 0.923 *** | (0.018) | |
| Self | 25. Being on methadone treatment makes me feel like I’m still a drug user. † | 0.859 *** | (0.028) | |
Factor loading is significant at 0.001 (***), 0.01(**), or 0.05(*) level (2-tailed).
Item removed in the sensitivity analyses.
Substance Use Stigma Mechanisms Scale
The 24-item SU-SMS (Measure S2) measured experiences of anticipated (9-items; “Family members will think that I cannot be trusted”), enacted (9-items; “Employers have discriminated against me”), and internalized stigma (6-items; “Having used alcohol/drugs makes me feel unclean”) regarding their substance use history. Anticipated and enacted items differentiated between three stigma sources (family, employers, and healthcare workers). The current analysis retains the employer stigma source subscales that were omitted in the initial published validation of the SU-SMS.41 Responses were given on a 5-point Likert-type scale; higher scores indicate greater substance use stigma.
Drug use history
Number of years using opioids and lifetime injection drug use (1=yes, 0=no) was assessed at baseline. Data reported at months 3, 6, and 12 was used to calculate the mean number of bags of heroin used in the past 12 months and a mean score for opioid withdrawal symptoms experienced the week before each assessment (5-point Likert-type scale: 0=not experiencing symptoms, 4=experiencing extreme symptoms). Recent opioid use, measured at month 12, included having injected heroin in the past 6 months or past 30 days, and the proportion of urine toxicology results negative for opiates. Two urines were collected each week. The proportion of toxicology results that were negative for opioids was calculated for the 48 tests collected between months 6 and 12. Readiness to reduce opioid or cocaine use (2 items) or adopt safer injection behaviors when injecting (2 items; not sharing needles; using a clean/new needle) was assessed at month 12. Responses were given on a 4-point Likert-type scale, where a lower number reflected less readiness to change the target behavior (1=Pre-contemplation, 4=Maintenance). Participants who did not report injection behaviors were not asked items regarding readiness to change injection behaviors. Responses were then averaged to reflect participants’ overall readiness to change across these two respective domains.
MMT history
Participants reported any previous use of MAT (including MMT) and the number of times they had previously enrolled in any MAT program (0=no prior MAT history) at baseline. Participants’ current methadone dose (mg/day) was abstracted from the clinic’s records at month 12.
Statistical Analysis
Descriptive statistics were used to characterize the study sample. MMT-SMS and SU-SMS item responses were averaged to create composite scores for each stigma mechanism scale and stigma source subscale. To facilitate comparisons of the MMT-SMS and the SU-SMS in this sample, all reliability and validity statistics were conducted for both measures, respectively.
Cronbach’s Alpha, a measure of internal consistency, assessed the reliability of the stigma mechanism scales and stigma source subscales.
Confirmatory Factor Analysis (CFA) tested the structural validity of the hypothesized 7-factor structure (Figure 1) for the three primary stigma mechanisms (2nd order scales; anticipated, enacted, internalized) and three stigma sources within the anticipated and enacted mechanisms (1st order subscales: FAM, EMP, HCW). Analyses were conducted in MPlus 7.0 based on a covariance matrix using maximum likelihood (ML) estimation.45 Correlated error variance was specified for experiences of anticipated and enacted stigma reported from the same stigma source (e.g. anticipated and enacted stigma from employers). Given potential concerns regarding our sample size and the number of items included in our CFA; sensitivity analysis was conducted removing 10 items to better inform the precision of our observed fit indices.
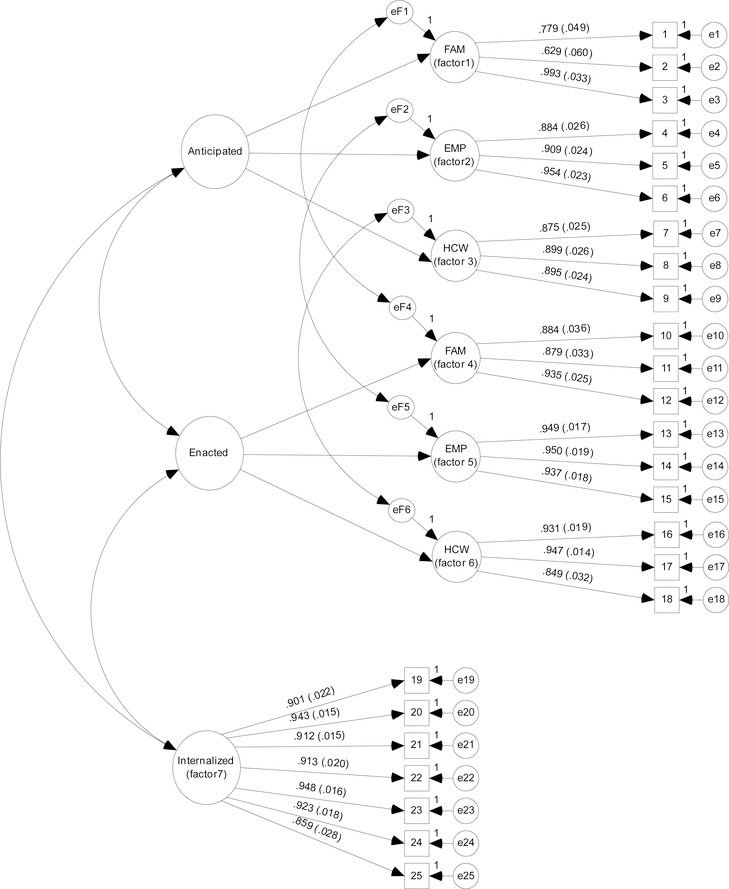
Figure 1.
Structural Validity: 7-factor latent variable measurement model with standardized factor loadings (SE) (N=93). MMT-SMS items (Table 2) correspond to the item numbers that appear in boxes (observed variables), MMT-SMS latent factors appear as circles, and measurement error variance ‘e’ is represented for each MMT-SMS item and first order factor. Stigma sources are: FAM=Family members, EMP=Employers, HCW=Healthcare Workers.
Structural, construct, and criterion validity were assessed via Pearson’s r and Kendall’s Tau-b to examine associations between the two stigma measures and continuous or binary/count variables, respectively. Evidence of construct validity was assessed via convergent and discriminant correlations between the stigma measures and theoretically related, but distinct constructs.
The MMT-SMS and SU-SMS did not have missing data. The number of participants with missing baseline demographics or 12-month follow-up data was small (≤ 10 participants). This was managed using pairwise deletion. Changes in sample sizes are noted in the tables where relevant.
Specific to the MMT-SMS, we sought to explore whether anticipated and enacted MMT stigma would be observed among those living with someone who still uses drugs. An additional association of interest was whether one’s length of time using opioids or having injected drugs would be associated with MMT stigma. We aimed to assess whether different stigma mechanisms (anticipated, enacted, internalized) would be differentially associated with having a MAT history and current daily methadone dose. Given that MMT can signal to others that an individual has an OUD, we expect experiences of substance use and MMT stigma as measured by the MMT-SMS and SU-SMS, respectively, to be related but distinct constructs. To assess the criterion validity of the MMT-SMS in understanding MMT-related outcomes, we assessed whether MMT stigma was associated with lower readiness to reduce drug-related behaviors and having engaged in greater opioid use while on MMT. These associations were also examined in relation to the SU-SMS to further compare the contributions of both scales in relation to MMT-related outcomes.
Post-hoc analyses were conducted to examine whether the observed associations between enacted MMT and substance use stigma from employers on the proportion of urine toxicology tests negative for opioids varied by employment status (employed, unemployed, on disability).
RESULTS
Participant characteristics
Ninety-three participants completed this cross-sectional sub-study. Participants reflected the affiliated methadone clinic’s patient population (Table 1). On average, participants were 38 years old (range: 20–56), half were male, most were non-Hispanic white (67.7%), and identified as heterosexual (91.7%). Approximately one-third had less than a high school education (32.3%). Most were unemployed (73.8%) earning less than $20,000 a year (88.1%), but stably housed (90.5%). No significant (p≤0.10) or clinically meaningful differences were observed for participants’ responses to the MMT-SMS on their randomization to the parent-study’s intervention or wait-list control arm.
Structural Validity
The 7-factor model converged suggesting good model fit to the observed data (RMSEA=0.076, CI 90%=0.061–0.090, p-close=0.003; CFI=0.974; TLI=0.971; Figure 1). Standardized factor loadings of the individual MMT-SMS items were significantly (p<0.001) and substantially (factor loadings range: 0.692–0.993) associated with their hypothesized construct(s) (Table 2). The model explained a substantial amount of item variance comprising the anticipated (R2 =0.479 – 0.974), enacted (R2 =0.722 – 0.902) and internalized (R2 =0.739 – 0.899) hypothesized constructs. Modification indices suggest that model fit would not be improved by altering the hypothesized 7-factor model. In our sensitivity analysis, good model fit (RMSEA=0.066, CI 90%=0.039–0.090, p-close=0.143; CFI=0.988; TLI=0.985) and strong factor loadings were maintained (range: 0.754–0.978). Combined, these data indicate that the MMT-SMS is a structurally valid measure.
The associations between the MMT-SMS stigma mechanism scales and stigma source subscales provide further support for the hypothesized 7-factor structure (Table 3). Specifically, the correlations between the anticipated and enacted stigma scales are significant, but below the 0.800 threshold, indicating that the two scales are measuring closely related but distinct constructs.46 In contrast, the correlations between internalized stigma and anticipated and enacted stigma is significant but modest in effect size (r<0.5), indicating internalized stigma is less closely related to anticipated and enacted stigma as hypothesized by the Stigma Framework.39 Similarly, the correlations between the anticipated and enacted stigma mechanisms subscales are significant but of moderate to large effect size (r=0.353–0.698), indicating the respective subscales generally capture distinct stigma-related experiences from each stigma source. The one exception being anticipated and enacted MMT stigma from HCW which exceeded the 0.800 threshold suggesting those in this sample who have experienced MMT stigma in the healthcare context anticipate it will occur again in the future.
Table 3.
Structural Validity: Associations within and between the Methadone Maintenance Treatment Stigma Mechanisms Scale (N = 93)
| MMT-SMS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stigma Mechanisms: | Anticipated | Enacted | Internalized | |||||||
| Scale (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total | |
| Anticipated | Total | 1.00 | ||||||||
| FAM subscale | .799*** | 1.00 | ||||||||
| EMP subscale | .830*** | .534*** | 1.00 | |||||||
| HCW subscale | .800*** | .432*** | .485*** | 1.00 | ||||||
| Enacted | Total | .756*** | .567*** | .581*** | .686*** | 1.00 | ||||
| FAM subscale | .654*** | .698*** | .467*** | .436*** | .822*** | 1.00 | ||||
| EMP subscale | .539*** | .318** | .595*** | .395*** | .810*** | .511*** | 1.00 | |||
| HCW subscale | .653*** | .353*** | .380*** | .833*** | .824*** | .479*** | .527*** | 1.00 | ||
| Internalized | Total | .428*** | .269** | .409*** | .352*** | .387*** | .325*** | .288** | .332*** | 1.00 |
FAM = ‘Family members’ stigma source subscale. EMP = ‘Employers’ stigma source subscale. HCW = ‘Healthcare Workers’ stigma source subscale. Correlation is significant at 0.001 (***), 0.01(**), or 0.05(*) level (2-tailed).
Scale Reliability and Mean Levels of MMT Stigma
High internal consistency was achieved across all MMT-SMS scales (α=0.886–0.952) and stigma source subscales (α=0.806–0.933) (Table 4). Modification indices suggested the internal reliability of the anticipated and the enacted stigma subscales from family and healthcare workers, respectively, might be slightly improved with the removal of one item. This improvement was not deemed clinically or psychometrically meaningful, and these items were retained in the final scales.
Table 4.
Scale Reliability: Mean stigma levels and internal consistency of Methadone Maintenance Treatment Stigma Mechanisms Scale (N = 93)
| Scales (subscales) | M (SD) | α | Modified α with item removed | |
|---|---|---|---|---|
| MMT-SMS | ||||
| Anticipated Stigma | (k=9) | 2.85 (0.89) | .886 | .887 (2. Family members will not support my methadone treatment)* |
| Family Members | (k=3) | 2.97 (1.07) | .806 | N/A |
| Employers | (k=3) | 2.76 (1.09) | .907 | N/A |
| Healthcare Workers | (k=3) | 2.81 (1.14) | .879 | N/A |
| Enacted Stigma | (k=9) | 2.55 (0.93) | .902 | N/A |
| Family Members | (k=3) | 2.78 (1.20) | .893 | N/A |
| Employers | (k=3) | 2.22 (1.02) | .933 | N/A |
| Healthcare Workers | (k=3) | 2.65 (1.18) | .899 | .912 (18. Healthcare workers have not prescribed me medication that I needed)* |
| Internalized | (k=7) | 2.33 (1.04) | .952 | N/A |
| SU-SMS | ||||
| Anticipated Stigma | (k=9) | 2.94 (1.05) | .932 | N/A |
| Family Members | (k=3) | 2.94 (1.24) | .939 | N/A |
| Employers | (k=3) | 3.03 (1.19) | .892 | N/A |
| Healthcare Workers | (k=3) | 2.86 (1.17) | .886 | N/A |
| Enacted Stigma | (k=9) | 2.76 (0.93) | .872 | N/A |
| Family Members | (k=3) | 3.29 (1.24) | .918 | .920 (10. Family members have thought I cannot be trusted)** |
| Employers | (k=3) | 2.44 (1.08) | .804 | .898 (15. Employers have not given me promotions)** |
| Healthcare Workers | (k=3) | 2.55 (1.23) | .878 | N/A |
| Internalized | (k=6) | 3.23 (1.04) | .917 | N/A |
k = number of items in the scale or subscale, respectively.
MMT-SMS item number is the same as listed in Table 2.
SU-SMS item number is the same as listed in supplemental material (Measure S2).
Overall, participants reported moderate levels of anticipated (M=2.85, SD=0.089), enacted (M=2.55, SD=0.93), and internalized (M=2.33, SD=1.04) MMT stigma. For both anticipated and enacted mechanisms, experiences of stigma from family members were greatest, followed by healthcare workers, then employers. Results indicate the MMT-SMS is an internally reliable measure among persons receiving MMT.
Construct Validity
Greater anticipated and enacted MMT stigma were experienced among those currently living with someone who uses drugs (Table 5). One’s length of time using opioids was not associated with MMT stigma. Lifetime injection history was only modestly associated with enacted MMT stigma. Similarly, stigma mechanisms were not associated with a prior MAT history. Greater internalized MMT stigma was associated with having a lower daily methadone dose.
Table 5.
Construct Validity: Convergent and divergent associations with related constructs (N = 93)
| MMT-SMS | |||||||||
| Stigma Mechanisms: | Anticipated | Enacted | Internalized | ||||||
| Scales (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total |
| Drug Use History | |||||||||
| Live with Others who Use Drugs | .233** | .187* | .216* | .244* | .222* | .212* | .103 | .257** | .165 |
| No. Years Illicit Opioid Use | −.192 | −.197 | −.154 | −.122 | −.052 | −.146 | −.072 | −.036 | −.009 |
| Lifetime Injection History | .080 | .041 | .148 | .058 | .187* | .143 | .230* | .138 | .147 |
| MAT Use History | |||||||||
| Previous MAT History (yes, no) | −.094 | −.114 | −.033 | −.084 | −.083 | −.060 | −.093 | −.057 | .048 |
| No. of Times Previously on MAT | −.108 | −.156 | −.071 | −.035 | .014 | −.061 | .047 | .053 | −.031 |
| Current Methadone Dose (n = 83) | .137 | .105 | .058 | .167 | .081 | .001 | .117 | .089 | −.230* |
| SU-SMS | |||||||||
| Stigma Mechanisms: | Anticipated | Enacted | Internalized | ||||||
| Scales (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total |
| Drug Use History | |||||||||
| Live with Others who Use Drugs | .203* | .189* | .207* | .169 | .179* | .152 | .120 | .171 | .114 |
| No. Years Illicit Opioid Use | −.037 | −.015 | −.010 | −.072 | −.055 | −.089 | −.023 | −.015 | −.118 |
| Lifetime Injection History | .139 | .211* | .096 | .083 | .163 | .219* | .175 | .066 | .145 |
| MAT Use History | |||||||||
| Previous MAT History (yes, no) | .026 | .087 | .038 | −.027 | −.060 | .022 | −.082 | −.062 | .018 |
| No. of Times Previously on MAT | −.076 | −.067 | −.065 | −.069 | −.058 | −.059 | −.100 | .015 | −.039 |
| Current Methadone Dose (n = 83) | .004 | −.043 | .005 | .052 | .058 | −.007 | .040 | .106 | −.065 |
| MMT-SMS and SU-SMS Correlation matrix | |||||||||
| Stigma Mechanisms: | MMT Anticipated | MMT Enacted | MMT Internalized | ||||||
| Scales (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total |
| SU Anticipated (Total) | .627*** | .395*** | .523*** | .592*** | .563*** | .405*** | .382*** | .585*** | .331*** |
| SU Anticipated FAM | .478*** | .360*** | .437*** | .361*** | .464*** | .405*** | .343*** | .387*** | .278** |
| SU Anticipated EMP | .536*** | .313** | .492*** | .484*** | .441*** | .277** | .356*** | .451*** | .265** |
| SU Anticipated HCW | .637*** | .359*** | .448*** | .722*** | .575*** | .379*** | .305*** | .709*** | .332*** |
| SU Enacted (Total) | .580*** | .372*** | .450*** | .767*** | .690*** | .549*** | .542*** | .602*** | .335*** |
| SU Enacted FAM | .401*** | .310** | .354*** | .308** | .474*** | .490*** | .334*** | .331*** | .316** |
| SU Enacted EMP | .420*** | .282** | .401*** | .332*** | .578*** | .510*** | .587*** | .338*** | .186 |
| SU Enacted HCW | .547*** | .287** | .317** | .709*** | .586*** | .308** | .382*** | .739*** | .279** |
| SU Internalized (Total) | .397*** | .348*** | .353*** | .262* | .395*** | .436*** | .269** | .255* | .521*** |
Correlation is significant at 0.001 (***), 0.01(**), or 0.05(*) level (2-tailed). MMT = Methadone Maintenance Treatment. MAT = Medication-assisted Treatment (includes methadone, buprenorphine, LAAM). SU = Substance Use; FAM = Family Members; EMP = Employers; HCW = Healthcare Workers. Inter-scale/subscale correlations along the diagonal appear in bold text.
The MMT-SMS was significantly associated with the corresponding SU-SMS stigma mechanisms scales and stigma source subscales. Effect sizes were predominantly moderate suggesting the two measures are assessing MMT and substance use stigma as distinct experiences. The largest effect sizes emerged for both anticipated (r=0.722) and enacted stigma (r=0.739) from healthcare workers, suggesting that an individual’s methadone status may directly relate to healthcare providers’ perceptions of them as substance users compared to other stigma sources.
Criterion Validity
Greater enacted MMT stigma from family members was significantly and uniquely associated with a lower readiness to reduce drug use or engage in safer injection behaviors (Table 6). Greater anticipated MMT stigma, particularly from family members, was significantly associated with greater heroin use and experiences of opioid withdrawal while on methadone over the previous 12 months. Experiences of MMT stigma were not associated with having injected heroin in the past 6-months, but anticipated MMT stigma was significantly associated with having injected heroin among those who injected in the past 30 days. Experiencing greater enacted MMT stigma, particularly from employers, was associated with a higher proportion of urine toxicology results negative for opioids in the past 6 months.
Table 6.
Criterion Validity: MMT-SMS associations with markers of substance use-related behaviors while receiving MMT
| MMT-SMS | |||||||||
| Stigma Mechanisms: | Anticipated | Enacted | Internalized | ||||||
| Scales (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total |
| Readiness to Change (n = 83, ^n=50) | |||||||||
| Reduce Drug Use | .053 | −.003 | .081 | .045 | −.135 | −.217* | −.088 | −.022 | −.011 |
| Use New/Clean Needle (among injectors^) | −.174 | −.242 | −.149 | −.048 | −.236 | −.360** | −.138 | −.077 | −.228 |
| Cumulative Opioid Experiences (n = 91) | |||||||||
| Mean No. Bags Heroin Used Past 12 mo. | .222* | .303** | .100 | .143 | .148 | .200 | .049 | .104 | −.026 |
| Mean Opioid Withdrawal Past 12 mo. | .242* | .247* | .174 | .170 | .172 | .174 | .072 | .167 | −.062 |
| Recent Opioid Use (n = 84) | |||||||||
| Injected Heroin Past 6 Months | −.039 | .009 | −.069 | .037 | .044 | .080 | .007 | .026 | −.019 |
| Injected Heroin Past 30 Days (n=18) | .420* | .285 | .222 | .490* | .303 | .194 | .269 | .269 | −.019 |
| % Negative UTox Past 6-months† | .137 | .052 | .208 | .074 | .231* | .149 | .320** | .118 | .055 |
| SU-SMS | |||||||||
| Stigma Mechanisms: | Anticipated | Enacted | Internalized | ||||||
| Scales (subscales): | Total | FAM | EMP | HCW | Total | FAM | EMP | HCW | Total |
| Readiness to Change (n = 83, ^n=50) | |||||||||
| Reduce Drug Use | .093 | −.073 | .026 | .081 | −.104 | −.201 | −.100 | −.052 | −.055 |
| Use New/Clean Needle (among injectors^) | −.263 | −.302* | −.236 | −.121 | −.202 | −.303** | −.202 | −.035 | −.368** |
| Cumulative Opioid Experiences (n = 91) | |||||||||
| Mean No. Bags Heroin Used Past 12 mo. | .163 | .173 | .155 | .101 | .062 | .130 | −.133 | .125 | .195 |
| Mean Opioid Withdrawal Past 12 mo. | .119 | .037 | .094 | .185 | .140 | .078 | .050 | .196 | .099 |
| Recent Opioid Use (n = 84) | |||||||||
| Injected Heroin Past 6 Months | .045 | .147 | .053 | −.058 | .035 | .177 | −.004 | −.072 | .098 |
| Injected Heroin Past 30 Days (n=18) | .296 | .156 | .301 | .194 | .546** | .543* | .358 | .207 | .403* |
| % Negative UTox Past 6-months† | .164 | .166 | .175 | .091 | .275* | .154 | .364*** | .156 | .069 |
Correlation is significant at 0.001 (***), 0.01(**), or 0.05(*) level (2-tailed).
= subsample of persons who injected
UTox = Urine Toxicology test for opioids
Participants provided twice-weekly urines for testing over a 6-month period (48 urines total).
Comparing MMT and Substance Use Stigmas
In this sample, both the MMT-SMS and SU-SMS demonstrated good reliability and validity. The SU-SMS, inclusive of the employer stigma source subscale, maintained structural validity (see supplemental material 3: Figure 2, Tables 7–8) and high internal consistency across all stigma mechanisms (α=0.872–0.932) and stigma sources (α=0.804–0.939; Table 4). Compared to the MMT-SMS, experiences of substance use stigma were slightly higher on average, particularly internalized stigma, anticipated stigma from employers, and enacted stigma from family members (Table 4).
As demonstrated in the correlation matrix (Table 5), the MMT-SMS and SU-SMS demonstrate good construct validity distinguishing between experiences of MMT vs. substance use stigma. Both scales had relatively similar patterns of association with the drug use and MAT use constructs, likely reflecting the similarities in the two scales converging around substance use and related treatment options. Notably, only internalized MMT stigma was associated with current methadone dose.
In terms of criterion validity (Table 6), the two scales were more differentiated. Specifically, MMT stigma was uniquely associated with cumulative opioid experiences while receiving MMT in the past 12 months. Having injected heroin in the past 30 days was associated with anticipated MMT stigma, as well as enacted and internalized substance use stigma. While enacted MMT stigma from family members was associated with readiness to change substance use behaviors more broadly, substance use stigma was uniquely associated with lower readiness to change injection-related behaviors. Both enacted MMT and enacted substance use stigma, from employers was associated with having a higher proportion of urine toxicology tests negative for opioids in the past 6-moths. In post-hoc analyses, the association for enacted MMT stigma from employers did not hold among those who were employed (r=0.264, p=0.266; n=20), unemployed (r=0.232, p=0.126; n=45), or on disability (r=0.017, p=0.948; n=17) at month 12. The association for enacted substance use stigma from employers was significant only among those on disability (r=0.697, p=0.008; employed: r=0.200, p=.399; unemployed: r=0.291, p=0.052) at month 12.
DISCUSSION
The current study offers initial evidence that the MMT-SMS is a valid and reliable measure of MMT stigma among persons receiving MMT. To our knowledge, this is the first study to evaluate the psychometric properties of a MMT stigma scale designed to differentiate between three well established manifestations of stigma (anticipated, enacted, internalized) across distinct stigma sources (family, employers, healthcare workers). Content and criterion validity suggests that MMT stigma is distinct from substance use stigma; internalized MMT stigma alone was associated with daily MMT dose.
MMT and substance use stigma appear to play unique roles in undermining MMT-related success. Specifically, anticipated MMT stigma was associated with opioid use, including recent injection of heroin, for participants receiving MMT. In comparison, substance use stigma had broader associations with injection behaviors, including recent injection while receiving MMT and lower readiness to reduce injection risk behaviors. Collectively, the observations across these domains indicate that stigma-reduction interventions to improve MMT success may need to co-address MMT and substance use stigmas. Future work is needed to illuminate the intersecting processes of these two stigmas,47,48 their respective effects on the initiation and retention of PWOUD in MMT over time, and continued substance use behaviors in the context of MMT.
While research on MMT stigma is limited, prior work has predominantly studied it as a continuation of substance use stigma among persons in treatment, versus stigma uniquely related to methadone.17–27,29,49,50 Our findings indicate that PLWOUD receiving MMT are likely contending with substance use and MMT stigma as two distinct but intersecting forces versus merely a continuation of one stigmatized identity. Aligned with efforts to support treatment options for PLWOUD in the US,51 the MMT-SMS could help inform efforts to reduce stigma’s impact on MMT access and improve MMT retention. The MMT-SMS could measure changes in MMT stigma as an outcome of MMT stigma reduction interventions which are currently limited to advocacy programs targeting patient-provider education and New York City’s recent public health campaign to promote MAT engagement, “Living Proof”.52,53
Future prospective work with the MMT-SMS should examine the unique predictive contributions of these three stigma mechanisms. For example, examining whether greater internalized MMT stigma is associated lower MMT dose because the patient is being prescribed an inadequate dose that leads to feelings of shame because they perceive their treatment efforts are not working, or if patients with greater shame towards their MMT seek out lower MMT doses. The Stigma Framework has been used to guide HIV stigma research, identifying how stigma mechanisms impact physical, emotional, and behavioral health.40 Similar applications likely exist for the MMT-SMS. Previous research in Taiwan observed that greater perceived criticism from family members was associated with significantly lower MMT retention.54 In this study, we found that a lower readiness to reduce substance use among participants was significantly associated with enacted MMT stigma from family. Greater anticipated MMT stigma from family members was also significantly associated with greater heroin use and self-reported opioid withdrawal over a 12-month period of receiving MMT. The MMT-SMS could help inform our understanding of family relationships among PLWOUD on maximizing the long-term therapeutic effects of MMT.
Improvement in employment status is an important MMT outcome.55,56 We observed that experiencing greater enacted MMT stigma or substance use stigma from employers was associated with negative urine toxicology tests for opioid use. In post-hoc analyses this association was only maintained for PWOUD on disability who had prior experiences of substance use stigma from employers. Our study was not designed to investigate differences in stigma experiences by employment status; thus, caution should be used in interpreting these results. Additional research is needed to elucidate the relationship between enacted MMT and substance use stigma from employers and MMT outcomes.
This study has several limitations despite the MMT-SMS’s theoretical and psychometric strengths. First, our relatively small sample size approached the ideal range for confirmatory factor analysis,57 yet achieved good model fit despite being modestly underpowered. As a cross sectional validation study, we were unable to assess test-retest reliability. Our sample was also limited to PLWOUD enrolled in MMT over a 12-month period in the Northeastern US and may not be generalizable to other US or international settings. Therefore, the MMT-SMS would benefit from being assessed at multiple time points in larger samples of MMT-experienced populations, including those not currently receiving MMT, with greater diversity in terms of age (younger persons with a history of MMT), culture/language, sexual orientation, opioid type (prescription, heroin), and route of administration (pills, recent injection).
The MMT-SMS is specific to MMT stigma. Current efforts to widely expand MAT access include a focus on other pharmaceutical treatments for OUD (i.e. buprenorphine, naltrexone), and future research will require an understanding of whether the MMT-SMS can be adapted to these other treatment modalities. Some important differences may exist between these treatment options. Therefore, current research from our group is adapting the MMT-SMS to examine this measures’ performance among buprenorphine patients.
Supplementary Material
Acknowledgments
CONFLICTS OF INTEREST AND SOURCES OF FUNDING
LRS (K01 DA039767), MLM (T32 DA023356; 3R01 DA040648–02S1), MMC (K02 DA033139), COC (K24 DA036955), and VAE (K01 DA042881) are currently funded by NIH through the National Institute on Drug Abuse. COC’s spouse is employed by Quest Diagnostics and they hold stocks and stock options in Quest Diagnostics. The other authors have no conflicts of interest to declare.
Contributor Information
Laramie R. Smith, Division of Infectious Diseases and Global Public Health, University of California San Diego, La Jolla, CA, United States
Maria Luisa Mittal, Division of Infectious Diseases and Global Public Health, University of California San Diego, La Jolla, CA, United States
Karla Wagner, School of Community Health Sciences, University of Nevada, Reno, Reno, NV
Michael M. Copenhaver, University of Connecticut, Storrs, CT, United States
Chinazo O. Cunningham, Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, United States
Valerie A. Earnshaw, Department of Human Development and Family Sciences, University of Delaware, Newark, DE, United States
REFERENCES
- 1.United Nations Office of Drugs and Crime. World drug report 2017. United Nations publication; 2017. [Google Scholar]
- 2.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;CD011117(5):1–65. doi: DOI: 10.1002/14651858.CD011117.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Whelan PJ, Remski K. Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45–50. doi: 10.4103/0976-3147.91934 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;6(2):CD002207. [DOI] [PubMed] [Google Scholar]
- 5.Gowing L, Farrel MF, Bronemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;10(8):CD004145. [DOI] [PubMed] [Google Scholar]
- 6.United States Government Accountability Office (GAO). Opioid use disorders: HHS needs measuers to assess the effectiveness of efforts to expand access to medication-assisted treatment. October 2017;GAO-18–44:1–36. https://www.gao.gov/assets/690/688047.pdf. Accessed 02/06/2018.
- 7.Volkow ND. Medications for opioid use disorder: Bridging the gap in care. Lancet. 2017;391(10118):285–287. doi: S0140–6736(17)32893–3. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JA, Schwartz RP, Mitchell SG, et al. Why don’t out-of-treatment individuals enter methadone treatment programmes? Int J Drug Policy. 2010;21(1):36–42. doi: 10.1016/j.drugpo.2008.07.004 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goffman E. Stigma: Notes on the management of spoiled identity. New York: Touchstone; 1963. [Google Scholar]
- 10.Link BG, Phelan JC. Conceptualizing stigma. Annual review of Sociology. 2001;27(1):363–385. [Google Scholar]
- 11.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: A conceptual framework and implications for action. Soc Sci Med. 2003;57(1):13–24. [DOI] [PubMed] [Google Scholar]
- 12.Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd C. The stigmatization of problem drug users: A narrative literature review. Drugs: education, prevention and policy. 2013;20(2):85–95. [Google Scholar]
- 14.Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88(2):188–196. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy-Hendricks A, Barry CL, Gollust SE, Ensminger ME, Chisolm MS, McGinty EE. Social stigma toward persons with prescription opioid use disorder: Associations with public support for punitive and public health–oriented policies. Psychiatric services. 2017;68(5):462–469. [DOI] [PubMed] [Google Scholar]
- 16.Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder—and its treatment. JAMA. 2014;311(14):1393–1394. [DOI] [PubMed] [Google Scholar]
- 17.Gourlay J, Ricciardelli L, Ridge D. Users’ experiences of heroin and methadone treatment. Subst Use Misuse. 2005;40(12):1875–1882. [DOI] [PubMed] [Google Scholar]
- 18.Anstice S, Strike CJ, Brands B. Supervised methadone consumption: Client issues and stigma. Subst Use Misuse. 2009;44(6):794–808. [DOI] [PubMed] [Google Scholar]
- 19.Murphy S, Irwin J. “Living with the dirty secret”: Problems of disclosure for methadone maintenance clients. J Psychoactive Drugs. 1992;24(3):257–264. [DOI] [PubMed] [Google Scholar]
- 20.Earnshaw V, Smith L, Copenhaver M. Drug addiction stigma in the context of methadone maintenance therapy: An investigation into understudied sources of stigma. International journal of mental health and addiction. 2013;11(1):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo J, Bhalerao A, Bawor M, et al. “Don’t judge a book by its cover”: A qualitative study of methadone patients’ experiences of stigma. Substance abuse: research and treatment. 2017;11:1–12. doi: DOI: 10.1177/1178221816685087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran BX, Vu PB, Nguyen LH, et al. Drug addiction stigma in relation to methadone maintenance treatment by different service delivery models in vietnam. BMC Public Health. 2016;16(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deering DE, Sheridan J, Sellman JD, et al. Consumer and treatment provider perspectives on reducing barriers to opioid substitution treatment and improving treatment attractiveness. Addict Behav. 2011;36(6):636–642. [DOI] [PubMed] [Google Scholar]
- 24.Conner KO, Rosen D. “You’re nothing but a junkie”: Multiple experiences of stigma in an aging methadone maintenance population. Journal of social work practice in the addictions. 2008;8(2):244–264. [Google Scholar]
- 25.Crawford S. Shouting through bullet-proof glass: Some reflections on pharmacotherapy provision in one Australian clinic. Int J Drug Policy. 2013;24(6):e14–7. doi: 10.1016/j.drugpo.2013.07.004 [doi]. [DOI] [PubMed] [Google Scholar]
- 26.Yarborough BJH, Stumbo SP, McCarty D, Mertens J, Weisner C, Green CA. Methadone, buprenorphine and preferences for opioid agonist treatment: A qualitative analysis. Drug & Alcohol Dependence. 2016;160:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer B, Chin AT, Kuo I, Kirst M, Vlahov D. Canadian illicit opiate users’ views on methadone and other opiate prescription treatment: An exploratory qualitative study. Subst Use Misuse. 2002;37(4):495–522. [DOI] [PubMed] [Google Scholar]
- 28.Luoma JB, Twohig MP, Waltz T, et al. An investigation of stigma in individuals receiving treatment for substance abuse. Addict Behav. 2007;32(7):1331–1346. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren B, Eklund M, Melin Y, Graneheim UH. From resistance to existence—Experiences of medication-assisted treatment as disclosed by people with opioid dependence. Issues Ment Health Nurs. 2015;36(12):963–970. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee TI, Wickersham JA, Desai MM, Pillai V, Kamarulzaman A, Altice FL. Factors associated with interest in receiving prison-based methadone maintenance therapy in malaysia. Drug Alcohol Depend. 2016;164:120–127. doi: 10.1016/j.drugalcdep.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayman DJ, Goldstein MF, Deren S, Rosenblum A. Predicting treatment retention with a brief “Opinions about methadone” scale. J Psychoactive Drugs. 2006;38(1):93–100. [DOI] [PubMed] [Google Scholar]
- 32.Hunt DE, Lipton DS, Goldsmith DS, Strug DL, Spunt B. “It takes your heart”: The image of methadone maintenance in the addict world and its effect on recruitment into treatment. Int J Addict. 1985;20(11–12):1751–1771. [DOI] [PubMed] [Google Scholar]
- 33.Stancliff S, Myers JE, Steiner S, Drucker E. Beliefs about methadone in an inner-city methadone clinic. Journal of Urban Health. 2002;79(4):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Nguyen H, Nguyen HLT, Mai HT, et al. Stigmatization among methadone maintenance treatment patients in mountainous areas in northern vietnam. Harm reduction journal. 2017;14(1):1. doi: DOI 10.1186/s12954-016-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luoma JB, Nobles RH, Drake CE, et al. Self-stigma in substance abuse: Development of a new measure. Journal of psychopathology and behavioral assessment. 2013;35(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etesam F, Assarian F, Hosseini H, Ghoreishi FS. Stigma and its determinants among male drug dependents receiving methadone maintenance treatment. Arch Iran Med. 2014;17(2):108–114. [PubMed] [Google Scholar]
- 37.Bozinoff N, Anderson BJ, Bailey GL, Stein MD. Correlates of stigma severity among persons seeking opioid detoxification. J Addict Med. 2018;12(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Link BG, Struening EL, Rahav M, Phelan JC, Nuttbrock L. On stigma and its consequences: Evidence from a longitudinal study of men with dual diagnoses of mental illness and substance abuse. J Health Soc Behav. 1997;38(2):177–190. [PubMed] [Google Scholar]
- 39.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: A review of HIV stigma mechanism measures. AIDS and Behavior. 2009;13(6):1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: A test of the HIV stigma framework. AIDS Behav. 2013;17(5):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith LR, Earnshaw VA, Copenhaver MM, Cunningham CO. Substance use stigma: Reliability and validity of a theory-based scale for substance-using populations. Drug Alcohol Depend. 2016;162:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith LR, Fisher JD, Cunningham CO, Amico KR. Understanding the behavioral determinants of retention in HIV care: A qualitative evaluation of a situated information, motivation, behavioral skills model of care initiation and maintenance. AIDS Patient Care and STDs. 2012;26(6):344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beatty PC, Willis GB. Research synthesis: The practice of cognitive interviewing. Public Opin Q. 2007;71(2):287–311. [Google Scholar]
- 44.Copenhaver MM, Lee I, Baldwin P. A randomized controlled trial of the community-friendly health recovery program (CHRP) among high-risk drug users in treatment. AIDS and Behavior. 2013;17(9):2902–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muthén L, Muthén B. Mplus user’s guide (1998–2012). Seventh Edition. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 46.Brown T. Confirmatory factor analysis for applied research: The guilford press; 2006. [Google Scholar]
- 47.Reidpath DD, Chan KY. A method for the quantitative analysis of the layering of HIV-related stigma. AIDS Care. 2005;17(4):425–432. [DOI] [PubMed] [Google Scholar]
- 48.Berger MT. Workable sisterhood: The political journey of stigmatized women with HIV/AIDS. Princeton: University Press; 2010. [Google Scholar]
- 49.Cooper S, Nielsen S. Stigma and social support in pharmaceutical opioid treatment populations: A scoping review. International Journal of Mental Health and Addiction. 2017;15(2):452–469. [Google Scholar]
- 50.Room R. Stigma, social inequality and alcohol and drug use. Drug Alcohol Rev. 2005;24(2):143–155. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 52.Woods JS, Joseph H. Reducing stigma through education to enhance medication-assisted recovery. Journal of addictive diseases. 2012;31(3):226–235. [DOI] [PubMed] [Google Scholar]
- 53.NYC DOH. Health department expands public education about the opioid overdose epidemic; launches “Living proof” campaign featuring new yorkers recovering from opioid addiction. New York City Department of Health and Mental Hygiene, New York: 2017. [Google Scholar]
- 54.Lee C, Wang T, Tang H, Liu Y, Bell J. Familial expressed emotion among heroin addicts in methadone maintenance treatment: Does it matter? Addict Behav. 2015;45:39–44. [DOI] [PubMed] [Google Scholar]
- 55.Zanis DA, Metzger DS, McLellan AT. Factors associated with employment among methadone patients. J Subst Abuse Treat. 1994;11(5):443–447. [DOI] [PubMed] [Google Scholar]
- 56.Richardson L, Wood E, Montaner J, Kerr T. Addiction treatment-related employment barriers: The impact of methadone maintenance. J Subst Abuse Treat. 2012;43(3):276–284. doi: 10.1016/j.jsat.2011.12.008 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelloway EK. Using Mplus for structural equation modeling: A researcher’s guide. SAGE Publications; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.