Mazzetti et al. reveal the presence of α-synuclein oligomers within synaptic terminals in the skin, and show that their levels are increased in patients with Parkinson’s disease. The sensitivity, specificity and positive predictive value of this assay suggest its potential suitability as a diagnostic tool.
Keywords: α-synuclein oligomers, Parkinson’s disease, skin biopsy, monozygotic twins, proximity ligation assay
Abstract
A variety of cellular processes, including vesicle clustering in the presynaptic compartment, are impaired in Parkinson’s disease and have been closely associated with α-synuclein oligomerization. Emerging evidence proves the existence of α-synuclein-related pathology in the peripheral nervous system, even though the presence of α-synuclein oligomers in situ in living patients remains poorly investigated. In this case-control study, we show previously undetected α-synuclein oligomers within synaptic terminals of autonomic fibres in skin biopsies by means of the proximity ligation assay and propose a procedure for their quantification (proximity ligation assay score). Our study revealed a significant increase in α-synuclein oligomers in consecutive patients with Parkinson’s disease compared to consecutive healthy controls (P < 0.001). Proximity ligation assay score (threshold value > 96 using receiver operating characteristic) was found to have good sensitivity, specificity and positive predictive value (82%, 86% and 89%, respectively). Furthermore, to disclose the role of putative genetic predisposition in Parkinson’s disease aetiology, we evaluated the differential accumulation of oligomers in a unique cohort of 19 monozygotic twins discordant for Parkinson’s disease. The significant difference between patients and healthy subjects was confirmed in twins. Intriguingly, although no difference in median values was detected between consecutive healthy controls and healthy twins, the prevalence of healthy subjects positive for proximity ligation assay score was significantly greater in twins than in the consecutive cohort (47% versus 14%, P = 0.019). This suggests that genetic predisposition is important, but not sufficient, in the aetiology of the disease and strengthens the contribution of environmental factors. In conclusion, our data provide evidence that α-synuclein oligomers accumulate within synaptic terminals of autonomic fibres of the skin in Parkinson’s disease for the first time. This finding endorses the hypothesis that α-synuclein oligomers could be used as a reliable diagnostic biomarker for Parkinson’s disease. It also offers novel insights into the physiological and pathological roles of α-synuclein in the peripheral nervous system.
Introduction
α-Synuclein oligomers have recently been indicated as ‘a new hope’ in the field of synucleinopathies, including Parkinson’s disease and dementia with Lewy bodies (Roberts et al., 2015; Bengoa-Vergniory et al., 2017). The oligomeric species of α-synuclein consist in small aggregates of the protein that have not yet acquired a fibrillar conformation, which occur in the early stage of the pathology, preceding and probably triggering the formation of pale bodies and Lewy bodies. In this field, Roberts et al. (2015) searched for oligomeric α-synuclein species in post-mortem brain from Parkinson’s disease patients using the proximity ligation assay (PLA), an innovative and simple approach capable of detecting in situ protein interactions (e.g. protein dimerization). The resulting α-synuclein PLA signal was significantly more abundant in patients than in healthy control subjects, confirming the wide distribution of α-synuclein oligomers in Parkinson’s disease-affected brains and suggesting the relevance of this approach for studying pathology progression starting from the early stage (Roberts et al., 2015).
Recently, Parkinson’s disease has been redefined as a multisystem disorder not limited to the CNS (reviewed in Chaudhuri and Sauerbier, 2016; Klingelhoefer and Reichmann, 2017). Several clues led to this statement. First, the prodromic stage of Parkinson’s disease is often characterized by autonomic non-motor symptoms including constipation and sweating disturbance (Cersosimo and Benarroch, 2008; Jost, 2010; Knudsen and Borghammer, 2018). Furthermore, the presence of pathological α-synuclein inclusions was confirmed not only along the enteric nervous system (Wakabayashi et al., 1990) and in the autonomic ganglia (den Hartog Jager and Bethlem, 1960), but also in other body regions (Beach et al., 2010; Gelpi et al., 2014; Braak and Del Tredici, 2017). Second, pure autonomic failure, a syndrome characterized by autonomic symptoms without motor features and due to neurodegeneration of neurons in sympathetic ganglia with α-synuclein-positive Lewy body-like inclusions (Singer et al., 2017), frequently evolves into Parkinson’s disease or multiple system atrophy. Furthermore, 123I-MIBG myocardial scintigraphy is able to distinguish Parkinson’s disease both from healthy subjects, with 95% sensitivity and 88.9% specificity (Shin et al., 2006), and from atypical parkinsonism, with a diagnostic sensitivity of 89.5% (Ryu et al., 2019), as the loss of postganglionic sympathetic fibres occurs exclusively in Parkinson’s disease and dementia with Lewy bodies patients, not in those affected by multiple system atrophy (Knudsen and Borghammer, 2018). The same denervation was observed in the autonomic structures of the skin, i.e. vessels and sweat glands in patients with Parkinson’s disease (Dabby et al., 2006; Navarro-Otano et al., 2015). This alteration translates into the autonomic dysfunction that may be observed in the prodromal stage of Parkinson’s disease, including sweating disturbance (Swinn et al., 2003).
Given the increasing body of evidence related to defects of the autonomic nervous system in Parkinson’s disease, many studies have investigated the distribution of α-synuclein pathology in the peripheral nervous system looking for an early marker of the pathology and focusing mainly on the presence and accumulation of total and phosphorylated α-synuclein (reviewed in Schneider et al., 2016). To date, the only study concerning α-synuclein oligomers in the autonomic nervous system was performed using the PLA technique in gastrointestinal biopsies of patients with Parkinson’s disease and healthy control subjects (Ruffmann et al., 2018). Despite the positive outcome of peripheral PLA staining, the detection of α-synuclein oligomers in gastrointestinal samples was not considered adequate to diagnose or predict Parkinson’s disease.
In this scenario, the aim of the present study was to search for α-synuclein oligomers in the peripheral nervous system by focusing on skin biopsies, which constitute a simple and minimally invasive model to detect α-synuclein-related pathology in living patients (Zange et al., 2015; Donadio et al., 2016; Gibbons et al., 2016). As studies on monozygotic twins offer the opportunity to control for many potential confounders encountered in general population studies [i.e. differences in genetic background, early-life environmental exposure, age and gender (Castillo-Fernandez et al., 2014)], we conducted a comparative analysis in a cohort of Parkinson’s disease patients and healthy subjects including a noteworthy subgroup of monozygotic twins discordant for the disease.
Materials and methods
Patients and clinical assessment
The case-control study population consisted of 105 subjects: 29 consecutive healthy controls, 38 consecutive Parkinson’s disease patients and 19 couples of monozygotic twins discordant for the disease (total number of Parkinson’s disease cases, n = 57; total number of healthy controls, n = 48). All subjects were enrolled at the Parkinson Institute (Milan, Italy) by neurologists experienced in movement disorders and contributed to the Parkinson Institute Biobank (Filocamo et al., 2013). In twin pairs, monozygosity was confirmed by carrying out a genotyping scan of DNA on peripheral blood samples (2 ml each). Specifically, DNA was extracted using a commercial isolation kit, performing a quantitative fluorescent polymerase chain reaction (QF-PCR) as previously reported (Fernández-Martínez et al., 2007). Patients and controls were balanced for age at assessment and gender. In addition to general demographic data, the following information was collected for all patients: disease duration, clinical rating of activities of daily living and motor symptoms [using parts II and III of the Unified Parkinson’s Disease Rating Scale (UPDRS), respectively (Fahn and Elton, 1987)], disease severity [using the Hoehn and Yahr staging system (Hoehn and Yahr, 1967)], presence of constipation [using Rome III criteria (Barichella et al., 2017)] and orthostatic hypotension [if the patients required the use of specific medication and/or experienced a fall in systolic blood pressure of at least 20 mmHg and diastolic blood pressure of at least 10 mmHg within 3 min of standing (The Consensus Committee of the American Autonomic Society and the American Academy of Neurology, 1996; Cilia et al., 2015)]. The demographic and clinical data of the investigated cohort are reported in Table 1. In addition, the Composite Autonomic Symptom Score 31 (COMPASS 31) (Sletten et al., 2012; Pierangeli et al., 2015), a 31-item self-administered questionnaire addressing six domains of autonomic functions (orthostatic intolerance, vasomotor, secreto-motor, gastrointestinal, bladder, and pupillomotor), was administered to the study population (n = 94; Table 2).
Table 1.
Demographic and clinical data
| Variable | Total PD cases | Total healthy controls | Consecutive idiopathic PD cases | Consecutive healthy controls | PD twins | Healthy twins |
|---|---|---|---|---|---|---|
| (n = 57) | (n = 48) | (n = 38) | (n = 29) | (n = 19) | (n = 19) | |
| Male gender, n (%) | 38 (67) | 26 (54) | 24 (63) | 12 (41) | 14 (74) | 14 (74) |
| Age at biopsy, years, mean (SD) | 61.6 (10.8) | 60.3 (12.0) | 61.4 (9.9) | 58.9 (11.6) | 61.8 (12.6) | 61.8 (12.6) |
| Disease duration, years, mean (SD) | 7.1 (5.6) | – | 7.4 (6.2) | – | 6.4 (4.1) | – |
| UPDRS part II score, mean (SD) | 6.6 (5.1) | – | 6.1 (5.6) | – | 7.4 (4.5) | – |
| UPDRS part III score, mean (SD) | 19.3 (12.4) | – | 20.2 (13.2) | – | 17.6 (10.9) | – |
| Hoehn and Yahr stage, mean (SD) | 1.9 (0.7) | – | 2.0 (0.7) | – | 1.8 (0.7) | – |
| Orthostatic hypotension, n (%) | 4 (7) | – | 4 (11) | – | 0 (0) | – |
| Constipation, n (%) | 34 (60) | – | 22 (58) | – | 12 (63) | – |
PD = Parkinson’s disease; SD = standard deviation.
Table 2.
COMPASS 31 data
| Domain | Total PD cases | Total healthy controls | Consecutive idiopathic PD cases | Consecutive healthy controls | PD twins | Healthy twins |
|---|---|---|---|---|---|---|
| (n = 51) | (n = 43) | (n = 32) | (n = 25) | (n = 19) | (n = 18) | |
| Orthostatic intolerance, mean (SD) | 5.64 (9.71) | 0.65 (3.01) | 4.25 (8.06) | 0.00 (0.00) | 8.00 (11.85) | 1.56 (4.58) |
| Vasomotor, mean (SD) | 0.54 (1.19) | 0.17 (0.67) | 0.55 (1.17) | 0.07 (0.33) | 0.53 (1.25) | 0.32 (0.95) |
| Secreto-motor, mean (SD) | 4.12 (4.06) | 1.30 (2.10) | 3.48 (3.92) | 1.71 (2.23) | 5.19 (4.18) | 0.71 (1.80) |
| Gastrointestinal, mean (SD) | 5.99 (4.07) | 3.74 (3.23) | 5.44 (4.12) | 3.57 (3.59) | 6.91 (3.92) | 3.97 (2.74) |
| Bladder, mean (SD) | 1.63 (1.71) | 1.16 (1.51) | 1.60 (1.54) | 0.8 (1.38) | 1.70 (2.01) | 1.67 (1.58) |
| Pupillo-motor, mean (SD) | 1.46 (1.39) | 0.76 (1.03) | 1.57 (1.41) | 0.8 (0.84) | 1.26 (1.38) | 0.70 (1.27) |
| Total, mean (SD) | 19.38 (14.1) | 7.78 (6.30) | 16.89 (12.12) | 6.95 (5.68) | 23.58 (16.54) | 8.93 (7.08) |
PD = Parkinson’s disease.
Skin biopsy
Volar forearm skin biopsies were fixed in Zamboni solution for 24 h at 4°C. Then the samples were paraffin-embedded and sliced in 3-μm thick serial sections using a microtome (MR2258, Histoline), and processed as follows: (i) one section per patient was stained with haematoxylin and eosin to verify the presence of the dermal autonomic structures we were interested in, i.e. blood vessels, arrector pilorum muscles and sweat glands; (ii) one section for each sample underwent immunohistochemistry assays to assess the presence of total α-synuclein within the synaptic terminal targeting the autonomic structures of the skin; and (iii) three sections for each sample underwent the PLA procedure.
Proximity ligation assay and immunofluorescence
Probe conjugation
Experiments were performed using a Duolink® kit (Sigma-Aldrich), as previously described (Roberts et al., 2015). According to the manufacturer’s instructions, 1 mg/ml rabbit anti-α-synuclein antibody S3062 (SynS3062, Sigma-Aldrich; directed to human α-synuclein, amino acids 111–132) was separately conjugated to the Probemaker oligonucleotides MINUS and PLUS. After probe conjugation, the resulting dilution of α-synuclein antibody was 1:20 of the original stock concentration.
Procedure
After deparaffinization and rehydration, 3-μm sections from skin biopsies were pretreated in 10% formic acid (10 min), then a 20-min blocking step was unrolled with 1% bovine serum albumin (BSA) in 0.01 M phosphate-buffered saline (PBS) plus 0.1% Triton™ X-100 for 30 min. Subsequently, sections were incubated with α-synuclein-PLUS and α-synuclein-MINUS probes (1:100 in PLA diluent) and mouse anti-synaptophysin (Dako, clone DakSynap, 1:100) for 2 h at 37°C. The amplification reaction was accomplished by serial incubation with: (i) ligase in Duolink® ligation solutions for 1 h at 37°C; and (ii) polymerase in Duolink® amplification reagents that were added with the secondary antibody donkey anti-mouse conjugated to Alexa Fluor® 568 (Molecular Probes) for 2 h at 37°C. Finally, samples were counterstained with TO-PRO®-3 (Molecular Probes; 1:1000, 10 min) and mounted using Mowiol®+DABCO®.
To confirm the specificity of probe-conjugated SynS3062, positive controls were obtained by staining mesencephalic brain sections of four patients with Parkinson’s disease, which had notable antigenicity for α-synuclein and present Lewy body pathology, and by comparing them to brain sections of three control subjects (Supplementary Fig. 1). Negative controls were carried out omitting one of the two α-synuclein probes.
Image acquisition and data analysis
Images including synaptophysin-positive autonomic structures (sweat glands, arrector pilorum muscles and arterioles) were collected at × 60 magnification (1024 × 1024) with a water-immersion 40 × objective, using a Leica Sp8 confocal microscope equipped with an Argon laser coupled with a Hybrid Detector (used to acquire PLA signal), a diode-pumped solid-state laser coupled with a photomultiplier tube and a helium/neon mixed gas laser coupled with a Hybrid Detector. For each sample, five-step images were collected, reconstructed in a z-stack and analysed with ImageJ software (NIH). We conducted the quantitative analysis in all the specimens containing the sweat gland (n = 105) that displayed the greatest quantity of synaptic terminals, while the qualitative analysis was performed on arrector pilorum muscles (n = 23 for overall controls; n = 21 for Parkinson’s disease) and arterioles (n = 10 for overall controls; n = 12 for Parkinson’s disease). The presence of α-synuclein oligomers was rated as the area of PLA signal within synapses (synaptophysin-positive), normalized for synaptic density. The synaptic localization of PLA signal was assessed by the superimposition of the mask of the synaptophysin-positive red channel and the PLA-positive green channel. Furthermore, synaptic density was evaluated as the ratio between the synaptophysin-positive area (corresponding to total synaptic terminals) and the total area of the sweat gland (as previously described by Navarro-Otano et al., 2015). The score obtained was used in statistical analysis. All procedures were performed blind to the disease (Parkinson’s disease versus healthy controls) and twin status (consecutive versus twins).
Statistical analysis
Continuous variables are described as mean and standard deviation (SD) or median and interquartile range (IQR) according to normality of distribution. Dichotomous data are reported as counts and percentages, as appropriate. The primary end point of the study was the comparison of PLA density score between consecutive Parkinson’s disease patients and consecutive healthy controls. It was performed using the Mann-Whitney test. Therefore, a multivariable model (with PLA score analysed on log scale) was built to adjust for age, gender and twin status. Three supportive comparisons were performed: (i) consecutive Parkinson’s disease cases versus Parkinson’s disease twins; (ii) consecutive healthy controls versus healthy twins; and (iii) affected twins versus healthy twins. This last comparison was performed using Wilcoxon’s test (non-parametric test for paired data). Similar between-group comparison was considered for the analysis of COMPASS 31 scores. Comparison of dichotomous variables was conducted using Fisher’s exact test. Then, receiver operating characteristic (ROC) curves were plotted to determine the best cut-off in terms of sensitivity and specificity in discriminating Parkinson’s disease cases from controls. The positive predictive value (PPV) and the area under the curve (AUC) as a measure of overall performance were calculated accordingly. The association between PLA scores and clinical parameters (disease duration, UPDRS part II and III scores, Hoehn and Yahr stage, COMPASS 31 and secreto-motor domain of COMPASS 31), as well as that between PLA area, i.e. the area covered by PLA staining inside synaptic terminals and synaptic density, was investigated in Parkinson’s disease patients with the calculation of Spearman’s rank correlation coefficients (ρ). Finally, independent variables [Model 1, disease status and PLA staining (>96); Model 2, disease status and twin status] associated with autonomic failure (COMPASS 31 total score and secreto-motor domain subscore) were investigated using linear regression analysis. The analysis was performed with the Stata 15.1 software (StataCorp). A two-tailed P-value < 0.05 was considered statistically significant.
Ethical approval
The study was performed in agreement with the principles of the Declaration of Helsinki. Subjects participated to the Parkinson Institute Biobank, approved by Local Ethics Committee. Written informed consent was obtained from all subjects. Human brain samples (controls and Parkinson’s disease cases) were obtained from Nervous Tissue Bank of Milan. Written informed consent was obtained prior to acquisition of tissue from all patients.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Results
PLA signal in skin biopsies increases in Parkinson’s disease compared to healthy controls
First, we assessed the specificity of the probe-conjugated-SynS3062 PLA technique in our experimental conditions and carried out the PLA technique in substantia nigra of human brain sections of control subjects (Supplementary Fig. 1A and E) and patients with Parkinson’s disease (Supplementary Fig. 1B and F). PLA signal was absent in 3/3 controls (Supplementary Fig. 1A and E), while it provided both a particular dot-like pattern around pale bodies and widespread staining in the neuronal cell bodies of Parkinson’s disease samples (Supplementary Fig. 1B and F). Total α-synuclein staining, which was assessed by classical immunohistochemistry, was instead present both in controls (Supplementary Fig. 1C and G) and cases (Supplementary Fig. 1D and H). Thus, widespread PLA staining, which was present in 8% of the neuronal cell bodies analysed (n = 91), indicates that PLA specifically recognizes the oligomeric form of α-synuclein, which represents the hallmark of the early stage of the pathology. Furthermore, the lack of PLA staining in the neuronal cell bodies of controls (n = 90) indicates that PLA does not reveal physiological α-synuclein. PLA staining occurs only in pathological aggregates, as previously demonstrated by Roberts et al. (2015).
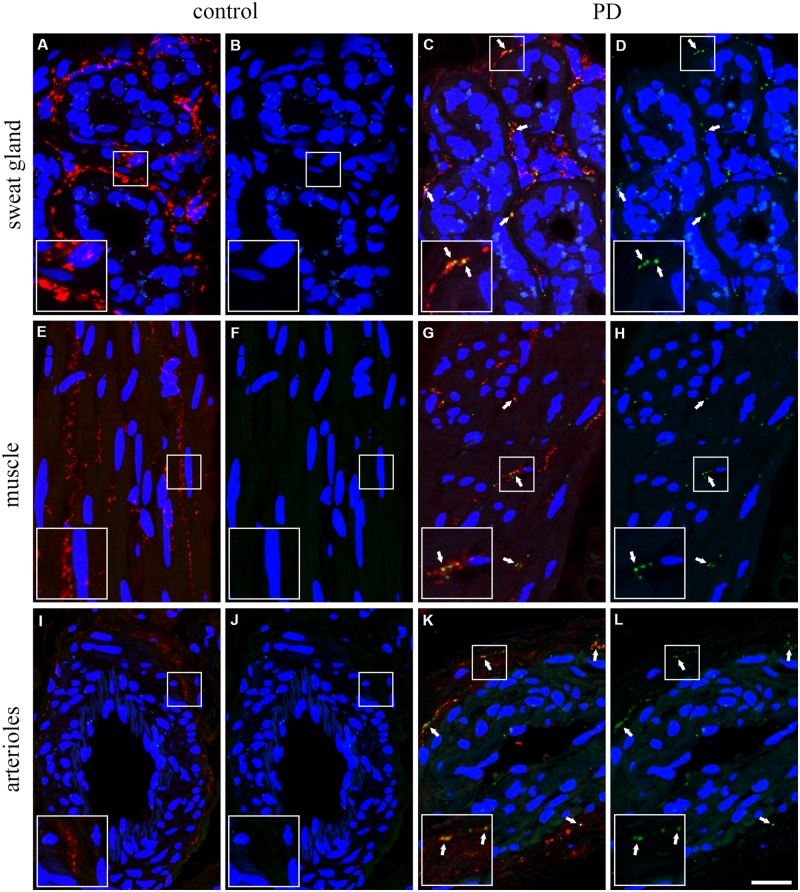
The assay was then performed on skin samples. We initially confirmed the presence of total α-synuclein in all the samples analysed (Supplementary Fig. 2; the specificity of S3062 antibody was verified in immunohistochemistry by preabsorption with purified α-synuclein, as described in the Supplementary material and shown in Supplementary Fig. 3A–F). Then, considering the localization of α-synuclein mainly at the synaptic level, we pursued a single-blind investigation for the presence of α-synuclein oligomers within the synaptic terminals of skin sections, which were specifically stained with an anti-synaptophysin antibody. This strategy enabled us to investigate exclusively the autonomic fibres in the skin based not only on their anatomy (Wang and Gibbons, 2013; Zange et al., 2015; Glatte et al., 2019) but also on the fact that the afferent sensitive terminals are synaptophysin-negative (Murthy and Camilli, 2003; Takamori et al., 2006; Györffy et al., 2018). The synaptophysin staining provided a plentiful and distinctive dot-like signal, highlighting the presence of synaptic terminals in all the autonomic structures of the skin (Fig. 1A, C, E, G, I and J). Negative control for PLA is shown in Supplementary Fig. 3G–I′. In control samples, little or no PLA signal was found within synaptophysin-positive fibres that make contacts with the observed structures (Fig. 1A, B, E, F, I and J). On the contrary, a greater amount of PLA-positive oligomeric species was present within the synaptic terminals targeting sweat glands (Fig. 1C and D), arrector pilorum muscle (Fig. 1G and H) and arterioles (Fig. 1K and L) in samples from Parkinson’s disease patients. The most robust PLA signal was observed in sweat glands, probably due to the greater amount of synaptic terminals featuring this structure (Supplementary Table 1). The staining pattern in twins discordant for Parkinson’s disease was consistent with those of consecutive patients and healthy controls.
Figure 1.
α-Synuclein-PLA staining within synaptic terminals in skin biopsies from controls and patients. In sweat glands (A–D), arrector pilorum muscles (E–H) and blood vessels (I and L–N) the particular dot-like pattern of PLA (green) was detectable in Parkinson’s disease patients (C, D, G, H, M and N; white arrows) within synaptic terminals (synaptophysin-positive, red), while it was mostly absent in healthy controls (A, B, E, F, I and L). Nuclei counterstained with TO-PRO®-3 (blue). Insets show ×2 magnified view of selected squared area. Scale bar = 20 μm. PD = Parkinson’s disease.
PLA score enables the distinction between Parkinson’s disease patients and healthy subjects
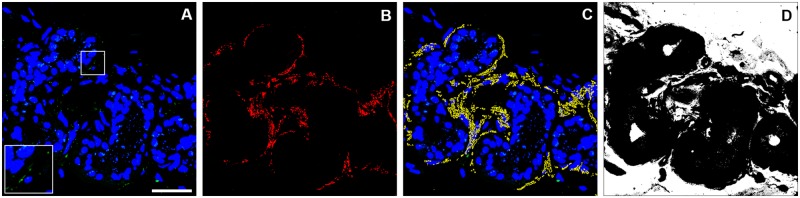
We aimed to quantify α-synuclein oligomers in the skin synaptic terminals. A first aspect to be considered is that, in accordance with a previously described reduction in the autonomic fibres in Parkinson’s disease patients (Dabby et al., 2006; Navarro-Otano et al., 2015), we observed, by semiquantitative analysis, a decrease in the number of synaptic terminals targeting the autonomic structures analysed in 26.3% (n = 15) of Parkinson’s disease samples and 20% (n = 21) of overall population samples (Supplementary Fig. 4), as revealed by the decrease in synaptophysin staining (consecutive controls versus overall Parkinson’s disease population, P = 0.009). Interestingly, we observed a comparable reduction in seven affected monozygotic twins (36.8%) and in four out of their seven healthy twins (21%), which is significantly greater than what was reported in the consecutive healthy cohort (3.4%). To normalize the amount of PLA signal for the presence of synaptic terminals, which reflects on the probability to find α-synuclein oligomers staining within, we performed a quantitative analysis based on the ratio between the area of PLA staining and the synaptic terminal density (Supplementary Table 2), namely PLA score, as visualized in Fig. 2.
Figure 2.
Analytical process in PLA score determination. (A) Representative image of α-synuclein-PLA staining to be quantified. (B) Mask of synaptophysin-positive signal. (C) Superimposition of mask of B (yellow) and A. (D) Total sweat gland area. α-Synuclein-PLA staining (A) was evaluated as the ratio between the area of PLA signal within synaptic terminals and the synaptic density, defined as the ratio between the area of synaptophysin-positive signal (B, red area) and total sweat gland area (D, black area). Inset shows ×2 magnified view of selected squared area. Scale bar = 20 μm. PD = Parkinson’s disease.
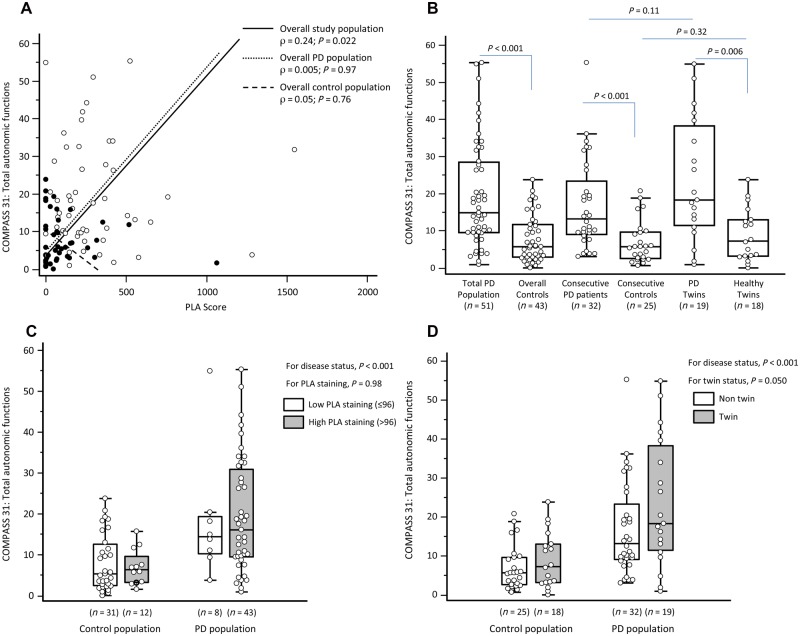
The analysis of the median values of PLA scores (Fig. 3) detected a significantly higher signal in the total affected population than in the overall controls, despite a right-skewed distribution in both groups with a wider dispersion in patients. A significant difference was also found in consecutive patients versus consecutive healthy controls and in affected twins versus healthy twins. PLA scores were similar in consecutive Parkinson’s disease patients and Parkinson’s disease twins, and no significant difference was detected between consecutive healthy controls and healthy twins, although a 2.5-fold higher score was observed in healthy twins. Multivariate analysis confirmed the difference found between patients and overall controls (P < 0.001).
Figure 3.
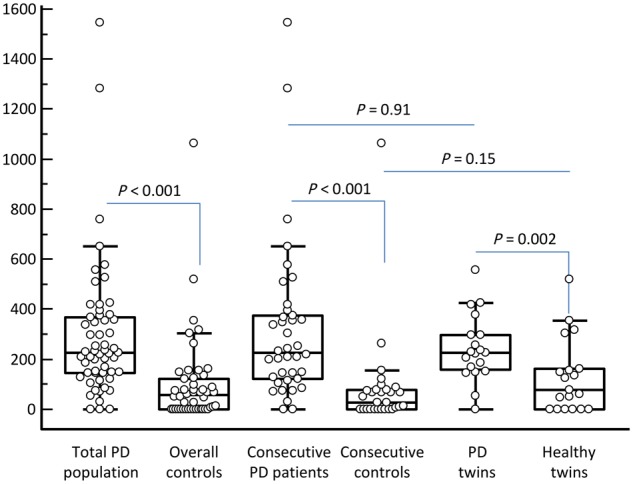
Box and whisker plots of PLA staining in the study population. The box represents the median value (middle line) and the interquartile range (IQR; 25–75th percentile). The external lines extend from the minimum to the maximum value, excluding ‘outside’ (±1.5 times the IQR) and ‘far out’ (±3 times the IQR) values, which are displayed as separate points. PD = Parkinson’s disease.
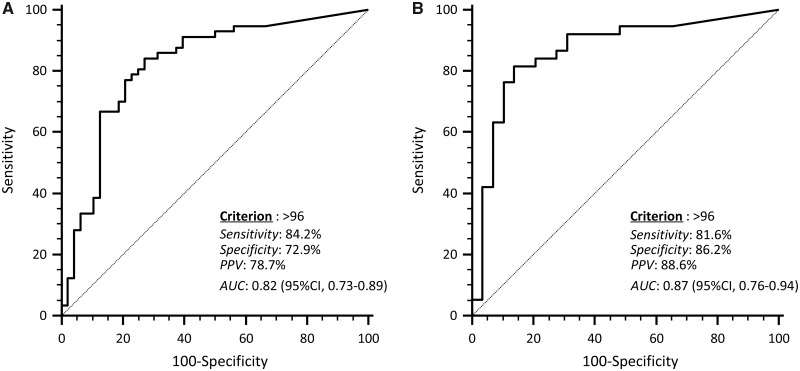
Based on ROC analysis (Fig. 4A), a PLA score >96 in the whole study population had the highest sensitivity and specificity in the identification of Parkinson’s disease patients. However, as Parkinson’s disease twins tended to present a higher PLA staining score, ROC curve was computed also in consecutive patients and consecutive healthy controls (Fig. 4B). The same cut-off presented an even higher performance in discriminating Parkinson’s disease patients from controls. Using this threshold value, the proportions of subjects marked positively were: consecutive Parkinson’s disease patients, 82%; consecutive healthy controls, 14%; Parkinson’s disease twins, 89%; healthy twins, 47% [versus consecutive healthy controls, P = 0.019 (Fisher’s exact test)].
Figure 4.
ROC curve analysis of PLA staining score in identifying Parkinson’s disease patients. The optimal criterion in the overall population (A) and in consecutive Parkinson’s disease cases and consecutive healthy controls (B) is indicated along with its sensitivity, specificity, positive predictive value (PPV) and the area under the curve (AUC).
We report no association between PLA score and clinical features of Parkinson’s disease such as disease duration, UPDRS part II and III scores, Hoehn and Yahr stage and constipation. On the other hand, a mild linear correlation was found between PLA score and both COMPASS 31 total score (Fig. 5A) and secreto-motor domain subscore in the overall study population (Supplementary Fig. 5A). However, no correlation was found between COMPASS 31 and PLA scores in the subgroups of patients and controls. Between-group comparison of the scoring of autonomic failure mainly replicated the findings observed for the analysis of PLA score (COMPASS 31 total score, Fig. 5B; secreto-motor domain score, Supplementary Fig. 5B). Multivariate analysis confirmed that the observed differences in both COMPASS 31 (Fig. 5C) total and secreto-motor domain (Supplementary Fig. 5C) scores mainly depended on disease status and not on increased PLA staining. However, analogue bivariate regression models showed that total COMPASS 31 score was independently associated also with twin status (P = 0.050; Fig. 5D), while no effect was found for secreto-motor domain (P = 0.61; Supplementary Fig. 5D).
Figure 5.
Autonomic function and PLA score. (A) Spearman’s rank correlation between Composite Autonomic Symptom Score 31 (COMPASS 31) and PLA (black dots, controls; white dots, patients). (B) Box and whisker plots of COMPASS 31 in the study population. (C) Box and whisker plots of COMPASS 31 in the study population by disease status and increased PLA staining according to linear regression analysis. (D) Box and whisker plots of COMPASS 31 in the study population by disease status and twin status according to linear regression analysis. For box and whisker plots: the box represents the median value (middle line) and the IQR (25–75th percentile); the external lines extend from the minimum to the maximum value, excluding ‘outside’ (±1.5 times the IQR) and ‘far out’ (±3 times the IQR) values, which are displayed as separate points. PD = Parkinson’s disease.
Discussion
In this study, we aimed to (i) test the hypothesis that patients with Parkinson’s disease are characterized by increased accumulation of α-synuclein oligomers in the synaptic terminals of the autonomic nerve fibres in skin biopsies; and (ii) disclose the role of putative genetic predisposition in contributing to synucleinopathy by evaluating the differential accumulation of oligomers in twins discordant for disease compared to consecutive unrelated Parkinson’s disease patients and healthy controls. Although recent studies have investigated the distribution of α-synuclein oligomeric pathology in the CNS in Parkinson’s disease (Roberts et al., 2015; Sekiya et al., 2019) and multiple system atrophy (Sekiya et al., 2019), proving that α-synuclein oligomers are significantly more abundant in affected brains than in controls, to date no evidence has been reported for the peripheral nervous system. Here, we disclose the significant increase in previously undetected α-synuclein oligomeric species within synaptic terminals of the autonomic nerve fibres in consecutive Parkinson’s disease patients, compared to consecutive healthy subjects. This finding makes α-synuclein oligomers a reasonably good candidate for becoming a reliable biomarker, at least for sporadic forms of Parkinson’s disease. Although no significant difference was detected between the median PLA score of consecutive healthy controls and of healthy twins, the increased prevalence of PLA-positive subjects among healthy twins enabled us to support the notion that genetic predisposition is an important but non-sufficient factor in the aetiology of the disease, and that additional environmental triggers are needed.
To date, peripheral α-synuclein oligomer pathology in Parkinson's disease has been studied by Ruffmann et al. (2018), who investigated the distribution of oligomeric species of α-synuclein within nerve fibres of gastrointestinal samples from patients and healthy controls using the PLA technique. Surprisingly, they did not find any significant difference between the two cohorts, probably because of a methodology-related misinterpretation of data. The authors selected the number of nuclei as a normalization factor in the quantitative analysis, which does not reflect the quantity of nerve fibres in the tissue and flattens any possible difference. Furthermore, they selected calretinin as neuronal marker, although this calcium-binding protein identifies only a subpopulation of neurons (Kunze and Furness, 1999). On the contrary, another investigation on gastrointestinal samples performed using the protein misfolding cyclic amplification technique, proved to be able to distinguish between Parkinson’s disease patients and healthy subjects based on the detection of α-synuclein aggregates (Fenyi et al., 2019) and similar results were obtained by means of real-time quaking-induced conversion (RT-QuIC) on CSF (Fairfoul et al., 2016) and olfactory mucosa (De Luca et al., 2019), thus supporting the presence of α-synuclein pathology both in the peripheral nervous system and in fluids. Here we analysed skin biopsies and showed that 82% of consecutive Parkinson’s disease patients displayed an increase in α-synuclein oligomers compared to consecutive healthy subjects, in 86% of whom oligomers were absent or present in low quantities. Previous studies investigated the distribution of α-synuclein in skin biopsy from living patients by immunohistochemical assays (Tolosa and Vilas, 2015), but different and controversial outcomes arose. Although total α-synuclein was detected both in patients with Parkinson’s disease and control subjects, its amount was greater in patients (Wang et al., 2013; Gibbons et al., 2016). In addition, Gibbons and colleagues reported that the biopsy site does not affect the potency of total α-synuclein detection (90% sensitivity and specificity); for this reason we selected the biopsy site that is best-tolerated by the patients according to our clinical experience, namely the volar forearm. Further studies in the field suggested that the accumulation of phosphorylated α-synuclein in skin biopsy could constitute a high-sensitivity biomarker, which successfully enables the distinction between Parkinson’s disease patients and healthy subjects (Zange et al., 2015; Donadio et al., 2016; Beach et al., 2018; Melli et al., 2018). Study findings on phosphorylated α-synuclein deposits in skin biopsies have provided different ranges of sensitivity and specificity. Donadio et al. (2016) reported a 100% specificity and a sensitivity that depends on the biopsy site (ranging from 31% in distal leg, to 100% in cervical site), whereas more recently, Melli et al. (2018) reported a value of 84% for both parameters, which is consistent with our results. The significance of phosphorylation in the biology and pathophysiology of the protein is controversial (Tenreiro et al., 2014). Despite some reports suggesting that phosphorylated α-synuclein promotes inclusion formation in vitro and in cellular models, other studies reported that, in human brain, phosphorylation of α-synuclein appears to take place in more advanced stages of disease (Zhou et al., 2011; Oueslati, 2016). On the other hand, α-synuclein oligomerization was described as an early event in the pathology (Roberts et al., 2015; Bengoa-Vergniory et al., 2017) that occurs independently of the phosphorylation process. Thus, our findings on α-synuclein oligomers reveal an intracellular event that may occur earlier than phosphorylation of α-synuclein aggregates. This is not in contrast to the current state of the art in the field, but rather it provides further information and insight into the mechanisms underlying onset and progression of α-synuclein pathology.
Our study cohort involves a group of twins discordant for the pathology never explored before, which has provided additional insights on the role played by genetic factors versus environmental factors in the development of Parkinson’s disease. We report that 89% of affected twins exhibited an increased presence of α-synuclein oligomers, findings similar to the consecutive Parkinson’s disease cohort. Interestingly, this pattern was also found in 47% of healthy twins, a significantly higher proportion than that in consecutive healthy subjects (14%). However, the comparison of median values showed only a trend towards significance, which was reasonably due to the skewness of data and the necessarily limited sample size, since the prevalence of Parkinson’s disease and twin pregnancies are 1–2% and 0.3%, respectively (Lees et al., 2009; Goldman et al., 2019). The prevalence of an increase of α-synuclein oligomers among our healthy cohort (14%) does not reflect the prevalence expected for Parkinson’s disease (1% in the selected age group), whereas it is in accordance with the prevalence of incidental Lewy body disease (8–12%; Beach et al., 2008; Dickson et al., 2008), which is characterized by the presence of α-synuclein inclusions in autoptic brain without clinical symptoms of either Parkinson’s disease or dementia with Lewy bodies. Genetic predisposition has proved to be a key element in Parkinson’s disease aetiology, both in genome-wide association studies (Chang et al., 2017) and in a prospective study involving monozygotic twins (Goldman et al., 2019). In this last study, monozygotic twins displayed a concordance of 20% in developing Parkinson’s disease. For these reasons, we can speculate that, among the percentage of healthy twins positive for α-synuclein-PLA (47%), not every subject is going to develop the pathology. Thus, the increased presence of α-synuclein oligomers within peripheral synaptic terminals of healthy twins does not seem to constitute a sufficient condition for the development of the pathology. However, the higher PLA score observed in healthy twins, compared to consecutive healthy controls, converges towards the evaluation of genetic predisposition as an important, but not independent factor responsible for Parkinson’s disease, uncovering the key role of environmental factors, which are more likely to be shared in early and mid-life by twin pairs. Nonetheless, there is evidence that gene–environment interactions should also be also taken into account (Pezzoli and Cereda, 2013).
Neither the meaning of the formation of α-synuclein oligomers in terms of neuronal health or disease, nor how this potentially contributes to α-synuclein pathology has been understood so far. On one side, the toxicity of the oligomeric species of α-synuclein is supported by numerous studies, both in in vitro and in cellular models (Wong and Krainc, 2017; Mor et al., 2019), which point to the identification of several putative targets that could play crucial roles in triggering cell death in Parkinson’s disease and, as a whole, in synucleinopathies. Among others, a dose-dependent increase in α-synuclein oligomerization has been indicated as the cause of axonal transport disruption, leading to synaptic terminal loss in an induced pluripotent stem cells model obtained from Parkinson’s disease patients carrying α-synuclein gene duplication or E46K mutation (Prots et al., 2018). An emerging target for α-synuclein in pathological processes is the microtubule cytoskeleton, given the evidence that α-synuclein interacts with microtubules and their dynamics, and that Parkinson’s disease-linked mutations in α-synuclein corrupt this interaction and interfere with microtubule behaviour in neurons (Cartelli et al., 2016). On the other side, α-synuclein aggregation has recently been proposed to be an epiphenomenon or a protective element, although ‘these conceptual frameworks are difficult to resolve because of the inability to probe brain tissue in real time’ (Espay et al., 2019). An intriguing point is the presence of very small amounts of staining for α-synuclein oligomers observed in control brains (Roberts et al., 2015; Sekiya et al., 2019) and the low percentage of positivity found in the autonomic nervous system of healthy patients, as revealed by our analyses. This indicates that the presence of small amounts of α-synuclein oligomers, insufficient to trigger the pathology, may be physiological, as suggested by the variability observed also in consecutive healthy controls. The correlation analyses between synaptic density and PLA staining—which appeared to be more consistent in Parkinson’s disease patients (Supplementary Fig. 6)—could potentially suggest a role for oligomer accumulation in synaptic degeneration and dysfunction. The detailed analysis of the chemical nature of α-synuclein oligomers in both patients and healthy controls might contribute to explain this picture. Indeed, two different species of α-synuclein oligomers, derived from familial α-synuclein mutants, have been reported (Paslawski et al., 2014). These species exert a different grade of toxicity, mediated by a highly lipophilic element in the structure of α-synuclein, which promotes membrane interactions and disrupts lipid bilayer integrity (Fusco et al., 2017). This is consistent with the fact that α-synuclein mutations involved in familial Parkinson’s disease (in particular A53T and E46K) are translated into vesicle interaction impairment (Auluck et al., 2010). In this context, Bartels et al. (2011) indicated that native human α-synuclein acquires a helically folded tetrameric conformation, which undergoes little or no amyloid-like aggregation.
We further explored the potential relationship between skin α-synuclein oligomer deposition and the neurodegenerative process by taking clinical features into consideration. Similarly, although we found a mild correlation between PLA score and autonomic dysfunction (including measures of the secreto-motor domain) in the overall study population, no relationship was detected in the subgroup of patients. Besides, multivariate analysis disclosed that this relationship substantially depends on the disease status (Parkinson’s disease versus healthy controls). Indeed, high PLA score is found both in Parkinson’s disease patients showing autonomic defects as well as in patients not reporting any autonomic dysfunction. Similar results have been reported for the deposition of total α-synuclein in patients with and without autonomic defects (Gibbons et al., 2016). Nonetheless, despite Parkinson’s disease twins and consecutive Parkinson’s disease patients displaying a comparable PLA score, multivariate analysis showed an independent worsening effect of twin status on autonomic dysfunction (COMPASS 31), which further enables one to argue that the amplification of the neurodegenerative burden is multifactorial (e.g. genetic, environmental, etc). In addition, we did not find any significant association between PLA score and clinical features of Parkinson’s disease, including disease duration, the severity and disability of motor features (as assessed by the UPDRS part II and III scores and the Hoehn and Yahr stage). However, the causal relationship between the severity of Lewy body pathology and neuronal death in the pathophysiology of Parkinson’s disease is still to be clarified (Surmeier et al., 2017; Lang and Espay, 2018). Taken as a whole, the present cross-sectional study design does not enable us to infer a cause-effect relationship between PLA-staining and neuronal synaptic density and clinical features. Therefore, the present results should be interpreted cautiously as the hypothesis of a putative toxic effect of α-synuclein oligomers needs to be addressed in a prospective study.
In conclusion, in this work we report the first evidence of previously undetected α-synuclein oligomers in the autonomic nervous system of the skin and propose their quantitative analysis, which could be a reliable diagnostic biomarker for Parkinson’s disease. Indeed, the sensitivity, specificity and positive predictive value of this parameter are similar to those of scintigraphy (Sudmeyer et al., 2011; Saeed et al., 2017), which is already taken into consideration for diagnostic purposes. In addition, our findings in discordant twins suggest the existence of a genetic predisposition, which increases the probability of developing the pathology. However, our data do not unveil the true pathological meaning of the oligomers, as their presence in pathology could be necessary, but not sufficient, in triggering fibre and neuron degeneration. We believe that further investigation of this cohort could be helpful in uncovering the pathological mechanisms underlying Parkinson’s disease.
Supplementary Material
Acknowledgements
The authors thank all patients and families for their contribution and ‘Fondazione Grigioni per il Morbo di Parkinson’ (Milan-Italy) for long-lasting support. We are grateful to Dr Serena Caronni and Prof. Michela Barichella for their contribution to clinical data interpretation and to Dr Jennifer S. Harthwig for reading and editing the manuscript.
Funding
This work was supported by ‘Fondazione Grigioni per il Morbo di Parkinson’, Milan-Italy, a charitable association linked to Italian Association Parkinsonian (http://www.parkinson.it/fondazione-grigioni.html). The ‘Fondazione Grigioni per il Morbo di Parkinson’ paid for part of laboratory expenses and for the salary of S.M., V.F., E.C., C.B. and support fellows of M.J.B., and A.M.C. The DNA samples and skin biopsies were obtained from the ‘Parkinson Institute Biobank’, member of the Telethon Network of Genetic Biobank (http://biobanknetwork.telethon.it) funded by TELETHON Italy.
Competing interests
The authors report no competing interests.
Glossary
Abbreviations
- PLA
proximity ligation assay
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- Auluck PK, Caraveo G, Lindquist S.. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol 2010; 26: 211–33. [DOI] [PubMed] [Google Scholar]
- Barichella M, Cereda E, Cassani E, Frazzitta G, Pezzoli G.. A focus on Rome III criteria for the assessment of constipation in Parkinson’s disease. Mov Disord 2017; 32: 630.. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ.. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011; 477: 107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 2008; 115: 445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL III, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010; 119: 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Serrano GE, Kremer T, Canamero M, Dziadek S, Sade H, et al. Immunohistochemical method and histopathology judging for the systemic synuclein sampling study (S4). J. Neuropathol Exp Neurol 2018; 77: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J.. Alpha-synuclein oligomers: a new hope. Acta Neuropathol 2017; 134: 819–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K.. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the Wheat from the Chaff. J Parkinsons Dis 2017; 7: S71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartelli D, Aliverti A, Barbiroli A, Santambrogio C, Ragg EM, Casagrande FVM, et al. α-Synuclein is a Novel Microtubule Dynamase. Sci Rep 2016; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Fernandez JE, Spector TD, Bell JT.. Epigenetics of discordant monozygotic twins: implications for disease. Genome Med 2014; 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE.. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008; 23: 1065–75. [DOI] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 2017; 49: 1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Sauerbier A.. Parkinson disease. Unravelling the nonmotor mysteries of Parkinson disease. Nat Rev Neurol 2016; 12: 10–11. [DOI] [PubMed] [Google Scholar]
- Cilia R, Cereda E, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, et al. Parkinson’s disease beyond 20 years. J Neurol Neurosurg Psychiatry 2015; 86: 849–55. [DOI] [PubMed] [Google Scholar]
- Dabby R, Djaldetti R, Shahmurov M, Treves TA, Gabai B, Melamed E, et al. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm 2006; 113: 1169–76. [DOI] [PubMed] [Google Scholar]
- De Luca CMG, Elia AE, Portaleone SM, Cazzaniga FA, Rossi M, Bistaffa E, et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl Neurodegener 2019; 8: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog Jager WA, Bethlem J.. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 1960; 23: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 2008; 115: 437–44. [DOI] [PubMed] [Google Scholar]
- Donadio V, Incensi A, Piccinini C, Cortelli P, Giannoccaro MP, Baruzzi A, et al. Skin nerve misfolded α-synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 2016; 79: 306–16. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Vizcarra JA, Marsili L, Lang AE, Simon DK, Merola A, et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology 2019; 92: 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton R.. Members of the UPDRS Development Committee Unified Parkinson’s disease rating scale In: Recent development in Parkinson disease. Florham Park, NJ: Macmillan Health care Information; 1987. p. 153–63. [Google Scholar]
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 2016; 3: 812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyi A, Leclair-Visonneau L, Clairembault T, Coron E, Neunlist M, Melki R, et al. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol Dis 2019; 129: 38–43. [DOI] [PubMed] [Google Scholar]
- Fernández-Martínez FJ, Galindo A, Moreno-Izquierdo A, Gómez-Rodríguez MJ, Moreno-García M, Grañeras A, et al. Application of QF-PCR for the prenatal assessment of discordant monozygotic twins for fetal sex. Prenat Diagn 2007; 27: 648–52. [DOI] [PubMed] [Google Scholar]
- Filocamo M, Baldo C, Goldwurm S, Renieri A, Angelini C, Moggio M, et al. Telethon network of genetic biobanks: a key service for diagnosis and research on rare diseases. Orphanet J Rare Dis 2013; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco G, Chen SW, Williamson PTF, Cascella R, Perni M, Jarvis JA, et al. Structural basis of membrane disruption and cellular toxicity by a-synuclein oligomers. Science 2017; 358: 1440–3. [DOI] [PubMed] [Google Scholar]
- Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 2014; 29: 1010–8. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R.. The diagnostic discrimination of cutaneous α-synuclein deposition in Parkinson disease. Neurology 2016; 87: 505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatte P, Buchmann SJ, Hijazi MM, Illigens B-W, Siepmann T.. Architecture of the cutaneous autonomic nervous system. Front Neurol 2019; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM, Marek K, Ottman R, Meng C, Comyns K, Chan P, et al. Concordance for Parkinson’s disease in twins: a 20-year update. Ann Neurol 2019; 85: 600–5. [DOI] [PubMed] [Google Scholar]
- Györffy BA, Kun J, Török G, Bulyáki É, Borhegyi Z, Gulyássy P, et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc Natl Acad Sci USA 2018; 115: 6303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD.. Parkinsonism: onset, progression, and mortality. Neurology 1967; 17: 427.. [DOI] [PubMed] [Google Scholar]
- Jost WH. Gastrointestinal dysfunction in Parkinson’s Disease. J Neurol Sci 2010; 289: 69–73. [DOI] [PubMed] [Google Scholar]
- Klingelhoefer L, Reichmann H.. Parkinson’s disease as a multisystem disorder. J Neural Transm 2017; 124: 709–13. [DOI] [PubMed] [Google Scholar]
- Knudsen K, Borghammer P.. Imaging the autonomic nervous system in Parkinson’s Disease. Curr Neurol Neurosci Rep 2018; 18: 79.. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB.. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 1999; 61: 117–42. [DOI] [PubMed] [Google Scholar]
- Lang AE, Espay AJ.. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov Disord 2018; 33: 660–77. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T.. Seminar Parkinson’s disease. Lancet 2009; 373: 2055–66. [DOI] [PubMed] [Google Scholar]
- Melli G, Vacchi E, Biemmi V, Galati S, Staedler C, Ambrosini R, et al. Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann Clin Transl Neurol 2018; 5: 1394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor DE, Daniels MJ, Ischiropoulos H.. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord 2019; 34: 167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Camilli PD.. Cell biology of the presynaptic terminal. Annu Rev Neurosci 2003; 26: 701–28. [DOI] [PubMed] [Google Scholar]
- Navarro-Otano J, Casanova-Mollà J, Morales M, Valls-Solé J, Tolosa E.. Cutaneous autonomic denervation in Parkinson’s disease. J Neural Transm 2015; 122: 1149–55. [DOI] [PubMed] [Google Scholar]
- Oueslati A. Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: what have we learned in the last decade? J Parkinsons Dis 2016; 6: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslawski W, Mysling S, Thomsen K, Jørgensen TJD, Otzen DE.. Co-existence of two different α-synuclein oligomers with different core structures determined by hydrogen/deuterium exchange mass spectrometry. Angew Chem Int Ed 2014; 53: 7560–3. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Cereda E.. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013; 80: 2035–41. [DOI] [PubMed] [Google Scholar]
- Pierangeli G, Turrini A, Giannini G, Del Sorbo F, Calandra-Buonaura G, Guaraldi P, et al. Translation and linguistic validation of the composite autonomic symptom score COMPASS 31. Neurol Sci 2015; 36: 1897–902. [DOI] [PubMed] [Google Scholar]
- Prots I, Grosch J, Brazdis R-M, Simmnacher K, Veber V, Havlicek S, et al. α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci USA 2018; 115: 7813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RF, Wade-Martins R, Alegre-Abarrategui J.. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain 2015; 138: 1642–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffmann C, Bengoa-Vergniory N, Poggiolini I, Ritchie D, Hu MT, Alegre-Abarrategui J, et al. Detection of alpha-synuclein conformational variants from gastro-intestinal biopsy tissue as a potential biomarker for Parkinson’s disease. Neuropathol Appl Neurobiol 2018; 44: 722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu DW, Kim JS, Lee JE, Oh YS, Yoo SW, Yoo IR, et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical parkinsonian syndromes. Clin Nucl Med 2019; 44: 282–8. [DOI] [PubMed] [Google Scholar]
- Saeed U, Compagnone J, Aviv RI, Strafella AP, Black SE, Lang AE, et al. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: current and emerging concepts. Transl Neurodegener 2017; 6: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SA, Boettner M, Alexoudi A, Zorenkov D, Deuschl G, Wedel T.. Can we use peripheral tissue biopsies to diagnose Parkinson’s disease? A review of the literature. Eur J Neurol 2016; 23: 247–61. [DOI] [PubMed] [Google Scholar]
- Sekiya H, Kowa H, Koga H, Takata M, Satake W, Futamura N, et al. Wide distribution of alpha-synuclein oligomers in multiple system atrophy brain detected by proximity ligation. Acta Neuropathol 2019; 137: 455–66. [DOI] [PubMed] [Google Scholar]
- Shin DH, Lee PH, Bang OY, Joo IS, Huh K.. Clinical implications of cardiac-MIBG SPECT in the differentiation of parkinsonian syndromes. J Clin Neurol 2006; 2: 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Berini SE, Sandroni P, Fealey RD, Coon EA, Suarez MD, et al. Pure autonomic failure predictors of conversion to clinical CNS involvement. Neurology 2017; 88: 1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W.. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc 2012; 87: 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudmeyer M, Antke C, Zizek T, Beu M, Nikolaus S, Wojtecki L, et al. Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med 2011; 52: 733–40. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM.. Parkinson’s disease is not simply a prion disorder. J Neurosci 2017; 37: 9799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N.. Sweating dysfunction in Parkinson’s disease. Mov Disord 2003; 18: 1459–63. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell 2006; 127: 831–46. [DOI] [PubMed] [Google Scholar]
- Tenreiro S, Eckermann K, Outeiro TF.. Protein phosphorylation in neurodegeneration: friend or foe? Front Mol Neurosci 2014; 7: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46: 1470. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Vilas D.. Peripheral synuclein tissue markers: a step closer to Parkinson’s disease diagnosis. Brain 2015; 138: 2120–2. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F.. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 1990; 79: 581–3. [DOI] [PubMed] [Google Scholar]
- Wang N, Gibbons CH.. Skin biopsies in the assessment of the autonomic nervous system In: Buijs RM, Swaab DF, editors. Handbook of clinical neurology. Amsterdam: Elsevier B.V.; 2013. p. 371–8. [DOI] [PubMed] [Google Scholar]
- Wang N, Gibbons CH, Laho J, Freeman R. a-Synuclein in cutaneous autonomic nerves. Neurology 2013; 81: 1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Krainc D.. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 2017; 23: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zange L, Noack C, Hahn K, Stenzel W, Lipp A.. Phosphorylated α-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain 2015; 138: 2310–21. [DOI] [PubMed] [Google Scholar]
- Zhou J, Broe M, Huang Y, Anderson JP, Gai W-P, Milward EA, et al. Changes in the solubility and phosphorylation of α-synuclein over the course of Parkinson’s disease. Acta Neuropathol 2011; 121: 695–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.