Substantial clinical heterogeneity in Parkinson’s disease makes diagnosis, treatment and prognosis challenging. Wilson et al. report that CSF soluble TREM2 correlates with abnormal CSF tau and may differentiate subgroups of patients with Parkinson’s disease according to the presence of comorbid pathologies.
Keywords: Parkinson’s disease, TREM2, tau, biomarker, cerebrospinal fluid
Abstract
Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease and affects 1% of the population above 60 years old. Although Parkinson’s disease commonly manifests with motor symptoms, a majority of patients with Parkinson’s disease subsequently develop cognitive impairment, which often progresses to dementia, a major cause of morbidity and disability. Parkinson’s disease is characterized by α-synuclein accumulation that frequently associates with amyloid-β and tau fibrils, the hallmarks of Alzheimer’s disease neuropathological changes; this co-occurrence suggests that onset of cognitive decline in Parkinson’s disease may be associated with appearance of pathological amyloid-β and/or tau. Recent studies have highlighted the appearance of the soluble form of the triggering receptor expressed on myeloid cells 2 (sTREM2) receptor in CSF during development of Alzheimer’s disease. Given the known association of microglial activation with advancing Parkinson’s disease, we investigated whether CSF and/or plasma sTREM2 differed between CSF biomarker-defined Parkinson’s disease participant subgroups. In this cross-sectional study, we examined 165 participants consisting of 17 cognitively normal elderly subjects, 45 patients with Parkinson’s disease with no cognitive impairment, 86 with mild cognitive impairment, and 17 with dementia. Stratification of subjects by CSF amyloid-β and tau levels revealed that CSF sTREM2 concentrations were elevated in Parkinson’s disease subgroups with a positive tau CSF biomarker signature, but not in Parkinson’s disease subgroups with a positive CSF amyloid-β biomarker signature. These findings indicate that CSF sTREM2 could serve as a surrogate immune biomarker of neuronal injury in Parkinson’s disease.
Introduction
Parkinson’s disease affects 1% of the population above 60 years old (de Lau and Breteler, 2006) and is the most common neurodegenerative disease after Alzheimer’s disease. While Parkinson’s disease is typically considered a disease of motor function, one in four patients meet criteria for mild cognitive impairment (MCI) at diagnosis (Muslimovic et al., 2005; Aarsland et al., 2009) and nearly 80% eventually develop dementia during the course of the disease (Aarsland et al., 2003; Hely et al., 2008; Cholerton et al., 2013). These non-motor symptoms do not improve with dopamine-enhancing therapies and contribute significantly to late morbidity, loss of quality of life, and mortality. Although at initial diagnosis, 6.5% of Parkinson’s disease patients show abnormal CSF levels of amyloid-β42 and tau (Marek et al., 2018), at autopsy, 60–80% of Parkinson’s disease subjects will have developed brain pathology consistent with Alzheimer’s disease, with prominent accumulation of amyloid plaques and/or tau containing neurofibrillary tangles (Tsuang et al., 2013; Dickson et al., 2018; Robinson et al., 2018). Progression of parkinsonism and cognitive decline are accelerated in individuals with Alzheimer’s disease and/or cerebrovascular disease (Tsuang et al., 2013; Dickson et al., 2018; Buchman et al., 2019). Identifying a biomarker that could help predict a change in cognitive function in Parkinson’s disease would be a valuable tool for clinical management and outcome measure for clinical trials. Given the heterogeneous nature of Parkinson’s disease, there is a critical need for novel biomarkers that could parse Parkinson's disease patient subgroups according to underlying co-pathologies to provide further information on individual clinical trajectory (Chen-Plotkin et al., 2018).
Triggering receptor expressed on myeloid cells 2 (TREM2) is an innate immune receptor expressed on the surface of brain microglia (Colonna, 2003). Functionally, TREM2 plays a central role in phagocytosis of apoptotic neurons, misfolded proteins, and cellular debris (Neumann and Takahashi, 2007; Wang et al., 2016; Yeh et al., 2016). TREM2 signalling also enhances microglial survival, proliferation, chemotaxis, and through effects on metabolism, inhibits the microglial proinflammatory response (Hamerman et al., 2006; Turnbull et al., 2006; Otero et al., 2009; Ulland et al., 2017; Parhizkar et al., 2019). Overexpression of TREM2 attenuates neuroinflammation and protects dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease (Ren et al., 2018). In human genetic studies, the loss-of-function p.R47H variant of TREM2 is a risk allele for Alzheimer’s disease (Guerreiro et al., 2013; Jonsson et al., 2013) and sporadic Parkinson’s disease (Benitez et al., 2013; Rayaprolu et al., 2013).
The soluble form of TREM2 (sTREM2) is produced via the cleavage of membrane-bound TREM2 by disintegrin and metalloproteinase domain-containing protein (ADAM) family members, including ADAM10 and ADAM17 (Wunderlich et al., 2013; Kleinberger et al., 2014), and by alternative splicing (Guerreiro et al., 2013). CSF levels of sTREM2 are a sensitive marker of microglial activation and correlate with tau-mediated neuronal injury in Alzheimer’s disease (Kleinberger et al., 2014; Henjum et al., 2016; Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016a, b, 2019). In this cross-sectional retrospective multicentre study, we tested whether CSF and/or blood sTREM2 correlated with CSF concentrations of amyloid-β1-42, amyloid-β1-40, total tau, and phospho-taup181, or with cognitive status.
Materials and methods
Study population
Participants (n = 165) were recruited from the Pacific Udall Center (PUC) cohort consisting of sites at Seattle (VA Puget Sound Health Care System/University of Washington) and Portland (Cholerton et al., 2013) and from the Stanford Movement Disorders Clinic. Participants were included if they were cognitively normal healthy adults or if they met UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria for Parkinson’s disease, had a cognitive diagnosis assigned (no cognitive impairment, MCI) (Cholerton et al.), Parkinson’s disease dementia (PDD) (Cholerton et al.), and if they completed lumbar puncture. Healthy control participants were recruited from the family of Parkinson’s disease participants and from the surrounding community. They had no history of Parkinson’s disease, other neurodegenerative diseases, or chronic neuropsychiatric disorders. They were neurologically normal on comprehensive neurological examination and within 1.5 standard deviations (SD) of age- and education-matched normative values on comprehensive neuropsychological testing. A full neuropsychological battery assessing multiple domains was given to assign a cognitive diagnosis. Participants from Stanford were defined as cognitively impaired if scores were ≥1.5 SD below age- and education-matched normative values on at least two separate neuropsychological measures, regardless of domain (Hendershott et al., 2019). The PUC cognitive diagnoses were assigned at a clinical consensus conference and required evidence of subjective and observed cognitive decline. For all Parkinson’s disease participants, cognitive impairment was further classified as PDD, as opposed to PD-MCI if the impairment was severe enough to interfere with daily activities (Emre et al., 2008). All study protocols were approved by Institutional Review Boards of Stanford University, Oregon Health and Science University, or VA Puget Sound Health Care System/University of Washington. In accordance with the Declaration of Helsinki, written informed consent was obtained from each study participant or their legally authorized representative.
Global cognitive function
Global cognitive function was assessed for all participants using the Montreal Cognitive Assessment (MoCA) test (Nasreddine et al., 2005). The MoCA is the most commonly used cognitive assessment in Parkinson’s disease and has a high sensitivity and specificity for identifying MCI in Parkinson’s disease (Hendershott et al., 2017). The MoCA is used in large multicentre Parkinson’s disease studies because it assesses primary cognitive domains at risk in Parkinson’s disease, has been validated in multiple languages, and requires substantially less time and training to properly administer and score than a full neuropsychological assessment (Chou et al., 2010).
Plasma and CSF collection
For the Stanford University cohort, fasted plasma was collected within 2 weeks of lumbar puncture. For the two PUC cohorts, centres in Seattle and Portland performed CSF and plasma blood draws on the same morning. Samples were collected between 2012 and 2017. All CSF samples were collected in polypropylene tubes and stored in externally threaded Thermo Scientific™ Nalgene™ General Long-Term Storage Cryogenic Tubes. No haemolysis or discoloration was apparent in any of the included CSF or plasma samples (visual inspection that the fluid was clear as water). CSF samples were subjected to a maximum of two freeze-thaw cycles, as recommended by Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry (Lewczuk et al., 2018).
Measurement of Alzheimer’s disease CSF core biomarkers
The following cut-off values for abnormal CSF biomarkers were used: ratio CSF amyloid-β1-42/amyloid-β1-40 <0.10, phospho-tau181 >40 pg/ml, and total tau >456 pg/ml, as measured using Lumipulse® G Assays (Fujirebio) on the Lumipulse® G fully automated platform (Bayart et al., 2019; Paciotti et al., 2019). CSF samples from all three cohorts were measured all together in 1 day by trained operators who were blinded to the clinical information.
CSF and plasma sTREM2 measurement
Electrochemiluminescent immunoassays (ECLIAs) on the Meso Scale Discovery platform were used to measure CSF and plasma sTREM2. MSD GOLD 96-well streptavidin plates were blocked with 3% bovine serum albumin (BSA) and coated with a solution containing 0.25 µg/ml biotinylated polyclonal goat anti-human TREM2 capture antibody (BAF1828, R&D Systems). CSF and plasma samples diluted 1:4 in 1% BSA were added to the prepared plates and incubated overnight at 4°C. Monoclonal mouse anti-human TREM2 detection antibody (B-3, sc373828, Santa Cruz Biotechnology) was added at a concentration of 1 µg/ml, followed by SULFO-TAG anti-mouse secondary antibody (1 µg/ml, R32AC-5, MSD). Plates were washed four times between incubations with phosphate-buffered saline (PBS)/0.05% Tween-20 (PBS-T) buffer. Samples were distributed in a randomized manner across plates and read in duplicate by an operator blinded to clinical information. Plates were read using a MESO QuickPlex SQ 120 running Discovery Workbench v4 software (MSD). CSF and plasma sTREM2 concentrations were calculated using the standard curve generated for each plate using recombinant human TREM2 protein (Sino Biological). A dedicated CSF and plasma sample was loaded onto all plates and used to normalize values. Interplate coefficients of variability were <15%. Samples were measured on the same day using the same reagents.
Statistical analysis
CSF (Shapiro-Wilk normality test, W = 0.8499, P < 0.0001) and plasma sTREM2 (W = 0.2202, P < 0.0001) did not follow a normal distribution and were log10-transformed to approach the assumptions of Gaussian normal distribution. Therefore, all statistical analyses described in this study are performed with the log10-transformed values. Categorical variables were assessed by performing the chi-square test. Association of sTREM2 with continuous variables was evaluated using a linear regression model. Demographic parameters between groups were evaluated using one-way ANOVA followed by Tukey corrected post hoc pairwise comparisons (for parametric data) and Mann-Whitney U-test or Kruskal-Wallis test followed by Dunn’s corrected post hoc comparisons (for non-parametric data). To determine whether CSF and plasma sTREM2 differed among clinically- and biomarker-defined groups, log10-transformed CSF sTREM2 levels were analysed using analysis of covariance (ANCOVA) with clinical diagnosis as fixed factor and age and gender as covariates. Tukey’s multiple comparisons test was performed for post hoc testing. Receiver operating characteristic (ROC) curve analysis was performed to assess CSF sTREM2 in differentiating Parkinson’s disease participants with abnormal CSF tau concentration from those with normal CSF tau concentration. Association between CSF sTREM2 and core Alzheimer’s disease CSF biomarker was assessed using linear mixed-effects model adjusting for age and gender as fixed effects and CSF sample freeze-thaw number as random effect. Association between CSF sTREM2 and MoCA score was assessed using a linear model adjusted for age, gender, years of education, and study site. To rule out the possibility that results were driven by extreme values, analyses were repeated with outliers removed (ROUT method Q = 1.000%) and the analysis yielded similar results. Analyses with outliers included and removed are presented in Supplementary Tables 2–7. Statistical tests were performed using GraphPad Prism software (GraphPad Inc, La Jolla, CA) and the freely available statistical software R (http://www.r-project.org/). All tests were two-sided and a significance level of α = 0.05 was adopted.
Data availability
The data that support the findings of this study are available from the corresponding author, upon request.
Results
Study participant characteristics
Demographic information for the study population is presented in Table 1. The clinically defined diagnostic groups consisted of healthy cognitively unimpaired age-matched controls (‘healthy control’), cognitively normal Parkinson’s disease subjects (PD-Normal), Parkinson’s disease subjects with mild cognitive impairment (PD-MCI) and Parkinson’s disease subjects with dementia (PDD). These groups differed in gender distribution (χ2 = 16.44, df = 1, P < 0.0001), with a larger proportion of male subjects in the PD-MCI and PDD groups. This is in line with previous data indicating that Parkinson’s disease patients who are male are more likely to be cognitively impaired (Cholerton et al., 2018). No differences were observed between any of the diagnostic groups for age [one-way ANOVA: F(3,161) = 2.526; P > 0.05] or disease duration as defined as time in years since diagnosis (Kruskal-Wallis χ2 = 4.767, df = 3, P = 0.092). As expected, performance on the MoCA, a test sensitive to global cognition and executive function in Parkinson’s disease (Hendershott et al., 2017), differed between clinical diagnostic groups (Kruskal-Wallis χ2 = 67.522, df = 3, P < 0.0001). While no significant difference was observed between healthy controls and PD-Normal, the MoCA score was significantly lower in the PD-MCI (P < 0.001) and PDD (P < 0.0001) groups. We observed significant differences in the OFF medicine Movement Disorders Society Unified Parkinson’s disease Rating Scale motor part III (MDS-UPDRS) across Parkinson’s disease diagnoses (Kruskal-Wallis test χ2 = 15.69, df = 3, P = 0.0004), which confirmed equal motor symptom severity in the PD-Normal and PD-MCI groups (P > 0.05), but increased motor symptoms in the PDD compared to both PD-Normal (P < 0.01) and PD-MCI (P < 0.001) groups. Finally, the percentage of APOE ε4 carriers was not significantly different in any of the diagnostic groups (χ2 = 0.419, P = 0.518).
Table 1.
Data summary
| Variable | Total | Healthy control | PD-Normal | PD-MCI | PDD | P-value |
|---|---|---|---|---|---|---|
| n = 165 | n = 17 | n = 45 | n = 86 | n = 17 | ||
| Gender, female/male, n (%) | 52 (32)/113 (68) | 10 (59)/7 (41) | 22 (49)/23 (51) | 17 (20)/69 (80) | 3 (18)/14 (82) | <0.0001a |
| Age, years, mean (SD) | 66.8 (8.32) | 65.06 (6.7) | 64.4 (7.6) | 67.8 (8.3) | 69.5 (10.3) | nsb |
| Disease duration, years, mean (SD) | – | – | 4.6 (3.9) | 5.6 (5.6) | 7.5 (4.8) | nsc |
| MoCA score, mean (SD) | 24.6 (3.9) | 27.4 (2.0) | 27.1 (2.3) | 24.1 (2.5)*, ^ | 17.8 (5.1)#, ^ ,† | <0.0001c |
| MDS-UPDRS, part III OFF, mean (SD) | 26.8 (16.4) | 1.18 (1.5) | 29.1 (14.6) | 27.5 (13.8) | 43.2 (12.5)$,† | <0.0001c,d |
| APOE ε4 allele, % carriage | 23.0 | 17.6 | 32.6 | 19.8 | 20.0 | nsa |
Chi-square test.
One-way ANOVA post hoc significances for age: PD-Normal versus PDD P < 0.05.
Kruskall-Wallis test.
Kruskall-Wallis test comparing Parkinson’s disease subgroups only.
P < 0.001 versus Control; #P < 0.0001 versus Control; ^P < 0.0001 versus PD-Normal; $P < 0.01 versus PD-Normal; &P < 0.01 versus PD-MCI; †P < 0.001 versus PD-MCI. ns = not significant.
Association between sTREM2 with demographic information
Overall, age was positively associated with CSF sTREM2 [β = +0.336, standard error (SE) = 0.006, P < 0.0001] but not plasma sTREM2 (β = −0.064, SE = 0.016, P = 0.412). We observed no association between disease duration and CSF sTREM2 (β = +0.029, SE = 0.010, P = 0.714) or plasma sTREM2 (β = +0.0166, SE = 0.027, P = 0.534). Furthermore, compared to female participants, male participants showed significantly higher CSF sTREM2 (Mann-Whitney U = 1660, n = 165, P < 0.0001) but not plasma sTREM2 (Mann-Whitney U = 2783, n = 165, P > 0.05). For these reasons, we included age and gender in our statistical models for CSF sTREM2. Including age and gender in statistical models evaluating plasma sTREM2 did not change the outcome of the analyses.
Baseline CSF and blood plasma sTREM2 levels in Parkinson’s disease
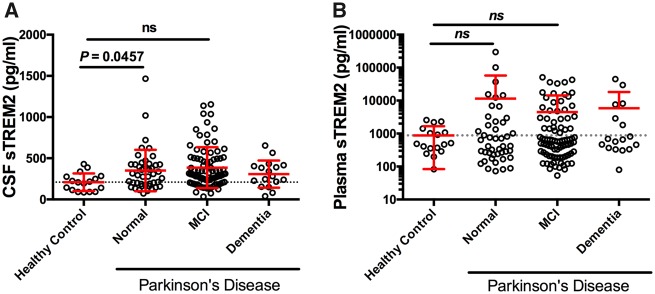
We first determined whether CSF and plasma levels of sTREM2 differed between PD-Normal, PD-MCI, PDD and healthy age-matched controls. Log10-transformed CSF sTREM2 levels were analysed using ANCOVA with clinical and cognitive diagnosis as fixed factor and age and gender, and study site as covariates. This revealed that CSF sTREM2 levels were significantly modulated in Parkinson’s disease [one-way ANCOVA: F(3,159) = 4.588; P = 0.004; Fig. 1A] with CSF sTREM2 significantly elevated in cognitively normal Parkinson’s disease participants compared to healthy control subjects (Tukey’s multiple comparisons test: P = 0.046). Higher concentrations of sTREM2 were observed in plasma compared to CSF overall; however, no differences were observed between PD-Normal, PD-MCI and PDD (Fig. 1B) [one-way ANCOVA: F(3,159) = 0.518; P = 0.671].
Figure 1.
CSF and plasma sTREM2 in healthy control and Parkinson's disease participants stratified according to cognitive status. (A) Scatter plot showing levels of CSF sTREM2 are elevated in PD-Normal subjects relative to healthy controls. (B) Plasma sTREM2 concentration is not significantly different across any of the diagnostic groups. Solid bars represent the mean and standard deviation (SD). CSF and plasma sTREM2 data were log-transformed and analysed using a one-way ANCOVA with age and gender as covariates, followed by post hoc multiple comparisons using Tukey contrasts. ns = not significant.
Heterogeneity in amyloid and tau co-pathologies in Parkinson’s disease
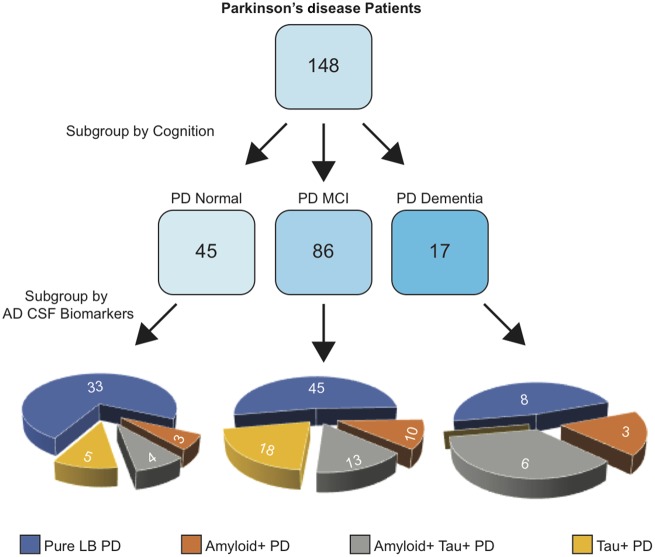
CSF amyloid-β and tau concentrations can reveal biomarker-defined Alzheimer’s disease (Jack et al., 2018), even within clinically-defined Parkinson’s disease populations (van Steenoven et al., 2016). Therefore, we used measurement of CSF amyloid and tau concentrations to stratify participants based on whether they had abnormally low CSF amyloid (amyloid+?>) or abnormally elevated CSF p-tau181 (tau+?>), thereby separating participants into five CSF biomarker-defined groups: (i) amyloid− and tau− control participants (Controls); (ii) amyloid− and tau− pure Lewy body Parkinson’s disease participants (LB-PD); (iii) amyloid+ Parkinson’s disease participants; (iv) amyloid?>+ and tau+ Parkinson’s disease participants; and (v) tau?>+ Parkinson’s disease participants. A flowchart describing the Parkinson’s disease patient subgroups is shown in Fig. 2 and levels of CSF amyloid and tau across each of the CSF biomarker-defined groups are shown in Supplementary Table 1.
Figure 2.
Flow chart depicting subgroups underlying the Parkinson’s disease participant population. At the first level, participants with Parkinson’s disease were stratified according to Montreal Cognitive Assessment (MoCA) to reveal underlying cognitive subgroups consisting of PD-Normal, PD-MCI, and PDD. Groups further stratified by Alzheimer’s disease (AD) CSF biomarkers to reveal further Parkinson's disease participant subgroups with pure Lewy Body pathology (pure LB PD), amyloid?>+ PD, amyloid?>+ tau?>+ PD, and tau?>+ PD.
CSF sTREM2 is elevated in Parkinson’s disease with abnormal CSF tau concentration
To determine the utility of a biomarker-defined diagnosis in Parkinson’s disease, we next compared CSF sTREM2 expression across biomarker-defined groups (Fig. 3A). After adjusting for age and gender, we found that CSF sTREM2 was significantly different between the biomarker-defined groups [F(4,158) = 8.926, P < 0.0001]. Post hoc testing revealed no significant differences between healthy controls, pure LB-PD, or amyloid+ Parkinson’s disease groups. In contrast, CSF sTREM2 was significantly elevated in the amyloid+ tau+ Parkinson’s disease group compared to the healthy control group (P < 0.001), pure LB-PD (P < 0.001), and amyloid+ Parkinson’s disease groups (P = 0.011). Moreover, CSF sTREM2 was significantly elevated in the tau+ Parkinson’s disease group compared to the control (P < 0.001) and pure LB-PD (P = 0.002) groups, and trended towards elevated relative to the amyloid+ Parkinson’s disease group (P = 0.057), indicating that even in the absence of abnormal CSF amyloid-β, a tau+ CSF signature associated with elevated CSF sTREM2. In support of this result, ROC curve analysis revealed that CSF sTREM2 could differentiate Parkinson’s disease participants with abnormal CSF tau concentration (n = 46) from those with normal CSF tau concentration (n = 119; Fig. 3B), with an area under curve (AUC) of 0.828 [95% confidence interval (CI) = 0.757–0.899].
Figure 3.
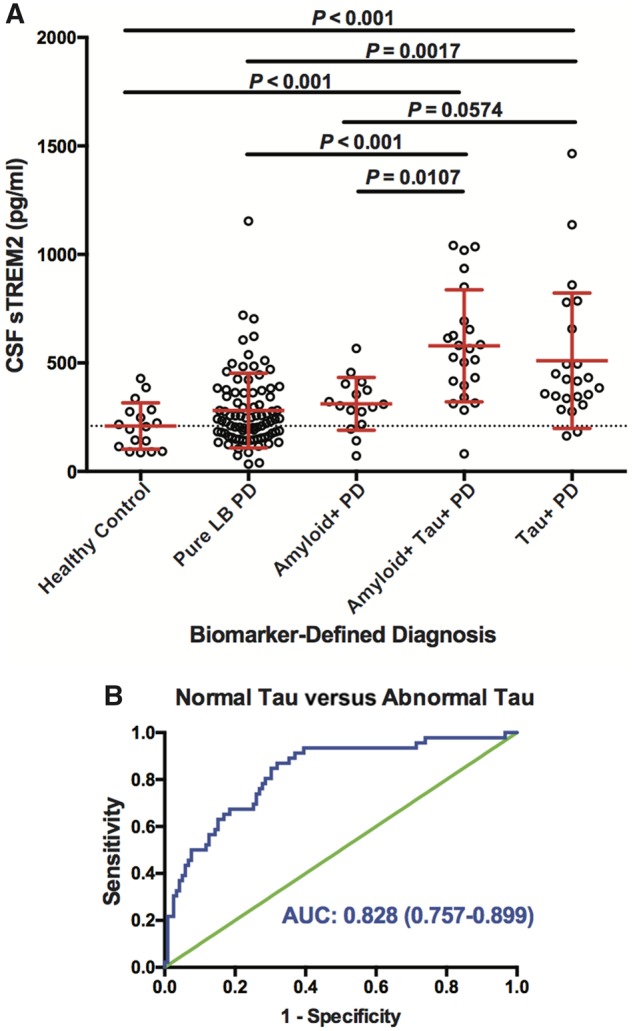
CSF sTREM2 concentration across biomarker-defined diagnostic profiles. (A) CSF amyloid and tau concentrations were used to stratify participants into one of five CSF biomarker-defined groups: (i) healthy control subjects with normal amyloid and tau; (ii) patients with Parkinson’s disease with normal amyloid and tau [pure Lewy body (LB)-PD]; (iii) patients with Parkinson’s disease with abnormal amyloid and normal tau (PD Amyloid+); (iv) patients with Parkinson’s disease with normal amyloid but abnormal tau (PD Tau+); and (v) patients with Parkinson’s disease with both abnormal amyloid and tau (PD Amyloid+ Tau+). Log-transformed CSF sTREM2 data were analysed using a one-way ANCOVA adjusted by age and gender followed by Tukey contrasts post hoc multiple comparisons. Red bars indicate mean and SD. (B) Receiver operating characteristic (ROC) curve analysis of CSF sTREM2 in discriminating Parkinson’s disease with tau co-pathology from Parkinson’s disease without tau pathology. The area under the curve was 0.828 (95% CI = 0.757–0.899).
CSF sTREM2 in biomarker-defined Parkinson’s disease groups across clinical diagnosis
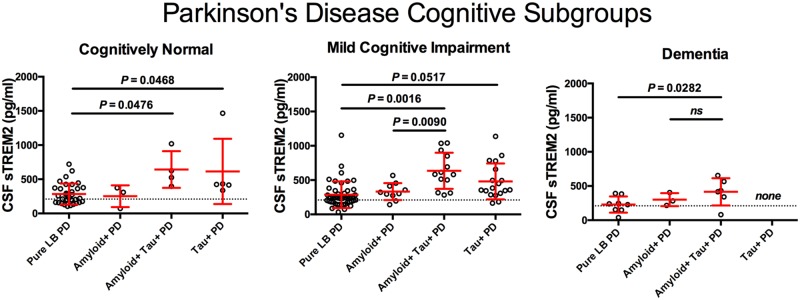
We next investigated whether the elevation of CSF sTREM2 in tau+ Parkinson’s disease subgroups occurred in each of the cross-sectional Parkinson’s disease cognitive subgroups. After adjusting for age and gender, we found that, once again, CSF sTREM2 concentrations were significantly different between biomarker-defined groups within the diagnoses PD-Normal [F(3,39) = 4.415, P = 0.009], PD-MCI [F(3,80) =6.626, P = 0.001], and PDD [F(2,12) = 4.498, P = 0.035] (Fig. 4). Post hoc analysis using Tukey contrasts revealed that CSF sTREM2 level was significantly higher in the amyloid+ tau+ Parkinson’s disease and tau+ Parkinson’s disease subgroups compared to the pure LB-PD subgroup in all three PD-Normal, PD-MCI and PDD cognitive diagnoses. This indicates that CSF sTREM2 is elevated in tau+ Parkinson’s disease participant subgroups, but not amyloid+Parkinson’s disease subgroups, not only in the Parkinson’s disease participant subgroups with MCI or dementia, but also in the cognitively unaffected Parkinson’s disease subgroup.
Figure 4.
CSF sTREM2 according to abnormal amyloid and tau biomarker profile subgroups within each of the clinically defined Parkinson’s disease diagnoses. Scatter plot representing levels of CSF sTREM2 (log-transformed) in participants for each of the four biomarker subgroups defined by abnormal amyloid and/or tau within corresponding cognitive diagnosis. The Parkinson’s disease cognitive subgroups included cognitively normal Parkinson’s disease participants, Parkinson’s disease with mild cognitive impairment, and Parkinson’s disease with dementia. Log-transformed CSF sTREM2 data were analysed using a one-way ANCOVA and were adjusted by gender and age followed by post hoc multiple comparisons using Tukey contrasts. Red bars indicate mean and SD. ns = not significant.
Association between CSF sTREM2 and CSF amyloid-β and tau concentrations
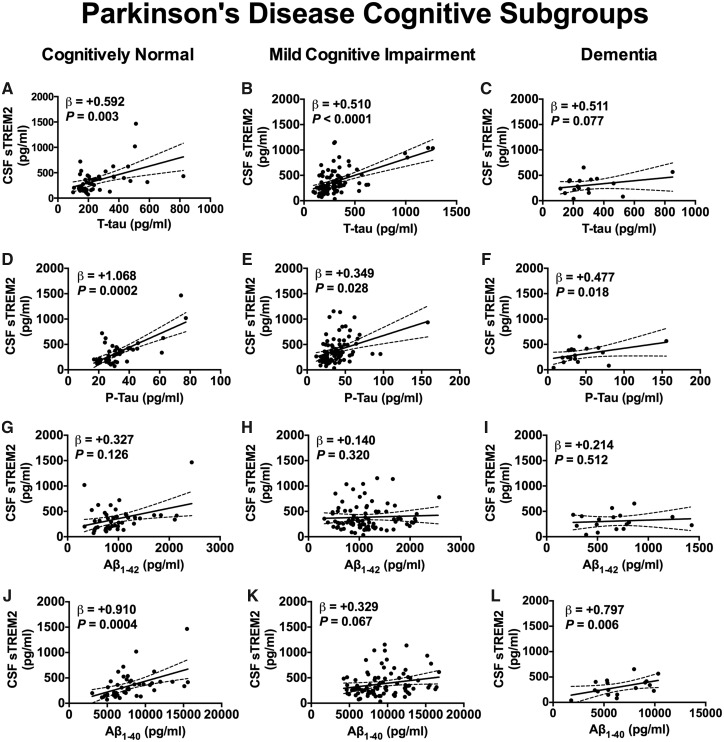
We next studied the relationship between CSF sTREM2 and the core Alzheimer’s disease CSF biomarkers phospho-taup181, total tau, amyloid-β1-42, and amyloid-β1-40. We found that in the pooled group of participants, CSF sTREM2 concentration was positively associated with CSF total tau concentration in PD-Normal (β = +0.592, SE = 0.186, P = 0.003), PD-MCI (β = +0.510, SE = 0.118, P < 0.0001), and despite relatively few cases, tended towards association in the PDD diagnosis (β = +0.511, SE = 0.267, P = 0.078) (Fig. 5A–C). Likewise, positive associations between CSF sTREM2 and CSF phospho-tau181 were detected in the PD-Normal (β = +1.068, SE = 0.262, P = 0.0002), PD-MCI (β = +0.3490, SE = 0.156, P = 0.028), and PDD groups (β = +0.477, SE = 0.177, P = 0.018; Fig. 5D–F). No association was observed between CSF sTREM2 and CSF amyloid-β1-42 in the PD-Normal (β = +0.327, SE = 0.209, P = 0.126), PD-MCI (β = +0.140, SE = 0.140, P = 0.320), or PDD diagnosis (β = +0.214, SE = 0.318, P = 0.512) (Fig. 5G–I). On the other hand, CSF sTREM2 was strongly associated with CSF amyloid-β1-40 in the PD-Normal (β = +0.910, SE = 0.234, P = 0.0004) and PDD groups (β = +0.797, SE = 0.243, P = 0.006), and tended towards a positive association in the PD-MCI group (β = +0.329, SE = 0.177, P = 0.067; Fig. 5J–L).
Figure 5.
Association between CSF sTREM2 and CSF amyloid-β and tau biomarkers. Significant association was observed between CSF sTREM2 levels and CSF total tau (A–C) and p-Tau181 (D–F) for all diagnostic groups. In no group did CSF sTREM2 associate with amyloid-β1–42 (G–I). Significant association was observed between CSF sTREM2 levels and CSF amyloid-β1–40 for PD-Normal (J) and PDD diagnoses (L), and trended towards significance in PD-MCI (K). Associations between CSF sTREM2 and biomarkers were assessed using linear mixed-effects model with age and gender as fixed effects and freeze-thaw cycle number as random effect. Biomarker values were log-transformed to reduce skewness. Plotted is the 95% confidence band of the best-fit line from the linear regression. β estimates and P-values from the linear model are shown. Aβ = amyloid-β.
CSF sTREM2 concentration is positively associated with MoCA in Parkinson’s disease with abnormal CSF tau concentration
Finally, we evaluated whether CSF sTREM2 levels were associated with performance on the MoCA using a linear model adjusted for age, gender, years of education, and study site. No association between CSF sTREM2 and MoCA score was detected within biomarker-defined Pure LB-PD (β = +0.079, SE = 0.012, P = 0.456) or amyloid+ Parkinson’s disease (β = +0.316, SE = 0.028, P = 0.348) subgroups (Fig. 6). In contrast, elevated CSF sTREM2 was associated with higher MoCA score in the amyloid+ tau+ Parkinson’s disease (β = +0.545, SE = 0.028, P = 0.021) and tau+ Parkinson’s disease (β = +0.538, SE = 0.059, P = 0.036) subgroups.
Figure 6.
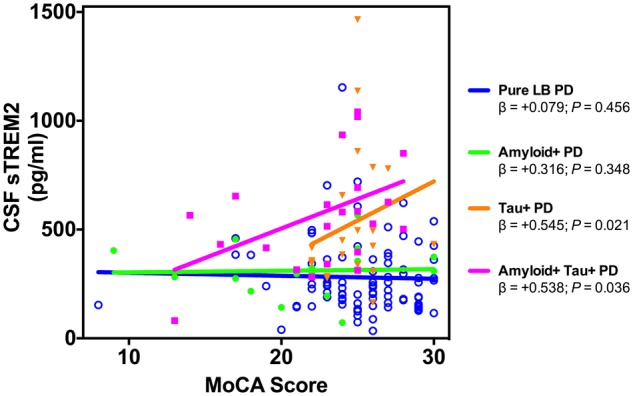
Association between CSF sTREM2 and MoCA score in the biomarker Parkinson’s disease participant subgroups. A significant positive association between CSF sTREM2 (log-transformed) and MoCA score was detected in both the tau+ Parkinson’s disease and amyloid+ and tau+ Parkinson’s disease subgroups. Plotted is the linear regression for each group. β estimates and P-values from the linear model adjusted by age, gender, years of education, and study site are shown.
Discussion
Substantial biological heterogeneity underlies the clinical presentation and progression of Parkinson’s disease and there is a significant need for markers that differentiate subgroups of Parkinson’s disease patients in their rate of progression along cognitive and motor trajectories, or in the types of co-morbid disease (Chen-Plotkin et al., 2018). We report here that CSF sTREM2 is increased in Parkinson’s disease patient subgroups with positive tau CSF biomarkers, with or without positivity for CSF amyloid.
Our observation that CSF sTREM2 is increased prior to cognitive symptoms in Parkinson’s disease stages is similar to that seen in the progression to late-onset Alzheimer’s disease (Kleinberger et al., 2014; Henjum et al., 2016; Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016b, 2019), and early-onset familial Alzheimer’s disease (Suarez-Calvet et al., 2016a). In Alzheimer’s disease, the increase in CSF sTREM2 concentration occurs after pathological decrease in CSF amyloid-β1-42 levels, and coincident with increased CSF tau concentration. In Parkinson’s disease, we observed a similarly strong association between CSF sTREM2 and phospho-tau181 and total tau concentrations, which are markers of neuronal and axonal cell injury and neurofibrillary tangles (Suarez-Calvet et al., 2019). We show that the elevation of CSF sTREM2 levels could differentiate Parkinson’s disease participants with or without abnormal CSF tau concentration with an AUC of 0.828 (CI = 0.757–0.899).
These results also suggest that CSF sTREM2 may serve as a general biomarker of neuronal injury across neurological diseases characterized by a neuroinflammatory component. Beyond Parkinson’s disease and Alzheimer’s disease, CSF sTREM2 is also elevated in HIV-1 infection (Gisslen et al., 2019), where high levels of hyperphosphorylated tau protein have been documented (Brew et al., 2005). CSF sTREM2 concentration is also elevated in relapsing-remitting multiple sclerosis, primary progressive multiple sclerosis, and other inflammatory neurological disease subjects (Piccio et al., 2008), and these forms of multiple sclerosis are also associated with tau pathology (Bartosik-Psujek and Stelmasiak, 2006; Anderson et al., 2008, 2010; Jaworski et al., 2012). We also found a positive association between CSF sTREM2 and amyloid-β1-40 concentrations across all diagnostic groups, an association previously observed in dementia patients (Henjum et al., 2018).
Soluble TREM2 is produced either from cleavage of surface-expressed TREM2 on brain microglia and parenchymal macrophages, or from alternative splicing in those cells; the relative proportion of these contributions is unknown, as it is not possible to estimate levels of surface TREM2 at this time in the brain. If sTREM2 is generated mainly through cleavage of functional surface TREM2, that would indicate that the beneficial TREM2 immune response is in decline, heralding progression to full dementia. Indeed, the findings in this study and those in Alzheimer’s disease, where sTREM2 peaks at the MCI stage and then declines, may be consistent with the idea that compensatory anti-inflammatory and pro-phagocytic TREM2 responses become limiting, allowing disease-causing processes to accelerate.
In plasma, we observed a 2.1-fold increase of sTREM2 concentration compared to CSF; however, no significant difference was observed in any of the Parkinson’s disease subgroups. This is possibly because of the cross-sectional nature of this study and the high level of variance in plasma sTREM2. Follow-up studies with longitudinal sampling may reduce the variance observed. For example, in a prospective longitudinal study, higher levels of sTREM2 in blood were associated with increased risk of developing dementia, Alzheimer’s disease, and vascular dementia in the general elderly Japanese population (Ohara et al., 2019).
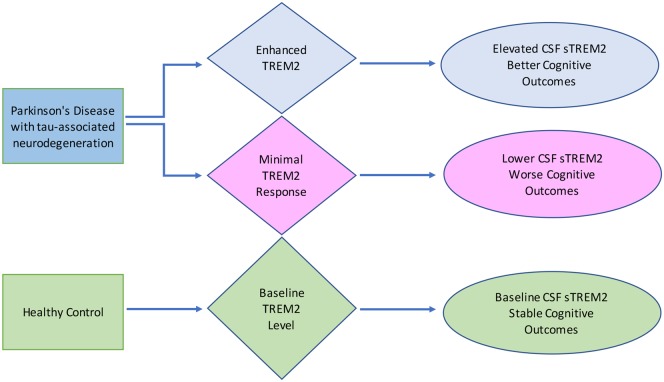
Finally, the finding that CSF sTREM2 is positively associated with MoCA score in Parkinson’s disease participants with elevated CSF tau concentration may seem counterintuitive, given that CSF sTREM2 is associated with higher CSF p-tau and total tau (Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016a, b). However, these observations are in fitting with a recent study on a large well-characterized sample of 385 elderly participants from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) reporting that higher CSF sTREM2 concentrations at baseline predicts a reduced rate of subsequent cognitive decline in subjects with a CSF biomarker profile of Alzheimer’s disease (Ewers et al., 2019). In addition, after controlling for biomarkers of Alzheimer’s disease pathology including CSF amyloid-β and p-tau181, a higher CSF sTREM2-to-p-tau ratio predicted a slower rate of conversion from cognitively normal to MCI or Alzheimer’s disease dementia. A separate study has shown a positive association between CSF sTREM2 and regional grey matter volume in early stage Alzheimer’s disease subjects, where CSF sTREM2 was associated with higher grey matter volume when controlled for CSF amyloid and tau (Gispert et al., 2016). Taken together with the results of this study, it appears that association between CSF sTREM2 and tau need not rule out association with better cognitive outcomes. This is depicted in Fig. 7, a hypothetical diagram showing that for a given level of tau pathology, a minimal TREM2 response could associate with lower CSF sTREM2 and therefore, worse cognitive outcomes. Alternatively, a high TREM2 response would associate with elevated CSF sTREM2 and better cognitive outcomes. A neuroprotective function of TREM2 signalling in microglia is supported by genome-wide association studies (Guerreiro et al., 2013; Jonsson et al., 2013) and preclinical research in mouse models of Alzheimer’s disease (Wang et al., 2016; Raha et al., 2017; Ulland et al., 2017; Cheng-Hathaway et al., 2018; Parhizkar et al., 2019). Moreover, direct injection of sTREM2 protein or viral-mediated overexpression of sTREM2 in hippocampus was associated with a reduction of amyloid plaque load and a rescue of spatial memory and long-term potentiation deficits (Zhong et al., 2019).
Figure 7.
Hypothetical diagram proposing that at a given level of tau pathology, a minimal TREM2 response could associate with lower CSF sTREM2 and worse cognitive outcomes. Alternatively, a high level of TREM2 response would associate with elevated CSF sTREM2 and better cognitive outcomes. In healthy control participants, baseline TREM2 associates with baseline levels of CSF sTREM2 and stable cognition.
There are limitations in this study. First, the number of Parkinson’s disease participants with dementia in the study population is limited. Nevertheless, we decided that including these few individuals was important for completeness of the analysis. Second, the results of this study were cross-sectional in nature and therefore we caution that longitudinal studies are required to examine association between TREM2 and cognitive progression in Parkinson’s disease. Third, while it is possible that increasing CSF sTREM2 levels in advancing cognitive decline might reflect prior or ongoing TREM2 signalling that is being terminated by cleavage of the membrane-bound TREM2 and release, further studies are required to better understand the temporal relationship between parenchymal microglial TREM2 expression and signalling and the appearance of the soluble form of TREM2.
In conclusion, we demonstrate that levels of CSF sTREM2 increase in Parkinson’s disease patient subgroups with a positive tau CSF biomarker signature, but not in Parkinson’s disease subgroups with a positive CSF amyloid-β biomarker signature, and this increase is observed in cognitively normal and MCI Parkinson’s disease subgroups. These findings suggest that CSF sTREM2 could serve as a surrogate biomarker of TREM2-mediated microglia function and neuronal injury in Parkinson’s disease.
Funding
E.N.W. holds the Stanford Medicine Dean’s Postdoctoral Fellowship. CSF and plasma samples were obtained from the Stanford ADRC (P50 AG047366) and the Pacific Udall Center (NINDS) P50 NS062684. This work was supported by NIH/NIA grant RF1AG053001 to K.I.A., NIH grants K23 NS075097 and R01 NS115114 and Michael J. Fox Foundation grant 6440.0 to K.P. This material is the result of work supported with resources and the use of facilities at the VA Puget Sound and VA Portland Health Care Systems. The authors would also like to acknowledge the generous support of the Jean Perkins Foundation and the Scully Research Initiative.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
Abbreviations
- LB-PD =
Lewy body Parkinson’s disease;
- MCI =
mild cognitive impairment;
- MoCA =
Montreal Cognitive Assessment;
- PDD =
Parkinson’s disease dementia;
- PD-MCI =
Parkinson’s disease patients with MCI;
- PD-Normal =
cognitively normal patients with Parkinson’s disease
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P.. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60: 387–92. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G.. Norwegian ParkWest Study G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009; 72: 1121–6. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Hampton DW, Patani R, Pryce G, Crowther RA, Reynolds R, et al. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain 2008; 131: 1736–48. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Patani R, Reynolds R, Nicholas R, Compston A, Spillantini MG, et al. Abnormal tau phosphorylation in primary progressive multiple sclerosis. Acta Neuropathol 2010; 119: 591–600. [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Stelmasiak Z.. The CSF levels of total-tau and phosphotau in patients with relapsing-remitting multiple sclerosis. J Neural Transm 2006; 113: 339–45. [DOI] [PubMed] [Google Scholar]
- Bayart JL, Hanseeuw B, Ivanoiu A, van Pesch V.. Analytical and clinical performances of the automated Lumipulse cerebrospinal fluid Abeta42 and T-Tau assays for Alzheimer’s disease diagnosis. J Neurol 2019; 266: 2304–11. [DOI] [PubMed] [Google Scholar]
- Benitez BA, Cooper B, Pastor P, Jin SC, Lorenzo E, Cervantes S, et al. TREM2 is associated with the risk of Alzheimer’s disease in Spanish population. Neurobiol Aging 2013; 34: 1711.e15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L.. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 2005; 65: 1490–2. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Wilson RS, Leurgans SE, Nag S, Shulman JM, et al. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology 2019; 92: e1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Albin R, Alcalay R, Babcock D, Bajaj V, Bowman D, et al. Finding useful biomarkers for Parkinson’s disease. Sci Transl Med 2018; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Hathaway PJ, Reed-Geaghan EG, Jay TR, Casali BT, Bemiller SM, Puntambekar SS, et al. The Trem2 R47H variant confers loss-of-function-like phenotypes in Alzheimer’s disease. Mol Neurodegener 2018; 13: 29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton B, Johnson CO, Fish B, Quinn JF, Chung KA, Peterson-Hiller AL, et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord 2018; 50: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, et al. Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis 2013; 3: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, et al. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord 2010; 25: 2501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol 2003; 3: 445–53. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM.. Epidemiology of Parkinson’s disease. Lancet Neurol 2006; 5: 525–35. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Heckman MG, Murray ME, Soto AI, Walton RL, Diehl NN, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018; 91: e1182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Mecocci P, Stender K.. Pooled analyses on cognitive effects of memantine in patients with moderate to severe Alzheimer’s disease. J Alzheimers Dis 2008; 14: 193–9. [DOI] [PubMed] [Google Scholar]
- Ewers M, Franzmeier N, Suarez-Calvet M, Morenas-Rodriguez E, Caballero MAA, Kleinberger G, et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci Transl Med 2019; 11: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert JD, Suarez-Calvet M, Monte GC, Tucholka A, Falcon C, Rojas S, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimer’s Dement 2016; 12: 1259–72. [DOI] [PubMed] [Google Scholar]
- Gisslen M, Heslegrave A, Veleva E, Yilmaz A, Andersson LM, Hagberg L, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm 2019; 6: e512.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med 2013; 368: 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL.. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol 2006; 177: 2051–5. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG.. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–44. [DOI] [PubMed] [Google Scholar]
- Hendershott TR, Zhu D, Llanes S, Poston KL.. Domain-specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson’s disease. Parkinsonism Relat Disord 2017; 38: 31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershott TR, Zhu D, Llanes S, Zabetian CP, Quinn J, Edwards KL, et al. Comparative sensitivity of the MoCA and Mattis Dementia Rating Scale-2 in Parkinson’s disease. Mov Disord 2019; 34: 285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjum K, Almdahl IS, Arskog V, Minthon L, Hansson O, Fladby T, et al. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimer’s Res Ther 2016; 8: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjum K, Quist-Paulsen E, Zetterberg H, Blennow K, Nilsson LNG, Watne LO.. CSF sTREM2 in delirium-relation to Alzheimer’s disease CSF biomarkers Abeta42, t-tau and p-tau. J Neuroinflamm 2018; 15: 304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener 2016; 11: 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Psujek M, Janczarek M, Szczerbo-Trojanowska M, Bartosik-Psujek H.. Total-tau in cerebrospinal fluid of patients with multiple sclerosis decreases in secondary progressive stage of disease and reflects degree of brain atrophy. Ups J Med Sci 2012; 117: 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 2013; 368: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Science Transl Med 2014; 6: 243ra86.. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Riederer P, O’Bryant SE, Verbeek MM, Dubois B, Visser PJ, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry 2018; 19: 244–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek K, Chowdhury S, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, et al. The Parkinson’s progression markers initiative (PPMI)-establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018; 5: 1460–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B.. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005; 65: 1239–45. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Neumann H, Takahashi K.. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol 2007; 184: 92–9. [DOI] [PubMed] [Google Scholar]
- Ohara T, Hata J, Tanaka M, Honda T, Yamakage H, Yoshida D, et al. Serum soluble triggering receptor expressed on myeloid cells 2 as a biomarker for incident dementia: the Hisayama study. Ann Neurol 2019; 85: 47–58. [DOI] [PubMed] [Google Scholar]
- Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol 2009; 10: 734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciotti S, Sepe FN, Eusebi P, Farotti L, Cataldi S, Gatticchi L, et al. Diagnostic performance of a fully automated chemiluminescent enzyme immunoassay for Alzheimer’s disease diagnosis. Clin Chim Acta 2019; 494: 74–8. [DOI] [PubMed] [Google Scholar]
- Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 2019; 22: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 2008; 131: 3081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Deming Y, Del-Aguila JL, Ghezzi L, Holtzman DM, Fagan AM, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol 2016; 131: 925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha AA, Henderson JW, Stott SR, Vuono R, Foscarin S, Friedland RP, et al. Neuroprotective Effect of TREM-2 in Aging and Alzheimer’s Disease Model. J Alzheimers Dis 2017; 55: 199–217. [DOI] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, et al. TREM2 in neurodegeneration: evidence for association of the p. R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 2013; 8: 19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Guo Y, Wei X, Yan S, Qin Y, Zhang X, et al. TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp Neurol 2018; 302: 205–13. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018; 141: 2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet M, Araque Caballero MA, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med 2016a; 8: 369ra178.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet M, Kleinberger G, Araque Caballero MA, Brendel M, Rominger A, Alcolea D, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med 2016b; 8: 466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet M, Morenas-Rodriguez E, Kleinberger G, Schlepckow K, Araque Caballero MA, Franzmeier N, et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-beta pathology. Mol Neurodegener 2019; 14: 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 2013; 70: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, et al. Cutting edge: tREM-2 attenuates macrophage activation. J Immunol 2006; 177: 3520–4. [DOI] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 2017; 170: 649–63 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenoven I, Aarsland D, Weintraub D, Londos E, Blanc F, van der Flier WM, et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of lewy body diseases: results from a large multicenter cohort. J Alzheimers Dis 2016; 54: 287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 2016; 213: 667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J.. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and gamma-secretase-dependent intramembranous cleavage. J Biol Chem 2013; 288: 33027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M.. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 2016; 91: 328–40. [DOI] [PubMed] [Google Scholar]
- Zhong L, Xu Y, Zhuo R, Wang T, Wang K, Huang R, et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat Commun 2019; 10: 1365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon request.