Abstract
Environmental exposures during pregnancy may increase breast cancer risk for mothers and female offspring. Tumor tissue assays may provide insight regarding the mechanisms. This study assessed the feasibility of obtaining tumor samples and pathology reports from mothers (F0) who were enrolled in the Child Health and Development Studies during pregnancy from 1959 to 1967 and their daughters (F1) who developed breast cancer over more than 50 years of follow-up. Breast cancer cases were identified through linkage to the California Cancer Registry and self-report. Written consent was obtained from 116 F0 and 95 F1 breast cancer survivors to access their pathology reports and tumor blocks. Of those contacted, 62% consented, 13% refused and 24% did not respond. We obtained tissue samples for 57% and pathology reports for 75%, and if diagnosis was made ⩽ 10 years we obtained tissue samples and pathology reports for 91% and 79%, respectively. Obtaining pathology reports and tumor tissues of two generations is feasible and will support investigation of the relationship between early-life exposures and molecular tumor markers. However, we found that more recent diagnosis increased the accessibility of tumor tissue. We recommend that cohorts request consent for obtaining future tumor tissues at study enrollment and implement real-time tissue collection to enhance success of collecting tumor samples and data.
Keywords: breast cancer prevention, Child Health and Development Studies, pathology reports, PEDIGREE study, tumor sample collection
Introduction
Pregnancy and intrauterine life are two critical periods when human breasts are most susceptible to environmental exposure.1,2 The Child Health and Development Studies (CHDS) has shown a strong association between breast cancer and p,p’dichlorodiphenyltrichloroethane (DDT) levels measured during pregnancy, particularly among women who were under the age of 14 in 1945 when DDT was introduced,3 and that DDT exposure in utero is associated with a nearly four-fold increased risk of breast cancer in daughters.4
The CHDS is comprised of parents and their offspring enrolled during the women’s pregnancies from 1959 to 1966. CHDS parents and offspring have been followed-up for cancer incidence over more than 57 years, presenting a unique opportunity for prospective studies of cancer beginning during pregnancy in mothers and during fetal life in offspring. Collection of tumor tissue and related pathology reports for information on tumor markers may help us understand the etiology of cancer. For this reason, we initiated a pilot study to determine the feasibility of collecting tumor tissue from breast cancer cases identified in the CHDS. This was the first attempt to collect pathology reports and tumor specimens from the CHDS, and we report on our experience in this article.
Methods
This study was made possible by a prospective follow-up of the CHDS, a pregnancy cohort of over 15,000 families who were enrolled between 1959 and 1966 during the mothers’ pregnancies (with deliveries extending into 1967). All mothers in the Child Health and Development Studies cohort were recruited from the Kaiser Foundation Health Plan in the East Bay of the San Francisco Bay Area. Cohort enrollment and baseline biospecimen and data collection methods have been described previously.5 In this study, we tested the feasibility of collecting tumor tissue samples and pathology reports from two generations of women, the founding mothers (F0) and their daughters (F1).
Eligible F0 cases were diagnosed by the age of 54 years. No age cut-off was imposed for the F1 generation, and the age range of F1 at diagnosis was 47–54 years. The years of diagnosis ranged from 1962 to 2012 in the F0 generation and from 1997 to 2013 in the F1 generation. Cases were identified by linkage to the California Cancer Registry (CCR) and the California Vital Status Records as well as by self-report during a survey of CHDS daughters conducted from 2010 to 2013. Essentially all eligible surviving F0 cases were identified through the CCR linkage. F1 living cases were identified by self-report to the CHDS only (n = 28) or by linkage to CCR only (n = 23), or both (n = 44). F0 diagnoses spanned over 50 years, the earliest diagnosis was 51 years before this study and the latest was 1 year before this study. In contrast, F1 diagnoses occurred within a much more compressed time span; the earliest diagnosis was 16 years before this study and the latest diagnoses were ascertained concurrent with this study. We invited surviving cases with a valid address (F0, n = 116; F1, n = 95) to provide access to their pathology reports and tumor samples. To determine the feasibility of acquiring tumor samples from deceased cases, we also attempted to collect tumor tissue and pathology reports for a small convenience sample of deceased cases in F0 (n = 26) and F1 (n = 10).
The present study was reviewed and approved by the Institutional Review Board (IRB) of the Public Health Institute and the California Committee for the Protection of Human Subjects. Informed consent was obtained from all participants, and we have complied with all federal guidelines governing the use of human participants.
Consent and tissue acquisition
To recruit living breast cancer cases to this study, we mailed participants an invitation packet consisting of an invitation letter describing the study, a ‘Consent to Authorize Use of Medical Information’ form, a ‘Consent to Authorize Release of Medical Information’ form and a ‘Consent to Participate in a Research Study’ form. To obtain death certificates for deceased cases, we submitted requests to the California Department of Health Vital Records Office.
Once consent from living participants or death certificates were received, tumor block and pathology report request forms were faxed to the pathology department of the hospital designated on the medical release form or the physician of record on the death certificate. Tissue sample and pathology request forms were accompanied by the study IRB approval letter, participant consent and authorization forms or death certificates. Faxes were followed-up by phone calls to pathology departments to determine sample availability.
Upon arrival, all identifying personal information was removed from tumor samples and pathology reports and replaced with a sample identification number. Pathology reports were received either in paper or in electronic form via fax, email and mail. Cancer classification variables (hormone receptor status, histopathology, grade and stage of breast cancer) were extracted from the reports. Estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 statuses were recorded when available.
Tumor block criteria
Samples were received in the form of formalin-fixed, paraffin-embedded tissues (tumor blocks) or slides. If slides were sent from the hospital, seven sections of tissue were requested for DNA extraction (7-μm thick, one section for hematoxylin and eosin staining), measuring 2 × 2-cm, assuming the tissue was >90% cancer. If the percentage of cancer tissue was <90%, two more sections were requested.
Results
Access to CHDS breast cancer cases via the CCR
The CCR provided contact information for less than half of the living F1 cases who were found on the CCR (n = 46 of 98 submitted) because they were already participants in a current CCR study. This is the main enrollment barrier for CCR-identified F1 participants in the CHDS. We did not encounter the same limitation for the F0 cancer cases as they were diagnosed many years ago and were less likely to be active participants in other CCR studies. As many of the F1 cases withheld by CCR self-reported their cancer directly to us in a recent survey and consented for future contact, we were able to include some of the women withheld by CCR in our study (n = 22). This result emphasizes the importance of active cohort follow-up for obtaining medical records and tumor tissue samples.
Deceased cases
We requested death certificates for 26 F0 cases and received 24 certificates (92%). A total of 13 certificates provided valid physician information. Among these, we acquired tumor specimens with pathology reports for four cases and only the pathology report for one case. We requested death certificates for ten F1 cases and received all certificates (100%). Nine provided valid physician information. Among those, we received tumor specimens with pathology reports for three cases.
Consent and tissue acquisition for surviving cases
The response rates are presented in Table 1 for both F0 and F1 living cases. The participants who consented had an age range of 57 to 93 years, with breast cancer diagnoses occurring as far back as 45 years. Race and location of participants are presented in Table 2. Among F0 with breast cancer, a total of 116 were eligible, and 69 were contacted; 46 of 69 participants (67%) provided consent (Table 1, F0). Of those, we received tissue samples for 14 cases (13 with pathology reports) and only pathology reports for 12 cases. Among F1 cases who were living, 95 were eligible, and 87 were successfully contacted; 52 of 87 participants (60%) provided consent (Table 1, F1). Of those, we received tissue samples with pathology reports for 42 and pathology reports only for six cases.
Table 1.
Response and consent rates for tumor sample and pathology report collection of living breast cancer cases
| Eligible (n) | Verified contact (n) | Pending at study closea [n (%)] | Refuseda [n (%)] | Consenteda [n (%)] | No report or tissueb [n (%)] | Pathologyb [n (%)] | Tissueb [n (%)] | |
|---|---|---|---|---|---|---|---|---|
| F0 (Mothers) | 116 | 69 | 10 (14%) | 13 (19%) | 46 (67%) | 20 (44%) | 25 (54%) | 14 (30%) |
| F1 (Daughters) | 95 | 87 | 27 (31%) | 8 (9%) | 52 (60%) | 4 (8%) | 48 (92%) | 42 (81%) |
| Both generations combined | 211 | 156 | 37 (24%) | 21 (13%) | 98 (63%) | 24 (25%) | 73 (75%) | 56 (57%) |
F0, mother generation; F1, daughter generation.
Percent calculated using category/verified contact.
Percent calculated using category/consented.
Table 2.
Location and race of study participants by category of participation among living breast cancer cases
| Verified contact | Consented | Tissue or pathology | |||||
|---|---|---|---|---|---|---|---|
| Generation | Demographic | n | % | n | % | n | % |
| F0 | Locationa | ||||||
| Bay Area | 47 | 67.1 | 30 | 65.2 | 17 | 65.4 | |
| Southern California (minus LA) | 1 | 1.4 | 1 | 2.2 | 1 | 3.9 | |
| Los Angeles County | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central Farm | 5 | 7.1 | 5 | 10.9 | 3 | 11.5 | |
| Northern California | 4 | 5.7 | 2 | 4.4 | 1 | 3.9 | |
| Central California | 7 | 10 | 6 | 13 | 3 | 11.5 | |
| Outside of California | 6 | 8.6 | 2 | 4.4 | 1 | 3.9 | |
| Raceb | |||||||
| White | 48 | 70.6 | 33 | 71.7 | 16 | 61.5 | |
| Black | 14 | 20.6 | 8 | 17.4 | 6 | 23.1 | |
| Hispanic | 2 | 2.9 | 1 | 2.2 | 1 | 3.9 | |
| Asian | 3 | 4.4 | 3 | 6.5 | 3 | 11.5 | |
| Other | 1 | 1.5 | 1 | 2.2 | 0 | 0 | |
| F1 | Locationa | ||||||
| Bay Area | 51 | 58.6 | 29 | 55.8 | 25 | 52.1 | |
| Southern California (minus LA) | 4 | 4.6 | 3 | 5.8 | 3 | 6.3 | |
| Los Angeles County | 3 | 3.5 | 2 | 3.9 | 2 | 4.2 | |
| Central Farm | 5 | 5.8 | 3 | 5.8 | 3 | 6.3 | |
| Northern California | 6 | 6.9 | 4 | 7.7 | 4 | 8.3 | |
| Central California | 7 | 8.1 | 6 | 11.5 | 6 | 12.5 | |
| Outside of California | 11 | 12.6 | 5 | 9.6 | 5 | 10.4 | |
| Raceb | |||||||
| White | 58 | 67.4 | 36 | 70.6 | 36 | 76.6 | |
| Black | 19 | 22.1 | 9 | 17.6 | 7 | 14.9 | |
| Hispanic | 3 | 3.5 | 2 | 3.9 | 2 | 4.3 | |
| Asian | 3 | 3.5 | 1 | 2.0 | 1 | 2.1 | |
| Other | 3 | 3.5 | 3 | 5.9 | 1 | 2.1 | |
F0, mother generation; F1, daughter generation.
Regions based on six-region definition recommended by the California Department of Social Services Research and Development Division Data Analysis and Publications Branch.
Race is unknown for one F0 in the verified contact category. Race is unknown for one F1 in all participation categories.
For tumor tissue collection, we contacted a total of 49 hospitals. Among them 38 hospitals were in California and 11 were out of state (over 90% of the samples were requested from California hospitals). It took an average of 4.5 (±2.3) provider/hospital contacts by phone and fax to receive tumor samples, pathology reports or notification that no samples were available. The cost of samples ranged from US$0 to $293 with an average per sample cost of $47, which was slightly under the budgeted sample cost of $50 (Supplementary Table S1).
Although we were able to collect tissue from cases diagnosed as far back as 1982, we found that tissue samples from cases diagnosed over 2 decades ago were difficult to obtain because the majority of hospitals only keep tumor samples and pathology reports for 10–15 years (Fig. 1). The majority of F1 breast cancer diagnoses fall within the last 10 years. As a result, 81% of F1 tumor samples were collected from cases who consented and only 30% for F0. Overall, for both F1 and F0 generations combined, we collected pathology reports for 75% of living cases requested and tissue samples for 57% of living cases requested.
Fig. 1.
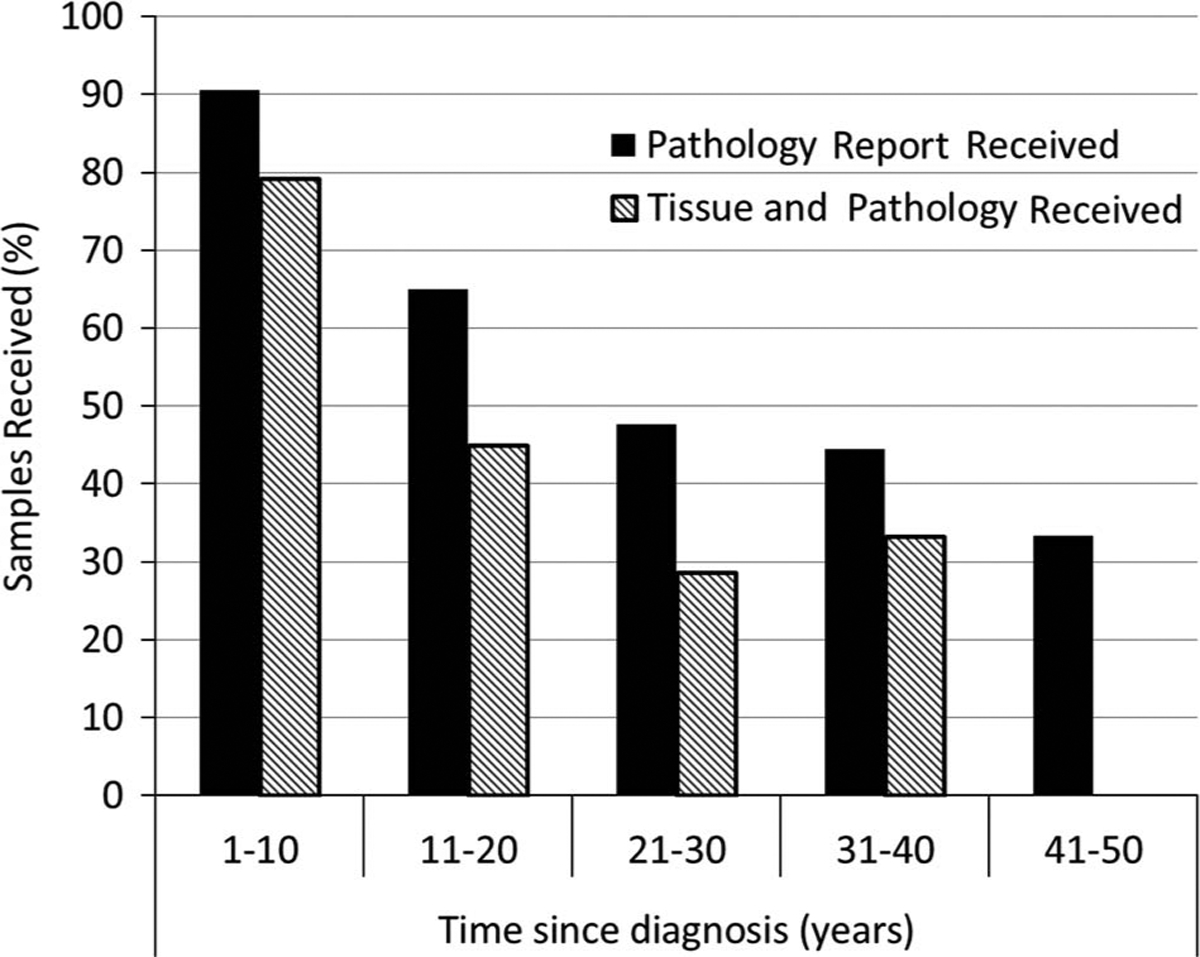
Percentage of tumor samples and pathology reports received (received/requested), stratified by years since breast cancer diagnosis (year of request – year of diagnosis) for both generations combined.
We gathered data from other prospective cancer cohort studies (Nurses’ Health Study, Health Professionals Follow-up Study, Women’s Health Initiative Cohort Study, Breast Cancer Family Registry Cohort and Sister Study) to compare tumor tissue collection consent rates.6–9 Even though these studies had different designs and not all had published tissue consent rates, the approximate tissue consent rates were reported by principal investigators and study managers. In studies that received consent at diagnosis or after, tumor tissue consent rates ranged from 50 to 96%.6–9 Studies that asked for consent before diagnosis reported a >90% consent rate. This suggests that obtaining broad consent before diagnosis results in greater access to tumor tissue than studies that sought tumor tissue consent at or after the time of diagnosis.
Discussion
This pilot study establishes the feasibility of obtaining IRB approval, obtaining participant consent and collecting tumor tissue samples for cancer cases in our long-term cohort that had cancer diagnoses at multiple facilities and belong to a variety health plans. This is consistent with another California study that successfully collected tumor samples going back 60 years among women who were members of the Kaiser Permanente Northern California health plan.10 In our study, we identified two substantial barriers to tumor collection. First, the CCR policy of only releasing contact of cancer survivors not already in another CCR study limited access to CHDS cohort cancer cases. Second, collecting samples when the diagnosis was made over a decade ago restricted access to tissue samples. Both these barriers can be addressed by active and ongoing follow-up of cohort members to obtain prompt self-report of cancer diagnosis.
We submitted all known breast cancer cases to the CCR in order to obtain contact information. CCR’s policy is to only release cases for contact who are not already part of an active CCR cancer study. They do this to prevent multiple researchers from contacting cancer patients within a short period to mitigate patient burden.11 Thus, CCR provided contact information about less than half of the living F1 cases submitted. Active follow-up provides the opportunity for collecting tumor samples from survivors that would have otherwise been inaccessible through CCR. It also empowers patients and survivors by giving them the ability to make an active choice to participate. The idea that cancer survivors want to participate in multiple studies is supported by the high participation rate of women who self-reported and were withheld by CCR. By obtaining trust and consent well in advance of diagnosis, we believe we can alleviate potential stress and inconvenience to cancer patients and survivors after diagnosis from being asked to participate in a cancer study.
Collection of samples and pathology reports for cancer diagnosed ⩽ 10 years ago has the highest feasibility as older samples and reports are more likely to have been discarded or lost. On the basis of our success in collecting pathology reports (92%) and tissue samples (81%) from F1 that have more recent diagnoses, collection of tumor samples from cases diagnosed within 10 years is highly achievable. We hope to incorporate routine procedures for obtaining advance participant consent for access to tumor tissue and pathology reports in CHDS routine follow-up.
Recommendations
On the basis of the tissue consent gained from participants in this and other studies,6–9 we recommend gaining broad consent from cohort members at enrollment (before cancer diagnosis), which includes permission for subsequent collection of tumor samples. Comparing consent rates from several cancer cohort studies, broad consent before diagnosis appears to increase the likelihood of obtaining consent to access records and samples compared with requesting consent after diagnosis.
We also recommend that future studies collect pathology reports and tumor specimens as they become available, because hospitals often destroy samples that are older than 10 years. Retention of histopathology slides and paraffin tumor blocks for ten years is consistent with guidelines set by the College of American Pathologists (CAP).12 Despite CAP guidelines, some hospitals keep samples for longer, although this was the exception rather than the rule. Recommendations for other investigators are summarized in Table 3.
Table 3.
Recommendations for long-term and cohort studies to collect tumor tissue samples
| Recommendation | Purpose |
|---|---|
| Gain broad consent for collection of samples from future diagnoses upon study enrollment | Broad consent that authorizes future contact and collection may be more likely to result in a higher tissue consent rate. Building a relationship before diagnoses may alleviate potential stress and inconvenience to cancer patients from being asked to participate in cancer studies during treatment and diagnosis |
| Target cases from within the last 10 years | Hospitals may be more likely to have samples from the last 10 years |
Conclusions
This study demonstrates the CHDS’s ability to obtain tissue samples and pathology reports in a long-term cohort study from participants who were diagnosed with breast cancer 0–32 years before collection attempts began. We were most successful in obtaining samples and pathology reports from cases diagnosed within the last 10 years. Obtaining consent for contact and tissue access at enrollment and maintaining active follow-up to identify new cases as they occur is recommended to maximize the acquisition of tumor tissue in long-term cancer cohorts. Where active follow-up is impractical, linkage to the CCR can identify contact information of subjects required to request consent and also institutional sources for pathology records and tissue samples. California has one of the most comprehensive tumor registries and it is likely that California cohorts can make an important contribution to understanding the etiology of cancer.
Supplementary Material
Acknowledgments
We thank all the CHDS staff for their contributions to this study: Lauren Zimmermann for her work on the CCR linkages, Eileen Johnson for her careful record keeping, and Sheena Cresswell who worked on IRB approval, recruitment and CCR linkages and requests. We also thank S.B.K., B.G.H. and F.G. for all their support.
Financial Support
This publication was made possible by the Breast Cancer and the Environment Research Program (BCERP) award number U01 ES019471 from the National Institute of Environmental Health Sciences (NIEHS) and the National Cancer Institute (NCI), NIH, DHHS, by funding from the California Breast Cancer Research Program through the Special Research Initiative under Grant 15ZB-0186, and by Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health, Department of Health and Human Services Contract HHSN275201100020C. The authors gratefully acknowledge the support from the Avon Foundation for this research. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862–04/DP003862; and the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California and contract HHSN261201000034C awarded to the Public Health Institute. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, the NIH, BCERP, State of California, Department of Public Health, and the CDC or their Contractors and Subcontractors.
Footnotes
Conflicts of Interest
None.
Ethical Standards
The present study was reviewed and approved by the Institutional Review Board (IRB) of the Public Health Institute and the California Committee for the Protection of Human Subjects. Informed consent was obtained from all participants, and we have complied with all federal guidelines governing the use of human participants.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S204017441700006X
References
- 1.Russo IH, Russo J. Pregnancy-induced changes in breast cancer risk. J Mammary Gland Biol Neoplasia. 2011; 16, 221–233. [DOI] [PubMed] [Google Scholar]
- 2.Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005; 7, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007; 115, 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn BA, La Merrill M, Krigbaum NY, et al. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. 2015; 100, 2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the school of public health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988; 2, 265–282. [DOI] [PubMed] [Google Scholar]
- 6.Personal communication with Lisa Godefroy Johnsonto receive the Women’s Health Initiative consent rates, 2015.
- 7.Personal communication with Meir Stampfer to receive the Health Professionals Follow-up Study consent rates, 2015.
- 8.Personal communication with Meir Stampfer to receive the Nurses’ Health Study consent rates, 2015.
- 9.Personal communication with Cynthia A. Kleeberger to receive The Sister Study consent rates, 2015.
- 10.Krieger N, Habel LA, Waterman PD, et al. Analyzing historical trends in breast cancer biomarker expression: a feasibility study (1947–2009). NPJ Breast Cancer. 2015; 1, 15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Policies and procedures for access to and disclosure of confidential data from the California Cancer Registry, 2014. Retrieved 23 September 2015 from http://www.ccrcal.org/pdf/Data_Statistics/CCRPoliciesProcedures_v05.1.pdf.
- 12.College of American Pathologists. Laboratory of Pathology Online Policy Manual, CAP & CLIA Retention Requirements. Retrieved 16 June 2014 from http://home.ccr.cancer.gov/lop/intranet/policymanual/generalpolicy/CAPCLIA.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


