Abstract
OBJECTIVES
The aim of our study was to examine pulmonary functions of patients from all stages of chronic obstructive pulmonary disease (COPD) according to their smoking status.
MATERIALS AND METHODS
A retrospective case-control study was carried out. Total of 148 patients were enrolled and divided into two groups in regards to their smoking status (quitters, n=68; non-quitters, n=80). Pulmonary function parameters, COPD assessment test score, Fagerström Nicotine Addiction Questionnaire score, smoking history and status were obtained from the electronic hospital data system. Patients’ admission and 12-month data were recorded.
RESULTS
In non-quitters, the mean FEV1 values decreased from 2.32±1.14 to 2.24±1.12 (p<0.001). Particularly, in Stage-0, in the early high-risk group of COPD, the reduction in FEV1 was 90 mL, while the reduction was 70, 60, 40, and 40 mL in Stage-I, -II, -III, and -IV, respectively. In quitters, the mean FEV1 levels increased from 2.10±1.19 to 2.19±1.20 (p<0.001). For COPD patients overall, an average increase in FEV1 of 80–110 mL was observed. At the end of the 12 months follow-up, 17 (27.5%) of the non-quitters showed deterioration, and five (7.3%) of the quitters showed improvement in COPD stage.
CONCLUSION
FEV1 decline was accelerated in COPD patients who continued to smoke, whereas this decline was not prevented by inhaler treatments. The Global Initiative for Chronic Obstructive Lung Disease Stage-0 group, which is not included in the current guidelines, needs to be redefined. This group appears to be the most important group for implementing the smoking cessation and prevention strategy.
Keywords: Chronic obstructive pulmonary disease, non-quitter, pulmonary function, smoker, quitter
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive and exacerbating, yet preventable and partially treatable disease, characterized by chronic airflow limitation [1]. The most common cause of the condition is cigarette smoking. However, air pollution, exposure to environmental or occupational dusts and gases, and consequently chronic systemic inflammation, also play a role in the development of the disease [1, 2]. It is estimated that in 2030, COPD will be the third leading cause of death worldwide [3].
For most patients, the first step in treatment is considered to be smoking cessation. Unfortunately, this is not achieved by many patients; patients are generally reluctant to quit smoking, especially in the early stages of COPD when their clinical symptoms are mild. In a study conducted in 2016, younger patients and socioeconomically disadvantaged individuals tended to be less likely to quit smoking in the early stages of COPD [4]. These patient groups tend to continue smoking even after starting inhaler treatment. A landmark study in patients with mild COPD at the age of 35–60 years showed that an aggressive smoking cessation program significantly reduced age-related decline in forced expiration volume in one second (FEV1) [5]. These patients were administered an inhaled anticholinergic bronchodilator, which resulted in a relatively small improvement in FEV1 after discontinuation of the drug [5]. After re-analysis of the drug’s effects on lung functions, a small improvement of 47 mL in FEV1 was reported in the first year after smoking cessation [6]. The average annual decline in FEV1 was 31 mL/year in sustained quitters, and 62 mL/year in continuing smokers. During the 5-year period of the study, the mean reduction in FEV1 was 77 mL in quitters, compared with 296 mL in continuing smokers [6]. These findings were important for the 5-year follow-up but include only a subset of COPD patients.
Furthermore, “healthy looking” individuals and patients who quit smoking have positive benefits in terms of future health. This was illustrated by Dhariwal et al. [7] who found an increase in FEV1 of 184 mL (7.2%) and 81 mL (3.3%) in the first 6 and 12 weeks, respectively, in COPD patients after the cessation of smoking. In addition, both COPD patients and those with normal initial respiratory function who quit smoking showed a significant improvement in the values of the transfer factor of the lung for carbon monoxide from the 6-week to the 1-year follow-up.
Improvement in respiratory function and FEV1 is expected in patients who quit smoking. Nevertheless, the gain is uncertain in patients with COPD on inhaler therapy and those who are active smokers. In our clinical practice, some COPD patients clearly state whether or not they are able to quit smoking. It is therefore debatable whether inhaler treatment is beneficial in patients who continue to smoke cigarettes.
The aim of our study was to examine respiratory functions according to smoking cessation status across all stages of COPD in patients who are currently smoking.
MATERIAL AND METHODS
Study Design
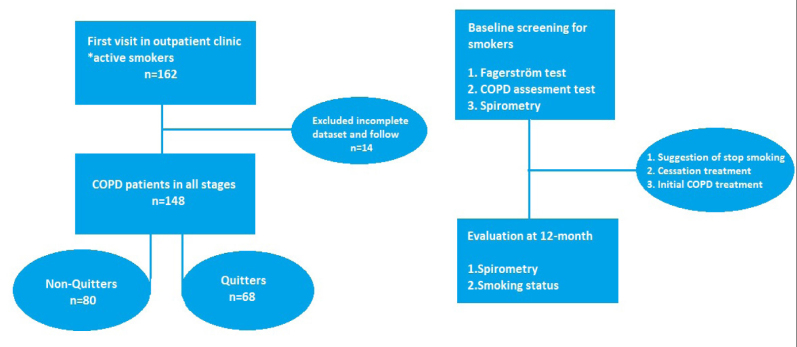
This retrospective case-control study was conducted between 2013 and 2015 in patients admitted to the chest disease polyclinic for smoking cessation in Izmir, Turkey. Patients’ referral and 12-month data were collected from electronic medical records. A total of 162 patients were included in the study. Patients were divided into two groups according to their smoking status such as: quitters (n=68) and non-quitters (n=80). The flowchart of the study is shown in Figure 1. This study was performed in accordance with the current Helsinki Declaration and good clinical practice guidelines. The study protocol was approved by the ethics committee of the Sifa University Medical Faculty (Nr, 198-60; Date, 29.10.2014).
Figure 1.
The flow of the study
Inclusion and Exclusion Criteria
Our inclusion criteria were as follows: (i) patients older than 18 years; (ii) actively smoking at referral; (iii) newly diagnosed or known COPD patients. Patients who met the following criteria were excluded from the study: (i) presence of restrictive lung disease (interstitial lung disease, sarcoidosis, etc.); (ii) diagnosed with asthma; (iii) bronchiectasis; (iv) chronic heart failure; (v) other acute diseases causing shortness of breath; and (vi) presence of active infection.
Data Collection
The data of all patients who applied to our outpatient clinic for smoking cessation assistance were obtained from the electronic hospital data system (Bizmed HBYS, Istanbul, Turkey) and were retrospectively evaluated. The following data were analyzed: (i) epidemiological data (age, sex, smoking history, age at onset of cigarette smoking, body mass index, etc.); (ii) clinical manifestations and active complaints; (iii) pulmonary function parameters such as FEV1, forced vital capacity (FVC), FEV1/FVC, peak expiratory flow (PEF), and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) (Spirolab III series, MIR, Rome, Italy); (iv) COPD stage according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 guidelines [3]; (v) Fagerström Nicotine Addiction Questionnaire (FNAQ) score [8]; (vi) COPD Assessment Test (CAT) score [9]. Patients’ admission and 12-month data were recorded. The FNAQ is the most widely used, validated questionnaire for measuring nicotine addiction [8]. The validity and reliability of the Turkish version of the scale has been confirmed previously by Uysal et al. [10]. The questionnaire consists of six questions, each rated individually. The highest score is 10. The CAT consists of eight items that assess patients according to their clinical symptoms (cough, sputum, dyspnea, and chest compressions), exercise capacity, self-confidence, sleep quality, and energy levels [9]. Patients mostly score between 0 and 40.
COPD Classification and Smoking Cessation Program
The diagnosis of COPD was made according to the GOLD 2018 criteria and pulmonary function parameters [3]. According to these criteria, patients with post-bronchodilator FEV1/FVC<70% were classified as follows: FEV1 >80%, Stage-I; 50–80%, Stage-II; 30–50%, Stage-III; and<30%, Stage-IV. Patients with chronic cough and sputum production, with a CAT score of >10, and a normal pulmonary function test were classified as Stage-0 [11]. Although this stage had been included in the first published GOLD guidelines, it was not included in the last few versions of the guidelines.
Patients were treated with a long-acting bronchodilator β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) in accordance with the GOLD guidelines for patients with newly diagnosed COPD GOLD Stages-III and IV. All patients had been enrolled in a smoking cessation program and had been informed of the diseases caused by cigarette smoking. The smoking cessation treatments, nicotine replacement, and medical treatment had been recommended in accordance with their levels of nicotine dependence. Smoking cessation statuses were analyzed based on data obtained at the end of the 3rd and 12th months.
Statistical Analyses
Study data are presented as descriptive statistics (mean±standard deviation for continuous variables and percentile for categorical variables). The chi-square test was used to compare demographic data and Student’s t-test was used to compare normally distributed data, whereas the Mann-Whitney U Test was used to compare non-normally distributed data. Statistical significance was set at p<0.05 for Student’s t-test and at p<0.01 for the Mann-Whitney U test. Differences between groups were analyzed by paired t-test. The cut-off value for smoking cessation on the CAT score was calculated by the receiver operating characteristic (ROC) curve analysis. All statistical analysis was performed using IBM Statistical Package for the Social Sciences version 19 (IBM SPSS Corp.; Armonk, NY, USA).
RESULTS
Demographic Data at Baseline
The mean age of the 148 patients (68 women and 80 men) was 48.6±15.1 years (range, 20–83 years). The mean age at which patients started smoking was 18.4±3.9 years (range, 9–40 years). The cumulative level of smoking was 24.9±16.4 pack-years. The daily average amount of smoking was 19.2±8.6 cig/day. Among the patients examined, 4.8% were mild smokers, 29.7% were moderate smokers, and 66.2% were heavy smokers. Pulmonary function tests revealed that the mean FEV1 value was 2.22±1.17 L (67.9±29.1%) and the FEV1/FVC value was 67.49±12.80%. The distribution of patients according to COPD GOLD stages was 43.9% for stage-0, 12.8% for Stage-I, 14.1% for Stage-II, 11.4% for Stage-III, and 17.5% for Stage-IV. The success rate of smoking cessation was 67.5% at 3 months and 45.9% at 12 months. At 12 months, the number of non-quitters was 80 (54.1%) (Table 1).
Table 1.
Patient demographics at baseline
| Number of Patients | 148 |
| Sex (Female/Male), n | 68/80 |
| Age, y | 48.6±15.1 |
| Age at start smoking, y | 18.4±3.9 |
| Level of smoking, pack/years | 24.9±16.4 |
| Daily number of cigarettes (cig/day) | 19.2±8.6 |
| Mild smoker (0–9) | 6 (4.8) |
| Moderate smoker (10–19) | 44 (29.7) |
| Heavy smoker (>20) | 98 (66.2) |
| FNAQ, points | 5.1±2.2 |
| low grade addiction (0–3) | 41 (27.7) |
| Intermediate grade addiction (4–6) | 70 (47.2) |
| high grade addiction (7–10) | 37 (25.0) |
| CAT scores, points | 19.8±9.0 |
| Pulmonary function test | |
| FEV1, L | 2.22±1.17 |
| FEV1, % | 67.9±29.1 |
| FVC, L | 3.17±1.38 |
| FVC, % | 81.24±27.97 |
| FEV1/FVC, % | 67.49±12.80 |
| PEF | 3.22±2.17 |
| PEF (%) | 43.64±23.25 |
| FEF25–75, L | 2.37±1.05 |
| FEF25–75, % | 53.38±26.04 |
| COPD GOLD status | |
| Stage-0 | 65/148 – (43.9) |
| Stage-I | 19/148 – (12.8) |
| Stage-II | 21/148 – (14.1) |
| Stage-III | 17/148 – (11.4) |
| Stage-IV | 26/148 – (17.5) |
| Smoking cessation treatments | |
| Only NRT | 50 (75.7) |
| NRT + Varenicline | 36 (24.3) |
| Patients with initial inhaler treatment | 43 (29.0) |
| Successfull smoking cessation at 3-M | 100 (67.5) |
| Quitters at 12-M | 68 (45.9) |
| Non-quitters at 12-M | 80 (54.1) |
Data are presented mean±SD or n (%).
FEV1: a forced expiration volume in 1 s; FVC: forced vital capacity; PEF: peak expiratory flow; FEF25–75: forced expiratory flow between 25% and 75% of vital capacity; COPD GOLD: Global Initiative for Chronic Obstructive Lung Disease; FNAQ: Fagerström Nicotine Addiction Questionnaire; CAT: chronic obstructive lung disease assessment test; M: month; L: liter; NRT: nicotine replacement treatment
When the baseline parameters of patients were compared according to COPD GOLD stages, the average ages of the patients were 40.7±12.0, 45.3±10.1, 45.1±14.3, 62.1±11.2 and 64.8±8.7 years for GOLD Stages-0, I, II, III, and IV, respectively. The average smoking consumption was 19.1±10.5 pack-years for Stage-0, 23.5±15.5 pack-years for Stage-I, 31.5±24.6 for Stage-II, 28.4±12.9 for Stage-III, and 32.8±18.5 pack-years for Stage-IV. The group-wise distribution of patients’ pulmonary function parameters and CAT scores is shown in Figure 2.
Figure 2.
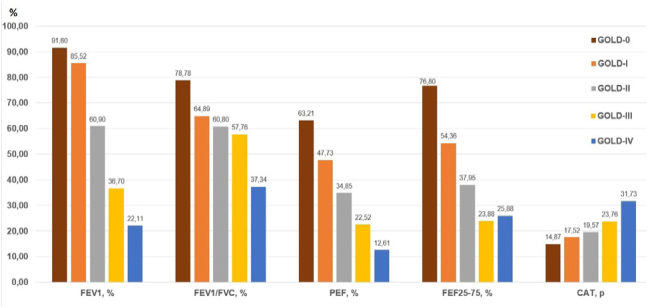
Comparison of baseline pulmonary function parameters according to chronic obstructive pulmonary disease severity
(FEV1: a forced expiration volume in 1 s; FVC: forced vital capacity; PEF: peak expiratory flow; FEF25–75: forced expiratory flow between 25% and 75% of vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; CAT: chronic obstructive lung disease assessment test)
When the initial parameters were compared according to smoking cessation status, there was no statistically significant difference between groups for age, smoking history, level of smoking consumption, FEV1, FVC, FEV1/FVC value, FNAQ score, or body-mass index (Table 2). The CAT score was 18.2±8.8 points in non-quitters and 21.7±8.9 points in quitters, and the difference was statistically significant (p=0.01). When ROC curve analysis was performed, the cut-off value for smoking cessation was CAT >20 (sensitivity, 60.3%; specificity, 63.7%; 95% CI, 0.53–0.69; p=0.01). The CAT score distribution in non-quitters and quitters is shown in Figure 3.
Table 2.
Comparison of baseline parameters according to smoking cessation status
| Characteristic | Non-quitters (n=80) | Quitters (n=68) | p |
|---|---|---|---|
| Age | 48.8±15.1 | 48.3±15.2 | 0.83 |
| Age of start smoking, y | 18.5±4.4 | 18.2±3.3 | 0.68 |
| Smoking history, py | 27.9±19.1 | 25.8±21.8 | 0.53 |
| Body mass index | 25.9±5.5 | 26.1±4.7 | 0.78 |
| Daily number of cigarettes (cig/day) | 20.1±9.1 | 18.1±7.8 | 0,18 |
| Light smoker (≤10 cig/day) | 9.3±1.7 | 9.4±1.5 | 0.34 |
| Heavy smoker (≥11 cig/day) | 23.0±8.1 | 22.1 ±6.1 | 0.89 |
| Very Heavy smoker (≥20 cig/day) | 24.1±8.0 | 22.9 ±5.9 | 0.04 |
| FEV1, L | 2.32 ±1.14 | 2.10±1.19 | 0.25 |
| FVC, L | 3.25±1.34 | 3.08±1.43 | 0.45 |
| FEV1/FVC, % | 68.52±12.81 | 66.41±12.86 | 0.32 |
| FNAQ, p | 5.1±2.2 | 5.0±2.2 | 0.85 |
| CAT, p | 18.2±8.8 | 21.7±8.9 | 0.01 |
Data are presented as mean±SD.
See Table 1 legend for expansion of other abbreviation
FEV1: a forced expiration volume in 1 s; FVC: forced vital capacity; FNAQ: Fagerström Nicotine Addiction Questionnaire; CAT: chronic obstructive lung disease assessment test
Figure 3. a, b.
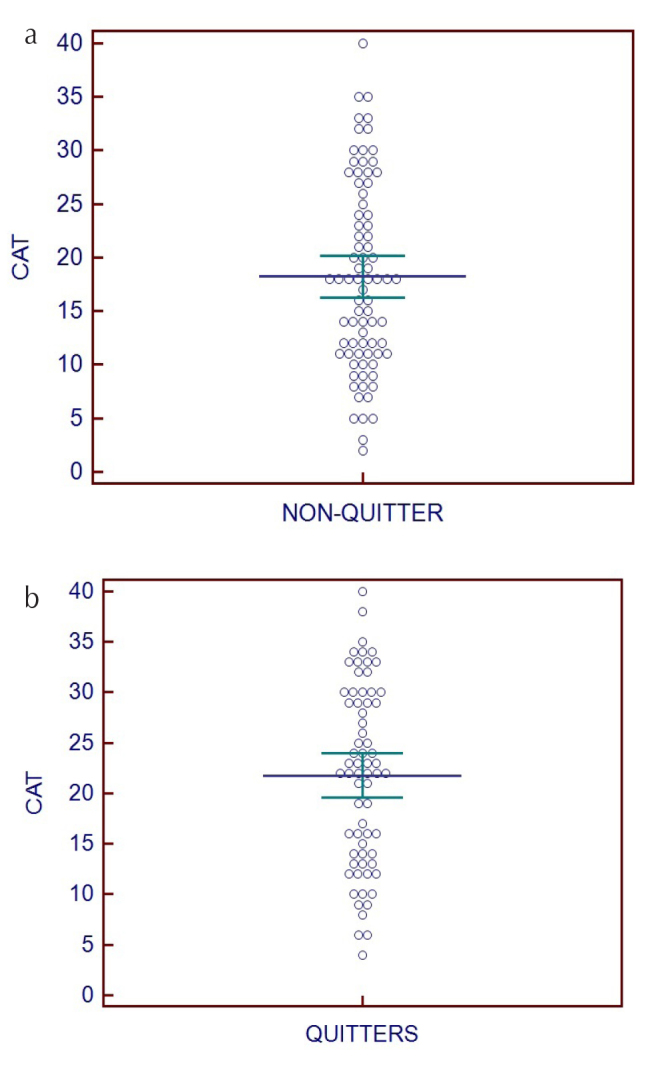
Chronic obstructive pulmonary disorder Assessment Test score distribution in quitters and non-quitters
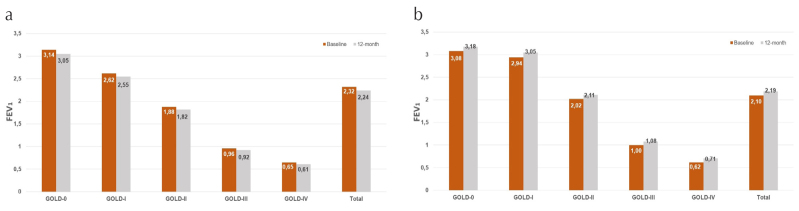
Baseline and 12-month FEV1 values were compared for non-quitters (n=80) and quitters (n=68) (Table 3). In non-quitters, the mean FEV1 values decreased from 2.32±1.14 to 2.24±1.12 (−80 mL; p<0.001). Particularly in Stage-0, in the early high-risk group of COPD, the reduction in FEV1 was 90 mL, while the reduction was 70, 60, 40, and 40 mL in Stages-I, -II, -III, and -IV, respectively (Figure 4a). In quitters, the mean FEV1 levels increased from 2.10±1.19 to 2.19±1.20 mL (+90 mL; p<0.001). For COPD patients overall, an average increase of 80–110 mL in FEV1 was observed (Figure 4b). At the end of the 12 months follow-up, 17 (27.5%) of the non-quitters showed deterioration in COPD stage, and five (7.3%) of the quitters showed improvement in COPD stage (Table 4).
Table 3.
Changes in FEV1 values at baseline and at 12 months
| GOLD Status | Non-Quitter | Quitter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| n | Baseline | 12-month | Δ, L | p | n | Baseline | 12-month | Δ, L | p | |
| Stage-0 | 36 | 3.14±0.79 | 3.05±0.78 | −0.08 | <0.001 | 29 | 3.08±0.73 | 3.18±0.72 | +0.10 | <0.001 |
| Stage-I | 14 | 2.62±0.65 | 2.55±0.64 | −0.07 | <0.001 | 5 | 2.94±0.68 | 3.05±0.69 | +0.11 | <0.001 |
| Stage-II | 11 | 1.88±0.49 | 1.82±0.50 | −0.06 | <0.001 | 10 | 2.02±0.63 | 2.11±0.62 | +0.09 | <0.001 |
| Stage-III | 8 | 0.96±0.27 | 0.92±0.28 | −0.04 | 0.10 | 9 | 1.00±0.14 | 1.08±0.13 | +0.08 | <0.001 |
| Stage-IV | 11 | 0.65±0.13 | 0.61±0.14 | −0.04 | <0.001 | 15 | 0.62±0.13 | 0.71±0.15 | +0.09 | <0.001 |
| Total | 80 | 2.32±1.14 | 2.24±1.12 | −0.08 | <0.001 | 68 | 2.10±1.19 | 2.19±1.20 | +0.09 | <0.001 |
Mean (median)±SD; Δ, change; L: liters
Figure 4. a, b.
Changes in forced expiration volume in 1 s value of non-quitters, and quitters
Table 4.
Change of GOLD Stages within 12 months
| Non-quitters (n=80) | Quitters (n=68) | ||
|---|---|---|---|
|
|
|
||
| GOLD Status | n | GOLD Status | n |
| Stage-0 to Stage-I | 5 | Stage-I to Stage-0 | 1 |
| Stage-I to Stage-II | 9 | Stage-II to Stage-I | 0 |
| Stage-II to Stage-III | 1 | Stage-III to Stage-II | 1 |
| Stage-III to Stage-IV | 2 | Stage-IV to Stage-III | 3 |
| Total worsening | 17–21.5% | Total improved | 5–7.3% |
DISCUSSION
This study investigated pulmonary function of COPD patients 12 months after quitting smoking as compared to those who did not quit smoking. Non-quitters generally experienced a reduction of 80 mL in FEV1 after 12 months. This reduction was seen as 40 mL in GOLD Stage-III and Stage-IV and was less remarkable than that in the other stages. These extremely low FEV1 values in patients with COPD may reflect the absence of healthy pulmonary parenchyma due to continued smoking.
In a previous study of a large COPD population, the mean FEV1 decline was reported to be 47–79 mL/year in GOLD Stage-II, 56–59 mL/year in Stage-III, and 35 mL/year in stage-IV [12]. In addition, there was insufficient data on Stage-I COPD and the smoking status of the patients was not evaluated. In our study, the mean FEV1 decline in non-quitters and GOLD Stage-I COPD patients was 70 mL/year. However, it has been reported that healthy subjects have a spontaneous average FEV1 decline of 25–29 mL/year after the age of 25 years, and an additional loss of 15 mL in medium-heavy smokers [13].
In a large study conducted by Scanlon et al. [14] in patients with mild-to-moderate COPD, it was reported that in those who continue to smoke, there was a decrease of 49 mL and 0.79% in FEV1 at 1 year and a decrease of 62±55 mL between 1 and 5 years. They reported that this decrease was two-fold greater than that observed in quitters. In our study, we noticed a similar decrease in FEV1 at an average of 80 mL (range, 40–80 mL), with rates or reduction varying according to the COPD groups. Scanlon et al. [14] also examined those who quit smoking in the same study and reported that they had an increase of 47 mL or 2% in FEV1 at 1 year; the FEV1 decline was 31±41 mL/year or 0.27% in sustained quitters between 1 and 5 years. These results suggest that if the patients quit smoking, the decline in FEV1 due to the increase in age would return to its natural course. In another study, individuals who followed a short-term cessation program reported a 100-mL increase in FEV1 after 3 months [15]. In our study, we observed an average increase in FEV1 of 90 mL (range, 80–110 mL) in COPD patients across all stages who quit smoking. This difference between the two studies can be explained by the inhaler treatments that had only just started and continued. Nevertheless, it is clear that quitting smoking resulted in a positive gain for the patients.
Results are ambiguous for patients who started inhaler treatment but continued to smoke. In our study, we have started all GOLD Stage-III and Stage-IV patients on combined inhaler therapy. Non-quitters in Stage-III and IV had a 40 mL reduction in FEV1; quitters showed an increase of 80 mL and 90 mL in FEV1 in Stage-III and Stage-IV, respectively. This indicates that the initiation of inhaler treatment (LABA + LAMA) is not particularly useful for patients who continue to smoke. A recent study of inhaled steroid (ICS) treatment in asthmatic patients found that smokers had eosinophilic airway inflammation plus neutrophilic airway inflammation [16]. In that study, smoking was shown to impair the effectiveness of ICS therapy in the treatment of mild-to-moderate asthma. In COPD patients, the relationship between smoking status and respiratory outcomes has recently been investigated [17]. That study compared former smokers and current smokers for a 12-month period. Former smokers had a mean difference of 30 mL (range, 9–51 mL) in patients receiving fluticasone furoate, 22 mL (range, 1–43 mL) in patients receiving fluticasone furoate + vilanterol, and no difference in patients using only vilanterol (range, −27 to 15 mL) [17]. However, that study also showed that current smokers had a blunted FEV1 response with ICS and a smaller decrease in the frequency of exacerbations with ICS/LABA than former smokers. Although these studies also addressed the need for inhaler therapy in patients who smoke, the results remained inconclusive. A more detailed and comprehensive study on this subject is therefore necessary.
Furthermore, smoking cessation outcomes in COPD patients vary widely. In our study, smoking cessation success was 67.5% at 3 months and 45.9% at 12 months. The initial CAT scores of quitters were also higher than those non-quitters (18.2±8.8 vs. 21.7±8.9). Patients with a CAT score of <20, i.e., who were clinically less symptomatic, should be monitored more carefully for smoking cessation. In a recent study, contrary to our findings, the baseline CAT score in quitters and non-quitters was found to be similar (18.5 vs. 16.5; p=not significant), but improvements in quitters (10.5 vs 15.5; p<0.01) were reported in controls [18]. However, in a 2012 study of 739 patients with COPD, smoking cessation success was 60.2% in males and 55.6% in females [19].
The COPD GOLD stage-0 patients in our study constituted an interesting research population. In this group of patients, although parameters such as FEV1, FVC, and FEV1/FVC were in the normal range, a mild reduction was detected in PEF and FEF25–75 parameters. A previous study reported that FEF25–75 value less than 80% was an early indicator of small airway impairment [20]. However, a cut-off point of 60% for FEF25–75 was used to identify the presence of small airway disease and increased airway resistance [21, 22]. In addition, forced expiration volume in 3 s (FEV3)/FVC can also be used to identify small airway disease [23]. Our finding indicated a cut-off point of 76.80% for FEF25–75; this may be indicative of the onset of small airway disease in COPD patients. In these patients, mean age (40.7±12.0 vs. 64.8±8.7; p<0.001), baseline FNAQ (4.69±2.31 vs. 6.03±1.98; p=0.01), and baseline CAT score (14.87±7.21 vs. 31.73±4.09; p<0.001) were lower than those in GOLD Stage-IV patients. Similarly, in a study conducted by Kömüs et al. [24] involving stage-0 patients, the PEF value was reported as 87.05±19.24% and the FEF25–75 value was reported as 56.56±16.59%; these values were lower than those of non-smokers. These findings emphasize the importance of early detection of patients in this risk group before they begin smoking cessation. Previously, this subclinical phase of COPD was defined as Stage-0 in the first GOLD manual, but it was not included in subsequent guidelines [3, 11]. Early detection of this patient group and initiation of smoking cessation program before end-organ damage occurs is extremely important [25]. Some studies have also suggested that this pre-clinical phase should be re-classified as early COPD or Stage-0, and that biomarker-based screening is required [26].
In addition, we observed COPD stage changes in patients according to smoking cessation status after 12 months. Among patients in the worst stage, 21.5% were non-quitters, whereas among those in the better stage, 7.3% were quitters. This may indicate that the condition of patients who have not quit smoking deteriorate over time, but it does not recover soon after quitting smoking, and that the damage may be permanent.
There are some limitations to our study. First, the study contained historical data and possible involuntary bias due to its retrospective nature. Second, the number of patients in the groups decreased because of the distribution of patients in the various COPD groups. Therefore, attention should be paid to the analysis of distributed data. Third, the obtained data may not be generalizable to COPD patients; nevertheless, significant results were obtained.
In conclusion, FEV1 decline is accelerated in COPD patients who continue to smoke, and this decline was not prevented by inhaler treatments. However, there was a significant improvement in FEV1 in all COPD patients who quit. The GOLD Stage-0 group, which is not included in the current guidelines, needs to be redefined, and this group is the most important in terms of prevention of the disease.
MAIN POINTS.
This study shows changes in pulmonary function tests in COPD patients if they continue to smoke or quit smoking within one year.
In non-quitters, the reduction in FEV1 was 40–90 mL, and 27.5% of patients had deterioration in the COPD stage.
In quitters, an average increase in FEV1 of 80–110 mL was observed, and 7.3% of the patients showed improvement in COPD stage.
Early COPD or GOLD Stage-0 patients, which is not included in the current guidelines, needs to be redefined. Early detection of this patient group and initiation of smoking cessation program before end-organ damage occurs is extremely important.
Footnotes
Ethics Committee Approval: The study protocol was approved by the ethics committee of the Sifa University Medical Faculty (Nr:198-60, and date:29.10.2014).
Informed Consent: Due to the retrospective design of the study, the informed consent forms were not able to be taken.
Conflict of interest: The author have stated explicitly that there are no conflicts of interest in connection with this article.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Vogelmeier CF, Criner GJ, Martínez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol. 2017;53:128–49. doi: 10.1016/j.arbres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 2007;85:141–7. doi: 10.1139/y06-093. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2018. Available from: http://www.goldcopd.org/
- 4.Tøttenborg SS, Thomsen RW, Johnsen SP, et al. Determinants of Smoking Cessation in Patients With COPD Treated in the Outpatient Setting. Chest. 2016;150:554–62. doi: 10.1016/j.chest.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–505. doi: 10.1001/jama.272.19.1497. [DOI] [PubMed] [Google Scholar]
- 6.Pride NB. Smoking cessation: effects on symptoms, spirometry and future trends in COPD. Thorax. 2001;56:7–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Dhariwal J, Tennant RC, Hansell DM, et al. Smoking cessation in COPD causes a transient improvement in spirometry and decreases micronodules on high-resolution CT imaging. Chest. 2014;145:1006–15. doi: 10.1378/chest.13-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with referebce to individualization of treatment. Addict Behav. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 10.Uysal MA, Kadakal F, Karşidağ C, et al. Fagerstrom test for nicotine dependence: reliability in a Turkish sample and factor analysis. Tuberk Toraks. 2004;52:115–21. [PubMed] [Google Scholar]
- 11.Pauwels RA, Buist AS, Calverley PM, et al. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 12.Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–9. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerstjens HA, Rijcken B, Schouten JP, et al. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax. 1997;52:820–7. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanlon PD, Connett JE, Waller LA, et al. Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–90. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 15.Maci E, Comito F, Frezza AM, et al. Lung nodule and functional changes in smokers after smoking cessation short-term treatment. Cancer Invest. 2014;32:388–93. doi: 10.3109/07357907.2014.919308. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda T, Obase Y, Kishikawa R, et al. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma. Allergy Asthma Proc. 2016;37:50–8. doi: 10.2500/aap.2016.37.3944. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt SP, Anderson JA, Brook RD, et al. Cigarette smoking and response to inhaled corticosteroids in COPD. Eur Respir J. 2018;51:1701393. doi: 10.1183/13993003.01393-2017. [DOI] [PubMed] [Google Scholar]
- 18.Pezzuto A, Stellato M, Catania G, et al. Short-term benefit of smoking cessation along with glycopirronium on lung function and respiratory symptoms in mild COPD patients: a retrospective study. J Breath Res. 2018;12:046007. doi: 10.1088/1752-7163/aad0a8. [DOI] [PubMed] [Google Scholar]
- 19.Kupiainen H, Kinnula VL, Lindqvist A, et al. Successful Smoking Cessation in COPD: Association with Comorbidities and Mortality. Pulm Med. 2012;2012 doi: 10.1155/2012/725024. 725024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marseglia GL, Cirillo I, Vizzaccaro A, et al. Role of forced expiratory flow at 25–75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007;28:74–8. doi: 10.2500/aap.2007.28.2920. [DOI] [PubMed] [Google Scholar]
- 21.McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J. 2014;1 doi: 10.3402/ecrj.v1.25898. doi: 10.3402/ecrj.v1.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir Med. 2016;116:19–27. doi: 10.1016/j.rmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Manoharan A, Anderson WJ, Lipworth J, et al. Assessment of spirometry and impulse oscillometry in relation to asthma control. Lung. 2015;193:47–51. doi: 10.1007/s00408-014-9674-6. [DOI] [PubMed] [Google Scholar]
- 24.Kömüs N, Tertemiz KC, Sevinç C. The importance of the at risk COPD patients (Stage 0) and clinical differences. Tuberk Toraks. 2008;56:382–9. [PubMed] [Google Scholar]
- 25.Vrbica Ž, Labor M, Gudelj I, et al. MARKO study group. Early detection of COPD patients in GOLD 0 population: an observational non-interventional cohort study - MARKO study. BMC Pulm Med. 2017;17:36. doi: 10.1186/s12890-017-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siafakas N, Bizymi N, Mathioudakis A, et al. EARLY versus MILD Chronic Obstructive Pulmonary Disease (COPD) Respir Med. 2018;140:127–31. doi: 10.1016/j.rmed.2018.06.007. [DOI] [PubMed] [Google Scholar]