Abstract
Purpose
To evaluate efficacy and safety of intravitreal injections of aflibercept (IVT-AFL) treat-and-extend (T&E) dosing regimens in treatment-naïve patients with exudative age-related macular degeneration (AMD).
Methods
Adults aged at least 50 years old with exudative AMD and best-corrected visual acuity (BCVA) of 73–25 Early Treatment Diabetic Retinopathy Study (ETDRS) letters were included. Patients received three monthly doses of IVT-AFL 2 mg. At week 16, patients were randomized 1:1 to IVT-AFL T&E with either 2- or 4-week adjustments. The primary endpoint was mean change in BCVA from baseline to week 52. Outcomes were assessed at weeks 52 and 96.
Results
Baseline characteristics were comparable between the groups (n = 123 each). Over 52 weeks, mean number of injections was 7.2 and 6.9 and mean last injection interval was 10.7 and 11.8 weeks, for the 2- and 4-week groups, respectively. From baseline, mean change in BCVA was + 9.0 and + 8.4 letters (week 52) and + 7.6 and + 6.1 letters (week 96); mean change in central retinal thickness was − 134.4 µm and − 126.1 µm (week 52) and − 130.5 µm and − 125.3 µm (week 96). Last injection interval before week 52 was at least 12 weeks in 42.3% and 49.6% of patients and 56.9% and 60.2% before week 96. Over 96 weeks, mean number of injections was 10.4 (both groups). The safety profile of IVT-AFL was consistent with previous reports.
Conclusions
IVT-AFL administered using two different T&E regimens for treatment-naïve exudative AMD improved functional and anatomic outcomes at week 52 and outcomes were maintained to week 96. Outcomes were similar between the 2- and 4-week groups.
Trial Registration
ClinicalTrials.gov identifier, NCT02305238.
A peer-reviewed video was retrospectively added to this publication (MP4 117051 kb)
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01236-x) contains supplementary material, which is available to authorized users.
Keywords: Aflibercept, Anti-vascular endothelial growth factor agents, Exudative age-related macular degeneration, Ophthalmology, Treat-and-extend
Key Summary Points
| Why carry out this study? |
| The goal of proactive flexible treat-and-extend (T&E) regimens is to reduce the treatment burden associated with anti-vascular endothelial growth factor (VEGF) therapy, while maintaining a patient’s visual acuity gains. |
| The aim of this study was to evaluate the efficacy and safety of intravitreal aflibercept (IVT-AFL) T&E dosing regimens in treatment-naïve patients with exudative age-related macular degeneration (AMD). |
| What was learned from the study? |
| IVT-AFL administered using either 2- or 4-week adjustment T&E regimens in treatment-naïve patients with exudative AMD improved functional (best-corrected visual acuity + 9.0 and + 8.4 letters) and anatomic outcomes (central retinal thickness − 134.4 and − 126.1 µm) at 52 weeks; functional and anatomic outcomes were maintained over 96 weeks. |
| A large proportion of patients (35.1% and 40.5%) had an intended injection interval of 16 weeks at week 52. |
| The incidence of treatment-emergent adverse events was consistent with the known safety profile of IVT-AFL. |
| IVT-AFL T&E regimens were efficacious and safe over 96 weeks of treatment using either 2- or 4-week adjustments. |
Digital Features
This article is published with digital features, including a summary slide and video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.11603511.
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide and accounts for 10.9% of visual impairment in Japan [1, 2]. AMD-related vision loss is primarily associated with the exudative form of AMD [3], which is characterized by abnormal growth of new blood vessels in the macula [4].
Overexpression of vascular endothelial growth factor (VEGF) plays a significant role in the development of new blood vessels [4, 5]. Treatment of exudative AMD involves administering anti-VEGF agents, such as aflibercept and ranibizumab, which are an effective treatment option for patients with exudative AMD [4]. Although rapid functional and anatomic improvements are evident in the first year of treatment, regression to baseline after initial gains is common in a clinical setting [6–10]. Improvements achieved during the first and second year of protocol-mandated treatment are difficult to maintain in clinical practice [6–8]. Anti-VEGF treatments are associated with high clinic and patient burden, which may reduce long-term compliance and increase healthcare costs [6, 7]. If the intervals between treatments are too short, patients may be overtreated, potentially leading to unnecessary treatment burden and an increased risk of adverse events (AEs) associated with intravitreal injection procedure (such as conjunctival hemorrhage and endophthalmitis). Conversely, if the intervals between treatments are too long, patients may be undertreated, potentially leading to inferior functional and anatomic outcomes [9]. Therefore, the goal of anti-VEGF treatment for exudative AMD is to improve and maintain functional and anatomic gains over and beyond the first year of treatment, while minimizing treatment burden on patients [7, 9].
The goal of flexible treatment strategies, including pro re nata (PRN) and proactive treat-and-extend (T&E) regimens, is to reduce the treatment burden associated with anti-VEGF therapy, while maintaining visual acuity (VA) gains [7, 9, 11]. T&E is a proactive, individualized dosing strategy whereby the injection interval can be gradually extended if functional and anatomic stability is maintained, and the interval shortened if deterioration is observed, in order to minimize the risk of disease recurrence rather than in response to it [6]. For intravitreal aflibercept (IVT-AFL) injection, proactive T&E approaches are effective for maintaining functional outcomes in patients with exudative AMD, while reducing the burden of clinic visits [6]. IVT-AFL T&E regimens offer patients an individualized treatment approach whereby the patient’s injection interval is decided on the basis of functional and anatomic evaluations. This flexible approach avoids both overtreatment and undertreatment, while optimizing functional and anatomic outcomes. Additionally, patients receive an injection at every visit and therefore physicians can adapt the treatment plan accordingly [7]. The functional and anatomic improvements achieved during the initial dosing phase of PRN regimens are often not sustained unless frequent monitoring and strict retreatment criteria are applied. Therefore, individualized proactive T&E regimens are generally preferred over PRN regimens [7].
The optimal T&E regimen for the treatment of exudative AMD is yet to be determined, particularly the ideal amount of time that the interval can be adjusted by at any given time and the maximum injection interval offered [7, 11]. Recommended guidelines for patients in the Asia–Pacific region suggest that the IVT-AFL injection interval can be extended by 4-week increments following the initial three doses, to a maximum interval of 12 weeks for patients with inactive disease [11]. To date, no randomized controlled studies have examined in detail the outcomes associated with 4-week adjustments and injection intervals beyond 12 weeks. Further evaluation could be beneficial for identifying patients that are suitable for 2- or 4-week adjustments and injection intervals of up to 16 weeks [11]. Given the lack of published evidence on this subject, a clinical study was required to explore the optimal T&E regimen(s) that could be applied in real-world clinical practice.
The aim of this randomized study was to examine the efficacy and safety of IVT-AFL administered in two different T&E regimens (2- and 4-week adjustments) in patients with exudative AMD, while allowing a minimum interval of 8 weeks and a maximum interval of 16 weeks.
Methods
Study Design
ALTAIR was a 96-week, randomized, open-label, phase 4 study (ClinicalTrials.gov identifier, NCT02305238) that investigated the efficacy and safety of repeated doses of IVT-AFL with two different T&E approaches in patients with exudative AMD. The study was conducted at 41 centers across Japan between December 2014 and November 2017, in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The protocol and any amendments were approved by the independent ethics committee or institutional review board (IRB) at each study site (see supplementary material). There was no central IRB involved in the study and the protocol was reviewed and approved by the IRB at each participating center. All patients provided written informed consent.
Participants
Adults aged at least 50 years old with exudative changes due to active subfoveal choroidal neovascularization (CNV) lesions secondary to AMD, including juxtafoveal lesions that affected the fovea, as evidenced by fluorescein angiography (FA) in the study eye, were included. Patients were required to have best-corrected visual acuity (BCVA) of 73–25 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (approximately 20/40–20/320 Snellen equivalent) in the study eye. If both eyes met the inclusion criteria, the eye with the worst BCVA was selected as the study eye. Both eyes could be treated, but only one eye per patient was analyzed. Exclusion criteria are listed in Table S1 in the supplementary material.
Randomization and Interventions
The study design is summarized in Fig. S1 in the supplementary material. This study was conducted as a randomized trial to enable a descriptive comparison of the two treatment arms without introducing selection bias for one T&E strategy over the other. Block randomization was stratified by baseline BCVA (< 55 and ≥ 55 ETDRS letters) and exudative AMD subtype (presence or absence of polypoidal choroidal vasculopathy [PCV], as decided by the investigator). Patients received three initial monthly doses of IVT-AFL 2 mg in the study eye. Patients received IVT-AFL at week 16 and were randomized 1:1 to receive IVT-AFL in a T&E regimen with either a 2-week (IVT-AFL-2W) or 4-week (IVT-AFL-4W) adjustment, to minimize potential imbalances during treatment initiation.
Patients received an IVT-AFL injection at every pre-scheduled treatment visit. The timing of treatment visits following IVT-AFL injection at week 16 was determined at the previous visit by the treating physician on the basis of evaluation and pre-defined treatment criteria. Patients were evaluated at weeks 52 and 96, regardless of treatment schedule. Ophthalmic evaluations were conducted at every treatment and evaluation visit, except for safety follow-up, using VA testing with ETDRS letter score, slit lamp and indirect ophthalmoscopy, and optical coherence tomography (OCT). Fundus photography, FA, and indocyanine green angiography were conducted at the screening visit and at weeks 52 and 96. Safety evaluations were conducted at every treatment and evaluation visit, including the follow-up.
Patients who met the criteria for treatment adjustment in the IVT-AFL-2W group had their injection interval extended or shortened by 2 weeks, while patients who met the criteria for treatment adjustment in the IVT-AFL-4W group had their injection interval extended or shortened by 4 weeks. If patients in the IVT-AFL-4W group underwent an interval shortening of 4 weeks (due to disease activity), any possible extension or shortening thereafter was set as 2 weeks as a conservative measure to ensure the best possible VA outcomes for these patients. The injection interval was maintained in both groups without change if the criteria for treatment adjustment were not met and residual fluid was decreased from the previous visit. The criteria for shortening, maintaining, or extending the injection interval are shown in Table 1. The minimum and maximum injection intervals were 8 and 16 weeks, respectively.
Table 1.
Criteria for shortening, maintaining, or extending the IVT-AFL injection interval
| Criteria for shortening the treatment interval |
|
When any of the following criteria are met for the study eye, the subsequent treatment interval was shortened: New or persistent fluid with unchanged or increased fluid volume from measurement at the previous treatment visit as indicated by OCT Loss of ≥ 5 ETDRS letters from the previous visit in conjunction with recurrent fluid on OCT An increase in CRT of ≥ 100 μm at the central 1 mm compared with the lowest previous value measured by OCT New-onset neovascularization as determined at the investigator’s discretion based on review of fundus examination and multi-imaging assessment if deemed necessary New macular hemorrhage New fluid or persistent intra- or subretinal fluid with unchanged or increased fluid volume from the previous visit as indicated by total OCT scan area (all volumetric fluid assessments were derived from multiple cross-sectional images and extracted from the OCT report) |
| Criteria for maintaining the treatment interval |
| If none of the criteria for shortening were met and residual fluid had decreased from the previous visit, then the treatment interval was maintained without change, even with persistent fluid |
| Criteria for extending the treatment interval |
| If none of the criteria for shortening were met and there was no fluid on OCT, then the interval was extended |
CRT central retinal thickness, ETDRS Early Treatment Diabetic Retinopathy Study, IVT-AFL intravitreal aflibercept, OCT optical coherence tomography
Study Endpoints
The primary endpoint was mean change in BCVA (ETDRS letters) from baseline to week 52. Secondary endpoints included, but were not restricted to, the proportion of patients who lost fewer than 15 letters (vision maintenance) or gained at least 15 letters, the proportion of patients without fluid on OCT, and the mean change in central retinal thickness (CRT) from baseline to week 52. The mean number of IVT-AFL injections, the mean injection interval, the last injection interval (the interval between the last two injections), and the intended injection interval (determined by the investigator as the next planned interval and based on pre-defined treatment criteria evaluating the efficacy of the previous injection) were recorded. Endpoints were assessed at weeks 52 and 96.
AEs were treatment-emergent events if they occurred or worsened after the first IVT-AFL dose and at most 30 days after the last dose. All AEs were reported in case report forms and coded using Medical Dictionary for Regulatory Activities version 19.1. An adjudication of AEs according to the Antiplatelet Trialists’ Collaboration (APTC) criteria was performed.
Statistical Analysis
The sample size of 240 randomized patients was calculated on the basis of an assumed standard deviation (SD) of 12.5 ETDRS letters in BCVA change from baseline to week 52, non-inferiority delta of 5 letters, and a two-sided alpha of 0.05 between the two treatment arms. While the objective of this study was to describe the outcomes achieved with both treatment regimens, the sample size was calculated with a power of 86%, which allowed for descriptive statistical comparison. The two-sided 95% confidence intervals (CI) of mean change in BCVA were estimated per treatment arm using one-sample t statistics. Any treatment differences for the two treatment arms were estimated using an analysis of covariance model with treatment arm and exudative AMD subtype as fixed effect and baseline BCVA as a covariate. Secondary visual outcomes were summarized descriptively, and their two-sided 95% CIs were estimated using normal approximation; any point estimate treatment differences were estimated using the Mantel–Haenszel (MH) method stratified by baseline BCVA (< 55 or ≥ 55 ETDRS letters) and exudative AMD subtype. Patients in the IVT-AFL-4W group who had their interval shortened remained in the 4-week group for data analysis. All statistical analyses were exploratory and outcomes were summarized descriptively, as no confirmatory statistical analysis was intended.
Statistical evaluation was performed using Statistical Analysis Software v9.2 or higher (SAS Institute Inc., Cary, NC, USA). Detailed statistical methodology is available in the supplemental methods.
Results
Patients
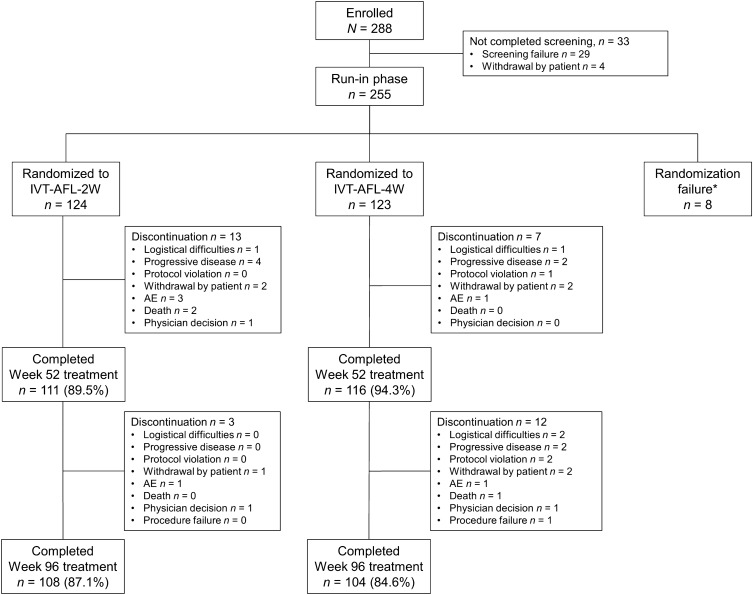
In total, 288 patients were enrolled and 255 patients completed screening. Of these, 254 patients received at least one of the three initial monthly doses of IVT-AFL and comprised the safety analysis set. In total, of 247 patients treated with IVT-AFL, 124 patients were randomized to the 2-week group and 123 patients to the 4-week group. Eight patients were not randomized. One patient in the IVT-AFL-2W group was not included in the full analysis set (FAS) as the post-randomization assessment was not available because of premature discontinuation (death) immediately after randomization at week 16 (Fig. 1). Overall, 85.8% (n = 212) of patients completed the study. The main reasons for discontinuation were progressive disease (n = 8), withdrawal by patient (n = 7), and AEs (n = 6) (Fig. 1).
Fig. 1.
Patient disposition. *A total of eight patients were not randomized because of withdrawal by patient (n = 3), protocol violation (n = 2), physician decision (n = 1), logistic difficulties (n = 1), or no IVT-AFL injection (n = 1). 2W/4W 2-/4-week adjustment, AE adverse event, IVT-AFL intravitreal aflibercept
Patient baseline demographics were similar between the two groups (Table 2). The overall mean (SD) age was 74.0 (8.0) years, baseline BCVA was 55.0 (12.5) ETDRS letters, and most patients were male (72.4%).
Table 2.
Patient baseline characteristics and demographics (FAS)
| Characteristic | IVT-AFL-2W adjustment | IVT-AFL-4W adjustment | Total |
|---|---|---|---|
| n = 123 | n = 123 | N = 246 | |
| Age, mean (SD), years | 73.0 (7.9) | 75.0 (8.1) | 74.0 (8.0) |
| Sex, n (%) | |||
| Male | 87 (70.7) | 91 (74.0) | 178 (72.4) |
| Baseline BCVA score, mean (SD) | 54.8 (13.1) | 55.3 (12.0) | 55.0 (12.5) |
| Baseline CRT, mean (SD), µm | 386.2 (159.2) | 370.3 (120.0) | 378.3 (141.0) |
| Type of exudative AMD, n (%)a | |||
| Typical AMD | 75 (61.0) | 75 (61.0) | 150 (61.0) |
| Polypoidal choroidal vasculopathy | 46 (37.4) | 44 (35.8) | 90 (36.6) |
| Retinal angiomatous proliferation | 4 (3.3) | 9 (7.3) | 13 (5.3) |
| Type of CNV lesions on FA, n (%)b | |||
| Classic CNV | 35 (28.5) | 42 (34.1) | 77 (31.3) |
| Classic CNV and occult CNV | 14 (11.4) | 17 (13.8) | 31 (12.6) |
| Occult CNV | 72 (58.5) | 62 (50.4) | 134 (54.5) |
| Pigment epithelial detachment, n (%) | 83 (67.5) | 78 (63.4) | 161 (65.4) |
| Subretinal fluid, n (%) | 104 (84.6) | 104 (84.6) | 208 (84.6) |
| Intraretinal fluid, n (%) | 43 (35.0) | 44 (35.8) | 87 (35.4) |
| Hemorrhage, n (%) | 67 (54.5) | 61 (49.6) | 128 (52.0) |
| Subretinal hemorrhage | 56 (45.5) | 50 (40.7) | 106 (43.1) |
| Intraretinal hemorrhage | 28 (22.8) | 30 (24.4) | 58 (23.6) |
2W/4W 2-/4-week adjustment, AMD age-related macular degeneration, BCVA best-corrected visual acuity, CNV choroidal neovascularization, CRT central retinal thickness, FA fluorescein angiography, FAS full analysis set, IVT-AFL intravitreal aflibercept, SD standard deviation
aPatients could be classified into more than one group
bUnknown in the IVT-AFL-2W (n = 2) and IVT-AFL-4W (n = 1) adjustment groups and one patient had no CNV in the IVT-AFL-4W group
Treatment Exposure
The mean (SD) number of IVT-AFL injections was 7.2 (0.9) in the IVT-AFL-2W group and 6.9 (1.0) in the IVT-AFL-4W group (baseline to week 52) and 3.6 (1.6) in the IVT-AFL-2W group and 3.7 (1.4) in the IVT-AFL-4W group (weeks 54–96). Overall, from baseline to week 96, the mean (SD) number of IVT-AFL injections was almost identical between the IVT-AFL-2W and IVT-AFL-4W groups (10.4 [2.6] and 10.4 [2.4], respectively).
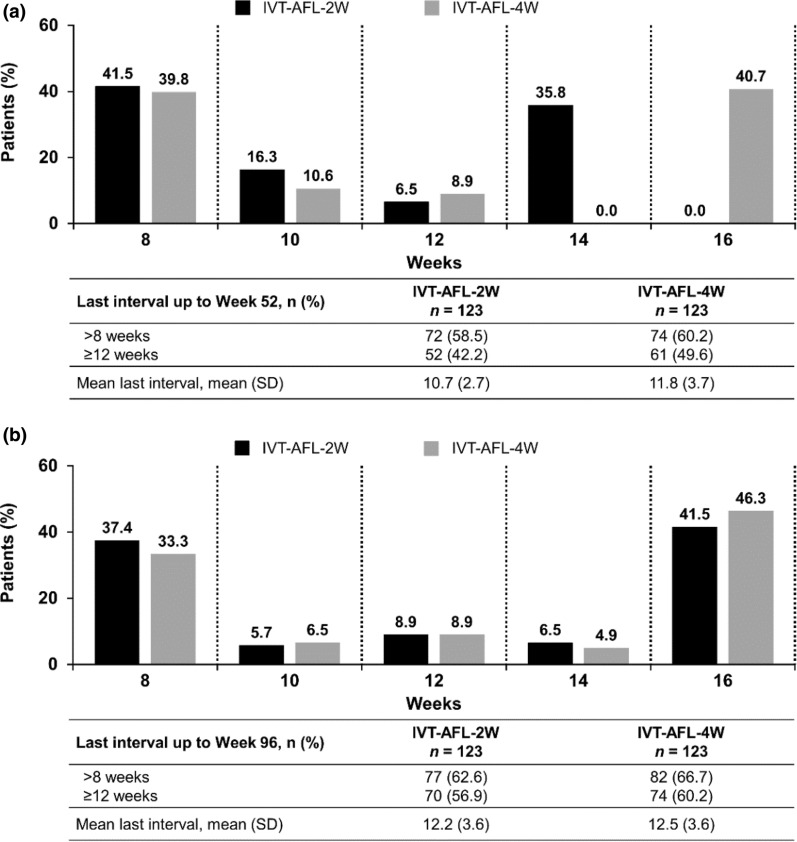
The mean (SD) last interval at week 52 was 10.7 (2.7) weeks and 11.8 (3.7) weeks, and at week 96 was 12.2 (3.6) weeks and 12.5 (3.6) weeks in the IVT-AFL-2W and IVT-AFL-4W groups, respectively. The last injection interval before week 52 was at least 12 weeks in 42.3% (IVT-AFL-2W) and 49.6% (IVT-AFL-4W) of patients and before week 96 was at least 12 weeks in 56.9% (IVT-AFL-2W) and 60.2% (IVT-AFL-4W) of patients. The last injection interval before week 52 was 16 weeks in 0.0% (IVT-AFL-2W) and 40.7% (IVT-AFL-4W) of patients and before week 96 was 16 weeks in 41.5% (IVT-AFL-2W) and 46.3% (IVT-AFL-4W) of patients (Fig. 2).
Fig. 2.
The last injection interval up to a Week 52 and b Week 96 (FAS)
The intended injection interval at the last visit up to week 52 was at least 12 weeks in 56.8% (n/N = 63/111; IVT-AFL-2W) and 57.8% (n/N = 67/116; IVT-AFL-4W) of patients and was extended to 16 weeks for 35.1% (n/N = 39/111; IVT-AFL-2W) and 40.5% (n/N = 47/116; IVT-AFL-4W) of patients.
Up to week 96, the injection interval in the IVT-AFL-2W and IVT-AFL-4W groups was extended to 16 weeks for 43.1% (n = 53) and 54.5% (n = 67) of patients, respectively, and maintained at 16 weeks until study completion for 41.5% (n = 51) and 42.3% (n = 52) of patients, respectively. The proportion of patients who stayed at an 8-week injection interval, who never had an extension, was 27.6% (n = 34) in the IVT-AFL-2W group and 22.0% (n = 27) in the IVT-AFL-4W group. Up to week 96, the proportion of patients with intervals of 12 weeks and beyond who maintained at least a 12-week interval was 48.0% (n = 59) for both treatment arms.
Efficacy
Functional Outcomes
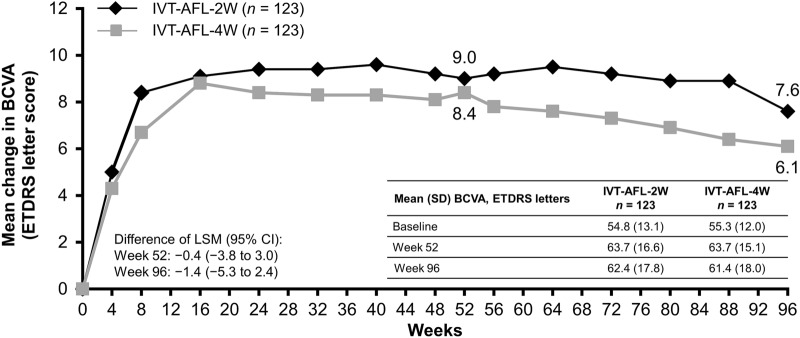
The mean change in BCVA (ETDRS letters) from baseline to week 52 was 9.0 letters (95% CI 6.4–11.5) in the IVT-AFL-2W group and 8.4 letters (95% CI 6.0–10.8) in the IVT-AFL-4W group (least squares [LS] mean difference of − 0.4 [95% CI − 3.8 to 3.0]). From baseline to week 96, the mean change in BCVA was 7.6 letters (95% CI 5.0–10.3) and 6.1 letters (95% CI 3.1–9.0) in the IVT-AFL-2W and IVT-AFL-4W groups, respectively (LS mean difference of − 1.4 [95% CI − 5.3 to 2.4]) (Fig. 3).
Fig. 3.
Mean change in BCVA (ETDRS letter score) in IVT-AFL-2W and IVT-AFL-4W groups from baseline to week 96 (FAS). Last observation carried forward analysis. 2W/4W 2-/4-week adjustment, BCVA best-corrected visual acuity, CI confidence interval, ETDRS Early Treatment Diabetic Retinopathy Study, FAS full analysis set, IVT-AFL intravitreal aflibercept, LSM least squares mean
The proportion of patients who lost fewer than 15 ETDRS letters was 96.7% (95% CI 93.6–99.9) in the IVT-AFL-2W group and 95.9% (95% CI 92.4–99.4) in the IVT-AFL-4W group at week 52 (MH-adjusted difference of − 0.9% [95% CI − 5.7 to 4.0]). At week 96, 95.1% (95% CI 91.3–98.9) and 91.9% (95% CI 87.0–96.7) of patients in the IVT-AFL-2W and IVT-AFL-4W groups lost fewer than 15 ETDRS letters, respectively (MH-adjusted difference of − 3.1% [95% CI − 9.5 to 3.2]) (Fig. S2a in the supplementary material). The proportion of patients who gained at least 15 ETDRS letters was 32.5% (95% CI 24.2–40.8) and 30.9% (95% CI 22.7–39.1) in the IVT-AFL-2W and IVT-AFL-4W groups, respectively, at week 52 (MH-adjusted difference of − 1.4% [95% CI − 12.7 to 9.8]). At week 96, 28.5% (95% CI 20.5–36.4) and 31.7% (95% CI 23.5–39.9) of patients in the IVT-AFL-2W and IVT-AFL-4W group, respectively, gained at least 15 ETDRS letters (MH-adjusted difference of 3.4% [95% CI − 7.9 to 14.7]) (Fig. S2b in the supplementary material).
Anatomic Outcomes
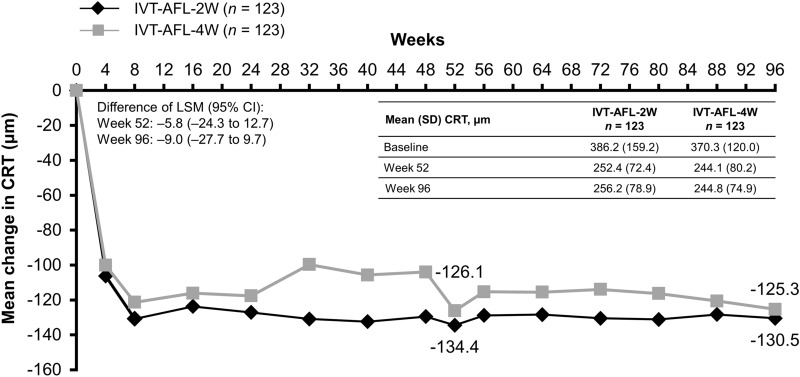
The mean change in CRT from baseline to week 52 was − 134.4 µm (95% CI − 162.2 to − 106.6) in the IVT-AFL-2W group and − 126.1 µm (95% CI − 147.1 to − 105.1) in the IVT-AFL-4W group (LS mean difference of − 5.8 µm [95% CI − 24.3 to 12.7]). Mean change in CRT from baseline to week 96 was − 130.5 µm (95% CI − 158.7 to − 102.4) in the IVT-AFL-2W group and − 125.3 µm (95% CI − 146.6 to − 104.1) in the IVT-AFL-4W group (LS mean difference of − 9.0 [95% CI − 27.7 to 9.7]) (Fig. 4).
Fig. 4.
Mean change in central retinal thickness (µm) from baseline to week 96 (FAS). Last observation carried forward analysis. 2W/4W 2-/4-week adjustment, CI confidence interval, CRT central retinal thickness, IVT-AFL intravitreal aflibercept, LSM least squares mean
The proportion of patients without fluid on OCT was 53.7% (IVT-AFL-2W) and 62.6% (IVT-AFL-4W) at week 16, and 68.3% (95% CI 60.1–76.5) (IVT-AFL-2W) and 69.1% (95% CI 60.9–77.3) (IVT-AFL-4W) at week 52 (MH-adjusted difference of − 1.0% [95% CI − 10.6 to 12.7]). At week 96, 67.5% (95% CI 59.2–75.8) of patients in both treatment arms had no fluid on OCT (MH-adjusted difference of 0.4% [95% CI − 11.4 to 12.2]).
Safety
An overview of the main safety findings at week 96 is provided in Table 3. The proportion of treatment-emergent AEs (TEAEs) was comparable between the IVT-AFL-2W and IVT-AFL-4W groups (68.5% and 69.9%, respectively), and these were predominantly mild and moderate in severity. The incidence of ocular TEAEs was higher in the IVT-AFL-4W group (30.9%) compared with the IVT-AFL-2W group (21.0%) (Table 3). However, the incidence of non-ocular TEAEs was similar between the IVT-AFL-2W and IVT-AFL-4W groups (52.4% and 56.1%, respectively). The most common ocular TEAEs in the IVT-AFL-2W and IVT-AFL-4W groups, respectively, were cataract (5.6% and 8.1%), conjunctival hemorrhage (3.2% and 6.5%), dry eye (2.4% and 4.9%), and retinal pigment epithelium tear (2.4% and 0%), whereas the most common non-ocular TEAEs were nasopharyngitis (21.0% and 16.3%) and constipation (3.2% and 5.7%). No cases of endophthalmitis were reported.
Table 3.
Safety overview at week 96 (SAS)
| Number of patients (%) | IVT-AFL-2W adjustment | IVT-AFL-4W adjustment | Randomization failurea |
|---|---|---|---|
| n = 124 | n = 123 | n = 7 | |
| Any TEAE | 85 (68.5) | 86 (69.9) | 5 (71.4) |
| Mild | 62 (50.0) | 55 (44.7) | 2 (28.6) |
| Moderate | 15 (12.1) | 22 (17.9) | 1 (14.3) |
| Severe | 8 (6.5) | 9 (7.3) | 2 (28.6) |
| Ocular TEAE (study eye) | |||
| Any ocular TEAE (study eye) ≥ 2%b | 26 (21.0) | 38 (30.9) | 0 |
| Cataract | 7 (5.6) | 10 (8.1) | 0 |
| Conjunctival hemorrhage | 4 (3.2) | 8 (6.5) | 0 |
| Dry eye | 3 (2.4) | 6 (4.9) | 0 |
| Retinal pigment epithelium tear | 3 (2.4) | 0 | 0 |
| Non-ocular TEAE | |||
| Any non-ocular TEAE ≥ 3%c | 65 (52.4) | 69 (56.1) | 5 (71.4) |
| Constipation | 4 (3.2) | 7 (5.7) | 0 |
| Large intestine polyp | 0 | 4 (3.3) | 0 |
| Nasopharyngitis | 26 (21.0) | 20 (16.3) | 0 |
| Influenza | 2 (1.6) | 4 (3.3) | 0 |
| Contusion | 1 (0.8) | 4 (3.3) | 0 |
| Hypertension | 1 (0.8) | 4 (3.3) | 1 (14.3) |
| Any serious TEAEs | 19 (15.3) | 20 (16.3) | 3 (42.9) |
| Ocular SAE in study eye | 3 (2.4) | 2 (1.6) | 0 |
| Non-ocular SAE | 16 (12.9) | 16 (13.0) | 3 (42.9) |
| Any TEAE leading to discontinuation of study drug | 1 (0.8) | 2 (1.6) | 0 |
| APTC arterial thromboembolic events | 1 (0.8) | 2d (1.6) | 0 |
| Non-fatal myocardial infarction | |||
| Acute myocardial infarction | 0 | 1 (0.8) | 0 |
| Myocardial infarction | 0 | 1 (0.8) | 0 |
| Non-fatal stroke | 0 | 1 (0.8) | 0 |
| Vascular death | 1 (0.8) | 0 | 0 |
| Any death | 2 (1.6) | 1 (0.8) | 0 |
2W/4W 2-/4-week adjustment, APTC Antiplatelet Trialists’ Collaboration, IVT-AFL intravitreal aflibercept, SAE serious adverse event, SAS safety analysis set, TEAE treatment-emergent adverse event
aRandomization failure was due to physician decision (n = 1), logistical difficulties (n = 1), protocol violation (n = 2), and withdrawal by patient (n = 3)
bOcular TEAEs ≥ 2% in either IVT-AFL treatment arm
cNon-ocular TEAEs ≥ 3% in either IVT-AFL treatment arm
dThree events were reported in two patients
The only serious ocular TEAE in the study eye was cataract, which was reported in 2.4% and 1.6% of patients in the IVT-AFL-2W and IVT-AFL-4W groups, respectively. Three patients (one patient in the IVT-AFL-2W group and two patients in the IVT-AFL-4W group) discontinued IVT-AFL because of a TEAE up to week 96. Two deaths (intracranial hemorrhage and completed suicide) were reported in the IVT-AFL-2W group, both of which were assessed as not related to IVT-AFL. One death (pneumonia) was reported in the IVT-AFL-4W group (not related to IVT-AFL). The proportion of APTC events was similar between the treatment arms (0.8% and 1.6%) and consistent with previous studies [12, 13].
Discussion
The ALTAIR study showed that IVT-AFL in a T&E regimen with 2-week or 4-week adjustments improved functional and anatomic outcomes in treatment-naïve patients with exudative AMD over 52 weeks, while reducing the treatment burden. The mean change in BCVA from baseline to week 52 was 9.0 (IVT-AFL-2W) and 8.4 (IVT-AFL-4W) letters and mean change in CRT from baseline to week 52 was − 134.4 µm (IVT-AFL-2W) and − 126.1 µm (IVT-AFL-4W). Functional and anatomic outcomes were maintained to week 96; the mean change in BCVA from baseline to week 96 was 7.6 (IVT-AFL-2W) and 6.1 (IVT-AFL-4W) letters and mean change in CRT from baseline to week 96 was − 130.5 µm (IVT-AFL-2W) and − 125.3 µm (IVT-AFL-4W). The mean number of IVT-AFL injections was the same in the two groups (10.4). More than half of patients (57% in the 2-week and 58% in the 4-week group) had an intended injection interval of at least 12 weeks up to week 52 and the majority of patients (57% [IVT-AFL-2W] and 60% [IVT-AFL-4W]) had a last injection interval of at least 12 weeks at week 96. More than 40% of patients (42% [IVT-AFL-2W] and 46% [IVT-AFL-4W] had a last injection interval of 16 weeks up to week 96. There were no new safety events with the IVT-AFL T&E regimens compared with previous studies [12, 13].
Although good outcomes have been achieved in clinical trials, numerous studies have shown the challenges of bringing this efficacy into the real world [14–17]. In clinical practice, fixed dosing is associated with burdens for both the patient and the clinic; therefore, in real-world practice, regimens such as T&E (proactive) and PRN (reactive) are often adopted to reduce treatment burden while maintaining functional outcomes [7]. Utilization of proactive IVT-AFL T&E regimens, further substantiated by the results of ALTAIR, allows for a pragmatic approach to treatment of exudative AMD and offers benefits to both physicians and patients [7].
The duration of VEGF-A suppression differs between patients [18, 19]; therefore, by titrating the injection interval on the basis of the individual patient’s functional and anatomic outcomes, and adjusting the treatment if necessary, physicians can achieve optimal functional outcomes for each patient while reducing the frequency of clinic visits. With proactive, individualized T&E dosing regimens, the need for interim monitoring is minimized. Reducing the number of appointments per patient and minimizing the need for monitoring visits could help to ease clinic flow and patient burden while maintaining functional outcomes. Furthermore, planning the next injection helps to minimize the possibility of treatment delays and facilitates clinic management [7]. The molecular attributes of aflibercept allow for extended injection intervals. Previous studies of IVT-AFL in patients with exudative AMD have reported a median aqueous half-life of at least 9 days [20] and a range of intraocular VEGF suppression times of up to 16 weeks [18, 19]. The results from ALTAIR indicate that, with IVT-AFL T&E, the injection interval can be extended to 12 weeks and beyond in approximately 57–60% of patients.
ALTAIR explored interval adjustments and outcomes using an IVT-AFL T&E regimen in patients with exudative AMD. Our study demonstrated that an IVT-AFL T&E regimen, following initial monthly dosing, can be effective in the first year of treatment and continuously efficacious in the second year using either a 2- or 4-week adjustment based on defined criteria for interval extension, maintenance, or shortening. In other studies, injection intervals were adjusted by 2-week increments with a set minimum injection interval of 4 weeks and a maximum of 12 weeks [21–23]. In ALTAIR, 57–60% of patients achieved a last injection interval of at least 12 weeks up to week 96, compared with 17–37% of patients in other prospective studies of ranibizumab and IVT-AFL T&E regimens [21, 24–26]. Up to week 96, 41–46% of patients in ALTAIR reached the maximum last injection interval of 16 weeks either with 2- or 4-week adjustments. Moreover, the results of the ALTAIR study suggest that IVT-AFL T&E regimens with a minimum injection interval of 8 weeks may provide patients with good functional outcomes. The good functional outcomes observed in the VIEW studies, in which IVT-AFL was given every 8 weeks, further supports this [12, 13]. The functional and anatomic outcomes in ALTAIR, using a T&E regimen, are comparable with those observed for the IVT-AFL every 4 or 8 weeks (q4 and q8; fixed dosing) arm in the VIEW study [12, 13]. The mean change in BCVA from baseline to week 52 was 8.7, 9.3, and 8.4 letters and from baseline to week 96 was 6.9, 7.6, and 7.6 letters in ALTAIR and VIEW q4 and q8 groups, respectively. The mean change in CRT from baseline to week 52 was − 130 µm, − 138 µm, and − 139 µm and from baseline to week 96 was − 128 µm, − 128 µm, and − 133 µm in ALTAIR and VIEW q4 and q8 groups, respectively. In ALTAIR, patients received fewer injections than in VIEW (10.4, 16.0 [q4], and 11.2 [q8], respectively). Extending the interval by 4 weeks, to beyond 12 weeks and up to a 16-week interval, offers potential advantages for both patients (reduced treatment burden) and healthcare providers (scheduling visits) compared with the alternative 2-week increments [7, 11].
While there was no reading center involved in the ALTAIR study, the investigators conducted ophthalmic examinations at the screening visit and at every treatment and evaluation, and fundus photography, FA, and indocyanine green angiography were conducted at the screening visit and at weeks 52 and 96. Patients in the 4-week group who had their injection interval shortened by 4 weeks and were then extended by 2-week increments remained in the 4-week group for analysis. All statistical analyses were exploratory, and no confirmatory statistical analysis was intended. Although the objective of this study was to describe the outcomes achieved with both treatment regimens, the sample size allowed for descriptive statistical comparison. There was a greater proportion of male patients than female patients in the ALTAIR study, which is consistent with the Japanese population of the VIEW 2 study [27]. Further analyses will evaluate the efficacy of the two different T&E regimens in subgroups of interest, such as those with PCV.
Conclusion
IVT-AFL administered to treatment-naïve patients with exudative AMD using two different T&E regimens, with a minimum injection interval of 8 weeks and a maximum interval of 16 weeks, improved and maintained functional and anatomic outcomes over 96 weeks, while minimizing the treatment burden on patients. To provide further practical and personalized IVT-AFL T&E treatment regimens for patients with exudative AMD, future investigations should address predictive factors (such as subtypes of AMD lesions and fluid status) of functional/anatomic outcomes and injection intervals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the patients and investigators who contributed to this study.
Funding
Funding for the study, medical writing and editorial assistance for this manuscript, and funding for the Rapid Service Fee and Open Access Fee was provided by Bayer. In conjunction with the ALTAIR steering committee, Bayer participated in the design of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Additionally, Bayer was responsible for the conduct of the study and oversight of the collection and management of data.
Medical Writing and Editorial Support
Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) was provided by Mia Cahill (ApotheCom, UK) and was funded by Bayer Consumer Care AG, Switzerland. Mia Cahill has no conflicts of interest to declare. The authors thank Daniel Janer, MD who provided input and expert medical guidance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Masahito Ohji has received grants, consultancy fees, honorarium, travel support and speaker fees from AbbVie Japan, Inc., Alcon Pharma K.K. (Japan), Allergan, B.L.J Ltd., Bayer Yakuhin, Ltd. (Japan), Chugai, HOYA, Kowa, Novartis Pharma K.K., Otsuka Pharmaceuticals, Pfizer Pharmaceuticals K.K, Santen Pharmaceuticals Co., Ltd., Senju Pharmaceutical Co., Ltd., and Topcon.
Kanji Takahashi has received consultancy fees, honoraria, travel support and speaker fees from Alcon Pharma K.K. (Japan), Bayer Yakuhin, Ltd. (Japan), B.L.J Ltd., Carl Zeiss Co., Ltd., Kowa, Kyowa Kirin Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceuticals, Pfizer Pharmaceuticals K.K., Santen Pharmaceuticals Co., Ltd., and Senju Pharmaceutical Co., Ltd.
Annabelle A. Okada has received personal fees from AbbVie Japan, Inc., Astellas Japan, Bayer Healthcare AG, Daiichi-Sankyo, and Senju Pharmaceutical Co., Ltd.; and grants and personal fees from Alcon Pharma K.K. (Japan), Bayer Yakuhin Ltd. (Japan), Mitsubishi Tanabe Pharma Corporation, and Santen Pharmaceuticals Co., Ltd. (Japan).
Masato Kobayashi, Yoshimi Matsuda and Yasuhiro Terano are employees of Bayer Yakuhin Ltd. (Japan).
Compliance with Ethics Guidelines
The study was conducted at 41 centers across Japan between December 2014 and November 2017, in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The protocol and any amendments were approved by the independent ethics committee or institutional review board (IRB) at each study site (see supplementary material). There was no central IRB involved in the study and the protocol was reviewed and approved by the IRB at each participating center. All patients provided written informed consent. A list of investigators who participated in the study is provided in the supplementary material.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Masahito Ohji, Email: eye.ohji@gmail.com.
for the ALTAIR Investigators:
Masahito Ohji, Tsukasa Hanemoto, Tatsushi Kaga, Takeya Kouno, Hirokuni Kitamei, Shinpei Sato, Kanji Takahashi, Ryoji Yanai, Eiichi Uchio, Kazunori Miyata, Yoshihiro Wakabayashi, Takatoshi Maeno, Tsutomu Yasukawa, Masayuki Horiguchi, Tetsuya Nishimura, Akiteru Kawahara, Yasuo Kurimoto, Kenichi Murai, Namie Kobayashi, Wataru Kimura, Eriko Matsushita, Tomohiro Iida, Kanako Yasuda, Yuji Kato, Masahiro Miura, Annabelle Ayame Okada, Ryusaburo Mori, Atsushi Sugiyama, Yasuo Ito, Daisaku Kimura, Kei Nakai, Chota Matsumoto, Shinobu Takeuchi, Kishiko Okoshi, Yoshihisa Nuno, Yohei Nomoto, Toshio Mori, Muneyasu Takeda, Noriko Yoshida, Mio Hosokawa, and Kohei Sonoda
References
- 1.World Health Organization. Prevention of blindness and visual impairment. WHO. 2019. http://www.who.int/blindness/causes/priority/en/. Accessed Dec 2019.
- 2.Yamada M, Hiratsuka Y, Roberts CB, et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol. 2010;17:50–57. doi: 10.3109/09286580903450346. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 6.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 7.Lanzetta P, Loewenstein A, Vision Academy Steering Committee Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–1273. doi: 10.1007/s00417-017-3647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maguire MG, Martin DF, Ying GS, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel PJ, Devonport H, Sivaprasad S, et al. Aflibercept treatment for neovascular AMD beyond the first year: consensus recommendations by a UK expert roundtable panel, 2017 update. Clin Ophthalmol. 2017;11:1957–1966. doi: 10.2147/OPTH.S145732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezaei KA. Global trends in retina. Chicago: American Society of Retina Specialists; 2016. [Google Scholar]
- 11.Koh A, Lanzetta P, Lee WK, et al. Recommended guidelines for use of intravitreal aflibercept with a treat-and-extend regimen for the management of neovascular age-related macular degeneration in the Asia–Pacific region: report from a consensus panel. Asia–Pacific J Ophthalmol. 2017;6:296–302. doi: 10.22608/APO.2016125. [DOI] [PubMed] [Google Scholar]
- 12.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SY, Mimoun G, Oubraham H, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33:474–481. doi: 10.1097/IAE.0b013e31827b6324. [DOI] [PubMed] [Google Scholar]
- 15.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group The neovascular age-related macular degeneration database: multicenter study of 92,976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Chew JK, Broadhead GK, et al. Intravitreal ranibizumab for neovascular age-related macular degeneration in clinical practice: five-year treatment outcomes. Graefes Arch Clin Exp Ophthalmol. 2015;253:1217–1225. doi: 10.1007/s00417-014-2799-8. [DOI] [PubMed] [Google Scholar]
- 18.Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol. 2016;100:1494–1498. doi: 10.1136/bjophthalmol-2015-308264. [DOI] [PubMed] [Google Scholar]
- 19.Fauser S, Schwabecker V, Muether PS. Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am J Ophthalmol. 2014;158:532–536. doi: 10.1016/j.ajo.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Do DV, Nguyen QD. Pharmacokinetics of free aflibercept in patients with neovascular age related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:406. [Google Scholar]
- 21.Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the Lucentis compared to Avastin study treat-and-extend protocol: two-year results. Ophthalmology. 2016;123:51–59. doi: 10.1016/j.ophtha.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125:57–65. doi: 10.1016/j.ophtha.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto A, Okada AA, Nakayama M, Yoshida Y, Kobayashi H. One-year outcomes of a treat-and-extend regimen of aflibercept for exudative age-related macular degeneration. Ophthalmologica. 2017;237:139–144. doi: 10.1159/000458538. [DOI] [PubMed] [Google Scholar]
- 24.Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137:372–379. doi: 10.1001/jamaophthalmol.2018.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126:723–734. doi: 10.1016/j.ophtha.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1:314–321. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Ogura Y, Terasaki H, Gomi F, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. Br J Ophthalmol. 2015;99:92–97. doi: 10.1136/bjophthalmol-2014-305076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.