Abstract
Introduction
Chronic venous disease (CVD) is a common condition associated with valvular dysfunction, venous hypertension and endothelial inflammation. Sulodexide facilitates the healing of venous ulcers and is frequently used in patients with CVD without ulcer. This review assessed the efficacy and safety of sulodexide for treatment of signs and symptoms of lower extremity CVD.
Methods
We searched MEDLINE, EMBASE, CINAHL and AMED as well as the Cochrane Central Register of Controlled Trials and the World Health Organisation (WHO) International Clinical Trials Registry Platform Search Portal. We also manually searched potentially relevant journals, conference proceedings and journal supplements. Any study monitoring any effect of sulodexide in patients with CVD at any stage of the disease, classified or non-classified, was considered. Treatment effects were estimated using standardised mean differences (SMDs), mean differences (MDs) and risk ratios (RRs), as appropriate. We calculated 95% confidence intervals (CIs) and heterogeneity (Q, tau and I2).
Results
The search found 64 studies, but only 23 provided data on 7153 participants (mean age 55 years; 68% female). The 13 studies providing extractable quantitative information included 1901 participants (mean age 55.2 years; 65% female). Sulodexide decreased the intensity of pain, cramps, heaviness, oedema and total symptom score and reduced inflammatory mediators in patients with CVD. The risk of adverse events (AEs) was not different between sulodexide and placebo or heparan sulphate (RR 1.31, 95% CI 0.74–2.32; I2 = 0%; 270 participants). The overall risk of AEs with sulodexide was low: 3% (95% CI 1–4%) estimated from 3656 participants.
Conclusion
Sulodexide was found to have a beneficial venoactive effect on the major signs and symptoms of CVD such as pain, cramps, heaviness and oedema without increasing the risk of AEs. It is also likely to exert a systemic effect on the course of CVD by interfering with inflammatory chemokines.
Keywords: Chronic venous disease, Efficacy, Meta-analysis, Safety, Sulodexide
Key Summary Points
| The majority of validated approaches for the management of chronic venous disease (CVD) are relatively invasive and may be limited by patient acceptance; compression is instead usually associated with poor compliance. |
| This systematic review assessed the efficacy and safety of sulodexide for treatment of signs and symptoms of lower extremity CVD by reviewing the literature and conducting a meta-analysis. |
| Sulodexide had a beneficial venoactive effect on the major signs and symptoms of CVD, such as pain, cramps, heaviness and oedema, without increasing the risk of adverse events. It is also likely to exert a systemic effect on the course of CVD by interfering with inflammatory chemokines. |
Introduction
Chronic venous disease (CVD)—in the more advanced stages also referred to as chronic venous insufficiency or CVI—is a multifactorial condition of the lower limbs related to endothelial dysfunction, inflammation, vein wall remodelling, incompetent valves, venous hypertension and reflux [1]. Other causes include venous outflow obstruction and failure of the calf muscle pump owing to obesity or leg immobility [2]. CVD is reported to affect from 30 to 50% of women and 10–30% of men in the Caucasian population [3], with slightly lower rates in Hispanic, African American and Asian individuals [4].
According to the causes, CVD can be defined as congenital, primary (not associated with an identifiable mechanism of venous dysfunction) [5] or secondary [6] (resulting from an antecedent event, usually an episode of acute deep venous thrombosis—DVT—or trauma or other) [2, 7].
Primary CVD is more common than secondary, with the latter being responsible for about 18–28% of limbs with CVD [8]; rarely, congenital malformations can cause CVD [9].
The pathogenesis of primary CVD remains unclear. The primary abnormality appears to be genetically determined alterations of constituents of the extracellular matrix, including collagen, elastin and especially proteoglycans (both at the valvular and vein wall level). Symptoms and/or signs may appear in the presence of stable or transient risk factors causing venous hypertension with distension of veins or distortion of venous valves, such as prolonged standing, obesity, pregnancy, sedentary habits and age-related weakening of calf muscles [10, 11].
Recent studies showed that venous hypertension and microcirculatory stasis with the attending change of shear stress damage the glycocalyx and elicit a pro-inflammatory response. This results in recruitment of leukocytes by increased expression of—among others—intercellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1) as well as release of pro-inflammatory agents such as interleukin-6 (IL-6), reactive oxygen species (ROS) and matrix metalloproteinases (MMP-2, MMP-9). These, in turn, contribute to tissue remodelling by promoting the degradation of extracellular matrix proteins, resulting in altered structural integrity and function of the venous wall and valves [1, 12, 13].
The main abnormality causing secondary CVD is the event leading to venous hypertension, in most cases DVT, causing blood flow obstruction in the location of acute thrombus and dilatation of the veins with slowing of the blood flow distal to the thrombus. The complex process of acute thrombus organisation and transformation with consecutive recanalisation also leads to increased inflammatory processes. This results in progressive vein wall damage and valvular incompetence [14].
The current (CEAP) classification for chronic venous disorders combines clinical signs (C), aetiology (E), anatomical distribution (A) and physiological conditions (P) for descriptive classification of CVD [15]. Clinical severity scoring and quality of life scores are instruments for longitudinal research to assess outcomes. The Revised Venous Clinical Severity Score (rVCCS) is the most advanced and combines symptoms (pain), signs (varicose veins, venous oedema, skin pigmentation, inflammation, induration, active ulcer number, duration and size) and use of compression therapy [16, 17].
CVD is associated with a reduced quality of life, particularly in relation to pain, physical function and mobility [2]. Advanced stages of chronic venous disease (CEAP classes 4–6) are associated with chronic disability and high healthcare costs [18].
The symptoms of CVD recently received specific attention and became the object of a consensus, which listed, among the more or less common symptoms, pain or aching, throbbing, tightness, heaviness, fatigue, feeling of swelling, cramps, itching, restless legs, tingling, and heat or burning sensation [19]. The effect of phlebotonics on symptoms was examined in a Cochrane review [20]. However, other treatments were also reported to have favourable effects on CVD symptoms, including sulodexide, for which a review has not yet been published.
Description of the Intervention
Guidelines indicate surgery, endovenous thermal ablation [21], cyanoacrylate adhesive closure [22] or sclerotherapy and mechanical compression for the treatment of CVD. However, these interventions are inconvenient for patients and the resulting effects are highly operator-dependent and could negatively affect the patients’ quality of life. The level of reimbursement by the different healthcare systems is limited, if there is any. Long-term compression is associated with very limited compliance [23–27]. Pharmacological treatments, on the contrary, are well accepted by the patients, easy to administer and the patients’ compliance is often good [28]. Among the pharmacological treatments, sulodexide deserves special attention because of its specific characteristics.
Sulodexide comprises two fractions: a fast-moving heparin fraction (80%), which has affinity for antithrombin III, and a dermatan sulphate fraction (20%) that has affinity for heparin cofactor II [29]. In clinical trials it has demonstrated efficacy in the treatment/prevention of vascular diseases associated with increased thrombotic risk, including peripheral arterial occlusive diseases [30, 31], post-myocardial infarction [32], recurrent deep vein thrombosis [33–35] and post-thrombotic syndrome [36, 37].
On the basis of sulodexide’s antiplatelet [38], anti-inflammatory [11, 39, 40] and anti-proteolytic [41] effects, as well as the protective effect on the glycocalyx layer [42], it may interfere with the pathogenesis of both primary and secondary disease in patients with CVD [43, 44]. In fact, sulodexide has been shown to have favourable effects on the most distressing clinical sign of CVD, venous ulcers, of different origins [45, 46].
Objectives
This systematic review was undertaken to examine the available information on the effect of sulodexide for the treatment of symptoms of lower extremity CVD. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Methods
Criteria for Considering Studies for this Review
We included all comparative and non-comparative trials assessing the efficacy of sulodexide in patients with CVD at any disease stage. We excluded studies that did not meet the above criteria as well as studies that exclusively monitored the size and/or frequency of venous ulcer healing, already examined in other meta-analyses [46, 47]. We imposed no limitation on language, journal, diagnostic criterion or CEAP class (for the studies carried out after the relevant consensus) or use of compression measures. No limitation was imposed on the use of concomitant medication either, since all studies contributing to the quali-quantitative assessment excluded the concomitant treatment with other venoactive medications/phlebotonics. The only exception was one study that used sulodexide in an open, non-parallel, add-on design with diosmin-hesperidin in the treatment of venous ulcers without reporting data relevant to this review [48].
Types of Participants
Participants included consenting males and females > 18 years of age suffering from any type of CVD, regardless of the method of diagnosis.
Types of interventions
We considered all studies in which at least one arm had been treated with oral sulodexide, alone or after a run-in period with intramuscular injections, at any dosage and for any duration of treatment.
Types of Outcome Measures
We included studies that assessed at least one of the following outcome measures:
Symptoms: pain [scored or reported as visual analogue scale (VAS)], cramps, paraesthesias, heaviness and total symptom score;
Signs: oedema (scored or reported as volume), discoloration and skin temperature (scored);
Biochemical markers (including matrix metalloproteinases and interleukins);
Adverse events: number of subjects who reported adverse events, regardless of whether indicated as potentially correlated with the treatment.
Search Methods
In the first instance we searched the electronic databases MEDLINE, EMBASE, CINAHL and AMED as well as the Cochrane Central Register of Controlled Trials and the World Health Organisation (WHO) International Clinical Trials Registry Platform Search Portal. To favour sensitivity, the search terms were “sulodexide”, “glycosaminoglycan”, “chronic venous disease”, “chronic venous insufficiency” and “vascular disease”. Additionally, we searched the reference lists of articles retrieved by electronic searches for additional citations and hand-searched potentially relevant journal, conference proceedings and journal supplements. The electronic search included papers published up to 30 April 2019; the complete search was concluded on 30 June 2019.
Data Collection and Analysis
The authors independently assessed the eligibility of studies identified by the searches; disagreements were resolved by consensus.
Data Extraction and Management
One author (AAB) extracted data from the studies; the other author (JM) checked the extracted data. Collected information includes characteristics of study participants, characteristics of intervention and control groups (where applicable) and outcome characteristics of every group of participants.
Measures of Treatment Effect
We estimated the effects of treatment using the package meta [49] in R [50]. Dichotomous variables were examined by meta-analysis of single proportions (function metaprop) for pooling non-comparative studies and meta-analysis of binary outcome data (metabin) for pooling RCTs. Similarly, continuous variables were examined by meta-analysis of single means (metamean) or meta-analysis of continuous outcome data (metacont), respectively. Results were reported with the relevant 95% confidence intervals. When the change of continuous variables at study end was not reported, it was estimated from the initial and final values, assuming a correlation of 0.750 [51]. Where needed, instead of the raw data we used the standardised mean difference [52] using the method of Algina and Keselman to estimate the variance [53]. All analyses were performed using the random effects model unless otherwise specified. Heterogeneity was estimated in each analysis since high heterogeneity suggests considering the results with care.
Results
Results of the Search
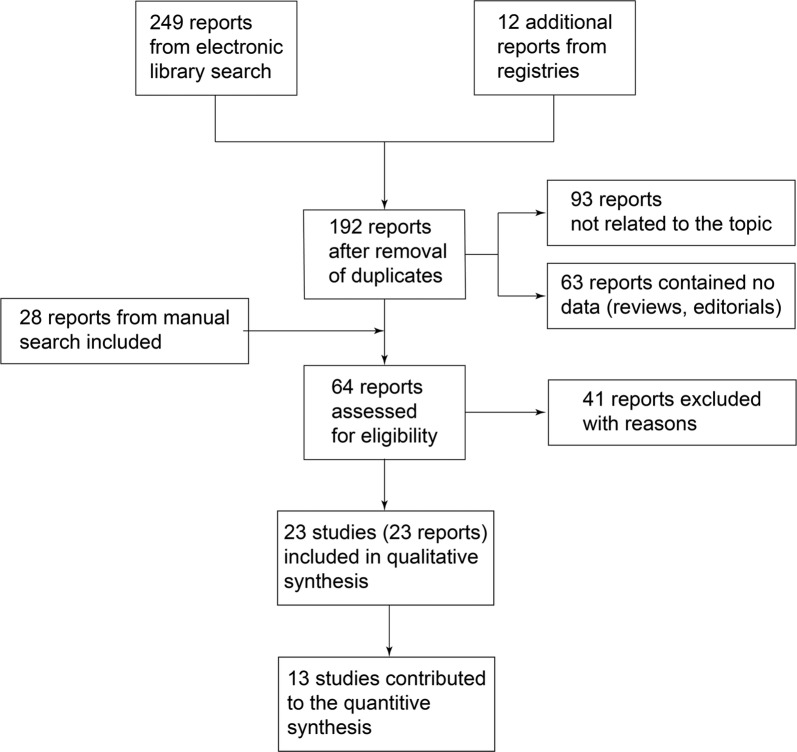
Figure 1 provides a summary of the literature search results and screening process.
Fig. 1.
Summary of the literature search and screening process
Included Studies
We identified 23 studies that could provide information on the effect of sulodexide in the management of CVD [42, 44, 45, 48, 54–72], with exclusion of the studies exclusively reporting the course of venous ulcers. Whenever possible, data were retrieved from the original tables; where data were reported as graphs only, numeric data were reconstructed by analysing the graphs at high magnification with a high definition software and applying standard algorithms in R. Studies were published in English, Italian, Spanish, Polish, Russian and Chinese but no limitation was imposed on the language. We instead excluded studies from the quantitative synthesis when no numeric data or graphs were presented in relation to the considered outcomes [48, 66, 72], when only mean data without an interpretable form of variance were reported [54, 55, 57, 58, 62, 69] and when data were reported but considered in one study only.
Overall, the studies considered in this review included 7153 participants (mean age 55 years; 68% female). The mean number of participants included per clinical trial was 300 (range 8–2263). Not all studies yielded data suitable for a quantitative synthesis; the studies providing quantitative information included 1901 participants (mean age 55.2 years; 65% female) with an average size of 140 patients per study (range 8–476).
All participants were reported to meet the respective CVD criteria of every study. Formal classification of CVD stage was reported only in seven of the most recent studies [43, 44, 66, 68–70, 72], according to the CEAP consensus [15]. The C3 and C4 classes accounted for almost 70% of classified cases: C0: 0.3%; C1: 3.0%; C2: 8.4%; C3: 31.8%; C4: 36.9% (with a prevalence of C4a where the stage was split); C5: 8.7%; C6: 10.9%.
Four studies specified that investigators recommended/prescribed compression treatment [43–45, 69], although one author specified that the compliance with compression was limited [69].
Symptoms and signs were almost exclusively monitored by means of four- or six-level scores. Only one study measured pain with a VAS [68]; the same study measured the distal oedema in cm3. Specialised techniques were used to measure the markers of inflammation in serum or tissue [43, 44, 68, 71].
Excluded Studies
We excluded 41 studies that were originally considered as potentially eligible. The reasons for exclusion were: no clinical data reported (n = 13) [12, 13, 29, 39–41, 73–79], indication other than CVD with data on CVD symptoms not available/not extractable (n = 13) [80–92], no extractable data on symptoms (n = 9) [36, 93–101], preliminary partial publication (n = 3) [102–104], data reported but not stratified by treatment (n = 1) [105], review (n = 1) [47] or duplicate (n = 1) [98].
Effects of Interventions
The effects of interventions were divided into three major groups: symptoms and signs of CVD, modifications of inflammatory markers and number of patients with adverse events. Except scoring of heaviness, biochemical markers and number of patients with adverse events, none of these variables were reported by at least two controlled studies. Consequently, the methods for combining single-arm data have been used for all variables.
Symptoms of CVD
Symptoms of CVD for which at least two studies provided interpretable data were: total symptoms score [63, 68], pain [56, 59, 61, 64, 65, 67, 68, 70], cramps [45, 56, 59, 63, 64, 67, 70], paraesthesia [45, 56, 59, 60, 64, 67] and heaviness [45, 60, 61, 67, 70]. All these variables were monitored by means of scales rating the intensity 0–3 except for one study where pain was measured with the VAS [68]. The total symptom score was computed by summing the individual score over a different number of symptoms in the two cases: investigator-defined number of symptoms [63] or VCSS symptoms and signs [68].
Total Symptom Score
The total symptom score was reported in two studies on a total 483 patients. One study used the sum of scores assigned to each of: heaviness; paraesthesias; cramps; pain that was orthostatic, clinostatic and on effort; oedema of the foot, leg and general; skin pigmentation; desquamation; eczema; alteration of annexes; hypodermitis; stasis ulcer; lymphangitis; cyanosis; venous ectasia [63]. The scale ranged from 0–3, so that the total score could range from 0–51. The other study instead used the VCSS scoring system, which could range from 0 to 21 (since patients with ulcers were excluded) [68]. We therefore used the standardised mean difference between baseline and month 2 [63] or month 3 [68] data and pooled the studies using the log-transformed means.
The result (Fig. 2) showed a definite effect associated with sulodexide, regardless of the measurement method, dosage and formulation used. The overall effect in terms of standardised mean difference was 1.63 SD [95% confidence interval (CI): 1.18; 2.25 SD] above zero. In absolute terms (raw change of total score), the effect can be indicated as a decrease of 7.57 (95% CI 5.92; 9.69) score points with significant heterogeneity (I2: 95.9%; P < 0.0001).
Fig. 2.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.1: mean decrease of total symptom score expressed as standardised mean difference using the inverse variance method
Pain
Pain was reported in 8 studies on a total of 927 patients [56, 59, 61, 64, 65, 67, 68, 70] monitored for 2 (N = 46) [56, 61], 3 (N = 866) [59, 64, 67, 68, 70] or 6 (N = 15) months [65]. Six studies used the conventional 0–3 scoring system [56, 59, 61, 64, 65, 67], one used a 0–5 scale [70] and one used the visual analogue scale (VAS) [68]. We used the standardised mean difference to estimate the effect, with the random effects model (Fig. 3).
Fig. 3.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.2: mean decrease of pain expressed as standardised mean difference using the inverse variance method
The overall effect could be estimated as 2.51 SD (95% CI 1.20; 3.82 SD) with high heterogeneity (I2 100%; P < 0.0001). In absolute terms, the effect estimated after the exclusion of the trial using the VAS could be reported as a decrease of 1.74 (95% CI 1.27; 2.22) score points with high heterogeneity (I2 98.2%; P < 0.0001).
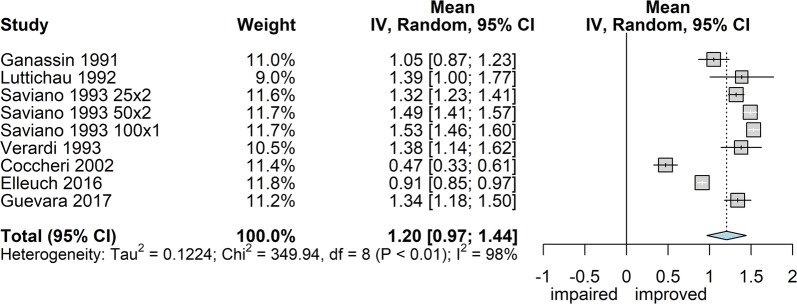
Cramps
Seven studies reported the intensity of cramps (frequently reported as “nocturnal” cramps) measured with the usual scale 0–3 [45, 56, 59, 63, 64, 67], except one that used the 0–5 scale [70]. Overall, 1427 patients were monitored, of whom 480 were treated for 2 months [56, 63] and the rest for 3 months. The overall effect estimate (Fig. 4) was 1.20 score points of improvement (95% CI 0.97; 1.44) with very high heterogeneity (I2 97.7%; P < 0.0001). In this case, however, large heterogeneity was expected since the dispersion measure within each study (homogeneous population and limited score span) was mostly smaller than the differences between populations across studies.
Fig. 4.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.3: mean decrease of cramps expressed as mean difference using the inverse variance method
Paraesthesia
Six studies reported the intensity of paraesthesia, measured with the usual 0–3 score scale [45, 56, 59, 60, 64, 67]. Overall, 747 patients were monitored, of whom 79 were treated for 2 months [56, 59] and the rest for 3 months. The overall effect estimate (Fig. 5) was 1.06 score points of improvement (95% CI 0.65; 1.48) with high heterogeneity (I2 98%; P < 0.0001).
Fig. 5.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.4: mean decrease of paraesthesia scores expressed as mean difference using the inverse variance method
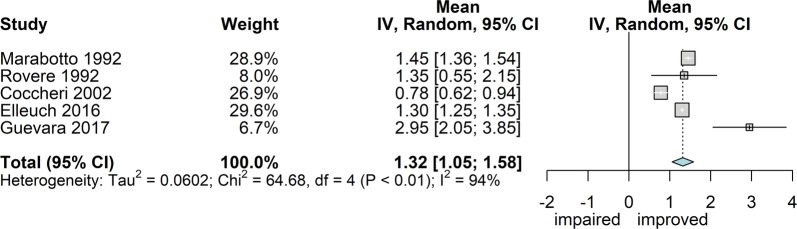
Heaviness
Five studies reported the intensity of heaviness, measured with the 0–3 score scale in four [45, 60, 61, 67] and 0–5 in the fifth [70]. Overall, 948 patients were monitored, treated for 2 (95 patients) [60, 61] or 3 months. The overall effect estimate (Fig. 6) was 1.32 score points of improvement (95% CI 1.05; 1.69) with high heterogeneity (I2 94%; P < 0.0001).
Fig. 6.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.5: mean decrease of heaviness scores expressed as mean difference using the inverse variance method
Two of the studies reporting this variable were comparative vs. calcium heparin [61] or placebo [45]. The result of the comparison showed a significantly greater effect with sulodexide (P = 0.01; Fig. 7), corresponding to a decrease of 0.28 score points (95% CI 0.07; 0.50).
Fig. 7.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.1: heaviness, mean change of intensity score expressed as mean difference using the inverse variance method
Signs of CVD
Signs of CVD for which at least two studies provided interpretable data were: oedema [45, 56, 61, 63–65, 68, 70], discoloration [56, 60, 64, 70] and skin temperature [45, 65, 67]. The variables were scored 0–3 except for one study using a 0–5 scale [70] and one in which oedema was measured as volume [68].
Oedema
Eight studies reported the intensity of oedema, measured with a 0–3 score scale in six [45, 56, 61, 63–65], with a 0–5 score scale in one [70] and as volume in one [68]. Overall, 1005 patients were monitored, of whom 522 were treated for 2 months [56, 61, 63, 65] and the rest for 3 months. The overall effect estimate used the standardised difference (Fig. 8) and yielded 1.54 SD of improvement (95% CI 0.97; 2.10 SDs) with high heterogeneity (I2 99%; P < 0.0001). The effect size in terms of score points could be estimated after exclusion of the study using actual volume and resulted in an improvement of 1.15 (95% CI 0.78; 1.52) score points with high heterogeneity (I2 98%; P < 0.0001).
Fig. 8.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.6: mean decrease of oedema measure expressed as mean standardised difference using the inverse variance method
Discoloration
Four studies reported data on skin discoloration over a total of 418 patients, of whom 87 were treated for 2 months [56, 60] and the rest for 3 months [64, 70]. The overall effect estimate yielded an improvement of 1.06 score points (95% CI 0.16; 1.96; Fig. 9); however, the heterogeneity was high (I2 97.6%; P < 0.0001).
Fig. 9.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.7: mean improvement of discoloration expressed as mean difference using the inverse variance method
Skin Temperature
Three studies reported data on skin temperature over a total of 571 patients treated for 2 (N = 15) [65] or 3 months [45, 67]. The overall effect estimate yielded an improvement of 1.11 score points (95% CI 0.48; 1.74; Fig. 10) with high heterogeneity (I2 98.5%; P < 0.0001).
Fig. 10.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.8: mean improvement of skin temperature expressed as mean difference using the inverse variance method
Modifications of Inflammatory Markers
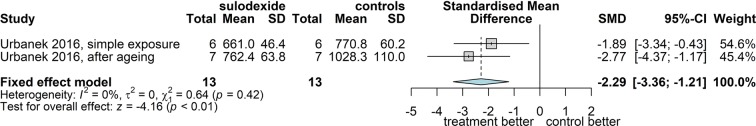
The only markers examined under the same conditions in at least two trials were the serum level of MMP-2 [68, 71] and MMP-9 [43, 68, 71]. The effect of exposure to sulodexide on IL-6, MCP-1, s-ICAM-1 and the generation of free radicals were tested under different conditions in 3–4 different experiments in two trials [43, 44].
Matrix Metalloproteinase-2
Two studies examined the level of MMP-2 in the serum of 50 patients treated for 3 (22 cases) [68] or 6 months (28 cases) [71]. Since both measuring methods used are known to be remarkably precise, the pooled analysis is better suited to the fixed effect model and yielded an estimate indicating a significant decrease of the MMP-2 of 30.5 ng/ml (95% CI 20.7; 40.3) (Fig. 11).
Fig. 11.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.9: mean decrease of matrix metalloproteinase 2 in serum of patients, expressed as mean difference using the inverse variance method and the fixed effect model
Matrix Metalloproteinase-9
Three studies examined the level of matrix metalloproteinase-9 in serum of 61 patients treated for 2 (N = 11) [43], 3 (N = 22) [68], or 6 (N = 28) months [71]. In view of the different levels of precision of the techniques used, we analysed the standardised mean difference. The combined outcome indicated a mean decrease of 2.43 (95% CI 1.33; 3.52) SDs (Fig. 12) or, in original units, 1.14 ng/ml (95% CI 0.95; 1.32) using the fixed effect model.
Fig. 12.
Forest plot of overall mean from studies reporting a single mean. 1. Sulodexide in CVD; outcome 1.10: mean decrease of matrix metalloproteinase 9 in serum of patients, expressed as standardised mean difference using the inverse variance method
Interleukin-6
The release of IL-6 was examined in three different experiments in the same study [44]. The three tests can be considered as three independent assays of the same phenomenon and can therefore be combined in a meta-analysis. The distribution of data suggested using the fixed effect model on the standardised mean difference to avoid heterogeneity. The outcome indicated a significant (P < 0.0001) decrease of IL-6 release in the cells exposed to serum from treated patients compared with the cells exposed to the serum from the same patients before treatment (controls with CVD; Fig. 13). In absolute terms, the treatment reduced the release of IL-6 by 611 (95% CI 517; 705) pg/105 cells.
Fig. 13.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.2: release of IL-6 in the medium, expressed as standardised mean difference using the inverse variance method and the fixed effect model
Monocyte Chemoattractant Protein-1
The release of MCP-1 was examined in two different experiments in the same study [44]. The outcome, based on the same approach as before (fixed effect model on the standardised mean difference, Fig. 14), indicated a significant (P < 0.0001) decrease of MCP-1 release in the cells exposed to serum from treated patients compared with the cells exposed to the serum from the same patients before treatment (controls with CVD). In absolute terms, the treatment reduced the release of MCP-1 by 156 (95% CI 105; 207) pg/105 cells.
Fig. 14.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.3: release of MCP-1 in the medium, expressed as standardised mean difference using the inverse variance method and the fixed effect model
Soluble Intercellular Adhesion Molecule-1
The release of s-ICAM-1 was examined in two different experiments in the same study [44]. Using the fixed effect model on the standardised mean difference, the outcome (Fig. 15) indicated a significant (P < 0.0001) decrease of s-ICAM-1 release in the cells exposed to serum from treated patients compared with the cells exposed to the serum from the same patients before treatment (controls with CVD). In absolute terms, the treatment reduced the release of s-ICAM-1 by 104 (95% CI 61; 148) pg/105 cells.
Fig. 15.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.4: release of s-ICAM-1 in the medium, expressed as standardised mean difference using the inverse variance method and the fixed effect model
Generation of Free Radicals
The release of free radicals into the medium was examined in two different experiments in the same study [44]. The outcome, according to the fixed effect model on the standardised mean difference (Fig. 16), indicated a significant decrease (P < 0.0001) of the generation of free radicals in the cell cultures exposed to serum from treated patients compared with the cells exposed to the serum from the same patients before treatment (controls with CVD). In absolute terms, the treatment reduced the generation of free radicals by 140 (95% CI 92; 186) events/105 cells.
Fig. 16.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.5: generation of free radicals from the culture, expressed as standardised mean difference using the inverse variance method and the fixed effect model
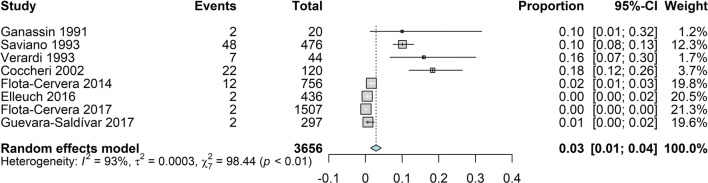
Number of Patients with Adverse Events
The number of patients reporting adverse events, regardless of their potential correlation with treatment, was reported in two comparative trials [45, 56] and in six non-comparative trials [63, 64, 66, 67, 69, 70]. We decided not to examine the data of routine haematology, clinical chemistry and vital signs, since in the few studies in which such data have been reported, only mean data have been published, whereas no mention was made of individual clinically relevant changes that could configure an adverse event.
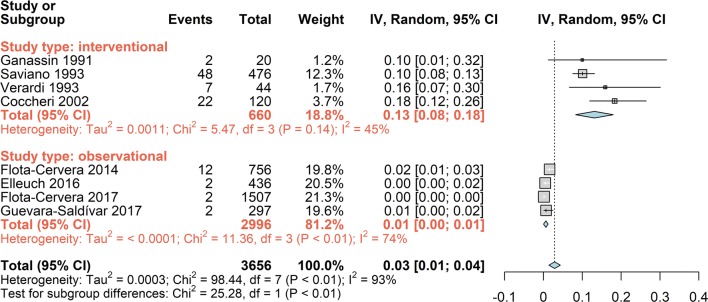
The analysis of all available data on the proportion of subjects reporting adverse events indicated a very low incidence: 3% (95% CI 1; 4; Fig. 17). However, the high heterogeneity (I2 93%) and the distribution of the events across studies suggested that the overall result was the composite of two different conditions. Stratifying the proportion of patients reporting adverse events by type of study (interventional or observational; Fig. 18) clearly showed two significantly different (P < 0.01) rates of reporting: 13% (95% CI 8; 18) in the interventional studies and 1% (95% CI 0; 1) in the observational studies.
Fig. 17.
Forest plot of overall proportion from studies reporting a single proportion. 1. Sulodexide in CVD; outcome 1.11: overall proportion of patients reporting adverse events, using the inverse variance method and the random effects model
Fig. 18.
Forest plot of overall proportion from studies reporting a single proportion. 1. Sulodexide in CVD; outcome 1.12: overall proportion of patients reporting adverse events stratified by type of study, using the inverse variance method and the random effects model
The significant difference between observational and interventional studies suggests that the observational studies can be useful to record rare adverse events but, unless well monitored, are likely to underestimate the incidence of the common adverse events.
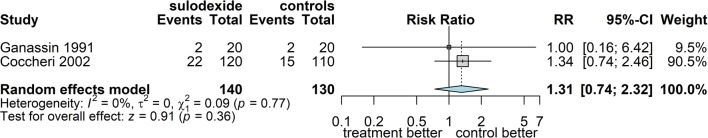
Among the considered studies, two were comparative vs. heparan sulphate and placebo, covering a total of 270 patients [45, 56]. The incidence of adverse events with sulodexide in these interventional trials, one of which was double-blind vs. placebo [45], did not differ from that among controls (Fig. 19): RR 1.31 (95% CI: 0.74; 2.32).
Fig. 19.
Forest plot of comparison: 2. Sulodexide vs. controls; outcome 2.6: proportion of patients with adverse events, expressed as risk ratio using the Mantel-Haenszel method and the random effects model
Discussion
Summary of Main Results
We examined and evaluated the existing evidence on the efficacy and safety of sulodexide on the symptoms and markers of chronic venous disease (CVD) with the exclusion of ulcers, which have already been reviewed elsewhere [46, 47]. This systematic review included 23 studies with 7153 participants; 13 studies, with 1901 participants, produced data suitable for the quantitative synthesis relevant to the efficacy evaluation; 8 studies with 3656 participants provided data for the quantitative synthesis relevant to the safety evaluation.
We could extract enough information to examine the overall effect of sulodexide on the variation of symptoms (pain, cramps, paraesthesias, heaviness and total symptom score) and of signs (oedema, discoloration and skin temperature) of CVD (Table 1). The interpretation of the results on symptoms and signs is that there is an effect of sulodexide in reducing the intensity of symptoms and signs of the size of approximately 1.5–2.5 standard deviations, with the exception of discoloration, which appeared unaffected by the treatment (the confidence interval included zero).
Table 1.
Effect of sulodexide on the symptoms and signs of CVD, expressed as standardised mean change from baseline and 95% confidence interval
| Symptom/sign | Cases | Standardised mean change from baseline | 95% CI | Notes |
|---|---|---|---|---|
| Total score | 483 | 1.63 | 1.18; 2.25 | Studies contributing to the measurement used different number of symptoms to compute the sum of scores |
| Pain | 910 | 2.51 | 1.20; 3.82 | |
| Cramps | 1427 | 1.59 | 1.16; 2.02 | |
| Paraesthesia | 747 | 1.13 | 0.76; 1.50 | |
| Heaviness | 948 | 1.95 | 1.23; 2.68 | |
| Oedema | 1005 | 1.54 | 0.97; 2.10 | One study used the measurement of volume instead of the score |
| Discoloration | 418 | 0.34 | 0.40; 1.08 | |
| Skin temperature | 571 | 1.62 | 0.82; 2.42 |
Consistent information could be gathered from in vitro studies examining the effect of pre-treatment with sulodexide on the release of inflammatory markers (Table 2), showing inhibition of the inflammatory markers associated with CVD.
Table 2.
Effect of sulodexide on the release of inflammatory markers in CVD, expressed as mean difference between CVD control serum and serum from sulodexide-treated CVD patients, and 95% confidence interval
| Marker | Cases | Mean difference | 95% CI | P (fixed effect model) |
|---|---|---|---|---|
| Interleukin-6 | 19 vs. 19 | − 611 pg/105 cells | − 705; − 517 | p < 0.0001 |
| Monocyte chemoattractant protein-1 | 13 vs. 13 | − 156 pg/105 cells | − 207; − 156 | P < 0.0001 |
| Soluble intercellular adhesion molecule-1 | 13 vs. 13 | − 104 pg/105 cells | − 147; − 61 | p < 0.0001 |
| Free radicals generation | 13 vs. 13 | − 140 events/105 cells | − 186; − 92 | p < 0.0001 |
Safety was examined via the number of patients reporting adverse events. The analysis (Table 3) showed a significant difference in proportion of patients reporting adverse events between interventional and observational studies. Although this may be expected, it also points to the limited reliability of observational studies, unless carefully monitored.
Table 3.
Incidence of patients with CVD reporting adverse events during treatment with sulodexide, expressed as proportion with 95% confidence interval
| Type of studies | Cases observed | Proportion reporting | 95% CI |
|---|---|---|---|
| Interventional | 660 | 0.13 | 0.08; 0.18 |
| Observational | 2996 | 0.01 | 0.00: 0.01 |
| Total | 3656 | 0.03 | 0.01; 0.04 |
Overall Completeness and Applicability of Evidence
Several limitations were identified in the included studies, some of which have already been mentioned. Only seven studies staged CVD according to the CEAP classification and, of these, two studies investigated inflammatory response markers [43, 44], while the other five were observational studies that failed to report data on symptoms stratified by CEAP class [66, 68–70, 72]. Therefore, homogeneity in diagnostic criteria is limited, and potential misclassification bias cannot be ruled out. Furthermore, we were unable to perform a subgroup analysis by CVD stage because data by class were not available.
Potential Biases in the Review Process
We did not perform sensitivity analyses because the available information could not change substantially. Two sensitivity analyses could have been appropriate, one to use different correlation factors in the calculation of the mean change from baseline and one to account for the effect of standard treatment provisions such as compression. However, using other (smaller) correlation factors could only have widened the confidence intervals but without affecting the point estimate. A sensitivity analysis for compression should account for three factors: in a study that recommended compression, not all patients actually received compression; all patients that used compression did not necessarily correctly follow the prescription; data were not stratified by use of compression. Consequently, we decided not to perform such analyses.
There was a high degree of heterogeneity in almost all analyses. Although there may be a clinical heterogeneity due to different diagnostic classification criteria, we believe that most of the heterogeneity is due to two major factors:
No standardisation was involved in measuring variables, given the different scales that have been used, and
Even when the same scale was used, the differences in perception across different countries and healthcare systems represented a source of heterogeneity across studies that were internally very consistent.
These considerations may explain why the accuracy of measurements within studies was generally very high, while the differences between different studies were often very large, thereby explaining the high heterogeneity seen in the analyses.
Agreements and Disagreements with Other Studies or Reviews
To date, there have been no reviews on sulodexide related to symptoms, only on ulcers [46, 47]. Reviews were published on phlebotonics [20, 106], but are not applicable to drugs with the profile of sulodexide.
Conclusions
Implications for Practice
Based on the currently available information, sulodexide is a useful venoactive treatment for the management of CVD because it is effective in ulcer healing, in reducing the major symptoms and signs of CVD (particularly total symptom intensity, pain, cramps, heaviness and oedema) and in decreasing the release of the inflammatory markers present in CVD. Furthermore, the use of sulodexide was safe. Additional randomised controlled trials should be performed to attain conclusive evidence.
Implications for Research
To date, the only validated approaches to CVD are invasive: surgery, sclerotherapy, endovascular thermal ablation, cyanoacrylate adhesive closure and compression treatment. They therefore may have limited patient acceptance. For compression, the rates of poor compliance are high. Pharmacological treatments that target the conditions altering the structural integrity of the venous wall and valves may be better tailored to the patients’ demands.
Further research should focus on high-quality and properly sized randomised controlled trials, using standard staging of patients and standardised assessment of symptom severity, reporting the results also stratified by stage. The trials should be designed with a clearly set primary endpoint from which the appropriate sample size can be calculated to reduce the risk of beta error, which has influenced many studies in the past. This programme should be applied to each medication or, at least, to each homogeneous class of medications to allow estimating the risk-to-benefit ratio. Finally, it would be of relevance to include properly designed studies of the cost-utility ratio, based on quality of life data.
Acknowledgements
Funding
The study was conducted with the support of Alfasigma, Italy, which did not affect the processing and the final results of this work. Alfasigma, Italy, also supported the journal’s Rapid Service and Open Access fee.
Medical Writing Assistance
The authors thank Matt Weitz of Springer Healthcare for reviewing the English and preparing the manuscript for submission. This assistance was funded by Alfasigma Italy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Angelo A. Bignamini received consultancy fees from Bayer Healthcare and from Alfasigma. Jiří Matuška has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data analysed in this article are present in the original papers mentioned and are shown in the figures as original or derived data.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11590920.
References
- 1.Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg. 2018;46:380–393. doi: 10.1016/j.avsg.2017.06.131. [DOI] [PubMed] [Google Scholar]
- 2.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 3.Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomized trial. Int Angiol. 2015;34:428–436. [PubMed] [Google Scholar]
- 4.Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158:448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meissner MH, Gloviczki P, Bergan J, et al. Primary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):54S–67S. doi: 10.1016/j.jvs.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):68S–83S. doi: 10.1016/j.jvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. Int Angiol. 1999;18:83–102. [PubMed] [Google Scholar]
- 8.Labropoulos N, Gasparis AP, Pefanis D, Leon LR, Jr, Tassiopoulos AK. Secondary chronic venous disease progresses faster than primary. J Vasc Surg. 2009;49:704–710. doi: 10.1016/j.jvs.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1–59. [PubMed] [Google Scholar]
- 10.Marinel.lo Roura J. [Etiology of CVI: Primary venous insufficiency. In Marinel.lo Roura, J ed. Ulcers of the Lower Limbs]. Barcelona: Editorial Glosa 2005:115–7. (Book in Spanish). https://editorialglosa.es
- 11.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96:1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 12.Mannello F, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide modulates inflammatory pathways in chronic venous disease. Int Angiol. 2014;33:236–242. [PubMed] [Google Scholar]
- 13.Andreozzi GM. Sulodexide in the treatment of chronic venous disease. Am J Cardiovasc Drugs. 2012;12:73–81. doi: 10.2165/11599360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SR, Mathes BM. Post-thrombotic (postphlebitic) syndrome. In: Collins KA (Ed.), UpToDate. Waltham, MA: UpToDate Inc. Available from from https://www.uptodate.com/contents/post-thrombotic-postphlebitic-syndrome Accessed 25 Apr 2019.
- 15.Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387–1396. doi: 10.1016/j.jvs.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 17.Marston WA, Vasquez MA, Lurie F, et al. Multicenter assessment of the repeatability and reproducibility of the revised Venous Clinical Severity Score (rVCSS) J Vasc Surg Venous Lymphat Disord. 2013;1:219–224. doi: 10.1016/j.jvsv.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 18.Mathes BM. Clinical manifestations of lower extremity chronic venous disease. In: Collins KA (Ed.), UpToDate. Waltham, MA: UpToDate Inc. 2019; Available from https://www.uptodate.com/contents/clinical-manifestations-of-lower-extremity-chronic-venous-disease Accesed 24 Apr 2019.
- 19.Perrin M, Eklof B, VANR A, et al. Venous symptoms: the SYM vein consensus statement developed under the auspices of the European Venous Forum. Int Angiol. 2016;35:374–398. [PubMed] [Google Scholar]
- 20.Martinez-Zapata MJ, Vernooij RW, Uriona Tuma SM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2016;4:CD003229. doi: 10.1002/14651858.CD003229.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2S–48S. doi: 10.1016/j.jvs.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 22.Myers K, Roberts S. ACP consensus on cyanoacrylate adhesive closure. Phlebolymphology. 2018;25:103. [Google Scholar]
- 23.Jull AB, Mitchell N, Arroll J, et al. Factors influencing concordance with compression stockings after venous leg ulcer healing. J Wound Care. 2004;13:90–92. doi: 10.12968/jowc.2004.13.3.26590. [DOI] [PubMed] [Google Scholar]
- 24.Raju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Ann Vasc Surg. 2007;21:790–795. doi: 10.1016/j.avsg.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Renner R, Gebhardt C, Simon JC. Compliance to compression therapy in patients with existing venous leg ulcers. Results of a cross-sectional study. Med Klin (Munich). 2010;105:1–6. doi: 10.1007/s00063-010-1001-2. [DOI] [PubMed] [Google Scholar]
- 26.Ayala A, Guerra JD, Ulloa JH, Kabnick L. Compliance with compression therapy in primary chronic venous disease: results from a tropical country. Phlebology. 2019;34:272–277. doi: 10.1177/0268355518798153. [DOI] [PubMed] [Google Scholar]
- 27.Kankam HKN, Lim CS, Fiorentino F, Davies AH, Gohel MS. A summation analysis of compliance and complications of compression hosiery for patients with chronic venous disease or post-thrombotic syndrome. Eur J Vasc Endovasc Surg. 2018;55:406–416. doi: 10.1016/j.ejvs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Branisteanu DE. Assessing compliance with nonoperative treatments of chronic venous disorders: the VEIN Act Program in Romania. Medicographia. 2016;38:175–180. [Google Scholar]
- 29.Coccheri S, Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Devel Ther. 2013;8:49–65. doi: 10.2147/DDDT.S6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaddi A, Galetti C, Illuminati B, Nascetti S. Meta-analysis of some results of clinical trials on sulodexide therapy in peripheral occlusive arterial disease. J Int Med Res. 1996;24:389–406. doi: 10.1177/030006059602400501. [DOI] [PubMed] [Google Scholar]
- 31.Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V. Sulodexide in the treatment of intermittent claudication. Results of a randomized, double-blind, multicentre, placebo-controlled study. Eur Heart J. 2002;23:1057–1065. doi: 10.1053/euhj.2001.3033. [DOI] [PubMed] [Google Scholar]
- 32.Condorelli M, Chiariello M, Dagianti A, et al. IPO-V2: a prospective, multicenter, randomized, comparative clinical investigation of the effects of sulodexide in preventing cardiovascular accidents in the first year after acute myocardial infarction. J Am Coll Cardiol. 1994;23:27–34. doi: 10.1016/0735-1097(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 33.Errichi BM, Cesarone MR, Belcaro G, et al. Prevention of recurrent deep venous thrombosis with sulodexide: the SanVal registry. Angiology. 2004;55:243–249. doi: 10.1177/000331970405500302. [DOI] [PubMed] [Google Scholar]
- 34.Cirujeda JL, Granado PC. A study on the safety, efficacy, and efficiency of sulodexide compared with acenocoumarol in secondary prophylaxis in patients with deep venous thrombosis. Angiology. 2006;57:53–64. doi: 10.1177/000331970605700108. [DOI] [PubMed] [Google Scholar]
- 35.Andreozzi GM, Bignamini AA, Davi G, et al. Sulodexide for the prevention of recurrent venous thromboembolism: The Sulodexide In Secondary Prevention Of Recurrent Deep Vein Thrombosis (SURVET) study: a multicenter, randomized, double-blind, placebo-controlled trial. Circulation. 2015;132:1891–1897. doi: 10.1161/CIRCULATIONAHA.115.016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cospite M, Milio G, Ferrara F, Cospite V, Palazzini E. Haemodynamic effects of sulodexide in post-thrombophlebitic syndrome. Acta Ther. 1992;18:149–161. [Google Scholar]
- 37.Luzzi R, Belcaro G, Dugall M, et al. The efficacy of sulodexide in the prevention of postthrombotic syndrome. Clin Appl Thromb Hemost. 2014;20:594–599. doi: 10.1177/1076029614533143. [DOI] [PubMed] [Google Scholar]
- 38.Adiguzel C, Iqbal O, Hoppensteadt D, et al. Comparative anticoagulant and platelet modulatory effects of enoxaparin and sulodexide. Clin Appl Thromb Hemost. 2009;15:501–511. doi: 10.1177/1076029609338711. [DOI] [PubMed] [Google Scholar]
- 39.Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease—part I: inflammatory biomarkers in wound healing. Biochim Biophys Acta. 2016;1862:1964–1974. doi: 10.1016/j.bbadis.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Mannello F, Ligi D, Canale M, Raffetto JD. Sulodexide down-regulates the release of cytokines, chemokines, and leukocyte colony stimulating factors from human macrophages: role of glycosaminoglycans in inflammatory pathways of chronic venous disease. Curr Vasc Pharmacol. 2014;12:173–185. doi: 10.2174/1570161111666131126144025. [DOI] [PubMed] [Google Scholar]
- 41.Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease—part II: proteolytic biomarkers in wound healing. Biochim Biophys Acta. 2016;1862:1900–1908. doi: 10.1016/j.bbadis.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Broekhuizen LN, Lemkes BA, Mooij HL, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbanek T, Zbigniew K, Begier-Krasinska B, Baum E, Breborowicz A. Sulodexide suppresses inflammation in patients with chronic venous insufficiency. Int Angiol. 2015;34:589–596. [PubMed] [Google Scholar]
- 44.Urbanek T, Krasinski Z, Suminska-Jasinska K, et al. Sulodexide reduces the inflammatory reaction and senescence of endothelial cells in conditions involving chronic venous disease. Int Angiol. 2016;35:140–147. [PubMed] [Google Scholar]
- 45.Coccheri S, Scondotto G, Agnelli G, Aloisi D, Palazzini E, Zamboni V. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost. 2002;87:947–952. [PubMed] [Google Scholar]
- 46.Coccheri S, Bignamini AA. Pharmacological adjuncts for chronic venous ulcer healing. Phlebology. 2016;31:366–367. doi: 10.1177/0268355515619562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu B, Lu J, Yang M, Xu T. Sulodexide for treating venous leg ulcers. Cochrane Database Syst Rev. 2016;6:CD010694. doi: 10.1002/14651858.CD010694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez Ochoa A. Sulodexide and phlebotonics in the treatment of venous ulcer. Int Angiol. 2017;36:82–87. doi: 10.23736/S0392-9590.16.03718-4. [DOI] [PubMed] [Google Scholar]
- 49.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 50.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017;18:https://www.R-project.org/. Accessed 22 Apr 2019.
- 51.Anon. Section 16.1.3.2 Imputing standard deviations for changes from baseline. In: Higgins JPT, Green S (Ed.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org; 2011. Accessed 23 Apr 2019.
- 52.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–177. [Google Scholar]
- 53.Algina J, Keselman HJ. Approximate confidence intervals for effect sizes. Educ Psychol Meas. 2003;63:537–553. [Google Scholar]
- 54.Ferrero S. Therapy of lower limbs phlebopathies with sulodexide. Clinical study of efficacy and tolerability. NAM. 1990;6:169–172. [Google Scholar]
- 55.Colombo F, Cristadoro F, Troyer L, Varini F. Superficial phlebopathies: tolerability and efficacy of the drug sulodexide. Geriatrics. 1991;8:45–48. [Google Scholar]
- 56.Ganassin L, Sogaro F. Galeazzi E [Evaluation of anti-thrombophilic properties of heparan sulfate in venous vascular pathology. A controlled clinical study versus active reference] Minerva Cardioangiol. 1991;39:275–283. [PubMed] [Google Scholar]
- 57.Pecis C, Giovilli M, Mezzanotte C, Pecis A, Sgroi G: Complementarity of the medical therapy in the surgical treatment of the varicose disease. Flebolinfologia. 1991; 3.4–3.6 (Article in Italian).
- 58.Petruzzellis V, Troccoli T, Florio T, Vadalà P. Sulodexide oral administration: therapeutic activity in chronic venous insufficiency. Giorn It Angiol. 1991;9:139–143. [Google Scholar]
- 59.Luttichau U, Palazzini E. Pharmacological treatment of post-phlebitic syndromes with sulodexide. Med Prax. 1992;13:1–2. [Google Scholar]
- 60.Marabotto M, Cavaliere P. Open trial on a new oral formulation of sulodexide for the treatment of post-phlebitic syndrome. Biol Med. 1992;14:1–12. [Google Scholar]
- 61.Rovere V, Amerio A, Mauro M, et al. Efficacy of action and tolerability of a new oral formulation of sulodexide in the treatment of the post-phlebitic syndrome. Controlled study vs. subcutaneous heparin. Nuova Stampa Med Ital. 1992;12:25–35. [Google Scholar]
- 62.Allegra C. Current role of glycosaminoglycans and perspectives in therapy. Minerva Angiol. 1993;18:45–49. [Google Scholar]
- 63.Saviano M, Maleti O, Liguori L. Double-blind, double-dummy, randomized, multi-centre clinical assessment of the efficacy, tolerability and dose-effect relationship of sulodexide in chronic venous insufficiency. Curr Med Res Opin. 1993;13:96–108. doi: 10.1185/03007999309111538. [DOI] [PubMed] [Google Scholar]
- 64.Verardi S, Ippoliti A, Ramundo A, Ranucci A, Tozzi A. Medium-term treatment of chronic venous insufficiency with oral sulodexide. Agg Med Chir. 1993;11:230–240. [Google Scholar]
- 65.Di Domenica M. Superficial veins thrombosis and varicophlebitides: antithrombotic therapy with sulodexide. Minerva Cardioangiol. 2000;48:152–154. [Google Scholar]
- 66.Flota-Cervera LF, Paz-Janeiro JL, Guevara-Saldívar MI, et al. Sulodexide in chronic venous disease. Clinical experience in Mexico. Rev Mex Angiol. 2014;42:28–37. [Google Scholar]
- 67.Elleuch N, Zidi H, Bellamine Z, Hamdane A, Guerchi M, Jellazi N. Sulodexide in patients with chronic venous disease of the lower limbs: clinical efficacy and impact on quality of life. Adv Ther. 2016;33:1536–1549. doi: 10.1007/s12325-016-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bogachev VI, Golovanova OV, Malysheva IN. Efficacy of sulodexide in treatment of chronic venous insufficiency. Results of the ACCORD trial. Angiol Sosud Khir. 2017;23:83–88. [PubMed] [Google Scholar]
- 69.Flota Cervera LF, Frati Munari AC, Velazquez Herrera AE, Carbajal Contreras A. Chronic venous disease treated with sulodexide: a survey among primary care physicians in Mexico. Int Angiol. 2017;36:558–564. doi: 10.23736/S0392-9590.17.03805-6. [DOI] [PubMed] [Google Scholar]
- 70.Guevara-Saldívar MI, Garza-Ruiz AF, Gonzáles-Ochoa A, et al. Sulodexide for the management of chronic venous disease in clinical stages C3 and C4 Open observational study. Rev Mex Angiol. 2017;17(45):15–22. [Google Scholar]
- 71.Serra R, Gallelli L, Conti A, et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des Devel Ther. 2014;8:519–527. doi: 10.2147/DDDT.S61770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chupin AV, Katorkin SE, Katel’nitskii II, et al. Sulodexide in treatment of chronic venous insufficiency. Results of the All-Russian multicenter programme ACVEDUCT. Angiol Sosud Khir. 2018;24:47–55. [PubMed] [Google Scholar]
- 73.Ligi D, Croce L, Mosti G, Raffetto JD, Mannello F. Chronic venous insufficiency: transforming growth factor-beta isoforms and soluble endoglin concentration in different states of wound healing. Int J Mol Sci. 2017;18:2206. doi: 10.3390/ijms18102206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreozzi GM. Role of sulodexide in the treatment of CVD. Int Angiol. 2014;33:255–262. [PubMed] [Google Scholar]
- 75.Frati Munari AC. Medical significance of endothelial glycocalyx. Part 2: its role in vascular diseases and in diabetic complications. Arch Cardiol Mex. 2014;84:110–116. doi: 10.1016/j.acmx.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez Jiménez OA, Frati-Munari A. Varices and chronic venous disease. General aspects and bases for treatment. Galenus. 2014;42:34–37. [Google Scholar]
- 77.Roztocil K, Antignani PL. Sulodexide: it is time for a program against chronic venous disease. Internat Angiol. 2014;33:209–211. [PubMed] [Google Scholar]
- 78.Frati-Munari AC. Medical significance of endothelial glycocalyx. Arch Cardiol Mex. 2013;83:303–312. doi: 10.1016/j.acmx.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 79.Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Curr Vasc Pharmacol. 2013;11:354–365. doi: 10.2174/1570161111311030010. [DOI] [PubMed] [Google Scholar]
- 80.González Ochoa A. Reducing hyperpigmentation after sclerotherapy using an antithrombotic drug. In: Greenhalgh RM, editor. Vascular and endovascular controversies update—40 years of looking forward. London: BIBA Publishing; 2018. pp. 491–496. [Google Scholar]
- 81.Mazzaccaro D, Muzzarelli L, Modafferi A, Righini PC, Settembrini AM, Nano G. Use of venoactive drugs after surgery for varicose veins: a preliminary study. Int Angiol. 2018;37:79–84. doi: 10.23736/S0392-9590.17.03875-5. [DOI] [PubMed] [Google Scholar]
- 82.Zakharova NO, Bulgakova SV, Katorkin SE, Melnikov MA, Treneva EV, Nikolaeva AV. The treatment of elderly and senile patients with venous trophic ulcers and type 2 diabetes mellitus. Adv Gerontol. 2017;30:917–924. [PubMed] [Google Scholar]
- 83.Apollonio A, Antignani PL, Di Salvo M, et al. A large Italian observational multicentre study on vascular ulcers of the lower limbs (Studio Ulcere Vascolari) Int Wound J. 2016;13:27–34. doi: 10.1111/iwj.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoiko YM, Gudymovich VG, Tsyplyashchuk AV. Current aspects of endothelial protection in treatment of patients with chronic venous insufficiency at the stage of trophic disorders. Angiol Sosud Khir. 2016;22:109–114. [PubMed] [Google Scholar]
- 85.Flota-Cervera LF, Nuricumbo-Vázquez A, Santana-Vega D, et al. Sulodexide for the venous leg ulcers: experience in Mexico. Rev Mex Angiol. 2015;43:131–137. [Google Scholar]
- 86.Katorkin SE. Significance of endothelial protection in treatment of patients with class c6 chronic venous disease and type 2 diabetes mellitus. Angiol Sosud Khir. 2015;21(99–102):4–6. [PubMed] [Google Scholar]
- 87.Katorkin SE. The evaluation of the effectiveness of the application of sulodexide for the combined treatment of the patients presenting with trophic ulcers on the lower extremities of venous etiology. Flebogiya. 2015;9:35–41. [Google Scholar]
- 88.Zou YX, Feng X, Jing ZP. Efficacy and safety of sulodexide in the treatment of venous ulcers of leg. Pharm Care Res. 2007;7:22–24. [Google Scholar]
- 89.Gacka M, Mastej K, Adamiec R. Thrombotic and fibrinolytic activity in patients with venous ulcer during sulodexide treatment. Przeglad Flehologiczny. 2004;12:97–100. [Google Scholar]
- 90.Mastej K, Gacka M, Adamiec R. Assessment of the healing of venous leg ulcer in post-thrombotic syndrome during combined therapy sulodexide. Przeglad Flehologiczny. 2004;12:131–134. [Google Scholar]
- 91.Kucharzewski M, Franek A, Koziolek H. Treatment of venous leg ulcers with sulodexide. Phlebologie. 2003;32:115–120. [Google Scholar]
- 92.Scondotto G, Aloisi D, Ferrari P, Martini L. Treatment of venous leg ulcers with sulodexide. Angiology. 1999;50:883–889. doi: 10.1177/000331979905001102. [DOI] [PubMed] [Google Scholar]
- 93.Aldrett E, Frati A. Sulodexide as and adjunctive management in patients with venous leg ulcers. Internat Angiol. 2018;37:73. [Google Scholar]
- 94.Casoni P, Villa F, Corona P. Sulodexide therapy after endovascular or hemodynamic treatment for varicose veins. Internat Angiol. 2018;37:73. [Google Scholar]
- 95.Gossetti B, Irace L, Benedetti-Valentini F. Use of glucosaminoglycans (GAG) in vascular surgery: assumption and clinical experience. Minerva Angiol. 1993;18:41–43. [Google Scholar]
- 96.Stagnaro-Neri M, Stagnaro S. Free radicals and microcirculatory alterations in the cinstitutional hypotonic phlebopathies. Minerva Angiol. 1993;18:105–108. [Google Scholar]
- 97.Cospite M, Ferrara F, Cospite V, Palazzini E. Sulodexide and the microcirculatory component in microphlebopathies. Curr Med Res Opin. 1992;13:56–60. doi: 10.1185/03007999209115223. [DOI] [PubMed] [Google Scholar]
- 98.Cospite M, Milio G, Ferrara F, Cospite V, Palazzini E. Haemodynamic effects of sulodexide in post-thrombophlebitic syndrome. Phlebologie. 1992;2:733–735. [Google Scholar]
- 99.Mauro M, Ferraro G, Palmieri G. Profibrinolytic and antithrombotic effects of sulodexide oral administration: a double-blind, crossover, placebo-controlled study. Curr Ther Res. 1992;51:342–350. [Google Scholar]
- 100.Del Guercio R, Siciliano G, Niglio A, Del Guercio M. Evaluations on the use of sulodexide in a group of patients with CVI. Minerva Angiol. 1991;16:141–142. [Google Scholar]
- 101.Di Stefano D, Vinci M. Antithrombotic therapy of phlebopathies with sulodexide. Controlled study of efficacy and tolerability. Eur Rev Med Pharmacol Sci. 1989;11:507–515. [Google Scholar]
- 102.Apollonio A, Antignani PL, Di Salvo M, et al. Epidemiology and healing of vascular ulcers in Italy. Preview of the SUV study results (AIUC September 2012): short report. Acta Phlebol. 2012;13:101–103. [Google Scholar]
- 103.Apollonio A, Antignani PL, Di Salvo M, et al. SUV2012. First results of the observational multicenter AIUC study for the epidemiology and treatment investigation of vascular ulcers of the lower limbs in Italy. Acta Vulnol. 2012;10:259–264. [Google Scholar]
- 104.Pecis C, Sgroi G, Giovilli M, Stringhi E, Mezzanotte C. Place of medical therapy with sulodexide in the operated patient with varicose veins. Flebolinfologia. 1990;16:51–53. [Google Scholar]
- 105.Apollonio A, Mosti G, Ricci E. Microcirculation and venous ulcers. Acta Vulnol. 2008;6:125–132. [Google Scholar]
- 106.Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (Daflon(R)) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37:143–154. doi: 10.23736/S0392-9590.18.03975-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed in this article are present in the original papers mentioned and are shown in the figures as original or derived data.