Abstract
Introduction:
Over 500,000 bone grafting procedures are performed every year in the United States for neoplastic and traumatic lesions of the craniofacial skeleton, costing $585 million in medical care. Current bone grafting procedures are limited, and full-thickness critical-sized defects (CSDs) of the adult human skull thus pose a substantial reconstructive challenge for the craniofacial surgeon. Cell-based strategies have been shown to safely and efficaciously accelerate the rate of bone formation in CSDs in animals. The authors recently demonstrated that supraphysiological transplantation of macrophages seeded in pullalan–collagen composite hydrogels significantly accelerated wound healing in wild type and diabetic mice, an effect mediated in part by enhancing angiogenesis. In this study, the authors investigated the bone healing effects of macrophage transplantation into CSDs of mice.
Methods:
CD1 athymic nude mice (60 days of age) were anesthetized, and unilateral full-thickness critical-sized (4 mm in diameter) cranial defects were created in the right parietal bone, avoiding cranial sutures. Macrophages were isolated from FVB-L2G mice and seeded onto hydroxyapatite-poly (lactic-co-glycolic acid) (HA-PLGA) scaffolds (1.0 × 106 cells per CSD). Scaffolds were incubated for 24 hours before they were placed into the CSDs. Macrophage survival was assessed using three-dimensional in vivo imaging system (3D IVIS)/micro-CT. Micro-CT at 0, 2, 4, 6, and 8 weeks was performed to evaluate gross bone formation, which was quantified using Adobe Photoshop. Microscopic evidence of bone regeneration was assessed at 8 weeks by histology. Bone formation and macrophage survival were compared at each time point using independent samples t tests.
Results:
Transplantation of macrophages at supraphysiological concentration had no effect on the formation of bones in CSDs as assessed by either micro-CT data at any time point analyzed (all P > 0.05). These results were corroborated by histology. 3D IVIS/ micro-CT demonstrated survival of macrophages through 8 weeks.
Conclusion:
Supraphysiologic delivery of macrophages to CSDs of mice had no effect on bone formation despite survival of transplanted macrophages through to 8 weeks posttransplantation. Further research into the physiological effects of macrophages on bone regeneration is needed to assess whether recapitulation of these conditions in macrophage-based therapy can promote the healing of large cranial defects.
Keywords: Critical-sized cranial defects, macrophage-based therapy, craniofacial, calvarium
Bone has an exceptional capacity to spontaneously regenerate following injury. When bone loss is excessive, following cancer, trauma, or osteomyelitis, or in certain comorbid states, including osteoporosis, infection, diabetes, and smoking, this capacity for regeneration is exceeded. Large boney defects of the adult craniofacial skeleton thus represent a substantial reconstructive challenge, and can result in dramatic deformities in aesthetically, functionally, and anatomically complex areas.1 Over 500,000 bone grafting procedures are performed every year in the United States, costing $585 million in medical care.2,3 Bone grafting procedures are the gold standard surgical approach to repair large boney defects but are limited by the availability of donor tissue and the morbidity associated with harvesting.
Tissue engineering (TE) techniques are emerging as new and promising therapies able to facilitate bone regeneration.4 Bone TE involves transplanting cells, scaffolding materials, and growth factors (GFs), in specific combinations to support regenerative repair, replace the missing bone tissue, and restore tissue function. Cells are isolated, cultivated ex vivo, and seeded into functional anatomically shaped bone scaffolds. In preclinical studies, scaffolds are placed into critical-sized bone defects (CSDs) to explore their osteogenic capability. A CSD is the smallest intraosseous wound size in a particular bone and species of animal that will not heal spontaneously during the lifetime of the animal or within the time period of scientific investigation.5 In standard mice models, a circular 4 mm CSD in the suture-free parietal bone is the most commonly used model.6 Numerous animal studies have shown that mesenchymal stromal cells (MSCs) derived from bone marrow (BMSCs),7–14 adipose tissue (ADSCs),15–19 and peripheral blood (BD-MSCs)20 can effectively and efficiently generate functional bone tissue in CSDs. Similar results have been found using stromal cells isolated from skeletal muscle,21 endometrial tissue,22 umbilical cord blood,23,24 umbilical cord Wharton jelly,25 dental pulp,26 and periosteal tissue.27 Preliminary results in humans suggest that autologous BMSCs28,29 and bone marrow30 can improve repair of large skeletal defects, but few clinical trials have been published and clinical success remains to be confirmed in a larger population of patients.4,31 In this original study, we aimed to investigate the bone healing effects of macrophage transplantation into CSDs of mice.
METHODS
Animals
FVB-L2G (FVB-Tg (CAG-luc,-GFP)L2G85Chco/J) and CD1 athymic nude mice were purchased from Charles River Laboratories. Mice were bred and maintained at the Stanford University Comparative Medicine Pavilion in accordance with Stanford University guidelines. All the animals were housed in light- and temperature-controlled facilities and given food and water ad libitum. All experiments followed the protocols approved by the Animal Facilities at Stanford University.
Cell Isolation and Culture
Adult mouse macrophages were generated using methods described previously.32 In brief, bone marrow cells were isolated from FVB-L2G mice and differentiated in IMDM + GlutaMax (Life Technologies, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, HyClone), 100 U/mL penicillin, 100 mg/mL streptomycin (Life Technologies), and 10 ng/mL mouse macrophage-colony stimulating factor (M-CSF) (Peprotech). Cells were cultured on 10 cm3 plates and incubated (37°C, 5% carbon dioxide) for 10 days, at which point the plated cells exhibited morphological changes characteristic of macrophages.33,34 Cells were then lifted by first washing the plates twice with phosphate-buffered saline (PBS) and incubating for 10 minutes with TrypLE (Life Technologies) at 37°C. The cells were then removed with cell lifters (Corning), and excess TrypLE was removed by the addition of serum-containing culture media and centrifugation. The macrophages were then counted and diluted to appropriate concentrations with PBS for transplantation. Macrophages were then seeded in PBS onto hydroxyapatite-poly (lactic-co-glycolic acid) (HA-PLGA) scaffolds (1.0 × 106 cells per CSD) and incubated for 24 hours before placement into the CSD (Fig. 1A).
FIGURE 1.
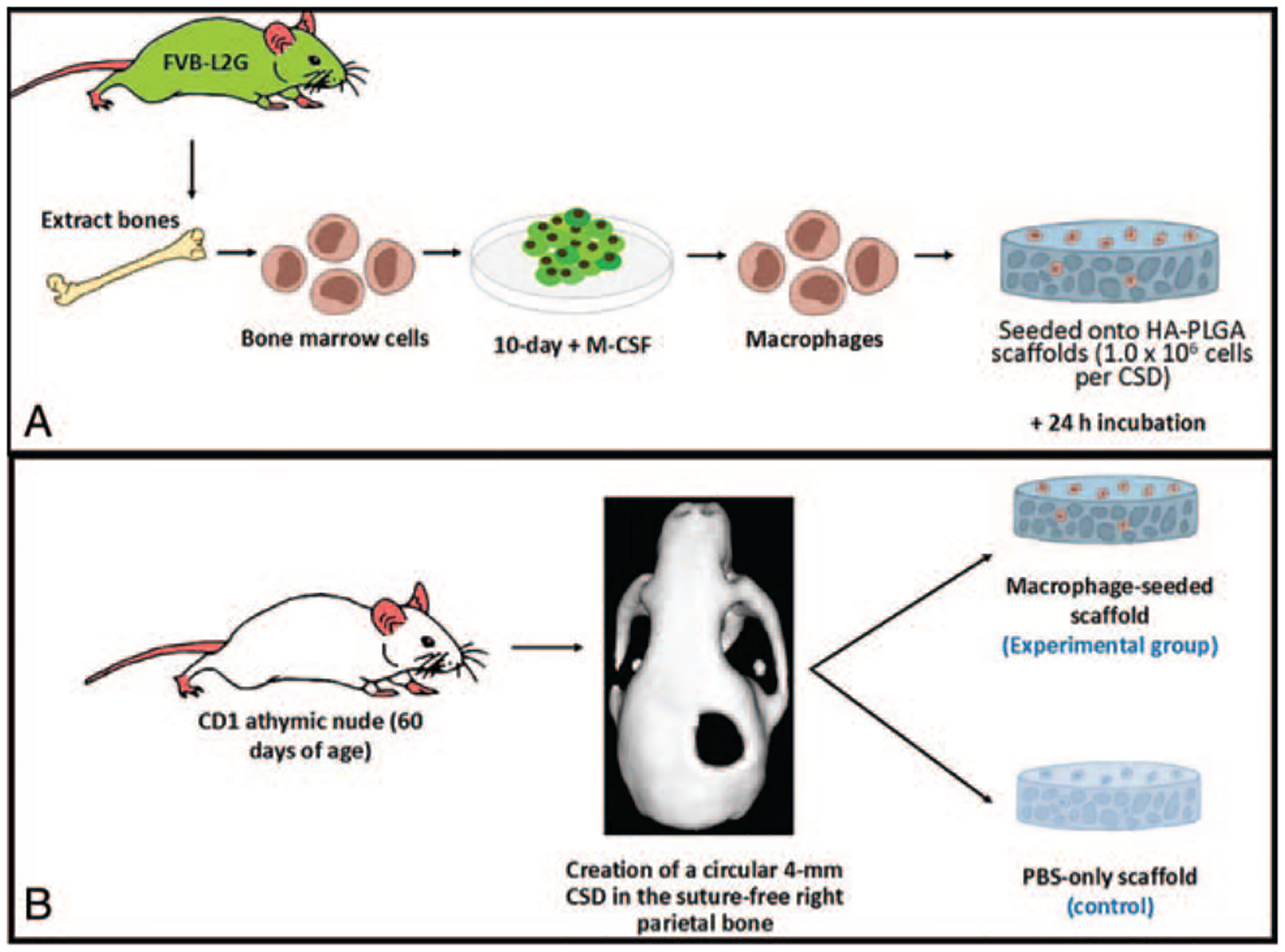
(A) To prepare the macrophages for transplantation, bone marrow was extracted from FVB-L2G mice and cultured for 2 d using a serum supplemented with macrophage-colony stimulating factor (M-CSF) to differentiate the cells into macrophages. This was confirmed histologically after 10 d in culture. The differentiated cells were then seeded onto hydroxyapatite-poly (lactic-co-glycolic acid) (HA-PLGA) scaffolds and incubated for 24 h. (B) Critical-sized defects (CSDs) were created in the suture-free parietal bone of CD1 athymic mice, 60 d of age. Into this defect the mice either received: Experimental group: A macrophage-seeded scaffold (n = 10); or Control group: A PBS soaked scaffold (n = 10)
Calvarial Defect Model
CD1 athymic nude mice, 60 days of age, were randomly divided into 2 groups: scaffolds with macrophages (experimental group, n = 15), and unseeded hydrogels (control group, n = 15) (Fig. 1B). Mice were anesthetized (isoflurane, 1–2%) and the skin overlying the parietal bone was sterilized with 70% alcohol. A midline longitudinal incision over the calvaria was used to expose the skull and cranial skin flaps were elevated. The subcutaneous fascia was divided, and periosteal flaps were reflected bilaterally. A single full-thickness critical-sized (4 mm) calvarial defect was created on the right suture-free parietal bone using a 4-mm drill. Defects were rinsed with PBS solution to clear debris, and care was taken not to damage the dura mater. The defects were immediately filled with the scaffolds. The skin flaps were closed with interrupted sutures (nylon 6–0, Ethicon). Warm saline boluses (100 cc/kg) were injected subcutaneously at the end of the procedure to maintain hydration. The day of surgery was designated as day 0. Mice in each group were euthanized at 0, 2, 4, 6, and 8 weeks (n = 2 mice per time point) via exposure to hyperbaric carbon dioxide.
Microcomputed Tomography
At weeks 0, 2, 4, 6, and 8 (n = 3 mice per group per time point), the whole calvarium was dissected and fixed in 2% paraformaldehyde (PFA) at 4°C for 12 to 16 hours. Skulls were scanned using calibrated X-ray micro-computerized tomography (micro-CT) equipment to assess bone formation (Micro XCT, Xradia Inc, Pleasanton, CA) at 4 magnification, 10 μm resolution with a peak voltage of 40 kVp, an LE #2 source filter, and a beam hardening constant of 2. Bone volume in the regions of interest (ROI) was quantified using Adobe Photoshop (Adobe) determined as a ratio to the inner diameter of the original CSD (4 mm). Individual CT slices (200–300) within the 4 mm CSD were binarized for the determination of bone formation.
Histology and Staining
Following micro-CT, fixed skulls were decalcified in 0.4 M EDTA in PBS (pH 7.2) at 4°C for 2 weeks. EDTA was changed at 1 week. Specimens were then dehydrated in 30% sucrose (in PBS) at 4°C for 24 hours and embedded in Tissue Tek O.C.T. (Sakura Finetek) under dry ice. Frozen blocks were mounted on a MicroM HM550 cryostat (MICROM International GmbH) and 7 to 8 μm thick sections were transferred to Superfrost/Plus adhesive slides (Fisher Scientific). Representative sections were stained with hematoxylin and eosin (H&E) dye using a standardized protocol. Bright-field images were taken with a Leica DM4000B microscope (Leica Microsystems) and RETIGA 2000R camera (QImaging Scientific Cameras).
H&E-stained sections were used for quantification of bone formation at 3 standardized locations within the defect following methods described previously.17 Using Bioquant software (R&M Biometrics), we drew a line through the original defect from 1 edge to the other edge. We measured the length of the line as it passed through bone versus the length through scaffold for each sample.
Three-Dimensional In Vivo Imaging/ Microcomputerized Tomography
The survival and localization of transplanted macrophages in vivo in the CSD site was characterized using three-dimensional in vivo imaging (3D IVIS) Lumina Imaging System (Xenogen Corporation, Alameda, CA) and bioluminescence imaging (BLI) at 0, 2, 4, 6, and 8 weeks.
Statistical Analysis
GraphPad Prism version 6.0c was used for analysis of data and generation of graphs.
Two-tailed unpaired Student t tests were used for comparisons of bone formation at 0, 2, 4, 6, and 8 weeks between the 2 groups. Results are expressed as mean ± standard error of the mean (SEM). A *P value of ≤0.05 was considered statistically significant.
RESULTS
All animals survived the 8-week postoperative period. No complications, including wound infections, were noted.
Microcomputerized Tomography Results
Defects treated with both PBS-scaffolds and macrophage-seeded scaffolds demonstrated minimal osseous healing over the 8-week course. Radiographical analysis of the calvarial defects revealed that no improvement in bone formation at any of the time points analyzed in mice treated with macrophage-seeded, compared with PBS-only scaffolds, all P > 0.05 (Figs. 2 and 3).
FIGURE 2.
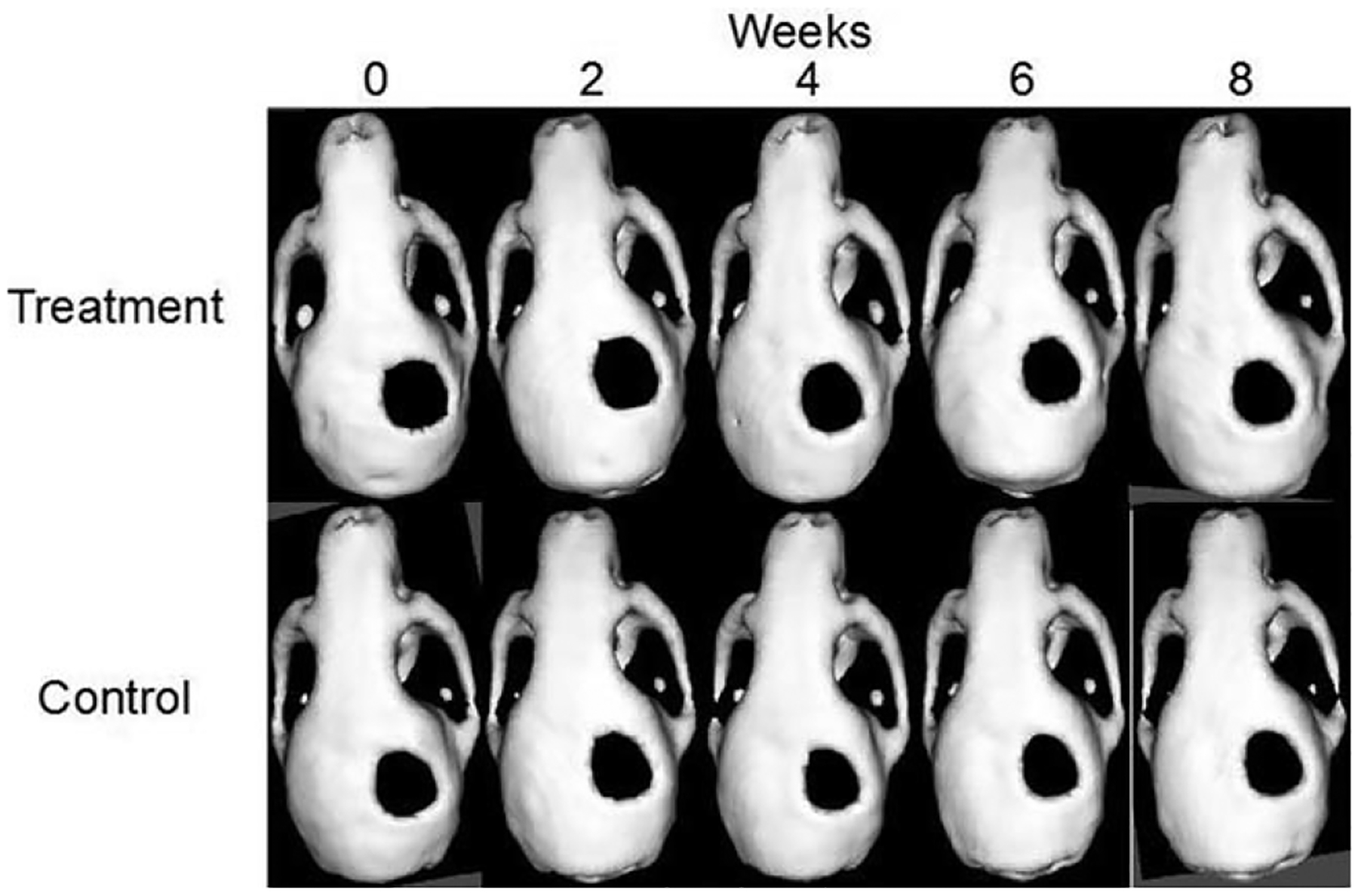
Microcomputerized tomography images of representative mouse in the treatment and control group.
FIGURE 3.
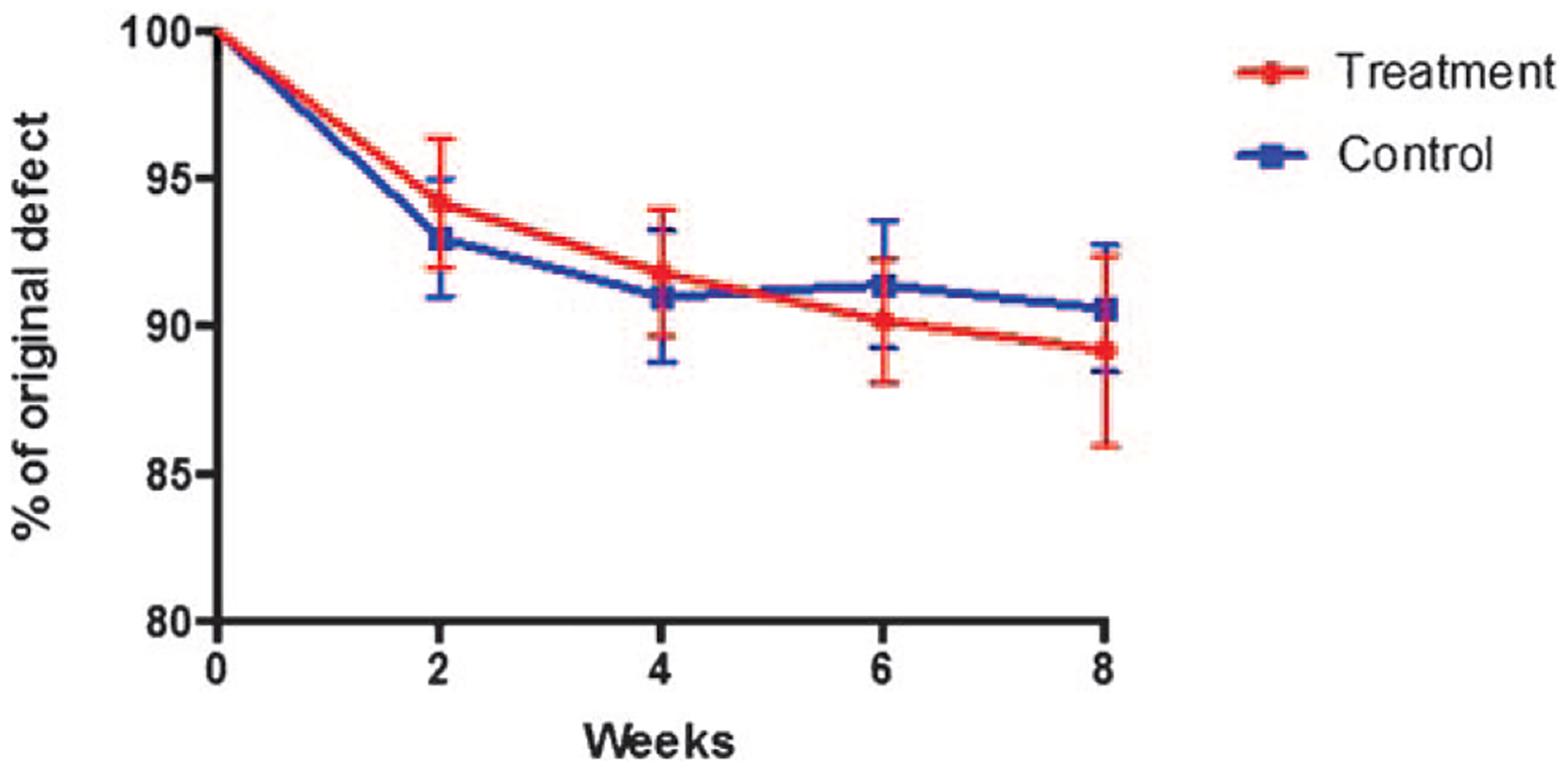
The calvarial defect healing curve showing the percent of original defect that remained at 0, 2, 4, 6, and 8 wk following scaffold transplantation.
Histology Results
Histology corroborated by the CT results and revealed no evidence of new bone formation 8 weeks following the transplantation of the scaffold in either the experimental or the control group (Fig. 4A, B). Nuclei in the scaffolds indicated the presence of viable macrophages up to 8 weeks post transplantation.
FIGURE 4.
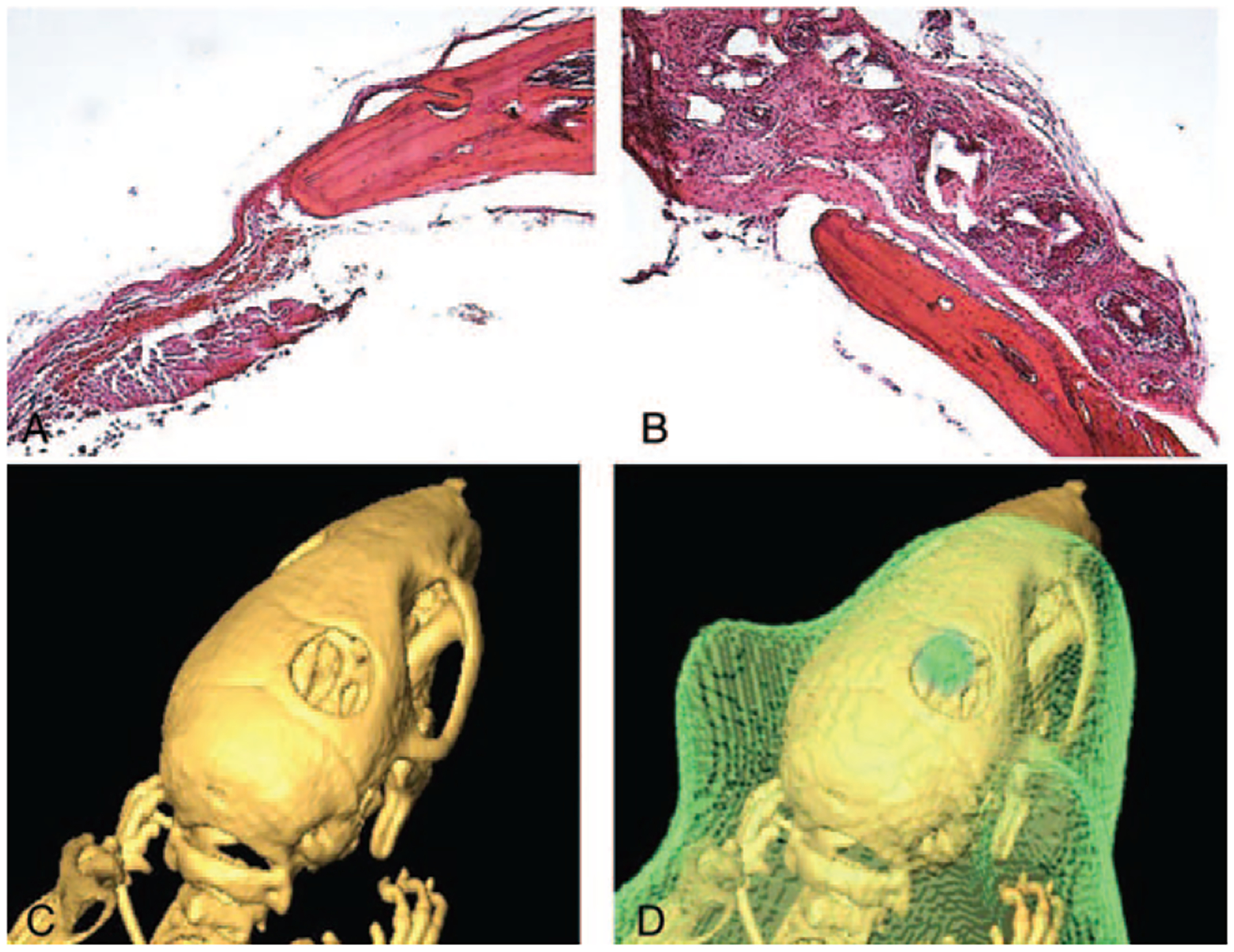
(A, B) Representative histological sections of the critical-sized defect in a mouse in the experimental group (A) and the control group (B). Both images show a sharp demarcation between the bone tissue and the scaffold with no evidence of new bone formation. Histology sections shown here were imaged at ×10 magnification. (C, D) A representative micro-CT (C) and corresponding IVIS/micro-CT image (D) of a mouse 8-wk posttransplantation. The green coloring indicates luciferase activity and therefore the presence of viable transplanted macrophages from EGFP-luciferase mice in the calvarial defect. CT, computerized tomography; IVIS, in vivo imaging system.
Three-Dimensional In Vivo Imaging System/ Microcomputerized Tomography Imaging
Three-dimensional in vivo imaging system/microcomputerized tomography imaging demonstrated survival of macrophages through the 8-week study period (Fig. 4C, D).
DISCUSSION
The exact mechanisms responsible for bone formation in cell-based therapies are unknown. Living cells, regardless of tissue source, have greater bone regenerative capacity than cell-free media and decellularized matrices.31 It is thought that enhancing cell survival, proliferation, and differentiation potential is desirable.35 The most commonly transplanted cells are MSCs which have long-term self-renewing and differentiation capabilities and may enable lasting therapeutic effects. These cells require appropriate conditions to direct their differentiation into the cell fates important in bone regeneration. It is interesting to explore whether the application of more differentiated cells normally involved in secondary bone healing may be of potential benefit in bone regeneration tissue engineering.
Macrophages are myeloid-derived innate immune cells which play an integral role in tissue homeostasis, repair, and regeneration. The primary function of macrophages is to phagocytose invading pathogens, and secondary functions including the release of growth factors, cytokines, interleukins, and nitric oxide to initiate inflammatory responses and repair damaged tissue.36,37 The bone tissue resident macrophages, “osteomacs,” reside in periosteal and end-osteal tissues in close association with osteoblasts38 and promote osteoblastogenesis in vivo and in vitro.39–41 Osteomacs mediate the anabolic effects of parathyroid hormone on bone,42 express proanabolic bone molecules including bone morphogenetic protein 2 (BMP2), BMP4, tumor growth factor beta-1 (TGF-ß1), Wnt proteins, and oncostatin M (OSM).43,44 Following fractures, osteomacs are activated and found in abundance in the healing callus.45,46 Bone marrow-derived recruited macrophages also mediate bone repair following fracture, and populate the fracture callus in the inflammatory phase of bone healing,47,48 and produce various osteoactive cytokines, including OSM, matrix metalloproteinases, and bone morphogenetic proteins.47,49–51 Harnessing this bone regenerative potential of macrophages may be a promising strategy for enhancing bone repair therapeutically.
Macrophage-based therapy has been long described in the cancer field.52 The exceptional ability of transplanted monocytes or macrophages to adopt the functions of local tissue-specific macrophages makes them promising candidates for cell-based therapies.53 Macrophages “activated” ex vivo by culture with either peripheral nerves54,55 or excised skin,56 and injected locally to the area of nerve or spinal cord damage induced partial motor recovery in animals. Injection of incubated autologous macrophages mediated clinically significant recovery of motor and sensory function in patients with acute complete spinal cord injury,57,58 however, a follow-up study failed to confirm these findings.59 Pulmonary macrophage transplantation therapy has been shown to correct lung disease in mice with results and macrophage survival persisting for at least 1 year.60,61 Cutaneous wound healing is another process highly dependent upon the coordinated interplay between macrophages along with resident dermal and infiltrating immune cells.36,62 We recently demonstrated that supraphysiological transplantation of macrophages seeded onto pullulan–collagen hydrogels and placed into splinted wounds in diabetic and non-diabetic mice significantly increased the rate of wound healing, with effects thought to be mediated by increased angiogenesis.33
The potential of macrophage-based therapy to promote bone regeneration has not been previously shown. Following our previous work33 we explored the potential of macrophage-based therapy to heal CSDs of mice. Monocytes were isolated from EGFP-luciferase mice to allow for cell tracking, as these mice express firefly luciferase and cytoplasmic EGFP constitutively in all cells.63 After 10 days in culture with macrophage colony stimulating factor (M-CSF), adherent cells exhibit the characteristic morphology of macrophage differentiation.33,34 Differentiated macrophages were seeded onto hydroxyapatite-poly (lactic-co-glycolic acid) (HA-PLGA) scaffolds and transplanted into CSDs of immunodeficient mice. The survival, localization, and behavior of transplanted macrophages in the wound site were characterized using 3D IVIS and histologic analysis.
This study demonstrates that allogenic monocytes, differentiated into macrophages ex vivo with M-CSF and seeded onto HA-PLGA scaffolds, did not promote de novo bone formation when transplanted into CSDs of immunodeficient mice. These results were found despite the transplanted macrophages surviving the 8-week postoperative study period. Although the sample sizes and observation period used here are comparable to those used in previous studies investigating the osteogenic potential of cell-based therapy in calvarial CSDs,17 bone healing effects of MSF-cultured macrophages were not seen as they are after transplantation of stem and progenitor cells.
An important question is whether the conditions used in the present study were able to provide an environment capable of promoting the bone-forming action of the transplanted macrophages. Bone TE involves the combination of cells, growth factors, and the biodegradable scaffolds used, which together recreate the necessary environmental cues and the suitable niche for bone regeneration.64 The monocytes used here were simply cultured in M-CSF and no attempt was made to bias macrophage differentiation along specific phenotypes through the use of additional culture media. When macrophages were used to treat nerve damage in animal models they were “educated” ex vivo by culturing with peripheral nerves54,55 or excised skin56 toward the wound healing phenotype before topical injection to areas of nerve injury. In our previous cutaneous wound healing model, the transplanted macrophages were only cultured in M-CSF prior to transplantation and it was thought the injured skin provided sufficient cues to activate the desired regenerative phenotypes of the transplanted macrophages.33 Following these findings, it was hypothesized that the injured bone environment would provide sufficient cues to activate the desired regenerative phenotypes of the transplanted macrophages. However, perhaps the environment inside the calvarial defect is less inflammatory and therefore unable to efficiently direct differentiation of the transplanted macrophages. Macrophage therapy for healing calvarial defects may require the addition of suitable growth factors and cytokines, such as macrophage chemotactic factor-1 and transforming growth factor which are made by osteocytes, to effectively exploit their bone regenerative potential.
Additionally, bone formation is known to be highly dependent upon adequate blood supply, and perhaps scaffolds seeded with factors that promote neovascularization, such as with vascular endothelial growth factor, may be necessary in addition to transplanted macrophages. The HA-based scaffold was used in this study because of its known beneficial effects in bone healing.65–67 HA belongs to a family of compounds known as apatites that closely resemble the mineral component of bone, and mimics natural bone at the nanoscale.68 HA-based scaffolds have promising results in bone regeneration due to their osteoconductive properties, unlimited availability, and absence of immune response and risk of virus transmission.65–67 HA scaffolds, however, may not be the most conducive material to macrophage function and future work may consider exploring different scaffolds materials. “Immuno-informed” biomaterials, for example, can induce macrophage polarization toward the M2 phenotype, or the “alternatively activated” anti-inflammatory macrophage type, to enable them to fulfil a regeneration function.69 For example, high surface wettability materials, such as titanium surfaces, can promote the M2 phenotype of macrophages in vitro.70 Future work should explore the effects of using different culture conditions, scaffolds, growth factors, and cytokines, to more definitively determine the therapeutic potential of macrophage-based therapy in the skeleton.
A second modification to the described protocol which may be of potential benefit is the cotransplantation of macrophages with other cells involved in bone healing. Unlike stem/progenitor cells, macrophages are terminally differentiated and are unable to give rise to other cell types. Although they are integral to bone healing it may be that the regenerative potential of macrophages is only realized upon the presence of cells, such as other immune or tissue-resident cells, which in turn may also provide the necessary supportive factors. Alternatively, the addition of macrophages may enhance MSC-based therapies, as M2 macrophages can promote the osteoblast differentiation of MSCs.71 Multicellular therapy is gaining popularity in medicine, and recent clinical trials have shown promising effects of the delivery of macrophages along with other mesenchymal cell types in reducing pathology and symptomatology of both critical limb ischemia72 and ischemic cardiomyopathy.73 Future work should consider the cotransplantation of macrophages with other cells such as MSCs.
CONCLUSIONS
Importantly, transplanted macrophages survive in scaffolds within CSD for at least 8 weeks, highlighting longevity that could ultimately be of clinical importance. This suggests that if their bone-forming potential can be enhanced, then similar pipelines can be used as described here. Macrophage-based therapies in cancer treatment suggest that outcomes are exquisitely sensitive to factors such as cell source and the methods used to collect, isolate, culture, and prepare the macrophages for transplantation.74 It is therefore essential that in future macrophage-based therapeutic investigations researchers are transparent in reporting the methodology concerning the collection of differentiation techniques, including the animal strain, any pretreatments, age, anatomic source of precursor cells/ monocytes/macrophages, culture conditions, and cytokine treatment.74
The versatility and plasticity of macrophages represent both an opportunity and a research challenge for the future development of macrophage-based therapy. Current understanding of macrophage biology and their role in bone homeostasis, repair, and regeneration is expanding. Macrophages are increasingly recognized to be an extremely heterogeneous population, and it is likely that recruited and resident tissue macrophages biased toward more pro- or anti-inflammatory states may have different functional roles, at distinct time points in the bone healing process. Increased understanding of the cellular and molecular mechanisms by which endogenous macrophages regulate bone metabolism is essential to exploit this knowledge therapeutically.
Acknowledgments
This work was supported by the California Institute for Regenerative Medicine (CIRM) Clinical Fellow training grant, Stanford University School of Medicine Transplant and Tissue Engineering Fellowship Award, American Society of Maxillofacial Surgeons (ASMS)/Maxillofacial Surgeons Foundation (MSF) Research Grant Award, Hagey Laboratory for Pediatric Regenerative Medicine, The Oak Foundation, a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu, and NIH grants R01 GM087609, R01 GM116892, and U01 HL099776.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1990;1:60–68 [DOI] [PubMed] [Google Scholar]
- 2.Greenwald AS, Boden SD, Goldberg VM, et al. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am 2001;83: S98–103 [DOI] [PubMed] [Google Scholar]
- 3.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract 2002;5:143–151 [PubMed] [Google Scholar]
- 4.Meijer GJ, de Bruijn JD, Koole R, et al. Cell-based bone tissue engineering. PLoS Med 2007;4:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomaxillofacial nonunions. (WALTER REED ARMY MEDICAL CENTER WASHINGTON DC, 1985). [Google Scholar]
- 6.Krebsbach PH, Mankani MH, Satomura K, et al. Repair of craniotomy defects using bone marrow stromal cells. Transplantation 1998;66:1272–1278 [DOI] [PubMed] [Google Scholar]
- 7.Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop Scand 1989;60:334–339 [DOI] [PubMed] [Google Scholar]
- 8.Blum JS, Barry MA, Mikos AG, et al. In vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarial defect model. Hum Gene Ther 2003;14:1689–1701 [DOI] [PubMed] [Google Scholar]
- 9.Bruder SP, Kraus KH, Goldberg VM, et al. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am 1998;80:985–996 [DOI] [PubMed] [Google Scholar]
- 10.Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 1998;16:155–162 [DOI] [PubMed] [Google Scholar]
- 11.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 2000;49:328–337 [DOI] [PubMed] [Google Scholar]
- 12.Petite H, et al. Tissue-engineered bone regeneration. Nat Biotechnol 2000;18:959–963 [DOI] [PubMed] [Google Scholar]
- 13.Shang Q, et al. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg 2001;12:586–593 [DOI] [PubMed] [Google Scholar]
- 14.Kruyt MC, et al. Bone tissue engineering in a critical size defect compared to ectopic implantations in the goat. J Orthop Res 2004;22:544–551 [DOI] [PubMed] [Google Scholar]
- 15.Yoon E, Dhar S, Chun DE, et al. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng 2007;13:619–627 [DOI] [PubMed] [Google Scholar]
- 16.Dudas JR, et al. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg 2006;56:543–548 [DOI] [PubMed] [Google Scholar]
- 17.Cowan CM, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004;22:560–567 [DOI] [PubMed] [Google Scholar]
- 18.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi B, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, et al. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med 2015;4:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taub PJ, Yau J, Spangler M, et al. Bioengineering of calvaria with adult stem cells. Plast Reconstr Surg 2009;123:1178–1185 [DOI] [PubMed] [Google Scholar]
- 22.Ai J, et al. Repair of critical size rat calvarial defects using endometrial-derived stem cells embedded within gelatin/apatite nanocomposite scaffold. Stem Cell Discovery 2013;3:37 [Google Scholar]
- 23.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301 [DOI] [PubMed] [Google Scholar]
- 24.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000;109:235–242 [DOI] [PubMed] [Google Scholar]
- 25.Mitchell KE, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 2003;21:50–60 [DOI] [PubMed] [Google Scholar]
- 26.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000;97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radtke CL, Nino-Fong R, Esparza Gonzalez BP, et al. Characterization and osteogenic potential of equine muscle tissue- and periosteal tissue-derived mesenchymal stem cells in comparison with bone marrow- and adipose tissue-derived mesenchymal stem cells. Am J Vet Res 2013;74:790–800 [DOI] [PubMed] [Google Scholar]
- 28.Quarto R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344:385–386 [DOI] [PubMed] [Google Scholar]
- 29.Marcacci M, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6-to 7-year outcome of a pilot clinical study. Tissue Eng 2007;13:947–955 [DOI] [PubMed] [Google Scholar]
- 30.Warnke P, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004;364:766–770 [DOI] [PubMed] [Google Scholar]
- 31.Fisher JN, Peretti GM, Scotti C. Stem cells for bone regeneration: from cell-based therapies to decellularised engineered extracellular matrices. Stem Cells Int 2016:20169352598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walmsley GG, et al. Delivery of macrophages in a biomimetic scaffold accelerates diabetic wound healing through enhanced angiogenesis. J Am Coll Surg 2015;221:S113–S114 [Google Scholar]
- 33.Hu MS, et al. Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair. JCI Insight 2017;2:96260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiskopf K, et al. Engineered SIRPa variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013;341:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, et al. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv Drug Delivery Rev 2015;91:125–140 [DOI] [PubMed] [Google Scholar]
- 36.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hume DA, Loutit J, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/ 80: macrophages of bone and associated connective tissue. J Cell Sci 1984;66:189–194 [DOI] [PubMed] [Google Scholar]
- 39.Chang MK, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232–1244 [DOI] [PubMed] [Google Scholar]
- 40.Vi L, et al. Macrophages promote osteoblastic differentiation in vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015;30:1090–1102 [DOI] [PubMed] [Google Scholar]
- 41.Alexander KA, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011;26:1517–1532 [DOI] [PubMed] [Google Scholar]
- 42.Cho SW, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A 2014;111:1545–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne C, Takebe J, Offenbacher S, et al. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002;30:26–31 [DOI] [PubMed] [Google Scholar]
- 44.Guihard P, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012;30:762–772 [DOI] [PubMed] [Google Scholar]
- 45.Abou-Khalil R, et al. Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy. J Bone Miner Res 2014;29:304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawao N, et al. The tissue fibrinolytic system contributes to the induction of macrophage function and CCL3 during bone repair in mice. PLoS One 2015;10:e0123982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glynne Andrew J, Andrew SM, Freemont AJ, et al. Inflammatory cells in normal human fracture healing. Acta Orthop Scand 1994;65:462–466 [DOI] [PubMed] [Google Scholar]
- 48.Oni O The early stages of the repair of adult human diaphyseal fractures. Injury 1997;28:521–525 [DOI] [PubMed] [Google Scholar]
- 49.Horwood NJ. Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol 2016;51:79–86 [DOI] [PubMed] [Google Scholar]
- 50.Kaneko M, et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology 2001;40:247–255 [DOI] [PubMed] [Google Scholar]
- 51.Fernandes TJ, et al. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS One 2013;8:e73266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacerna LV, Stevenson G, Stevenson HC. Adoptive cancer immunotherapy utilizing lymphokine activated killer cells and gamma interferon activated killer monocytes. Pharmacol Ther 1988;38:453–465 [DOI] [PubMed] [Google Scholar]
- 53.Martinez FO, Gordon S. The evolution of our understanding of macrophages and translation of findings toward the clinic. Expert Rev Clin Immunol 2015;11:5–13 [DOI] [PubMed] [Google Scholar]
- 54.Rapalino O, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med 1998;4:814. [DOI] [PubMed] [Google Scholar]
- 55.Lazarov-Spiegler O, et al. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J 1996;10:1296–1302 [DOI] [PubMed] [Google Scholar]
- 56.Bomstein Y, et al. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol 2003;142:10–16 [DOI] [PubMed] [Google Scholar]
- 57.Knoller N, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine 2005;3:173–181 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma 2006;23:360–370 [DOI] [PubMed] [Google Scholar]
- 59.Lammertse D, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord 2012;50:661. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki T, et al. Pulmonary macrophage transplantation therapy. Nature 2014;514:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Happle C, et al. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Sci Transl Med 2014;6:250ra113–250ra113 [DOI] [PubMed] [Google Scholar]
- 62.MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care (New Rochelle) 2016;5:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Y-A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A 2004;101:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2011;2:403–430 [DOI] [PubMed] [Google Scholar]
- 65.Heise U, Osborn J, Duwe F. Hydroxyapatite ceramic as a bone substitute. Int Orthop 1990;14:329–338 [DOI] [PubMed] [Google Scholar]
- 66.Oonishi H Orthopaedic applications of hydroxyapatite. Biomaterials 1991;12:171–178 [DOI] [PubMed] [Google Scholar]
- 67.Sartoris D, Holmes R, Resnick D. Coralline hydroxyapatite bone graft substitutes: radiographic evaluation. J Foot Surg 1992;31:301–313 [PubMed] [Google Scholar]
- 68.Rho J-Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys 1998;20:92–102 [DOI] [PubMed] [Google Scholar]
- 69.Sridharan R, Cameron AR, Kelly DJ, et al. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today 2015;18:313–325 [Google Scholar]
- 70.Hotchkiss KM, et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater 2016;31:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong L, Zhao Y, Zhang Y, et al. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci 2016;46:65–71 [PubMed] [Google Scholar]
- 72.Powell RJ, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther 2012;20:1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henry TD, et al. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ Res 2014;115:730–7 [DOI] [PubMed] [Google Scholar]
- 74.Lee S, Kivimäe S, Dolor A, et al. Macrophage-based cell therapies: the long and winding road. J Control Release 2016;240:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]


