Abstract
Obesity and atrial fibrillation (AF) are growing epidemics with significant overlap in comorbidities. Multiple smaller studies have evaluated the effects of weight loss and risk factor modification on recurrence of AF, reduction in AF burden and improvement in AF symptom severity. The objective of this study was to determine if a modest weight loss of ≥10% of initial body weight is enough to improve outcomes in overweight or obese patients with established AF. We performed an extensive literature search and systematic review of studies that compared weight loss of ≥10% versus weight loss of less than 10% or weight gain and assessed outcomes including recurrence of AF as determined via a Holter monitor, AF burden and improvement in AF symptom severity. Risk ratio (RR) 95% confidence intervals were measured for dichotomous variables and mean difference (MD) 95% confidence intervals were measured for continuous variables, where MD > 0 favors the group with ≥10% weight loss. Five studies with a total of 548 patients were included. Patients who lost ≥10% of their initial body weight experienced less recurrence of AF (RR 0.29; 95% CI 0.19–0.44) and a larger reduction in reported event frequency (MD 1.74; 95% CI 0.70–2.79), episode duration (MD 2.14; 95% CI 0.04–4.23), global episode severity (MD 1.89; 95% CI 1.34–2.45), and symptom severity (MD 5.36; 95% CI 3.75–6.97). In conclusion, weight loss is associated with less risk of recurrent AF, reduction in AF burden and improvement in AF symptom severity.
Keywords: Atrial Fibrillation, Weight Loss, Overweight, Obese
INTRODUCTION
Atrial fibrillation (AF) is a growing epidemic with 15.9 million individuals projected to be affected by the arrhythmia in the United States by 2050.1,2 This rise in incidence and prevalence is accompanied by almost a doubling in AF-related mortality over the last two decades.2 Historically, the predominant management strategy in AF encompasses the three pillars of rate control, rhythm control, and stroke prevention. Recently, lifestyle interventions have been proposed as an important fourth pillar. The prevalence of obese individuals in the United States has tripled since the 1960s with one third of the population being obese.3 Numerous studies have demonstrated a strong and consistent association between obesity and AF.4,5 While there is some data to suggest benefit of weight loss in the primary prevention of AF,6 there is already a large population of patients who suffer from this arrhythmia. The purpose of our current study was to perform a systematic review of literature and meta-analysis to compare recurrence of AF, reduction of AF burden and improvement in AF symptom severity from baseline to follow up between those who lost ≥10% of their body weight versus those who lost less than 10% of their body weight or gained weight.
METHODS
We searched PubMed, EMBASE, clinicaltrials.gov, Medline, Google scholar and the Cochrane Central Register of Clinical Trials (Cochrane Library, Issue 09, 2017). This was assessed up to March 2019. No language restriction was applied. The reference list of all eligible studies was also reviewed. Search terms included (Weight Loss OR Obesity) AND (Atrial Fibrillation).
Studies were selected by two independent reviewers. The PRISMA statement for reporting systemic reviews and meta-analyses was applied to the methods for this study. The studies had to fulfill the following criteria to be considered in the analysis:
1) Studies had to have evaluated outcomes between those who lost ≥10% of their body weight and those who lost less than 10% of their body weight or gained weight in patients with established AF; 2) Studies had to have reported the percentage of patients who experienced freedom from AF or AF burden scores; 3) Studies with a minimum follow up of 12 months; 4) Study must have been published in a peer-reviewed scientific journal.
We aimed to compare the recurrence of AF, reduction in AF burden and improvement in AF symptom severity from baseline to follow up in patients who lost who lost ≥10% of their body weight versus those who lost less than 10% of their body weight or gained weight.
Two authors (O.M.A. and F.L.) independently performed literature search and extracted data from eligible studies. Outcomes were extracted from original manuscripts and supplementary data. Information was gathered using standardized protocol and reporting forms. Disagreements were resolved by consensus. Two reviewers (O.M.A. and F.L.) independently assessed the quality items and discrepancies were resolved by consensus or involvement of a third reviewer (J.C.H), if necessary.
Two authors (O.M.A. and F.L.) independently assessed the risk of bias of the included trials using standard criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions. Discrepancies were resolved by discussion or adjudication by a third author (J.C.H.).
Data was summarized across treatment arms using the Mantel-Haenszel risk ratio (RR) and inverse variance mean difference (MD), where a MD > 0 favored the group with ≥10% weight loss. Heterogeneity of effects was evaluated using the Higgins I-squared (I2) statistic. Random effects models for analyses were used with high heterogeneity (defined as I2 > 25%), otherwise fixed effects models of DerSimonian and Laird were used. Funnel plot analysis was used to address publication bias. The statistical analysis was performed by the Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Descriptive statistics are presented as means and standard deviations (SD) for continuous variables or number of cases (n) and percentages (%) for dichotomous and categorical variables.
RESULTS
Initial search resulted in 1,912 abstracts. 610 were duplicates and 1262 were excluded based on titles and abstracts (Figure 1). We included five studies in our final analysis; one randomized control trial,7 three prospective nonrandomized studies,8–10 and one retrospective, subgroup analysis.11
Figure 1.

Selection of studies
Baseline demographics and characteristics of the five studies are summarized in Table 1 and 2. We included a total of 548 patients. Among these, 232 patients (42%) had ≥10% weight loss and 316 (58%) had less than 10% weight loss or weight gain from baseline to follow-up. The risk of bias is summarized in figures 2 and 3. Four studies were considered to be at “high risk” for selection bias due to the study design.8–11 Three studies were considered to have “unclear risk” for attrition bias given the data on attrition after final cohorts were selected is unavailable.9–11 Finally, one study was considered to have “unclear risk” of other biases, such as recall bias, as it was not mentioned how data was obtained retrospectively.11
Table 1.
Patient Demographics and Characteristics
| Study (ref) | Abed et al.7 | ARREST-AF8 | LEGACY9 REVERSE-AF11 |
CARDIO-FIT10 | ||||
|---|---|---|---|---|---|---|---|---|
| ≥10% WL | <10% WL | ≥10% WL | <10% WL | ≥10% WL | <10% WL | ≥10% WL | <10% WL | |
| Patients - n | 75 | 75 | 61 | 88 | 135 | 220 | 28 | 34 |
| Age (years) | 60±10 | 60±10 | 58±11 | 57±10 | 65±11 | 62±11 | NR | NR |
| Men | 51 (68%) | 50 (67%) | 34 (56%) | 61 (69%) | 86 (64%) | 148 (67%) | NR | NR |
| Anthropometric values | ||||||||
| Waist circumference, cm | 110±10 | 112±11 | NR | NR | NR | NR | NR | NR |
| Weight (kg) | 99±13 | 101±16 | 101±18 | 97±17 | 101±17 | 99±17 | NR | NR |
| Body mass index (kg/m2) | 33±4 | 34±4 | 34±5 | 32±5 | 34±5 | 33±5 | NR | NR |
| Body surface area (m2) | 2.1±0.2 | 2.2±0.2 | NR | NR | NR | NR | NR | NR |
| Paroxysmal atrial fibrillation | 44 (59%) | 42 (56%) | 49 (56%) | 40 (65%) | 71 (53%) | 117 (53%) | NR | NR |
| Number of antiarrhythmic agents | 1.3±0.6 | 1.4±0.5 | 1.1±0.3 | 1.0±0.2 | 1.1±0.7 | 0.9±0.8 | NR | NR |
| Risk Factors | ||||||||
| Hypertension | 62 (83%) | 65 (87%) | 53 (87%) | 73 (83%) | 109 (81%) | 165 (75%) | NR | NR |
| Hyperlipidemia | 45 (60%) | 51 (68%) | 39 (64%) | 47 (53%) | 66 (49%) | 101 (46%) | NR | NR |
| Diabetes mellitus | 18 (24%) | 21 (28%) | 9 (15%) | 17 (19%) | 41 (30%) | 62 (28%) | NR | NR |
| Apnea hypopnea index >30 | NR | NR | 32 (53%) | 55 (62%) | 69 (51%) | 113 (51%) | NR | NR |
| Coronary artery disease | 7 (9%) | 10 (13%) | 10 (16%) | 10 (11%) | 21 (16%) | 23 (10%) | NR | NR |
| Valvulopathy | 5 (7%) | 4 (5%) | NR | NR | 8 (6%) | 11 (5%) | NR | NR |
| Alcohol (>30g/week) | 26 (35%) | 26 (35%) | 11 (18%) | 24 (27%) | 42 (31%) | 69 (31%) | NR | NR |
| Smoker | 32 (43%) | 30 (40%) | 20 (33%) | 31 (35%) | 50 (37%) | 88 (40%) | NR | NR |
Values are presented as ± SD or as n (%)
Abbreviations: NR = not reported
Table 2.
Study Characteristics
| Study | Abed et al.7 | ARREST-AF8 | LEGACY9 | REVERSE-AF11 | CARDIO-FIT10 |
|---|---|---|---|---|---|
| Study design | Prospective Randomized Partially blinded | Prospective Nonrandomized | Prospective Nonrandomized | Retrospective Sub-analysis | Prospective Nonrandomized |
| Mean follow up (months) | 15 | 42 | 48 | 49 | |
| Study population | Overweight/obese patients with symptomatic AF | Initial ablation with BMI ≥ 27 kg/m2 and ≥ 1 risk factor | Symptomatic paroxysmal or persistent AF who had a BMI ≥ 27 kg/m2 | Symptomatic paroxysmal or persistent AF who had a BMI ≥ 27 kg/m2 | |
| Intervention | 2 phase weight management program | Attended a physician-directed risk factor management clinic | Optional physician-led or self-managed weight loss program. | Physician-led risk factor management clinic and a tailored exercise program. | |
| Comparator arm | Written and verbal nutrition and exercise advice | Given information on management of risk factors | No comparator arm. Groups based on degree of weight loss. | No comparator arm. Groups based on fitness and degree of weight loss. | |
| Primary Outcome | AF burden as determined by the AFSS | Procedural success | AF burden as determined by the AFSS and AF freedom | AF burden as determined by the AFSS and AF freedom | |
| AF recurrence | 7-day Holter | Symptoms, ECG and Holter | 7-day Holter | 7-day Holter | |
Abbreviations: AF = atrial fibrillation; AFSS = Atrial Fibrillation Symptom Severity Score; BMI = body mass index
Figure 2.
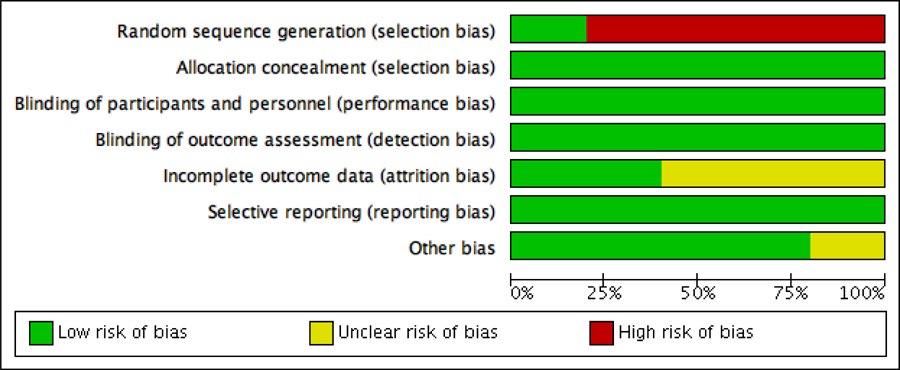
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies, according Cochrane Handbook for Systematic Reviews of Interventions.
Figure 3.

Risk of bias summary: review authors’ judgements about each risk of bias item for each included study, according Cochrane Handbook for Systematic Reviews of Interventions.
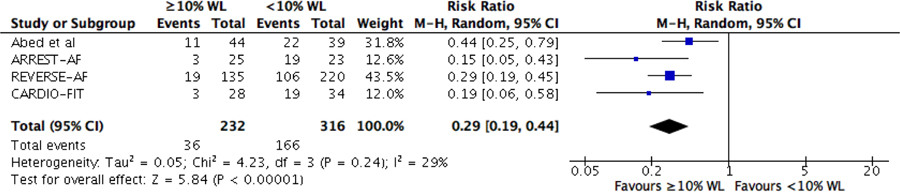
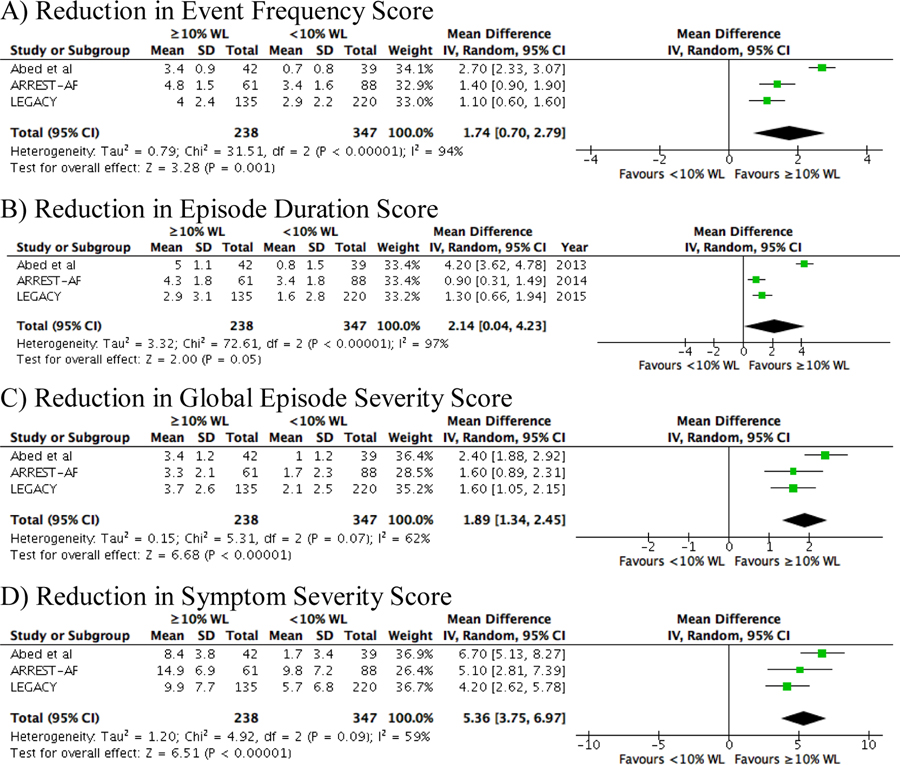
Patients who lost ≥10% of their initial body weight experienced less recurrence of AF (RR 0.29; 95% CI 0.19–0.44) at final follow up (Figure 4). Additionally, there was a statistically significant reduction in reported event frequency (MD 1.74; 95% CI 0.70–2.79), episode duration (MD 2.14; 95% CI 0.04–4.23), global episode severity (MD 1.89; 95% CI 1.34–2.45), and symptom severity (MD 5.36; 95% CI 3.75–6.97) (Figure 5).
Figure 4.
Forrest plots and funnel plots for the comparative analysis of risk of recurrent atrial fibrillation in those who lost 10% or more of their body weight compared to those who lost less than 10% of their body weight or gained weight from baseline to follow up.
Figure 5:
Forrest plots for the comparative analysis of the reduction in the various components of the Atrial Fibrillation Severity Scale scores in those who lost 10% or more of their body weight compared to those who lost less than 10% of their body weight or gained weight from baseline to follow up. A) event frequency, B) episode duration, C) global episode severity, D) symptom severity.
Funnel plot analysis of the included studies showed no evidence of publication bias on recurrence of AF, AF burden or improvement in AF symptom severity (Figure 4 and Supplementary data – Figure 1).
DISCUSSION
This is the first systematic review and meta-analysis of studies comparing outcomes between symptomatic AF patients who lost ≥10% of their initial body weight compared to those who lost less than 10% of their body weight or gained weight. The results of this meta-analysis show that a reduction of 10% or more of initial body weight is associated with less recurrence of AF, as well as improvements in AF burden and AF symptom severity. While recent guidelines have already been updated to advocate for weight loss in the management of AF,12 these findings are significant given that they show a modest weight loss of ≥10% is associated with improved outcomes, providing physicians with a practical target to counsel their patients.
Obesity is an independent risk factor for AF, with a 4 to 5% increased risk of developing AF with each unit increase in body mass index.4,5 Fortunately, weight loss has been shown to reduce AF recurrence in a dose dependent manner, with a greater freedom from AF with greater weight loss.8,9 Additionally, weight loss seems to slow or reverse the progression of paroxysmal AF to persistent and permanent AF seen in sustained obesity.11
This strong association between obesity and AF can in part be explained by the structural and electrical remodeling of the atria seen in obesity and its association with a multitude of proarrhythmic changes including diastolic dysfunction, atrial enlargement, autonomic tone abnormalities, and a systemic proinflammatory state.7,13,14 Furthermore, obesity has been shown to be associated with a unique atrial substrate, linking epicardial or pericardial fat with the presence, severity, and outcomes of AF.14 This extensive remodeling is reversible with weight loss, which has been shown to reduce left atrial volumes and left ventricular hypertrophy.9
The benefits of weight loss extend past reversal of the remodeling seen in obesity; the greater freedom from AF was also accompanied by less antiarrhythmic drug7–10 use and fewer ablation10,11 procedures in studies that recorded this information. This is important given that long-term outcomes of ablation including freedom from AF have not improved in proportion to the advances in ablative techniques and technologies.15 Late recurrence of AF following ablation procedures was initially presumed to be secondary to persistent pulmonary vein conduction. However, this explanation would better support mechanisms for early recurrence of AF. Furthermore, progressive atrial substrate is observed even after successful ablation.16 All of this argues in favor of the notion that an underlying substrate responsible for AF is promoted by unrecognized and undertreated risk factors such as obesity and its related comorbidities. This is further supported by the variety of cardiac risk factors that have been found to be present more frequently in patients with late recurrence of AF following ablation.17 This may also explain the improved outcomes post-ablation among patients who had lost more than 10% of their body weight.8
While obesity is a crucial modifiable risk factor in the management of atrial fibrillation, it is by no means the only one. Hypertension, diabetes mellitus, dyslipidemia, sleep apnea and elevated levels of c-reactive protein have all been shown to be associated with AF, with improved outcomes when these cardiac risk factors are treated.18–24 Even though it is out of the scope of this study to determine the relative contribution of each of these risk factors, it is well established that weight loss in obese patients reduces all of these comorbid conditions.25–29
However, the sustainability of weight loss remains a controversial topic. This is especially pertinent given that fluctuations in weight have been shown to attenuate the benefits of weight loss, which is expected given weight fluctuation has been shown to increase the risk of various cardiometabolic risk factors.25–29 Still, participation in dedicated, physician-led weight loss and risk factor modification clinics has shown promising results.9 Additionally, incorporation of tailored exercise programs to improve cardiorespiratory fitness has been shown to augment the effects of weight loss.10 Similarly, other elements of AF care that may be associated with weight loss such as medication compliance, management of comorbidities, and other lifestyle changes have been associated with better outcomes.30 All of this stresses the importance of a holistic approach to AF management. Targeting obesity and other cardiac risk factors and comorbidities should be considered in secondary and tertiary prevention of AF.
The current systematic review and meta-analysis has several important limitations that should be acknowledged. First, the studies included were comprised of patients from a single center, which significantly restricts the generalizability of the results and contain one study using a retrospective study design in which patient overlap cannot be ruled out. Second, there were different study protocols, with both randomized and non-randomized trials included. Third, the AFSS scores used to assess AF burden has the potential to underestimate arrhythmia burden. Similarly, the extended 7-day Holter used in all of the included studies may have potentially missed episodes of AF. However, both the AFSS and extended Holter monitors were used in both groups and were thus a limitation for all groups and would be expected to be non-differential to the outcomes studied.
In conclusion, in patients with symptomatic AF, weight loss of ≥10% is associated with less recurrence of AF, larger reduction in AF burden and improvement in AF symptom severity relative to patients who lost less weight or gained weight.
Supplementary Material
Acknowledgments
Conflicts of Interest:
Dr. Hsu reports receiving honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Janssen Pharmaceuticals, and Bristol-Myers Squibb, and research grants from Biotronik and Biosense-Webster.
Dr. Ho reports receiving research grants from the American Heart Association (19CDA34760021) and Abbott, equity in Vektor Medical and fellowship support from Medtronic, Abbott, Boston Scientific, and Biotronik.
Abbreviation list
- AF
atrial fibrillation
- AFSS
atrial fibrillation symptom severity
- CI
confidence interval
- MD
mean difference
- RR
risk ratio
REFERENCES
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–497. [DOI] [PubMed] [Google Scholar]
- 4.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Parise H, Levy D, D’Agostino RB Sr., Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 6.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjostrom L, Karason K. Bariatric Surgery and the Risk of New-Onset Atrial Fibrillation in Swedish Obese Subjects. J Am Coll Cardiol 2016;68:2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 8.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 9.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 10.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 11.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, McEvoy RD, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Calkins H, Field ME, Chen LY, Furie KL, Cigarroa JE, Heidenreich PA, Cleveland JC Jr., Murray KT, Ellinor PT, Shea JB, Ezekowitz MD, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019.
- 13.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7:835–846. [DOI] [PubMed] [Google Scholar]
- 16.Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Morton JB, Sanders P, Kalman JM. Long-term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm 2012;9:473–480. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty S, Mohanty P, Di Biase L, Bai R, Pump A, Santangeli P, Burkhardt D, Gallinghouse JG, Horton R, Sanchez JE, Bailey S, Zagrodzky J, Natale A. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J Am Coll Cardiol 2012;59:1295–1301. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 19.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009;119:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300–305. [DOI] [PubMed] [Google Scholar]
- 21.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 23.Pena JM, MacFadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J 2012;33:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol 2005;95:764–767. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JW, Brinkman-Kaplan VL, Lee H, Wood CL. Relationship of weight loss to cardiovascular risk factors in morbidly obese individuals. J Am Coll Nutr 1994;13:256–261. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res 2001;9 Suppl 4:326S–334S. [DOI] [PubMed] [Google Scholar]
- 27.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr 1992;56:320–328. [DOI] [PubMed] [Google Scholar]
- 28.Fagerberg B, Berglund A, Andersson OK, Berglund G. Weight reduction versus antihypertensive drug therapy in obese men with high blood pressure: effects upon plasma insulin levels and association with changes in blood pressure and serum lipids. Journal of hypertension 1992;10:1053–1061. [PubMed] [Google Scholar]
- 29.MacMahon S, Cutler J, Brittain E, Higgins M. Obesity and hypertension: epidemiological and clinical issues. Eur Heart J 1987;8 Suppl B:57–70. [DOI] [PubMed] [Google Scholar]
- 30.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved Outcomes by Integrated Care of Anticoagulated Patients with Atrial Fibrillation Using the Simple ABC (Atrial Fibrillation Better Care) Pathway. Am J Med 2018;131:1359–1366 e1356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.