Abstract
Background:
Lung transplantation (LTx) offers a survival benefit for patients with end-stage lung disease. When suitable donors are identified, centers must accept or decline the offer for a matched candidate on their waitlist. The degree to which variability in per-center offer acceptance practices impacts candidate survival is not established. The purpose of this study was to determine the degree of variability in per-center rates of LTX offer acceptance, and to ascertain the associated contribution to observed differences in per-center waitlist mortality.
Methods:
We performed a retrospective cohort study of candidates waitlisted for LTx in the US using registry data. Logistic regression was fit to assess the relationship of offer acceptance with donor-, candidate-, and geographic factors. Listing center was evaluated as a fixed effect to determine the adjusted per-center acceptance rate. Competing risks analysis employing the Fine-Gray model was undertaken to establish the relationship between adjusted per-center acceptance and waitlist mortality.
Results:
Of 15,847 unique organ offers, 4,735 (29.9%) were accepted for first-ranked candidates. After adjustment for important covariates, transplant centers varied markedly in acceptance rate (9% to 67%). Higher cumulative incidence of 1-year waitlist mortality was associated with lower acceptance rate. For every 10% increase in adjusted center acceptance rate, the risk of waitlist mortality decreased by 36.3% (subdistribution hazard ratio 0.637; 95% CI 0.592, 0.685).
Conclusions:
Variability in center-level behavior represents a modifiable risk factor for waitlist mortality in LTx. Further intervention is needed to standardize center-level offer acceptance practices and minimize waitlist mortality.
Keywords: Lung Transplantation, Organ Allocation, Organ Offer Acceptance, Waitlist Survival
Introduction
Transplantation is the gold-standard and increasingly-utilized therapy for patients with end-stage lung disease, and confers considerable survival and quality of life advantages to patients who often are afforded no other treatment options(1–3). As acceptable allografts remain a limited resource, considerable attention is paid to the equitable and judicious allocation of allografts, and efforts are made continuously to optimize distribution of allografts. In lung transplantation, this effort has resulted in a system in which – after geographic consideration – urgency is balanced by projected transplant benefit as a means to allocate organs to those who both need them urgently, and are simultaneously also most likely to benefit from the procedure. The system in place remains appropriately under intense scrutiny, such that identified disparities with respect to transplantation on account of disease etiology and geographic location might be best addressed and remediated(4–8). While the rate of lung transplantation is presently at an all-time high of 157 transplants per 100 waitlist years, the number of candidates added to the waitlist annually has also risen, contributing to an all-time high in the waitlist mortality of 16.5 deaths per 100 waitlist years(1).
When a candidate for lung transplantation becomes the highest-priority candidate and is the first to receive candidate donor lung allograft offers, the center acting on his or her behalf must elect to accept or decline the matched offer and subsequently either proceed with lung transplantation or return to the waitlist to await a later offer. Though there exists relative consensus with respect to suitable candidate donor allografts and the appropriateness of a candidate to proceed with transplantation, only 24% of offers are accepted for the first-ranked lung transplant candidate, suggesting significant variability in the decision to accept an organ offer(9, 10). The relationship between center-level organ-offer acceptance practices and waitlist outcomes was first established in liver and kidney transplantation, demonstrating that candidates listed at programs with low rates of acceptance of first-ranked organ offers were at elevated risk of waitlist mortality(11, 12). In heart and lung transplantation, Wey et al. were the first to establish the relationship between organ offer acceptance practices and the associated impact on survival to transplantation(13). At the program level, variability in this rate of acceptance remains a critically under-examined behavior, and the degree to which the variability in center-level organ offer acceptance relates to center-level waitlist mortality is not yet established. As such, while the lung allocation system intends to allocate allografts to the highest-priority candidates each time an allograft becomes available, variability in acceptance of these offers results in an inefficiency of the system at the national level.
To establish the degree of variability in organ offer acceptance practices across the United States, as well as ascertain the strength of the relationship between center-level organ offer acceptance and waitlist mortality, we performed a retrospective registry analysis employing organ offer data for individual candidates for isolated lung transplantation. As all offers permit the opportunity for a lung transplantation candidate to proceed to transplantation, center-level acceptance rates represent a modifiable behavior that may significantly impact equitable organ allocation and survival to transplantation.
Methods
Data Source
This study was approved by our institution’s Institutional Review Board prior to study initiation. We performed a retrospective cohort analysis using United Network of Organ Sharing (UNOS) Standard Analysis and Research (STAR) data. These data were subsequently linked with information from the Potential Transplant Recipient (PTR) file, which provides match run information for every offer made for each candidate donor lung allograft that is ultimately employed for use in transplantation. Further discussion of the mechanics of lung allocation in the United States is provided in the supplementary material. These dastasets have previously been comprehensively described(14). The data source used for this analysis included all candidates waitlisted for lung transplantation from May, 2007 through March, 2017.
Study Population and Cohort Determination
The Potential Transplant Recipient file was queried for all match runs for adult and adolescent (age ≥ 12 years old at the time of listing, as offers are made in order of LAS to these two populations) candidates listed for isolated lung transplantation that received a genuine first-ranked offer for a lung allograft between May, 2007 (the earliest date for which data were available) and March, 2017. Only match runs that resulted in transplantation were considered. Offers that were determined to be bypassed, an uncommon event in which an organ is offered first to a lower rank-order candidate, whether due to directed donation, natural disaster, or donor medical urgency, were not considered genuine. Offers from donors in which critical donor-specific information (donor lung PO2, tobacco history, diabetes status, and PHS increased infection risk) was missing were excluded. Finally, transplant centers that received fewer than 10 first-ranked offers in a year were excluded, as acceptance patterns may be artificially variable due to small sample size (12 centers during the study period) (Figure 1).
Figure 1.
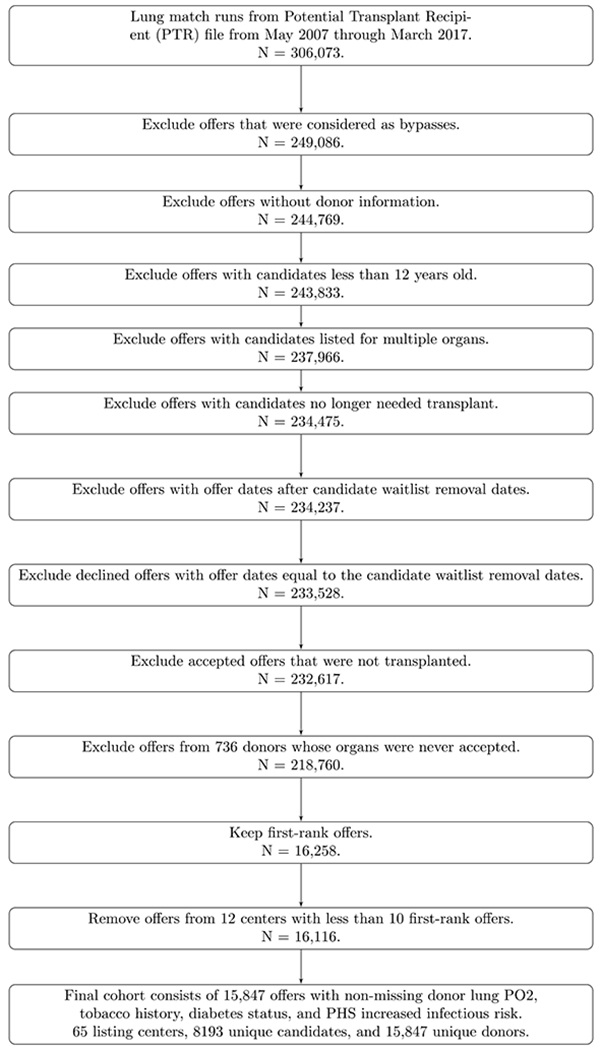
Flow diagram of cohort selection.
Model of First-Rank Offer Acceptance
The unique first-rank offers were the units of analysis. As the primary analysis, we modeled the outcome of acceptance of the first-ranked offer using logistic regression and included listing center as the primary exposure. Factors included in adjustment of acceptance rate were based on publicly-available Scientific Registry of Transplant Recipients (SRTR) models, including the following donor-, candidate-, and geographic factors: listing center, candidate age, candidate sex, candidate race, primary lung diagnosis group, LAS (as a restricted cubic spline with 4 knots), blood type compatibility, candidate need for ventilator, candidate need for Extra-Corporeal Membrane Oxygenation (ECMO), prospective crossmatch requirement, donor age, donor sex, donor PO2, donor tobacco history, donor confirmed pulmonary infection, donor death mechanism, donor diabetes status, donor CDC risk, organ share type, and donor-candidate pTLC ratio (as a restricted cubic spline with 4 knots). We employed marginal standardization to estimate the adjusted acceptance rate for each center and to permit inference to the total population from which the data are drawn(15). We predicted the counterfactual acceptance probability for each offer as if it was listed at all 65 transplant centers, and then we averaged the predicted probability for each center. As a sensitivity analysis, we fit a generalized linear mixed-effect model for the outcome of acceptance of the first-rank offer for a given candidate with a logit link function and treated listing center as a random effect. Because a candidate could potentially receive multiple first-rank offers and candidates were clustered within transplant centers, the model included candidate-level and center-level random intercepts to separately account for correlation due to clustering within candidates and centers respectively.
Model of Waitlist Mortality
The unique candidates were the units of analysis. We followed candidates from the time of receiving their first first-rank offer, as opposed to the time of listing, in order to assess candidate waitlist mortality from the time that the first first-rank offers were declined. Removal reasons of death and decompensation precluding transplantation were considered as surrogates for waitlist mortality. Competing risk analysis using a Fine-Gray subdistribution hazards model was performed to assess the relationship between the primary exposure - adjusted center acceptance rate - with the waitlist morality outcome. Because the occurrence of lung transplantation can hinder the observation of death on the waitlist, removal from waitlist due to lung transplantation or condition improved was considered as a competing risk and was distinguished from administrative censoring. The cumulative incidence of waitlist mortality was assessed at 1 year after listing. As the listing center was considered a fixed effect and each center had a unique adjusted center acceptance rate, adjusted center acceptance rate was included as a continuous covariate in the waitlist mortality model. To improve interpretability, we also grouped adjusted center acceptance rate based on quartiles and estimated the cumulative incidence function for each group. Confounders of waitlist mortality were adjusted for in the model using guidance from SRTR, including candidate age at listing (continuous), sex, race, blood type, primary lung diagnosis group, LAS (as a restricted cubic spline with 4 knots), prospective crossmatch requirement, need for mechanical support (ventilator, ECMO) at listing, and double lung preference. The unadjusted overall, as well as the center-specific, cumulative incidence functions of waitlist mortality were estimated. Adjusted cumulative incidence for each listing center was estimated using the marginal standardization method as performed in the acceptance rate model.
Measurement of Post-Transplantation Outcomes.
Fundamentally, the decision to decline an available offer for a candidate represents a decision to continue on the waitlist to await a superior offer at an uncertain duration of time later. To ascertain if allografts accepted at later positions varied significantly in post-transplant performance than allografts accepted at the first position, we compared post-transplant graft failure outcomes between allografts accepted at the first position vs. those accepted later in the match run. The event of interest was graft failure, whereas death (with functioning allograft) and re-transplantation were considered as competing risks. Unadjusted cumulative incidences of graft failure were estimated yearly up to 5-year post-transplantation for allografts accepted by first-rank or lower-rank patients. Gray’s test was used to compare cumulative incidence functions of graft failure between organs accepted by first-rank and accepted by lower-rank candidates. Adjusted cumulative incidences of graft failure were estimated using the Fine-Gray subdistribution hazard model. Candidate covariates were selected from published SRTR models of allograft survival and modeled based on functional form. Variables included donor to candidate predicted total lung capacity ratio (pTLC ratio), FEV1 at transplant, LAS at match, recipient age at transplantation, dialysis occurring between listing and transplantation, recipient serum creatinine at transplantation, and prior transplantation. The adjusted cumulative incidence of graft failure was estimated using marginal standardization as described above. As a sensitivity analysis, we assessed the event-free survival (freedom from graft failure, death, and re-transplantation) using Kaplan-Meier and Cox regression models.
Results
Study Population
Across 65 listing centers, a total of 8,193 candidates received first-ranked offers from 15,847 unique donors after application of our inclusion criteria (see Figure 1 for patient selection). The overall acceptance rate for the first-ranked offer was 29.9% (4735/15,847). Complete demographic information for candidates and donors is reported in table 1. Candidates who accepted the first-ranked offer tended to have a lower LAS at the time of match (median 47.5 for accept vs. 50.5 for decline) and were less likely female (42.1% for accept vs 55.9% for decline). Of the 5,157 candidates that declined the first-ranked offer, 1,965 candidates (38.1%) never received a subsequent first-ranked offer, and 97 (1.8%) never received a subsequent offer at any sequence position. Of these 1,965 candidates that did not receive a subsequent first-ranked offer, 1,301 (66.2%) underwent later transplantation, and 474 (24.1%) were ultimately removed from the waitlist due to death or decompensation.
Table 1.
Demographics by First Rank Offer Acceptance
| Accepted (N=4735) | Declined (N=11112) | Total (N=15847) | |
|---|---|---|---|
| Candidate age | 57 (44.0, 63.0) | 55 (43.0, 62.0) | 56 (43.0, 62.0) |
| Candidate female sex | 1993 (42.1%) | 6212 (55.9%) | 8205 (51.8%) |
| Candidate ethnicity | |||
| White | 3648 (77.0%) | 8035 (72.3%) | 11683 (73.7%) |
| Black | 520 (11.0%) | 1474 (13.3%) | 1994 (12.6%) |
| Hispanic | 431 (9.1%) | 1164 (10.5%) | 1595 (10.1%) |
| Other | 136 (2.9%) | 439 (4.0%) | 575 (3.6%) |
| Lung Disease Diagnostic Group | |||
| A: Obstructive | 796 (16.8%) | 1298 (11.7%) | 2094 (13.2%) |
| B: Vascular | 239 (5.0%) | 618 (5.6%) | 857 (5.4%) |
| C: Cystic Fibrosis | 699 (14.8%) | 1418 (12.8%) | 2117 (13.4%) |
| D: Restrictive | 2729 (57.6%) | 6905 (62.1%) | 9634 (60.8%) |
| Lung re-transplantation | 202 (4.3%) | 701 (6.3%) | 903 (5.7%) |
| Other | 70 (1.5%) | 172 (1.5%) | 242 (1.5%) |
| 47.5 | 50.5 | 49.6 | |
| Allocation LAS at time of match | (38.6, 71.2) | (40.9, 72.4) | (40.2, 71.8) |
| Identical blood type | 4547 (96.0%) | 10906 (98.1%) | 15453 (97.5%) |
| Mechanical ventilation at listing | 254 (5.4%) | 474 (4.3%) | 728 (4.6%) |
| ECMO at listing | 117 (2.5%) | 132 (1.2%) | 249 (1.6%) |
| Bilateral lung preference at listing | 3973 (83.9%) | 9588 (86.3%) | 13561 (85.6%) |
| Prospective crossmatch requirement | 519 (11.0%) | 1958 (17.6%) | 2477 (15.6%) |
| Donor to candidate predicted TLC ratio | 1.0 (0.2) | 1.1 (0.3) | 1.1 (0.3) |
| Donor age | 30 (21.0, 44.0) | 33 (22.0, 47.0) | 32 (22.0, 46.0) |
| Donor female sex | 2143 (45.3%) | 4231 (38.1%) | 6374 (40.2%) |
| Donor lung PO2 on 100% | |||
| <= 225 | 1157 (24.4%) | 2604 (23.4%) | 3761 (23.7%) |
| >225 - 350 | 453 (9.6%) | 1142 (10.3%) | 1595 (10.1%) |
| 350 - 475 | 1752 (37.0%) | 4140 (37.3%) | 5892 (37.2%) |
| >475 | 1373 (29.0%) | 3226 (29.0%) | 4599 (29.0%) |
| Donor positive tobacco history | 327 (6.9%) | 1082 (9.7%) | 1409 (8.9%) |
| Donor confirmed pulmonary infection | 1847 (39.0%) | 4377 (39.4%) | 6224 (39.3%) |
| Death mechanism | |||
| Seizure | 52 (1.1%) | 118 (1.1%) | 170 (1.1%) |
| Drug Intoxication | 249 (5.3%) | 717 (6.5%) | 966 (6.1%) |
| Asphyxiation | 174 (3.7%) | 442 (4.0%) | 616 (3.9%) |
| Cardiovascular | 299 (6.3%) | 750 (6.7%) | 1049 (6.6%) |
| Gunshot Wound | 909 (19.2%) | 2035 (18.3%) | 2944 (18.6%) |
| Blunt Injury | 1159 (24.5%) | 2747 (24.7%) | 3906 (24.6%) |
| Other | 79 (1.7%) | 153 (1.4%) | 232 (1.5%) |
| Anoxia | 80 (1.7%) | 209 (1.9%) | 289 (1.8%) |
| Cerebrovascular/Stroke | 1601 (33.8%) | 3600 (32.4%) | 5201 (32.8%) |
| Head Trauma | 102 (2.2%) | 275 (2.5%) | 377 (2.4%) |
| CNS Tumor | 31 (0.7%) | 66 (0.6%) | 97 (0.6%) |
| Donation after circulatory death | 78 (1.6%) | 265 (2.4%) | 343 (2.2%) |
| Donor ethnicity | |||
| White | 2837 (59.9%) | 6922 (62.3%) | 9759 (61.6%) |
| Black | 926 (19.6%) | 2099 (18.9%) | 3025 (19.1%) |
| Hispanic | 759 (16.0%) | 1624 (14.6%) | 2383 (15.0%) |
| Other | 213 (4.5%) | 467 (4.2%) | 680 (4.3%) |
| Donor positive history of diabetes | 257 (5.4%) | 856 (7.7%) | 1113 (7.0%) |
| Donor PHS increased infectious risk | 529 (11.2%) | 1535 (13.8%) | 2064 (13.0%) |
| Donor history of hypertension | 1042 (22.0%) | 2638 (23.8%) | 3680 (23.3%) |
| Share type | |||
| Common OPO | 1072 (22.6%) | 2244 (20.2%) | 3316 (20.9%) |
| OPO | 2747 (58.0%) | 5918 (53.3%) | 8665 (54.7%) |
| Zone A | 878 (18.5%) | 2775 (25.0%) | 3653 (23.1%) |
| Zone B | 22 (0.5%) | 139 (1.3%) | 161 (1.0%) |
| Zone C | 10 (0.2%) | 26 (0.2%) | 36 (0.2%) |
| Zone D | 6 (0.1%) | 10 (0.1%) | 16 (0.1%) |
| Share type | |||
| OPO | 3819 (80.7%) | 8162 (73.5%) | 11981 (75.6%) |
| Regional Share | 916 (19.3%) | 2950 (26.5%) | 3866 (24.4%) |
| OPO Competition | |||
| 1 Center | 1906 (40.3%) | 4244 (38.2%) | 6150 (38.8%) |
| 2 Centers | 1313 (27.7%) | 3395 (30.6%) | 4708 (29.7%) |
| 3 Centers | 1516 (32.0%) | 3473 (31.3%) | 4989 (31.5%) |
Demographics reported based on acceptance or rejection of first-ranked offer. Continuous variables reported as Median (IQR); Categorical variables described as N (%). Continuous variables compared using the Student’s t-test; Categorical variable compared using X2 test. LAS: Lung Allocation Score, ECMO: Extra-Corporeal Membrane Oxygenation, CNS: Central Nervous System, PHS: Public Health Service, OPO: Organ Procurement Organization
Variability of Per-Center Organ-Offer Acceptance Rate
The unadjusted rate at which centers accepted a first-ranked offer for a candidate on their waitlist varied significantly, from 9% to 72%. The unadjusted per-center organ offer acceptance rate is depicted in Figure 2, with centers categorized by UNOS region and each representative point scaled by the number of first-rank offers that center received during the study period. As described in methods, our model was then fit to adjust for the pre-specified donor-, candidate-, and geographic factors to establish the per-center adjusted organ-offer acceptance rate. A significant listing center effect was detected after adjusting for confounders of offer acceptance, indicating offer acceptance rates differed significantly across centers (Wald Chi-square = 701.3, df = 64, p<0.001). In the sensitivity analysis that treated listing center as a random effect, we also detected significant center variability by rejecting the null hypothesis that the variance of the random center effect was zero (likelihood ratio test p<0.001), which was consistent with the main analysis. Using estimates of variance components, approximately 20.7% of the variability in the offer acceptance rate was accounted for by the unobserved center-specific attributes that were not adjusted for in the model. See supplemental table 1 for complete regression results.
Figure 2.
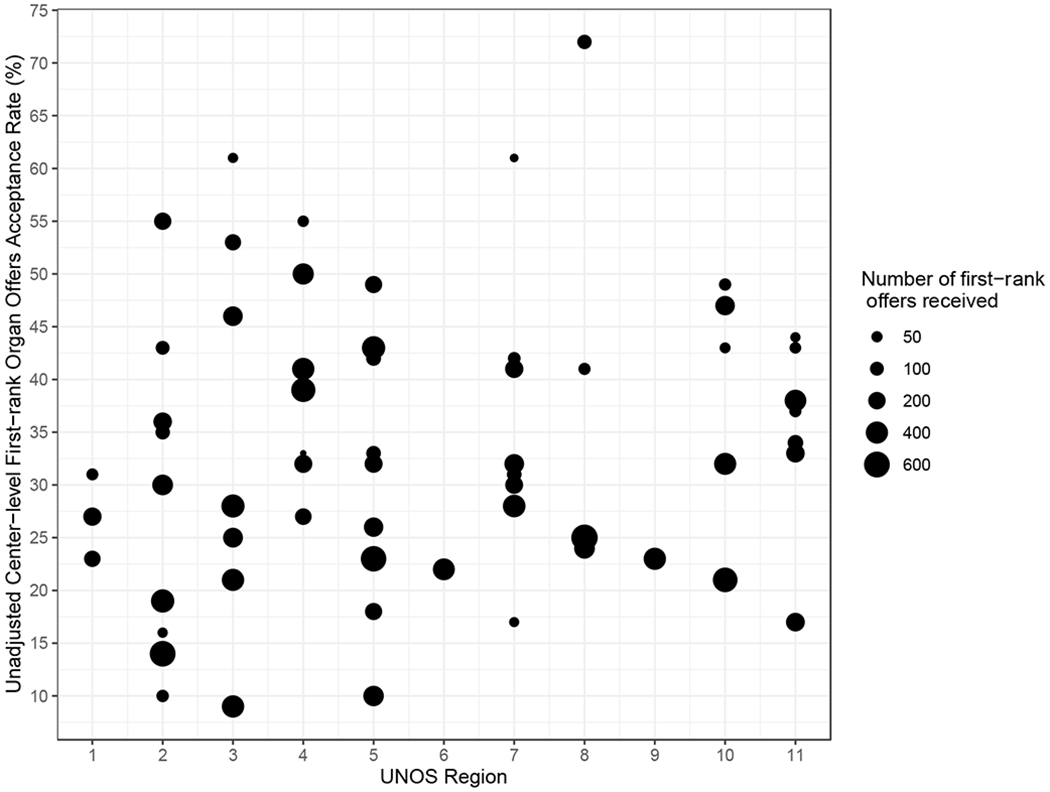
Distribution of unadjusted per-center offer acceptance rate. Centers are stratified by UNOS region. The diameter of each circle corresponds to the number of offers received by the program during the study period.
Analysis of Per-Center Waitlist Mortality and Transplantation
The 1-year unadjusted cumulative incidence of transplantation for the entire cohort was 82.97% (95% CI 82.13 to 83.78%), and for waitlist mortality was 10.9% (95% CI 10.22 to 11.58%). Across centers, unadjusted waitlist mortality ranged from 0.03% to 37%. Figure 3 demonstrates the observed relationship between unadjusted per-center acceptance rate and per-center 1-year cumulative incidence of waitlist mortality. Adjusted center acceptance rate was examined as a continuous variable in the subdistribution hazard model of waitlist mortality. A 10% increase in adjusted center acceptance rate was associated with a 36.3% decrease in the risk of waitlist mortality (subdistribution hazard ratio 0.64, 95% CI 0.59 to 0.69). Similarly, we fit the subdistribution hazards model for transplantation at one year. An increase of 10% in adjusted center acceptance rate increased the likelihood of transplantation by 29.5% (subdistribution hazard ratio (SHR 1.30, 95% CI 1.27 to 1.33). To better facilitate interpretability, centers were then stratified by quartiles of adjusted acceptance rate to further report the impact of per-center acceptance rate on waitlist mortality. The cumulative incidence function of waitlist mortality, stratified by quartiles of per-center adjusted acceptance rate, is depicted in Figure 4. The incidence of waitlist mortality varied significantly by center acceptance rate, with an estimate of 1-year waitlist mortality of 20.2% (95% CI 18.3% to 22.4%) for candidates at centers with an acceptance rate < 25%, to 4% (95% CI 3% to 5.4%) for candidates at centers with an acceptance rate > 40% (P < 0.001 by Gray’s test). See supplemental tables 2 and 3 for complete regression results. Similarly, the incidence of transplantation varied significantly by acceptance rate, as depicted in Figure 5. The cumulative incidence of transplantation, stratified by per-center adjusted acceptance rate, is reported in Figure 6. The 1-year rate of transplantation ranged from 71.3% (95% CI 69.5% to 73.2%) for programs with an adjusted acceptance rate < 25%, to 90.9% (95% CI 89.9% to 91.9%) for centers with an adjusted acceptance rate ≥40% (P < 0.001 by Gray’s test).
Figure 3.
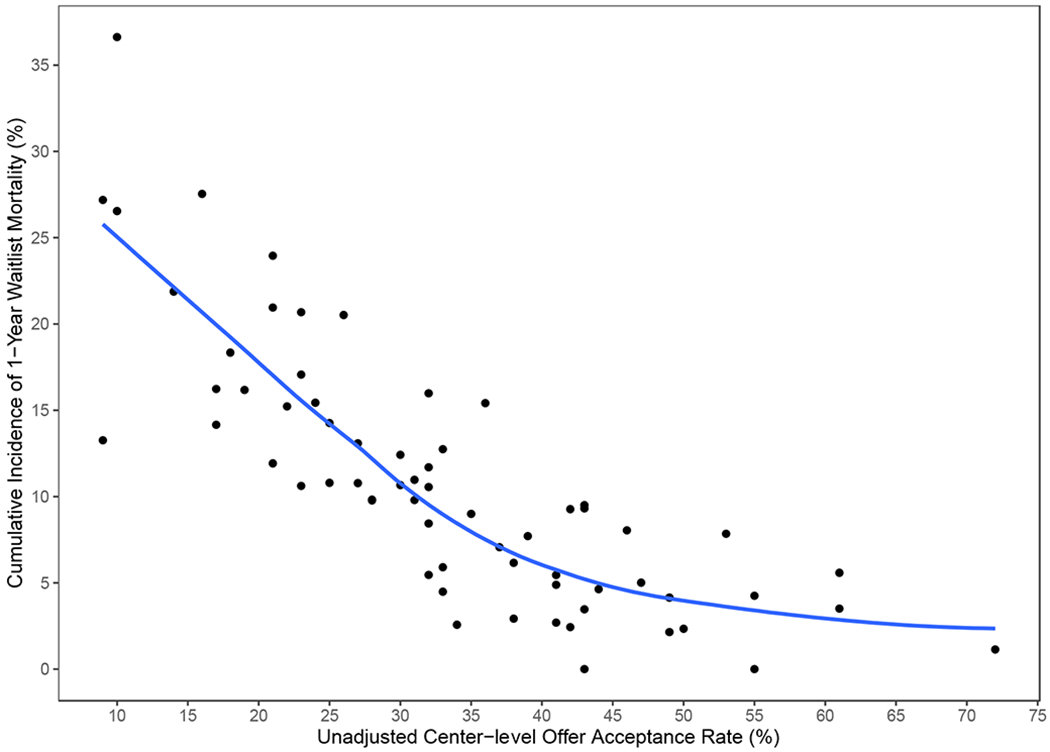
Relationship between per center acceptance rate and per center 1-year cumulative incidence of waitlist mortality. LOESS (locally weighted scatterplot smoothing) curve is added to the plot to help visualize the relationship.
Figure 4.
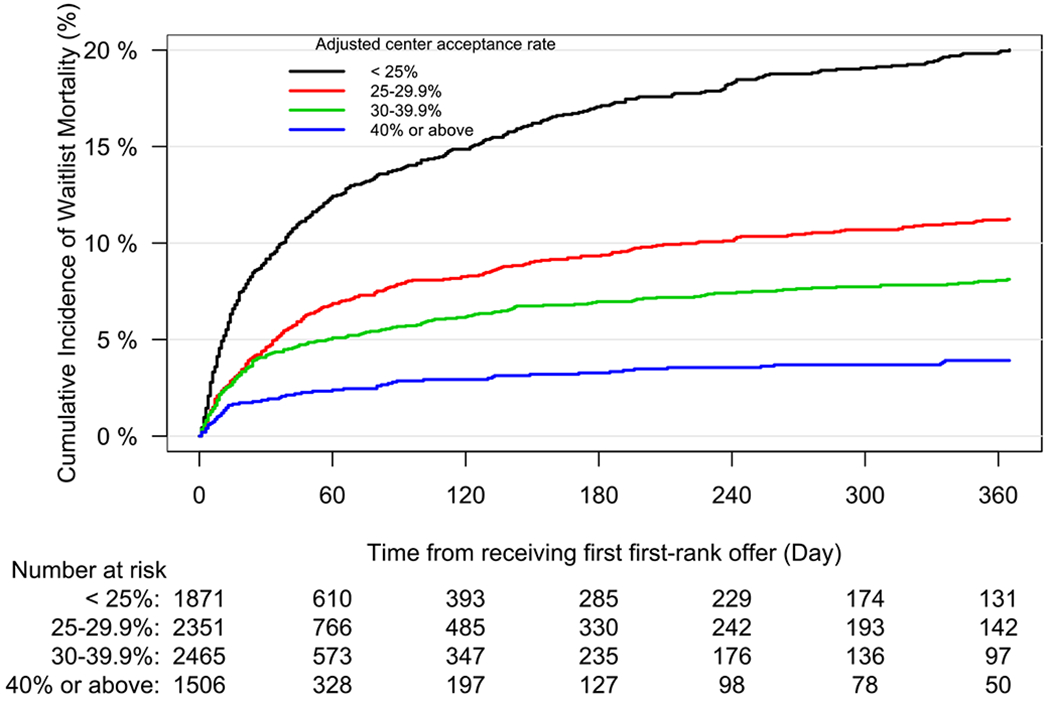
Cumulative incidence function of waitlist mortality for candidates listed for lung transplantation, stratified by listing center adjusted acceptance rate. Table reflects the number of candidates at risk.
Figure 5.
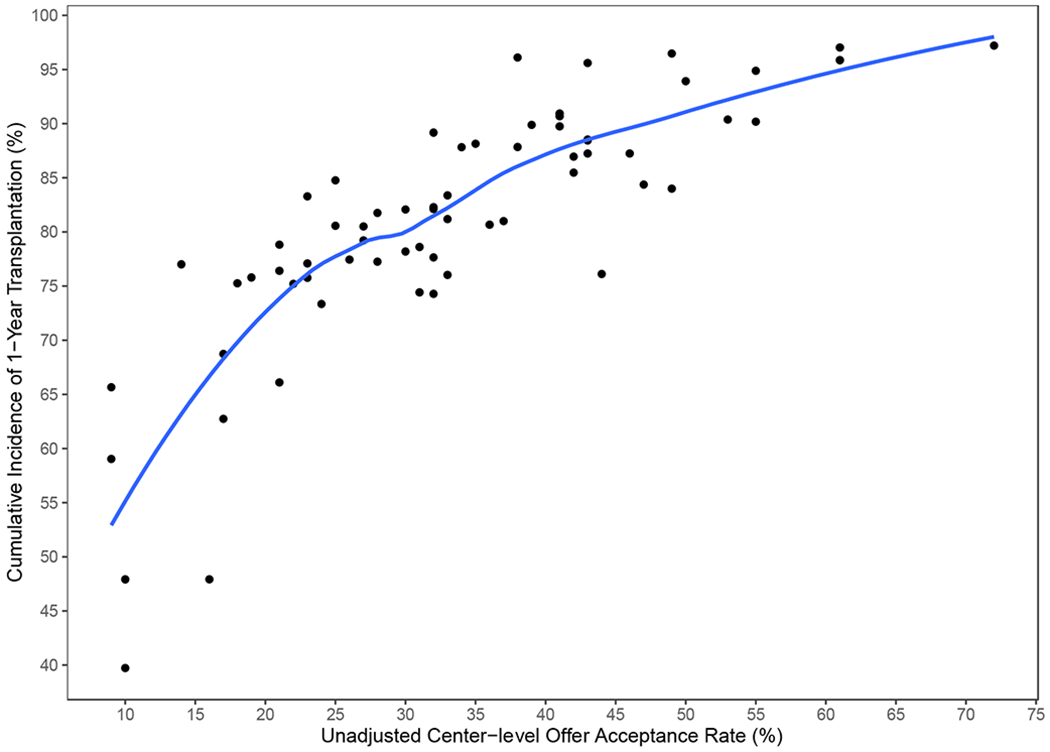
Relationship between per center acceptance rate and per center 1-year cumulative incidence of transplantation. LOESS (locally weighted scatterplot smoothing) curve is added to the plot to help visualize the relationship.
Figure 6.
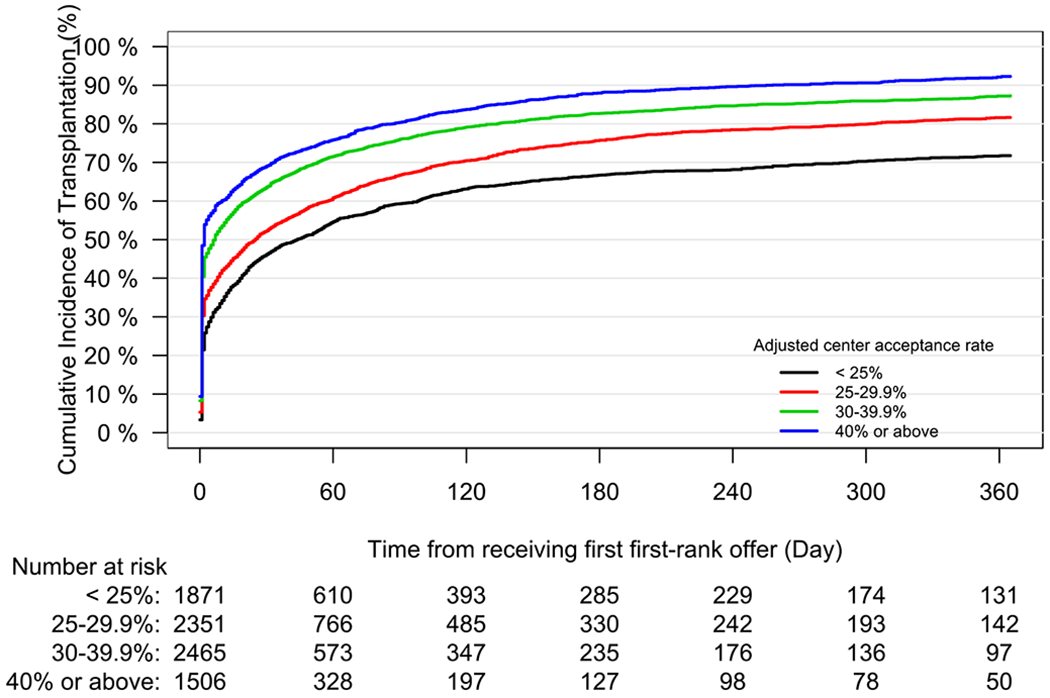
Cumulative incidence function of transplantation for candidates listed for lung transplantation, stratified by listing center adjusted acceptance rate. Table reflects the number of candidates at risk.
Post-Transplantation Outcomes
The 1-, 3-, 5-year unadjusted cumulative incidences of graft failure were 4.5% (95% CI 3.9% to 5.1%), 12.2% (95% CI 11.2% to 13.3%), and 17.5% (95% 16.3% to 18.8%), respectively, for allografts accepted at the first position. These are similar to the 1-, 3-, 5-year unadjusted cumulative incidence of graft failure of 4.3% (95% CI 4.0% to 4.7%), 11.7% (95% CI 11.0% to 12.4%), and 17.0% (95% CI 16.2% to 17.8%), respectively, for allografts accepted at later positions. There was no significant difference in the 5-year cumulative incidence function of graft failure between allografts accepted by first-rank and lower-rank recipients (Gray’s test, P = 0.41). The Fine-Gray model described in methods was fit using complete cases (15455 of 15847 allografts). The adjusted subdistribution hazard ratio of graft failure between allografts accepted at the first-rank vs lower-rank positions was 1.03 (95% CI 0.94 to 1.14, P = 0.51, supplemental table 4), suggesting no significant difference in the risk of graft failure between allografts accepted at the first-rank and lower-rank sequences. The adjusted cumulative incidence function of graft failure by sequence priority at transplantation is shown in Supplementary Figure 1. The adjusted event-free survival function, stratified by allograft acceptance at the first-rank or lower-rank position is shown in Supplementary Figure 2 (supplementary table 5).
Discussion
Cardiothoracic transplantation is appropriately one of the most closely-scrutinized disciplines in medicine. The combination of a scarce resource and a medically-comorbid patient population demands that outcomes at both the local and national levels be closely measured. In this retrospective analysis of US-based registry data, we find that organ offer acceptance patterns vary significantly by transplant center, a variation that contributes significantly to observed per-center differences in waitlist mortality for candidates awaiting lung transplantation. These data have important ramifications for candidate-, center-, and national-level decision-making with respect to the optimal allocation and standardization of practices in thoracic transplantation. Specifically, center-level organ-offer acceptance practices offers an avenue to elicit meaningful improvements to the distribution of lung allografts to candidates awaiting transplantation.
In the current Lung Allocation System, allografts are offered to candidates based on consideration of the risk of waitlist mortality and expected post-transplantation benefit. The most salient effect of the LAS has been a reduction in the rate of waitlist mortality for candidates awaiting lung transplantation, while more modest impacts in post-transplant survival for recipients have been reported(8, 16–18). When a candidate receives an offer of a lung allograft, the candidate (or the center acting on his or her behalf) must select between accepting the offer, or declining and awaiting a later offer. The data reported here demonstrate that for a significant fraction of candidates, those that decline a first-ranked offer never receive a subsequent first-ranked offer (38.1%), and many do not proceed to transplantation at any time. Given that outcomes between candidates that accepted a first-ranked offer do not differ significantly from those that accept an allograft at a lower position (allografts declined by higher-priority candidates), these data suggest that broader standardization of offer acceptance practices may contribute meaningfully to the reduction of waitlist mortality by re-directing allografts to the highest-priority candidates. These findings reflect those recently reported with respect to the decline of specific increased-risk donors in both heart and lung transplantation(19, 20).
Assessment of outcomes that include time points in the pre-transplant setting highlights the need to account for candidates that, while cared for by the organ-failure team and potentially considered for transplantation, do not proceed with transplantation. Measures such as an “intent to treat” approach may likewise better account for the work that transplant centers perform in the pre-transplant setting(21). The SRTR has begun making offer-acceptance data available to lung transplant programs in order to permit objective assessment of these practices(22). As these measures are further emphasized by the transplant community, individual centers must likewise incorporate these measures in to clinical practice.
The variability observed in the acceptance and decline of offers in this study speaks to the latitude that transplant programs may take in considering allografts suitable for transplantation(23–27). While certain donor-candidate relationships may impact the suitability of an allograft for a particular candidate recipient, these data demonstrate that in the current era of lung transplantation significant differences still exist in the decision to accept an allograft for transplantation. As these behaviors are (1) modifiable at the center level, and (2) lead to the distribution of allografts to lower-priority candidates, targeted intervention may help to standardize acceptance practices and improve the efficacy of organ allocation under the current system(4, 28). Establishment of better consensus of “acceptable” allografts may then further help optimize the allocation of allografts in the US. Similarly, increased reporting of center-level acceptance rates by the SRTR (and associated changes in response to that reporting) will likely help to identify what factors currently un-reported to the registry impact acceptance rates.
Several limitations bear review in consideration of this analysis. As with all retrospective studies employing large national databases, there exists the possibility of potential unmeasured confounders for which we cannot account. The data source is US-specific, and as such, these data may be most applicable to settings with similar donor, recipient, and organ allocation features. Several limitations specific to organ offer acceptance data bear consideration: first, organ offer data are presently only available for offers that are eventually accepted, and second, the ability of programs to screen-out certain offers from match runs based on donor characteristics may alter program-level acceptance practices without necessarily impacting overall candidate access to transplantation. That all offers examined here were employed for transplantation suggests that at least one center determined that the allograft was suitable for transplantation, but unfortunately no parallel conclusion can be made for the un-examined offers declined by all centers. In addition, while justification is provided to UNOS by all centers that decline offers (included in supplemental table 6), these justifications are subjective, lack concordance between centers, and lack adequate granularity to ascertain the reasons for offer decline. Finally, as alluded to by Wey, et al, higher offer acceptance likely contributes to a decreased incidence of waitlist mortality due to the higher transplant rate, while offer acceptance may not materially impact the per-center rate of waitlist mortality(13). These factors must be considered in future analyses of organ offer practices, particularly as the community considers how best to judge programs based on their offer acceptance practices.
These data support the conclusion that despite general consensus across many facets of donor selection in lung transplantation, there persists significant variability in organ offer acceptance practices across transplant centers in the United States, a relationship which contributes significantly to observed differences in the rate of transplantation and incidence of waitlist mortality at the center level. Organ offer acceptance practices represent a distinctly modifiable behavior, such that further examination of these practices across centers may help to better re-distribute lung allografts to the highest-priority candidates and reduce waitlist mortality, and thereby improve both the justice and efficacy of the Lung Allocation System as presently implemented. Moving forward, center-level performance would be best judged in the context of overall performance that considers outcomes of both candidates and recipients.
Supplementary Material
Acknowledgements
The authors report no relevant conflicts of interests as described by ICMJE.
MSM is supported by the National Heart, Lung, and Blood Institute F32HL132460-02.
This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (MLC), 5U01HL088953-05 and the National Institutes of Health TL-1 clinical and translational science award (CTSA), UL1TR0025531 (NCATS).
Abbreviations
- PTR
Potential Transplant Recipient
- OPO
Organ Procurement Organization
- OPTN
Organ Procurement Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
- LAS
Lung Allocation Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valapour M, Skeans MA, Smith JM, Edwards LB, Cherikh WS, Uccellini K, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Lung. Am J Transplant 2017; 17 Suppl 1: 357–424. [DOI] [PubMed] [Google Scholar]
- 2.Singer JP, Katz PP, Soong A, Shrestha P, Huang D, Ho J, Mindo M, Greenland JR, Hays SR, Golden J, Kukreja J, Kleinhenz ME, Shah RJ, Blanc PD. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant 2017; 17: 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vock DM, Durheim MT, Tsuang WM, Finlen Copeland CA, Tsiatis AA, Davidian M, Neely ML, Lederer DJ, Palmer SM. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc 2017; 14: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pullen LC. Lung Allocation Sequence: The System Evolves. Am J Transplant 2018; 18: 1037–1038. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuto LJ, Anderson DR, Kim HP, Hook JL, Shah L, Robbins HY, D’Ovidio F, Bacchetta M, Sonett JR, Arcasoy SM, From the Columbia University Lung Transplant P. Geographic disparities in donor lung supply and lung transplant waitlist outcomes: A cohort study. Am J Transplant 2017. [DOI] [PubMed] [Google Scholar]
- 6.Sell JL, Bacchetta M, Goldfarb SB, Park H, Heffernan PV, Robbins HA, Shah L, Raza K, D’Ovidio F, Sonett JR, Arcasoy SM, Lederer DJ. Short Stature and Access to Lung Transplantation in the United States. A Cohort Study. Am J Respir Crit Care Med 2016; 193: 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberlein M, Hunsicker L, Reed RM. Short Stature and Access to Lung Transplantation: Reducing Disparities by Changing to a Lung Size-based Allocation Mechanism. Am J Respir Crit Care Med 2016; 194: 642–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford TC, Grimm JC, Magruder JT, Ha J, Sciortino CM, Kim BS, Bush EL, Conte JV, Higgins RS, Shah AS, Merlo CA. Lung Transplant Mortality Is Improving in Recipients With a Lung Allocation Score in the Upper Quartile. Ann Thorac Surg 2017; 103: 1607–1613. [DOI] [PubMed] [Google Scholar]
- 9.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, Snell GI, Verleden GM, Zamora MR, Glanville AR. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34: 1–15. [DOI] [PubMed] [Google Scholar]
- 10.Snell GI, Paraskeva M, Westall GP. Donor selection and management. Seminars in respiratory and critical care medicine 2013; 34: 361–370. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DS, French B, Lewis JD, Scott FI, Mamtani R, Gilroy R, Halpern SD, Abt PL. Liver transplant center variability in accepting organ offers and its impact on patient survival. Journal of hepatology 2016; 64: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wey A, Salkowski N, Kasiske BL, Israni AK, Snyder JJ. Influence of kidney offer acceptance behavior on metrics of allocation efficiency. Clinical transplantation 2017; 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wey A, Valapour M, Skeans MA, Salkowski N, Colvin M, Kasiske BL, Israni AK, Snyder JJ. Heart and lung organ offer acceptance practices of transplant programs are associated with waitlist mortality and organ yield. Am J Transplant 2018; 18: 2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014; 14: 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014; 43: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016; 35: 433–439. [DOI] [PubMed] [Google Scholar]
- 17.Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, Shah AS. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant 2009; 28: 769–775. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, Garrity E. The Impact of the Lung Allocation Score. Seminars in respiratory and critical care medicine 2010; 31: 108–114. [DOI] [PubMed] [Google Scholar]
- 19.Mulvihill MS, Cox ML, Bishawi M, Osho AA, Yerokun BA, Wolfe CR, DeVore AD, Patel CB, Hartwig MG, Milano CA, Schroder JN. Decline of Increased Risk Donor Offers on Waitlist Survival in Heart Transplantation. J Am Coll Cardiol 2018; 72: 2408–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox ML, Mulvihill MS, Choi AY, Bishawi M, Osho AA, Haney JC, Daneshmand M, Klapper JA, Wolfe CR, Hartwig M. Implications of declining donor offers with increased risk of disease transmission on waiting list survival in lung transplantation. J Heart Lung Transplant 2019; 38: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado DA, RoyChoudhury A, Lederer DJ. A novel patient-centered “intention-to-treat” metric of U.S. lung transplant center performance. Am J Transplant 2018; 18: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder JJ, Salkowski N, Zaun D, Leppke SN, Leighton T, Israni AK, Kasiske BL. New quality monitoring tools provided by the Scientific Registry of Transplant Recipients: CUSUM. Am J Transplant 2014; 14: 515–523. [DOI] [PubMed] [Google Scholar]
- 23.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, Matthay M. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 2002; 360: 619–620. [DOI] [PubMed] [Google Scholar]
- 24.Van Raemdonck D, Neyrinck A, Verleden GM, Dupont L, Coosemans W, Decaluwe H, Decker G, De Leyn P, Nafteux P, Lerut T. Lung donor selection and management. Proc Am Thorac Soc 2009; 6: 28–38. [DOI] [PubMed] [Google Scholar]
- 25.Meers C, Van Raemdonck D, Verleden GM, Coosemans W, Decaluwe H, De Leyn P, Nafteux P, Lerut T. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transplant international : official journal of the European Society for Organ Transplantation 2010; 23: 628–635. [DOI] [PubMed] [Google Scholar]
- 26.Verleden SE, Martens A, Ordies S, Heigl T, Bellon H, Vandermeulen E, Van Herck A, Sacreas A, Verschakelen J, Coudyzer W, Van Raemdonck DE, Vos R, Weynand B, Verleden GM, Vanaudenaerde B, Neyrinck A. Radiological Analysis of Unused Donor Lungs: A Tool to Improve Donor Acceptance for Transplantation? Am J Transplant 2017; 17: 1912–1921. [DOI] [PubMed] [Google Scholar]
- 27.Peritore D, Rizzato L, Trapani S, Morabito V, Fiaschetti P, Cacciotti A, Montemurro A, Del Sordo E, Ricci A, Nanni Costa A. Optimizing the Use of Available Lungs. Transplantation proceedings 2017; 49: 692–694. [DOI] [PubMed] [Google Scholar]
- 28.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL. Development of the New Lung Allocation System in the United States. Am J Transplant 2006; 6: 1212–1227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


