Abstract
Rationale and Objective:
Patients on dialysis report very low physical activity. We implemented a pilot trial to assess the feasibility of a pedometer-based intervention to gather preliminary evidence about its impact on physical activity, symptoms, and surrogates of cardiovascular risk.
Study Design:
Pilot randomized controlled trial.
Setting and Participants:
Sixty dialysis patients from San Francisco dialysis clinics.
Intervention:
Participants were randomly assigned 1:1 to receiving pedometers with weekly step goals or usual care for 3 months.
Outcomes:
The primary outcome was step counts, measured using pedometers. Secondary outcomes included physical performance using the Short Physical Performance Battery, the Physical Function and Vitality scales of the SF-36, the Dialysis Symptoms Index, and the Center for Epidemiologic Studies–Depression, with endothelial function as a secondary and heart rate variability (HRV) as an exploratory surrogate measure of cardiovascular risk. Targeted enrollment was 50% and targeted completion was 85%.
Results:
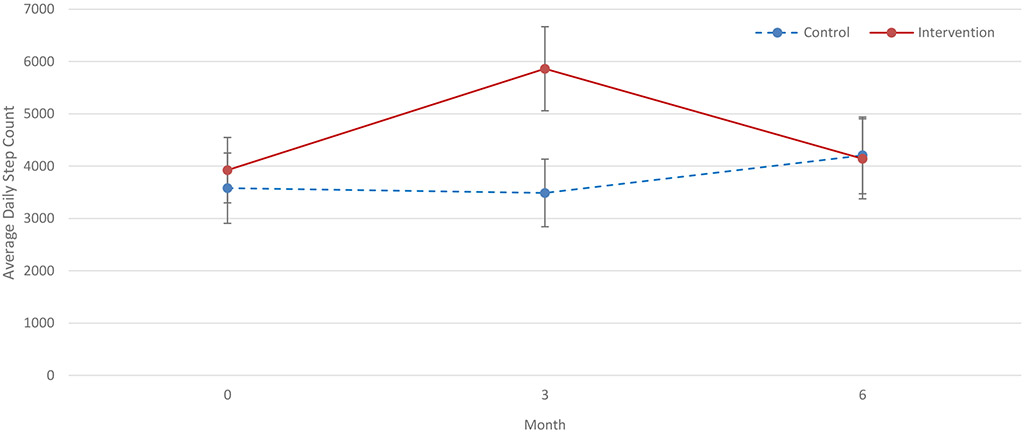
Forty-nine percent of approached patients were enrolled, and 92% completed the study. After 3 months, patients randomized to the intervention (n=30) increased their average daily steps by 2,256 (95% CI, 978-3537) more than the 30 controls (P<0.001). HRV (standard deviation of N-N intervals) increased by 14.94 ms (95% CI, 0.31-33.56; P=0.05) in the intervention group as compared to controls. There were no statistically significant differences across the intervention groups in symptoms, physical performance, or endothelial function. Participants in the intervention group reverted to baseline steps during the post-intervention follow-up.
Limitations:
The Northern California study setting may limit generalizability. Walking does not capture the full spectrum of physical activity.
Conclusion:
A short-term pedometer-based intervention led to increased step counts in dialysis patients, but the increase was not sustained. Pedometer-based interventions are feasible for dialysis patients, but future studies are needed to address whether more prolonged interventions can improve physical function or symptoms.
Funding:
Supported by grants from AKF, NIH-NIDDK, and ISN.
Trial Registration:
Registered at ClinicalTrials.gov with study identifier NCT02623348.
Keywords: physical activity, physical function, dialysis, symptoms, quality of life (QoL), walking, exercise, sedentary lifestyle, physical function, step counts, pedometer, physical performance, activity patterns, end-stage renal disease (ESRD), heart rate variability (HRV), endothelial function, randomized controlled trial (RCT)
Introduction
Patients treated with dialysis report very low levels of total physical activity (including activity as part of daily living and purposeful exercise) that are well below recommended1-3 levels and even below levels reported by many healthy sedentary individuals, by as much as 35%.4-6 These extremely low levels of activity are associated with poor functional status and higher mortality,7,8 even when compared to patients who are more active but still low.9 Patients on dialysis also have impaired physical function10,11 and experience a heavy burden of symptoms including fatigue, weakness, and muscle cramps.12-14 These symptoms are worse among more sedentary patients,15,16 and it is possible that increasing activity could alleviate them. It is also possible that increasing activity could decrease cardiovascular events in this high-risk population.
Studies of moderate or vigorous exercise training in patients treated by maintenance hemodialysis have demonstrated improvement in physical functioning and symptoms.17-19 However, only a fraction of patients have been willing and able to participate in these interventions,11 and patients who are inactive and/or unable to participate in more vigorous interventions may be those who could benefit most.17 It is not clear that vigorous interventions are needed. In fact, less vigorous interventions may be more accessible to a larger number of dialysis patients.
We conducted a 3-month randomized controlled trial comparing pedometers and weekly step goals to usual care among 60 patients treated with dialysis, with a 3-month post-intervention follow up without counselling or pedometers to study maintenance of effect. The study was a pilot to determine feasibility and participation rates and a preliminary efficacy trial to examine whether patients in the pedometer group would increase their step counts and whether any increases would be sustained after the intervention. To gather preliminary evidence about whether this less vigorous intervention would be sufficient to provide cardiovascular benefit, we also examined changes in surrogate measures of cardiovascular risk including endothelial function and heart rate variability (HRV).
Methods
Inclusion and exclusion criteria
We enrolled patients from three San Francisco dialysis clinics. Inclusion criteria were age ≥18 years, receiving in-center hemodialysis (HD) or any form of peritoneal dialysis (PD), having telephone access, and being ambulatory. Patients using a cane or other assistive device were eligible, but those using wheelchairs or scooters were excluded. Patients provided informed consent to participate. The study was approved by the UCSF Committee on Human Research (14-13175) and was registered at ClinicalTrials.gov ( NCT02623348).
Baseline testing
Participants were asked their race and ethnicity, and medical records were reviewed for information about dialysis prescription, laboratory results, comorbid conditions, and medications.
Primary Outcome
The primary outcome was measured step count at 3 months after the beginning of the intervention using pedometers (Accusplit AE120, Livermore, CA).4,20-22 Patients were asked to wear the pedometer at their waist continuously during waking hours for one week and to record their daily steps in a diary. Step counts were relayed to study personnel in person or by telephone.
Secondary outcomes
Secondary outcomes, also measured at 3 months after the beginning of the intervention, included physical performance using the Short Physical Performance Battery, the Physical Function and Vitality scales of the SF-36, the Dialysis Symptoms Index, and the Center for Epidemiologic Studies –Depression, with endothelial function as a secondary and heart rate variability (HRV) as an exploratory surrogate measure of cardiovascular risk. Physical function was assessed immediately prior to a mid-week HD session or on the day of a regularly scheduled PD clinic visit using the Short Performance Physical Battery (SPPB),23 an objective assessment of lower extremity function, and the Physical Functioning (PF) Scale of the SF-36.24
We used the Vitality scale from the SF-3625 as well as the modified Dialysis Symptoms Index (DSI).26 We also assessed depressive symptoms using the Center for Epidemiologic Studies –Depression instrument (CES-D).27
Endothelial function was measured noninvasively as the reactive hyperemia index with peripheral arterial tonometry (RHI-PAT) using the EndoPAT 2000 (Itamar Medical), according to their published protocols.28 Measurement was performed on a digital artery in the non-access hand for HD patients.
Heart rate variability was measured during the five minutes of baseline recording for endothelial function testing during which we also ascertained the SDNN (standard deviation of NN intervals on an electrocardiography waveform) and the LF/HF (ratio of low-to-high frequency power) as measures of HRV.29 Patients who had baseline arrhythmia were excluded from analyses of HRV (n=10). Complete methods are included in the supplementary material (S1).
Randomization
Patients were randomly assigned to participate in a 3-month intervention program or control group in a 1:1 ratio, stratified by dialysis modality. We targeted enrollment of 12 PD patients and 48 HD patients. This sample size was chosen to provide 80% power to detect an increase of 1,000 steps or greater in the intervention group compared to the control group despite predicted levels of dropout (assuming a standard deviation of 1178.5 steps per day),30 which we felt would be clinically significant because differences in activity even below recommended levels are associated with better outcomes.9 Randomization was performed using the website Randomization.com using variable block sizes from 2-6, with assignments placed into sequentially-numbered opaque envelopes that were opened by study personnel and assigned after recruitment and baseline assessment.
Intervention
Our intervention consisted of providing pedometers in conjunction with weekly semi-scripted counselling sessions in which a member of the study team called the participant at a scheduled time each week. Participants in the intervention group were asked to continue wearing their pedometers and to record their step counts for 3 months. During the weekly counselling session, participants reported their step counts, and research personnel provided specific step goals for the upcoming week and advised about ways to incorporate more walking into participants’ daily routine. Patients were encouraged to walk at a comfortable pace. The first counselling session took place one week after baseline assessment and subsequent randomization.
We recommended that participants in the intervention group increase their steps by 10% compared to the prior week. If patients did not meet their weekly target, we did not set a higher target for the subsequent week. For patients who had periods of reduced activity (e.g., after hospitalizations), we revised their goals (i.e., increasing in 10% increments of their new “baseline” daily steps).
Patients in the control group were asked to return the pedometers after recording steps during the initial week of data collection and were not contacted during the intervention portion of the study.
After the 3-month assessment, pedometers were returned to study personnel by both groups. In order to study whether any gains in walking were maintained without active intervention, we measured step counts and our other outcomes again after an additional 3 months.
Safety Monitoring and Adverse Events:
Participants were given contact information for study personnel to report any issues or concerns and were asked about any issues in the preceding week at the time of their weekly session. Patients’ nephrologists and dialysis clinic staff were informed of patients’ participation at the time of enrollment.
Statistical Analysis
Patients’ baseline characteristics were summarized as median (IQR) for continuous variables or frequency and percentage for categorical variables. For step counts, we calculated average daily steps over the week prior to each assessment for each participant and reported the mean of those average daily steps. Other outcomes were reported as mean ±SD for each time period. The primary outcome was between-group difference in change in step count. We used mixed effects linear regression analyses to assess changes at 3 and 6 months for steps, physical performance and function, symptoms, endothelial function, and HRV. We also analyzed an “activity-relevant” subset of the DSI that included symptoms we felt may improve with activity as well as the individual components of that subset.14,16,31-33 We adjusted for the stratification factor (dialysis modality), and sex, in each model. We also examined whether outcomes differed among HD and PD patients in a pre-specified subgroup analysis via a group by subgroup interaction test. We performed post-hoc analyses using linear regression to examine factors associated with percentage of weekly goals achieved, and logistic regression to examine factors associated with achieving the overall step target at 3 months as set after baseline assessment.
Two-sided p-values <0.05 were considered statistically significant. Statistical analyses were performed using Stata, version 14 (StataCorp, College Station, TX).
Results:
Baseline characteristics, step counts, and symptoms
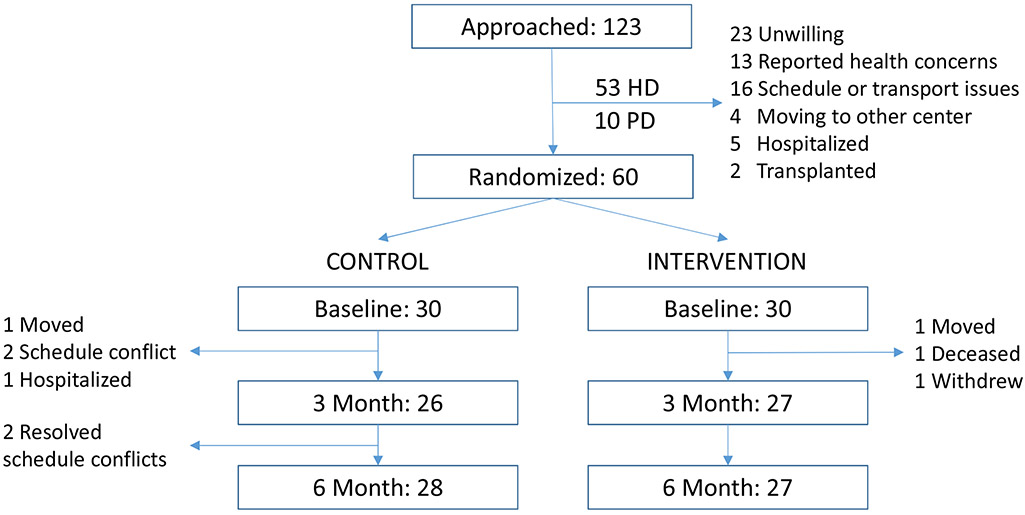
We approached 123 potentially eligible patients in order to reach the target of 48 HD and 12 PD participants (Figure 1). The median age of participants was 58 years (IQR, 53-66), and 78% were men. The racial and ethnic distribution and burden of comorbidity were similar between groups, but there were more men in the intervention group (Table 1). The only potential imbalance between groups was in sex, which we adjusted for in our analysis. Demographic characteristics of those who did not participate were similar to those enrolled in the study (Table S1).
Figure 1. Recruitment and Randomization.
All patients available for follow-up were included in the primary analysis.
Table 1.
Patient Characteristics at Baseline for 48 Hemodialysis Patients and 12 Peritoneal Dialysis Patients
| Characteristic | Control (n=30)* |
Intervention (n=30)* | p-value |
|---|---|---|---|
| Age, years | 56 (51, 65) | 60 (53, 66) | 0.4 |
| Male Sex, % | 63 | 93 | 0.005 |
| Hispanic, % | 17 | 17 | 0.9 |
| Race, % | |||
| White | 17 | 13 | 0.7 |
| Black | 37 | 47 | 0.4 |
| Asian | 20 | 20 | 0.9 |
| Native Hawaiian/Pacific | 10 | 7 | 0.6 |
| Islander | |||
| More than one race | 7 | 7 | 0.9 |
| Unknown/Unreported | 3 | 7 | 0.2 |
| BMI, kg/m2 | 31.6 (26.7, 34.6) | 26.9 (25.3, 32.9) | 0.5 |
| Comorbidities, % | |||
| HTN | 93 | 93 | 0.9 |
| DM | 40 | 33 | 0.6 |
| CAD | 27 | 37 | 0.4 |
| CHF | 27 | 30 | 0.8 |
| Stroke | 13 | 7 | 0.4 |
| Peripheral Vascular | 3 | 13 | 0.2 |
| Disease | |||
| HIV | 3 | 0 | 0.3 |
| Arrhythmia | 13 | 20 | 0.5 |
| Dialysis Vintage, years | 1.9 (0.95, 4.7) | 3.7 (1.5, 7.2) | 0.3 |
| Hemoglobin, g/dL | 10.9 (9.8, 11.7) | 10.6 (9.6, 11.7) | 0.9 |
| Serum Albumin, g/dL | 3.9 (3.7, 4.1) | 3.9 (3.6, 4.1) | 0.6 |
| Std Kt/V | 2.37 (2.1, 2.5) | 2.30 (2.07, 2.44) | 0.6 |
| Education, % | |||
| High School or Less | 37 | 37 | 0.9 |
| Vocational or Some | 25 | 33 | 0.8 |
| College | |||
| College Degree | 20 | 13 | 0.5 |
| Professional or Graduate | 10 | 20 | 0.3 |
| Degree | |||
| Currently smoking, % | 20 | 10 | 0.3 |
| Use of Assistive Device, % | 17 | 30 | 0.2 |
| Cane | 13 | 20 | 0.5 |
| Walker | 3 | 10 | 0.3 |
Data expressed as percentage or Median [IQR] as appropriate.
The mean of participants’ average daily steps at baseline was 3578 ± 3680 in the control group and 3924 ± 3422 in the intervention group (Table S2). Physical function, symptom burden and severity on the DSI, and CESD scores were similar between the groups, but participants in the control group had lower baseline Vitality scores than those in the intervention group. Median endothelial function and indicators of HRV were low in both groups relative to the general population.
Participation and Dropout Rates:
Forty-nine percent of patients approached for the study were enrolled, and 92% of participants completed the study. Ninety percent of patients in the intervention completed the 3-month program, and 83% of all calls were completed as planned. All participants in the intervention who completed the 3-month program went on to complete the 6-month follow-up.
Change in outcomes at three months
As shown in Table 2, at 3 months, patients in the intervention group increased their steps by 2,256 (95% CI, 978-3537) more than participants in the control group (p<0.001). There was no statistically significant difference in change in SPPB score between groups at 3 months. There was also no statistical difference in change in symptom burden or severity on the DSI (including an “activity-relevant” subset of the DSI [Tables S3, S4]), Vitality Scale, or depressive symptoms. SDNN increased nominally, by 14.94 (95% CI, 0.31-33.56) in the intervention group as compared to controls, but this was of borderline statistical significance (p=0.05). There was no statistical difference between groups in the change in LF/HF or in endothelial function. The effect of the intervention did not differ significantly by modality (HD versus PD) for any outcome. Within-group changes are included in Table S5.
Table 2.
Step Counts, Physical Function, Questionnaire Scores, Endothelial Function, and Heart Rate Variability in 48 Hemodialysis Patients and 12 Peritoneal Dialysis Patients
| Difference in change between groups, at 3 mo* |
Difference in change between groups, from 3 to 6 mo* |
Difference in change between groups, from baseline to 6 mo* |
||||
|---|---|---|---|---|---|---|
| Difference (95% CI) |
p- value** |
Difference (95% CI) |
p- value** |
Difference (95% CI) |
p- value** |
|
| Primary outcome | ||||||
| Average Daily Step Count*** | 2256 (978, 3537) | 0.001 | −2294 (−3593, −991) | 0.001 | −34 (−1179, 1111) | 0.9 |
| Symptom or Score † | ||||||
| SPPB Score | −0.04 (−0.8, 0.73) | 0.9 | −0.71 (−1.49, 0.07) | 0.07 | −0.69 (−1.40, 0.02) | 0.06 |
| PF Score | −1.28 (−11.79, 9.23) | 0.8 | −4.37 (−15.0, 6.25) | 0.4 | −9.83 (20.53, 0.86) | 0.07 |
| DSI Total Symptom Burden | 1.32 (−1.33, 3.98) | 0.3 | 2.70 (0.02, 5.38) | 0.05 | 4.90 (2.60, 7.20) | <0.01 |
| DSI Total Symptom Severity | 2.31 (−6.48, 11.1) | 0.6 | 11.28 (2.39, 20.17) | 0.01 | 16.36 (8.35, 24.37) | <0.01 |
| CESD Total Score | 2.54 (−2.14, 7.23) | 0.3 | 0.44 (−4.29, 5.17) | 0.9 | 3.29 (−1.07, 7.66) | 0.1 |
| Vitality Score | 2.83 (−9.98, 15.64) | 0.7 | −13.20 (−26.15, −0.38) | 0.04 | −13.74 (−25.0, −2.53) | 0.02 |
| Surrogate for cardiovascular risk ‡ | ||||||
| RHI | 0.16 (−0.33, 0.65) | 0.5 | −0.29 (−0.35, 0.15) | 0.3 | −0.09 (−0.54, 0.36) | 0.7 |
| SDNN | 14.94 (0.31, 33.56) | 0.05 | −5.13 (−23.75, 13.49) | 0.6 | 9.45 (−5.93, 24.83) | 0.2 |
| LF/HF | −0.12 (−0.96, 0.73) | 0.8 | 0.20 (−0.65, 1.04) | 0.7 | 0.06 (−0.66, 0.78) | 0.9 |
Difference in change between groups modeled through mixed effects linear regression analysis, adjusted for sex and by stratification factor (modality).
p-value for between-group comparison of change, adjusted for sex and by stratification factor (modality).
Average daily step count was calculated by averaging individual daily step counts over the week prior to each assessment.
SPPB, Short Physical Performance Battery; PF, Physical Functioning Scale of the SF-36; DSI, Dialysis Symptom Index; CESD, Centers for Epidemiologic Studies Depression Scale.
Endothelial Function and Heart Rate Variability recorded in 40 HD patients and 10 PD patients without arrhythmia. RHI, Reactive Hyperemia Index; SDNN, Standard Deviation of N-N intervals; LF/HF, Ratio of Low Frequency Power to High Frequency Power.
Change in outcomes from three to six months
During the 3 months after the active intervention phase, patients in the intervention group decreased 2,294 steps per day (95% CI, −3,593 to −991) as compared to controls, returning to baseline step counts. There was no significant between-group difference in change in objective or self-reported physical function from 3 to 6 months. However, changes in total symptom burden and severity on the DSI from 3 to 6 months tended to be greater among those in the intervention group than among controls (difference in change of 2.70 [95% CI, 0.02-5.38] for symptom burden [p=0.05] and 11.28 [95% CI, 2.39-20.17] for symptom severity [p=0.01]). relative to controls, the intervention group also increased fatigue on the DSI (difference in change of 1.26; 95% CI, 0.46, 2.05], p=0.02) and decreased Vitality scores (difference in change of −13.2 [95% CI −26.15, −0.38], p=0.04) from 3 to 6 months. There were no statistically significant difference in changes from 3 to 6 months in any other outcome.
Post-hoc analysis of factors associated with meeting goals in the intervention group
Of the 28 participants who completed the intervention, 11 (37%) were able to reach the overall target of 10,000 steps, or an increase of 10% each week over the entire 12-week intervention. The median percentage of weekly goals achieved by participants was 33% (IQR, 25%-67%).
Factors associated with better achievement of weekly goals were higher baseline step counts (5.9% [95% CI, 3.2%-8.5%] per 1,000 steps greater), SPPB score (7.1% [95% CI, 1.0%-13.3%] per 1-point greater), and PF Score (4.5% [95% CI, 0.5%-8.5%] per 10 points greater) (Table 3). Only baseline step count was associated with a higher odds of meeting target steps at the end of the intervention (OR of 1.29 [95% CI, 1.00-1.65] per 1,000 steps greater) (Table 4).
Table 3.
Post-hoc Linear Regression Analysis of Factors Associated with Meeting Weekly Goals (N=30)
| Baseline characteristic* | Difference in weekly goal completion (95% CI)** |
p-value |
|---|---|---|
| Age, per 10 y older | −0.4% (−1.4, 0.5) | 0.3 |
| Dialysis vintage, per 1-y older | −1.6% (−5.0, 1.7) | 0.3 |
| Hemoglobin, per 1-g/dL greater | 0.04% (−2.6, 2.7) | 0.9 |
| Serum Albumin, per 1-g/dL greater | 24.7% (−8.5, 57.9) | 0.1 |
| Std Kt/V, per 1-unit greater | −13.4% (−46.9, 20.1) | 0.4 |
| Baseline step count, per 1,000 steps greater | 5.9% (3.2, 8.5) | <0.001 |
| SPPB Score, per 1 point greater | 7.1% (1.0, 13.3) | 0.03 |
| PF Score, per 10 points greater | 4.5% (0.5, 8.5) | 0.03 |
| Vitality Score, per 10 points greater | 1.6% (−2.8, 6.0) | 0.46 |
| DSI Total Symptom Burden, per 1 point greater | 0% (−2.0, 1.8) | 0.9 |
| DSI Total Symptom Severity, per 1 point greater | −0.1% (−0.6, 0.4) | 0.6 |
| CESD Score, per 1 point greater | 0.5% (−1.5, 0.5) | 0.3 |
SPPB, Short Physical Performance Battery; PF, Physical Functioning Scale of the SF-36; DSI, Dialysis Symptom Index; CESD, Centers for Epidemiologic Studies Depression Scale.
Univariable analysis.
Table 4.
Post-hoc Logistic Regression Analysis of Factors Associated with Likelihood of Meeting Overall Targets (N=30)
| Baseline characteristic* | OR (95% CI)** | p-value |
|---|---|---|
| Age, per 10 y older | 0.54 (0.27, 1.08) | 0.08 |
| Dialysis vintage, per 1-y older | 0.92 (0.74, 1.15) | 0.5 |
| Hemoglobin, per 1-g/dL greater | 0.98 (0.12, 1.17) | 0.8 |
| Serum Albumin, per 1-g/dL greater | 6.83 (0.57, 82.14) | 0.1 |
| Std Kt/V, per 1-unit greater | 1.85 (0.21, 16.16) | 0.6 |
| Baseline step count, per 1,000 steps greater | 1.29 (1.00, 1.65) | 0.05 |
| SPPB Score, per 1 point greater | 1.40 (0.86, 2.27) | 0.2 |
| PF Score, per 10 points greater | 1.08 (0.81, 1.43) | 0.6 |
| Vitality Score, per 10 points greater | 0.94 (0.72, 1.23) | 0.6 |
| DSI Total Symptom Burden, per 1 point greater | 0.98 (0.87, 1.1) | 0.7 |
| DSI Total Symptom Severity, per 1 point greater | 0.99 (0.96, 1.03) | 0.7 |
| CESD Score, per 1 point greater | 1.03 (0.97, 1.10) | 0.3 |
SPPB, Short Physical Performance Battery; PF, Physical Functioning Scale of the SF-36; DSI, Dialysis Symptom Index; CESD, Centers for Epidemiologic Studies Depression Scale.
Univariate analysis.
Safety Monitoring and Adverse Events
Eight participants (27%) reported symptoms related to the intervention. Symptoms included shortness of breath (10%), soreness (10%), lower extremity pain (7%), cramping (14%), and fatigue during or after walking (10%). Two patients reported chest pain with walking and were advised to limit their walking to usual levels until consulting their cardiologists. One patient died during the study. The patient was in the intervention group, but the death was determined not to be related to the intervention. There were no hospitalizations related to the intervention, and at study completion there was no significant difference between groups in hospitalizations within the last 6 months.
Discussion:
Our intervention successfully increased patients’ step counts during the intervention period, but the increase was not sustained after the intervention was discontinued (Figure 2). The intervention did not improve symptoms or endothelial function, but HRV improved in patients assigned to the intervention compared with controls. In post-hoc analysis, patients with higher baseline step count, physical function, and Vitality were more likely to achieve weekly goals, though only higher baseline step count was associated with higher odds of meeting overall target steps.
Figure 2. Average Daily Step Count at Baseline, 3 months, and 6 months.
Forty-nine percent of patients approached ultimately enrolled in the study, and 92% of enrolled participants completed the study, which met our targeted completion rate of 85%, but was less than our targeted enrollment of 50%. Concern about possibility of being assigned to control may have limited participation. On the other hand, performing this intervention outside the context of a clinical trial may result in higher enrollment but might have a lower completion rate if potentially less motivated patients were included. We observed a considerably higher rate of participation and completion than has been observed in studies of more vigorous intensity exercise in this population.17,34,35 It is also higher than was reported in two other studies of walking in dialysis patients, which reported retention rates of 66% over 4 months21 and 77% over 6 months respectively.36 However, participants in our study returned to their baseline step counts after the active period of the intervention. Thus, although walking may be a more acceptable intervention than other forms of exercise, engaging with patients to encourage and monitor walking appears to have been an important contributor to retaining patients in the study and to successfully increasing step counts.
We did not find any difference in changes between the two groups in physical function, fatigue, or other symptoms from baseline to 3 months. However, from 3 to 6 months, patients in the intervention group reported a primarily fatigue-driven increase in total symptom burden and severity on the DSI, which is reflected by the decline in the Vitality scale and increase in fatigue specifically on the DSI. Although the differences between groups from 3 to 6 months arose in part because of improvement among patients in the control group as well as because of worsening in the intervention group (Tables S3, S5), the consistent finding of a difference in fatigue deserves consideration. We had hypothesized that increasing step counts would improve fatigue, as higher physical activity is associated with higher levels of energy or decreased levels of exhaustion in the dialysis population.37-39 It may be that patients in our intervention group “overtrained,” resulting in fatigue, which can occur even in highly conditioned athletes.40 However, we would have expected to observe this at the 3-month assessment when participants in the intervention group were at their highest step counts rather than at 6 months when they had regressed to baseline. It is notable that there was not a concomitant decrease in physical function at 6 months. Therefore, it may be that the reported increase in fatigue is related in part to a change in perception after a decline in walking from their 3-month peak.
We also examined patient characteristics associated with meeting weekly and overall step goals. Patients with higher baseline step counts, physical performance, and PF score had better achievement of weekly goals. Patients with higher baseline step counts were also more likely to meet their overall targets for the intervention. We did not observe any differences based on age, dialysis vintage, hemoglobin, or dialysis adequacy. These findings may be important for development and dissemination of physical activity interventions in the dialysis population. For example, less active patients may do better if counselled to increase steps in smaller increments than the 10% increases that we recommended. Of note, the narrow targets for hemoglobin and Kt/V may reduce overall variability, and the lack of association in our study may not mean that severe anemia or underdialysis would not negatively impact patients’ ability or willingness to exercise. However, the lack of association with dialysis vintage is reassuring as it may indicate that even long-term dialysis patients can meet step targets.
We measured HRV as a surrogate for cardiovascular morbidity and mortality. SDNN was low in both groups at baseline but increased significantly in the intervention group compared with the controls over the 3-month intervention period, approaching mean values for short-term HRV recordings observed among healthy individuals.41 In one observational study of patients with CKD not yet on dialysis, a change of similar magnitude was associated with lower risk of hospitalization.42 Other studies of dialysis patients have shown improvement in HRV after one year of moderate to vigorous exercise,43 but to our knowledge this is the first study showing improvement in HRV with low-intensity interventions over a shorter time scale.
We did not observe any difference in change in endothelial function between the groups at either 3 or 6 months. Effecting change in endothelial function may require longer or more intense activity, or there may be factors unique to dialysis patients (e.g., damage to the endothelial glycocalyx44 or chronic inflammation and oxidative stress45-47) that attenuate the response of endothelial function to increasing walking.
Strengths of our study were the randomized design, the relatively high rates of participation and retention, and particularly the post-intervention follow-up. Most studies of exercise interventions in the dialysis population have not examined the durability of exercise behavior or of any associated benefits,48-50 with the exception of one small study (n=15 completing follow-up) that prescribed metronome-based walking sessions and showed improvement in participants’ 6-minute walking distance post-intervention that regressed to baseline approximately one year later.51 Our study shows that regression in walking may occur much sooner post-intervention if no maintenance phase is incorporated. The fact that patients did not sustain their higher step counts has important implications for more widespread application of walking interventions. It will be important to conduct larger studies to assess whether more automated or technology-driven methods can be implemented to allow for longer interventions without requiring weekly personal contact. A future study should incorporate more gradual increases in steps for more sedentary participants and consider incorporating a directed maintenance phase after the intervention to sustain higher step counts. A smaller increment in step count per week (e.g., 5%) would also result in a lower overall target, which may be easier to maintain for patients with more limitations.
Our study also has several limitations that should be acknowledged. Because the study participants were all given pedometers initially, this could have been a motivation to increase walking.22 However, the control group did not have access to their pedometers during the intervention period and did not use pedometers off-protocol to our knowledge. Because the intervention included pedometers and advice to increase walking, we cannot ascertain the relative benefit of each of these strategies individually. Because our results are based on differences in measures during one week at the beginning and one at the end of each time period, there may have been conditions that we did not account for (e.g., weather, personal issues) that would cause measurements not to represent participants’ typical step counts. However, such issues would tend to bias results towards the null, and we do not have reason to suspect that these would affect one group more than another. Study participants were selected from dialysis facilities in Northern California, which may limit generalizability of physical activity to the broader US dialysis population. Despite randomization, baseline endothelial function was better in the intervention group, and HRV was better in the control group. However, any difference in these characteristics are the result of chance.52 Longer epochs of recording such as 24-hour HRV monitoring may provide a more complete picture of sympathetic and parasympathetic activity than short-term recordings. Finally, step counts only measure walking (a light intensity activity) and do not capture the full range of physical activity. However, walking is reported to be the most common form of physical activity for most dialysis patients.47
Our study shows that a walking intervention increased patients’ step counts among patients on dialysis and improved HRV. However, the increase in walking was not sustained after the intervention. A home-based pedometer intervention is feasible for ambulatory dialysis patients, but continued feedback and encouragement from providers may be necessary to achieve durable change in activity. It is possible that patients who are able to successfully and sustainably increase walking will also have improvements in physical function or symptoms, although further study will be needed.
Supplementary Material
Item S1: Supplemental methods for measurements for endothelial function and heart rate variability.
Table S1. Demographics of patients included and excluded from the study.
Table S2. Step counts, physical function, questionnaire scores, endothelial function, and heart rate variability.
Table S3. Activity-relevant subset of DSI and individual symptoms on the DSI.
Table S4. Activity-relevant subset of DSI and individual symptoms on the DSI.
Table S5. Within-group changes in step counts, physical function, questionnaire scores, endothelial function, and heart rate variability.
Acknowledgments
Support: Dr. Sheshadri’s effort was supported by an American Kidney Fund Clinical Scientist in Nephrology Fellowship and a Ruth L. Kirschstein National Research Service Award (NRSA) Individual Postdoctoral Fellowship (F32 DK111154-02). Dr. Kittiskulnam received support from an International Society of Nephrology fellowship. Dr. Johansen’s effort was supported by a Midcareer Investigator Award in Patient Oriented Research (K24-DK085153). None of the funders had a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Data Sharing: Individual participant data that underlie the results reported in this article (text, tables, figures, and supplementary material) will be shared, after deidentification, beginning 9 months and ending 36 months following article publication, with investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for the purpose of individual participant data meta-analysis. Proposals may be submitted up to 36 months following article publication and should be directed to the corresponding author).
References
- 1.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston j, et al. Frailty in Older Adults: Evidence for a Phenotype. Journal of Gerontology: Medical Sciences. 2001;56A(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 3.Hayhurst WS, Ahmed A. Assessment of physical activity in patients with chronic kidney disease and renal replacement therapy. Springerplus. 2015;4:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med. 2009;49(1):3–11. [DOI] [PubMed] [Google Scholar]
- 5.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78(11):1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;Vol. 57 2564–2570. [DOI] [PubMed] [Google Scholar]
- 7.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–212. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL. Exercise in the End-Stage Renal Disease Population. Journal of the American Society of Nephrology. 2007;18(6):1845–1854. [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL, Kaysen GA, Dalrymple LS, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8(2):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kittiskulnam P, Sheshadri A, Johansen KL. Consequences of CKD on Functioning. Semin Nephrol. 2016;36(4):305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zukiman WZHW, Yaakup H, Zakaria NF, Bin Shah SA. Symptom Prevalence and the Negative Emotional States in End-Stage Renal Disease Patients with or without Renal Replacement Therapy: A Cross-Sectional Analysis. Journal of Palliative Medicine. 2017;20(10):1127–1134. [DOI] [PubMed] [Google Scholar]
- 14.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2487–2494. [DOI] [PubMed] [Google Scholar]
- 15.Gordon PL, Doyle JW, Johansen KL. Postdialysis fatigue is associated with sedentary behavior. Clinical nephrology. 2011;75(5):426–433. [PubMed] [Google Scholar]
- 16.Sheshadri A, Kittiskulnam P, Johansen KL. Higher Physical Activity Is Associated With Less Fatigue and Insomnia Among Patients on Hemodialysis. Kidney Int Rep. 2019;4(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Painter P, Carlson L, Carey S, Paul SM, Myll J. Low-functioning hemodialysis patients improve with exercise training. Am J Kidney Dis. 2000;36(3):600–608. [DOI] [PubMed] [Google Scholar]
- 18.Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant. 2005;20(7):1429–1437. [DOI] [PubMed] [Google Scholar]
- 19.Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. American Journal of Kidney Diseases. 2000;35(3):482–492. [DOI] [PubMed] [Google Scholar]
- 20.Tudor-Locke C, Jr DRB. How Many Steps/Day Are Enough? Preliminary Pedometer Indices for Public Health. Sports Med. 2004;34(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Nowicki M, Murlikiewicz K, Jagodzińska M. Pedometers as a means to increase spontaneous physical activity in chronic hemodialysis patients. J Nephrol. 2010;23(3):297–305. [PubMed] [Google Scholar]
- 22.Bravata DM, Smith-Spangler C, Sundaram V, al. e. Using pedometers to increase physical activity and health: A Systematic Review. JAMA. 2007;298(19):2296–2304. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health economics. 1993;2(3):217–227. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1994;3(5):329–338. [DOI] [PubMed] [Google Scholar]
- 26.Weisbord SD, Fried LF, Arnold RM, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27(3):226–240. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. . Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 28.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp. 2010;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder JR, Stauss HM, Gindes H, et al. Finger volume pulse waveforms facilitate reliable assessment of heart rate variability, but not blood pressure variability or baroreflex function. BMC Cardiovascular disorders. 2014;14(180):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamojska S, Szklarek M, Niewodniczy M, Nowicki M. Correlates of habitual physical activity in chronic haemodialysis patients. Nephrol Dial Transplant. 2006;21(5):1323–1327. [DOI] [PubMed] [Google Scholar]
- 31.Anand S, Johansen KL, Dalrymple LS, et al. Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: The comprehensive dialysis study. Hemodialysis International. 2013;17:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunlop DD, Song J, Semanik PA, Sharma L, Chang RW. Physical activity levels and functional performance in the osteoarthritis initiative: a graded relationship. Arthritis Rheum. 2011;63(1):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall M, Hinman RS, van der Esch M, et al. Is the relationship between increased knee muscle strength and improved physical function following exercise dependent on baseline physical function status? Arthritis Res Ther. 2017;19(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalom R, Blumental JA, Sanders Williams R, McMurray RG, Dennis VW. Feasibility and benefits of exercise training in patients on maintenance dialysis. Kidney Int. 1984;25:958–963. [DOI] [PubMed] [Google Scholar]
- 35.Molsted S, Eidemak I, Sorensen HT, Kristensen JH. Five months of physical exercise in hemodialysis patients: effects on aerobic capacity, physical function and self-rated health. Nephron Clin Pract. 2004;96(3):c76–81. [DOI] [PubMed] [Google Scholar]
- 36.Manfredini F, Mallamaci F, D'Arrigo G, et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J Am Soc Nephrol. 2017;28(4):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SH, Do JY, Jeong HY, Lee SY, Kim JC. The Clinical Significance of Physical Activity in Maintenance Dialysis Patients. Kidney Blood Press Res. 2017;42(3):575–586. [DOI] [PubMed] [Google Scholar]
- 38.Kouidi E. Health-related quality of life in end-stage renal disease patients: the effects of renal rehabilitation. Clinical nephrology. 2004;61 Suppl 1:S60–71. [PubMed] [Google Scholar]
- 39.Tentori F, Elder SJ, Thumma J, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25(9):3050–3062. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor PJ, Puetz TW. Chronic Physical Activity and Feelings of Energy and Fatigue. Medicine & Science in Sports & Exercise. 2005;37(2):299–305. [DOI] [PubMed] [Google Scholar]
- 41.Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol. 2010;33(11):1407–1417. [DOI] [PubMed] [Google Scholar]
- 42.Brotman DJ, Bash LD, Qayyum R, et al. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol. 2010;21(9):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouidi E, Karagiannis V, Grekas D, et al. Depression, heart rate variability, and exercise training in dialysis patients. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17(2):160–167. [DOI] [PubMed] [Google Scholar]
- 44.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23(11):1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stinghen AE, Pecoits-Filho R. Vascular damage in kidney disease: beyond hypertension. International journal of hypertension. 2011;2011:232683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid Med Cell Longev. 2017;2017:3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. [DOI] [PubMed] [Google Scholar]
- 48.Bohm C, Stewart K, Onyskie-Marcus J, Esliger D, Kriellaars D, Rigatto C. Effects of intradialytic cycling compared with pedometry on physical function in chronic outpatient hemodialysis: a prospective randomized trial. Nephrol Dial Transplant. 2014;29(10):1947–1955. [DOI] [PubMed] [Google Scholar]
- 49.van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20(1):141–146. [DOI] [PubMed] [Google Scholar]
- 50.Konstantinidou Erasmia, Koukouvou Georgia, Kouidi Evangelia, Deligiannis Asterios, Tourkantonis A. EXERCISE TRAINING IN PATIENTS WITH END-STAGE RENAL DISEASE ON HEMODIALYSIS: COMPARISON OF THREE REHABILITATION PROGRAMS. Journal of Rehabilitation Medicine. 2002;34:40–45. [DOI] [PubMed] [Google Scholar]
- 51.Anna Maria Malagoni Luigi Catizone, Mandini Simona, et al. Acute and long-term effects of an exercise program for dialysis patients prescribed in hospital and performed at home. JNephrol. 2008;21:871–878. [PubMed] [Google Scholar]
- 52.Altman DG, Dore CJ. Randomisation and baseline comparisons in clinical trials. Lancet (London, England). 1990;335(8682):149–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Supplemental methods for measurements for endothelial function and heart rate variability.
Table S1. Demographics of patients included and excluded from the study.
Table S2. Step counts, physical function, questionnaire scores, endothelial function, and heart rate variability.
Table S3. Activity-relevant subset of DSI and individual symptoms on the DSI.
Table S4. Activity-relevant subset of DSI and individual symptoms on the DSI.
Table S5. Within-group changes in step counts, physical function, questionnaire scores, endothelial function, and heart rate variability.