Abstract
In this paper, we present an overview and descriptive results from the first egocentric network study of men who have sex with men (MSM) from across the United States: the ARTnet study. ARTnet was designed to support prevention research for human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) that are transmitted across partnership networks. ARTnet implemented a population-based egocentric network study design that sampled egos from the target population and asked them to report on the number, attributes, and timing of their sexual partnerships. Such data provide the foundation needed for parameterizing stochastic network models that are used for disease projection and intervention planning. ARTnet collected data online from 2017 to 2019, with a final sample of 4904 participants who reported on 16198 sexual partnerships. The aims of this paper were to characterize the joint distribution of three network parameters needed for modeling: degree distributions, assortative mixing, and partnership age, with heterogeneity by partnership type (main, casual and one-time), demography, and geography. Participants had an average of 1.19 currently active partnerships (“mean degree”), which was higher for casual partnerships (0.74) than main partnerships (0.45). The mean rate of one-time partnership acquisition was 0.16 per week (8.5 partners per year). Main partnerships lasted 272.5 weeks on average, while casual partnerships lasted 133.0 weeks. There was strong but heterogenous assortative mixing by race/ethnicity for all groups. The mean absolute age difference for all partnership types was 9.5 years, with main partners differing by 6.3 years compared to 10.8 years for casual partners. Our analysis suggests that MSM may be at sustained risk for HIV/STI acquisition and transmission through high network degree of sexual partnerships. The ARTnet network study provides a robust and reproducible foundation for understanding the dynamics of HIV/STI epidemiology among U.S. MSM and supporting the implementation science that seeks to address persistent challenges in HIV/STI prevention.
Keywords: Men who have sex with men, Sexual networks, Mathematical modeling, Network modeling, Network science
1. Introduction
Human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) continue to present significant public health challenges. In the United States, HIV and STI incidence disparities are linked to demographics (Singh et al., 2014), risk behavior (Goldstein et al., 2017), clinical care access (Beer et al., 2017), and geography (Oster et al., 2015). Of the estimated 40,000 new HIV infections occurring in 2017, two-thirds were among men who have sex with men (MSM) (Centers for Disease Control and Prevention, 2019b). The large disparities in HIV/STI cases by race and age have worsened, with incidence increasing among younger non-white MSM while decreasing in other MSM groups (Rosenberg et al., 2018). Syphilis has also concentrated among MSM (de Voux et al., 2015), following similar demographic and geographic patterns as HIV (Grey et al., 2017; Sullivan et al., 2018). Understanding the persistent and emerging drivers of HIV/STI transmission dynamics among MSM is critical to prevention.
Sexual partnership networks are the mechanism through which all STI and most HIV transmissions circulate. The pathogens are transmitted by sexual acts embedded within partnerships, and circulation through the population depends on how those partnerships form and dissolve — a highly structured and population-specific dynamic process (Morris et al., 2009; Goodreau et al., 2012; Jenness et al., 2016a). While sexual network structure can be measured and analyzed either cross-sectionally or dynamically (Morris, 1997), dynamic networks determine how infectious diseases spread when the average duration of infection is longer than the average duration of partnerships (Jenness et al., 2018). This is often the case for HIV/STIs in the context of persistent sexual partnerships.
A large body of research over the past three decades has investigated which network features are most important in determining the size and speed of HIV/STI epidemics within and across populations (Carnegie and Morris, 2012). Three key features are the distribution of degree (number of ongoing partners), assortative mixing (selecting partners based on attributes similar to one’s own), and the distribution of partnership durations (Jenness et al., 2018). Partnership concurrency (2+ ongoing partners) amplifies the speed of HIV/STI transmission by allowing for backward paths of transmission (from infected partners acquired later to earlier uninfected partners) and shorter waiting times before onward transmission (Morris et al., 2009). Heterogeneity in network degree and duration, paired with assortative mixing by demographic attributes, may also play an important role in generating persistent disparities in HIV/STI incidence between MSM subgroups (Janulis et al., 2018; Sullivan et al., 2014). Network definitions also implicitly underlie recommendations for HIV/STI prevention tools, including guidelines for HIV preexposure prophylaxis (PrEP) that outline indications based on network degree, partnership type, and mixing by disease status (US Public Health Service, 2018).
Recent methodological advances have removed the primary obstacle to network measurement for HIV/STI epidemiology: the absence of a general statistical framework for temporal network model estimation from sampled network data (Krivitsky and Handcock, 2014). Traditional descriptive network analysis has relied on the collection of data on a complete cross-sectional network census — measurement of all nodes (persons) and edges (partnerships) in a population. While this design has been attempted in a couple of high-profile sexual network studies (Helleringer et al., 2009; Klovdahl et al., 1982), cost and implementation challenges make it infeasible for routine public health use. Various forms of adaptive sampling have also been developed, of which Respondent-Driven Sampling (Heckathorn, 1997) and partial sociometric designs (Birkett et al., 2015) are most well-known. However, methods for recovering valid estimates of the population network structure from these designs are limited and do not include temporal networks needed for disease modeling (Delva et al., 2016). The methodological advance that changed the field was principled estimation of Exponential-family Random Graph Models and their temporal counterparts from egocentrically sampled network data (Krivitsky and Morris, 2017). Egocentric network sampling draws egos (study participants) from the population of interest and then asks them to report on the number, attributes, and timing of their partnerships (Jenness et al., 2016a). It requires no link tracing, and can be integrated into a standard survey sampling design. Statistical methods can then be used to infer the complete dynamic network structure consistent with the sampled network data.
Temporal exponential random graph models (TERGMs) are an inferential statistical framework for complete or egocentrically sampled network data. They can be used to estimate the population parameters of network models from egocentric sample statistics on the incidence, duration, and heterogeneity in sexual partnerships at the dyadic (partnership) level (Krivitsky and Handcock, 2014). Once estimated, TERGMs can then be used for simulating from the temporal network defined by the model. The simulated networks will reproduce, on average, the observed (or scaled) network statistics from the sample. This provides an empirically grounded approach to simulating the partnership networks needed for mathematical projection models of infectious disease dynamics (Krivitsky and Morris, 2017). Software such as EpiModel provides flexible tools for building network-based epidemic models with egocentrically sampled network data (Jenness et al., 2018).
Egocentric data provide directly observed information on a limited but key set of network structural features: degree distributions, assortative mixing by nodal attributes, and partnership duration, all of which can be stratified by demographic and partnership attributes. However, not all structural features can be observed in this design, including those one or two edges removed from the sampled ego. Unobserved features include assortative mixing by degree (that goes beyond mixing by measurable attributes) and higher-order aggregate network features, like geodesics and component size distributions. However, many of these aggregate network features are largely determined by the lower order features that are observable — they are emergent properties of the local rules people use for partner selection — so the loss of information is less than it might appear.
Egocentric data have been successfully used to model the drivers of HIV transmission, racial disparities in disease incidence, and optimal strategies for HIV/STI prevention (Jenness et al., 2017a, 2019; Jenness et al., 2016b). These empirically grounded network models depend on egocentric network data. The Demographic and Health Surveys have been a useful data source for populations in Sub-Saharan Africa (Delva et al., 2016), but to date there have been few network data sources for MSM that broadly span across demographics and geography (Rosenberg et al., 1999). The National HIV Behavioral Surveillance (NHBS) system in the United States and the NATSAL and EMIS studies in Europe have provided key behavioral indicators on MSM, but these studies were not designed for network modeling and therefore have limited or no sexual network data (Whitham et al., 2017; Mitchell et al., 2013; Kramer et al., 2016). MSM network data commonly used in network-based epidemic models (including our own) have been collected in cohort studies with restrictive eligibility criteria (Sullivan et al., 2014), aggregated across multiple small studies in ways that may impact inference (Beck et al., 2015), or transported across studies of populations not represented in the models (Marshall et al., 2018). The HIV/STI modeling field needs up-to-date estimates of commonly used network parameters from study designs without restricted behavioral eligibility criteria for parameterizing network models for HIV/STI at multiple scales and in multiple geographic settings.
In this paper, we present descriptive results from the ARTnet study, the first egocentric network study of MSM from across the United States. Our analytic aims were to characterize the distribution and heterogeneity in network degree, assortative mixing, and partnership age (an unbiased estimator of partnership duration) (14). We estimated these three outcomes with stratifications by geography, demographics, and HIV status for different sexual partnership types (main, casual, and one-time) and different definitions of what type of sexual intercourse constitutes a sexual partnership (anal versus oral intercourse). The results in this paper provide summary network statistics that can be used in future epidemic modeling applications. We will also be making the full data available for primary analysis by external researchers, so these results represent just a small selection of the potential model parameterizations that may be evaluated.
2. Methods
2.1. Study design
This analysis used data collected in the ARTnet study of MSM in the United States in 2017–2019. MSM were recruited directly after participating in the American Men’s Internet Study (AMIS) (Zlotorzynska et al., 2019), a parent web-based study about MSM sexual health that recruited through banner ads placed on websites or social network applications. At the completion of AMIS, MSM were asked to participate in ARTnet, which focused on sexual network features. ARTnet data collection occurred in two waves (following AMIS): July 2017 to February 2018 and September 2018 to January 2019.
Eligibility criteria for ARTnet were male sex at birth, current male cisgender identity, lifetime history of sexual activity with another man, and age between 15 and 65. Respondents were deduplicated within and across survey waves based on IP and email addresses, resulting in a final sample of 4904 participants who reported on 16,198 sexual partnerships see Fig. 1). In the case of duplicates across waves, we used the more recent survey record. The Emory University Institutional Review Board approved the study. Due to the sensitive nature of the data and small sample sizes of participants in some rural geographies, a data use agreement with the ARTnet Principal Investigator (the corresponding author of this paper) will be required before data sharing with external researchers.
Fig. 1.
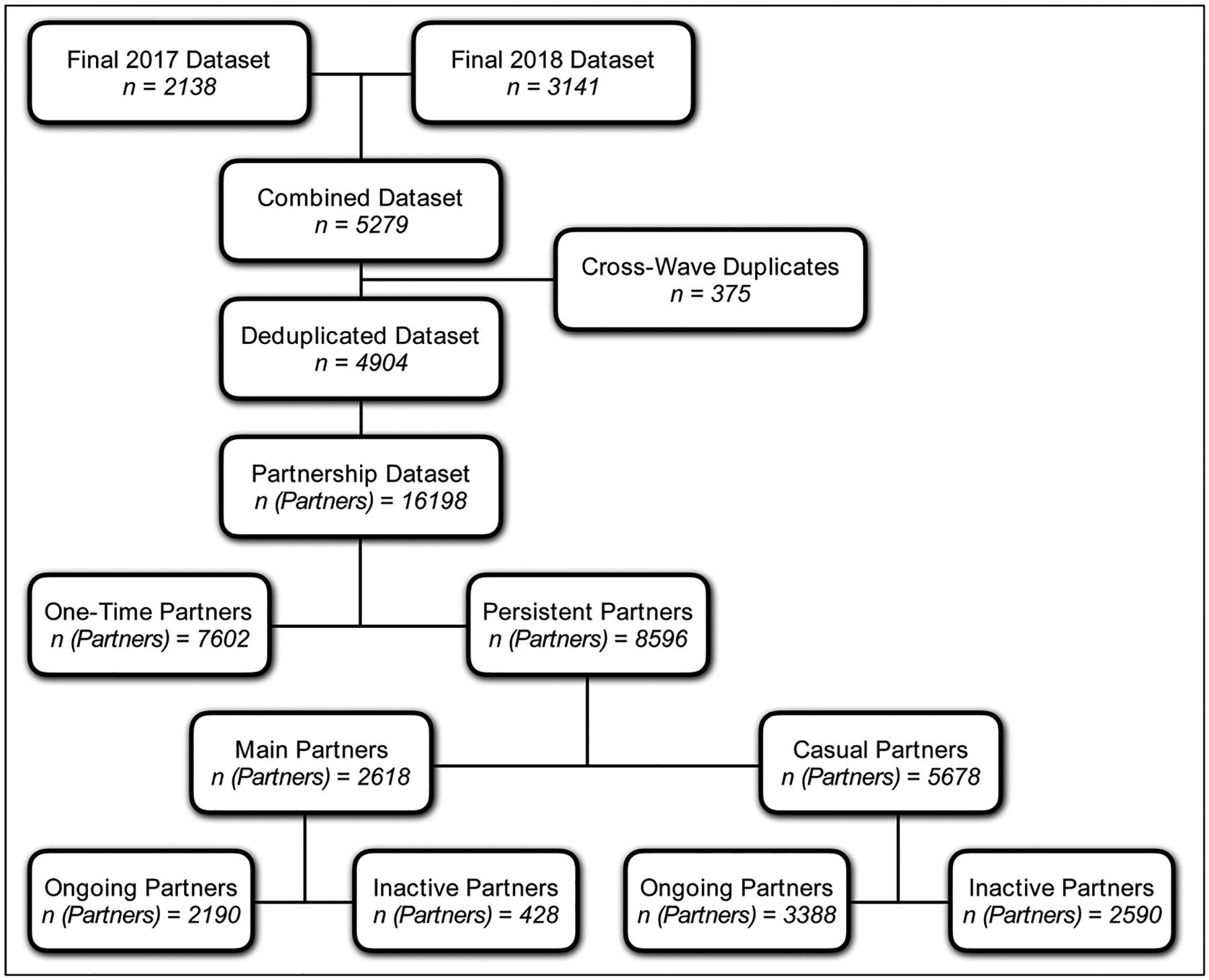
ARTnet Recruitment and Data Type Diagram, with Partnership Stratification by One-Time versus Persistent Partners, Partnership Type, and Ongoing Status of Partnerships.
2.2. Measures
ARTnet participants were first asked about demographic and health-related information. Covariates used in this analysis included race, age, ZIP Code of residence, and current HIV status. ZIP Codes were transformed into Census regions/divisions and urbanicity levels by matching against county databases. Participants reporting as never testing for HIV, having indeterminate test results, or never receiving test results were classified as having an unknown HIV status.
Participants were asked summary questions about the overall number of partnerships within each type in the past year and then asked detailed partner-specific questions for up to most recent 5 partners. The detailed partner-specific questions included attributes of the partner and details about the partnership itself. Partner attributes considered here included age, race/ethnicity, and HIV status. Participants were allowed to report any partner attribute as unknown. When partner age was unknown, age was imputed based on a response to a categorical question (e.g., 5–10 years younger/older, 2–5 years younger/older). Partnerships were classified into three types: “main” (respondent reported they considered this partner a “boyfriend, significant other, or life partner”), casual (someone they have had sex with more than once, but not a main partner), and one-time (Sullivan et al., 2014). For one-time partners, we asked for the date that sexual activity occurred. For persistent (main and casual) partnerships, we asked for the date of most recent sex, the date first sex (which could have been prior to the past year), and whether the partnership was ongoing (if the participant expected sexual activity would occur in the future). For each partnership, we asked whether (for one-time) or how frequently (for persistent) oral and anal sex occurred. For this analysis, these questions classified the partnership as oral only, anal only, or both oral and anal.
Outcome measures reported in this analysis include descriptive statistics for characteristics of participants and their reported partnerships, and the aggregate network statistics used to estimate the TERGMs underlying epidemic simulations on dynamic networks. The network statistics include ego degree, attribute mixing in partnerships, and the current age of ongoing partnerships (i.e., how long partnerships have lasted up to the point of the survey date), stratified by the attributes of persons and partnerships. Degree is a property of individuals, whereas mixing and age are properties of partnerships. Degree was defined as the number of persistent partners measured on the day of the survey (includes main and casual partnerships). Degree is not defined for one-time partnerships, so for these we instead calculated a weekly rate of new contacts by subtracting the total main and casual partners from the total past-year partners, and dividing by 52. In other words, estimates of the one-time partnership acquisition rate was not subject to truncation to the last 5 past-year partners. Partnership age for extant main and casual partnerships was calculated by taking the difference between the survey date and the partnership start date. The mean age of ongoing partnerships is the network statistic needed for TERGM estimation as an unbiased estimator of partnership duration assuming geometrically distributed durations; the logic and derivation have been explained in a prior study (Jenness et al., 2018). Mixing was measured by the relative frequency of partnerships that occurred within and between groups defined by race/ethnicity, age, and HIV status.
2.3. Statistical analysis
Descriptive analyses of demographic and behavioral characteristics are presented using proportions, means, standard deviations, and medians. Degree was stratified by ego-level characteristics and partnership type, and Poisson regression was used to estimate the mean and 95 % confidence intervals. Partnership age was estimated by partnership type for ego-level characteristics and dyadic matching on those characteristics. Age mixing was estimated for both categorical age groups and continuously using absolute differences in age. Mixing was analyzed using joint or row conditional proportions (for categorical attributes), and means and standard deviations for the continuous age difference measure. Analyses were performed in R 3.6.0. The analysis code is available at https://github.com/EpiModel/NetStats.
3. Results
Table 1 presents comparative descriptive statistics of deduplicated, unique men who completed the ARTnet study n = 4904 and the parent AMIS study n = 21,522. ARTnet participants were mainly white non-Hispanic 71.8 %), half (52.9 %) were aged 15–34, and one-third (36.3 %) were from the South. Participants were concentrated in urban counties (Large Central Metro: 43.1 %; Large Fringe Metro: 21.5 %). Participants reported an HIV prevalence of 8.7 %, with 15.3 % having an unknown HIV status (mostly due to never HIV testing). Comparing the demographics of ARTnet to AMIS, the race and regional distributions were similar, while ARTnet had more middle-aged participants and more participants from large central metropolitan areas. AMIS had no upper age eligibility restriction, whereas ARTnet had a maximum eligible age of 65. The proportion reporting as HIV-positive was similar, but AMIS had a higher level of unknown HIV status participants (because a higher proportion of AMIS respondents reported never testing for HIV).
Table 1.
Sample Characteristics of ARTnet Study Participants Compared to and AMIS Study Participants.
| ARTnet Complete N (%) |
AMIS Complete N (%) |
|
|---|---|---|
| Total Sample | 4904 (100.0) | 21522 (100.0) |
| Race/Ethnicity | ||
| Black, Non-Hispanic | 266 (5.4) | 1283 (6.0) |
| White, Non-Hispanic | 3523 (71.8) | 14901 (69.2) |
| Hispanic | 676 (13.8) | 3374 (15.7) |
| Other/Multiple | 439 (9.0) | 1964 (9.1) |
| Age | ||
| 15–24 | 1324 (27.0) | 7855 (36.5) |
| 25–34 | 1268 (25.9) | 4270 (19.8) |
| 35–44 | 694 (14.2) | 2429 (11.3) |
| 45–54 | 833 (17.0) | 3051 (14.2) |
| 55–65 | 785 (16.0) | 2883 (13.4) |
| 66 + | 0 (0.0) | 1034 (4.8) |
| Census Region and Division | *241 Missing | |
| West | 1246 (25.4) | 5050 (23.7) |
| Pacific | 842 (17.2) | 3339 (15.7) |
| Mountain | 404 (8.2) | 1711 (8.0) |
| Midwest | 994 (20.3) | 4415 (20.7) |
| West North Central | 296 (6.0) | 1442 (6.8) |
| East North Central | 698 (14.2) | 2973 (14.0) |
| South | 1782 (36.3) | 8152 (38.3) |
| West South Central | 498 (10.2) | 2329 (10.9) |
| East South Central | 222 (4.5) | 1168 (5.5) |
| South Atlantic | 1062 (21.7) | 4655 (21.9) |
| Northeast | 882 (18.0) | 3664 (17.2) |
| Middle Atlantic | 632 (12.9) | 2637 (12.4) |
| New England | 250 (5.1) | 1027 (4.8) |
| Urbanicity | *241 Missing | |
| Large Central Metro | 2116 (43.1) | 8003 (37.6) |
| Large Fringe Metro | 1056 (21.5) | 4741 (22.3) |
| Medium Metro | 921 (18.8) | 4533 (21.3) |
| Small Metro | 421 (8.6) | 1871 (8.8) |
| Micropolitan | 230 (4.7) | 1302 (6.1) |
| Noncore | 160 (3.3) | 831 (3.9) |
| HIV Status (Self-Reported) | ||
| Negative | 3726 (76.0) | 13885 (64.5) |
| Positive | 428 (8.7) | 1620 (7.5) |
| Unknown | 750 (15.3) | 6017 (28.0) |
Over 16,000 partnerships were reported in the detailed partnership modules, an average of 3.3 partners per participant Fig. 1). One-time (7602) partners make up almost half of all partnerships reported there, and about twice as many casual partnerships (5978) were reported there than main (2618) partnerships. For the persistent partnerships, the majority (65 %) were reported to be ongoing. Main partners were five times more likely to be ongoing than inactive, but they still comprised the minority (about 40 %) of all ongoing partnerships.
In Table 2 we move to the first set of network summary statistics: mean degree — the mean number of ongoing persistent partnerships — and weekly rate of one-time partnerships stratified by attributes of participants, overall, and by partnership type (main or casual) for all (oral or anal sex) partnerships. Supplemental Table 1 provides network degree with sexual partnerships separately categorized by any AI and any OI. Participants reported an average of 1.19 (95 % CI = 1.16–1.23) ongoing persistent partners, with higher mean degree for casual partnerships (0.74) than main (0.45). By race/ethnicity, white non-Hispanic MSM had the highest persistent partner mean degree (1.22). Black MSM had the lowest main mean degree (0.32), but the highest casual mean degree (0.81).
Table 2.
Heterogeneity in Mean Degree and Weekly Rates by Demographics and HIV Status, Stratified by Partnership Type, for All Anal or Oral Sex Partnerships among MSM in the United States.
| Main and Casual Mean (95 % CI) |
Main Mean (95 % CI) |
Casual Mean (95 % CI) |
One-Time Mean (95 % CI) |
|
|---|---|---|---|---|
| Total | 1.19 (1.16, 1.23) | 0.45 (0.43, 0.47) | 0.74 (0.72, 0.77) | 0.163 (0.152, 0.175) |
| Race/Ethnicity | ||||
| Black, Non-Hispanic | 1.12 (1.00, 1.26) | 0.32 (0.25, 0.39) | 0.81 (0.70, 0.92) | 0.117 (0.080, 0.163) |
| White, Non-Hispanic | 1.22 (1.18, 1.26) | 0.46 (0.44, 0.49) | 0.76 (0.73, 0.78) | 0.172 (0.159, 0.186) |
| Hispanic | 1.14 (1.06, 1.22) | 0.45 (0.40, 0.50) | 0.45 (0.63, 0.76) | 0.156 (0.128, 0.187) |
| Other/Multiple | 1.11 (1.01, 1.21) | 0.41 (0.35, 0.47) | 0.70 (0.62, 0.78) | 0.134 (0.102, 0.171) |
| Age | ||||
| 15–24 | 0.82 (0.77, 0.87) | 0.42 (0.38, 0.45) | 0.40 (0.37, 0.44) | 0.102 (0.086, 0.121) |
| 25–34 | 1.09 (1.03, 1.15) | 0.52 (0.48, 0.56) | 0.57 (0.53, 0.62) | 0.135 (0.116, 0.157) |
| 35–44 | 1.40 (1.31, 1.49) | 0.51 (0.46, 0.56) | 0.89 (0.82, 0.96) | 0.202 (0.170, 0.237) |
| 45–54 | 1.54 (1.46, 1.63) | 0.45 (0.40, 0.50) | 1.09 (1.02, 1.16) | 0.229 (0.198, 0.263) |
| 55–65 | 1.46 (1.37, 1.54) | 0.35 (0.31, 0.39) | 1.11 (1.03, 1.18) | 0.209 (0.179, 0.243) |
| Census Region and Division | ||||
| West | 1.17 (1.11, 1.23) | 0.43 (0.40, 0.47) | 0.74 (0.69, 0.79) | 0.175 (0.153, 0.200) |
| Pacific | 1.29 (1.21, 1.36) | 0.44 (0.40, 0.49) | 0.84 (0.78, 0.91) | 0.195 (0.167, 0.227) |
| Mountain | 0.94 (0.85, 1.04) | 0.42 (0.36, 0.49) | 0.52 (0.45, 0.59) | 0.134 (0.101, 0.173) |
| Midwest | 1.18 (1.12, 1.25) | 0.46 (0.42, 0.50) | 0.72 (0.67, 0.78) | 0.154 (0.131, 0.179) |
| West North Central | 1.16 (1.04, 1.29) | 0.49 (0.42, 0.58) | 0.67 (0.58, 0.76) | 0.152 (0.111, 0.200) |
| East North Central | 1.19 (1.11, 1.28) | 0.45 (0.40, 0.50) | 0.75 (0.68, 0.81) | 0.155 (0.127, 0.186) |
| South | 1.19 (1.14, 1.24) | 0.46 (0.43, 0.49) | 0.73 (0.69, 0.77) | 0.152 (0.134, 0.170) |
| West South Central | 1.23 (1.13, 1.33) | 0.47 (0.41, 0.53) | 0.76 (0.69, 0.84) | 0.143 (0.112, 0.179) |
| East South Central | 1.19 (1.06, 1.34) | 0.48 (0.40, 0.58) | 0.71 (0.61, 0.83) | 0.162 (0.115, 0.221) |
| South Atlantic | 1.17 (1.11, 1.24) | 0.45 (0.41, 0.49) | 0.72 (0.67, 0.78) | 0.153 (0.131, 0.178) |
| Northeast | 1.25 (1.18, 1.32) | 0.44 (0.40, 0.49) | 0.80 (0.75, 0.87) | 0.182 (0.155, 0.211) |
| Middle Atlantic | 1.27 (1.18, 1.36) | 0.44 (0.39, 0.50) | 0.83 (0.76, 0.90) | 0.176 (0.146, 0.211) |
| New England | 1.20 (1.07, 1.34) | 0.45 (0.37, 0.54) | 0.75 (0.65, 0.86) | 0.194 (0.145, 0.254) |
| Urbanicity (County) | ||||
| Large Central Metro | 1.26 (1.22, 1.31) | 0.45 (0.43, 0.48) | 0.81 (0.77, 0.85) | 0.196 (0.177, 0.215) |
| Large Fringe Metro | 1.18 (1.12, 1.25) | 0.42 (0.38, 0.46) | 0.76 (0.71, 0.81) | 0.158 (0.135, 0.183) |
| Medium Metro | 1.14 (1.07, 1.21) | 0.48 (0.44, 0.53) | 0.65 (0.60, 0.71) | 0.131 (0.109, 0.155) |
| Small Metro | 1.12 (1.03, 1.23) | 0.46 (0.40, 0.53) | 0.66 (0.59, 0.74) | 0.114 (0.085, 0.149) |
| Micropolitan | 1.06 (0.93, 1.19) | 0.42 (0.34, 0.51) | 0.63 (0.54, 0.74) | 0.132 (0.090, 0.184) |
| Noncore | 1.11 (0.96, 1.28) | 0.40 (0.31, 0.51) | 0.71 (0.59, 0.85) | 0.141 (0.091, 0.208) |
| HIV Status | ||||
| Negative | 1.24 (1.20, 1.27) | 0.46 (0.44, 0.48) | 0.78 (0.75, 0.81) | 0.172 (0.159, 0.185) |
| Positive | 1.59 (1.47, 1.71) | 0.50 (0.43, 0.57) | 1.09 (1.00, 1.19) | 0.269 (0.223, 0.321) |
| Unknown | 0.75 (0.69, 0.82) | 0.38 (0.34, 0.42) | 0.37 (0.33, 0.42) | 0.063 (0.046, 0.082) |
| Partnership Concurrency | ||||
| Concurrent (n, %) | 1290 (26.3 %) | 95 (1.9 %) | 952 (19.4 %) | – |
For persistent partnerships, mean degree increased with age, ranging from 0.82 in MSM aged 15–24 to 1.46 in men aged 55–65. This age trend was driven mostly by the increases in reported ongoing casual partners among older participants. Mean degree for main partners did not differ greatly across age categories, with a maximum of 0.52 among 25–34-year-old MSM and minimum of 0.35 among 55–64-year-old MSM. In contrast, the number of casual partnerships increased substantially with age: 15–24: 0.40; 55–65: 1.11.
MSM in the Middle Atlantic and Pacific census divisions had the highest total degree (1.27 and 1.29, respectively), while men in the Mountain division had the lowest (0.94). MSM in more urban areas had a higher degree (Large Central Metro: 1.26, Large Fringe Metro: 1.18), while MSM in rural areas had the lowest (Micropolitan: 1.06, Noncore:1.11). HIV-positive men had a higher total (1.59) and casual (1.09) degree than HIV-negative (1.24, 0.78) or status-unknown (0.75, 0.37) MSM. Overall, 1290 (26.3 %) MSM reported being in at least two persistent relationships concurrently (degree 2+ for any combination of main and casual partners). Only 1.9 % of MSM reported concurrent main partnerships, while 19.4 % reported concurrent casual partnerships.
The mean rate of one-time partnership acquisition was 0.163 per week (95 % CI = 0.152–0.175), which translates into 8.5 partners per year. The empirical distribution of rates was highly right-skewed, with 25 %, 50 %, and 75 % quantiles of the distribution at 0.000, 0.038, and 0.154 (Supplemental Fig. 1). One-time rates were highest among older and HIV-positive MSM. Supplemental Table 2 provides weekly one-time partnership rates with sexual partnerships separately categorized by any anal intercourse (AI) versus any oral intercourse (OI).
Table 3 presents the cross-tabulation of participants’ degree for main partnerships by degree for casual partnerships. Most MSM (79.2 %) had a degree of either 0 or 1 across both main and casual partnerships. There was generally a negative correlation between degree in one partnership type and degree in the other, as shown in the marginal summaries. Mean degree for casual partnerships was 0.84, 0.47, and 0.86 for MSM with a main mean degree of 0, 1, and 2, respectively. Although the mean casual degree was highest for MSM with a main degree of 2, this only represented 2 % of MSM. Main mean degree was highest (0.53) among MSM with a casual degree of 0, and was consistently lower for MSM with higher casual degrees.
Table 3.
Cross Tabulation of Participants by Degree for Across Main and Casual Partnership Type, for All Anal or Oral Sex Partnerships among MSM in the United States.
| Casual Degree | Marginal Summary | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Frequency | Mean Casual Degree | ||
| Main Degree | 0 | 1432 | 719 | 320 | 338 | 57% | 0.84 |
| 1 | 1463 | 271 | 136 | 130 | 41% | 0.47 | |
| 2 | 48 | 19 | 21 | 7 | 2 % | 0.86 | |
| Marginal Summary | Frequency | 60 % | 21 % | 10 % | 10 % | ||
| Mean Main Degree | 0.53 | 0.31 | 0.37 | 0.30 | |||
Table 4 presents estimates of the mean age (as an estimator of duration) of the 5578 ongoing persistent sexual partnerships by participant characteristics. Partnership ages were right-skewed, with an exponential decay shape, and the mean age exceeding the median age in every case. Supplemental Figure 2 shows the empirical density of ages stratified by partnership type. On average, main partnerships had been ongoing for 272.5 weeks (over 5 years), while casual partnerships were 133.0 weeks old (approximately 2.5 years). The average age of persistent partnerships was longest among white MSM (192.5 weeks) and shortest among Hispanic MSM (158.7 weeks). Hispanic MSM had the shortest ongoing partnerships for both main (219.3 weeks) and casual partners (119.0 weeks); white MSM had the longest ongoing main partnerships (286.1 weeks) and black men had the longest ongoing casual partnerships (154.6 weeks). The mean age of total, main, and casual partnerships increased with participant age, increasing from 71.1 weeks in MSM aged 15–24 to 274.4 weeks in MSM aged 55–65.
Table 4.
Heterogeneity in Age (in Weeks) of Ongoing Sexual Partnerships by Demographics and HIV Status of Ego Participant, for All Anal or Oral Sex Partnerships among MSM in the United States.
| Ongoing Partnerships N (%) |
All Mean (SD, Med) |
Main Mean (SD, Med) |
Casual Mean (SD, Med) |
|
|---|---|---|---|---|
| Total | 5837 (100.0) | 185.6 (267.2, 87.9) | 272.5 (336.8, 140.2) | 133.0 (196.5, 70.1) |
| Race/Ethnicity | ||||
| Black, Non-Hispanic | 297 (5.1) | 186.3 (234.4, 96.1) | 267.9 (286.1, 151.3) | 154.6 (203.0, 82.0) |
| White, Non-Hispanic | 4284 (73.4) | 192.5 (277.5, 90.9) | 286.1 (349.5, 153.1) | 134.9 (201.3, 69.8) |
| Hispanic | 771 (13.2) | 158.7 (231.1, 78.9) | 219.3 (291.7, 103.9) | 119.0 (169.8, 62.6) |
| Other/Multiple | 485 (8.3) | 166.8 (242.5, 82.0) | 241.6 (301.6, 126.9) | 122.6 (186.4, 67.0) |
| Age | ||||
| 15–24 | 1079 (18.5) | 71.1 (78.8, 46.6) | 74.6 (72.3, 52.2) | 67.4 (84.9, 37.9) |
| 25–34 | 1382 (23.7) | 142.7 (163.4, 83.9) | 195.9 (177.5, 152.7) | 94.8 (132.5, 49.4) |
| 35–44 | 966 (16.5) | 198.7 (239.8, 104.1) | 321.1 (287.2, 248.0) | 128.8 (172.9, 75.6) |
| 45–54 | 1273 (21.8) | 239.9 (312.5, 117.7) | 430.9 (397.3, 287.4) | 160.5 (226.4, 86.0) |
| 55–65 | 1137 (19.5) | 274.4 (379.7, 119.4) | 576.4 (535.5, 376.9) | 179.5 (248.8, 94.1) |
| HIV Status | ||||
| Negative | 4602 (78.8) | 186.8 (265.4, 90.4) | 281.0 (334.9, 153.0) | 131.2 (193.7, 70.1) |
| Positive | 677 (11.6) | 223.3 (309.3, 100.9) | 359.6 (414.3, 220.6) | 161.6 (222.1, 79.4) |
| Unknown | 558 (9.6) | 130.0 (212.6, 61.1) | 156.3 (242.2, 70.1) | 102.8 (173.2, 50.0) |
HIV-positive MSM had longer persistent partnerships (223.3 weeks) than HIV-negative MSM (186.8 weeks) or MSM with unknown HIV status (130.0 weeks). However, this was strongly confounded by participant age. The average participant ages of HIV-negative, HIV-positive, and HIV-unknown MSM were 37.5, 46.7, and 25.9. In a linear regression model of mean partnership age with only HIV status as the predictor (Supplemental Table 4), HIV-positive MSM had total partnership age that were 36.6 weeks longer than HIV-negative MSM. However, in a model with both HIV status and participant age as predictors, the difference was only 5.0 weeks longer for HIV-positive MSM; participant age was associated with both HIV status and partnership age.
Table 5 presents the age of ongoing sexual partnerships by partnership-level characteristics. The main comparisons for race/ethnicity, age group, and HIV status group are between partnerships in which the participant and partner have the same attributes compared to partnerships in which individuals have different attributes. For all main and casual partnerships, those in which both partners were black or those in which both partners were white had the longest partnership age (210.1 and 214.2 weeks, respectively), while partnerships between Hispanic MSM were the shortest (141.5 weeks). Black-black casual partnerships were longer than white-white casual partnerships, but the direction was reversed for main partnerships. By participant age, there was a monotonic increase in the age of all, main, and casual partnerships in matched groups, with main and casual partnerships having similar mean partnership ages for the youngest participant age category (66.1 and 65.6, respectively), but with main partnership age increasing more substantially across participant age groups.
Table 5.
Heterogeneity in Age (in Weeks) of Ongoing Sexual Partnerships by Demographics and HIV Status of Ego Participant and Partner, for All Anal or Oral Sex Partnerships among MSM in the United States.
| Ongoing Partnerships N (%) |
All Mean (SD, Med) |
Main Mean (SD, Med) |
Casual Mean (SD, Med) |
|
|---|---|---|---|---|
| Total | 5837 (100.0) | 185.6 (267.2, 87.9) | 272.5 (336.8, 140.2) | 133.0 (196.5, 70.1) |
| Race/Ethnicity | *113 Missing | *113 Missing | *15 Missing | *98 Missing |
| Black-Black | 147 (2.6) | 210.1 (239.3, 119) | 244.9 (231.0, 169.9) | 196.6 (242.1, 106.4) |
| White-White | 2795 (48.8) | 214.2 (304.6, 97.4) | 313.0 (375.4, 163.5) | 143.1 (214.7, 74.3) |
| Hispanic-Hispanic | 290 (5.1) | 141.5 (190.9, 79.6) | 190.1 (230.6, 105.7) | 104.8 (144.5, 61.3) |
| Other-Other | 88 (1.5) | 160.7 (213.9, 92.4) | 214.9 (260.0, 126.9) | 101.4 (126.4, 70.2) |
| Unmatched | 2404 (42.0) | 160.4 (228.6, 80.8) | 230.4 (289.8, 116.7) | 125.2 (180.7, 66.7) |
| Age | *48 Missing | *48 Missing | *5 Missing | *43 Missing |
| 15–24 | 666 (11.5) | 65.9 (71.7, 41.7) | 66.1 (67.6, 45.4) | 65.6 (77.5, 34.6) |
| 25–34 | 813 (14.0) | 149.7 (155.1, 94.8) | 200.0 (160.8, 167.3) | 93.1 (126.6, 49.0) |
| 35–44 | 309 (5.3) | 266.9 (275.7, 164.9) | 395.5 (283.6, 363.5) | 148.7 (207.5, 81.6) |
| 45–54 | 355 (6.1) | 339.0 (370.7, 173.7) | 540.3 (406.0, 440.3) | 221.3 (290.4, 111.7) |
| 55–65 | 217 (3.7) | 443.8 (533.4, 141.7) | 843.2 (587.9, 835.6) | 227.3 (345.4, 95.9) |
| Unmatched | 3429 (59.2) | 177.5 (253.7, 87.7) | 286.9 (343.7, 153.1) | 131.7 (186.5, 71.1) |
| HIV Status | *10 Missing | *10 Missing | *4 Missing | *6 Missing |
| Negative-Negative | 3817 (65.5) | 192.5 (271.9, 92.1) | 283.8 (337.9, 153.3) | 130.8 (193.2, 69.7) |
| Positive-Positive | 214 (3.7) | 269.3 (328.8, 143) | 366.5 (379.8, 280.4) | 210.2 (278.8, 87.6) |
| Unknown-Unknown | 202 (3.5) | 136.0 (230.4, 64.2) | 176.2 (310.9, 61.0) | 107.4 (143.5, 65.9) |
| Unmatched | 1594 (27.4) | 163.6 (246.6, 79.9) | 235.9 (322.7, 117.8) | 131.2 (143.5, 65.9) |
Table 6 presents estimates of mixing by race/ethnicity, age, and HIV status. Among all partnerships, most included a white partner (84.6 %). Of 2199 partnerships involving a black partner, 369 (16.8 %) were with another black partner. Of 4349 partnerships involving a Hispanic partner, 796 (18.3 % were with another Hispanic partner. Of 12,911 partnerships involving a white partner, 7094 54.9 %) were with another white partner. Assortative mixing is shown in a matrix format in Supplemental Table 5. Within-race mixing there is expressed as the proportion of egos with a same-race reported partner (row conditional proportions): 45.3 % of black, 34.5 % of Hispanic, 16.3 % of other, and 60.9 % of white MSM had partners within their own group.
Table 6.
Partnership Mixing by Demographics and HIV Status, Stratified by Partnership Type, for All Anal or Oral Sex Partnerships among MSM in the United States.
| All N (%) |
Main N (%) |
Casual N (%) |
One-Time N (%) |
|
|---|---|---|---|---|
| Total | 16198 (100.0) | 2618 (100.0) | 5978 (100.0) | 7602 (100.0) |
| Race/Ethnicity | *947 Missing | *18 Missing | *175 Missing | *754 Missing |
| Black-Black | 369 (2.4) | 57 (2.2) | 168 (2.9) | 144 (2.1) |
| Black-Hispanic | 308 (2.0) | 25 (1.0) | 126 (2.2) | 157 (2.3) |
| Black-Other | 181 (1.2) | 22 (0.8) | 78 (1.3) | 81 (1.2) |
| Black-White | 1341 (8.8) | 159 (6.1) | 558 (9.6) | 624 (9.1) |
| Hispanic-Hispanic | 796 (5.2) | 155 (6.0) | 285 (4.9) | 356 (5.2) |
| Hispanic-Other | 453 (3.0) | 70 (2.7) | 161 (2.8) | 222 (3.2) |
| Hispanic-White | 2792 (18.3) | 399 (15.3) | 1053 (18.1) | 1340 (19.6) |
| Other-Other | 233 (1.5) | 51 (2.0) | 75 (1.3) | 107 (1.6) |
| Other-White | 1684 (11.0) | 295 (11.3) | 648 (11.2) | 741 (10.8) |
| White-White | 7094 (46.5) | 1367 (52.6) | 2651 (45.7) | 3076 (44.9) |
| HIV Status | *20 Missing | *4 Missing | *10 Missing | *6 Missing |
| Negative-Negative | 8752 (54.1) | 1793 (68.6) | 3600 (60.3) | 3359 (44.2) |
| Negative-Positive | 1013 (6.3) | 239 (9.1) | 476 (8.0) | 298 (3.9) |
| Negative-Unknown | 4632 (28.6) | 351 (13.4) | 1249 (20.9) | 3032 (39.9) |
| Positive-Positive | 367 (2.3) | 95 (3.6) | 175 (2.9) | 97 (1.3) |
| Positive-Unknown | 551 (3.4) | 26 (1.0) | 220 (3.7) | 305 (4.0) |
| Unknown-Unknown | 863 (5.3) | 110 (4.2) | 248 (4.2) | 505 (6.6) |
| Age (Both Partners) | *643 Missing | *9 Missing | *101 Missing | *533 Missing |
| 15–24 | 2289 (14.7) | 529 (20.3) | 656 (11.2) | 1104 (15.6) |
| 25–34 | 2116 (13.6) | 501 (19.2) | 685 (11.7) | 930 (13.2) |
| 35–44 | 685 (4.4) | 165 (6.3) | 246 (4.2) | 274 (3.9) |
| 45–54 | 747 (4.8) | 138 (5.3) | 322 (5.5) | 287 (4.1) |
| 55–65 | 489 (3.1) | 92 (3.5) | 204 (3.5) | 193 (2.7) |
| Different Groups | 9229 (59.3) | 1184 (45.4) | 3764 (64.0) | 4281 (60.6) |
| Age (Mean, SD, Median) | *643 Missing | *9 Missing | *101 Missing | *533 Missing |
| Absolute Difference | 9.5 (9.3, 6.0) | 6.3 (7.4, 4.0) | 10.8 (9.8, 8.0) | 9.6 (9.3, 6.0) |
| Abs. Diff. of Square Roots | 0.78 (0.74, 0.53) | 0.52 (0.58, 0.32) | 0.87 (0.78, 0.62) | 0.80 (0.74, 0.56) |
By HIV status, most relationships (89.0 %) involved an HIV-negative partner (Table 6. Of 14,397 partnerships involving a HIV-negative partner, 8752 60.8 %) were with another HIV-negative partner. Of 1931 partnerships involving a HIV-positive partner, 367 (19.0 %) were with another HIV-positive partner, while 551 (28.5 %) were with a partner of unknown status. The proportion of relationships with a partner of unknown HIV status was lowest within main partnerships (487, 18.6 %), higher among casual partnerships (1717, 28.7 %), and greatest within one-time partnerships (3842, 54.4 %). Supplemental Table 6 presents a mixing matrix version of these results. Supplemental Table 7 compares the empirical distribution of partnership mixing by HIV status for main and casual partnerships against expected values under reference distributions generated by statistical models (exponential random graph models) assuming the marginal distribution of HIV status and heterogeneity in mean degree by HIV status.
Finally, by age, 59.3 % of partnerships were among partners in different age categories, ranging from 45.4 % among main partnerships to 64.0 % among casual partnerships. Across all partnerships, the mean difference in age between ego and alter was 9.5 years, with main partners only differing by 6.3 years on average compared to an average of 10.8 years among casual partners. The absolute age differences in one-time partnerships was slightly lower than those in casual partnerships.
4. Discussion
In this paper, we present the first detailed egocentric network statistics of MSM from across the United States. This analysis highlighted three network features critical for driving the prevalence and dynamics of HIV/STI epidemics in this target population: network degree, assortative mixing, and partnership age. This analysis suggests that MSM across geographies and demographics may be at sustained risk for HIV/ STI acquisition and transmission through a combination of both high cumulative numbers of sexual partners and high mean degree among persistent partnerships. The reported partnerships involve high levels of disassortative mixing across HIV statuses, and between race and age groups with large preexisting differences in HIV/STI prevalence. Overall, these network descriptive statistics provide a foundation for understanding the latest trends in HIV/STI epidemiology among U.S. MSM, to be supplemented by future statistical and mathematical modeling to investigate the population-level implications for disease transmission dynamics and prevention strategies.
HIV/STI risk in MSM sexual networks are influenced by degree, mixing, and duration in combination (Jenness et al., 2018). The past decade has seen a stabilization of HIV incidence despite expanding access to and use of highly effective HIV prevention tools (Cohen et al., 2011), and an increase in three major bacterial STIs (gonorrhea, chlamydia, and syphilis) despite the availability of antibiotics (Workowski, 2015). Recent national analyses have shown a broad increase in total and casual past-year partners among MSM that could explain these disease trends (Chapin-Bardales et al., 2019). However, cumulative statistics like total past-year partnerships are not fully informative of current and future epidemic potential due to the offsetting effects of degree and duration in dynamic partnership networks (Bansal et al., 2010). Lower mean degree (such as that observed among younger MSM in our study) does not necessarily translate to lower risk. Because of their shorter partnership durations, younger MSM have higher partnership turnover rates than older MSM. Dynamic network statistics such as the “forward reachable path” (Armbruster et al., 2017) and dynamic network-based mechanistic models (Jenness et al., 2018) are needed to disentangle degree and duration to fully understand these results.
These findings also contribute to our understanding of HIV racial disparities. HIV incidence has remained stable among black MSM, who account for the greatest number of new HIV diagnoses, and increased 12 % among Hispanic MSM despite decreasing 14 % among white MSM (Centers for Disease Control and Prevention, 2019b). A long-standing puzzle has been to explain the causes of these disparities in light of evidence finding equal or lower risk levels for the race groups (black MSM) with the highest incidence (Sullivan et al., 2014; Beer et al., 2014; Millett et al., 2006). One hypothesis has been that assortative mixing by race may drive disparities: black MSM could be at greater risk for HIV because they are exposed to HIV at a higher rate when most of their contacts are with black MSM in whom prevalence is higher (Matthews et al., 2016). While assortative mixing may result in higher HIV exposure cross-sectionally, modeling suggests assortative mixing alone cannot generate or sustain disparities without differences in risk between groups (Goodreau et al., 2017a). A combination of preferential mixing plus risk-amplifying network features (e.g., higher mean degree) would be needed to explain the disparity over time. In our study, we found minor differences in mean degree by race/ethnicity, with the fewest ongoing main and casual partnerships among black and Hispanic men, respectively, and a white/black difference of nearly 3 one-time partners per year. By this measure alone, network risks were lower among black MSM, consistent with previous research (Mustanski et al., 2019; Uong et al., 2019). However, we found other network features that could be contributing to these disparities. Black MSM consistently had longer casual partnerships compared to white MSM, lengthening the opportunities for disease exposure.
We found some evidence for serosorting (mixing by known HIV status). For main and casual partnerships, 8.3 % occurred between HIV-positive and HIV-negative MSM, but the expected value in a proportional mixing model that accounted for the marginal distribution of HIV and heterogeneity in mean degree by HIV status was 18.3 %. The proportion of positive-positive main and casual partnerships was 2.9 %, whereas expected model value was 1.3 %. The latter is not necessarily a sign of intentional serosorting (preferential mixing in partnership formation), however, because the homophily may also reflect HIV transmissions that occur within a partnership that started as discordant (Khosropour et al., 1999). Notably, the proportion of partnerships involving an unknown partner was higher among casual and one-time partnerships, making up more than half of all one-time partnerships.
One strength of the ARTnet survey is that it included MSM across a wide age range (15–65). We found that HIV-positive respondents reported the longest partnerships, but this was strongly confounded by age. As an incurable infection, HIV prevalence increases with age (Mitsch et al., 2018; Centers for Disease Control and Prevention, 2019a), but younger MSM account for most new HIV diagnoses (Mitsch et al., 2018). Therefore, explanatory factors for increased risk acquisition among younger MSM are needed. Age was associated with an increase in casual and one-time partners, with the rate of partner acquisition among 35 to 65-year-old MSM twice that of those aged 15–24 years. There was a strong association between age and the duration of main partnerships, with a weaker association for casual partners. Younger MSM in our study may have reported lower degree, but the relatively weak age-based homophily and shorter partnerships could result in greater partner turnover with and increased risk of HIV exposure through contacts with older partners.
By geography and urbanicity, we found small differences in network risk that do not reflect the underlying spatial distribution of HIV burden. The distribution of HIV diagnoses (52 % in 2017) and HIV prevalence (46 % in 2015) is greatest in the South region, but this is also confounded by race/ethnicity (Centers for Disease Control and Prevention, 2019b). We did not observe higher mean degree of persistent partners or a higher rate of one-time partners in the South. Men from the Pacific and Middle Atlantic divisions had the greatest number of ongoing partnerships, driven by more ongoing casual partnerships. These are also regions where access to and use of prevention tools (such as PrEP) are the highest (Finlayson et al., 2019). This may reflect two fundamental changes in the epidemiological context for MSM over the past decade. First, in the era of pharmacological elimination of HIV acquisition and transmission risk (Eisinger et al., 2019), the spatial distribution of HIV may be driven more by heterogeneity in the use of prevention tools rather than differences behavioral risk (Beer et al., 1999). Second, the greater availability of prevention tools in some areas may cause an increase in behavioral risk through the knowledge that HIV biological risk is low or eliminated (Jenness et al., 2017b).
Finally, with respect to urbanicity, differences in population size, as might be observed in urban areas compared to rural areas, change the ratio of edges (partnerships) to nodes (individuals). This means that either the density of the network or the mean degree will differ in networks (or subnetworks) of different sizes (Jenness et al., 2018). Some studies have interpreted differences in network density, by itself and without consideration to network size, to have meaning in the HIV/ STI context (Mustanski et al., 2019). However, HIV/STI modeling studies typically assume frequency dependence, which preserves mean degree over network density when network size fluctuates (Krivitsky et al., 2011; Kolaczyk and Krivitsky, 2015). In this study, however, we found some evidence of a correlation between mean degree and population size of MSM, primarily with respect to differences in casual mean degree and one-time partnership rates (8–10 in Large Central and Large Fringe Metros versus 6–7 in less urban counties). For meta-population models, such as those that consider migration between rural and urban areas (Khanna et al., 2014), the frequency dependency assumption and corresponding preservation of mean degree may not be warranted.
While the ARTnet study was primarily designed to generate data to be used in network-based models using TERGMs, these data may also be used with minimal transformation in other partner-based mathematical models like pair-formation ordinary differential equation models (Powers et al., 2011). Partnership acquisition rates (key parameters in these compartmental models), for example, may be calculated as a function of mean degree and partnership age, following the general formula: incidence (partnership acquisition rate) = prevalence (mean degree) / duration (partnership age). The overall partnership acquisition rate of main partnerships (see Tables 2 and 4) is thus: 0.45 / 272.5 weeks = 0.00165 new main partners per week. Regarding partnership duration, which would also inform dissolution rates in pair-formation models, we note that complete partnership duration is not measurable directly from any empirical data, because of right-censoring (ongoing partnerships have an undefined duration) and length-biased sampling (the probability of observing a partnership is positively correlated with its duration) (14). However, partnership age is an unbiased estimator of partnership duration under the assumption of geometrically distributed partnership ages. Individual-based network models and compartmental models that assume memoryless transition processes for partnership dissolution may incorporate these relational age estimates as durations (with their reciprocals as rates) directly.
Limitations.
There are some limitations to this analysis. As a self-reported survey, there are concerns about possible recall and self-report biases associated with answering questions about sexual behavior, including reporting detailed information on all partners in the prior year or the age of a partnership. Certain partner information necessary for this analysis, such as HIV status, is influenced by disclosure and may not have been known to survey respondents. However, in most cases we allowed a “don’t know” or “prefer not to answer” survey response. Second, egocentric data on partnerships was limited to the most recent 5 partners in the past year. This could underestimate main and casual degree in cases where MSM had a large number of these persistent partnerships. As noted in the methods, the one-time partnership acquisition rates were not subject to this truncation. Third, the recruitment method resulted in a convenience sample of MSM that may not be representative of all MSM, particularly racial/ethnic minority MSM who may be under-represented in online studies (Sanchez et al., 2018; Sullivan et al., 2011). In the U.S., there are no population-based probability samples of MSM, with the largest ongoing study (the National HIV Behavioral Surveillance study) relying on venue-based recruitment that does not sample MSM who do not frequent gay-oriented social venues (Chapin-Bardales et al., 2019). However, our ARTnet estimates of diagnosed HIV prevalence based on self-report (10.3 % in Table 1, after removing unknown responses) were very close to recent estimates of diagnosed HIV prevalence for MSM in an analysis of national HIV case surveillance data (with additional adjustment to account for underreporting) of 10.6 % (Rosenberg et al., 2018). Furthermore, just as there are methods for weighting venue-based data (Jenness et al., 2011), our web-based ARTnet data may be weighted to account for biases in sampling. We did not weight currently as a first descriptive analysis of this dataset, but future localized analyses of ARTnet can and should weight.
Conclusions.
Estimates of network summary statistics among MSM that are up to date and broad in both demographic and geographic scopes, such as ARTnet, are essential. They provide the necessary empirical foundation for understanding population level HIV/STI transmission dynamics, as individual-level risks alone do not explain the size or heterogeneity in HIV/STI burden among MSM in the U.S. (Chapin-Bardales et al., 2019; Goodreau et al., 2017b). ARTnet demonstrates the feasibility of collecting these data quickly and nationally. Unlike other forms of network data collection, this type of egocentric sampling can be used for routine public health monitoring purposes. These network statistics do not tell the full epidemiological story, because they interact with the many factors that determine engagement in HIV-related prevention and care to generate epidemic dynamics. Mathematical modelling, and specifically network-based modeling that incorporates principled statistical estimation from egocentrically sampled network data, is needed to fully represent this transmission system. The descriptive statistics presented here are a first but important step in the process. These study designs and data, combined with the software tools for network model estimation and simulation (Jenness et al., 2018), provide a promising new framework for investigating and addressing, the critical challenges in HIV/STI prevention science.
4.1. Author statement
KMW and SMJ developed and executed the study, conducted the analyses, and wrote the manuscript. SMG and MM provided input on the study design, provided critical input on the analysis, and critically reviewed and edited the manuscript. PP, RR, and TS provided input on the study design and critically reviewed and edited the manuscript
Supplementary Material
Funding
This work was supported by National Institutes of Health grants R21 MH112449 and a grant from the MAC AIDS Fund.
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.epidem.2020.100386.
References
- Armbruster B, Wang L, Morris M, 2017. Forward reachable sets: analytically derived properties of connected components for dynamic networks. Netw Sci 5 (September (3)), 328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Read J, Pourbohloul B, Meyers LA, 2010. The dynamic nature of contact networks in infectious disease epidemiology. J. Biol. Dyn 4 (September (5)), 478–489. [DOI] [PubMed] [Google Scholar]
- Beck EC, Birkett M, Armbruster B, Mustanski B, 2015. A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs. J. Acquir. Immune Defic. Syndr 70 (October (2)), 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL, et al. , 1999. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J. Acquir. Immune Defic. Syndr 73 (December (4)), 446–453 2016 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer L, Oster AM, Mattson CL, Skarbinski J, 2014. Disparities in HIV transmission risk among HIV-infected black and white men who have sex with men, United States, 2009. AIDS 28 (1), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer L, Mattson CL, Bradley H, Shouse RL, 2017. Trends in ART prescription and viral suppression among HIV-positive young adults in care in the United States, 2009–2013. J. Acquir. Immune Defic. Syndr 1 April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett M, Kuhns LM, Latkin C, Muth S, Mustanski B, 2015. The sexual networks of racially diverse young men who have sex with men. Arch. Sex. Behav 44 (October (7)), 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie NB, Morris M, 2012. Size matters: concurrency and the epidemic potential of HIV in small networks. PLoS One 7 (8), e43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019a. HIV Infection Risk, Prevention, and Testing Behaviors Among Men Who Have Sex With Men—National HIV Behavioral Surveillance, 23 U.S. Cities, 2017. [Google Scholar]
- Centers for Disease Control and Prevention, 2019b. HIV Surveillance Report 30. pp. 1. (accessed .24 January 2020). http://www.cdc.gov/hiv/library/reports/hivsurveillance.html. [Google Scholar]
- Chapin-Bardales J, Rosenberg ES, Sullivan PS, Jenness SM, Paz-Bailey G, 2019. NHBS study group. Trends in number and composition of sex partners among men who have sex with men in the United States, national HIV behavioral surveillance, 2008–2014. JAIDS J Acquir Immune Defic Syndr. 1 (July (3)), 257–265 1;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. , 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med 365 (August (6)), 493–505 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Voux A, Kidd S, Grey JA, Rosenberg ES, Gift TL, Weinstock H, et al. , 2015. State-specific rates of primary and secondary syphilis among men who have sex with men — united States. MMWR Morb. Mortal. Wkly. Rep 66 (13), 349–354 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva W, Leventhal GE, Helleringer S, 2016. Connecting the dots: network data and models in HIV epidemiology. AIDS. 30 (June (13)), 2009–2020. [DOI] [PubMed] [Google Scholar]
- Eisinger RW, Dieffenbach CW, Fauci AS, 2019. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA 5 (Feburary (5)), 451–452 321. [DOI] [PubMed] [Google Scholar]
- Finlayson T, Cha S, Xia M, Trujillo L, Denson D, Prejean J, et al. , 2019. Changes in HIV preexposure prophylaxis awareness and use among men who have sex with men −20 urban areas, 2014 and 2017. MMWR Morb. Mortal. Wkly. Rep 12 (July (27)), 597–603 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein ND, Burstyn I, Welles SL, 2017. Bayesian approaches to racial disparities in HIV risk estimation among men who have sex with men. Epidemiology 28 (March (2)), 215–220. [DOI] [PubMed] [Google Scholar]
- Goodreau SM, Cassels S, Kasprzyk D, Montaño DE, Greek A, Montano DE, et al. , 2012. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS Behav. 16 (2), 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau SM, Rosenberg ES, Jenness SM, Luisi N, Stansfield SE, Millett GA, et al. , 2017a. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. Lancet HIV 4 (July (7)) e311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau SM, Rosenberg ES, Jenness SM, Luisi N, Stansfield SE, Millett GA, et al. , 2017b. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. Lancet HIV 4 (July (7)), e311–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey JA, Bernstein KT, Sullivan PS, Kidd SE, Gift TL, Hall EW, et al. , 2017. Rates of primary and secondary syphilis among white and black non-hispanic men who have sex with men, US States, 2014. J. Acquir. Immune Defic. Syndr 1 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn DD, 1997. Respondent-driven sampling: a new approach to the study of hidden populations. Soc. Probl 1 (May (2)), 174–199 44. [Google Scholar]
- Helleringer S, Kohler HP, Chimbiri A, Chatonda P, 2009. Mkandawire J. The likoma network study: context, data collection, and initial results. Demogr. Res 21, 427–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulis P, Phillips G, Birkett M, Mustanski B, 2018. Sexual networks of racially diverse young MSM differ in racial homophily but not concurrency. J. Acquir. Immune Defic. Syndr 77 (April (5)), 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Neaigus A, Murrill CS, Gelpi-Acosta C, Wendel T, Hagan H, 2011. Recruitment-adjusted estimates of HIV prevalence and risk among men who have sex with men: effects of weighting venue-based sampling data. Public Health Rep. 126 (5), 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, SMSM G, Morris M, Cassels S, 2016a. Effectiveness of combination packages for HIV-1 prevention in sub-Saharan Africa depends on partnership network structure: a mathematical modelling study. Sex. Transm. Infect 9 (June (8)), 619–624 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Goodreau SM, Rosenberg E, Beylerian EN, Hoover KW, Smith DK, et al. , 2016b. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Inf Dis. 214 (July (12)), 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Weiss KM, Goodreau SM, Gift T, Chesson H, Hoover KW, et al. , 2017a. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin. Infect. Dis 65 (5), 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Sharma A, Goodreau SM, Rosenberg ES, Weiss KM, Hoover KW, et al. , 2017b. Individual HIV Risk versus population impact of risk compensation after HIV preexposure prophylaxis initiation among men who have sex with men. PLoS One 12 (1), e0169484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness S, Goodreau SM, EpiModel MM, 2018. An r package for mathematical modeling of infectious disease over networks. J. Stat. Softw 84 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Maloney KM, Smith DK, Hoover KW, Goodreau SM, Rosenberg ES, et al. , 2019. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. Am. J. Epidemiol 188 (April (4)), 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna AS, Dimitrov DT, Goodreau SM, 2014. What can mathematical models tell us about the relationship between circular migrations and HIV transmission dynamics? Math. Biosci. Eng 11 (5), 1065–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosropour CM, Dombrowski JC, Swanson F, Kerani RP, Katz DA, Barbee LA, et al. , 1999. Trends in Serosorting and the association with HIV/STI risk over time among men who have sex with men. J. Acquir. Immune Defic. Syndr 2016 (2), 189–197 01;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klovdahl AS, Potterat JJ, Woodhouse DE, Muth JB, Muth SQ, Darrow WW, 1982. Social networks and infectious disease: the Colorado Springs Study. Soc Sci Med 38 (January (1)), 79–88 1994. [DOI] [PubMed] [Google Scholar]
- Kolaczyk ED, Krivitsky PN, 2015. On the question of effective sample size in network modeling: an asymptotic inquiry. Stat Sci Rev J Inst Math Stat. 1 (May (2)), 184–198 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer SC, Schmidt AJ, Berg RC, Furegato M, Hospers H, Folch C, et al. , 2016. Factors associated with unprotected anal sex with multiple non-steady partners in the past 12 months: results from the European Men-Who-Have-Sex-With-Men Internet Survey (EMIS 2010). BMC Public Health 19 (January 16), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivitsky PN, Handcock MS, 2014. A separable model for dynamic networks. J R Stat Soc Ser B Stat Methodol. 76 (1), 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivitsky PN, Morris M, 2017. Inference for social network models from egocentrically sampled data, with application to understanding persistent racial disparities in HIV prevalence in the US. Ann. Appl. Stat 11 (March (1)), 427–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivitsky PN, Handcock MS, Morris M, 2011. Adjusting for network size and composition effects in exponential-family random graph models. Stat. Methodol 8 (July (4)), 319–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BDL, Goedel WC, King MRF, Singleton A, Durham DP, Chan PA,et al. , 2018. Potential effectiveness of long-acting injectable pre-exposure prophylaxis for HIV prevention in men who have sex with men: a modelling study. Lancet HIV 5 (9), e498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DD, Smith JC, Brown AL, Malebranche DJ, 2016. Reconciling epidemiology and social justice in the public health discourse around the sexual networks of black men who have sex with men. Am. J. Public Health 106 (May (5)), 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett GA, Peterson JL, Wolitski RJ, Stall R, 2006. Greater risk for HIV infection of black men who have sex with men: a critical literature review. Am. J. Public Health 96 (6), 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KR, Mercer CH, Ploubidis GB, Jones KG, Datta J, Field N, et al. , 2013. Sexual function in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet Lond Engl. 30 (November (9907)), 1817–1829 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsch A, Singh S, Li J, Balaji A, Linley L, Selik R, 2018. Age-associated trends in diagnosis and prevalence of infection with HIV among men who have sex with men — united States, 2008–2016. MMWR Morb. Mortal. Wkly. Rep 21 (September (37)), 1025–1031 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, 1997. Sexual networks and HIV. AIDS(11 Suppl A) S209–16. [PubMed] [Google Scholar]
- Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S, 2009. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am. J. Public Health 99 (6), 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Morgan E, D’Aquila R, Birkett M, Janulis P, 2019. Newcomb ME. Individual and network factors associated with racial disparities in HIV among young men who have sex with men. J. Acquir. Immune Defic. Syndr 80 (January (1)), 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster AM, Wertheim JO, Hernandez AL, Ocfemia MCB, Saduvala N, Hall HI, 2015. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. J. Acquir. Immune Defic. Syndr 70 (4), 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. , 2011. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 378 (9787), 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Rothenberg RB, Kleinbaum DG, Stephenson RB, Sullivan PS, 1999. The implications of respondent concurrency on sex partner risk in a national, web-based study of men who have sex with men in the United States. J. Acquir. Immune Defic. Syndr 2013 (August(4)), 514–521 1;63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Purcell DW, Grey JA, Hankin-Wei A, Hall E, Sullivan PS, 2018. Rates of prevalent and new HIV diagnoses by race and ethnicity among men who have sex with men, U.S. states, 2013–2014. Ann. Epidemiol 28 (Decmber (12)), 865–873. [DOI] [PubMed] [Google Scholar]
- Sanchez TH, Zlotorzynska M, Sineath RC, Kahle E, Tregear S, Sullivan PS, 2018. national trends in sexual behavior, substance use and HIV testing among United States men who have sex with men recruited online, 2013 through 2017. AIDS Behav. 12 (August (8)), 2413–2425 22. [DOI] [PubMed] [Google Scholar]
- Singh S, Hu X, Wheeler W, Hall HI, 2014. HIV diagnoses among men who have sex with men and women-United States and 6 dependent areas, 2008–2011. Am. J. Public Health 104 (September(9)), 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Khosropour CM, Luisi N, Amsden M, Coggia T, Wingood GM, et al. , 2011. Bias in online recruitment and retention of racial and ethnic minority men who have sex with men. J. Med. Internet Res. 13 (May (2)), e38 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Peterson J, Rosenberg ES, Kelley CF, Cooper H, Vaughan A, et al. , 2014. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS One 9 (3), e90514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Purcell DW, Grey JA, Bernstein KT, Gift TL, Wimbly TA, et al. , 2018. Patterns of Racial/Ethnic disparities and prevalence in HIV and syphilis diagnoses among men who have sex with men, 2016: a novel data visualization. Am. J. Public Health 108 (November (S4)) S266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uong S, Rosenberg ES, Goodreau SM, Luisi N, Sullivan PS, Jenness SM, 2019. Assessment of Bias in estimates of sexual network degree using prospective cohort data. medRxiv. 14, 19003830 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Public Health Service, 2018. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update: a Clinical Practice Guideline. [Google Scholar]
- Whitham HK, Sansom SL, Wejnert C, Finlayson T, Y-LA H, An Q, et al. , 2017. Sex practices by HIV awareness and engagement in the continuum of care among MSM: a national HIV behavioral surveillance analysis in 21 U.S. Cities. AIDS Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workowski KA, 2015. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin. Infect. Dis 61 (December (suppl 8)), S759–S762. [DOI] [PubMed] [Google Scholar]
- Zlotorzynska M, Sullivan P, Sanchez T, 2016. The annual american men’s internet survey of behaviors of men who have sex with men in the United States. Key Indicators Report. JMIR Public Health Surveill. 2019 (October (1)), e11313 20;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


