Abstract
Introduction:
Prenatal alcohol exposure is associated with adverse pregnancy outcomes such as fetal alcohol spectrum disorders. The study characterizes the pattern and risk factors of alcohol use during pregnancy for American Indian and Caucasian mothers in the Northern Plains.
Methods:
A general population of pregnant women was recruited from five sites, including 2,753 Caucasians and 2,124 American Indians (2006–2017). Alcohol consumption was based on self-report using a modified Timeline Followback interview, administered three to four times during pregnancy and 1-month postpartum. Risk for prenatal drinking was calculated using logistic regression models after controlling for demographics, reproductive history, prenatal care, mental health, and SES. The analysis was conducted in 2019.
Results:
More Caucasian mothers consumed alcoholic beverages during pregnancy than American Indians (63% vs 52%), whereas more American Indian mothers were binge drinkers than Caucasians (41% vs 28%). American Indian mothers had a lower risk of drinking in the second and third trimesters and postpartum, but a higher risk of binge drinking in the first trimester compared with Caucasians. Frequent relocation increased the risk of prenatal alcohol use among American Indian mothers, whereas age, marriage, income, parity, and fertility treatment affected the risk of prenatal drinking among Caucasian mothers.
Conclusions:
Alcohol use was more prevalent among Caucasian mothers. Among those who consumed alcohol during pregnancy, American Indian mothers consumed larger quantities. Change of residence was found to be the sole risk factor for prenatal drinking among American Indian mothers, whereas different and multiple risk factors were found for Caucasian mothers.
INTRODUCTION
Prenatal alcohol exposure (PAE) is linked to a wide spectrum of adverse consequences. Fetal alcohol spectrum disorders are caused by PAE and encompass various types of neurodevelopmental disabilities, craniofacial anomalies, and growth impairments.1,2 Fetal alcohol spectrum disorder prevalence estimates are as high as one to five per 100 U.S. schoolchildren.3–6 Furthermore, PAE may increase the risk for fetal and infant mortality, including miscarriage, stillbirth, and sudden infant death syndrome.7–10 These adverse outcomes disproportionately affect socioeconomically disadvantaged populations and minority groups, including American Indians.11
Alcohol use by pregnant women is a significant public health concern. Based on the 2015–2017 data from Behavioral Risk Factor Surveillance System collected from phone survey of the U.S. population, 11.5% of pregnant women reported drinking and 3.9% reported binge drinking (consuming four or more drinks on at least one occasion) in the past 30 days.12 These estimates were slightly higher than those in 2011–2013 (10.2% drinking and 3.1% binge drinking).13 Analysis of the National Birth Defects Prevention Study data from phone interviews found that 30.3% of mothers who delivered live infants without birth defects reported drinking alcohol at some time during pregnancy, and 8.3% reported binge drinking.14
Maternal self-report is commonly used to collect information on alcohol consumption. Self-report methods are cost effective, especially on a large scale. Limitations include recall bias and under-reporting.15,16 Owing to concerns about confidentiality, social stigma, and punitive state laws,17 it is often felt that drinking in pregnancy is underestimated. Another reason for under-reporting is that some women only count alcohol use after pregnancy recognition. Even with self-reporting, prevalence estimates of prenatal drinking vary depending on how the data are collected.
In the Northern Plains (South Dakota, North Dakota), alcohol use is high among women of childbearing age. According to the 2016 self-reported data from the Behavioral Risk Factor Surveillance System, the prevalence estimates of any alcohol use ranged from 60% to 66% during the last 30 days, and the rates of binge drinking was reported at 23%–26%. The Northern Plains has a large American Indian population. It is the common belief that American Indian mothers drink alcohol at higher rates during pregnancy compared with Caucasian mothers. Some studies conducted in this region supported this notion.18,19 However, other studies found lower overall rates of alcohol use among American Indian pregnant women than other racial groups either nationally or in the Northern Plains.20,21
The Safe Passage Study, conducted by the Prenatal Alcohol in Sudden Infant Death Syndrome and Stillbirth Network, was a large longitudinal prospective cohort study designed to investigate the association between PAE and stillbirth and sudden infant death syndrome.11 Pregnant women were enrolled from the Northern Plains and South Africa, where residents are historically at higher risk for prenatal drinking and poorer pregnancy outcomes. Detailed information on the quantity, frequency, and timing of drinking was captured.15 The current analysis focuses on the Northern Plains cohort by comparing prenatal drinking between American Indian and Caucasian mothers. Improving the estimate of prenatal drinking can improve allocation of resources for public health prevention efforts and referral for treatment.
METHODS
Study Sample
The Safe Passage Study enrolled 4,968 unique pregnancies from the Northern Plains (2006–2017). A general population was recruited from five sites, including two on American Indian Reservations.11,22 The inclusion criteria for this analysis were women: (1) enrolled from the Northern Plains, (2) with drinking information, and (3) either Caucasian or American Indian. A total of 4,877 pregnant women met eligibility criteria, including 2,753 (56%) Caucasians and 2,124 (44%) American Indians. Ninety-one subjects were excluded: four without information on alcohol consumption and 87 self-identified as other races.
Measures
The Safe Passage Study used a modified Timeline Followback method (TLFB) to capture alcohol use during pregnancy.15 Specially, at the visit, drinking information on alcohol type, container size, sharing, and duration was captured for 30 days prior to the last drinking day. The TLFB interview was administered up to five times: at recruitment (6 gestational weeks to delivery), at up to three prenatal visits (20–24, 28–32, and >34 gestational weeks), and at 1 month post-delivery. At recruitment, alcohol consumption 15 days before and after the last menstrual period (LMP) was also collected. Alcohol use (yes/no) at 1 year prior to pregnancy was collected as well. The number of standard drinks was computed using information from the modified TLFB.11 Binge drinking was defined as four or more standard drinks per occasion.23
Analyzed maternal characteristics included demographics, reproductive history, prenatal care, psychological (e.g., depression, anxiety), and SES (e.g., income, relocation) (Table 1). This information was self-reported and collected at enrollment.11
Table 1.
Maternal Characteristics Potentially Confounding Racial Differences in Prenatal Alcohol Use
| Characteristics | American Indian, n (%)a 2,124 (44) | Caucasian, n (%) 2,753 (56) |
|---|---|---|
| Demographics | ||
| Ethnicity | ||
| Not Hispanic or Latina | 1,973 (93) | 2,701 (98) |
| Hispanic or Latina | 151 (7) | 52 (2) |
| Age, years | ||
| <20 | 364 (17) | 110 (4) |
| 20–34 | 1,630 (77) | 2,369 (86) |
| ≥35 | 130 (6) | 274 (10) |
| Marital status | ||
| Single | 583 (27) | 181 (7) |
| Married or partnered | 1,539 (73) | 2,572 (93) |
| Education | ||
| Any primary school | 76 (4) | 10 (0) |
| Some high school | 815 (38) | 98 (4) |
| Completed high school | 606 (29) | 358 (13) |
| Beyond high school | 625 (29) | 2,287 (83) |
| Partner education | ||
| Any primary school | 30 (2) | 7 (0) |
| Some high school | 432 (30) | 88 (3) |
| Completed high school | 631 (43) | 526 (21) |
| Beyond high school | 369 (25) | 1,914 (76) |
| Infant sex | ||
| Male | 960 (49) | 1,301 (49) |
| Female | 981 (51) | 1,350 (51) |
| Reproductive history | ||
| Gravidity | ||
| 1 | 442 (22) | 794 (29) |
| 2 | 486 (24) | 814 (30) |
| 3 | 360 (18) | 525 (20) |
| >3 | 738 (36) | 564 (21) |
| Parity | ||
| 0 | 532 (26) | 999 (37) |
| 1 | 501 (25) | 938 (35) |
| 2 | 373 (18) | 492 (18) |
| >2 | 620 (31) | 268 (10) |
| Fertility treatmentb | ||
| No | 1,944 (100) | 2,502 (94) |
| Yes | 5 (0) | 154 (6) |
| Prenatal care | ||
| Prenatal care in all trimesters | ||
| No | 789 (40) | 202 (8) |
| Yes | 1,174 (60) | 2,455 (92) |
| Mental health | ||
| Anxietyc | ||
| No | 1,262 (71) | 2,300 (87) |
| Yes | 504 (29) | 332 (13) |
| Depressiond | ||
| No | 1,481 (83) | 2,467 (94) |
| Yes | 306 (17) | 169 (6) |
| SES | ||
| Health coveragee | ||
| Commercial insurance | 43 (2) | 1,924 (73) |
| Self-pay | 1 (0) | 41 (2) |
| Public assistance | 1,725 (98) | 662 (25) |
| Government supportf | ||
| None | 50 (4) | 1,724 (75) |
| Prenatal only | 5 (1) | 19 (1) |
| Postnatal only | 62 (5) | 104 (5) |
| Both | 1,042 (90) | 434 (19) |
| Household monthly income, dollars | ||
| 250 | 324 (19) | 35 (1) |
| 750 | 493 (28) | 91 (4) |
| 1,500 | 518 (30) | 293 (11) |
| >2,500 | 402 (23) | 2,181 (84) |
| Relocation within a year | ||
| None | 766 (45) | 1,770 (68) |
| Once | 502 (29) | 558 (22) |
| More than once | 441 (26) | 262 (10) |
Note: Pearson’s chi-squared test to test race differences in the variables: p<0.05 for all variables except infant sex.
Race information was collected in the following categories: American Indian or Alaska Native, white, Asian, black or African American, Native Hawaiian or other Pacific Islander, and other/unknown. Only American Indian or Alaska Native and white were included in this study.
Fertility treatment included fertility treatment included assisted reproductive technology (in vitro fertilization, intracystoplasmic sperm injection, zygote intrafallopian transfer, gamete intrafallopian transfer) and ovulation induction with or without artificial insemination.
Anxiety was defined as having a STAI State Anxiety Cutoff Score >40 or STAI Trait Anxiety Cutoff Score >40 at any time point during pregnancy.
Depression was defined as having an Edinburgh Depression Scale >13 at any time point during pregnancy.
Public assistance included statewide Medicaid program, other statewide option, Veteran’s Benefits, and Indian Health Services.
Government support referred to Electronic Benefit Transfer (EBT)/Food Stamps, or Temporary Assistance for Needy Families (TANF), or Women, Infants and Children (WIC).
Statistical Analysis
Analyses were performed using R, version 3.5.1 in 2019. Chi-square tests were used to evaluate racial differences in maternal characteristics. The Wilcoxon rank sums test was employed for a two-sided comparison of the amount of alcohol consumed by American Indians and Caucasians. An effect was considered as statistically significant if the p-value of the test was <0.05.
Simple logistic regression analyses were performed; drinking or binge drinking was modeled as the outcome, and race or other maternal characteristics that showed significant differences between Caucasian and American Indian mothers as the exposure (Appendix Table 1). ORs and the 95% CIs were derived from the logistic regression. Multiple logistic regression analyses were further performed by including all variables to obtain AORs. Multicollinearity of variables was evaluated by computing the variance inflation factors. Every variable in each model has a variance inflation factor value <5, which is a commonly used threshold.24 Significance of an OR was defined as the 95% CI not including 1.
Regression models for drinking or binge drinking were built at each of the timeframes: 15 days before and after LMP, first trimester, second trimester, third trimester, and 1-month post-delivery. The analysis was performed by trimester, concordant with the visit schedule of the Safe Passage Study. Sensitivity analyses using the model accounting for the entire pregnancy was compared to individual timeframe analyses. Because of the imbalanced data sets, models were not built for drinking 1-year prior to pregnancy and binge drinking in the second and third trimesters. To evaluate race-specific factors that associate with drinking, the entire cohort was stratified into two groups based on race. Separate multiple logistic regression models were built for American Indians and Caucasians. Sensitivity analyses were performed to determine whether the models based on racial stratification were similar to those from the entire cohort.
Human subject studies were performed in accordance with the ethical guidelines and approved by the Sanford IRB, Avera IRB, Oglala Sioux Tribe Research Review Board, and Great Plains Indian Health Service IRB.
RESULTS
Compared with Caucasian mothers, a higher percentage of American Indian mothers were Hispanic, aged <20 years, and single. Most American Indian women and their partners received education at or below the level of high school, whereas the majority of the Caucasian counterparts had education beyond high school. The number of pregnancies was greater among American Indian mothers than among Caucasian mothers. Around 6% of Caucasian women underwent assisted reproductive technology or ovulation induction, in contrast to 0.3% for American Indians. More than 90% of Caucasian mothers had prenatal care in all trimesters whereas only 60% of American Indian mothers received prenatal care in all trimesters. American Indian mothers showed higher prevalence of anxiety and depression than Caucasians. Most American Indian mothers had a lower SES, reflected in health coverage from public assistance, government support, and low income. In addition, American Indian mothers relocated more often: 26% moved more than once within a year in contrast to 10% for Caucasian mothers. Significant differences were found in all demographics and social indicators between the two races except the infant sex (Table 1).
During the entire pregnancy, Caucasian mothers were more likely to consume alcohol than American Indians (63% vs 52%). Conversely, American Indian mothers were more likely to binge drink than Caucasians (41% vs 28%). This was also true at individual timeframes: 1 year prior to pregnancy, LMP, three trimesters, and 1 month postpartum (Figure 1, Appendix Tables 2 and 3). Most women consumed alcohol in the year prior to pregnancy. Around half of the women reported alcohol use at LMP and first trimester. Few reported drinking in the second and third trimesters. However, alcohol use rose 1 month after delivery. The trend of binge drinking across pregnancy was similar to that of any drinking, but with lower prevalence rates.
Figure 1.
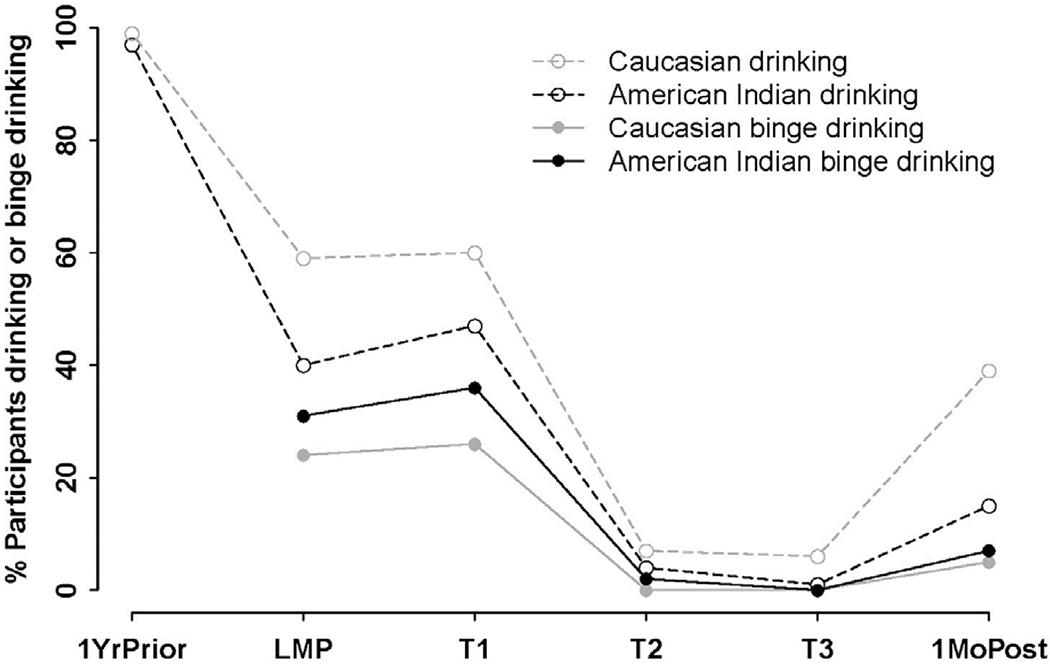
Prevalence of drinking and binge drinking before, during, and after pregnancy by race.
Notes: At each time point, a significant difference in dichotomous drinking was found between American Indian and Caucasian mothers (p<0.05, Chi-squared test). A significant difference was also found in binge drinking between the two races at each time point except T3 (p<0.05 Chi-squared test).
1YrPrior, 1 year prior to pregnancy; LMP, last menstrual period; T1, first trimester; T2, second trimester; T3, third trimester, 1MoPost, 1 month post-delivery.
Among women who reported any alcohol use, Caucasian mothers had a higher number of drinking days than American Indians during and after pregnancy. Some women consumed alcohol every day around LMP and after delivery (Appendix Table 4). However, the quantity of drinks consumed by American Indians was greater than Caucasians. There were individuals of both races who consumed alcohol heavily around the LMP and in the first trimester (Appendix Figure 1). Among binge drinkers, American Indian mothers reported more binge episodes than Caucasian mothers around the LMP and first trimester. A few women reported heavy binge drinking in these timeframes independent of race (Appendix Figure 2).
The ORs of drinking for American Indians (Caucasians as the reference) were calculated during and after pregnancy (Figure 2, Appendix Table 5). The crude ORs and 95% CIs were <1 at each time period. After the adjustment of covariates, American Indian mothers were found to be less likely to consume alcohol compared with Caucasians in the second and third trimesters as well as post-delivery. Specifically, there was a twofold decreased risk of any alcohol use for American Indian mothers compared with Caucasians in the second trimester (AOR=0.52). The risk was further reduced in the third trimester and after delivery (AOR=0.10, AOR=0.30). Sensitivity analyses using the model accounting for the entire pregnancy found no significant race differences in alcohol consumption (Table 2).
Figure 2.
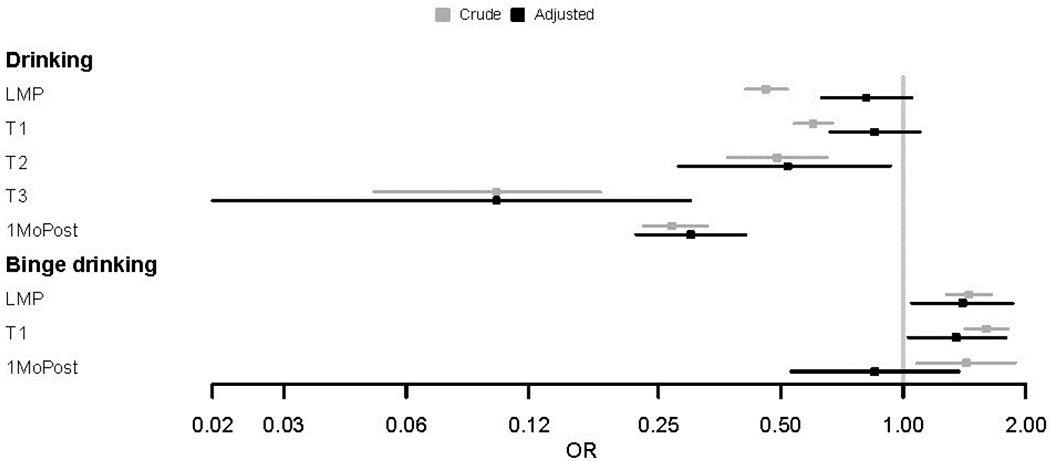
Association of drinking or binge drinking during and after pregnancy with being American Indian.
Notes: The associations were estimated without adjustment (crude OR) and with adjustment (AOR) for factors showing significant differences between American Indians and Caucasians in Appendix Table 1. Due to the reduced sample size of women who were binge drinkers in the second and third trimester, race differences in binge drinking were not determined in the two time frames.
LMP, last menstrual period; T1, first trimester; T2, second trimester; T3, third trimester, 1MoPost, 1 month post-delivery.
Table 2.
Association of Alcohol Consumption During the Entire Pregnancy With Maternal Characteristics by Race
| Variablesa | Drinking | Binge drinking | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| American Indian | Caucasian | Entire cohortb | American Indian | Caucasian | Entire cohort | |||||||
| OR (95% CI) | AORc (95% CI) | OR (95% CI) | AOR (95% CI) | OR (95% CI) | AOR (95% CI) | OR (95% CI) | AOR (95% CI) | OR (95% CI) | AOR (95% CI) | OR (95% CI) | AOR (95% CI) | |
| American Indian, ref=Caucasian | NA | NA | NA | NA | 0.64 (0.57, 0.72) | 0.81 (0.63, 1.05) | NA | NA | NA | NA | 1.86 (1.64, 2.11) | 1.38 (1.05, 1.83) |
| Hispanic, ref=no | 1.21 (0.86, 1.72) | 1.10 (0.61, 1.97) | 1.00 (0.57, 1.84) | 1.60 (0.74, 3.70) | 0.99 (0.74, 1.34) | 1.21 (0.76, 1.92) | 1.20 (0.84, 1.70) | 0.97 (0.51, 1.76) | 0.90 (0.45, 1.69) | 0.76 (0.30, 1.74) | 1.37 (1.01, 1.85) | 0.89 (0.53, 1.44) |
| Age | 1.03 (0.85, 1.24) | 1.38 (0.97, 1.98) | 1.41 (1.13, 1.75) | 1.58 (1.19, 2.11) | 1.30 (1.12, 1.49) | 1.59 (1.27, 1.98) | 0.99 (0.82, 1.21) | 1.26 (0.87, 1.82) | 0.92 (0.73, 1.17) | 1.12 (0.81, 1.53) | 0.83 (0.72, 0.97) | 1.25 (0.99, 1.57) |
| Married or partnered, ref=single | 0.73 (0.60, 0.89) | 1.06 (0.74, 1.53) | 1.11 (0.81, 1.52) | 0.91 (0.53, 1.53) | 1.00 (0.85, 1.17) | 1.02 (0.76, 1.38) | 0.71 (0.58, 0.87) | 0.91 (0.63, 1.33) | 0.52 (0.38, 0.71) | 0.58 (0.34, 0.99) | 0.53 (0.45, 0.63) | 0.80 (0.59, 1.09) |
| Education | 0.98 (0.88, 1.08) | 0.99 (0.83, 1.16) | 1.54 (1.32, 1.79) | 1.22 (0.96, 1.55) | 1.25 (1.16, 1.34) | 1.02 (0.89, 1.17) | 0.89 (0.80, 0.99) | 0.97 (0.81, 1.15) | 1.12 (0.94, 1.35) | 1.00 (0.77, 1.32) | 0.79 (0.73, 0.84) | 0.96 (0.83, 1.11) |
| Partner education | 0.92 (0.81, 1.06) | 0.87 (0.73, 1.05) | 1.27 (1.09, 1.47) | 0.96 (0.79, 1.17) | 1.30 (1.19, 1.41) | 0.93 (0.81, 1.06) | 0.86 (0.75, 0.99) | 0.85 (0.70, 1.02) | 1.00 (0.84, 1.18) | 0.95 (0.77, 1.17) | 0.81 (0.74, 0.88) | 0.90 (0.79, 1.04) |
| Gravidity | 0.87 (0.80, 0.94) | 0.94 (0.68, 1.30) | 0.80 (0.74, 0.86) | 0.97 (0.83, 1.13) | 0.80 (0.77, 0.85) | 0.95 (0.83, 1.09) | 0.92 (0.85, 0.99) | 0.99 (0.70, 1.38) | 0.84 (0.77, 0.91) | 1.01 (0.86, 1.19) | 0.92 (0.87, 0.97) | 0.99 (0.85, 1.15) |
| Parity | 0.88 (0.81, 0.95) | 0.92 (0.66, 1.28) | 0.76 (0.70, 0.82) | 0.70 (0.59, 0.84) | 0.79 (0.75, 0.83) | 0.79 (0.68, 0.91) | 0.92 (0.85, 0.99) | 0.96 (0.68, 1.36) | 0.79 (0.72, 0.86) | 0.73 (0.60, 0.89) | 0.93 (0.88, 0.99) | 0.83 (0.71, 0.98) |
| Fertility treatment, ref=no | 1.44 (0.24, 10.93) | 1.14 (0.13, 10.09) | 0.47 (0.34, 0.66) | 0.38 (0.26, 0.54) | 0.60 (0.44, 0.83) | 0.41 (0.29, 0.59) | 0.37 (0.02, 2.51) | 0.00 (NA) | 0.32 (0.18, 0.53) | 0.33 (0.19, 0.55) | 0.26 (0.15, 0.42) | 0.34 (0.19, 0.56) |
| Prenatal care, ref=no | 0.88 (0.73, 1.06) | 0.75 (0.56, 1.00) | 1.08 (0.80, 1.45) | 0.87 (0.58, 1.29) | 1.17 (1.01, 1.35) | 0.76 (0.60, 0.96) | 0.86 (0.71, 1.05) | 0.85 (0.63, 1.16) | 1.09 (0.77, 1.57) | 0.97 (0.62, 1.53) | 0.69 (0.59, 0.81) | 0.86 (0.68, 1.11) |
| Anxiety, ref=no | 1.25 (1.02, 1.55) | 1.13 (0.80, 1.60) | 0.79 (0.63, 1.00) | 0.88 (0.65, 1.21) | 0.90 (0.77, 1.05) | 1.00 (0.79, 1.26) | 1.41 (1.14, 1.75) | 1.33 (0.93, 1.90) | 1.09 (0.84, 1.41) | 0.94 (0.66, 1.32) | 1.44 (1.22, 1.69) | 1.11 (0.87, 1.42) |
| Depression, ref=no | 1.19 (0.93, 1.53) | 1.05 (0.70, 1.58) | 0.81 (0.59, 1.11) | 0.90 (0.58, 1.40) | 0.89 (0.74, 1.08) | 1.00 (0.75, 1.35) | 1.21 (0.93, 1.55) | 1.00 (0.65, 1.51) | 1.13 (0.79, 1.59) | 1.10 (0.68, 1.75) | 1.36 (1.11, 1.66) | 1.05 (0.77, 1.43) |
| Health coverage | 0.96 (0.71, 1.31) | 0.91 (0.59, 1.39) | 0.87 (0.80, 0.96) | 1.13 (0.95, 1.34) | 0.78 (0.74, 0.83) | 1.03 (0.89, 1.20) | 1.28 (0.92, 1.83) | 1.26 (0.79, 2.10) | 1.10 (1.00, 1.22) | 1.14 (0.95, 1.37) | 1.28 (1.20, 1.37) | 1.10 (0.93, 1.29) |
| Government support | 0.89 (0.74, 1.06) | 0.83 (0.67, 1.04) | 0.84 (0.78, 0.90) | 0.88 (0.78, 0.99) | 0.80 (0.76, 0.83) | 0.84 (0.75, 0.93) | 1.16 (0.96, 1.42) | 0.96 (0.76, 1.22) | 1.02 (0.94, 1.10) | 0.96 (0.83, 1.10) | 1.13 (1.07, 1.19) | 0.93 (0.83, 1.05) |
| Household income | 0.97 (0.88, 1.06) | 1.08 (0.94, 1.24) | 1.30 (1.13, 1.49) | 1.31 (1.05, 1.65) | 1.22 (1.15, 1.30) | 1.13 (1.01, 1.27) | 0.92 (0.83, 1.01) | 1.07 (0.93, 1.24) | 0.94 (0.81, 1.10) | 1.20 (0.94, 1.56) | 0.81 (0.76, 0.87) | 1.09 (0.96, 1.23) |
| Relocation | 1.38 (1.22, 1.55) | 1.38 (1.16, 1.64) | 0.96 (0.85, 1.08) | 0.95 (0.82, 1.11) | 1.05 (0.96, 1.14) | 1.12 (1.00, 1.26) | 1.34 (1.19, 1.51) | 1.29 (1.08, 1.54) | 1.16 (1.02, 1.32) | 0.97 (0.82, 1.15) | 1.35 (1.24, 1.47) | 1.12 (0.99, 1.27) |
Note: Boldface indicates statistical significance (95% CI not including 1).
Values assigned to each variable are in Appendix Table 1.
The entire cohort includes both American Indian and Caucasian mothers.
AOR was calculated from multivariate logistic regression models by including all variables in the table.
NA, not applicable.
Based on crude ORs, the risk of binge drinking was higher for American Indian mothers compared with Caucasians in individual timeframes (Figure 2, Appendix Table 6). After the adjustment of confounding variables, increased risk of binge drinking was observed around the LMP and first trimester for American Indian mothers. The risk increased modestly in these two timeframes (AOR=1.40, AOR=1.35). Sensitivity analyses using the model for the entire pregnancy showed a similar result (AOR=1.38) (Table 2), indicating American Indian mothers were more likely to binge drink during pregnancy.
Drinking behaviors were also be influenced by social and economic factors (Table 2). For Caucasian mothers, the risk of alcohol consumption increased with age (AOR=1.58), and the risk of binge drinking decreased for those who were married or partnered (AOR=0.58). Mothers with more pregnancies or those who received fertility treatment had decreased odds of drinking and binge drinking (parity: AOR=0.70, AOR=0.73; fertility treatment: AOR=0.38, AOR=0.33). In addition, the risk for alcohol use increased with higher household income (AOR=1.31) and minimal government support (AOR=0.88).
For American Indian mothers, the risk factors for alcohol use during pregnancy were very different from those among Caucasian mothers. Frequent change of residence was the only factor that significantly increased the risk of drinking and binge drinking among American Indian mothers (AOR=1.38, AOR=1.29). Sensitivity analyses showed that the models using the entire cohort identified similar risk factors for prenatal drinking to those based on racial stratification. (Table 2).
DISCUSSION
This study analyzed the alcohol use among 4,877 pregnant women from the Northern Plains. Fifty-nine percent of women reported alcohol consumption at some time during pregnancy and 33% reported binge drinking. These rates are higher than the estimates from two national studies.12,14 Although the general population was recruited, the Northern Plains historically has had more alcohol use than the national average. Data collection methods also play a role in the higher drinking rates from this study. The Safe Passage study collected alcohol use data via in-person in-depth interviews using TLFB approaches,15 whereas the two national studies conducted phone surveys.12,14
Decreased drinking rates after the first trimester were found. This is consistent with results from the National Birth Defects Prevention Study.14 Late recognition of pregnancy is likely the reason for a higher alcohol use rate in the first trimester than later trimesters. Binge drinking during early pregnancy has a profound effect on brain and organ development.25 The fact that binge drinking was higher in the first trimester for American Indians compared with Caucasians has important consequences.
This analysis illustrates the opposite of commonly held stereotypes or misconceptions about racial differences in prenatal alcohol use in the Northern Plains. First, this analysis demonstrates that more American Indian women abstained from drinking during pregnancy than Caucasian women. Second, the decline in the rate of drinking over the course of pregnancy was comparable between American Indian and Caucasian mothers. The finding of lower rates of alcohol use for American Indian pregnant women is consistent with the result from an earlier study that used the 2005–2009 data from National Survey of Drug Use and Health.21 Similar to the Safe Passage study, that was an in-person survey, which can potentially produce different results than telephone interviewing or mail survey.
Others studies conducted in the Northern Plains have found either no difference or more prenatal alcohol use by American Indians. One study reported no difference in alcohol consumption by race via mailing surveys to South Dakota mothers who gave birth in 2014.20 Based on the 2007–2012 birth certificates self-reported by North Dakota women, American Indians had more alcohol use during pregnancy than Caucasians.19 Another Northern Plains study asked women to complete a Prenatal Questionnaire at their first prenatal care visit and found that American Indian women had a higher risk for alcohol use during pregnancy compared with other racial groups.18 Therefore, the discrepancies in prenatal drinking between races may be due in part to the timing of assessments, variations in self-report methodology, and sample size.
American Indian mothers were found to be younger and have fewer years of education and lower household income than Caucasians, similar to the findings from other studies.19,20 Some of these demographic and social factors significantly impacted alcohol use during pregnancy. For American Indian mothers, the change of residence was the single most important risk factor for prenatal drinking and binge drinking. Frequent relocation is usually associated with the financial stress as well as lack of social support. For American Indians specifically, relocation occurs between homes on a reservation or between reservations and cities. People who experience changes of residence are prone to stress and poor mental health,26,27 which may contribute to heavier drinking behaviors. Most risk factors were not significant for American Indian women. The SES status of American Indians was relatively uniform and located in the lower category of government support and health coverage.
Risk factors of prenatal alcohol use for Caucasian mothers were substantially different from those for American Indians. Older Caucasian women from higher-income families were more likely to drink during pregnancy. This result has confirmed findings from other studies conducted in the Northern Plains, U.S., United Kingdom, Netherlands, and Norway that prenatal drinking is more common among older women.20,28 Women of higher SES are older and more likely to participate in social activities involved in alcohol consumption.
Limitations
The current study has several limitations. First, alcohol consumption was self-reported and could be complemented by objectively measured biomarkers. Several biomarkers have been developed to evaluate PAE by measuring ethanol metabolites in specimens from either the mother or infant or measuring offspring DNA methylation.28–32 Second, although a general population in the Northern Plains was recruited, the participants who visited the clinic sites may be subject to sampling bias. Third, the race information was self-reported, which could lead to misclassification. Fourth, addresses of relocation were not collected. Thus, for American Indians, it is unknown whether relocation occurred more frequently on reservations or between reservations and cities. Finally, interaction terms for maternal variables need to be investigated further.
CONCLUSIONS
This is the first study of this size to comprehensively characterize the alcohol use among pregnant women in the Northern Plains. Although alcohol use during pregnancy was less common among American Indians than Caucasians, among those who consumed alcohol, American Indian mothers consumed larger quantities. Relocation was found to be the sole risk factor for American Indian mothers, whereas different risk factors were found for Caucasian mothers. Understanding racial differences in risk factors and patterns of alcohol consumption will help tailor prevention efforts toward specific racial groups and may lead to greater acceptance of prevention efforts. In the past, health promotion campaigns focused exclusively on complete avoidance of alcohol by women when pregnant or attempting to become pregnant have faced resistance.33 Future programs and interventions for prenatal alcohol use must consider contextual factors. For American Indian mothers specifically, programs should be in place to provide housing and social assistance, and education should focus on the important consequences of binge drinking.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the cooperation of the study participants, Prenatal Alcohol in Sudden Infant Death Syndrome and Stillbirth Network investigators, and numerous study staff. The authors would like to acknowledge Dr. Christine Hockett for reviewing the manuscript.
The research presented in this paper is that of the authors and does not reflect the official policy of the NIH. The study sponsor had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
The research reported in this publication was supported by NIH grants U01HD055154 (Dukes), U01HD045935 (Elliott), U01HD055155 (Fifer), U01HD045991 (Kinney), and U01AA016501 (Odendaal) funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115(1):39–47. 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coriale G, Fiorentino D, Di Lauro F, et al. Fetal alcohol spectrum disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment. Riv Psichiatr. 2013;48(5):359–369. 10.1708/1356.15062. [DOI] [PubMed] [Google Scholar]
- 3.May PA, Chambers CD, Kalberg WO, et al. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA. 2018;319(5):474–482. 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May PA, Gossage JP, Kalberg WO, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3): 176–192. 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 5.May PA, Baete A, Russo J, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134(5):855–866. 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001. ;25(3): 159–167. [PMC free article] [PubMed] [Google Scholar]
- 7.Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake during pregnancy and the risk of stillbirth and death in the first year of life. Am J Epidemiol. 2002;155(4):305–312. 10.1093/aje/155.4.305. [DOI] [PubMed] [Google Scholar]
- 8.Strandberg-Larsen K, Gronboek M, Andersen AM, Andersen PK, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884–891. 10.1097/ede.0b013e3181bbd46c. [DOI] [PubMed] [Google Scholar]
- 9.Bailey BA, Sokol RJ. Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Iyasu S, Randall LL, Welty TK, et al. Risk factors for sudden infant death syndrome among Northern Plains Indians. JAMA. 2002;288(21):2717–2723. 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- 11.Dukes KA, Burd L, Elliott AJ, et al. The Safe Passage Study: design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol. 2014;28(5):455–465. 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny CH, Acero CS, Naimi TS, Kim SY. Consumption of alcohol beverages and binge drinking among pregnant women aged 18–44 years — United States, 2015–2017. MMWR Morb Mortal Wkly Rep. 2019;68(16):365–368. 10.15585/mmwr.mm6816a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2015;64(37): 1042–1046. 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- 14.Ethen MK, Ramadhani TA, Scheuerle AE, et al. Alcohol consumption by women before and during pregnancy. Matem Child Health J. 2009;13(2):274–285. 10.1007/sl0995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dukes K, Tripp T, Petersen J, et al. A modified Timeline Followback assessment to capture alcohol exposure in pregnant women: application in the Safe Passage Study. Alcohol. 2017;62:17–27. 10.1016/j.alcohol.2017.02.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DE, Naimi TS, Brewer RD, Roeber J. US state alcohol sales compared to survey data, 1993–2006. Addiction. 2010;105(9): 1589–1596. https://doi.org/l0.1111/j.1360-0443.2010.03007.x. [DOI] [PubMed] [Google Scholar]
- 17.Substance Use During Pregnancy State laws and policies as of July 1. www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Guttmacher Institute Accessed July 3, 2019.
- 18.Leonardson GR, Loudenburg R. Risk factors for alcohol use during pregnancy in a multistate area. Neurotoxicol Teratol. 2003;25(6):651–658. 10.1016/j.ntt.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Danielson RA, Wallenborn JT, Warne DK, Masho SW. Disparities in risk factors and birth outcomes among American Indians in North Dakota. Matern Child Health J. 2018;22(10): 1519–1525. 10.1007/sl0995-018-2551-9. [DOI] [PubMed] [Google Scholar]
- 20.Specker BL, Wey HE, Minett M, Beare TM. Pregnancy survey of smoking and alcohol use in South Dakota American Indian and white mothers. Am J Prev Med. 2018;55(1):89–97. 10.1016/j.amepre.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Watt TT. Alcohol use and cigarette smoking during pregnancy among American Indians/Alaska Natives. J Ethn Subst Abuse. 2012;11(3):262–275. 10.1080/15332640.2012.701570. [DOI] [PubMed] [Google Scholar]
- 22.Dukes K, Tripp T, Willinger M, et al. Drinking and smoking patterns during pregnancy: development of group-based trajectories in the Safe Passage Study. Alcohol. 2017;62:49–60. 10.1016/j.alcohol.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute on Alcoholism and Alcohol Abuse. Council approves definition of binge drinking. NIAAA Newsletter. 2004;3 10.1037/e306662005-004. [DOI] [Google Scholar]
- 24.Kock N, Lynn GS. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. Journal of the Association for Infomiation Systems. 2012;13(7):2 10.17705/ljais.00302. [DOI] [Google Scholar]
- 25.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25(3):168–174. [PMC free article] [PubMed] [Google Scholar]
- 26.Lin KC, Twisk JW, Huang HC. Longitudinal impact of frequent geographic relocation from adolescence to adulthood on psychosocial stress and vital exhaustion at ages 32 and 42 years: the Amsterdam growth and health longitudinal study. J Epidemiol. 2012;22(5):469–476. 10.2188/jea.je20110141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costlow K, Parmelee PA. The impact of relocation stress on cognitively impaired and cognitively unimpaired long-term care residents. Aging Ment Health. In press. Online August 30, 2019. 10.1080/13607863.2019.1660855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp GC, Arathimos R, Reese SE, et al. Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics. 2018;10(1):27–42. 10.2217/epi-2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concheiro-Guisan A, Concheiro M. Bioanalysis during pregnancy: recent advances and novel sampling strategies. Bioanalysis. 2014;6(23):3133–3153. 10.4155/bio.14.278. [DOI] [PubMed] [Google Scholar]
- 30.Lussier AA, Morin AM, Maclsaac JL, et al. DNA methylation as a predictor of fetal alcohol spectrum disorder. Clin Epigenetics. 2018;10:5 10.1186/sl3148-018-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portales-Casamar E, Lussier AA, Jones MJ, et al. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin. 2016;9:25 10.1186/s13072-016-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laufer BI, Kapalanga J, Castellani CA, Diehl EJ, Yan L, Singh SM. Associative DNA methylation changes in children with prenatal alcohol exposure. Epi genomics. 2015;7(8): 1259–1274. 10.2217/epi.15.60. [DOI] [PubMed] [Google Scholar]
- 33.Seiler NK. Alcohol and pregnancy: CDC’s health advice and the legal rights of pregnant women. Public Health Rep. 2016;131(4):623–627. 10.1177/0033354916662222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


