Abstract
Chemotherapy-related cognitive impairment and associated brain changes may reflect accelerated brain aging; however, empirical evidence for this theory is limited. The purpose of this study was to measure brain aging in newly diagnosed patients with breast cancer treated with chemotherapy (n=43) and compare its longitudinal change to that of controls (n=50). Brain age indices, derived from cortical measures, were compared between women with breast cancer and matched healthy controls across 3 timepoints (Time 1: pre-surgery, Time 2: 1 month following chemotherapy completion, and Time 3: 1-year post chemotherapy). The breast cancer group showed a significant decrease in cortical thickness across the 3 timepoints (p<.001) and a trend toward significant increase in predicted brain age especially from pre-treatment (Time 1) to post-chemotherapy (Time 2) compared to controls (p = 0.08). Greater increase in predicted brain age was related to several clinical factors (HER-2 status, surgery type, and history of neoadjuvant chemotherapy) and greater decrease in cortical thickness was associated with greater decrease in performance on a verbal learning task from Time 1 to Time 3 (r = −0.48, p <.01). This study demonstrated evidence of increased cortical brain aging in middle-aged patients with breast cancer following chemotherapy treatment that was associated with decreased verbal memory performance.
Keywords: chemotherapy-related cognitive impairment, neuroimaging, MRI, cortical brain age, breast cancer, prospective study
Introduction
Cancer and its treatments are associated with an increased risk for cognitive impairment. Our group and others have shown that patients who undergo chemotherapy treatment demonstrate measurable brain injury and associated cognitive deficit compared to chemotherapy naïve patients and controls (Deprez et al. 2014; Kesler and Blayney 2016; Kesler et al. 2013b; McDonald et al. 2013). Cognitive impairment occurs in approximately 60% or more of patients with breast cancer (BC) following chemotherapy treatment and shows little if any recovery over time (Kesler et al. 2017a; Wefel et al. 2015). Several mechanistic pathways have been proposed to explain this phenomenon such as the direct and indirect neurotoxic effects of chemotherapy, cytokine dysregulation, and accumulation of reactive oxygen species (Ahles et al. 2012; Ahles and Saykin 2007). It is likely that the etiology of cancer related cognitive impairment (CRCI) is multifactorial and could represent cumulative cellular toxic or aging processes.
Age is an established risk factor for the development of cancer (Dale et al. 2012; Podolskiy et al. 2016) and it is likely that the relationship between cancer and aging is bidirectional. The mechanisms of action of chemotherapy mimic those underlying cellular aging, neurodegeneration and inflammatory diseases (Ahles et al. 2012; Conroy et al. 2013; Kesler et al. 2013a; Mandelblatt et al. 2013; Sosa et al. 2013). Thus, it has been suggested that chemotherapy may accelerate the trajectory of biological aging, including brain age (Mandelblatt et al. 2014). Accordingly, our group has shown that chemotherapy-treated BC survivors who have a particular profile of brain structure may have a higher predicted probability of Alzheimer’s Disease (AD) diagnosis, a neurodegenerative condition associated with advanced aging (Kesler et al. 2017c). We also demonstrated that chemotherapy treated patients show reduced resilience to computationally simulated aging/neurodegeneration compared to healthy controls (Kesler et al. 2015). Others have demonstrated gray matter atrophy associated with brain aging cross sectionally (Koppelmans et al. 2012b) and elevated biological markers of cellular senescence in patients treated with chemotherapy (Pare et al. 2016; Sanoff et al. 2014; Wood et al. 2016). Recently, Scuric and colleagues ( 2017) reported higher levels of DNA damage and lower telomerase activity, both markers of cellular aging, in BC survivors with a history of chemotherapy and/or radiation treatment compared to survivors who had surgery alone. However, no studies to date have more directly evaluated brain age from pre-chemotherapy to post-chemotherapy.
Several changes to brain structure and function are associated with advancing age and these changes can be associated with cognitive decline and neurodegenerative diseases (Cole and Franke 2017). However, there are significant individual variations in terms of how aging can affect cognition and behavior. Brain age can be estimated from neuroimaging data and discrepancies between estimated brain age and chronological age are suggestive of atypical development (Cole and Franke 2017) or could be the result of exposure to environmental stressors such as cancer treatment. Individual brain age estimation may represent a unique biomarker with clinical utility including evaluating risk for neurodegenerative disease or cognitive impairment (Liem et al. 2017) and providing a practical, accessible metric of neurologic injury severity.
Structural brain data combined with brain age algorithms have been used to successfully measure accelerated brain age in persons with AD (Franke and Gaser 2012) and traumatic brain injury (Cole et al. 2015). This method has also been used to predict conversion from mild cognitive impairment (MCI) to AD (Gaser et al. 2013), but it has not been applied to CRCI research. The objective of this study was to compare longitudinal change in brain age in women with BC before and after chemotherapy compared to age-matched healthy peers and to examine relationships between brain age, cognitive impairment, and medical and demographic variables.
Material and Methods
Participants
We enrolled newly diagnosed patients with primary BC (stages I-IIIA) and frequency matched healthy controls as part of our ongoing prospective study of BC and cognition (Kesler et al. 2017a; Kesler et al. 2017b). Patients were assessed at 3 time points— prior to any treatment including surgery with general anesthesia (Time 1), 1 month after completing chemotherapy (Time 2), and 1 year after Time 2 (Time 3). Controls were assessed at yoked intervals. All participants were between the ages of 40 and 65 years which are the peak ages of BC diagnoses and exclude women who are more likely to have incipient neurodegenerative disease and are less likely to receive chemotherapy. Participants with BC had a minimum Karnofsky score of 70, which indicates adequate physical ability for participation (Mor et al. 1984; Yates et al. 1980). Women were excluded for any prior history of cancer, psychiatric, neurologic or comorbid medical conditions that are known to affect cognitive function and healthy women were excluded for any history of these conditions. All participants were excluded for MRI contraindications and/or major sensory impairments (e.g. blindness) that would preclude completion of cognitive tests. Medical information for patients was extracted from the Electronic Medical Record including disease stage, treatment regimen and course, and tumor pathology (hormone receptor, human epidermal growth factor receptor 2 [HER2]). This study was carried out in accordance with the recommendations of the Stanford University Institutional Review Board with written informed consent from all participants. All participants gave written informed consent in accordance with the Declaration of Helsinki.
Cognitive Assessment
Cognitive function was measured using a battery of standardized neuropsychological tests including the Rey Auditory Verbal Learning Test (RAVLT) for verbal learning and memory (Schmidt 2012), which includes Immediate Recall, Delayed Recall, and Interference. Trails 1 and 5 of the Comprehensive Trail Making Test (CTMT) were used to measure attention, processing speed and executive functioning (Moses 2004) and the Controlled Oral Word Association test (COWA) was used to measure verbal fluency (Ruff et al. 1996). We have shown this battery to be sensitive to CRCI in several previous studies (Henneghan 2018; Henneghan et al. 2018; Kesler et al. 2017a; Kesler et al. 2017b). Participants also completed an experimental battery of computerized cognitive tests that are not reported here.
We also measured psychological distress (depression, anxiety, fatigue) using the Total Score from the Clinical Assessment of Depression (CAD) (Aghakhani and Chan 2007). Further, we assessed subjective, real-world executive function and self-regulation with the Global Executive Composite of the Behavioral Rating Inventory of Executive Function- Adult (BRIEF-A), which we have shown to be sensitive to both the behavioral and neurofunctional consequences of chemotherapy (Kesler et al. 2011). Subjective memory function was measured using the Prospective and Retrospective Memory Questionnaire (PRMQ) (Crawford et al. 2006).
MRI Acquisition
MRI data were acquired on the same day as cognitive testing using a GE Discovery MR750 3.0 Tesla whole body scanner (GE Medical Systems, Milwaukee, WI). High-resolution T1-weighted images were acquired with 3D spoiled gradient echo pulse sequence: TR = 8.524 ms, TE = 3.396 ms, Inversion time = 400 ms, flip angle = 15 degrees, Field of view = 220 mm, phase field of view= 75%, slice thickness = 1.6 mm number of excitation =1, acquisition matrix = 256 × 256. In total, 124 contiguous coronal slices were with in-plane resolution of 0.859 mm × 0.859 mm. Participants also underwent resting state fMRI and diffusion tensor imaging during this session but these data are not reported here.
Neuroimaging Processing
The FreeSurfer software package version 5.3 (Fischl 2012) was used to measure cortical thickness and cortical surface area. Surface-based analysis in FreeSurfer involves the removal of non-brain tissue from the T1-weighted MRI, followed by an automated Talairach transformation, segmentation of subcortical white matter and cortical gray matter, intensity normalization, tessellation of gray/white-matter boundary, automated correction of topological defects and surface deformation to form the gray- and white matter boundary. Cortical thickness was determined as the difference between the pial and white-matter surface (Fischl and Dale 2000). Subcortical volumes were obtained from the automated procedure for volumetric measures of brain structures implemented in FreeSurfer. The details of these procedures are described in prior publications (Dale et al. 1999; Fischl et al. 2002). Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al. 2002) and manual measurements (Kuperberg et al. 2003; Salat et al. 2004). Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Han et al. 2006; Reuter et al. 2012). We performed visual quality checks to ensure no major errors within the FreeSurfer automated processing. Gray matter metrics including cortical surface area (mm2), cortical thickness (mm) and subcortical volumes (mm3) were extracted for each participant.
Predicted Brain Age (PBA) Calculation
Gray matter measures were then entered into Brain-Age Regression Analysis and Computation Utility Software (BARACUS version V1.1.2), which calculates PBA in years from 1) cortical thickness, 2) cortical surface area, 3) subcortical volumes, and 4) stacked anatomy) resulting in four different estimated brain ages for each participant (Liem 2017).
Statistical Analyses
Group demographics
Group differences in demographic and treatment variables were assessed with t-tests for continuous variables. P values less than 0.05 were considered statistically significant.
Predicted Brain Age and Cortical Thickness Group Comparison
Cortical thickness (CT) has been identified as an estimate of brain age in CRCI research (Koppelmans et al. 2012b), therefore we began our statistical analyses by comparing mean cortical thickness between BC patients and controls then moved on to compare PBA between the groups. We used separate linear mixed models to compare these metrics between the groups across all 3 timepoints, fully utilizing outcome data repeatedly measured for most participants. For estimation of mixed effect models, maximum likelihood estimation was used. Data points that were missing due to subject attrition or unusable imaging data were handled assuming that data were missing at random (Little and Rubin 2002). All available cases including the ones with missing information were included in the analyses. In mixed effects analyses each variable, CT and PBA, was modeled as a separate dependent variable predicted by the group membership (BC patients, Healthy Controls). Post-hoc linear mixed models were used to evaluate differences in CT and/or PBA between time 1 and time 2, then between time 2 and time 3 to better understand group differences in CT and PBA, along with student t tests at each time point to compare mean difference at time 1, time 2, and time 3.
Associations between Predicted Brain Age, Cortical Thickness, Cognitive Variables, Individual and Clinical Variables
Correlations were explored between change in CT from Time 1 to Time 3, change in PBA from Time 1 to Time 3, and individual and clinical variables: age (years), education (years), menopausal status (1 = post-menopause, 0 = pre-menopause), cancer stage at diagnosis (I,II,III), anthracycline chemotherapy (1 = yes, 0 = no), radiation treatment (1 = yes, 0 = no), hormonal blockade treatment (1 = yes, 0 = no), hormone receptor status (1 = positive, 0 = negative), HER 2 receptor status (1 = positive, 0 = negative), surgery type (1 = mastectomy, 0 = lumpectomy), and neoadjuvant chemotherapy (1 = yes, 0 = no), using two separate multiple linear regression models.
Two-tailed Pearson’s correlations were also examined among CT from Time 1 to Time 3, change in PBA, chronological age and cognitive performance on RAVLT-Immediate Recall, RAVLT-Interference, RAVLT-Delayed Recall, CTMT1 (similar to Trail Making Test A), CTMT5 (similar to Trail Making Test B), COWA Adjusted Score, and self-report data (BRIEF-A Global Score, PRMQ Total Score) using change scores (Time 3 minus Time 1) to account for time. These exploratory analyses were conducted for the purpose of hypothesis generation, therefore no correction for multiple comparisons was made and the p value was set at 0.05, thus risk for false positive findings is higher.
Results
Group Demographic Comparisons
43 women with newly diagnosed BC were enrolled in the study and 50 matched controls. Both groups were on average 50 years old and college educated. In the BC group, the majority had a history of stage II BC, and received radiation and hormonal treatment in addition to chemotherapy. See Table 1 for demographics and clinical variables. At Time 1, 43 BC patients and 50 controls completed data collection. At Time 2, 27 BC patients and 44 controls completed data collection and at Time 3, 34 BC patients and 44 controls completed data collection.
Table 1.
Baseline demographic and clinical characteristics. Data are shown as mean (standard deviation) unless otherwise indicated.
| Demographic Variable | BC (n=43) | Controls (n=50) | p value |
|---|---|---|---|
| Age | 49.44 (8.92) | 49.67 (9.99) | 0.91 |
| Age Range | 31.3–65.73 | 25.78–64.24 | |
| Education | 17.04 (3.19) | 17.52 (2.28) | 0.42 |
| Post-Menopause | 46.5% | 40% | .567 |
| Months Between T1 and T2 assessment | 5.98 (1.05) | 5.31 (0.89) | .004 |
| Months Between T2 and T3 assessment | 12.38 (1.74) | 12.61 (1.01) | 0.50 |
| Months Between T1 and T3 assessment | 18.32 (1.98) | 17.91 (1.38) | 0.33 |
| Number of chemotherapy cycles | 7.03 (4.39) | ||
| Radiation therapy | 65% | ||
| Hormone Blockade | 68.42% | ||
| Stage at diagnosis (I, II, III) | 11.6%, 67.5%, 20.9% | ||
| Estrogen receptor positive | 81% | ||
| Progesterone receptor positive | 65% | ||
| Human epidermal growth factor receptor 2 positive | 25.6% | ||
| Neoadjuvant chemotherapy | 48.8% | ||
| Lumpectomy | 46.5% | ||
| Mastectomy | 48.8% |
Cortical Thickness (CT) and Predicted Brain Age (PBA)
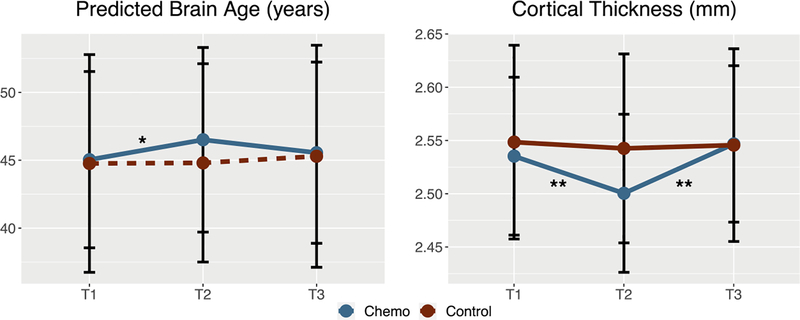
BC patients showed a significant difference in CT across the 3 time points compared to controls (p <.001). The slopes from time 1 to time 2 and from time 2 to time 3 were both significantly different between groups. (p’s<.01, Table 2, Figure 1a). Student’s t tests revealed a significant group difference in mean cortical thickness at time 2 only (t = −2.0859, p<.05). BC patients showed a trend towards significant differences in PBA (calculated from cortical thickness measures only) across the 3 time points (p = 0.085, Table 2, Figure 1b). The slope from time 1 to time 2 was significantly different between the groups (p<.05, Table 2, Figure 1b). Student’s t tests revealed no significant group difference in PBA at any time points (p<‘s>05).
Table 2.
Brain metric data across time points for breast cancer and healthy control groups. Data are shown as mean (standard deviation)
| Group | Timepoint | Cortical Thickness (mm) | Predicted Brain Age (years) |
|---|---|---|---|
| Chemo | T1 | 2.53 (0.07) | 45 (6.5) |
| Chemo | T2 | 2.50 (0.07) | 47 (6.8) |
| Chemo | T3 | 2.55 (0.07) | 46 (6.7) |
| Control | T1 | 2.55 (0.09) | 45 (8) |
| Control | T2 | 2.54 (0.09) | 45 (7.3) |
| Control | T3 | 2.55 (0.09) | 45 (8.2) |
Figure 1.
Predicted brain age and mean cortical thickness across time in patients with breast cancer (Chemo) and healthy female controls (Control). T1 = pre-treatment, T2 = 1 month post-chemotherapy, T3 = 1 year post-chemotherapy (or yoked intervals for controls). Predicted brain age changed significantly from T1 to T2 and cortical thickness changed across all three time points in the breast cancer group compared to controls. * p < 0.05, ** p < 0.001.
The assessment interval between times 1 and 2 differed significantly between groups (p = 0.004, Table 1). This represented a difference of only 0.68 months, or approximately 20 days, so was not likely a clinically meaningful difference. However, we conducted a post hoc general linear model analysis of PBA slope between times 1 and 2 covarying for assessment interval. The group effect remained significant (t = 2.75, p = 0.01) and assessment interval was not a significant covariate (p = 0.99).
Correlations Among CT, PBA, Demographic, Clinical, and Cognitive Variables
No significant correlations were found among demographic and clinical variables and difference in CT from Time 1 (pre-surgical baseline) to Time 3 (1-year post chemotherapy treatment). Difference in PBA from Time 1 (pre-surgical baseline) to Time 3 (1-year post chemotherapy treatment) was significantly related to HER 2 status (ß=−.45, p<.05), surgery type (ß=.53, p<.05), and neoadjuvant chemotherapy (ß=.41. p<.05). The relationship between PBA and hormone receptor status approached significance (ß=.38, p=.05). These regression models are displayed in Table 2.
A significant Pearson’s correlation was found between mean CT and RAVLT-Interference scores across time (r=.48, p<.01). The correlation between PBA and RAVLT-Interference scores approached significance (r=−.36, p=.052). These correlations are displayed in Table 3. A post hoc analysis was conducted examining RAVLT-Interference score differences between groups (BC, control) across all timepoints using linear mixed modeling. We found significant group differences across time (Chi Sq = 5.952, p = .014). Independent T tests revealed that group differences in RAVLT-Interference were only significant at Time 3 (t=2.347, p = .022). Cognitive test scores for BC patients and healthy controls at each time point are displayed in Supplementary Table 1 and plotted for BC patients in Supplementary Figure 1.
Table 3.
Relationships between clinical/demographic variables and changes in brain metrics from Time 1 to 3 for the breast cancer group
| Regression Coefficient | ||
|---|---|---|
| Mean Cortical Thickness | Predicted Brain Age | |
| Years Education | .07 | −.11 |
| Postmenopausal | −.22 | .35 |
| Cancer Stage at Diagnosis | −.37 | .31 |
| Anthracycline Chemotherapy | .08 | −.09 |
| Hormone Receptor Positive | −.33 | .24 |
| HER2 Receptor Positive | .02 | −.06 |
| Mastectomy | −.03 | .38 |
| Neoadjuvant Chemotherapy | −.15 | −.45* |
| Hormonal Blockade | −.10 | .53* |
| Radiation Treatment between T2-T3 | −.20 | .41* |
p < 0.05
Discussion
In this study we extended prior research that cross sectionally (Koppelmans et al. 2012b) and prospectively (McDonald et al. 2010) found evidence of decreased gray matter in BC survivors compared to controls. We also implemented a previously established and validated neuroimaging-based machine learning algorithm to longitudinally examine predicted brain age, in BC patients for the first time. Finally, we found correlations among brain aging metrics and cognitive impairment, specifically verbal memory interference, in chemotherapy treated patients with BC. We demonstrated evidence of an acute decrease in cortical thickness along with accelerated predicted brain age from time 1 to time 2 in patients with BC compared to controls. Our findings provide preliminary empirical support for the theory that accelerated aging is one of the underlying mechanisms of CRCI (Mandelblatt et al. 2014). These findings are especially important considering increased brain age has been associated with greater risk of developing Alzheimer’s disease in people with mild cognitive impairment (Gaser et al. 2013; Lowe et al. 2016), and reports that for every year an individual’s brain is predicted to be older than their chronological age, there is a 6% increased risk of death (Cole et al. 2018). Notably, BC patients’ mean cortical thickness and predicted brain age “normalized” from Time 2 to Time 3. This may be explained by the fact that our cohort was highly educated (with approximately 17 or more years of education) and years of education is often used as a proxy for cognitive reserve. Thus the sample in this study may have had high cognitive reserve making them more resilient to cognitive aging effects and facilitating brain recovery post treatment (Ferreira et al. 2016), representing a potential sampling bias in this study. Nonetheless, the BC group demonstrated a significant decreased cortical thickness and a trend towards significantly higher brain age, especially from time 1 to time 2, suggesting “brain aging” over time compared to the control group.
The decrease in cortical thickness and increase in predicted brain age across time in the BC group was specifically observed in the time between diagnosis and immediately following the completion of chemotherapy. These findings suggest that chemotherapy and surgical treatments received during this time may have an accelerating cortical brain aging effect or may prevent repair from tumor/treatment-related brain injury and consistent with previous reports of decreased gray matter volume in BC survivors compared to controls immediately following the completion of chemotherapy with partial recovery 1 year later (McDonald et al. 2010). However, the stability of this effect between 1 year post chemotherapy completion and 20 years post chemotherapy (Koppelmans et al. 2012b) remains unclear. Cognitive changes have been observed to persist for variable periods of time following the completion of adjuvant treatment (Janelsins et al. 2014; Koppelmans et al. 2012a). It is unclear whether the cortical brain changes we found represent true brain aging. Future studies could determine if assays of cellular senescence correspond to predicted brain age. Further, our results suggest that increased cortical brain aging may be a temporary phenomenon that resolves over time.
For many years it was assumed that aging is an inevitable process; however, recent research on aging, specifically epigenomic animal research suggests that cellular effects of aging may be reversible (Jaskelioff et al. 2011; Maherali et al. 2007; Ocampo 2016; Okita et al. 2007). For example, shortening of telomere length, a proxy for cellular aging in humans (Mather et al. 2011), has been reversed in populations at risk for prostate cancer (Ornish et al. 2008). Alternatively, it is possible that these cortical changes do not reflect true aging, but rather some other type of injury that is collinear with age. Prospective research evaluating cortical thickness and predicted brain age in a larger prospective cohort that extends beyond 1-year post chemotherapy treatment is needed to better understand the patterns of cortical brain aging in this population.
We did not find significant increases in predicted brain age as measured by subcortical volumes or cortical surface area, despite the fact that cortical thickness, cortical surface area, subcortical volume and stacked anatomy metrics were highly correlated at each time point (See Supplementary Table 2). We know that subcortical structures of the human brain are essential for various cognitive and social functions (Koshiyama et al. 2018), yet little is known about how cancer and/or cancer treatment effects these structures. CRCI neuroimaging studies have consistently identified cancer and/or chemotherapy related brain changes (de Ruiter and Schagen 2013; Simo et al. 2013) in both cortical and subcortical structures (Saykin 2003; Simo et al. 2013). It is possible that cancer and/or chemotherapy may specifically affect aging in cortical gray matter but not subcortical structures. In other studies that have utilized the BARACUS algorithm, authors typically report and use one of the four neuroimaging metrics as a proxy for “predicted brain age” such as stacked anatomy (Beheshti et al. 2018; Cole et al. 2018; Hatton et al. 2018). Hatton et al. (2018) comment that the simplicity of using stacked anatomy as a general measure of brain age inhibits the ability to identify specific locations of pathology in the brain. Furthermore, the stacked anatomy metric includes more features, and it is possible that the model used in the present study did not have the power to detect changes in this metric. Since we only found evidence of increased PBA using cortical thickness, it is possible that gray matter in the cortex ages faster than subcortical regions. In the general population, it appears that gray matter volume loss is greater in the cortex than in the subcortical structures as people age (Jernigan et al. 2001; Walhovd et al. 2005; Zheng et al. 2018).
Our findings suggest that decreased cortical thickness and subsequent increased brain age may contribute to neurocognitive dysfunction following BC chemotherapy. This is consistent with the literature demonstrating increased discrepancies between predicted and chronological ages in other neurologic and neuropsychiatric diseases. These conditions include HIV (Cole et al. 2017; Kuhn et al. 2018), mild cognitive impairment, obesity, Alzheimer’s disease and traumatic brain injury (Cole and Franke 2017). The pathophysiology of these diseases are distinct, however they appear to have secondary effects on the brain that likely share common neurobiological pathways such as neuroinflammation, oxidative stress, and mitochondrial dysfunction (Cole and Franke 2017). As noted above in the Introduction section, these are also candidate mechanisms of CRCI (Mandelblatt et al. 2014). Other age-related brain changes that overlap with those associated with CRCI include decreased brain volume (Chen et al. 2018; Inagaki et al. 2007; Koppelmans et al. 2014), and alterations in neuroprotective proteins like catechol-o-methyltransferase (COMT) and brain derived neurotrophic factor (BDNF). For example, decreased neurogenesis, tropomyosin-related kinase B, and Ca2 retention in the hippocampus have been shown in mice treated with chemotherapy compared to controls (Park et al. 2018).
Cortical thickness was associated with worsening verbal memory, specifically proactive interference, which refers to difficulties with learning new information due to competition from previously presented material. We have previously shown that our prospective cohort of BC patients show deficits in proactive interference compared to healthy controls (Kesler et al. 2017b). Aging adults are particularly susceptible to proactive interference (Lustig and Jantz 2015). Previous studies have demonstrated that proactive interference is a highly sensitive measure of MCI, is associated with regional amyloid burden in cognitive normal adults and also associated with MRI volumetric biomarkers of AD (Crocco et al. 2014; Loewenstein et al. 2016; Loewenstein et al. 2017).
Our multiple linear modeling analyses revealed no correlations between change in cortical thickness from pre-treatment to immediately following chemotherapy completion and any of the clinical or demographic variables in the patients with BC. However, when we modeled change in predicted brain age from pre-treatment to 1 year following the completion of treatment chemotherapy, we found a negative relationship between HER2 receptor status, suggesting that those patients with HER2+ status demonstrated improved, or “younger” brain age over time. Although HER2 + breast tumors are highly proliferating and clinically aggressive (Bianchini and Gianni 2014; Dieci et al. 2016). HER2 + breast cancers are treated with targeted therapies (e.g. trastuzumab); these have poor penetrance of the blood brain barrier, contrary to many cytotoxic and hormonal therapies, potentially limiting their impact on brain age. Notably, additional adjuvant treatments including radiation treatment and hormonal treatment occurred in all patients with BC, but there were no specific relationships observed between predicted brain age and these variables. These relationships may be confounded with disease severity and tumor receptor status, both of which influence therapeutic approach. Hormone receptor status has also been found to have variable influence on certain cognitive domains (Li et al. 2017), which may account for the relationship between hormone receptor status and predicted brain age approaching significance.
We found a relationship between history of neoadjuvant chemotherapy and change in predicted brain age, suggesting that those who received neoadjuvant chemotherapy demonstrated worsening, or “older”, brain age over time. Neoadjuvant chemotherapy is commonly used for the treatment of patients with high-risk operable primary breast cancer (Untch et al. 2014). Our findings are consistent with (Lyon et al. 2016) who reported that the receipt of neoadjuvant chemotherapy had negative effects on multiple cognitive domains that persisted over time in breast cancer survivors. Finally, we found a relationship between surgery type and change in predicted brain age over time, suggesting that that those who had a mastectomy demonstrated worsening, or “older”, brain age overtime than those who had a lumpectomy. Considering that the choice of mastectomy or lumpectomy depends on the staging of disease— mastectomies are chosen for breast tumors that are larger and involve more lymph nodes, and lumpectomies are chosen for local and smaller tumors (Newman 2017)—this finding may reflect the different disease severity in the surgical subgroups that was not captured by categorizing patients into three groups based on cancer stage (I,II,III). Furthermore, mastectomies are more invasive surgeries and often result in a secondary reconstruction surgery. Thus, these findings may reflect the association between disease severity and greater brain age, in addition to the effects of a longer and more aggressive surgery on brain aging.
Interestingly, we did not find significant relationships between chemotherapy type and changes in predicted brain age. Since breast cancer chemotherapy consists of combined chemotherapy regimens, we evaluated chemotherapy type by grouping patients as having had anthracycline based chemotherapy or non-anthracycline chemotherapy. This is one of the most common compounds used to treat breast cancer and the most consistently linked to chemo-related neurotoxicity (Kesler and Blayney 2016). Taken together, these findings suggest that breast cancer severity and subsequent treatments are associated with worsening predicted brain age over time. In this exploratory analysis, we did not correct for multiple comparisons for the separate multiple regression models that were run, and therefore our findings could represent type I error. It is also possible that these results reflect unknown multivariate, mediating/moderating effects involving treatment variables that we lack statistical power to adequately test, and thus these associations need to be replicated in a larger sample.
In addition to our small and highly educated sample, there were other limitations to this study. We utilized gray matter metrices to calculate and estimate brain age, however white matter and functional connectivity are more consistently reported to be affected by chemotherapy as discussed above in this section (Kesler 2014; Saykin et al. 2013). Therefore, we may have underestimated the brain aging effect of chemotherapy. Validated resting state fMRI/DTI brain age prediction algorithms are not currently publicly available for use. Additionally, the BARACUS algorithm we used was derived from cross-sectional data rather than longitudinal data (Liem 2017). However, neuropsychological normative data are also cross-sectional and yet are used to standardized longitudinal data. For study feasibility, given that we were recruiting newly diagnosed patients prior to treatment, we only included a limited number of behavioral and psychosocial variables in this study, so it is possible that there are relationships between predicted brain age and other factors not measured in this study. Lastly, we did not collect or store blood as part of this study, and are unable to provide insides on underlying biological mechanisms of the cortical changes found. Future neuroimaging studies should consider adding biomarker analyses which may contribute additional neurobiological insights of cancer related brain changes.
Despite these limitations, the study had several notable strengths. This is the first study to our knowledge to evaluate predicted brain age in patients with cancer. We conducted longitudinal assessment of patients compared to controls from a unique presurgical baseline whereas most previous prospective studies employ a post-surgical baseline. Our findings provide increased insight regarding the neural mechanisms underlying CRCI and lends empirical support to the hypothesis of accelerated aging associated with cancer and chemotherapy. Future research should extend the longitudinal follow up to beyond 1 year in order to determine the trajectory of brain aging or if different patterns of aging emerge (such as late onset of brain aging or brain aging in subcortical structures). Back-translating these findings into animal models in order to examine histological differences such as amyloid plaques, tangles, neuroinflammation, genomic alterations, demyelination, changes to cellular metabolism and mitochondrial dysfunction (Blalock et al. 2003) could potentially reveal biological mechanisms underlying accelerated brain age resulting from chemotherapy treatment.
Supplementary Material
Table 4.
Relationships between cognitive and brain changes (slopes) from Time 1 to 3 for the breast cancer group
| Pearson Correlation Coefficient | ||
|---|---|---|
| Mean Cortical Thickness | Predicted Brain Age | |
| RAVLT Immediate Recall | −.07 | −.11 |
| RAVLT- Interference | .48** | −.36* |
| RAVLT-Delayed Recall | −.08 | .17 |
| CTMT 1 | −.22 | .13 |
| CTMT 5 | −.25 | −.05 |
| COWA | .28 | −.18 |
| BRIEF-A | .01 | −.02 |
| PRMQ | −.24 | .15 |
p < 0.05
p < 0.01
RAVLT = Rey Auditory Verbal Learning Test; CTMT = Comprehensive Trail Making Test; COWA = Controlled Oral Word Association, BRIEF-A = Behavioral Rating Inventory of Executive Function (Adult Version); PRMQ = Prospective and Retrospective Memory Questionnaire
Acknowledgements
This research was funded by a grant from the National Cancer Institute (National Institutes of Health, 1R01CA172145, MPIs: SK, OP). The authors wish to thank the faculty and staff at the Stanford University Richard M. Lucas Center for their assistance with neuroimaging acquisitions.
Footnotes
Compliance with Ethical Standards
Disclosure of potential conflicts of interest: The authors have no conflicts of interest to disclose.
Research involving Human Participants and/or Animals: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Stanford Institutional Review Board, # 14623) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aghakhani A, & Chan EK (2007). Test Reviews: Bracken, B. A., & Howell, K. (2004). Clinical Assessment of Depression. Odessa, FL: Psychological Assessment Resources. Journal of Psychoeducational Assessment, 25(4), 416–422. doi: 10.1177/0734282907300383. [DOI] [Google Scholar]
- Ahles TA, Root JC, & Ryan EL (2012). Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol, 30(30), 3675–86. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, & Saykin AJ (2007). Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer, 7(3), 192–201. doi:nrc2073 [pii] 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheshti I, Maikusa N, & Matsuda H (2018). The association between “Brain-Age Score” (BAS) and traditional neuropsychological screening tools in Alzheimer’s disease. Brain Behav, 8(8), e01020. doi: 10.1002/brb3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G, & Gianni L (2014). The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol, 15(2), e58–68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, et al. (2003). Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci, 23(9), 3807–19. https://www.ncbi.nlm.nih.gov/pubmed/12736351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Sethi SK, Jin T, Patel SK, Ye N, Sun CL, et al. (2018). Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res, 20(1), 38. doi: 10.1186/s13058-018-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, & Franke K (2017). Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci, 40(12), 681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Cole JH, Leech R, Sharp DJ, & Alzheimer’s Disease Neuroimaging I (2015). Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol, 77(4), 571–81. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Ritchie SJ, Bastin ME, Valdes Hernandez MC, Munoz Maniega S, Royle N, et al. (2018). Brain age predicts mortality. Mol Psychiatry, 23(5), 1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, et al. (2017). Increased brain-predicted aging in treated HIV disease. Neurology, 88(14), 1349–1357. doi: 10.1212/WNL.0000000000003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, McDonald BC, Smith DJ, Moser LR, West JD, Kamendulis LM, et al. (2013). Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat, 137(2), 493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Henry JD, Ward AL, & Blake J (2006). The Prospective and Retrospective Memory Questionnaire (PRMQ): latent structure, normative data and discrepancy analysis for proxy-ratings. Br J Clin Psychol, 45(Pt 1), 83–104. doi: 10.1348/014466505X28748. [DOI] [PubMed] [Google Scholar]
- Crocco E, Curiel RE, Acevedo A, Czaja SJ, & Loewenstein DA (2014). An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features of Alzheimer’s disease. Am J Geriatr Psychiatry, 22(9), 889–97. doi: 10.1016/j.jagp.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale W, Mohile SG, Eldadah BA, Trimble EL, Schilsky RL, Cohen HJ, et al. (2012). Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst, 104(8), 581–9. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, & Schagen SB (2013). Functional MRI studies in non-CNS cancers. Brain Imaging Behav, 7(4), 388–408. doi: 10.1007/s11682-013-9249-9. [DOI] [PubMed] [Google Scholar]
- Deprez S, Vandenbulcke M, Peeters R, Emsell L, Smeets A, Christiaens MR, et al. (2014). Longitudinal Assessment of Chemotherapy-Induced Alterations in Brain Activation During Multitasking and Its Relation With Cognitive Complaints. J Clin Oncol. doi: 10.1200/JCO.2013.53.6219. [DOI] [PubMed] [Google Scholar]
- Dieci MV, Griguolo G, Miglietta F, & Guarneri V (2016). The immune system and hormone-receptor positive breast cancer: Is it really a dead end? Cancer Treat Rev, 46, 9–19. doi: 10.1016/j.ctrv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Ferreira D, Bartres-Faz D, Nygren L, Rundkvist LJ, Molina Y, Machado A, et al. (2016). Different reserve proxies confer overlapping and unique endurance to cortical thinning in healthy middle-aged adults. Behav Brain Res, 311, 375–383. doi: 10.1016/j.bbr.2016.05.061. [DOI] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage, 62(2), 774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images (Research Support, U.S. Gov’t, P.H.S.). Proc Natl Acad Sci U S A, 97(20), 11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. http://www.ncbi.nlm.nih.gov/pubmed/11832223. [DOI] [PubMed] [Google Scholar]
- Franke K, & Gaser C (2012). Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease. The Journal of Gerontopsychology and Geriatric Psychiatry, 25(4). [Google Scholar]
- Gaser C, Franke K, Kloppel S, Koutsouleris N, Sauer H, & Alzheimer’s Disease Neuroimaging I (2013). BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS One, 8(6), e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage, 32(1), 180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hatton SN, Franz CE, Elman JA, Panizzon MS, Hagler DJ Jr., Fennema-Notestine C, et al. (2018). Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol Aging, 67, 1–9. doi: 10.1016/j.neurobiolaging.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneghan A, Palesh O, Harrison M, Kesler S (2018). Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10 years post chemotherapy using machine learning. Journal of Neuroimmunology, 320, 38–47. doi: 10.1016/j.jneuroim.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneghan AM, Carter P, Stuifbergan A, Parmelee B, & Kesler S (2018). Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology. doi: 10.1002/pon.4745. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, et al. (2007). Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer, 109(1), 146–56. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17131349 [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, & Morrow GR (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment (Article). Int Rev Psychiatry, 26(1), 102–13. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature, 469(7328), 102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. (2001). Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging, 22(4), 581–94. https://www.ncbi.nlm.nih.gov/pubmed/11445259. [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. (2013a). Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t). Brain Behav Immun, 30 Suppl, S109–16. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Rao A, Blayney DW, Oakley Girvan I, Karuturi M, & Palesh O (2017a). Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front Human Neurosci, 11, 555. doi:doi: 10.3389/fnhum.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR (2014). Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging, 35 Suppl 2, S11–9. doi: 10.1016/j.neurobiolaging.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams M, Packer M, Rao V, Henneghan AM, Blayney DW, et al. (2017b). Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav, 7(3), e00643. doi: 10.1002/brb3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, & Blayney DW (2016). Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol, 2(2), 185–92. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, & O’Hara R (2011). Prefrontal cortex and executive function impairments in primary breast cancer. JAMA Neurol, 68(11), 1447–53. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Rao V, Ray WJ, & Rao A (2017c). Probability of Alzheimer’s disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 9, 67–75. doi: 10.1016/j.dadm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Watson CL, & Blayney DW (2015). Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging, 36(8), 2429–42. doi: 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, & Hoeft F (2013b). Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A, 110(28), 11600–5. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, & Schagen SB (2012a). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol, 30(10), 1080–6. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, de Groot M, de Ruiter MB, Boogerd W, Seynaeve C, Vernooij MW, et al. (2014). Global and focal white matter integrity in breast cancer survivors 20 years after adjuvant chemotherapy. Hum Brain Mapp, 35(3), 889–99. doi: 10.1002/hbm.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, et al. (2012b). Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat, 132(3), 1099–106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. (2018). Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep, 8(1), 1183. doi: 10.1038/s41598-017-18950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T, Kaufmann T, Doan NT, Westlye LT, Jones J, Nunez RA, et al. (2018). An augmented aging process in brain white matter in HIV. Hum Brain Mapp. doi: 10.1002/hbm.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. (2003). Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of general psychiatry, 60(9), 878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Li W, Gan C, Lv Y, Wang SH, & Cheng HD (2017). Chemotherapy-induced prospective memory impairment in breast cancer patients with different hormone receptor expression. Medicine, 96(13). doi:ARTN e6514 10.1097/MD.0000000000006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F, Gorgolewski C (2017). BIDS-Apps/baracus: v1.1.2. https://zenodo.org/record/1018841#.WvtveC-ZPNI Accessed.
- Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. (2017). Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage, 148, 179–188. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Little R, & Rubin D (2002). Statistical analysis with missing data. New York, NY: Wiley. [Google Scholar]
- Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, et al. (2016). A Novel Cognitive Stress Test for the Detection of Preclinical Alzheimer Disease: Discriminative Properties and Relation to Amyloid Load. Am J Geriatr Psychiatry, 24(10), 804–13. doi: 10.1016/j.jagp.2016.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein DA, Curiel RE, Wright C, Sun X, Alperin N, Crocco E, et al. (2017). Recovery from Proactive Semantic Interference in Mild Cognitive Impairment and Normal Aging: Relationship to Atrophy in Brain Regions Vulnerable to Alzheimer’s Disease. J Alzheimers Dis, 56(3), 1119–1126. doi: 10.3233/JAD-160881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe LC, Gaser C, Franke K, & Alzheimer’s Disease Neuroimaging I (2016). The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS One, 11(7), e0157514. doi: 10.1371/journal.pone.0157514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, & Jantz T (2015). Questions of age differences in interference control: When and how, not if? Brain Res, 1612, 59–69. doi: 10.1016/j.brainres.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Lyon DE, Cohen R, Chen H, Kelly DL, Starkweather A, Ahn HC, et al. (2016). The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: a 2-year longitudinal study. J Cancer Res Clin Oncol, 142(7), 1461–74. doi: 10.1007/s00432-016-2163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. (2007). Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell, 1(1), 55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW, et al. (2013). Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know?. Semin Oncol, 40(6), 709–25. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, et al. (2014). Cognitive Impairment in Older Patients With Breast Cancer Before Systemic Therapy: Is There an Interaction Between Cancer and Comorbidity? J Clin Oncol. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Parslow RA, & Christensen H (2011). Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci, 66(2), 202–13. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, & Saykin AJ (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Research and Treatment, 123(3), 819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, & Saykin AJ (2013). Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun, 30 Suppl, S117–25. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V, Laliberte L, Morris JN, & Wiemann M (1984). The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer, 53(9), 2002–7. http://www.ncbi.nlm.nih.gov/pubmed/6704925. [DOI] [PubMed] [Google Scholar]
- Moses J (2004). Comprehensive Trail Making Test (CTMT)By Cecil R. Reynolds. Austin, Texas: PRO-ED, Inc., 2002. Archives of Clinical Neuropsychology, 19(5), 703–708. doi: 10.1016/j.acn.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Newman LA (2017). Decision Making in the Surgical Management of Invasive Breast Cancer-Part 1: Lumpectomy, Mastectomy, and Contralateral Prophylactic Mastectomy. Oncology (Williston Park), 31(5), 359–68. https://www.ncbi.nlm.nih.gov/pubmed/28512732. [PubMed] [Google Scholar]
- Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JL, Xu J, Rodriguez Esteban C, Nuñez G, Nuñez-Delicado E, Campistol JM, Guillen I, Guillen P and Izpisua Belmonte JC (2016). In vivo amelioration of aging hallmarks by partial reprogramming. Cell, 167(7), 1719–1733.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, & Yamanaka S (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448(7151), 313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. (2008). Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol, 9(11), 1048–57. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Pare R, Shin JS, & Lee CS (2016). Increased expression of senescence markers p14(ARF) and p16(INK4a) in breast cancer is associated with an increased risk of disease recurrence and poor survival outcome. Histopathology, 69(3), 479–91. doi: 10.1111/his.12948. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim CJ, Kwak HB, No MH, Heo JW, & Kim TW (2018). Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology, 133, 451–461. doi: 10.1016/j.neuropharm.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Podolskiy DI, Lobanov AV, Kryukov GV, & Gladyshev VN (2016). Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nat Commun, 7, 12157. doi: 10.1038/ncomms12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. (2002). Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology, 58(5), 695–701. http://www.ncbi.nlm.nih.gov/pubmed/11889230. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, & Levin HS (1996). Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol, 11(4), 329–38. https://www.ncbi.nlm.nih.gov/pubmed/14588937. [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. (2004). Thinning of the cerebral cortex in aging. Cerebral cortex, 14(7), 721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, et al. (2014). Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst, 106(4), dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Ahles TA, Schoenfield JD (2003). Gray matter reduction on voxel-based morphometry in chemotherapy-treated cancer survivors. Journal of the International Neuropsychology Society, (9), 246. [Google Scholar]
- Saykin AJ, de Ruiter MB, McDonald BC, Deprez S, & Silverman DH (2013). Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: current status and recommendations for future research. Brain Imaging Behav, 7(4), 363–73. doi: 10.1007/s11682-013-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M (2012). Rey Auditory Verbal Learning Test (RAVLT): A Handbook. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Scuric Z, Carroll JE, Bower JE, Ramos-Perlberg S, Petersen L, Esquivel S, et al. (2017). Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer, 3, 50. doi: 10.1038/s41523-017-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, & Bruna J (2013). Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev, 37(8), 1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, & ME LL (2013). Oxidative stress and cancer: an overview. Ageing Res Rev, 12(1), 376–90. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Untch M, Konecny GE, Paepke S, & von Minckwitz G (2014). Current and future role of neoadjuvant therapy for breast cancer. Breast, 23(5), 526–37. doi: 10.1016/j.breast.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. (2005). Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging, 26(9), 1261–70; discussion 1275–8. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, & Schagen SB (2015). Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin, 65(2), 123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WA, Krishnamurthy J, Mitin N, Torrice C, Parker JS, Snavely AC, et al. (2016). Chemotherapy and Stem Cell Transplantation Increase p16(INK4a) Expression, a Biomarker of T-cell Aging. EBioMedicine, 11, 227–238. doi: 10.1016/j.ebiom.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JW, Chalmer B, & McKegney FP (1980). Evaluation of patients with advanced cancer using the Karnofsky performance status (Research Support, U.S. Gov’t, P.H.S.). Cancer, 45(8), 2220–4. http://www.ncbi.nlm.nih.gov/pubmed/7370963. [DOI] [PubMed] [Google Scholar]
- Zheng F, Liu Y, Yuan Z, Gao X, He Y, Liu X, et al. (2018). Age-related changes in cortical and subcortical structures of healthy adult brains: A surface-based morphometry study. J Magn Reson Imaging. doi: 10.1002/jmri.26037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.