Abstract
Episodic memory, or the ability to remember past events with specific detail, is central to the human experience and is related to learning and adaptive functioning in a variety of domains. In typically developing children, episodic memory emerges during infancy and improves during early childhood and beyond. Developmental processes within the hippocampus are hypothesized to be primarily responsible for both the early emergence and persistence of episodic memory in late infancy and early childhood. However, these hypotheses are based on non-human models. In-vivo investigations in early human development of hippocampal processes have been significantly limited by methodological challenges in acquiring neuroimaging data, particularly task-related functional neuroimaging data, from infants and toddlers. Recent studies in adults have shown neural activity in the brain regions supporting episodic memory during slow-wave sleep using functional magnetic resonance imaging (fMRI), and fMRI has been increasingly utilized in infancy and early childhood to address other research questions. We review initial evidence and present preliminary data showing the promise of this approach for examining hippocampal contribution to how infants and toddlers remember individual events, and their association with information about the context in which the event occurred. Overall, our review, integrated with the presentation of some preliminary data provides insight on leveraging sleep to gain new perspectives on early memory functioning.
Keywords: Memory development, episodic memory, functional magnetic resonance imaging, sleep, infancy, toddlers
1. Introduction
Episodic memory is the ability to remember past events with specific detail including elements of the spatio-temporal context in which the events occurred (Tulving, 2002). This ability is central to the human experience and allows us to reflect on the past and inform our current decisions, to consider alternative scenarios, and to plan for the future. To successfully remember an event, one must encode and retrieve the individual details specific to the event as well as elements of the context in which the event occurred, thereby allowing one to distinguish aspects of this event from other similar ones. The ability to episodically remember a past experience, therefore, allows us to mentally place ourselves within that past experience. The retrieval of these event details is typically associated with the subjective feeling of memory vividness (Tulving, 1985). Given the highly adaptive function of episodic memory, researchers have long been interested in studying its emergence and early development, both to examine how these early forming abilities arise to support continued development into childhood and how its early qualities might explain why a limited number of memories survive past the first few years of life (e.g. Tustin & Hayne, 2010).
However, given the inherent methodological difficulties associated with assessing detailed memory reports in infants and toddlers, researchers interested in the early emergence of episodic memory have focused on characterizing whether memories at that early age capture the unique spatio-temporal features of past episodes instead. Although a wealth of findings supports the ability of infants and toddlers to reproduce specific features of events (Bauer et al., 2011), it has been more difficult to conclusively demonstrate that this age group experiences episodic memory in the same way adults do. The question of how abilities supporting episodic memory first emerge in infancy (0–2 years) and develop through the toddler years (roughly 18 months to 3 years) has been a topic of substantial debate. A complicating factor is that methods used to assess these skills early in life cannot rely on verbal report due to language limitations (Olson & Newcombe, 2013). In contrast, studies in older children and adults can use verbal report and provide a direct and uncontroversial demonstration of memory ability (Mullally & Maguire, 2014). Across a variety of methodologies, we have gained substantial insight into the ability of infants and toddlers to demonstrate retention of details associated with past experiences.
In the first section of this review, we provide an overview of behavioral methods and the overall conclusions about principles of memory development drawn from them. Although behavioral studies have provided important insight into the early development of episodic memory, little is known about how changes in neural substrates in early development support behavioral improvements and whether there is continuity in neural regions and networks over the course of childhood and into adulthood. In adults however, the hippocampus has been conclusively associated with episodic memory capabilities (e.g. Moscovitch et al., 2016), and given its central role in episodic memory processes, there is interest in understanding the role of the hippocampus in supporting early forms of memory abilities in infants and toddlers. Based primarily on evidence with work from non-human animals, developmental processes within the hippocampus have been hypothesized to be primarily responsible for both the early emergence and improvement of episodic memory capabilities (Frankland et al., 2013; Lavenex & Lavenex, 2013). Despite the traction that these theories have gained, little in-vivo work has directly addressed these questions in humans. In the second section of this review, we discuss current evidence from human and animal literature about neural substrates that underlie early episodic memory abilities and outline open questions for human research.
Investigations of the development of hippocampal processes in infants and toddlers have been significantly limited by the methodological challenge of acquiring neuroimaging data, particularly task-related functional neuroimaging data. Recent studies in adults have provided a potential avenue to mitigate these challenges. These studies have shown neural activity in brain regions supporting episodic memory during slow-wave sleep using functional magnetic resonance imaging (fMRI) (Rasch et al., 2007; Van Dongen et al., 2012). Similar sleep-based fMRI methods have been used to study research questions outside of memory in infancy and the toddler years (Nordahl et al., 2008; Redcay et al., 2007). These studies reflect significant advances in the application of neuroimaging methods to study novel populations. They would allow researchers to identify neural signatures of early behavior and, in the realm of memory, provide a more rigorous approach to understand the neural underpinnings of early behavioral presentations of memory and to identify enduring or diverging neural signatures of memory from early development into adulthood. As a result, because fMRI methods during sleep have been successfully implemented in adults to assess episodic memory processes in adults, they are ripe for measuring whether similar neural patterns of activation in response to memory-related stimuli are reflected in the infant and toddler years as well. In the third section of this manuscript, we discuss initial evidence and present preliminary data as an example of the promise of this approach for examining hippocampal contribution to early memory development. In addition, we discuss applications of our approach for the examination of forgetting rates in infants and toddlers, which might explain infantile amnesia, as well as other processes that might depend or benefit from hippocampal contributions, including memory consolidation and word learning.
2. Assessments of episodic memory in infancy and early childhood: Insight from behavioral research
Research on episodic memory abilities in adults utilizes methods that capture a broad range of behavioral manifestations of memory (Tulving, 2002). These methods measure the fidelity of recall or recognition of features of past experiences, as well as the phenomenological quality of verbal reports of one’s own past. These methods are not well suited for the examination of the earliest manifestations of episodic memory, and researchers have had to overcome constraints in language development and participant’s tolerance of demanding experimental sessions (Bauer et al., 2011; Hartshorn et al., 1998). To address these challenges, researchers over the past several decades have created developmentally appropriate paradigms to investigate some of the core processes that support episodic memory as it emerges in infancy and undergoes important development through the toddler years and beyond. Specifically, these paradigms have focused on capturing the ability to encode and retain memory representations that link together the elements of an experience (e.g., Olson & Newcombe, 2013).
For example, imagine a toddler that has been asked by their parent about what games they played in daycare earlier that same day. To be able to accurately report features from this specific experience, the toddler needs to be able to retrieve a memory that contains multiple elements (e.g., toys, friends) and place these elements in space (e.g., on the rainbow mat inside or outside on the playground) and time (e.g., the games were played before lunch or after naptime). Furthermore, toddlers also need to appreciate associations between elements (e.g., that they played blocks with Omar before naptime on the rainbow mat and played toy cars with Isabella after naptime outside on the playground). To be able to assess these different aspects of episodic memory in toddlers, and even infants, researchers have primarily relied on three paradigms: imitation (e.g., Bauer, 2004), conjugate reinforcement (e.g., Rovee-Collier et al., 1980), and visual paired comparison (e.g., Rose et al., 2004). These paradigms do not require participants to provide verbal reports of their past experiences, but nevertheless demonstrably engage processes that underlie how young children might retrieve aspects of events and envision them within their context. As such, research using these paradigms provide converging evidence about the development of these processes throughout infancy and the toddler years. These paradigms are typically applicable to both infants and toddlers; however, distinctions will be made as necessary.
2.1. Imitation paradigms
Imitation paradigms assess infants and toddlers’ ability to reproduce actions demonstrated by an experimenter after a delay (e.g., Bauer, 2004). First, experimenters demonstrate to infants a sequence of actions carried out with several objects. After learning this sequence, infants are given the opportunity to imitate those actions. The ability to accurately imitate the sequence is seen as an indication of memory for the experience. This imitation provides insight on infants’ ability to retain actions precisely (e.g., Barr et al., 2016) as well as the temporal order with which they were enacted (e.g., Bauer & Mandler, 1992) or the spatial context that the task is carried out within (e.g., Burns et al., 2015). Although imitation does not involve verbal reports on memory, it is thought to be a useful proxy for episodic memory because it shares some of its functioning principles (e.g., Bauer et al., 2011), such as demonstrating decay over delays and suffering if there are mismatches between encoding and retrieval context (e.g., Bauer et al., 2007). This paradigm is appropriate for use in infants and toddlers, and it seems to provide insight particularly into the ability to retain temporal aspects of memory (Bauer, et al., 2000).
2.2. Conjugate reinforcement paradigms
The conjugate reinforcement paradigm has also been used extensively with infants. This paradigm assesses infants’ recognition of the connection between their body movement and the resulting motion of an item (Rovee-Collier et al., 1980). In this paradigm infants are typically taught that moving their legs can result in the desirable movement of a mobile. This is achieved by tying the infant’s leg to a mobile hung in clear sight and allowing them the opportunity to kick their legs and watch the mobile move. After the infant has learned this connection, the end of the ribbon previously attached to the mobile is removed, and thus leg kicking no longer produces movement in the mobile. Researchers then record the infant’s kicking frequency after a delay. Kicking while the mobile is no longer attached and providing immediate visual feedback is viewed as an indication of the infant’s retention of this conditioned response. Although memory manifests as a conditioned response, some researchers posit that infants demonstrate the engagement of processes that are shared with episodic memory, because the infant needs to reinstate a specific event representation in order to recognize that the conditioned response is relevant (e.g., Rovee-Collier, 1997). The results obtained from this task largely match the functioning principles of the formation and retrieval of declarative memories, focusing especially on context-dependency (e.g., Rovee-Collier, 1997) and the effects of reminders on memory retention (e.g. Rovee-Collier et al., 1980). The mobile conjugate task becomes developmentally inappropriate by the second half of the first year of life. However, older infants have been successfully taught to associate pressing a button with the movement of a toy train (Hartshorn & Rovee-Collier, 2003). Overall, results obtained with this paradigm have greatly contributed to our understanding of memory capabilities in infants.
2.3. Visual paired comparison paradigms
The visual paired comparison is another paradigm that has been successfully applied to infants as well as toddlers (Fantz, 1964). In the visual paired comparison paradigm, infants’ looking behavior when presented with old and new information provides information about their memory (Rose et al., 2004). In the encoding phase of this paradigm, infants view and encode a set of stimuli (e.g., pictures). During a later test phase, infants are presented with pairs of pictures such that each pair consists of a picture they had previously seen in the encoding phase, as well as a novel picture. Memory for the old picture is inferred by infants’ looking behavior, with looking times toward the new picture compared to the old picture serving as evidence of memory for the old picture (Rose et al., 2004). Performance on the visual paired comparison can also be effectively assessed in older children (e.g., Morgan & Hayne, 2011) and adults (e.g., Richmond et al., 2004), which has served to bolster the applicability of the findings. Impaired performance in amnesic adults indicates that the task assesses episodic memory, which is impaired in hippocampal amnesia (e.g., Pascalis et al., 2004). Although this paradigm was initially developed to examine the ability to discriminate between an old and new item, more recent instantiations have examined infants’ ability to form associations between multiple features of the memory, including assessments of item-context relations with great specificity (reviewed in: Pathman & Ghetti, 2016). As such, this paradigm provides a non-verbal measure that can examine how infants make associations between features of a memory, including between items and: their spatial location (e.g., Richmond et al., 2015), temporal order (e.g., Tummeltshammer et al., 2017), and other items (e.g., Johnson et al., 2019). A particular benefit of the visual paired comparison paradigm is that only a single presentation of stimuli is necessary, which bolsters the case that information retained is represented by episodic mechanisms, and that responses are evidence of reflecting on a single event instead of a learned association or a fact. Overall, a wealth of studies manipulating different aspects of the context of a memory have contributed to the understanding of what kinds of information can be encoded and retained in infancy and well beyond.
2.4. Convergence across behavioral paradigms
Despite their differences, these paradigms have largely converged in showing a range of memory capabilities in infancy and the toddler years (Mullally & Maguire, 2014). However, although it is evident that young infants can retain elements of their past experiences, prior to age 2 years they seemingly fail to form and retrieve memories that fully capture arbitrary associations, which is foundation for the ability to remember episodically (Ghetti, 2017). For example, even though infants can reproduce the temporal order of sequences of demonstrated actions (Carver & Bauer, 1999), these action sequences typically involve enabling relations. Enabling relations are adjoining actions in a sequence wherein an action is both temporally prior to and necessary for a subsequent action to be properly enacted (e.g., steps to make a gong from novel object parts). Enabling relations support successful retrieval, suggesting that retrieval benefits from functional relations among actions in addition to temporal relations (Bauer, 1992). Retention of fully arbitrary associations between actions and their temporal order in a sequence after one single demonstration is not reliable until the end of the second year of life, and it is not until 28 months that this order can be retained over a delay (Bauer et al., 1998). The timing of this emergence is remarkably similar across tasks and types of relations (Johnson et al., 2019; Newcombe et al., 2014; Richmond et al., 2015), but the absence of studies examining these relations with the same paradigm and participants prevents firm conclusions and begs for more work examining memory in the toddler years.
In addition to improvements in the ability to track contextual relations such as space and time, there is clear evidence across paradigms of improvements in the length of retention over the course of infancy and the toddler years (e.g. Cuevas & Sheya, 2018). Various conjugate reinforcement studies demonstrate that the kicking association is retained longer in older infants (e.g., Hartshorn et al., 1998; Hill et al., 1988). The same result is confirmed with visual paired comparison paradigms showing that preferential looking to novel items is observed over longer delays in older infants and toddlers (Hayne et al., 2016; Morgan & Hayne, 2011). Similar patterns of results have been found in studies using the imitation paradigm (Barr et al., 1996; Bauer et al., 2000).
Despite this strong tradition of behavioral research, we still have little understanding of how change in neural structure and function supports these behavioral improvements. This research would be critical to elucidate changes in the trajectory of brain-behavior associations over the course of early childhood. In the next section, we examine the current proposal that the early development of the hippocampus may be centrally responsible for behavioral presentation of episodic memory in the toddler years.
3. Hippocampal contribution to the emergence and persistence of episodic memory
The hippocampus is a structure in the medial temporal lobe that has long been considered critical for the formation and reinstatement of arbitrary associations between events and their spatio-temporal context, which is a key aspect of episodic memory (Cohen & Eichenbaum, 2001). Prior research has shown that anatomically distinctive subfields within the hippocampus have been tied to aspects of relational binding (Copara et al., 2014; Henke, 2010), which reflects the ability to form these arbitrary associations. This research, however, has been carried out predominately in adult organisms, including extensive work in rodents and humans (Burgess et al., 2002; Moser & Moser, 1998).
We have learned from a variety of developmental studies that episodic memory abilities develop during infancy and the toddler years, and changes in the hippocampus appear to mirror these behavioral changes. For example, the hippocampus undergoes considerable change postnatally (Insausti et al., 2010; Seress, 2001), through early childhood (Lee et al., 2017; Riggins et al., 2015), well into the adolescent years (DeMaster et al., 2013; Selmeczy et al., 2019), and in adulthood (Bellander et al., 2016; Boldrini et al., 2018). The role of hippocampal development in supporting the emergence and development of episodic memory has been extensively theorized. Hypotheses have primarily focused on the full integration of the dentate gyrus into the tri-synaptic circuit and neurogenetic processes.
3.1. The development of the dentate gyrus and tri-synaptic circuitry
The dentate gyrus is a hippocampal subfield, which in close connection with the cornu ammonis 3 (CA3), has been related to the ability to form and support distinct memory representations of complex and arbitrary relations (Leutgeb et al., 2007; Nakashiba et al., 2008). Animal research has indicated that the greatest postnatal growth in the hippocampus is within the dentate gyrus (Jabès et al., 2010; Jabès & Nelson, 2015). Other portions of the hippocampus were found in post-mortem research in humans to have comparatively less growth postnatally (Insausti et al., 2010; Seress, 2001). Consistent with this, Lavenex and Lavenex (2013) proposed that earlier developing memory capabilities, which may allow, for example, to represent the spatial context only from one’s own perspective, may be based on the recruitment of the more rudimentary monosynaptic path. This path develops early and does not include the CA3 and dentate gyrus, but only CA1 and subiculum. The development of the later emerging trisynaptic path, which involves the CA3 as well as the dentate gyrus, affords more sophisticated abilities including the ability to represent spatial contexts more flexibly without the need for self-reference (Ribordy et al., 2013), as well as the ability to effectively bind distinct temporal and associative features of an event. While this work makes claims that changes in hippocampal development are associated with improvements in memory abilities, especially along the transition from infancy into the toddler years (e.g. Ribordy et al., 2013), these associations with behavior have yet to be directly demonstrated in humans.
3.2. Neurogenetic processes
Another line of research focuses on the consequences of neurogenesis in the hippocampus, suggesting that neurogenesis may be both helpful and detrimental to memory, with high rates of neurogenesis leading both to increased learning abilities and a tendency to forget information encoded earlier in life (Frankland et al., 2013). The hippocampus is unique in that it undergoes neurogenesis in early life in mammals (Ming & Song, 2011). Research had long focused on the beneficial effects of hippocampal neurogenesis for memory formation in adult animals (e.g., Deng et al., 2010; Sahay et al., 2011), however it was recently discovered that reconfiguration of synaptic relations following integration of new neurons in the circuity can also disrupt previously encoded memories (Frankland et al., 2013), and that experimentally reducing levels of neurogenesis in infant mice promoted memory persistence (Akers et al., 2014). This tendency to forget information after neurogenesis may result in difficulty retrieving memories, but not necessarily the outright deletion of the memory trace, because rodents have been shown to recover these disrupted memories after chemical manipulation (Travaglia et al., 2016). Changes in the rate of neurogenesis are thought to be one explanation for age-related improvements in memory retention, which track improvements in episodic memory from infancy onwards (Ramsaran et al., 2018).
Taken together, these results provide a potential explanation for memory improvement and steep forgetting rates in infancy and the toddler years: high levels of neurogenesis, and the growth of portions of the hippocampus relevant to more complex aspects of episodic memory, may confer both rapidly improving episodic memory capabilities as well as issues with longer term retention of this episodic information. However, this theoretical model has also not been tested or validated in humans. Additional neuroimaging work in human infants and young children is required to address theories largely supported by animal data.
4. Probing changes in hippocampal function with neuroimaging: Challenges and solutions
The past several years have witnessed significantly increased interest in investigating how changes in brain function support behavioral development in infancy and the toddler years. These questions have also involved the investigation of early memory functioning. Various techniques have been used to pursue this line of investigation, each of which have their own strengths and limitations. For example, Event-Related Potentials (ERPs) data provide excellent temporal resolution when measuring brain activity and have been collected from infants and toddlers in numerous studies (e.g., Bauer, 2004; Riggins et al., 2009; Wiebe et al., 2006). In the context of early memory functioning, ERP studies have revealed evidence of brain activity in infants associated with memory encoding and retrieval (Bauer, 2004). For example, researchers have shown that the ERP signal varies when infants are viewing familiar versus novel stimuli (e.g., Wiebe et al., 2006), and in toddlers during retrieval of temporal order information associated with a deferred imitation task (Riggins et al., 2009). However, ERP methods are not ideally suited to interrogate processes supported by subcortical structures such as the hippocampus.
Similarly, Functional Near-Infrared Spectroscopy (fNIRS) has been used to successfully measure neural activity in infants and toddlers (for a review: Aslin et al., 2015) to examine language, (e.g., Fló et al., 2019), executive function (e.g., Perlman et al., 2014) and cortical signatures of memory for words (e.g., Benavides-Varela et al., 2012). FNIRS serves as another useful index of cortical function, and its utilization is rapidly increasing, however its limited penetration beyond the cortex prevents examination of hippocampal functioning.
The limitation of these neuroscientific tools most commonly used in infants to provide insight onto hippocampal functioning has led to interest in alternative approaches. For example, conditioned eyeblink has been proposed as a behavioral proxy for hippocampal function in young children based on work in humans and non-human animals (Vieites et al., 2019), and this is a technique that may prove useful in the future since eye-tracking data is more readily collected from infants and toddlers. However, once again, this paradigm does not directly examine hippocampal function.
Functional Magnetic Resonance Imaging (fMRI), however, is a technique that can successfully probe hippocampal function (e.g., Davachi, 2006; Rugg et al., 2012), and shows some promise, albeit with specific considerations to be taken into account for data collection. A challenging aspect of fMRI data collection in children is the considerable acoustic noise produced by the scanner (Edelstein et al., 2002) and the necessity that the participant stays very still during targeted behavioral tasks, which frequently last upwards of 30 minutes (e.g. Friston et al., 1996). One alternative is to focus on structural MRI, which has excellent spatial resolution and thus can elucidate details about the anatomy and pathology of cortical and subcortical structures. In addition, it is obtained while the participant is not actively engaged in a task and thus these images can be collected during sleep or while children are engaged with materials that they find interesting (e.g., cartoons). Also, several studies have now shown that collecting structural MRI data during natural sleep is viable in infants and toddlers (Nordahl et al., 2008; Raschle et al., 2012). Structural data can be informative about the shape and volume of the hippocampus and its subfields, as well as the rates of growth of these areas, and several studies have already looked at the association between hippocampal volume and memory abilities later in childhood (e.g. Lee et al., 2014; Riggins et al., 2015; Riggins et al., 2018). However, it does not tell us about the functional properties of the hippocampus. Theories about the developmental immaturity of the hippocampus in this age range may suggest that the hippocampus is not recruited in memory tasks or does not contribute as efficiently as in older children to the encoding and retrieval of memory episodes (e.g., Gómez & Edgin, 2016). In order to answer these questions, one needs to address hippocampal function, and to look at hippocampal function the most applicable imaging method is functional MRI (fMRI).
FMRI methods have long been used to assess hippocampal function. (e.g., Ranganath, 2010). In these studies, fMRI data are collected either while participants are engaged in encoding of new stimuli, while they attempt to remember them, or both (Davachi, 2006). Hippocampal activations have been consistently recorded (Chadwick et al., 2010) providing insight on the role of this structure during episodic memory acquisition and retrieval (e.g. Moscovitch et al., 2016; Rugg & Vilberg, 2013). These paradigms have been successfully adapted for the examination of developmental differences in neural substrates of memory formation and retrieval in older children (e.g., DeMaster & Ghetti, 2013; Ofen et al., 2007), who can be instructed to stay still or helped to control their motion with the use of mock scanners for practice. Children as young as four have been successfully assessed with resting-state connectivity procedures (obtained while awake) to link hippocampus-cortex connectivity with performance on episodic memory tasks (e.g. Geng et al., 2019); however, no data are currently available with infants and toddlers. Despite the typically limited number of overt responses elicited in these paradigms as compared to adult studies, researchers have been able to draw some important insight on the development of episodic memory, particularly from middle childhood into adolescence (for review see: Ofen, 2012).
When it comes to functional changes in infancy and the toddler years, however, research to date has focused on examining behavioral implications of functional hippocampal-cortex connectivity with memory tasks collected outside the scanner (e.g., Riggins et al., 2016). These lines of work are informative, but also expose the clear difficulties of obtaining task-related data on early hippocampal functioning. Recent studies in adults, however, have shown that task-related fMRI data collection is possible during natural nocturnal sleep (e.g., Van Dongen et al., 2012), which might afford important flexibility for populations, such as infants and toddlers, who may struggle to withstand the demands of fMRI experimental designs during wakefulness. Preexisting fMRI data collected in infants and toddlers during natural sleep also point towards the promise of this approach.
4.1. FMRI during natural nocturnal sleep: A window into early neurocognitive development
A study by Van Dongen et al. (2012) has provided insight on experimental approaches that promise to contribute to the examination of early hippocampal function and other neurocognitive mechanisms. In their study, adult participants encoded a series of visually presented images, such that each image appeared in a designated location on the screen along with a specific sound (e.g., a “meow” sound was associated with an image of a cat). Later, fMRI data were collected while participants were asleep and auditory stimuli were delivered to them. Specifically, during slow-wave sleep, participants heard sounds that had been previously paired with the studied images, as well as sounds that had not been heard previously. Greater activation was found in the left parahippocampal gyrus as well as other cortical regions typically involved in memory for previously paired sounds as compared to novel sounds. These results suggested that recent memories may be reactivated during sleep resulting in an ability to distinguish old stimuli from new stimuli. The finding that memories can be reactivated during sleep (Cairney et al., 2016; Oudiette & Paller, 2013) and that sleep reactivation can be captured experimentally with fMRI (Shanahan et al, 2018; Van Dongen et al., 2012) raises the question of whether these methods could be extended to assess hippocampal processes supporting memory in infants and toddlers.
Studies examining other cognitive processes have suggested the feasibility of this approach. In the last two decades a small number of studies have used fMRI methods to examine primarily phonological and auditory processing in infants and toddlers (e.g., Dehaene-Lambertz et al., 2002; Redcay et al., 2007). These studies have typically employed block designs to discover whether activation in target cortical regions could discriminate between different auditory conditions or whether activation could be localized in similar brain regions recruited by adults. For example, researchers have examined brain activity for: human language as compared to other sounds (Redcay et al., 2007; Shultz et al., 2014), mother’s voice as compared to the voice of a new adult (Dehaene-Lambertz et al., 2010), and differing linguistic content compared against each other (Dehaene-Lambertz et al., 2002). These various studies have demonstrated the striking ability of the sleeping brain to discriminate auditory information (and even respond to visual stimuli in the form of lights being shone on sleeping infants; e.g., Redcay et al., 2007), and have found that the nature of basic auditory processing is very similar to that of adults upon direct comparison (Wild et al., 2017). Overall, these studies have consistently reported experimentally induced cortical activations in infants and toddlers. Together with studies with adults showing memory reactivations, they have supported our efforts to examine memory-related activation in the hippocampus during sleep.
4.2. Memory-related fMRI activation in the sleeping hippocampus
Over the past few years, we have begun to pursue this new line of investigation seeking to examine hippocampal contribution to early memory functioning. We designed a novel paradigm to assess whether hippocampal activation differentiated between auditory stimuli that were or were not associated with previously experiences.
Specifically, in our first study (Prabhakar et al., 2018), we had 2-year-old participants involved in three visits to the laboratory over the course of roughly a week, during which they were involved in play sessions in two different rooms (A and B). These experiences were distinctive but designed to be roughly comparable; however, each room had a unique theme (e.g., bedtime, backyard, playground) with toys that were relevant to the theme, as well as a distinct puppet. Critically, while toddlers played with the toys and puppet in each room, they heard a unique African lullaby (e.g., room A had the bedtime game, with distinct toys, a unique character A, and song A playing in the background; in contrast, room B had the backyard game, with distinctly different toys, a unique character B, and song B playing in the background). After the third visit, one of the songs was played in a hallway outside the two play rooms (e.g., Song A), and toddlers’ memory was tested for the association between that song and the character (from game A) as well as the room in which they had heard the song (room A). Toddlers’ associations were only tested for one of the learned songs to reduce task demands, but the fact that unique characters and locations had been associated with two distinct songs provided reassurance about the unique and arbitrary association between a unique song and unique elements of the toddlers’ experiences. We could then use toddlers’ performance in this task as the behavioral indication of memory for the song-play experience and examine whether it was associated with the strength of hippocampal activation in response to the learned song during the nighttime fMRI session.
Over the course of the laboratory sessions, parents and their toddlers were familiarized with the MRI safety protocol and hearing protection necessary for neuroimaging data collection. At home, parents prepared their toddlers nightly by putting practice earplugs and headphones on their toddlers during sleep. Alongside this, they played sample audio of MRI sounds at high volumes to ensure that their toddlers were both comfortable with the hearing protection and not overly sensitive to these noised produced by the MRI. Parents were encouraged to incorporate these steps into their nightly routine so that their toddlers would not find these elements strange on the night of the actual functional neuroimaging session. Furthermore, we developed a plan for the day of neuroimaging so that toddlers would be optimally prepared. For some toddlers, this meant withholding an afternoon nap on the day of the neuroimaging session or choosing a daily activity that ensured toddlers would sleep readily by the time the neuroimaging session occurred. Altogether, an individualized plan was developed with each family to follow on the day and night of the neuroimaging session to ensure maximum comfort and efficiency, and to approximate the toddlers’ normal bedtime routines as closely as possible.
On the night of the neuroimaging session (on average 1.5 days after the last laboratory session), the MRI room was always set up with memory foam mattresses, pillows, blankets, stuffed animals, sheets hung up on the walls, and any pre-determined safety-screened personal items (often a special blanket), to make the neuroimaging process as comfortable and familiar for the toddler as possible. Although we would ideally have used a preparation protocol that was identical across participants, flexibility was critical to achieve successful data collection. For example, some parents arrived with their toddlers already asleep. In contrast, other parents used a room made available at our imaging center with an air mattress and a night lamp to be able to mimic the toddlers’ night routine. Others used the MRI bed to help their toddler fall asleep inside the imaging room itself. Regardless, we recorded the length of time toddlers were asleep and ensured that no toddlers began neuroimaging earlier or later than approximately 20 minutes into the start of sleep. Once toddlers had been asleep for roughly 20 minutes and were in a supine position, we placed on all hearing protection and began the neuroimaging session. Parents were reminded of the rough length of the session, put on their own hearing protection, and then were joined by a researcher, who was present in the MRI room with the parent for the duration of imaging to be able to quickly respond if toddlers showed signs of waking up. If toddlers seemed close to awakening, the experimenter immediately stopped the MRI and assessed whether the session could continue without waking the toddlers.
In this first study (Prabhakar et al., 2018), during the neuroimaging phase, toddlers were presented with nine randomized 20-second blocks of audio that included the learned song from the laboratory experience (song A), a reversed version of the learned song, and a completely novel African lullaby. Each active block was separated by 20-second silent blocks that served as a baseline. We predicted that a general contrast including both the previously heard song (song A) as well as the reversed version of this song would reveal hippocampal activation as both sound conditions included content associated with toddlers’ laboratory experiences, whereas the novel song did not.
Consistent with expectation, we found bilateral activation (Fig 1.A) using left and right hippocampal masks obtained from an infant and toddler brain atlas (Shi et al., 2011). Thus, this presentation of previously encountered auditory information which had been tied to a rich episodic experience, led to reliable recruitment of the hippocampus even during sleep. Additionally, the parameter estimates from the cluster in the right hippocampus for learned song > novel song showed a significant positive relation with toddlers’ ability to correctly identify the character and/or room associated with the learned song, song A (Fig 1.B+C). These connections between behavioral and neural evidence of memory suggest that sleep-MRI techniques can be used to assess the function of brain areas associated with episodic memory after a recent experience.
Figure 1.
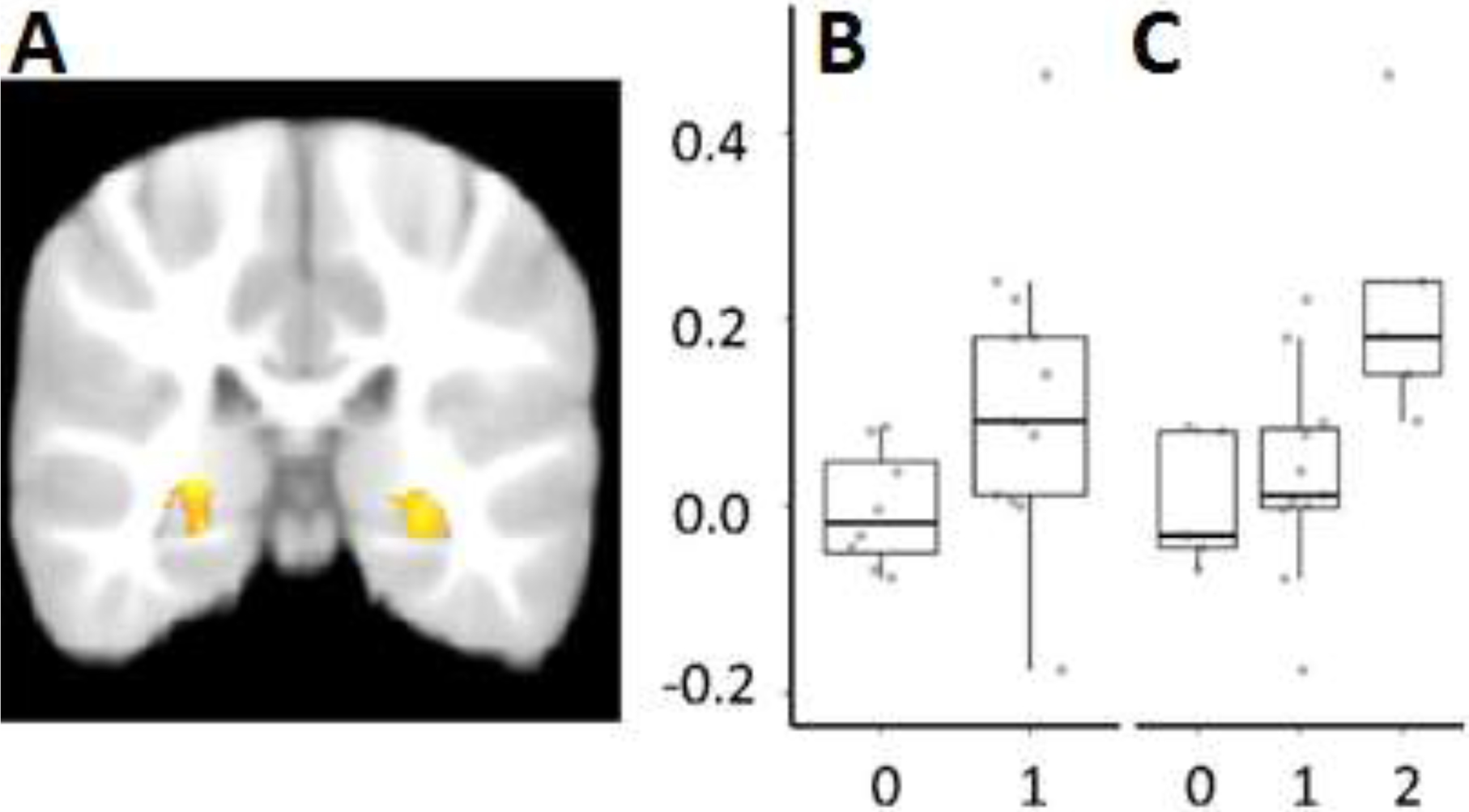
Functional MRI analysis for recent memory. Group analyses were constrained to the left (LH) and right hippocampus (RH) using age-appropriate hippocampal templates and a cluster thresholding process (z = 2.30, cluster corrected, P < 0.025). (A) These analyses isolated active bilateral clusters for the memory (target + reversed) > novel contrast (left, max: x = −26, y = −33, z = 1; 1,839 voxels; right, max: x = 23, y = −27, z = 1; 1,107 voxels). (B and C) Mean parameter estimates (PE) were extracted for the T > N contrast. The relation between PE (y axis) for T > N in the RH and the number of correct items in our two behavioral measures (x axis), room choice (B) [r = 0.43, t(19) = 2.10, P = 0.049, d = 0.77] and the composite memory measure (C) (ρ = 0.60, P = 0.004) was significant. Adapted from “Memory-Related Hippocampal Activation in the Sleeping Toddler.” By J. Prabhakar, E. G. Johnson, C.W. Nordahl, and S. Ghetti, 2018, Proceedings of the National Academy of Sciences, 115(25), p. 6502. Copyright 2018 National Academy of Sciences. Adapted with permission.
5. Infant memory research with fMRI: Enduring questions and promising applications
Developmental theories about the hippocampus have primarily been formulated using rodent models and post mortem histological studies; this work has contributed substantial understanding about neurogenesis rates as well as the development of the dentate gyrus and the trisynaptic path (Frankland et al., 2013; Gómez & Edgin, 2016; Insausti et al., 2010; Lavenex & Lavenex, 2013; Seress, 2001; Travaglia et al., 2016). However, none of these theories have been directly translated to human development in infancy and the toddler years, nor to our understanding of human memory development. To date, theories from the human developmental literature have suggested that cortical regions of the temporal lobe (and not the hippocampus) exclusively process memory for specific events in infancy, and possibly the toddler years as well, due to insufficient development of the trisynaptic path (Gómez & Edgin, 2016). Prabhakar et al. (2018) represents the first study to explore the functional role of the hippocampus in the early development of episodic memory in human toddlers, however the connection remains to be made in infants. As such, this work begins to validate non-human animal models that highlight the functional properties of the hippocampus and its contribution to early memory abilities. As a result of this work, the hippocampus can now be more effectively integrated into theories about early human memory development. Using this paradigm, future work can begin to explore the role of the emerging hippocampus in supporting other facets of the development of the human episodic memory system, including forgetting rate, consolidation, and learning abilities.
5.1. Forgetting and persistence of early memories
It is well known that infants and toddlers lose their memories at a fast rate, resulting in the phenomenon known as infantile amnesia (Bauer, 1997). Recent evidence in rodents has linked hippocampal processes to this forgetting (Frankland et al., 2013; Travaglia et al., 2016). A promising application of fMRI methods concerns the examination of the fate of early memories and the investigation of hippocampal activation over time. We describe the approach here in the context of a pilot study, as an illustration of the potential questions that this approach may help address. One question that could be addressed is whether evidence of memory reactivation could be observed in the hippocampus even when young children fail to recall the past behaviorally. This pattern of hypothetical results would suggest that the loss of early memories would primarily reflect a difficulty with successfully retrieving the memory as opposed to indicating a complete loss of the memory trace. A pilot study provides an illustration of this point. Seventeen toddlers from Prabhakar et al. (2018) (M age = 33.8 months, SD = 2.4, age range = 28.7 – 36.9 months) were brought back to the laboratory after approximately 4 to 8 months (M = 5.3 months, SD = 1.7) after their initial participation. Before the new nighttime fMRI session, we tested their memory for the previous experience, giving a very similar test of the association between a song and an associated room/puppet as Prabhakar et al. (2018). This time however, we used the song that was played in the second room (song B in room B) as memory for this song was not tested, either behaviorally or during the neuroimaging session, during the previous (i.e., the first) visit. This also meant that the song previously targeted in the MRI had not been heard since the previous neuroimaging session. Behavioral performance was expectedly poor, with the group choosing either the correct room (M = .67, SD = .49) or the correct puppet (M = .54, SD = .52) at chance levels. Then, toddlers underwent an fMRI paradigm which was similar to that used in Prabhakar et al. (2018) and included 20 second blocks targeting the old song last heard during the previous MRI scan, as well as a novel song. To establish whether reactivation occurred, one can then assess the levels of hippocampal activation in response to the old song compared to the new song. Exploratory analysis was carried out using a threshold purposefully more liberal than Prabhakar et al (2018) (p < .05, with no correction for multiple comparison), which showed two small clusters of hippocampal activation associated with the old song, learned 4 to 8 months earlier, compared to a completely novel song (Fig 2). The observation of these kinds of results with a proper sample size and statistical tests, would allow researchers to draw conclusions about the state of long-term memories. Additional extensions might include the comparison of memory and hippocampal activation between recent and remote memories. Overall, although we do not intend to draw any conclusions about the role of the hippocampus in long-term memory recall from this evidence, the observation of clusters of activation in the vicinity of the large cluster identified in Prabhakar et al (2018) provides a foundation for future studies. Future work will be able to determine more conclusively whether a functional index of long-term memory processing is apparent in the hippocampus and reflects the role of the hippocampus as an enduring neural signature of episodic memory processing between two and three years of age.
Figure 2.
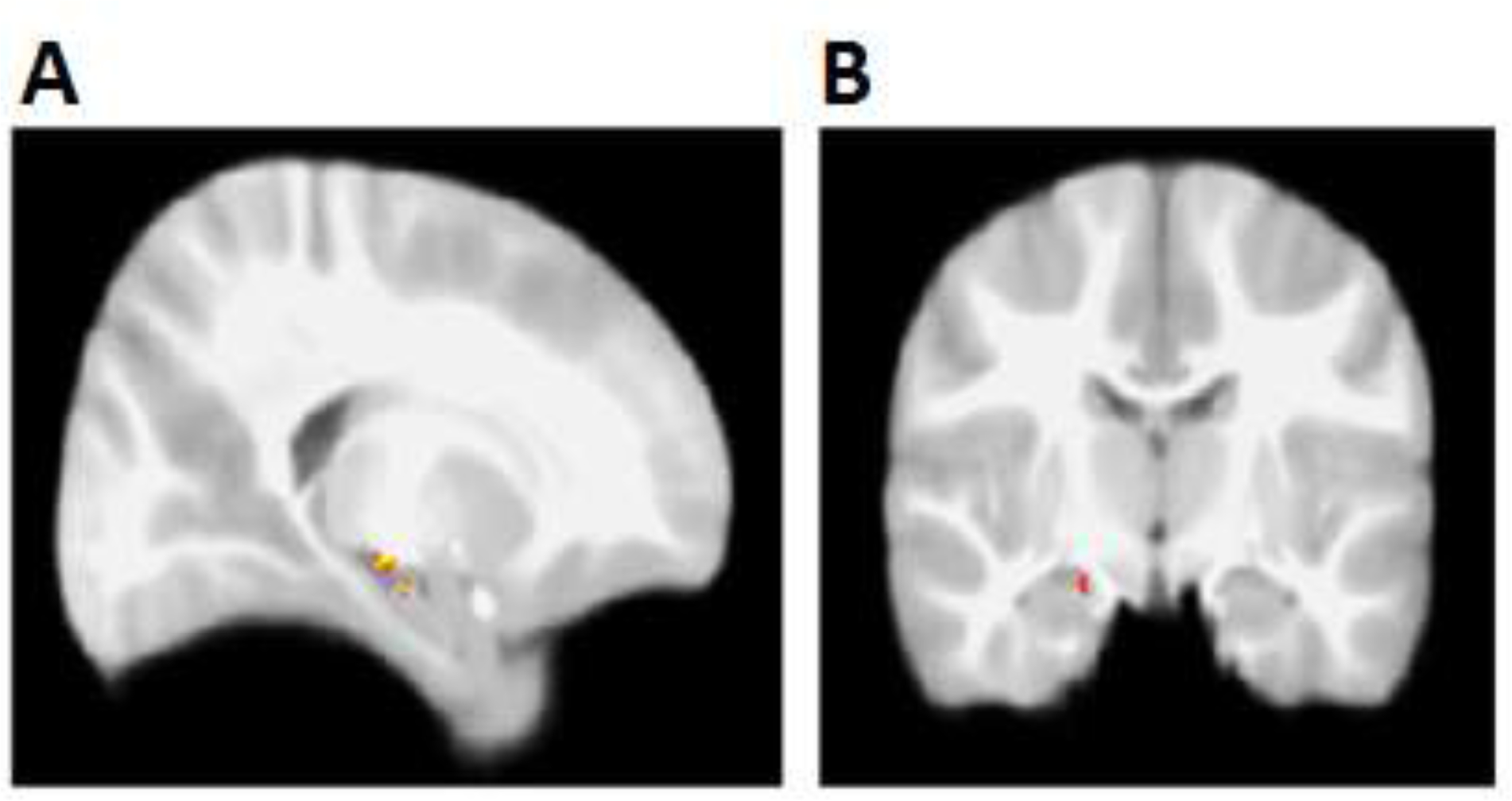
Preliminary functional clusters in the hippocampus for remote memory. Contrast comparison of previously heard song to a novel song resulted in two clusters in the right hippocampus [Z > 1.97, no cluster correction (Cluster 1, max: x = 18, y = −17, z = −9; 19 voxels) (Cluster 2, max: x = 18, y = −11, z = −13; 10 voxels)]. (A) Sagittal view, x = 18; (B) Coronal view, y = −11.
All the previously mentioned behavioral forms of episodic memory assessment in early life (deferred imitation, mobile conjugate, and visual paired comparison) can also feasibly be leveraged for assessment of memory retention, since each have been utilized to show age-related improvements in memory (e.g., Bauer et al., 2000; Hartshorn et al., 1998; Morgan & Hayne, 2011). In order to collect memory-related fMRI data during natural sleep in infants and toddlers, the major consideration is to find a way to integrate an auditory “tag” into the behavioral paradigm which can later be probed with the fMRI. Imitation tasks have been used successfully to assess infants’ ability to retain temporal (Bauer & Mandler, 1992) and toddlers’ spatial (Burns et al., 2015) contextual details, for example. Because there is reason to believe that temporal, spatial, and associative capabilities develop at different rates over the course of infancy and toddler years, as well as beyond into childhood (Ghetti, 2017; Olson & Newcombe, 2013), these developing abilities could be behaviorally assessed in pairing with unique sounds (i.e. spatial task paired with sound 1, temporal order task paired with song 2) to examine unique associations with hippocampal activity. Visual paired comparison tasks examining novelty preference for items or associations between items could also be associated with sounds (later played in the MRI). The association with a sound might be an incidental pairing with the experience (like in Van Dongen et al., 2012, where images were paired with animal sounds) or fundamentally incorporated into a multimodal associative memory task in which sounds are explicitly taught to be associated with an item on a screen and these associations are explicitly tested. Additionally, one could manipulate the depth of encoding by giving some children additional exposure to the unique sounds and visual stimuli in order to determine the neural mechanisms that support learning processes in infancy and early childhood. Finally, a focus on early infancy could probe the use of conjugate reinforcement techniques for age-appropriate behavioral assessment. Since conjugate reinforcement performance in young infants has been shown to be easily disrupted by changes in context (e.g., Borovsky & Rovee-Collier, 1990), researchers could creatively utilize music, or even simpler sounds, within this paradigm (e.g., Fagen et al., 1997). As with other tasks, a major focus of assessment should be on changes in retention as a result of age change, with variable delay, as indexed by both behavioral and fMRI data. We note that the longstanding debate about whether the conjugate reinforcement task recruits hippocampal processes or other neural substrates of episodic memory may be adjudicated through the inclusion of these fMRI methods.
Although connecting hippocampal activation with behavior is a fundamental promise of fMRI studies in infants and young children, fMRI approaches may also hold promise in the absence of behavioral outcomes. For example, this approach could allow researchers to manipulate factors critical to a memory representation (e.g. number of features, delay) and identify neural patterns of activation as a result of varying the content of memory representations even when participants do not produce a behavioral response. Additionally, neuroimaging methods could help address questions about shared processes between different methods used in infants (e.g. conjugate reinforcement or imitations tasks). Overall, fMRI studies during sleep can offer valuable and complementary information to the traditional behavioral methods.
5.2. Consolidation
The examination of consolidation processes could also benefit from incorporating these fMRI methods. Memory consolidation has been associated with slow wave sleep (Marshall & Born, 2007). FMRI studies during sleep in adults have examined brain activation associated with recently learned material and have shown that the hippocampus is active in response to this previously experienced auditory or olfactory stimuli (Diekelmann et al., 2011; Rasch et al., 2007; Van Dongen et al., 2012). Work exploring the effects of naps on memory consolidation in young children has demonstrated that naps after learning improve memory performance (Desrochers et al., 2016; Gómez et al., 2006). Given that consolidation during sleep has a benefit for memory retention in children and adults, a promising avenue for future research would be to adapt the sleep fMRI paradigm presented here to study the neural indices of consolidation during sleep. Critically, sleep fMRI can also be paired with electroencephalography, which provides a precise indication of sleep stage (Duyn, 2012). Together, these two modalities show promise for the examination of consolidation in infants and toddlers. They will allow researchers to identify the precise window during sleep and the specific neural regions, such as the hippocampus in combination with cortical regions, that support consolidation of memories during sleep in the infant and toddler years.
5.3. Word learning
Although language development early in childhood has captured the attention of researchers for decades (Barrett, 2017), only relatively recently have memory abilities been considered in the context of word learning ability in infants and toddlers (e.g., Gordon et al., 2016; Vlach & Debrock, 2017). Cross-situational word learning paradigms utilize repeated (often ambiguous) experiences with a word label and the item referent instead of direct instruction (Smith & Yu, 2008). This type of learning suggests that language acquisition processes benefit heavily from the ability to encode and retrieve experiences in which a word was learned (Vlach & Debrock, 2017). The involvement of the hippocampus in language processing has become more clear with recent adult work showing word learning difficulties in hippocampal amnesia (Warren & Duff, 2014), and hippocampal structural changes in response to intense vocabulary learning of a foreign langue (Bellander et al., 2016). Although vocabulary has been traditionally conceptualized as an aspect of semantic memory because it represents knowledge (Tulving, 1972), hippocampal processes may contribute to the initial acquisition of words, when infants or toddlers learn for the first time the initially arbitrary association between a novel label and a novel referent, before the information is consolidated into semantic networks. Accordingly, rapid development of the hippocampus overlaps with, and may partially explain, the concomitant vocabulary burst that occurs in late infancy and onwards (Goldfield & Reznick, 1990). For example, such work may inform the question of whether language acquisition processes mirror those of adults, starting in the hippocampus and moving with consolidation to the cortex (Davis & Gaskell, 2009), or whether extra-hippocampal medial temporal lobe processing is required at an age before the hippocampus can effectively contribute (e.g., perirhinal cortex: Sharon et al., 2011).
5.4. Caveats
Although we contend that the use of fMRI methods during natural sleep is quite promising, we recognize a few important caveats. Specifically, the methods described in the previous section require further investigation to establish a few but critical boundary conditions. One question is whether the auditory information that is presented is recognized as sufficiently similar to the initial episode, despite the unavoidable addition of MRI sounds during the course of the imaging procedure. To our knowledge, direct work has not been carried out to compare recognition of sounds inside versus outside the MRI with infants and toddlers. However, we fall back on the extensive work looking at language and social processing in infants, during which sounds presented in the MRI were localized in expected cortical areas based on their linguistic relevance, or the identity of the speaker (e.g., Dehaene-Lambertz, et al., 2002; Redcay et al., 2007). Direct comparisons of the extent of brain activation associated with encountered sounds at various levels of degradation would be informative. Intriguingly, the extent to which hippocampal activity is disrupted by changes to sound qualities may be an indication of the rigidity of context-dependence of memory in infancy (Robinson & Pascalis, 2004), and we may expect younger infants to be more likely to exhibit disruption as compared to toddlers. In our minds these fMRI methods ought to be developed in tandem with behavioral assessments of auditory memory in infants and toddlers, and fMRI may provide important insights.
Another question is whether brain function during sleep is fundamentally different from function during wakefulness? Whereas research has attempted to address this concern by localizing brain activity in infants associated with visual and auditory stimuli during sleep (e.g., Redcay et al., 2007), the fact that the activation occurs in the expected areas does not guarantee that auditory stimuli such as those presented in Prabhakar et al. (2018) prompt memory retrieval in the same way as they would during wakefulness. In a study relevant to this question, Diekelman et al. (2011) associated an odor with an object location memory task performed by adults, and then later presented this odor either during waking or sleeping. Presentation of the odor during sleep improved subsequent performance on the object location memory task, whereas presentation during waking was associated with a decline in performance. It is possible that memories are reactivated by stimuli presented during sleep, and that hippocampal activity is evidence of consolidation processes helping to strengthen a memory (e.g., Diekelmann & Born, 2010). This possibility is not necessarily damaging to the interpretability of imaging data collected during sleep, but more knowledge is necessary to facilitate interpretation of these data. Because of the limited number of studies directly comparing memory-related hippocampal activity during waking and sleep, more work is needed in humans to understand the nature of activity during sleep.
Another limitation of fMRI research conducted during natural sleep is the inability to measure brain activity associated with memory encoding, or with overt assessments or decisions of memory evidence during retrieval. The behavioral tasks used to encode memories in infants and toddlers constrain the investigation of encoding because these tasks are very active and not easily incorporated into the MRI scanner (i.e. deferred imitation, conjugate reinforcement), or simply require a subject to be awake (i.e. visual paired comparison). However, some insight could still be gained. For example, as is the case in behavioral research, the consequences of encoding can still be examined indirectly by manipulating encoding processes during wakefulness and then examining the impact of these manipulations during sleep. Despite remaining questions about fMRI data collected during sleep, and limitations in terms of what aspects of memory can be probed, this method can provide valuable insights into the development of episodic memory.
6. Conclusion
Behavioral data have consistently shown that episodic memory can be effectively assessed in infants and toddlers, and that these abilities improve with age. While data from older children and adults indicates the importance of hippocampal recruitment for episodic memory, and data primarily from animal research suggests that the hippocampus may undergo rapid development in infants and toddlers, neuroimaging data collected from humans are sparse. Despite the difficulty associated with data acquisition, such data show the potential for improving our understanding of memory retention, memory consolidation, and word learning capabilities. Other applications of this technique are limited only by the ability of researchers to incorporate auditory elements into their work, which can be probed in the MRI. While very early life fMRI research remains in its infancy, a great deal of growth lies ahead. Overall, our review integrated with the presentation of some preliminary data provides insight on leveraging sleep and neuroimaging to gain insight on early memory functioning.
Table 1.
Summary of future directions.
| Questions to address with fMRI methodology | |
|---|---|
| Forgetting and Retention |
|
| Consolidation |
|
| Word Learning |
|
Highlights.
Episodic memory develops considerably during infancy and the toddler years.
Development of the hippocampus is hypothesized to be critical.
In vivo investigation in humans has been limited due to methodological challenges.
Functional MRI increasingly used in early childhood and can image the hippocampus.
Functional MRI data promises to connect brain and memory development at this age.
Acknowledgements
We would like to thank Naoya Tani and Ethan Fox, and numerous undergraduate research assistants for help with data collection. We are very grateful to the parents and toddlers who participated in this study. This work was supported by a grant from the National Institute of Child Health and Development [R21HD088928] to Simona Ghetti.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from.
Data Statement
The data that support the findings of this study are available from the corresponding author upon request.
References
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, Cristofaro AD, Hsiang L, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, & Frankland PW (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science, 344(6184), 598–602. doi: 10.1126/science.1248903 [DOI] [PubMed] [Google Scholar]
- Aslin RN, Shukla M, & Emberson LL (2015). Hemodynamic correlates of cognition in human infants. Annual Review of Psychology, 66(1), 349–379. doi: 10.1146/annurev-psych-010213-115108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R, Dowden A, & Hayne H (1996). Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior and Development, 19(2), 159–170. doi: 10.1016/s0163-6383(96)90015-6 [DOI] [Google Scholar]
- Barr R, Moser A, Rusnak S, Zimmermann L, Dickerson K, Lee H, & Gerhardstein P (2016). The impact of memory load and perceptual cues on puzzle learning by 24-montholds. Developmental Psychobiology, 58(7), 817–828. doi: 10.1002/dev.21450 [DOI] [PubMed] [Google Scholar]
- Barrett M (2017). Early lexical development In Fletcher P & MacWhinney B (Eds.), The handbook of child language (pp. 361–392). Wiley. [Google Scholar]
- Bauer PJ (1992). Holding it all together: How enabling relations facilitate young children’s event recall. Cognitive Development, 7(1), 1–28. doi: 10.1016/0885-2014(92)90002-9 [DOI] [Google Scholar]
- Bauer PJ (1997). Development of memory in early childhood In Cowan N & Hulme C (Eds), The development of memory in childhood (pp. 83–111). Psychology Press. [Google Scholar]
- Bauer PJ (2004). Getting explicit memory off the ground: Steps toward construction of a neuro-developmental account of changes in the first two years of life. Developmental Review, 24(4), 347–373. doi: 10.1016/j.dr.2004.08.003 [DOI] [Google Scholar]
- Bauer PJ, Hertsgaard LA, Dropik P, & Daly BP (1998). When even arbitrary order becomes important: Developments in reliable temporal sequencing of arbitrarily ordered events. Memory, 6(2), 165–198. doi: 10.1080/741942074 [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Larkina M, & Deocampo J (2011). Early memory development In Goswami U (Ed), The Wiley-Blackwell handbook of childhood cognitive development (2nd ed., pp.153–179). Wiley-Blackwell. [Google Scholar]
- Bauer PJ, & Mandler JM (1992). Putting the horse before the cart: The use of temporal order in recall of events by one-year-old children. Developmental Psychology, 28(3), 441–452. doi: 10.1037/0012-1649.28.3.441 [DOI] [Google Scholar]
- Bauer PJ, Wenner JA, Dropik PL, Wewerka SS, & Howe ML (2000). Parameters of remembering and forgetting in the transition from infancy to early childhood. Monographs of the Society for Research in Child Development, 65(4, Serial No. 263). [PubMed] [Google Scholar]
- Bellander M, Berggren R, Mårtensson J, Brehmer Y, Wenger E, Li TQ, Bodammer NC, Shing Y-L, Werkle-Bergener M, & Lövdén M (2016). Behavioral correlates of changes in hippocampal gray matter structure during acquisition of foreign vocabulary. NeuroImage, 131, 205–213. doi: 10.1016/j.neuroimage.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Benavides-Varela S, Hochmann J, Macagno F, Nespor M, & Mehler J (2012). Newborns brain activity signals the origin of word memories. Proceedings of the National Academy of Sciences, 109(44). 17908–17913. doi: 10.1073/pnas.1205413109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arrango V, Dwork AJ, Hen R, & Mann JJ (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell, 22(4), 589–599. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky D, & Rovee-Collier C (1990). Contextual constraints on memory retrieval at six months. Child Development, 61(5), 1569. doi: 10.2307/1130765 [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, & O’Keefe J (2002). The Human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641. doi: 10.1016/s0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- Burns P, Russell C, & Russell J (2015). Preschool children’s proto-episodic memory assessed by deferred imitation. Memory, 23(8), 1172–1192. doi: 10.1080/09658211.2014.963625 [DOI] [PubMed] [Google Scholar]
- Cairney SA, Lindsay S, Sobczak JM, Paller KA, & Gaskell MG (2016). The benefits of targeted memory reactivation for consolidation in sleep are contingent on memory accuracy and direct cue-memory associations. Sleep, 39(5), 1139–1150. doi: 10.5665/sleep.5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, & Bauer PJ (1999). When the event is more than the sum of its parts: 9-month-olds long-term ordered recall. Memory, 7(2), 147–174. doi: 10.1080/741944070 [DOI] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Weiskopf N, & Maguire EA (2010). Decoding individual episodic memory traces in the human hippocampus. Current Biology, 20(6), 544–547. doi: 10.1016/j.cub.2010.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N, & Eichenbaum H (2001). From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press. [Google Scholar]
- Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, & Ekstrom AD (2014). Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. Journal of Neuroscience, 34(20), 6834–6842. doi: 10.1523/jneurosci.5341-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, & Sheya A (2018). Ontogenesis of learning and memory: Biopsychosocial and dynamical systems perspectives. Developmental Psychobiology, 61(3), 402–415. https://doi.org/10/1002/dev.21817 [DOI] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16(6), 693–700. [DOI] [PubMed] [Google Scholar]
- Davis MH, & Gaskell MG (2009). A Complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1536), 3773–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, & Hertz-Pannier L (2002). Functional neuroimaging of speech perception in infants. Science, 298(5600), 2013–2015. doi: 10.1126/science.1077066 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, & Dehaene S (2010). Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain and Language, 114(2), 53–65. doi: 10.1016/j.bandl.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Demaster DM, & Ghetti S (2013). Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex, 49(6), 1482–1493. doi: 10.1016/j.cortex.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Demaster D, Pathman T, Lee JK, & Ghetti S (2013). Structural development of the hippocampus and episodic memory: Developmental differences along the anterior/posterior axis. Cerebral Cortex, 24(11), 3036–3045. doi: 10.1093/cercor/bht160 [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, & Gage FH (2010). New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience, 11(5), 339–350. doi: 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers PC, Kurdziel LB, & Spencer RM (2016). Delayed benefit of naps on motor learning in preschool children. Experimental Brain Research, 234(3), 763–772. doi: 10.1007/s00221-015-4506-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, & Born J (2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2), 114. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Büchel C, Born J, & Rasch B (2011). Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. E-Neuroforum, 17(2). doi: 10.1515/nf-2011-0207 [DOI] [PubMed] [Google Scholar]
- Duyn JH (2012). EEG-fMRI methods for the study of brain networks during sleep. Frontiers in Neurology, 3, 100. doi: 10.3389/fneur.2012.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein WA, Hedeen RA, Mallozzi RP, El-Hamamsy S, Ackermann RA, & Havens TJ (2002). Making MRI quieter. Magnetic Resonance Imaging, 20(2), 155–163. doi: 10.1016/s0730-725x(02)00475-7 [DOI] [PubMed] [Google Scholar]
- Fagen J, Prigot J, Carroll M, Pioli L, Stein A, & Franco A (1997). Auditory context and memory retrieval in young infants. Child Development, 68(6), 1057. doi: 10.2307/1132291 [DOI] [PubMed] [Google Scholar]
- Fantz RL (1964). Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science, 146(3644), 668–670. [DOI] [PubMed] [Google Scholar]
- Fló A, Brusini P, Macagno F, Nespor M, Mehler J, & Ferry AL (2019). Newborns are sensitive to multiple cues for word segmentation in continuous speech. Developmental Science, e12802. doi: 10.1111/desc.12802 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Köhler S, & Josselyn SA (2013). Hippocampal neurogenesis and forgetting. Trends in Neurosciences, 36(9), 497–503. doi: 10.1016/j.tins.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, & Turner R (1996). Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. doi: 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- Geng F, Redcay E, & Riggins T (2019). The influence of age and performance on hippocampal function and the encoding of contextual information in early childhood. NeuroImage, 195, 433–443. 10.1016/j.neuroimage2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S (2017). Development of item–space and item–time binding. Current Opinion in Behavioral Sciences, 17, 211–216. doi: 10.1016/j.cobeha.2017.09.002 [DOI] [Google Scholar]
- Goldfield BA, & Reznick JS (1990). Early lexical acquisition: Rate, content, and the vocabulary spurt. Journal of Child Language, 17(1), 171–183. doi: 10.1017/s0305000900013167 [DOI] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, & Nadel L (2006). Naps promote abstraction in language-learning infants. Psychological Science, 17(8), 670–674. doi: 10.1111/j.1467-9280.2006.01764.x [DOI] [PubMed] [Google Scholar]
- Gómez RL, & Edgin JO (2016). The extended trajectory of hippocampal development: Implications for early memory development and disorder. Developmental Cognitive Neuroscience, 18, 57–69. doi: 10.1016/j.dcn.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KR, Mcgregor KK, Waldier B, Curran MK, Gómez RL, & Samuelson LK (2016). Preschool children’s memory for word forms remains stable over several days, but gradually decreases after 6 months. Frontiers in Psychology, 7, 1439. doi: 10.3389/fpsyg.2016.01439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K, & Rovee-Collier C (2003). Does infant memory expression reflect age at encoding or age at retrieval? Developmental Psychobiology, 42(3), 283–291. doi: 10.1002/dev.10101 [DOI] [PubMed] [Google Scholar]
- Hartshorn K, Rovee-Collier C, Gerhardstein P, Bhatt RS, Wondoloski TL, Klein P, Gilch J, Wurtzel N, & Campos-de-Carvalho M (1998). The ontogeny of long- term memory over the first year- and- a- half of life. Developmental Psychobiology, 32(2), 69–89. [PubMed] [Google Scholar]
- Hayne H, Jaeger K, Sonne T, & Gross J (2016). Visual attention to meaningful stimuli by 1- to 3-year olds: Implications for the measurement of memory. Developmental Psychobiology, 58(7), 808–816. doi: 10.1002/dev.21455 [DOI] [PubMed] [Google Scholar]
- Henke K (2010). A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience, 11(7), 523–532. doi: 10.1038/nrn2850 [DOI] [PubMed] [Google Scholar]
- Hill WL, Borovsky D, & Rovee-Collier C (1988). Continuities in infant memory development. Developmental Psychobiology, 21(1), 43–62. doi: 10.1002/dev.420210104 [DOI] [PubMed] [Google Scholar]
- Insausti R, Cebada-Sánchez S, & Marcos P (2010). Postnatal development of the human hippocampal formation. Advances in Anatomy, Embryology, and Cell Biology, 206, 1–86. [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, & Lavenex P (2010). Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. European Journal of Neuroscience, 31(2), 273–285. doi: 10.1111/j.1460-9568.2009.07061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès A, & Nelson CA (2015). 20 years after “The ontogeny of human memory.” International Journal of Behavioral Development, 39(4), 315–317. doi: 10.1177/0165025415573646 [DOI] [Google Scholar]
- Johnson EG, Leckey S, Davison K, & Ghetti S (2019). Associative binding in early childhood: Evidence from a preferential looking paradigm. Developmental Psychobiology. Advance online publication. https://doi.org.10.1002.dev.21904 [DOI] [PubMed] [Google Scholar]
- Lavenex P, & Lavenex PB (2013). Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioural Brain Research, 254, 8–21. doi: 10.1016/j.bbr.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Lee JK, Ekstrom AD, & Ghetti S (2014). Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage, 94, 162–171. doi: 10.1016/j.neuroimage.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Lee JK, Johnson EG, & Ghetti S (2017). Hippocampal development: structure, function and implications In: Hannula DE, & Duff MC (Eds.), The Hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition (pp. 141–166). Springer. [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, & Moser EI (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966. [DOI] [PubMed] [Google Scholar]
- Marshall L, & Born J (2007). The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences, 11(10), 442–450. doi: 10.1016/j.tics.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Ming GL, & Song H (2011). Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron, 70(4), 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, & Hayne H (2011). Age-related changes in visual recognition memory during infancy and early childhood. Developmental Psychobiology, 53(2), 157–165. doi: 10.1002/dev.20503 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic memory and beyond: The hippocampus and neocortex in transformation. Annual Review of Psychology, 67(1), 105–134. doi: 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, & Moser EI (1998). Functional differentiation in the hippocampus. Hippocampus, 8(6), 608–619. doi: 10.1002/(sici)1098-1063(1998)8:63.0.co;2-7 [DOI] [PubMed] [Google Scholar]
- Mullally SL, & Maguire EA (2014). Learning to remember: The early ontogeny of episodic memory. Developmental Cognitive Neuroscience, 9, 12–29. doi: 10.1016/j.dcn.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, Mchugh TJ, Buhl DL, & Tonegawa S (2008). Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science, 319(5867), 1260–1264. doi: 10.1126/science.1151120 [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Balcomb F, Ferrara K, Hansen M, & Koski J (2014). Two rooms, two representations? Episodic- like memory in toddlers and preschoolers. Developmental Science, 17(5), 743–756. 10.1111/desc.12162 [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, & Amaral DG (2008). Methods for acquiring structural MRI data in very young children with autism without the use of sedation. Journal of Autism and Developmental Disorders, 38(8), 1581–1590. doi: 10.1007/s10803-007-0514-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N (2012). The development of neural correlates for memory formation. Neuroscience & Biobehavioral Reviews, 36(7), 1708–1717. doi: 10.1016/j.neubiorev.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao Y, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, & Gabrieli JD (2007). Development of the declarative memory system in the human brain. Nature Neuroscience, 10(9), 1198–1205. doi: 10.1038/nn1950 [DOI] [PubMed] [Google Scholar]
- Olson IR, & Newcombe NS (2013). Binding together the elements of episodes: Relational memory and the developmental trajectory of the hippocampus In Bauer PJ & Fivush R (Eds.), Handbook on the development of children’s memory, (Vol. 1, pp. 285–308). Wiley-Blackwell. doi: 10.1002/9781118597705.ch13 [DOI] [Google Scholar]
- Oudiette D, & Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences, 17(3), 142–149. doi: 10.1016/j.tics.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin N, Holdstock J, Isaac C, & Mayes A (2004). Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition. Neuropsychologia, 42(10), 1293–1300. doi: 10.1016/j.neuropsychologia.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Pathman T, & Ghetti S (2016). More to it than meets the eye: How eye movements can elucidate the development of episodic memory. Memory, 24(6), 721–736. doi: 10.1080/09658211.2016.1155870 [DOI] [PubMed] [Google Scholar]
- Perlman SB, Luna B, Hein TC, & Huppert TJ (2014). FNIRS evidence of prefrontal regulation of frustration in early childhood. NeuroImage, 85, 326–334. doi: 10.1016/j.neuroimage.2013.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar J, Johnson EG, Nordahl CW, & Ghetti S (2018). Memory-related hippocampal activation in the sleeping toddler. Proceedings of the National Academy of Sciences, 115(25), 6500–6505. doi: 10.1073/pnas.1805572115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsaran AI, Schlichting ML, & Frankland PW (2018). The ontogeny of memory persistence and specificity. Developmental Cognitive Neuroscience, 36, 100591. doi: 10.1016/j.dcn.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C (2010). A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus, 20(11), 1263–1290. doi: 10.1002/hipo.20852 [DOI] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, & Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315(5817), 1426–1429. doi: 10.1126/science.1138581 [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, & Gaab N (2012). Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences, 1252(1), 43–50. doi: 10.1111/j.1749-6632.2012.06457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, & Courchesne E (2007). FMRI during natural sleep as a method to study brain function during early childhood. NeuroImage, 38(4), 696–707. doi: 10.1016/j.neuroimage.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Ribordy F, Jabès A, Lavenex PB, & Lavenex P (2013). Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cognitive Psychology, 66(1), 1–29. doi: 10.1016/j.cogpsych.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Richmond J, Sowerby P, Colombo M, & Hayne H (2004). The effect of familiarization time, retention interval, and context change on adults’ performance in the visual paired-comparison task. Developmental Psychobiology, 44(2), 146–155. doi: 10.1002/dev.10161 [DOI] [PubMed] [Google Scholar]
- Richmond JL, Zhao JL, & Burns MA (2015). What goes where? Eye tracking reveals spatial relational memory during infancy. Journal of Experimental Child Psychology, 130, 79–91. doi: 10.1016/j.jecp.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Riggins T, Blankenship SL, Mulligan E, Rice K, & Redcay E (2015). Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Development, 86(6), 1710–1718. doi: 10.1111/cdev.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, & Redcay E (2016). Hippocampal functional connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience, 19, 58–69. doi: 10.1016/j.dcn.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Botdorf M, Canada K, Cox L, & Hancock G (2018). Protracted hippocampal development is associated with age-related improvements in memory during early childhood. NeuroImage, 174, 127–137. 10.1016/j.neuroimage.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Miller NC, Bauer PJ, Georgieff MK, & Nelson CA (2009). Electrophysiological indices of memory for temporal order in early childhood: Implications for the development of recollection. Developmental Science, 12(2), 209–219. doi: 10.1111/j.1467-7687.2008.00757.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AJ, & Pascalis O (2004). Development of flexible visual recognition memory in human infants. Developmental Science, 7(5), 527–533. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2004). Infant visual recognition memory. Developmental Review, 24(1), 74–100. doi: 10.1016/j.dr.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C (1997). Dissociations in infant memory: Rethinking the development of implicit and explicit memory. Psychological Review, 104(3), 467–498. doi: 10.1037//0033-295x.104.3.467 [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C, Sullivan M, Enright M, Lucas D, & Fagen J (1980). Reactivation of infant memory. Science, 208(4448), 1159–1161. doi: 10.1126/science.7375924 [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, & Suzuki M (2012). Item memory, context memory, and the hippocampus: FMRI evidence. Neuropsychologia, 50(13), 3070–3079. doi: 10.1016/j.neuropsychologia.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, Ocarroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, & Hen R (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 472(7344), 466–470. doi: 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmeczy D, Fandakova Y, Grimm KJ, Bunge SA, & Ghetti S (2019). Longitudinal trajectories of hippocampal and prefrontal contributions to episodic retrieval: Effects of age and puberty. Developmental Cognitive Neuroscience, 36, 100599. doi: 10.1016/j.dcn.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L (2001). Morphological changes of the human hippocampal formation from midgestation to early childhood In Nelson CA & Luciana M (Eds.), Handbook of developmental cognitive neuroscience, (pp. 45–58). MIT Press. [Google Scholar]
- Shanahan LK, Gjorgieva E, Paller KA, Kahnt T, & Gottfried JA (2018). Odor-evoked category reactivation in human ventromedial prefrontal cortex during sleep promotes memory consolidation. eLife, 7, e39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon T, Moscovitch M, & Gilboa A (2011). Rapid neocortical acquisition of long-term arbitrary associations independent of the hippocampus. Proceedings of the National Academy of Sciences, 108(3), 1146–1151. doi: 10.1073/pnas.1005238108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap P, Wu G, Jia H, Gilmore JH, Lin W, & Shen D (2011). Infant brain atlases from neonates to 1- and 2-year-olds. PLoS ONE, 6(4). doi: 10.1371/journal.pone.0018746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Vouloumanos A, Bennett RH, & Pelphrey K (2014). Neural specialization for speech in the first months of life. Developmental Science, 17(5), 766–774. doi: 10.1111/desc.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, & Yu C (2008). Infants rapidly learn word-referent mappings via cross-situational statistics. Cognition, 106(3), 1558–1568. doi: 10.1016/j.cognition.2007.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A, Bisaz R, Sweet ES, Blitzer RD, & Alberini CM (2016). Infantile amnesia reflects a developmental critical period for hippocampal learning. Nature Neuroscience, 19(9), 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1972). Episodic and semantic memory In Tulving E & Donaldson W (Eds.), Organization of memory (pp. 381–403). Academic Press. [Google Scholar]
- Tulving E (1985). Memory and consciousness. Canadian Psychology, 26, 1–12. [Google Scholar]
- Tulving E (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53(1), 1–25. doi: 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- Tummeltshammer K, Amso D, French RM, & Kirkham NZ (2017). Across space and time: Infants learn from backward and forward visual statistics. Developmental Science, 20(5). doi: 10.1111/desc.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustin K, & Hayne H (2010). Defining the boundary: Age-related changes in childhood amnesia. Developmental Psychology, 46(5), 1049 10.1037/a0020105 [DOI] [PubMed] [Google Scholar]
- Van Dongen EV, Takashima A, Barth M, Zapp J, Schad LR, Paller KA, & Fernandez G (2012). Memory stabilization with targeted reactivation during human slow-wave sleep. Proceedings of the National Academy of Sciences, 109(26), 10575–10580. doi: 10.1073/pnas.1201072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieites V, Pruden SM, Shusterman A, & Reeb-Sutherland BC (2019). Using hippocampal-dependent eyeblink conditioning to predict individual differences in spatial reorienting strategies in 3- to 6-year-olds. Developmental Science. Advance online publication 10.1111/desc.12867 [DOI] [PubMed] [Google Scholar]
- Vlach HA, & Debrock CA (2017). Remember dax? Relations between children’s cross-situational word learning, memory, and language abilities. Journal of Memory and Language, 93, 217–230. doi: 10.1016/j.jml.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, & Duff MC (2014). Not so fast: Hippocampal amnesia slows word learning despite successful fast mapping. Hippocampus, 24(8), 920–933. doi: 10.1002/hipo.22279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Cheatham CL, Lukowski AF, Haight JC, Muehleck AJ, & Bauer PJ (2006). Infants ERP responses to novel and familiar stimuli change over time: Implications for novelty detection and memory. Infancy, 9(1), 21–44. doi: 10.1207/s15327078in0901_2 [DOI] [Google Scholar]
- Wild CJ, Linke AC, Zubiaurre-Elorza L, Herzmann C, Duffy H, Han VK Lee DSC, & Cusack R (2017). Adult-like processing of naturalistic sounds in auditory cortex by 3- and 9-month old infants. NeuroImage, 157(15), 623–634. 10.1016/j.neuroimage.2017.06.038 [DOI] [PubMed] [Google Scholar]


