Abstract
Vagus nerve stimulation (VNS) has rapidly gained interest as a treatment for a variety of disorders. A number of methods have been employed to stimulate the vagus nerve, but the most common relies on a cuff electrode implanted around the cervical branch of the nerve. Recently, two non-invasive methods have increased in popularity: transcutaneous cervical VNS (tcVNS) and transcutaneous auricular VNS (taVNS). Despite promising clinical results, there has been little direct comparison of these methods to stimulation delivered via an implanted device. In this study, we directly compared both non-invasive strategies to stimulation with an implanted cuff electrode on activation of the Hering-Breuer (HB) reflex, a non-invasive biomarker of A-fiber activation in the vagus. Stimulation was delivered across a wide range of parameters using tcVNS, taVNS, and an implanted cuff electrode in female rats. Activation of the HB reflex, changes in heart rate, and neck muscle twitch force were recorded. Consistent with low thresholds reported in previous studies, we found that the threshold to activate the HB reflex using an implanted cuff electrode was 0.406 ± 0.066 mA. tcVNS was capable of activating the HB reflex, but the threshold was 34.18 ± 1.86 mA, over 15 fold higher than the stimulation intensity that caused twitching of the neck muscles (2.09 ± 0.16 mA). No activation of the HB reflex was observed with taVNS at any parameters. These results describe activation of the HB reflex with each strategy and provide initial evidence regarding differences in the activation of the vagus nerve with invasive and non-invasive methods.
Keywords: vagus nerve stimulation, non-invasive, transcutaneous, auricular
Introduction
Vagus nerve stimulation (VNS) has gained interest as a treatment for a variety of disorders [1]–[6]. Originally approved for the treatment of epilepsy and depression, recent studies have shown promising clinical results for applications ranging from stroke recovery to the treatment of rheumatoid arthritis [3], [7]. In order to maximize clinical benefits, it is important to optimize the method of stimulation delivery.
Multiple strategies have been employed to electrically stimulate the vagus nerve. Implanted VNS (iVNS) relies on a surgically implanted cuff electrode placed around the left cervical branch of the vagus [8]–[12]. iVNS has been employed in over 100,000 individuals and provides effective seizure control in many patients [13]. Although it is generally well tolerated, a non-invasive alternative would provide advantages by avoiding the risks and costs associated with surgical implantation and battery replacement. Leveraging these advantages, two non-invasive methods of VNS have gained popularity.
The first strategy, transcutaneous cervical VNS (tcVNS), uses surface electrodes on the skin of the neck to target the cervical branch of the vagus nerve, the same region activated by implanted VNS [14]. tcVNS has been cleared by the FDA for the treatment of migraine and cluster headaches, and a recent large clinical study demonstrates that tcVNS significantly reduces headache frequency and severity compared to sham stimulation [15]. The second method, transcutaneous auricular VNS (taVNS), targets the auricular branch of the vagus via stimulation of the concha of the outer ear [16]. Clinically, taVNS has been primarily applied for epilepsy and depression [17], [18]. In addition, a variety of potential therapeutic applications have been investigated using taVNS in preclinical models of stroke, diabetes, and heart failure [19]–[22]. Both transcutaneous strategies display promising results, but there has been little direct comparison of non-invasive and implanted stimulation or characterization of the effect of stimulation parameters.
Direct measurement of activation of fibers in the vagus nerve in response to stimulation with these strategies would be ideal. However, recording methods that provide direct characterization of fiber activation are themselves invasive and may therefore interfere with accurate measurement of the nerve in response to non-invasive stimulation. To circumvent these issues and provide an initial characterization of these stimulation strategies, we recorded activation of the Hering-Breuer (HB) reflex. The HB reflex is a well-characterized response to stimulation of a subset of vagal A-fibers including the pulmonary stretch receptors [23], [24]. In animals under general anesthesia, stimulation of these fibers temporarily prevents inhalation and causes correspondent decreases in blood oxygen saturation (SpO2) [9].
Although the HB reflex itself does not have any known or presumed relevance to the clinical effects of VNS, it is mediated by activation of low-threshold, large diameter fibers. Moreover, HB activation is well-correlated with VNS-driven neural activity in the locus coeruleus (LC), as both arise from activation of afferent fibers that project to brainstem networks [24], [25]. The LC is the primary source of norepinephrine in the brain and is widely-regarded as a critical mediator of many clinical effects of VNS in the central nervous system [25], [26]. Thus, although it does not provide a full characterization of activation of the vagus nerve and is not itself predictive of clinical effects, the HB reflex represents a simple, non-invasive biomarker to garner insight into the presumptive activation of low-threshold fibers.
In the present study, we directly compared iVNS, tcVNS, and taVNS stimulation strategies in rats. Using each electrode configuration, we varied multiple stimulation parameters across a wide range and assessed activation of the HB reflex. The results from this study describe activation of the HB reflex with each of these strategies and provide a framework for selecting stimulation parameters and implementations for future studies.
Materials and Methods
Animals
All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee. Twenty-three Sprague Dawley female rats (Charles River, 3 to 6 months old, 250 to 500g) were housed in a 12:12 h reverse light-dark cycle. Fifteen rats were tested with non-invasive stimulation using tcVNS at various parameters. Of these 15, 8 were also tested with taVNS. Eight rats were implanted with a cuff electrode and tested with iVNS after a week of recovery.
Implanted Cervical VNS
Cuff implantation was performed as previously described [27]. Eight rats were anesthetized with ketamine hydrochloride (80 mg/kg, IP) and xylazine (10 mg/kg, IP) and placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lamboid suture and over the cerebellum. A two-channel connector was mounted to the screws using acrylic. An incision was made on the left side of the neck and the overlying musculature was blunt dissected to reveal the vagus nerve. The nerve was gently dissected away from the carotid artery. A cuff electrode, described in detail previously, was implanted surrounding the vagus nerve with two leads tunneled subcutaneously and connected to the two-channel connector on the skull [9]. Nerve activation was confirmed peri-surgically by observation of a ≥ 5% drop in blood oxygen saturation (SpO2) in response to a 5 s stimulation train of VNS consisting of 0.8 mA, 0.1 ms biphasic pulses at 30 pulses per second (PPS). Once cuff efficacy was confirmed, head and neck incisions were sutured closed and treated with antibiotic ointment. Rats received subcutaneous injections of 5 mL 50:50 0.9% saline 5% dextrose solution. A five day recovery period followed surgery and rats were given one Baytril tablet per day (2 mg/tablet, BioServ, Frenchtown, NJ).
Stimulation testing commenced approximately one week after surgical implantation. Rats were anesthetized with isoflurane mixed with pressurized room air (0.5 L/min, 1–3% isoflurane), placed in the supine position, and connected via the two-channel connector to an isolated programmable stimulator (Model 4100; A-M Systems™; Sequim, WA). Comparison of animals from previous experiments tested under isoflurane, ketamine/xylazine, and pentobarbital indicates that HB reflex measurement is not dependent on the anesthetic type (Fig. S4). Rats were stimulated with 5 second trains of biphasic current-controlled square waves (0.1 ms per phase) at 30 PPS across intensities ranging from 0.1 mA to 2.5 mA. All stimulation and recording were controlled with custom MATLAB software. SpO2 and heart rate were recorded using a pulse oximeter (Starr Life Sciences™, MouseOx Plus®) as previously described [23]. Data were read into MATLAB using a Starr Link Plus™ with the outputs connected to analog channels on an Arduino Mega 2560. To measure muscle activation, a force-transducer (2kg EBB Load Cell; Transducer Techniques; Temecula, CA) was placed on the surface of the neck directly over the implant and connected to another analog channel on the Arduino. Stimulation trains were delivered every 60 seconds, but subsequent stimulation trains were delayed if needed to allow SpO2 to recover. To avoid any effects of HB reflex sensitization or habituation, stimulation parameters were randomly interleaved and repeated twice in each preparation [28]. Data were sampled at 100 Hz.
Transcutaneous Cervical VNS
Fifteen rats were anesthetized with isoflurane mixed with pressurized room air (0.5 L/min, 1–3% isoflurane) and placed in the supine position with their upper limbs secured to the table. An experimental non-invasive stimulator developed to deliver tcVNS in rats (gammaCore; electroCore, LLC; Basking Ridge, NJ) was placed on the skin centered over the left or right cervical vagus nerve (parallel to the sagittal plane, 3 to 6 mm lateral from midline, 8 to12 mm rostral of the manubrium). As an active control, in some experiments the stimulator was placed on the medial surface of the left hind limb. The stimulator consisted of two surface disc electrodes (5 mm diameter, 10 mm separation center to center). Leads from the electrodes were connected to the A-M Systems stimulator. Square waves were generated using the built in stimulation waveform library. Sine waves were generated using a digital processor with a digital-to-analog converter (RP2; Tucker-Davis Technologies; Alachua, FL) and amplified to the desired current or voltage by the A-M systems stimulator. Prior to stimulation, the hair on the neck and left hind limb was removed with a chemical depilatory, and conducting gel (Signa gel; Parker Laboratories; Fairfield, NJ) was applied to the electrodes to ensure good contact with the skin.
Electrical stimulation consisted of 5 second trains at 10 to 480 PPS, where each pulse was a 1 ms sine wave at 1, 5, or 25 kHz with amplitude ranging from 0.5 to 48 mA or 1 to 64 V. Both current-controlled and voltage-controlled stimulation configurations were tested. To compare 5 kHz sine waves to the more commonly used square waves, a subset of four animals were also stimulated with biphasic current controlled square waves (5 second trains, 30 PPS, 0.5 to 48 mA, 0.5 ms per phase). SpO2 and heart rate were measured as described above. Muscle activation was measured via the force transducer attached to the tcVNS device directly over the electrodes. Stimulation trains were delivered every 60 seconds, but subsequent stimulation trains were delayed if needed to allow SpO2 to recover. Stimulation parameters were randomly interleaved and repeated twice in each preparation. Data were sampled at 100 Hz.
Transcutaneous Auricular VNS
Eight rats were anesthetized with isoflurane mixed with pressurized room air (0.5 L/min, 1–3% isoflurane) and placed in the prone position. Acupuncture needles (0.14 mm diameter; J-Type No. 01; Seirin America; Weymouth, MA) were placed on the auricular concha and connected to the A-M Systems stimulator, similar to previous studies [29], [30]. Electrical stimulation consisted of 5 second trains at 30 to 120 PPS, where each pulse was a current controlled biphasic square wave with current ranging from 0.1 to 2.5 mA and pulse-width ranging from 0.1–0.5 ms. SpO2 and heart rate were measured as described above. The threshold at which ear twitching could be observed was visually noted and recorded in each experiment. Stimulation was delivered every 60 seconds, but subsequent stimulation trains were delayed if needed to allow SpO2 to recover. Stimulation parameters were randomly interleaved and repeated twice. Data were sampled at 100 Hz.
Analysis and Statistical Comparisons
The magnitude of the HB reflex and heart rate response to each stimulation was measured as the maximum change from baseline in a 20 second window starting at the onset of stimulation. The baseline was defined as the average of the 20 seconds of data prior to the onset of each train of stimulation. The magnitude of the muscle response was measured as the maximum change from baseline in a 5 second window starting at the onset of stimulation. Baseline was defined as the average of the 5 seconds of force data prior to the onset. The window lengths were selected to effectively capture the peak response for each measure. The threshold for each measure in each animal was defined as the lowest current amplitude that resulted in a change in signal greater than 5x the standard deviation of the baseline for all stimulations at that intensity. The measured response for each stimulation and threshold for each experiment were calculated automatically by analysis software. Manual quality control was performed by three experimenters blinded during analysis to identify and rectify any trials with errors, such as a momentary loss of pulse oximeter signal.
Data reported in the text and figures represent mean ± standard error of the mean (SEM). For dose-response curves, a one-way repeated measures ANOVA was performed to determine if there was an effect of stimulation. Individual comparisons for each stimulation intensity were made using a post hoc Dunnett’s test compared to zero. When comparing dose-response curves for left and right tcVNS, a two-way repeated measures ANOVA was performed. Muscle and HB reflex thresholds were compared using a paired t-test. Thresholds for the various stimulation rates and sine wave frequencies were compared using a one-way ANOVA with individual comparisons made using post hoc Tukey’s tests. Thresholds for square waves and sine waves were compared using a two-sample t-test. The statistical test used for each comparison is noted in the text. All calculations were performed in MATLAB.
Results
Implanted cervical VNS activates the Hering-Breuer reflex with low intensity stimulation
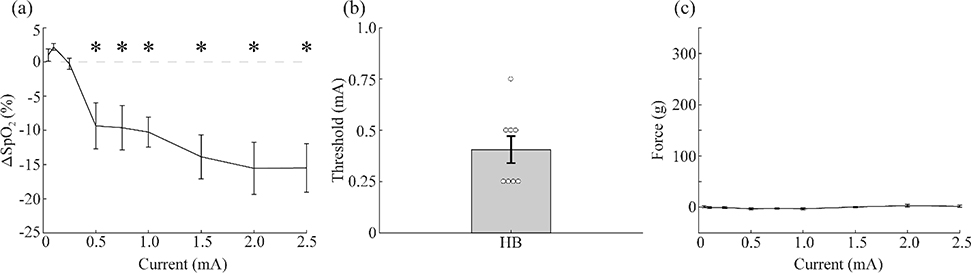
To benchmark the non-invasive methods, we first implanted eight rats and measured muscle contraction and changes in SpO2 and heart rate in response to various intensities of VNS delivered to the left cervical vagus nerve with a standard implanted cuff electrode. The average threshold intensity to cause a significant reduction in SpO2 was 0.406 ± 0.066 mA. There was no overall effect of stimulation on neck muscle twitch force (Fig. 1c). No significant reductions in heart rate were observed across the tested range of parameters, although a trend towards bradycardia is apparent at higher intensities (Fig. S1). These results demonstrate that iVNS yield reliable, low threshold activation of the HB reflex and minimal activation of neck muscles.
Figure 1: Implanted VNS activates the vagus nerve at low intensities.
a) VNS delivered via an implanted cuff electrode produces reductions in SpO2 as a function of stimulation intensity (n=8). b) The threshold intensity for generating a significant drop in SpO2 was approximately 0.5 mA. c) VNS delivered via an implanted cuff electrode produces minimal muscle activation as a function of stimulation intensity (n=8). * denotes intensities at which the mean response significantly differed from zero with p<0.05.
tcVNS is capable of activating the Hering-Breuer reflex
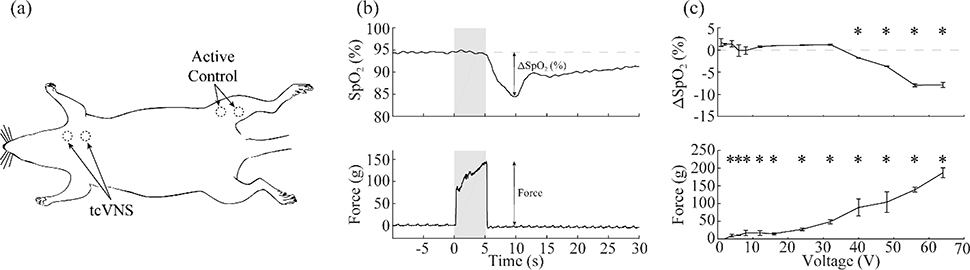
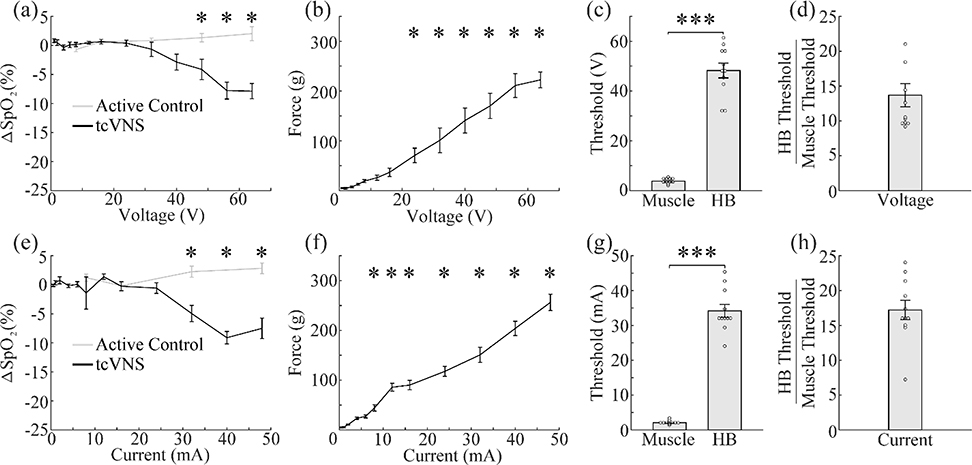
We performed tcVNS while parametrically varying the stimulation parameters to identify the intensities required to generate reliable activation of the fibers mediating the HB reflex. Using 5-second stimulation trains at 30 PPS (1 ms, 5 kHz sine waves), we applied both current-controlled and voltage-controlled stimulation with varying intensities to the surface of the neck overlying the left cervical vagus nerve (Fig. 2). The threshold for activating the muscles in the neck was 2.09 ± 0.16 mA and 3.82 ± 0.31 V for current- and voltage-controlled stimulation, respectively. The threshold for activating the HB reflex was substantially higher, at 34.18 ± 1.86 mA and 48.24 ± 2.98 V (Fig. 3c,g). For both current-controlled and voltage-controlled stimulation, the HB threshold was significantly higher than the muscle threshold (current-controlled: paired two-tailed t-test, p=1.14×10−8; voltage-controlled: paired two-tailed t-test, p=3.51×10−8). To quantify the fold-increase in stimulation intensity necessary to activate the HB reflex compared to muscle activation, we calculated an average ratio of the HB threshold divided by the muscle threshold. The ratio was 17.22 ± 1.39 for current-controlled stimulation and 13.69 ± 1.66 for voltage-controlled stimulation (Fig. 3d,h).
Figure 2: Experimental procedure for tcVNS.
a) Schematic diagram of the experimental setup denoting electrode placement. b) A representative drop in SpO2 (top) and change in force (bottom) at a stimulation intensity of 56 V. c) Representative dose-response curves (from the same experiment shown in panel b) of the change in SpO2 (top) and force (bottom) as a function of stimulation intensity. * denotes intensities at which stimulation produced a significant change from baseline in SpO2 or force.
Figure 3: tcVNS requires higher stimulation intensities to activate the vagus nerve.
a) tcVNS over the left cervical vagus nerve produces reductions in SpO2 as a function of voltage, but stimulation at the active control site does not. b) tcVNS activates the muscles in the neck at low voltages and the amplitude increases nearly linearly with increasing voltage. c) The threshold for activating the vagus nerve is significantly higher than the threshold to activate the neck muscles. d) The vagus activation threshold was over 10 fold higher than the muscle activation threshold. e-h) Same as a-d, but using current controlled stimulation instead of voltage controlled. * in (a-b and e-f) denotes intensities at which the mean response significantly differed from zero. *** denotes a difference between groups with p<0.001
To confirm that reductions in SpO2 were due to activation of the HB reflex and not simply a response to pain or somatic stimulation, we used the tcVNS device to apply stimulation to the medial surface of the left hind limb. We observed no reductions in SpO2 even with the most intense stimulation (Fig. 3a,e). The absence of changes in SpO2 with active stimulation on another skin surface supports the notion that tcVNS-dependent reductions in SpO2 arise from activation of the HB reflex via the vagus nerve rather than a non-specific mechanism, such as cutaneous stimulation.
We observed no overall effect of tcVNS on heart rate (Fig. S2a,b, one-way repeated-measures ANOVA, p=0.071).
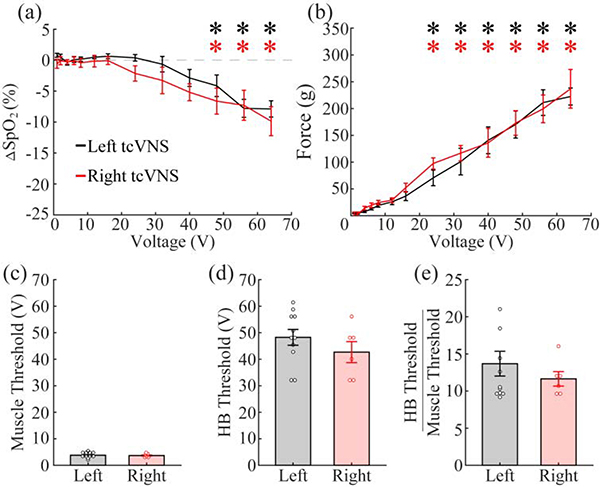
Certain applications of VNS, especially those designed to influence heart function, target the right cervical branch of the vagus. We used the same tcVNS method to assess HB reflex engagement within the right vagus nerve. Similar to stimulation of the left branch, the average muscle activation threshold was 3.69 ± 0.28 V (Fig. 4c). The average threshold for activation of the HB reflex was 42.67 ± 3.96 V (Fig. 4d). The threshold ratio was 11.64 ± 0.98 (Fig. 4e). No differences in thresholds were found (muscle threshold: unpaired two-tailed t-test, p=0.80; HB threshold: unpaired two-tailed t-test, p=0.28; threshold ratio: unpaired two-tailed t-test, p=0.41). Additionally, a two-way repeated measures ANOVA found no difference between the dose-response curves (SpO2: two-way repeated-measures ANOVA, p=0.18; Force: two-way repeated-measures ANOVA, p=0.72). Similar effects to left tcVNS were seen with heart rate. There was no overall effect of tcVNS on heart rate (Fig. S2a,b; one-way repeated measures ANOVA, p=0.500). These results suggest that left and right tcVNS are equally effective at activating the HB reflex and causing heart rate changes.
Figure 4: Left and Right tcVNS produce equivalent activation of the vagus nerve and neck muscles.
a) tcVNS of the left and right branches of the vagus nerve produce comparable activation of the HB reflex. b) Similarly, no difference in muscle activation was observed between left and right tcVNS. c-e) There is no difference in the muscle activation thresholds, vagus activation thresholds, or the ratio between the two for left and right tcVNS. * denotes intensities at which the mean response significantly differed from zero.
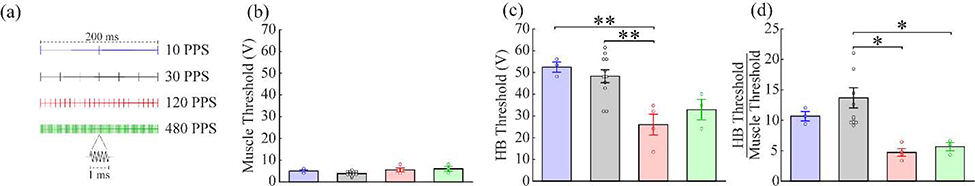
To identify paradigms that may more effectively induce nerve activation, we tested a range of stimulation parameters beyond those commonly used in clinical applications. First, we varied the stimulation rate using trains delivered at 10, 120, or 480 PPS (Fig. 5a). Increasing the rate significantly lowered the threshold voltage required to activate the HB reflex (Fig. 5c; 10 PPS: 52.44 ± 2.35 V, 30 PPS: 48.24 ± 2.98 V, 120 PPS: 26 ± 4.79 V, 480 PPS: 32.89 ± 4.70 V; one-way ANOVA, F(3, 17)=8.11, p=0.0014), but not the muscle activation threshold (Fig. 5b; 10 PPS: 5.0 ± 0.58 V, 30 PPS: 3.82 ± 0.31, 120 PPS: 5.50 ± 0.87 V, 480 PPS: 6 ± 1.15 V; one-way ANOVA, F(3, 17)=1.31, p=0.054). Consequently, increasing the rate led to significant reductions in the threshold ratio (Fig. 5d; 10 PPS: 10.67 ± 0.77, 30 PPS: 13.69 ± 1.66, 120 PPS: 4.72 ± 0.64, 480 PPS: 5.67 ± 0.69; one-way ANOVA, F(3, 17)=5.66, p=0.0071). Unexpectedly, stimulation at 10 PPS engendered a small, but significant increase in SpO2. Stimulation at 120 PPS significantly lowered the HB threshold compared to 30 PPS and 10 PPS (10 vs 120: Tukey’s test, p=0.0071; 30 vs 120: Tukey’s test, p=0.0032). Stimulation at 120 PPS and 480 PPS significantly lowered the threshold ratio compared to 30 PPS (30 vs 120: Tukey’s test, p=0.012; 30 vs 480: Tukey’s test, p=0.049). Stimulation at 120 PPS and 480 PPS significantly affected heart rate, but still only at intensities much higher than the HB reflex threshold (Fig. S2c; 120 PPS: one-way repeated measures ANOVA, p=6.98×10−4; 480 PPS: one-way repeated measures ANOVA, p=0.0031). These findings indicate that increasing the stimulation rate can lower the threshold to activate the HB reflex and cause a change heart rate without changing the muscle activation threshold.
Figure 5: Increasing tcVNS stimulation rate lowers intensity required to activate the vagus nerve.
a) Schematic diagrams of the waveform for each stimulation type. b) Stimulation rate does not affect the muscle activation threshold. c) Higher stimulation rates lower the threshold intensity to activate the vagus nerve. d) As a result, higher stimulation rates lower the ratio between the vagus activation threshold and the muscle activation threshold. * denotes a difference between groups with p<0.05. ** denotes a difference between groups with p<0.01.
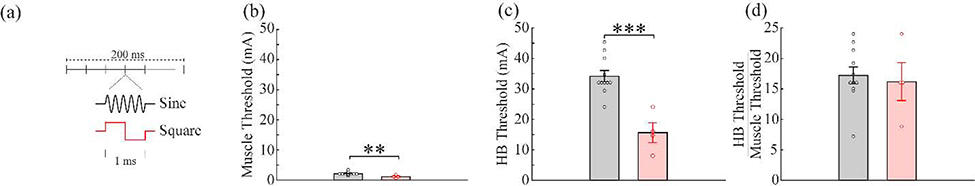
Next, we varied pulse shape to compare the sine wave commonly employed for tcVNS to square waves typically used in other nerve stimulation applications. Stimulation was delivered using biphasic current-controlled square-waves of equal duration (0.5 ms per phase) and compared to standard sine waves (Fig. 6a). As expected, square waves activated both the HB reflex (Fig. 6c; Sine: 34.18 ± 1.86 mA, Square: 15.67 ± 3.28 mA, unpaired t-test, p=2.19×10−4) and the muscles (Fig. 6b; Sine: 2.09 ± 0.16 mA, Square: 1.04 ± 0.24 mA, unpaired t-test, p=0.0037) at significantly lower thresholds compared to sine waves, consistent with greater charge delivery per phase. However, the threshold ratio for the pulse shapes was not different (Fig. 6d; Sine: 17.22 ± 1.39, Square: 16.2 ± 3.10 mA, unpaired t-test, p=0.73). This is consistent with a fixed increase in charge delivery and indicates that although square waves reduce the threshold to activate the HB reflex, they also reduce the threshold to activate the muscles by a similar amount and thus do not change the threshold ratio. Square waves did not significantly affect heart rate (Fig. S2d, one-way repeated measures ANOVA, p=0.747).
Figure 6: tcVNS using biphasic square waves reduces both muscle and vagus activation thresholds.
a) Schematic diagrams of the two waveform shapes. b) Square waves of equal duration (0.5 ms per phase) produce muscle activation at a significantly lower stimulation intensity threshold than 5 kHz sine waves. c) Similarly, square waves produce vagus nerve activation at a significantly lower stimulation intensity threshold. d) Due to the similar effect on both muscle and nerve thresholds, the ratio of the vagus nerve and muscle activation threshold was unchanged. ** denotes a difference between groups with p<0.01. *** denotes a difference between groups with p<0.001.
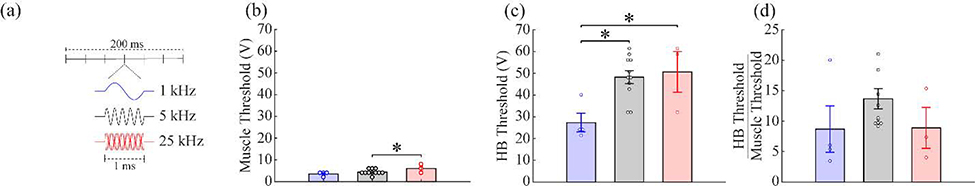
Lastly, we varied the frequency of the sine wave comprising each pulse to 1 kHz and 25 kHz (Fig. 7a). Frequency had a significant effect on the HB threshold (Fig. 7c; 1 kHz: 27.33 ± 4.27 V, 5 kHz: 48.24 ± 2.98 V, 25 kHz: 50.67 ± 9.37 V; one-way ANOVA, F(2, 15)=6.30, p=0.01) and the muscle threshold (Fig. 7b; 1 kHz: 4.25 ± 1.03 V, 5 kHz: 3.82 ± 0.31 V, 25 kHz: 6.67 ± 1.33 V; one-way ANOVA, F(2, 15)=4.22, p=0.035), but not the threshold ratio (Fig. 7d; 1 kHz: 8.69 ± 3.81, 5 kHz: 13.69 ± 1.66, 25 kHz: 8.89 ± 3.36 V; one-way ANOVA, F(2, 15)=1.42, p=0.27). No effect was seen on heart rate for any frequency sine wave (Fig. S2e; 1 kHz: one-way repeated measures ANOVA, p=0.992; 25 kHz: one-way repeated measures ANOVA, p=0.154). These results suggest that lower frequency sine waves are more effective at driving recruitment, but changes to the frequency affect both the HB activation threshold and muscle activation threshold equally.
Figure 7: Reducing the frequency of the tcVNS sine wave reduces both muscle and vagus activation thresholds.
a) Schematic diagram of the three waveform frequencies. b) Higher stimulation frequencies require greater stimulation intensities to activate the neck muscles. c) Similarly, higher frequencies have a higher threshold for vagus nerve activation. d) The ratio of the vagus nerve and muscle activation threshold was unchanged. * denotes a difference between groups with p<0.05
taVNS does not activate the Hering-Breuer reflex
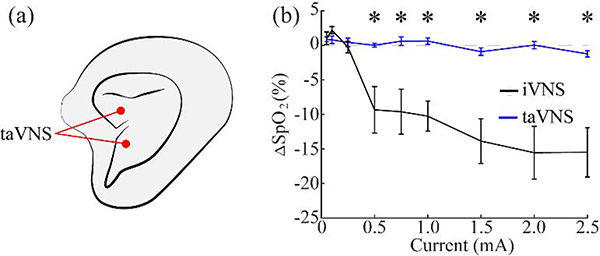
We next sought to explore whether auricular transcutaneous VNS would activate the HB reflex. To do so, we stimulated the concha of the outer ear, an area innervated by the auricular branch of the vagus nerve. A wide range of stimulation parameters was tested including intensities substantially higher than those which have been shown to be effective [19], [31]–[33]. Changes in SpO2 or heart rate were not observed with any stimulation parameters (Fig. 8b, S3). Similar to previous studies, ear twitching was observed on average at 0.65 ± 0.061 mA, confirming that stimulation was delivered effectively [34]. These findings demonstrate that activation of the auricular branch of the vagus nerve does not activate the canonical HB reflex like stimulation of the cervical branch.
Figure 8: taVNS does not activate the Hering-Breuer reflex.
a) Schematic diagram of the electrode locations. b) iVNS produces robust reductions in SpO2 as a function of stimulation intensity, but no reductions in SpO2 were observed at any intensity with taVNS. * denotes intensities at which the mean response significantly differed from zero.
Discussion
In this study, we directly compared three common invasive and non-invasive vagus nerve stimulation strategies by measuring activation of the Hering-Breuer (HB) reflex across a range of stimulation parameters. The HB reflex arises from activation of afferent pulmonary stretch receptor A-fibers in the vagus nerve, which produce a cessation of breathing and consequent reduction in oxygen saturation [23], [24]. As a result, the HB reflex represents a non-invasive biomarker of activation of a population of low-threshold fibers in response to VNS. While not presumed to be directly related to any clinical effects of VNS, the HB reflex is well-correlated with VNS-evoked neural activity in the locus coeruleus, a key nucleus in the therapeutic action of VNS [25], [26]. Additionally, because tcVNS and taVNS are non-invasive, the non-invasive nature of the HB reflex measurement precludes the need for dissection or other invasive experimental manipulations that could interfere with the action of the non-invasive stimulation methods. The results reported here provide an initial comparison of three commonly employed VNS techniques and support the need for further investigation into the mechanisms engaged by each of these stimulation strategies.
A large number of studies demonstrate the efficacy of tcVNS in a number of conditions, but there has been little experimental investigation into the threshold necessary to activate the vagus nerve and the effect of stimulation parameters. Using a common waveform (30 PPS, 1 ms sine wave at 5 kHz), we found that tcVNS could activate the HB reflex. The absence of changes in SpO2 in response to stimulation of the leg provides supporting evidence that tcVNS dependent reductions in SpO2 arise from activation of the HB reflex within the cervical branch of the vagus nerve, rather than non-specific cutaneous stimulation. However, the stimulation intensity required to activate the HB reflex with tcVNS was over 10 fold higher than that needed to evoke contraction of the overlying neck musculature. The majority of human studies that employ tcVNS use a maximum stimulation intensity of 24 V [15], [35]–[38]. Based on our findings, stimulation at these levels is unlikely to directly activate the vagus nerve. There are clear differences in the depth of the vagus nerve from the skin surface in rats (approximately 10 mm) and humans (approximately 35 mm) which could impact the response to stimulation [39]. However, the greater depth of the vagus nerve in humans would suggest that higher stimulation intensities than those used in this study may be required to generate consistent activation. The findings reported here raise the possibility that the therapeutic effects of tcVNS may arise from activation of the skin surface, overlying musculature, or other nerves or ganglia in the neck, as has previously been speculated [40]. Additional studies that incorporate direct measurement of targets benchmarked to iVNS are merited in order to gain a clear understanding of the mechanisms engaged by tcVNS.
Similar to tcVNS, taVNS has shown promising effects in both clinical and pre-clinical studies. We failed to observe activation of the HB reflex at any stimulation parameters with taVNS. These results raise two possible interpretations. First, the fibers of the auricular branch of the vagus may not follow the canonical pathway of cervical vagus nerve stimulation and thus may not supply innervation to the areas necessary to induce the HB reflex. Supporting this notion, a recent study demonstrated that the cardiovascular autonomic effects of taVNS, which are assumed to be due to activation of the vagus nerve and the nucleus tractus solitarius, are instead mediated largely by sensory afferents projecting to the upper cervical spinal cord [41]. Second, given the relatively sparse innervation via the auricular branch compared to fibers in the cervical branch of the vagus, stimulation may be insufficient to drive a significant response. This interpretation is consistent with studies reporting that neuroprotective and anti-inflammatory effects were present, but weaker, with taVNS compared to iVNS [19], [34]. It should be noted that these findings do not indicate that taVNS fails to drive activity in the central nervous system or other fibers within the vagus nerve. Rather, they suggest that the auricular and cervical branches of the vagus may produce differing central responses. Further characterization of the mechanism of activation of taVNS, including inactivation and nerve transection studies, are merited.
A number of studies utilize VNS to modulate cardiovascular function [1], [42], [43]. While the HB reflex arises primarily from activation of low threshold afferent A-fibers, bradycardia is ascribed to stimulation of efferent B-fibers [23], [24], [44]. We observed reliable tcVNS-dependent bradycardia only with higher stimulation rates of either 120 or 480 PPS. Because stimulation rate within this range does not influence the fiber activation threshold, these findings are consistent with integration at the end organ, such that higher rates reduce the number of activated fibers necessary to drive a significant response. We observed a similar reduction in the threshold for HB reflex activation at these higher stimulation rate. While stimulation of the right vagus nerve is often utilized for cardiovascular modulation, we did not observe substantial differences in either HB reflex engagement or bradycardia between right and left tcVNS. Thus, our findings suggest tcVNS can generate sufficient nerve activation to induce bradycardia at high stimulation intensities and rates, consistent with the temporal summation of B-fiber activation in this effect. No bradycardia was observed in response to taVNS at any parameters tested. However, other stimulation paradigms, such as those that employ longer stimulation trains than tested here, may be necessary to reveal taVNS effects on heart rate [45].
While the results of this study are important for guiding non-invasive stimulation parameters and methods, the use of the HB reflex as the sole measure of nerve activation provides several limitations. First, the HB reflex does not provide a comprehensive representation of activation of all of the fibers in the vagus nerve. Although the exact diameters of the fibers mediating this reflex have not been reported, their conduction velocity suggests that they are not the largest fibers in the nerve [23], [24]. Thus, there is likely some fiber activity induced below the threshold to activate the HB reflex. Moreover, while the HB reflex is a reasonable proxy of activation of some A-fibers, it fails to report B- or C-fiber activation. The correlation between VNS-dependent SpO2 and respiratory rate changes confirm that SpO2 reductions arise from a decrease in respiration rate caused by HB reflex activation (Fig. S5). Additionally, it is unclear what role, if any, the fibers mediating this reflex play in the effects of VNS. While the therapeutic effects of VNS are almost certainly not directly due to activation of the HB reflex, a number of studies evaluating plasticity and neurological recovery with iVNS indicate that the stimulation intensity needed to activate the HB reflex is below the effective range of parameters that induce plasticity when using the same cuff electrodes and animal model. [46]–[51]. Thus, if a given stimulation intensity does not activate the HB reflex, it is unlikely to improve neurological recovery. Additionally, the position of the rat could potentially affect VNS-dependent activation of the HB reflex. In the present study, to provide effective access to the stimulation sites, rats were prone when tested with taVNS and supine when tested with tcVNS or iVNS. While we cannot directly rule out a positional effect on HB reflex engagement, our previous studies using iVNS in the prone position while tested are similar to those observed in the present study in the supine position. Future studies that employ alternative methods to record nerve activation, including compound action potential recordings or immunohistochemistry, may offer greater insight into the differences between each stimulation route and the mechanism of action.
The present study provides an initial comparison of three commonly used VNS strategies. While more studies are needed to provide a full characterization of the actions of these stimulation methods on their direct targets, the results presented here raise the possibility that the reported behavioral and physiological effects of tcVNS and taVNS may arise from activation of classic sensory pathways (e.g., cervical spinal nerves). A clear description of the pathways engaged by each stimulation method is necessary to optimize the clinical utility of these promising therapeutic strategies.
Supplementary Material
Acknowledgements
We would like to thank Nikki Simmons, Collin Chandler, Robert Rennaker, and Michael Kilgard for help with figure creation, experimental design, and manuscript preparation.
Funding
This work was supported by the National Institutes of Health R01 NS094384, and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber and Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 and N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Doug Weber and Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
Footnotes
Declaration of Interest
All authors report no financial interests or potential conflicts of interest.
Supplementary data
Supplementary material
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].De Ferrari GM et al. , “Chronic vagus nerve stimulation : a new and promising therapeutic approach for chronic heart failure,” Eur. Heart J, vol. 32, pp. 847–855, 2011. [DOI] [PubMed] [Google Scholar]
- [2].Hays SA, Rennaker RL, and Kilgard MP, Targeting Plasticity with Vagus Nerve Stimulation to Treat Neurological Disease, 1st ed., vol. 207 Elsevier B.V., 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, and Schuurman PR, “Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis,” PNAS, vol. 113, no. 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mauskop A, “Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches,” Cephalalgia, vol. 25, no. 2, pp. 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- [5].Morris GL et al. , “A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group.,” Neurology, vol. 45, no. 2, pp. 224–30, Feb. 1995. [DOI] [PubMed] [Google Scholar]
- [6].Noble LJ et al. , “Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats,” Transl. Psychiatry, vol. 7, no. 8, p. e1217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kimberley TJ et al. , “Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke,” Stroke, vol. 49, pp. 1–4, 2018. [DOI] [PubMed] [Google Scholar]
- [8].Woodbury JW and Woodbury DM, “Vagal Stimulation Reduces the Severity of Maximal Electroshock Seizures in Intact Rats: Use of a Cuff Electrode for Stimulating and Recording,” Pacing Clin. Electrophysiol, vol. 14, no. 1, pp. 94–107, 1991. [DOI] [PubMed] [Google Scholar]
- [9].Rios MU, Bucksot JE, Rahebi KC, Engineer CT, and Michael P, “Protocol for Construction of Rat Nerve Stimulation Cuff Electrodes,” Methods Protoc., vol. 2, no. 19, pp. 1–27, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].El Tahry R et al. , “Repeated assessment of larynx compound muscle action potentials using a self-sizing cuff electrode around the vagus nerve in experimental rats,” J. Neurosci. Methods, vol. 198, no. 2, pp. 287–293, 2011. [DOI] [PubMed] [Google Scholar]
- [11].Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, and Jensen RA, “Posttraining Electrical Stimulation of Vagal Afferents with Concomitant Vagal Efferent Inactivation Enhances Memory Storage Processes in the Rat,” Neurobiol. Learn. Mem, vol. 70, no. 3, pp. 364–73, 1998. [DOI] [PubMed] [Google Scholar]
- [12].Somann JP et al. , “Chronic cuffing of cervical vagus nerve inhibits efferent fiber integrity in rat model,” J. Neural Eng, vol. 15, no. 3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ben-menachem E, Revesz D, Simon BJ, and Silberstein S, “Surgically implanted and non-invasive vagus nerve stimulation : a review of efficacy, safety and tolerability,” Eur. J. Neurol, vol. 22, pp. 1260–1268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goadsby PJ, Grosberg BM, Mauskop A, and Cady R, “Effect of noninvasive vagus nerve stimulation on acute migraine : An open-label pilot study,” Cephalalgia, vol. 34, no. 12, pp. 986–993, 2014. [DOI] [PubMed] [Google Scholar]
- [15].Silberstein SD et al. , “Non – Invasive Vagus Nerve Stimulation for the Acute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study,” Headache, vol. 56, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frangos E, Ellrich J, and Komisaruk BR, “Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear : fMRI Evidence in Humans,” Brain Stimul., vol. 8, no. 3, pp. 624–636, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stefan H et al. , “Transcutaneous vagus nerve stimulation ( t-VNS ) in pharmacoresistant epilepsies : A proof of concept trial,” Epilepsia, vol. 53, no. 7, pp. 115–118, 2012. [DOI] [PubMed] [Google Scholar]
- [18].Hein E, Nowak M, and Kiess O, “Auricular transcutaneous electrical nerve stimulation in depressed patients : a randomized controlled pilot study,” J. Neural Transm, vol. 120, pp. 821–827, 2013. [DOI] [PubMed] [Google Scholar]
- [19].Ay I, Napadow V, and Ay H, “Electrical Stimulation of the Vagus Nerve Dermatome in the External Ear is Protective in Rat Cerebral Ischemia,” Brain Stimul., vol. 8, no. 1, pp. 7–12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang S, Zhai X, Li S, Mccabe MF, and Wang X, “Transcutaneous Vagus Nerve Stimulation Induces Tidal Melatonin Secretion and Has an Antidiabetic Effect in Zucker Fatty Rats,” PLoS One, pp. 1–12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Z et al. , “Chronic Intermittent Low-Level Transcutaneous Electrical Stimulation of Auricular Branch of Vagus Nerve Improves Left Ventricular Remodeling in Conscious Dogs With Healed Myocardial Infarction,” Circ. Hear. Fail, no. 238, pp. 1014–1021, 2014. [DOI] [PubMed] [Google Scholar]
- [22].Yu L et al. , “Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve : A noninvasive approach to treat the initial phase of atrial fibrillation,” Hear. Rhythm, vol. 10, no. 3, 2013. [DOI] [PubMed] [Google Scholar]
- [23].McAllen RM, Shafton AD, Bratton BO, Trevaks D, and Furness JB, “Calibration of thresholds for functional engagement of vagal A, B and C fiber groups in vivo,” Bioelectron. Med, vol. 1, no. 1, pp. 21–27, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chang R, Strochlic D, Williams E, Umans B, and Liberles S, “Vagal Sensory Neuron Subtypes that Differentially Control Breathing,” Cell, vol. 161, no. 3, pp. 622–633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, and Hays SA, “Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation,” Exp. Neurol, vol. 289, pp. 21–30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hulsey DR et al. , “Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation,” Brain Stimul., vol. 9, no. 2, pp. 174–181, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, and Hays SA, “Vagus nerve stimulation intensity in fluences motor cortex plasticity,” Brain Stimul., vol. 12, no. 2, pp. 256–262, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Siniaia MS, Young DL, and Poon C, “Habituation and desensitization of the Hering-Breuer reflex in rat,” J. Physiol, vol. 523, no. 2, pp. 479–492, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].He W et al. , “The auriculo-vagal afferent pathway and its role in seizure suppression in rats,” BMC Neurosci, vol. 14, no. 85, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li S et al. , “Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats,” Mol. Pain, pp. 1–21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cai L, Lu K, Chen X, Huang J, Zhang B, and Zhang H, “Auricular vagus nerve stimulation protects against postoperative cognitive dysfunction by attenuating neuroinflammation and neurodegeneration in aged rats,” Neurosci. Lett, vol. 703, no. December 2018, pp. 104–110, 2019. [DOI] [PubMed] [Google Scholar]
- [32].He W, Wang X, Shi H, Su Y, Jing X, and Zhu B, “The Anticonvulsant Effect of Transcutaneous Auricular Vagus Nerve Stimulation is Associated with Balancing the Autonomic Dysfunction in Rats,” World J. Tradit. Chinese Med, vol. 2, no. 3, pp. 48–53, 2016. [Google Scholar]
- [33].Jiang Y, Li L, Ma J, Zhang L, Niu F, and Feng T, “Auricular vagus nerve stimulation promotes functional recovery and enhances the post-ischemic angiogenic response in an ischemia / reperfusion rat model,” Neurochem. Int, vol. 97, pp. 73–82, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Zhao YX et al. , “Transcutaneous Auricular Vagus Nerve Stimulation Protects Endotoxemic Rat from Lipopolysaccharide-Induced Inflammation,” Evidence-Based Complement. Altern. Med, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gaul C, Diener H, Silver N, Magis D, Reuter U, and Andersson A, “Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache ( PREVA ): A randomised controlled study,” Cephalalgia, vol. 36, no. 6, pp. 534–546, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kinfe TM, Pintea B, Guresir E, and Vatter H, “Partial Response of Intractable Cluster-Tic Syndrome Treated by Cervical Non-Invasive Vagal Nerve Stimulation ( nVNS ),” Brain Stimul., vol. 8, pp. 2013–2015, 2015. [DOI] [PubMed] [Google Scholar]
- [37].Silberstein SD et al. , “Chronic migraine headache prevention with noninvasive vagus nerve stimulation The EVENT study,” Neurology, vol. 87, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Trimboli M, Al-kaisy A, Andreou AP, Murphy M, and Lambru G, “Non-invasive vagus nerve stimulation for the management of refractory primary chronic headaches : A real-world experience,” Cephalalgia, vol. 38, no. 7, pp. 1276–1285, 2018. [DOI] [PubMed] [Google Scholar]
- [39].Hammer N et al. , “Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation - A cadaveric study,” Sci. Rep, vol. 8, no. 1, p. 7997, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Henssen DJHA et al. , “Vagus nerve stimulation for primary headache disorders : An anatomical review to explain a clinical phenomenon,” Cephalalgia, vol. 0, no. 0, pp. 1–15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mahadi KM, Lall VK, Deuchars SA, and Deuchars J, “Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways,” Brain Stimul., 2019. [DOI] [PubMed] [Google Scholar]
- [42].Tosato M, Yoshida K, Toft E, Nekrasas V, and Struijk J, “Closed-loop control of the heart rate by electrical stimulation of the vagus nerve,” Med. Biol. Eng. Comput, vol. 44, pp. 161–169, 2006. [DOI] [PubMed] [Google Scholar]
- [43].Ardell JL et al. , “Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control,” J. Physiol, vol. 595, no. 22, pp. 6887–6903, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qing KY et al. , “B fibers are the best predictors of cardiac activity during Vagus nerve stimulation,” Bioelectron. Med, vol. 4, no. 5, pp. 1–11, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Badran BW et al. , “Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate,” Brain Stimul., vol. 11, no. 4, pp. 699–708, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Borland MS et al. , “Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity,” Brain Stimul, vol. 9, no. 1, pp. 117–123, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Loerwald KW, Borland MS, Rennaker RL, Hays SA, and Kilgard MP, “The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation,” Brain Stimul., vol. 11, no. 2, pp. 271–277, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Buell EP et al. , “Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity,” Neuroscience, vol. 406, pp. 290–299, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Buell EP, Loerwald KW, Engineer CT, Borland MS, and Buell JM, “Cortical map plasticity as a function of vagus nerve stimulation rate,” Brain Stimul., vol. 11, no. 6, pp. 1218–1224, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Helmers SL et al. , “Application of a computational model of vagus nerve stimulation,” Acta Neurol. Scand, vol. 126, no. 5, pp. 336–343, 2012. [DOI] [PubMed] [Google Scholar]
- [51].Labiner DM and Ahern GL, “Vagus nerve stimulation therapy in depression and epilepsy: Therapeutic parameter settings,” Acta Neurol. Scand, vol. 115, no. 1, pp. 23–33, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.