Abstract
Mitochondrial dysfunction is a pivotal event in many neurodegenerative disease states including traumatic brain injury (TBI) and spinal cord injury (SCI). One possible mechanism driving mitochondrial dysfunction is glutamate excitotoxicity leading to Ca2+-overload in neuronal or glial mitochondria. Therapies that reduce calcium overload and enhance bioenergetics have been shown to improve neurological outcomes. Pioglitazone, an FDA approved compound, has shown neuroprotective properties following TBI and SCI, but the underlying mechanism(s) are unknown. We hypothesized that the interaction between Pioglitazone and a novel mitochondrial protein called mitoNEET was the basis for neuroprotection following CNS injury. We discovered that mitoNEET is an important mediator of Ca2+-mediated mitochondrial dysfunction and show that binding mitoNEET with Pioglitazone can prevent Ca2+-induced dysfunction. By utilizing wild-type (WT) and mitoNEET null mice, we show that Pioglitazone mitigates mitochondrial dysfunction and provides neuroprotection in WT mice, though produces no restorative effects in mitoNEET null mice. We also show that NL-1, a novel mitoNEET ligand, is neuroprotective following TBI in both mice and rats. These results support the crucial role of mitoNEET for mitochondrial bioenergetics, its importance in the neuropathological sequelae of TBI and the necessity of mitoNEET for Pioglitazone-mediated neuroprotection. Since mitochondrial dysfunction is a pathobiological complication seen in other diseases such as diabetes, motor neuron disease and cancer, targeting mitoNEET may provide a novel mitoceutical target and therapeutic intervention for diseases that expand beyond TBI.
Keywords: NL-1, Controlled Cortical Impact, Calcium Overload, Mitochondrial Bioenergetics, Seahorse, Beam Walk, Novel Object Recognition
Introduction
Traumatic brain injury (TBI) is a serious health concern that effects at least 2.8 million Americans annually (CDC, 2015). The disease is initiated by an impact to the head, causing axonal shearing, neurovascular disruption and cell death at the injury site. This primary injury activates a complex signaling cascade resulting in a secondary non-mechanical injury. It is thought that by targeting the secondary injury cascade, the expansive neuronal degeneration that occurs following the impact can be minimized. Within this secondary injury cascade, a period of hyperglycolysis has been observed followed by prolonged suppression of glucose metabolism (Prins and Matsumoto, 2014). Well-aligned is a period of mitochondrial dysfunction which becomes significant at 1 hour post-injury and peaks at 12 to 72 hours post-injury depending on injury severity (Gilmer et al., 2009a; Hill et al., 2017; Kulbe et al., 2017; Singh et al., 2006). Targeting mechanisms that drive mitochondrial dysfunction has traditionally shown therapeutic benefits, including improved tissue sparing and neurological recovery.
A class of drugs, which have received much attention in the field of neuronal insult and injury, are thiazolidinediones, or glitazones. These peroxisome proliferator activated receptors (PPAR) agonists have previously been used for the treatment of type 2 diabetes. Pioglitazone, one of these glitazones, has shown neuroprotective effects through the attenuation of mitochondrial dysfunction, though its mechanism of neuroprotection has not been elucidated (Rabchevsky et al., 2017; Sauerbeck et al., 2011a; Thal et al., 2011). Previous studies hypothesized that Pioglitazone’s therapeutic effect was mediated via neuroinflammatory modulation based on interactions with PPAR-γ. However, studies examining PPAR-γ activation after TBI later discredited this theory (Sauerbeck et al., 2011a; Thal et al., 2011). Additionally, Pioglitazone has been shown to attenuate mitochondrial dysfunction within the first 24 hours following a brain or spinal cord injury (SCI), which hints to a fast-acting mechanism compared to the extended time required for gene expression changes through PPAR activation (Besson et al., 2005; Hyong et al., 2008; Kapadia et al., 2008; Montcalm-Smith et al., 2007). Recently, Pioglitazone has been used for chronic treatment of TBI for improvement of cognitive outcomes, highlighting an extended therapeutic window that would not be dependent on mitigation of early inflammation (McGuire et al., 2019).
An additional mechanism that has been credited for Pioglitazone’s therapeutic effects is its ability to inhibit pyruvate carrier complexes (Divakaruni et al., 2013; Shannon et al., 2017). However, the observed inhibition is only seen at high, non-physiologically relevant concentrations, limiting the relevance of this mechanism in vivo. At lower, physiologically-relevant concentrations, Pioglitazone improves TBI- and SCI-induced mitochondrial dysfunction providing support that interactions, independent of pyruvate carrier inhibition, are contributing to the neuroprotective properties of this compound (Patel et al., 2017; Rabchevsky et al., 2017; Sauerbeck et al., 2011a). Previous reports also highlight that Pioglitazone interacts with a novel mitochondrial membrane protein called mitoNEET (Bieganski and Yarmush, 2011; Zuris et al., 2011). mitoNEET is known to contribute to the formation of inter-mitochondrial junctions (Vernay et al., 2017) and to directly modulate mitochondrial bioenergetics (Wang et al., 2017). To better understand these observations, we put forth the hypothesis that mitoNEET modulates TBI-induced mitochondrial dysfunction and that this protein is the therapeutic target of Pioglitazone and the basis for neuroprotection. Work from our group (Rabchevsky et al., 2017), further supported this hypothesis when showing that Pioglitazone lost its neuroprotective effects in mitoNEET null mice following SCI.
To understand the role of mitoNEET in Pioglitazone-mediated neuroprotection, we used wild-type (WT) and mitoNEET null mice to study mitochondrial bioenergetics after controlled cortical impact (CCI) brain injury to induce TBI-mediated mitochondrial dysfunction. Since mitoNEET has been directly linked to mitochondrial bioenergetics, we were also interested in whether the removal of mitoNEET altered mitochondrial calcium buffering and consequently altered mitochondrial susceptibility to injury in mitoNEET null mice. We then targeted this dysfunction with Pioglitazone or our novel mitoNEET specific ligand, NL-1 (Geldenhuys et al., 2010; Geldenhuys et al., 2014; Geldenhuys et al., 2016; Logan et al., 2015). Results from these studies show that Pioglitazone loses its neuroprotective effects in TBI-injured mitoNEET null animals. Additionally, we show that NL-1 treatment following a moderate brain injury in WT mice improves mitochondrial dysfunction, cortical sparing and functional recovery. Further, NL-1 is effective in multiple rodent species, imparting cortical sparing in brain-injured rats. These results provide support that the neuroprotective effects of NL-1 transcend species. In summary, these findings may be an important step in revealing a novel target mechanism for treatment of TBI while providing important insight into the secondary events that occur after traumatic injury. Since mitochondrial dysfunction is a pathological hallmark of diseases expanding beyond TBI, including SCI, diabetes, motor neuron disease, cancer and other neurodegenerative disorders, this mechanism may also provide a therapeutic target for many other human diseases.
Materials and Methods
Animals
All of the studies performed were approved by the University of Kentucky Institutional Animal Care and Usage Committee. Additionally, the Division of Laboratory Animal Resources at the University is accredited by the Association for the Assessment and Accreditation for Laboratory Animal Care, International (AAALAC, International) and all experiments were performed with its guidelines. All animal experiments are in compliance with ARRIVE guidelines and experiments are carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All experiments were conducted using either male or female adult (3 to 5 months) wild-type C57BL/6 (Jackson Laboratories, Bar Harbor, ME) or mitoNEET null mice with an average mass of 30 grams. mitoNEET null mice were gifted to the lab and the generation of the mouse model was previously described (Wiley et al., 2007). In summary, the mitoNEET null mice were developed through disruption of the mitoNEET gene on the C57Bl/6 (albino) × 129 SvEv Brd (agouti) background acquired from the Lexicon Genetics Omnibank program which they developed using their proprietary gene trap technology (Zambrowicz et al., 1998). Experiments using NL-1 also involved the usage of Sprague Dawley rats (Charles River; ~300 g; male) at 2mo of age.
Animals were randomly assigned to groups and all data analysis was performed blinded to treatment groups. The animals were housed 5 (mice) or 2 (rats) per cage and maintained in a 14-hr light/10hr dark cycle for mice and 12-hr light/12hr dark cycle for rats. All animals were fed a balanced diet ad libitum and water was reverse osmosis generated. For mitochondrial bioenergetics assessment, experiments were conducted with n=3–6 biological replicates and for each biological replicate there were technical replicates of n≥3. For Pioglitazone cortical sparing assessment in WT versus mitoNEET null mice, n=6/group. For NL-1 functional assessments in WT versus mitoNEET null mice, n=13–16/group. For chronic NL-1 mouse study and NL-1 rat efficacy experiment, n=4–8/group. The exact number of animals per study are reported within the figure legends.
Controlled Cortical Impact Brain Injury
All surgical procedures were performed as previously described and were classified as moderate (0.75 mm depth of contusion) to severe (1.0 mm depth of contusion) in mice or severe (2.0 mm depth of contusion) in rats at 3.5 meters/second for 500ms (Sullivan et al., 1999; Sullivan et al., 2000; Sullivan et al., 2011). Prior to injury, animals were anesthetized using 2% isoflurane, animals were weighed and their head was shaved to remove hair to avoid infection. Animals were then placed in a Kopf stereotaxic frame for proper positioning under a pneumatic impactor (Precision Science Instruments) and body temperature was maintained at 37°C with the use of an isothermal pad. A 3mm (mice) or 6mm (rats) craniotomy was performed lateral to the central fissure on the left side of the skull centered between lambda and bregma, with much care taken to not disrupt the dura. Animals with damage to the dura were excluded. Injury groups then received a unilateral injury directly to the surface of the brain using a 2mm (mice) or 5mm (rats) impactor rod tip. Sham animals received a craniotomy but did not receive an impact to the brain. Following the injury, the craniotomy was covered with a piece of hardened dental acrylic, made the night before, and secured with sterile surgical adhesive. Incisions were then closed with sutures. After the craniotomies were covered and the incisions were closed on all animals, isoflurane exposure was ended and the animals were removed from the stereotaxic frame. They were then placed in a clean cage that was temperature controlled at 37°C with a heating pad until the animals were mobile and fully responsive.
To investigate the neuroprotective effects of either Pioglitazone or NL-1 after TBI, animals received a CCI (as described above) and then given an IP injection of either Pioglitazone or NL-1 as described in figure legends. Both drugs were solubilized immediately before injections in vehicle (25 μl of 100% Ethanol, 1 μl 38% HCl, and 225μl of 0.9% saline for Pioglitazone and 100% DMSO for NL-1) to yield a concentration which would require 100 μl of compound injected at the desired dosage (Sauerbeck et al., 2011a). WT animals that received continuous administration of NL-1 for 1 week received one injection of NL-1 at 15minutes after injury and then the osmotic pump was inserted at the nape of the neck to release 3ug/μl of NL1 at a rate of 1.0 μL an hour for 7 days. Osmotic pumps were removed at 7 days post-injury and animals were allowed to recover.
Mitochondrial isolation
Mitochondria were isolated using previously employed differential mitochondrial isolation methods (Pandya et al., 2007; Pandya et al., 2009; Pandya et al., 2016). Animals were asphyxiated with CO2 and rapidly decapitated. Following decapitation, the brain was rapidly removed and placed in a beaker of ice-cold mitochondrial isolation buffer (MIB) composed of 215 mM Mannitol, 75 mM Sucrose, 0.1% BSA, 1 mM EGTA, and 20 mM HEPES at pH 7.2. Cortical tissue samples were homogenized and then centrifuged at 1300 × G for 3 min. The supernatant was placed in a fresh tube and the pellet was resuspended in isolation buffer to be spun again at 1300 × G for 3 min. The supernatants from the first and second spins were collected in separate tubes and spun at 13,000 × G for 10 min. The pellets from both tubes were combined, resuspended in 500 μl isolation buffer and placed in a nitrogen bomb at 1,200 psi for 10 min to release trapped mitochondria within synaptosomes. The pressure in the nitrogen bomb was rapidly released after 10 min.
The samples were placed as the top layer on a Ficoll separation column which consisted of a 10% Ficoll layer (bottom) and a 7.5% Ficoll layer (top). The Ficoll column with sample was centrifuged at 32,000 × G for 30 min at 4 °C. Following the Ficoll purification, the mitochondrial pellet was resuspended in isolation buffer without EGTA and centrifuged at 10,000 × G for 10 min at 4 °C in order to remove residual Ficoll from the purified mitochondrial sample. The final mitochondrial pellet was resuspended in isolation buffer without EGTA to yield a final concentration of approximately 10 mg/ml, and stored immediately on ice. Protein concentrations for each sample were determined with all the samples on the same plate using the BCA protein assay kit and measuring absorbance at 560 nm with a Biotek Synergy HT plate reader (Winooski, VT).
Measurement of mitochondrial bioenergetics
Measurements of mitochondrial bioenergetics in isolated mitochondrial were completed using a Seahorse XFe24 Flux Analyzer as published previously using slight modifications (Hubbard et al., 2019; Pandya et al., 2016; Sauerbeck et al., 2011b). Stock mitochondrial substrates of 500 mM pyruvate, 250 mM malate, 30 mM ADP, 1 mg/ml oligomycin-A, 1 mM FCCP, 1 mM rotenone and 1 M succinate were prepared and the pH was adjusted to pH 7.2. The day before the planned experiment, a 24 well dual-analyte sensor cartridge was hydrated and kept in a non-CO2 incubator at 37 °C. On the scheduled day of the experiment, the sensor cartridge ports A through D were loaded with the appropriate mitochondrial substrates or inhibitors (10 × working concentration made from stocks) and injected into the assay plate according to protocol procedure to reach the final concentration of the compound (1 ×) in each well. All mitochondrial working stocks were prepared in respiration buffer (RB) composed of 215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 2 mM MgCl, 2.5 mM KH2PO4 at pH 7.2 and stored at 4 °C. The amount of substrates/inhibitors loaded for each port is based upon the initial 500 μl RB volume in the mitochondrial plate as follows: Port A – 50 μl (mixture of pyruvate, malate and ADP), Port B – 55 μl (Oligomycin A), Port C – 60 μl (FCCP), and Port D – 65 μl (rotenone and succinate). Once the sensor cartridge was loaded with all of the experimental reagents it was placed into the Seahorse XFe24 Flux Analyzer for automated calibration.
Seahorse Standard XFe24 flux assay plates were utilized for mitochondrial analysis. Mitochondria (5 μg) from every experimental group were analyzed together on a single plate. Mitochondrial samples were resuspended in 50 μl RB and added in experimental wells whereas background control wells contained 50 μl of RB without mitochondria. Once every well was loaded on XFe24 plate, it was then centrifuged at 3,000 rpm for 4 min at room temperature. Following the centrifugation of the plates, 450 μl (37 °C) of pre-warmed RB was gently added to each well for a final volume of 500 μl per well. Plates were then placed into the calibrated Seahorse XFe24 flux analyzer for mitochondrial bioenergetics analysis after the sensor cartridge calibration was concluded.
An optimized protocol was utilized for the analysis of bioenergetics in purified mitochondria using the Seahorse Biosciences XFe24 Flux Analyzer. The protocol contains sequential and/or cyclic steps of a cartridge probe calibration, mixing substrates in the assay system, a delay for some time, injections of substrates/inhibitors and then measurement of the oxygen consumption rates (OCR) as elaborated upon previously.(Pandya et al., 2016; Sauerbeck et al., 2011b) Mitochondrial oxygen consumption rates (OCR) were recorded in the absence or the presence of various mitochondrial substrates/inhibitors which were added from port A to port D. State III response in presence of 5 mM pyruvate, 2.5 mM malate and 1 mM ADP (Port A) was measured followed by State IV response in presence of 1 μM oligomycin A (Port B). Sequentially, State VFCCP and State VSucc OCR rates were recorded automatically in presence of 4 μM FCCP (Port C); 0.1 μM rotenone and 10 mM of succinate (Port D) respectively. The data files collected from each experiment were analyzed and reported here as percent OCR (% OCR). In this study, we reported on State V(FCCP) rates since as this parameter is indicative of impairment of maximal mitochondrial function and mitochondrial reserve capability following TBI.
Western Blot Analysis
Cortical tissue was isolated from both mitoNEET null and WT mice and homogenized in cold lysis buffer supplemented with 1X protease inhibitor. The homogenate was sonicated for 10 seconds at 10% amplitude and then centrifuged at 4°C for 15minutes at 13,000g. The supernatant was removed and protein concentration was quantified using a BCA protein assay kit (23227, Peirce BCA Protein Assay, ThermoFisher Scientific). Ten micrograms of each sample was loaded into a 10% mini-PROTEAN precast gel running and ran at 165 V for 60minutes (Bio-Rad). The protein was transferred to a poly(vinylidene fluoride) (PVDF) membrane at 100 V for 1 h. The membrane was blocked for 1 hour with reconstituted 5% nonfat dry milk in 0.2% TBST (Tris-buffered saline, 0.1% Tween 20). The membrane was then blocked for 1hr with 5% nonfat dry milk in 0.2% TBST (Tris-buffered saline, 0.1% Tween 20). mitoNEET Protein-Tech Primary antibody (16006–1-AP) was diluted at 1:500 in TBST and the mixture was incubated on the membrane for 1hr at room temperature. The membrane was then washed with PBS and incubated with secondary antibody Anti-Rabbit IgG-HRP (#7074S Cell Signaling) diluted at 1:5000 followed by 3 washes of TBST. The blots were imaged using Chemidoc-XRS system (Bio-Rad).
Histological Analysis
Animals were euthanized using FATAL-Plus (NAC No.: 10980012, Vortech Pharmaceuticals, Dearborn, Michigan 48126) and immediately perfused with saline followed by cold 4% paraformaldehyde, which was freshly prepared one-day prior. The brains were removed and placed in 4% paraformaldehyde and 30% sucrose solution in PBS for 24hrs. After 24hrs, the brains were transferred to a 30% sucrose PBS buffer solution without paraformaldehyde. 35uM coronal sections were cut with a freezing microtome throughout the rostral caudal extent of the damaged cortex. Sections were stained with cresyl violet and subjected to image analysis for lesion volume assessment. Quantitative assessment of cortical damage employed a blinded unbiased stereological protocol using the Cavalieri method as previously described (Sullivan et al., 1999; Sullivan et al., 2000). All slides were assessed blindly with respect to treatment group using ImageJ software (NIH, USA) for simple ROI analysis of 12 sections per animal to measure cortical sparing.
Adjusted Neurological Severity Score
Injured wild-type or mitoNEET null mice had functional motor recovery tested using the adjusted neurological severity score (aNSS). This 14-point test measures coordinated motor function and balance as a measurement of motor function/recovery following injury. This score is based upon functional ability measured during the beam walk test. One day prior to injury, all animals were trained to traverse elevated beams of 3, 2, 1, and 0.5 cm width and a 0.5 cm diameter rod. At 1 hour, 24 hours, 72 hours and 120 hours post-injury, mice were allowed to traverse the beams and scored as follows. The 4 largest beams had a maximum score of 3-points and the smallest, 0.5cm diameter, rod had a maximum score of 2-points. For the largest 4 beams, one point was deducted if the animal had one or more footfalls or became inverted during the test. For the 0.5cm rod, 1-point was deducted for inversion on the rod. For all beams/rods, if an animal was unwilling to traverse the beam or fell off during the test, the animal received a score of 0 for that beam.
Novel Object Recognition
Injured WT mice underwent cognition testing following injury using the novel object recognition (NOR) test. Prior to surgery, each animal tested received their own 10.5” x 19” x 8” plastic box which was not cleaned to maintain a familiar environment for the length of the study. During this time, animals were allowed to acclimate to their box for 1hr. On testing day, 6 weeks post-injury, vehicle and drug treated mice were place back into their designated box that had two identical novel objects placed 1inch from two diagonal corners. The objects used for this study were plastic children toys (Fisher-Price® Little People®) approximately 3 × 2 inches. The animals were allowed to explore their box and the novel objects for 5 minutes. After the acclimation period, the mice were removed from their designated boxes and then tested 4 hours later. During the test period, mice were placed back into their boxes where one of the previous objects was replaced with novel object of equal size and shape. Objects were not shared between groups and were thoroughly cleaned with soap and water after usage. The amount of time that the animal spent investigating each object was recorded by an evaluator who was blinded to the injury and treatment of each mouse. The discrimination index was then calculated by subtracting the time the animal spent investigating the familiar object from the time that the animal spent investigating the novel object. This value was then divided by the total time of investigation, calculated by adding the time the animal spent investigating the familiar object and the novel object.
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging was conducted using an approach similar to our previously published work (Patel et al., 2017). In summary, a 7T Bruker/Siemens Clinscan MRI scanner was used to image rat brains at 14 days post-injury following either vehicle or NL-1 treatment. During the imaging, animals were placed under continuous isoflurane anesthesia which was introduced through a nose cone and the animals head was placed under a 4-channel rat brain coil. After inserting the animal and coil into the MRI scanner, localizers in the three orthogonal planes were used to focus and homogenize the magnetic field around the injured brain tissue. Following localization, T2-weighted images were acquired in the coronal plane yielding 12 subsequent slices centered around the injury site with a field-of-view (FOV) of 40 mm × 40 mm x 0.7 mm with a pixel resolution of 384 × 512. The imaging parameters were: Excitation Time (TE) = 42 ms, Relaxation Time (TR)= 2070 ms, with the phase encoding direction set to Anterior-Posterior and a total acquisition time of 11.25 minutes. MRI images were generated using Syngo Medical Imaging software (Siemens AG, Germany). Quantification of these images were conducted in a similar manner to those reported previously (Patel et al., 2016). In summary, all images were post-processed using FSL FMRIB Software Library v5.0 (Analysis Group; FMRIB, Oxford, UK). (Jenkinson et al., 2012) For each of the 12 coronal sections, the mean and standard deviation of the T2 parameter throughout the imaged brain (including the injury) was calculated. A threshold was then applied where any voxel greater than 3 standard deviations (SD) above the total mean signal was considered T2 hyper-intense “injured tissue”. The T2-weighted cortical sparing was then calculated by the total volume of tissue on the injured hemisphere minus the T2 hyper-intense voxels on the injured hemisphere divided by the total volume of tissue on the contralateral hemisphere minus T2 hyper-intense voxels.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, CA, USA). Animal numbers were powered according to published experimental data and anticipated effect sizes; experiments were not powered to detect for sex differences where applicable. For all analyses, the significance of differences among groups was set at p < 0.05. For each measure, data were measured using interval/ratio scales. The Brown-Forsythe and Bartlett’s tests were performed to ensure homogeneity of variance. Furthermore, the Shapiro-Wilk test was completed to ensure normality. As these criteria were met for all experimental data, parametric statistics were employed for all data analyses. The statistical test employed for each study can be found within the figure legend however in summary, the following applied. For mitochondrial assessments, data sets were evaluated using either a t-test or a one-way analysis of variance and, when appropriate, post-hoc comparisons employing the Dunnett and Bonferoni test. Two-way ANOVA were run to analyze differences in cortical sparing and aNSS between mitoNEET null mice and wild-type mice with Pioglitazone or NL-1 treatment. For Novel Object Recognition and MRI-weight cortical sparing studies with continuous NL-1 treatment for 7 days in WT mice, a t-test was performed to measure differences between groups.
Results
Pioglitazone increases mitochondrial respiration following calcium insult in WT mice while there was a lack of Ca2+-mediated effects in mitoNEET null mice
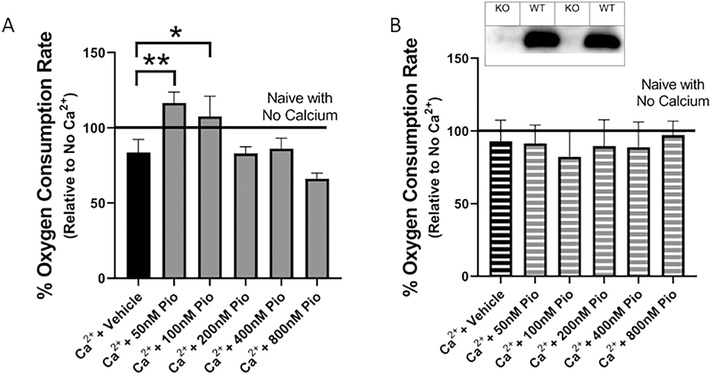
The mitochondrial effects of Pioglitazone have been debated in the literature (Divakaruni et al., 2013; Patel et al., 2017; Sauerbeck et al., 2011a; Thal et al., 2011). Traditionally, Pioglitazone was marketed within a class of drugs called PPAR agonists, but researchers found that this thiazolidinedione was interacting with cellular proteins besides PPAR. To date, Pioglitazone has been shown to interact with multiple proteins at the cytosolic, nuclear and mitochondrial level (Bieganski and Yarmush, 2011; Colca et al., 2004; Divakaruni et al., 2013; Kapadia et al., 2008; Panzer et al., 2002; Shannon et al., 2017). In order to study the importance of mitochondria bioenergetics in Pioglitazone-mediated neuroprotection, we isolated mitochondria, removing any interactions due to cytosolic or nuclear proteins such as the PPARs. This allows for insight into what is occurring at the mitochondrial level by eliminating non-mitochondrial interactions. Previous studies have shown that micromolar concentrations of Pioglitazone are able to decrease ex vivo mitochondrial respiration through interactions with the pyruvate carrier complex (Divakaruni et al., 2013; Shannon et al., 2017). However, results from Sauerbeck et al. showed that lower concentrations of Pioglitazone, within the nanomolar to micromolar range, can increase mitochondrial respiration when administered in vivo (Sauerbeck et al., 2011a). To properly address this discrepancy, cortical mitochondria were isolated from naive C57BL/6 mice and then administered a calcium insult to induce mitochondrial dysfunction (150nm Ca2+/mg of mitochondrial protein). These isolated mitochondria were then treated with various concentrations of Pioglitazone and mitochondrial bioenergetics were analyzed (Figure 1A). Calcium insult provides an ex vivo effect that is similar to what is seen within the excitotoxicity mechanism following a TBI (i.e. increased mitochondrial calcium cycling and loading). This approach also builds upon a previous report from our laboratory demonstrating direct effects of calcium cycling/loading on mitochondrial respiration in the absence of mitochondrial permeability transition pore (mPTP) opening and reactive oxygen species production or oxidative damage (Pandya et al., 2013). The current results demonstrate that 50nM Pioglitazone can increase mitochondrial respiration 12.7% compared to naïve non-insulted mitochondria and 32.8% compared to vehicle calcium-insulted mitochondria. Also, a dose-dependent response was evident where lower dosages of Pioglitazone (50 and 100nM) increased mitochondrial bioenergetics and higher concentrations (200, 400, and 800nM) decreased mitochondrial bioenergetics. This data supports recent reports where lower concentrations of Pioglitazone increase mitochondrial respiration and higher concentrations decreased mitochondrial respiration (Divakaruni et al., 2013; Rabchevsky et al., 2017; Shannon et al., 2017).
Figure 1. Pioglitazone restores mitochondrial bioenergetics, dependent upon the presence of mitoNEET, following ex vivo calcium insult.
Naive cortical mitochondria were isolated from adult wild-type and mitoNEET null mice and challenged with calcium; dose response with Pioglitazone was conducted to examine bioenergetic rescue. (A) Pioglitazone (50 nM) is able to increase State V complex I-mediated maximal mitochondrial respiration in mitochondria isolated from wild-type C57BL/6 mice cortex exposed to a high calcium (Ca2+; 150nm/mg) environment. (B) However, in mitochondria isolated from the cortical tissue of mitoNEET null mice, the same calcium insult (150nm/mg protein) has no significant effect and ex vivo addition of Pioglitazone does not increase State V complex I-mediated maximal mitochondrial respiration. Line of 100% OCR corresponds to the normalized average respiration of isolated mitochondria isolated from both wild-type (A) and mitoNEET null (B) mice in the absence of Ca2+. Western blots insert shows presence of mitoNEET in wild-type C57BL/6 mice but not in mitoNEET null mice. One-way ANOVA, Compared Drug Treated to Ca2+ + Vehicle, Bonferroni Post-Hoc, N=3/group, mean ± SEM, F5,17 = 15.77, **p=0.0015, *p=0.016 in wild-type mitochondria; F5,17 = 0.10, mitoNEET null mitochondria.
Pioglitazone has also been shown to bind mitoNEET but the importance of this interaction within the model of TBI remains unclear. mitoNEET is a novel mitochondrial membrane protein which has been shown to modulate mitochondrial respiration (Geldenhuys et al., 2010; Kusminski et al., 2012; Tamir et al., 2015; Wiley et al., 2007). Western blots were performed in WT versus mitoNEET null cortical homogenate to confirm the presence, or lack, of mitoNEET (Figure 1B inset). As shown in Figure 1A, calcium is able to decrease mitochondrial respiration in wild-type mitochondria and Pioglitazone is able to reverse calcium-induced mitochondrial dysfunction. However, in mitochondria isolated from the cortical tissue of mitoNEET null mice, the same concentration of calcium (150nM/mg of protein) had no significant effect on mitochondrial respiration and the use of Pioglitazone provided no increased respiration (Figure 1B). Combined, these results demonstrate the novel finding that mitoNEET is pivotal in calcium-mediated alterations in mitochondrial bioenergetics and verify that mitoNEET is a specific mitochondrial target for Pioglitazone to increase respiration capacity.
Pioglitazone loses its bioenergetic and neuroprotective effects in mitoNEET null animals following CNS injury
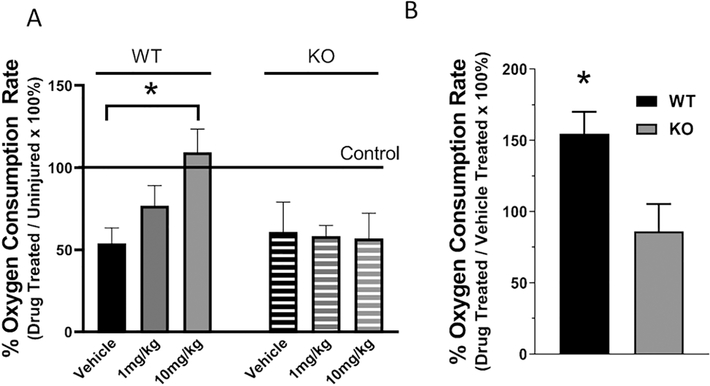
Previous work from our laboratory and others have shown that mitochondrial dysfunction is chronic and progressive over the first 72hrs following a control cortical impact (Gilmer et al., 2009a; Hill et al., 2017; Kulbe et al., 2017; Singh et al., 2006). Mitochondrial dysfunction has been shown to be amenable to various targeted therapeutic interventions and to promote improvements in tissue sparing and functional outcomes following an injury (Davis et al., 2008; Gilmer et al., 2009a; Hubbard et al., 2018; Kulbe et al., 2017; Pandya et al., 2007; Pandya et al., 2009; Pandya et al., 2014; Readnower et al., 2011; Sauerbeck et al., 2011a; Scheff and Sullivan, 1999). Pioglitazone has been shown to improve mitochondrial dysfunction, afford neuroprotection and improve functional outcomes (Sauerbeck et al., 2011a). In order to test the importance of mitoNEET in Pioglitazone’s ability to increase mitochondrial bioenergetics following injury, mitoNEET null and wild-type mice received a moderate CCI and received IP injections of vehicle or Pioglitazone (1mg/kg or 10mg/kg) beginning at 15 mins post-injury and every 6 hrs thereafter until 24 hrs post-injury. This dosing paradigm was used because we have shown that Pioglitazone needed to be pharmacologically active within the mitochondria to impart improved bioenergetics, which is supported by Figure 1A and past research (Sauerbeck et al., 2011a). At 25hrs post-injury, mitochondria were isolated from the injured cortex and bioenergetics were assessed. As illustrated in Figure 2A, and in agreement with previous publications (Sauerbeck et al., 2011a), 10 mg/kg administration beginning 15 mins post-injury restored State V, complex 1-mediated mitochondrial respiration in wild-type animals. It is important to note that State V respiration was used as our indicator for mitochondrial bioenergetics since this parameter is indicative of maximal mitochondrial function and mitochondrial reserve capability rendering high sensitivity to mitochondrial bioenergetic disruption following TBI. Interestingly, no effect was demonstrated in mitoNEET null animals suggesting the mechanism of improved bioenergetics observed previously is dependent upon presence of mitoNEET.
Figure 2. Pioglitazone improves mitochondrial function, even at delayed therapeutic initiation, following TBI in wild-type but not mitoNEET null animals.
Mitochondria were isolated from the ipsilateral cortex of adult wild-type and mitoNEET null mice at 25 hrs post-injury and State V complex I-mediated maximum mitochondrial respiration was assessed. Wild-type and mitoNEET null mice received a moderate CCI followed by serial IP injections of either vehicle or Pioglitazone (1mg/kg or 10mg/kg) at 15 mins, 6 hrs, 12 hrs, 18 hrs, and 24 hrs post-injury (A). Pioglitazone administration (10 mg/kg) significantly improved mitochondrial function in wild-type animals but no effect was demonstrated in mitoNEET null mice. One-way ANOVA, Compared Drug Treated to Vehicle, Bonferroni Post-Hoc, N=3/group, mean ± SEM, F2,8 = 5.30, *p=0.035 for wild-type; F2,8 = 0.01, for mitoNEET null mice. Data expressed as percentage of average sham control group. (B) State V complex I-mediated maximal mitochondrial oxygen consumption rate in wild-type and mitoNEET null mice. Pioglitazone (1 IP injection, 10mg/kg) was administered at 12 hrs post-injury with mitochondrial isolations at 13 hrs post-injury. Mitochondria from wild-type mice treated with Pioglitazone had significantly increased bioenergetics compared to mitoNEET null mice. N=4/group, mean ± SEM, Unpaired t-test, t = 2.77, *p=0.032, two-tailed. Data expressed as percentage of vehicle-treated injured group.
To examine the therapeutic window of opportunity for Pioglitazone, we assessed delayed treatment at 12 hours post-injury following a CCI in wild-type and mitoNEET null mice. In this study, animals received a moderate CCI injury with treatment at 12 hours post-injury and cortical mitochondria were isolated at 13 hours post-injury. Mitochondria isolated from mice treated with Pioglitazone (10mg/kg) had a 54% increase in mitochondrial bioenergetics compared to vehicle treated wild-type mice (Figure 2B). On the contrary, mitoNEET null mice experienced no increase in mitochondrial bioenergetics compared to vehicle treated animals of the same genotype. This data provides further support that the ability of Pioglitazone to improve mitochondrial dysfunction following TBI is directly dependent on the presence of mitoNEET. Furthermore, it indicates that Pioglitazone has a remarkable therapeutic window of opportunity following TBI. Importantly, these data suggest that mitochondrial dysfunction following TBI may not be due to overt mitochondrial failure due to oxidative damage as previous hypothesized. Rather, it appears that mitochondria may be inhibited and this inhibition can be either reversed or bypassed with proper interventions. Additionally, the observed improvement in mitochondrial dysfunction at 13 hrs post-injury, following delayed treatment initiated at 12 hrs post-injury, further supports our hypothesis that Pioglitazone directly targets mitochondrial function through mitoNEET, and not via transcriptional regulation mediated through PPARs activation.
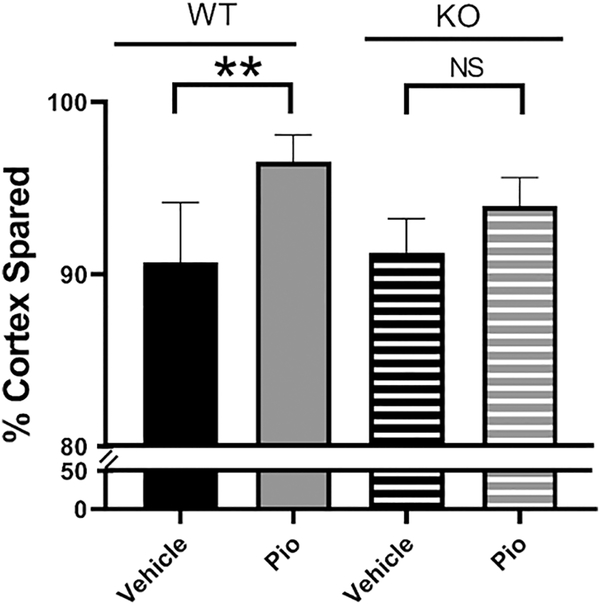
To directly test whether Pioglitazone’s neuroprotective properties are dependent upon its interaction with mitoNEET, adult wild-type and mitoNEET null mice received a moderate contusion injury before dosing with either vehicle or 10mg/kg of Pioglitazone through IP injection at 15min and followed by once daily administration. Cortical tissue sparing was assessed at 7 days post-injury and, as shown in Figure 3, Pioglitazone treatment significantly increases tissue sparing in WT mice but has no significant effect in mitoNEET null mice following injury. It is also important to note that no significant differences were measured between vehicle-treated animals of either genotype, showing that the removal of mitoNEET does not increase the animals’ susceptibility to injury.
Figure 3: Pioglitazone provides neuroprotective effects following TBI in wild-type but not mitoNEET null mice.
Adult wild-type C57BL/6 or mitoNEET null mice received a moderate CCI followed by serial Pioglitazone injections (10 mg/kg) at 15 mins and every 24 hrs until euthanasia at 7 days post-injury. Cortical tissue sparing was assessed using a stereological approach. Pioglitazone treatment significantly increases tissue sparing in wild-type but not mitoNEET null mice. N=6/group, mean ± SEM, One-way ANOVA, Compared Drug Treated to Vehicle within same genotype and vehicle in different genotypes, Tukey Post-Hoc, F3,23 = 8.26, **p=0.0014.
The mitoNEET specific ligand, NL-1, increases cortical sparing and improves functional outcome following a TBI
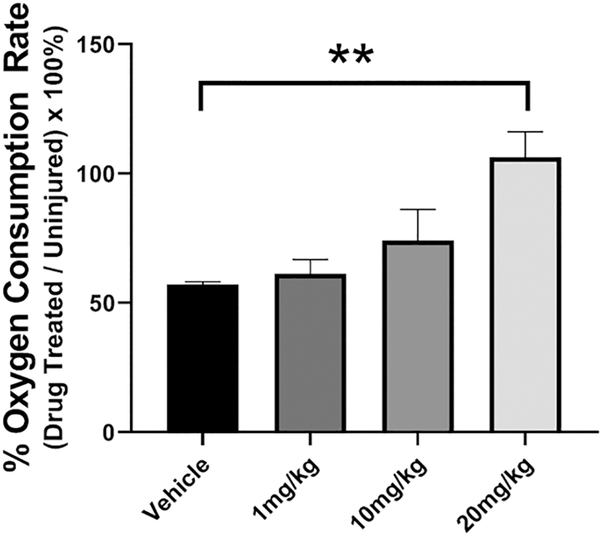
Based on our results using Pioglitazone, we decided to further probe the importance of mitoNEET as a therapeutic target for TBI. We hypothesized that mitoNEET binding with a novel ligand would provide the same degree of neuroprotection as was observed with Pioglitazone. Therefore, our collaborators synthesized a compound that binds to mitoNEET, NL-1. Pharmacodynamic studies have shown that this drug is very similar to Pioglitazone, retaining the traditional glitazone structure and binding to mitoNEET with equal affinity as Pioglitazone. Importantly, NL-1 lacks the PPAR binding region (Geldenhuys et al., 2010; Logan et al., 2015; Pedada et al., 2014). Using mitochondrial bioenergetics as our outcome measure, WT and mitoNEET null mice received a severe CCI and either vehicle or IP injections of NL-1 (1mg/kg, 10mg/kg or 20mg/kg) at 15 mins and 24 hrs post-injury. Mitochondria were isolated from the injured cortex at 25 hrs post-injury and mitochondrial respiration measured. As demonstrated in Figure 4, NL-1 improves State V (complex 1-mediated maximum respiration) mitochondrial respiration in a dose-dependent manner.
Figure 4: NL-1, a mitoNEET ligand, increases mitochondrial bioenergetics following TBI.
Adult C57BL/6 mice received varying dosages of a novel mitoNEET ligand, NL-1, at 15 mins, 6 hrs, 12 hrs, 18 hrs, and 24 hrs following severe CCI. Mitochondria were isolated at 25 hrs post-injury. State V mitochondrial respiration (mediated through complex 1) was significantly elevated in mitochondria derived from NL-1 treated (20 mg/kg) mice compared to that from vehicle-treated mice. In addition, compared to vehicle, the 10mg/kg had an effect size of 7.43. Data expressed by percentage of average sham. N=4/group, mean ± SEM, One-way ANOVA, Compared Drug Treated to Vehicle, Dunnett Post-Hoc, F3,15 = 7.32, **p=0.0032.
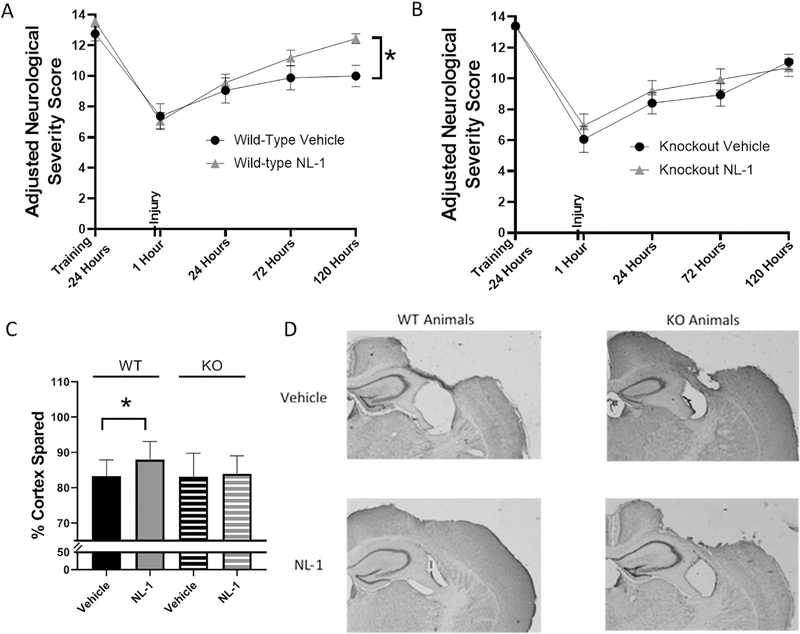
Our initial cortical sparing studies began with the use of 20 mg/kg of NL-1 which was given via IP injection at 15minutes post-injury and then daily for the first 7 days. This dosage paradigm allowed NL-1 to be present within the mitochondria during the time points of documented mitochondrial dysfunction. However, we speculate that NL-1 may have off-target effects, similar to those observed with rosiglitazone (block of KATP and cardiotoxicity) (Varga et al., 2015), and at 20 mg/kg, we encountered toxicity in the NL-1 treated mice so we then decreased the dosage of NL-1 to 10mg/kg for our future studies. After determining this optimal dosage of NL-1, which improved TBI-induced mitochondrial dysfunction (10 mg/kg NL-1 effect size = 7.43) and did not result in any toxicity issues, we began long-term neuroprotection studies. WT or mitoNEET null mice received a severe CCI injury and an IP injection of either 10mg/kg NL-1 or vehicle at 15 minutes and then daily for the first 7 days post-injury. During this 7 day timeline, animals were scored according to the aNSS using the beam walk test. With the model of injury used, animals experience damage to regions of the motor cortex leading to motor deficits. To test these deficits, animals were trained before their TBI surgery to traverse 5 varying sized beams. Foot faults on the beam were recorded at 1 hour, 1 day, 3 days and 5 days post-injury. Injections on behavioral test days were administered following the behavioral tests in order to avoid any confounding stresses. The WT mice treated with NL-1 had a significant increase in functional recovery at day 5 compared to vehicle treated. In contrast, no significant effect on functional recovery was demonstrated in mitoNEET null mice that received NL-1 compared to vehicle treated animals (Figure 5A and 5B). At 7 days post-injury, animals were euthanized and tissue sparing was calculated. As seen in Figure 5C and 5D, NL-1 administration following a severe injury significantly increases (p=0.042, n=15 to 16/group) cortical tissue sparing (~37% reduction in lesion volume) compared to vehicle treated animals. There is no neuroprotective effect of NL-1 in mitoNEET null mice. The neuroprotective effects seen with NL-1 also correlated with increased functional recovery following injury.
Figure 5: NL-1 produces significant functional recovery and neuroprotection, an effect lost in mitoNEET null mice.
Adult wild-type and mitoNEET null mice were trained on the beam walk test, the main component used for adjusted neurological severity score (aNSS), and then received a severe CCI injury 24 hours after training. Mice were given serial IP injections of NL-1 (10 mg/kg) at 15 mins and then every 24 hrs thereafter for the first 7 days post-injury. At 1 hr, 1 day, 3 days and 5 days post-injury, the beam walk test was performed and foot faults, inversions and ability to traverse the beams were measured and used to calculate aNSS (see Methods). (A) Wild-type mice treated with NL-1 had significantly improved performance at 5 days (120 h) post-injury compared to vehicle-treated mice. (B) There were no significant differences between NL-1 treatment and vehicle treatment in mitoNEET null mice. n=15–16/group, mean ± SEM, Two-way ANOVA, Compared Drug Treated to Vehicle within same genotype, Sidak Post-Hoc, F4,120 = 1.96, * p=60.025 (5 days post-injury) for wild-type animals; F4,116 = 0.48 for mitoNEET null animals. (C) Wild-type mice administered NL-1 (10 mg/kg) had a significant increase in cortical sparing (37%) compared to vehicle-treated mice at 7 days post-injury, an effect that was lost in the mitoNEET null animals. (D) Representative images of NL-1 mediated cortical sparing are provided. n=15–16/group, mean ± SEM, One-way ANOVA, Compared Drug Treated to Vehicle within same genotype, Dunnet Post-Hoc, F3,62 = 2.91, * p=0.042.
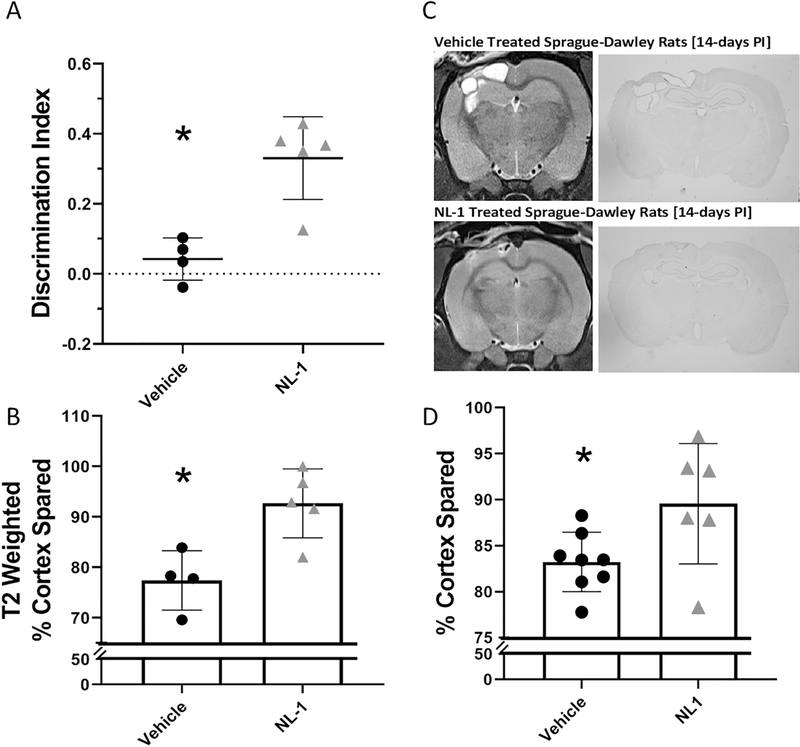
In a follow-up study, we then assessed the effects of NL-1 when introduced continuously for 7 days following a moderate CCI injury. Following injury, animals received an injection of NL-1 at 10mg/kg at 15minutes post-injury and then had an osmotic pump inserted to introduce 3ug/μl of NL-1 continuously at a rate of 1.0 μL an hour for 7 days. Chronic cognitive function, which is also a feature of this model of TBI, was measured using a Novel Object Recognition (NOR) Paradigm. At six weeks post-injury, animals (n=4–5/group) that received NL-1 or vehicle were tested. Figure 6A illustrates that specific mitoNEET-targeting drug NL-1 can improve the discrimination factor during the NOR Test at 6 weeks post-injury in TBI in mice. These animals also had T2 weighted MRI scans completed after their behavior testing in order to determine the degree of damage within the tissue. As opposed to early time points after TBI, the 6 week post-injury time point showed less edema and swelling, which allowed for a more discrete T2-signal for quantification. As seen in Figure 6B, NL-1 provided long-term T2-weighted cortical sparing compared to vehicle treated animals.
Figure 6: NL-1 administration following TBI is neuroprotective chronically across species.
(A) Wild-type mice treated with NL-1 continuously (one injection at 15 mins post-injury followed by release of 3ug/μl at a rate of 1.0 μL/hour by osmotic pump) for 1 week following a moderate CCI injury had improved cognitive performance measured with the Novel Object Recognition task compared to vehicle treated animals when tested at 6 weeks post-injury. Within this test, the amount of time that each animal spent investigating a novel object compared to a familiar object within a 5-minute period was measured. Discrimination index is calculated by subtracting the time the animal spent investigating the familiar object from the time that the animal spent investigating the novel object divided by the total time of object investigation. NL-1 treated mice spent significantly more time investigating the novel object compared to vehicle-treated mice. N=4–5/group, mean ± SEM, Unpaired two-tailed t-test, Compared Vehicle to NL-1 Treated, t = 4.40, * p = 0.0032. (B) Mice who showed improvements in memory/cognition also showed improved cortical sparing which was measured by T2-weighted MRI at 6 weeks post-injury. n=4–5/group, mean ± SEM, Unpaired two-tailed t-test, Compared Vehicle to NL-1 Treated. t = 3.54, * p = 0.0095. (C) In a separate experiment, Sprague Dawley rats were given a moderate CCI and administered 10mg/kg of NL-1 via IP injection at 15minutes, 6hrs and then daily for 7 days post-injury. At 14 days after injury, rats were scanned using T2-weighted MRI. Representative images of MRI scans and brain morphology demonstrating cortical sparing provided by NL-1. (D) T2-weighted scans were quantified and cortical sparing was measured. NL-1 administration provides significantly increased levels of cortical sparing compared to vehicle-treated rats. n=6–8/group, mean ± SEM, Unpaired two-tailed t-test, Compared Vehicle to NL-1 Treated, t = 2.40, * p = 0.034.
We then questioned whether the efficacy of NL-1 transcended beyond mice into rats. To test this, we assessed NL-1 in adult Sprague-Dawley rats (n=6–8/group) that were injured (severe CCI). Following injury, these animals were treated with either vehicle or NL-1 at 10mg/kg given via IP injection at 15minutes, 6hrs and then daily for 7 days post-injury. MRI scans were completed 14 days post-injury and animals were immediately euthanized for histological analysis. As illustrated in Figure 6, NL-1 administration significantly increases cortical tissue sparing (38% lesion reduction) after TBI proving that the neuroprotection observed is not limited only to mice.
Discussion
Mitochondrial dysfunction is a hallmark feature within the secondary injury cascade in models of rodent TBI. Although there are several decades of research in this topic, it was only recently that a careful time course study was completed in order to give an exact temporal profile of this dysfunction. These studies have shown that mitochondrial failure within the ipsilateral cortex is significant at 3 hours post-injury, peaks at 12 hours post-injury and persists out to 72 hours post-injury (Singh et al., 2006). Other studies have also shown that when looking more focally to the lesion, within the penumbra and injury core of the cortical tissue, the loss in mitochondrial bioenergetics occurs much more rapidly showing significance at 1 hour post-injury and persisting till at least 72 hours post-injury (Gilmer et al., 2009b; Hill et al., 2017; Kulbe et al., 2017; Pandya et al., 2007; Pandya et al., 2009).
In addition to the temporal profile, emerging questions have risen as to the mechanism of this dysfunction. Research has shown that mitochondrial calcium load is able to initiate mPTP formation and that intra-mitochondrial calcium load increases within the same timeframe as a loss of bioenergetics (Pandya et al., 2009). Both loss in mitochondrial bioenergetics and increased calcium load are amenable to treatment with mitochondrial uncoupling agents when given at a delayed period post-injury having a therapeutic window of opportunity showing effects out to 24 hours post-injury (Hubbard et al., 2018; Pandya et al., 2009). In the current study, we demonstrate that Pioglitazone can overcome Ca2+-induced mitochondrial dysfunction in a cell-free system (Figure 1). Similarly aligned and shown within Figure 2, Pioglitazone can restore mitochondrial dysfunction when administered at 12 hours after a TBI. Together, these findings may indicate that TBI-induced mitochondrial dysfunction is reversible and not only due to direct oxidative damage to mitochondrial enzymes as previously hypothesized. Additionally, these effects were not present in mitoNEET null mice indicating a pivotal role for mitoNEET in mediating mitochondrial respiration following Ca2+ insult and TBI.
This hypothesis is further supported by previous work demonstrating therapeutic windows of 15 minutes to 24 hours post-injury for other mitochondrial-targeted therapeutics (Davis et al., 2008; Pandya et al., 2007; Pandya et al., 2009; Sauerbeck et al., 2011b). In contrast, mitochondrial oxidative damage has been documented to peak at 3 hours within the ipsilateral cortex (Opii et al., 2007). From the literature, we see that calcium load increases following TBI and that calcium burden can inhibit mitochondrial respiration without altering ROS production and increasing markers of oxidative damage (Pandya et al., 2013; Pandya et al., 2007). All in all, it appears that oxidative damage to mitochondrial proteins, lipids, RNA and DNA may not be the primary cause of TBI-induced mitochondrial dysfunction, at least within the first hours post-injury, which has been previously hypothesized.
Metabolic dysfunction is a finding not limited to preclinical studies of TBI but is also apparent in clinical reports. FDG-PET studies in humans who have sustained a TBI have demonstrated an acute period of hyperglycolysis followed by an extended period of hypoglycemia (Bergsneider et al., 1997; Glenn et al., 2015). This effect is similar to what has been seen within previous calcium studies in animal models, where increasing calcium load leads to an initial increase in respiration followed by a decrease in respiration (Pandya et al., 2013).
The exact mechanism of Pioglitazone-mediated neuroprotection has been highly debated in the literature. Historically, the effect of Pioglitazone has been thought to be mediated thorough interactions with PPAR-γ and subsequent alterations in inflammation. However, Pioglitazone is able to retain its therapeutic effects in the presence of PPAR inhibitors (Sauerbeck et al., 2011a; Thal et al., 2011). Additionally, researchers have found that Pioglitazone improves mitochondrial bioenergetics in vivo and that this improvement is also seen when used ex vivo, where contributions from the cytosol or nucleus are removed. Therefore, with this study, we attempted to understand the mechanism of Pioglitazone neuroprotection as it relates to a known mitochondrial target of Pioglitazone, mitoNEET, in the context of neuronal injury.
Interestedly, under varying conditions, Pioglitazone appears to increase and decrease mitochondrial bioenergetics depending on the concentrations used. In previous studies, mitochondrial bioenergetics were decreased in permeabilized cells when incubated with micromolar concentrations of Pioglitazone, by inhibiting the mitochondrial pyruvate carrier (Divakaruni et al., 2013; Shannon et al., 2017). However, research from Sauerbeck et. al. (Sauerbeck et al., 2011a) found that Pioglitazone can increase mitochondrial bioenergetics in isolated mitochondria from animals administered Pioglitazone following TBI. Results from this study provide possible insight into where this discrepancy may arise in that Pioglitazone has a biphasic effect that increases mitochondrial bioenergetics at low concentrations but can decrease bioenergetics by limiting substrates to mitochondria when concentrations become too high.
In the literature, Pioglitazone is described as a PPAR agonist that provides neuroprotection through changes in gene expression of a plethora of important proteins such a cytokines and various endogenous antioxidants. These changes are extremely important when treating TBI and should not be disregarded. However, improvements of mitochondrial dysfunction that have been seen following an injury to the brain occur on a timescale much more rapid than the effects seen with changes in gene expression (Sauerbeck et al., 2011a). As seen with the previous data, animals treated with Pioglitazone at 12 hours post-injury and killed 1 hour later had increased mitochondrial function compared to vehicle treated animals. Since the drug was introduced only one hour before the mitochondria were isolated, the effects are thought to be based on direct interactions and not through changes in gene expression. Additionally, these data may indicate that Pioglitazone may have an extended therapeutic window of opportunity to target mitochondrial dysfunction following TBI and ongoing work in our laboratory is addressing this question.
This observation raises the question, if Pioglitazone is not providing neuroprotection through changes in gene expression, then what is Pioglitazone interacting with directly? Insight into this question may have been proposed by Colca et al and Bieganski et. al. who both concluded that Pioglitazone can bind to mitoNEET, a novel mitochondrial membrane protein that has been shown to alter mitochondrial bioenergetics (Bieganski and Yarmush, 2011; Colca et al., 2004). Corroborating these findings, Kusminksi et al. (Kusminski et al., 2012) showed that reducing mitoNEET expression in vitro leads to increased mitochondrial respiration, demonstrating that mitoNEET can directly alter mitochondrial function. Additionally, a recent study by Zuris et al. showed that Pioglitazone’s interactions with mitoNEET are able to inhibit [2Fe-2S] cluster transfer upon binding however the role of such a mechanism in providing therapy in CNS injury is unknown (Zuris et al., 2011). Tamir et. al (Tamir et al., 2015) has also proposed mitoNEET to be a sensor for oxidative stress.
From the current data, we see that Pioglitazone is able to increase mitochondrial bioenergetics by over 54% in wild-type mice who express mitoNEET in their mitochondria compared to vehicle treated, but it has no effect in mitoNEET null mice. To further support this, wild-type mice that were injured with a moderate-severe CCI injury and administered Pioglitazone had a significant (~65% compared to vehicle) reduction in lesion volume. This was not observed in the mitoNEET null mice who had no significant changes in lesion volume compared to vehicle-treated mice. This provides evidence that mitoNEET is crucial for Pioglitazone-mediated neuroprotection.
To further test that hypothesis that interactions with mitoNEET lead to increased mitochondrial bioenergetics, NL-1 was synthesized as a truncated glitazone, retaining a structure similar to Pioglitazone, which allows it to bind mitoNEET but not interact with the PPAR isoforms. This drug was not only found to provide a significant increase in cortical sparing following a severe TBI but was able to improve motor and cognitive function in wild-type mice, which was not seen in mitoNEET null mice. Furthermore, NL-1 was also able to increase cortical tissue sparing (38% decrease in lesion) in rats following a severe TBI, showing cross species efficacy.
In conclusion, the data provided from this study provides strong evidence that Pioglitazone-mediated neuroprotection following a contusion injury in rodent models, including increasing tissue sparing and improving functional outcomes, is dependent on interactions with mitoNEET. It also appears that mitoNEET plays a pivotal role in mediating Ca2+ and TBI-induced mitochondrial dysfunction. This data also supports mitoNEET as a potential therapeutic target for TBI as well as other disease states where mitochondrial dysfunction is prevalent.
Acknowledgements
We acknowledge David Powell, Ph.D. at the Magnetic Resonance Imaging and Spectroscopy Center (MRISC), which is a service and consultation center supporting basic and clinical research at the University of Kentucky, for assistance in MRI scanning.
Funding
This work was supported by the Kentucky Spinal Cord and Head Injury Research Trust #15-14A (PGS), National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000117). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH CTSA UL1TR000117 (PGS). This work was supported in part by a Merit Review Award # I01BX003405 to (PGS) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The work was also supported by F31NS086395 through the NIH (HMY) and by the National Institute of General Medical Sciences, U54GM104942 and Stroke CoBRE P20 GM109098 (WJG). The work was also supported, in part, by grant 1 S10 OD023573-01 (MRISC) used to purchase 7T small animal scanner.
Footnotes
Author Disclosure Statement
The authors involved within the preparation of this manuscript certify that no competing financial interests exist.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, Mcarthur DL, Caron MJ, Kraus JF, Becker DP, Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. Journal of neurosurgery, 86 (1997), pp. 241–251 [DOI] [PubMed] [Google Scholar]
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C, Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neuroscience letters, 388 (2005), pp. 7–12 [DOI] [PubMed] [Google Scholar]
- Bieganski RM, Yarmush ML, Novel ligands that target the mitochondrial membrane protein mitoneet. J Mol Graph Model, 29 (2011), pp. 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cdc, Report to congress on traumatic brain injury in the united states: Epidemiology and rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention, Atlanta, GA: (2015), pp. [Google Scholar]
- Colca JR, Mcdonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR, Identification of a novel mitochondrial protein (“mitoneet”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab, 286 (2004), pp. E252–260 [DOI] [PubMed] [Google Scholar]
- Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG, Fasting is neuroprotective following traumatic brain injury. J Neurosci Res, 86 (2008), pp. 1812–1822 [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, Mcdonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN, Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America, 110 (2013), pp. 5422–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldenhuys WJ, Funk MO, Barnes KF, Carroll RT, Structure-based design of a thiazolidinedione which targets the mitochondrial protein mitoneet. Bioorg Med Chem Lett, 20 (2010), pp. 819–823 [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Leeper TC, Carroll RT, Mitoneet as a novel drug target for mitochondrial dysfunction. Drug Discov Today, 19 (2014), pp. 1601–1606 [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Yonutas HM, Morris DL, Sullivan PG, Darvesh AS, Leeper TC, Identification of small molecules that bind to the mitochondrial protein mitoneet. Bioorg Med Chem Lett, 26 (2016), pp. 5350–5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Joy K, Sullivan PG, Scheff SW, Early mitochondrial dysfunction after cortical contusion injury. Journal of neurotrauma, 26 (2009a), pp. 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Sullivan PG, Miller K, Scheff S, Early mitochondrial dysfunction following cortical contusion injury. Journal of neurotrauma, (2009b), pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TC, Martin NA, Mcarthur DL, Hovda DA, Vespa P, Johnson ML, Horning MA, Brooks GA, Endogenous nutritive support after traumatic brain injury: Peripheral lactate production for glucose supply via gluconeogenesis. Journal of neurotrauma, 32 (2015), pp. 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RL, Singh IN, Wang JA, Hall ED, Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochemistry international, (2017), pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WB, Harwood CL, Geisler JG, Vekaria HJ, Sullivan PG, Mitochondrial uncoupling prodrug improves tissue sparing, cognitive outcome, and mitochondrial bioenergetics after traumatic brain injury in male mice. J Neurosci Res, 96 (2018), pp. 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WB, Joseph B, Spry M, Vekaria HJ, Saatman KE, Sullivan PG, Acute mitochondrial impairment underlies prolonged cellular dysfunction after repeated mild traumatic brain injuries. Journal of neurotrauma, 36 (2019), pp. 1252–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J, Rosiglitazone, a ppar gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain research, 1215 (2008), pp. 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, Fsl. Neuroimage, 62 (2012), pp. 782–790 [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R, Mechanisms of anti-inflammatory and neuroprotective actions of ppar-gamma agonists. Frontiers in bioscience : a journal and virtual library, 13 (2008), pp. 1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbe JR, Hill RL, Singh IN, Wang JA, Hall ED, Synaptic mitochondria sustain more damage than non-synaptic mitochondria after traumatic brain injury and are protected by cyclosporine a. Journal of neurotrauma, 34 (2017), pp. 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, Mcclain DA, Li C, Scherer PE, Mitoneet-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med, 18 (2012), pp. 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SJ, Yin L, Geldenhuys WJ, Enrick MK, Stevanov KM, Carroll RT, Ohanyan VA, Kolz CL, Chilian WM, Novel thiazolidinedione mitoneet ligand-1 acutely improves cardiac stem cell survival under oxidative stress. Basic research in cardiology, 110 (2015), pp. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire JL, Correll EA, Lowery AC, Rhame K, Anwar FN, Mccullumsmith RE, Ngwenya LB, Pioglitazone improves working memory performance when administered in chronic tbi. Neurobiol Dis, 132 (2019), pp. 104611. [DOI] [PubMed] [Google Scholar]
- Montcalm-Smith E, Caviness J, Chen Y, Mccarron RM, Stress biomarkers in a rat model of decompression sickness. Aviation, space, and environmental medicine, 78 (2007), pp. 87–93 [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA, Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. Journal of neurotrauma, 24 (2007), pp. 772–789 [DOI] [PubMed] [Google Scholar]
- Pandya JD, Nukala VN, Sullivan PG, Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Frontiers in neuroenergetics, 5 (2013), pp. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG, Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. Journal of neurotrauma, 24 (2007), pp. 798–811 [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Sullivan PG, The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler fccp. Experimental neurology, 218 (2009), pp. 381–389 [DOI] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG, N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following tbi. Experimental neurology, 257 (2014), pp. 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Sullivan PG, Leung LY, Tortella FC, Shear DA, Deng-Bryant Y, Advanced and high-throughput method for mitochondrial bioenergetics evaluation in neurotrauma. Methods in molecular biology, 1462 (2016), pp. 597–610 [DOI] [PubMed] [Google Scholar]
- Panzer U, Schneider A, Guan Y, Reinking R, Zahner G, Harendza S, Wolf G, Thaiss F, Stahl RA, Effects of different ppargamma-agonists on mcp-1 expression and monocyte recruitment in experimental glomerulonephritis. Kidney international, 62 (2002), pp. 455–464 [DOI] [PubMed] [Google Scholar]
- Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG, Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Experimental neurology, 293 (2017), pp. 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Smith TD, Vanrooyen JL, Powell D, Cox DH, Sullivan PG, Rabchevsky AG, Serial diffusion tensor imaging in vivo predicts long-term functional recovery and histopathology in rats following different severities of spinal cord injury. Journal of neurotrauma, 33 (2016), pp. 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedada KK, Zhou X, Jogiraju H, Carroll RT, Geldenhuys WJ, Lin L, Anderson DJ, A quantitative lc-ms/ms method for determination of thiazolidinedione mitoneet ligand nl-1 in mouse serum suitable for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci, 945–946 (2014), pp. 141–146 [DOI] [PubMed] [Google Scholar]
- Prins ML, Matsumoto JH, The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. J Lipid Res, 55 (2014), pp. 2450–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Patel SP, Sullivan PG, Targeting mitoneet with pioglitazone for therapeutic neuroprotection after spinal cord injury. Neural Regen Res, 12 (2017), pp. 1807–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Pandya JD, Mcewen ML, Pauly JR, Springer JE, Sullivan PG, Post-injury administration of the mitochondrial permeability transition pore inhibitor, nim811, is neuroprotective and improves cognition after traumatic brain injury in rats. Journal of neurotrauma, 28 (2011), pp. 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG, Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Experimental neurology, 227 (2011a), pp. 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbeck A, Pandya J, Singh I, Bittman K, Readnower R, Bing G, Sullivan P, Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J Neurosci Methods, 198 (2011b), pp. 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG, Cyclosporin a significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. Journal of neurotrauma, 16 (1999), pp. 783–792 [DOI] [PubMed] [Google Scholar]
- Shannon CE, Daniele G, Galindo C, Abdul-Ghani MA, Defronzo RA, Norton L, Pioglitazone inhibits mitochondrial pyruvate metabolism and glucose production in hepatocytes. FEBS J, 284 (2017), pp. 451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED, Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J Cereb Blood Flow Metab, 26 (2006), pp. 1407–1418 [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW, Exacerbation of damage and altered nf-kappab activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci, 19 (1999), pp. 6248–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW, Dietary supplement creatine protects against traumatic brain injury. Ann Neurol, 48 (2000), pp. 723–729 [PubMed] [Google Scholar]
- Sullivan PG, Sebastian AH, Hall ED, Therapeutic window analysis of the neuroprotective effects of cyclosporine a after traumatic brain injury. Journal of neurotrauma, 28 (2011), pp. 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir S, Paddock ML, Darash-Yahana-Baram M, Holt SH, Sohn YS, Agranat L, Michaeli D, Stofleth JT, Lipper CH, Morcos F, Cabantchik IZ, Onuchic JN, Jennings PA, Mittler R, Nechushtai R, Structure-function analysis of neet proteins uncovers their role as key regulators of iron and ros homeostasis in health and disease. Biochim Biophys Acta, 1853 (2015), pp. 1294–1315 [DOI] [PubMed] [Google Scholar]
- Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K, Pioglitazone reduces secondary brain damage after experimental brain trauma by ppar-gamma-independent mechanisms. Journal of neurotrauma, 28 (2011), pp. 983–993 [DOI] [PubMed] [Google Scholar]
- Varga ZV, Ferdinandy P, Liaudet L, Pacher P, Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol, 309 (2015), pp. H1453–H1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay A, Marchetti A, Sabra A, Jauslin TN, Rosselin M, Scherer PE, Demaurex N, Orci L, Cosson P, Mitoneet-dependent formation of intermitochondrial junctions. Proceedings of the National Academy of Sciences of the United States of America, 114 (2017), pp. 8277–8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Landry AP, Ding H, The mitochondrial outer membrane protein mitoneet is a redox enzyme catalyzing electron transfer from fmnh2 to oxygen or ubiquinone. J Biol Chem, 292 (2017), pp. 10061–10067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley SE, Murphy AN, Ross SA, Van Der Geer P, Dixon JE, Mitoneet is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proceedings of the National Academy of Sciences of the United States of America, 104 (2007), pp. 5318–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Friedrich GA, Buxton EC, Lilleberg SL, Person C, Sands AT, Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature, 392 (1998), pp. 608–611 [DOI] [PubMed] [Google Scholar]
- Zuris JA, Harir Y, Conlan AR, Shvartsman M, Michaeli D, Tamir S, Paddock ML, Onuchic JN, Mittler R, Cabantchik ZI, Jennings PA, Nechushtai R, Facile transfer of [2fe-2s] clusters from the diabetes drug target mitoneet to an apo-acceptor protein. Proceedings of the National Academy of Sciences of the United States of America, 108 (2011), pp. 13047–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]