Abstract
In humans and other primates, 1,25(OH)2vitamin D3 regulates the expression of the cathelicidin antimicrobial peptide (CAMP) gene via toll-like receptor (TLR) signaling that activates the vitamin D pathway. Mice and other mammals lack the vitamin D response element (VDRE) in their CAMP promoters. To elucidate the biological importance of this pathway, we generated transgenic mice that carry a genomic DNA fragment encompassing the entire human CAMP gene and crossed them with Camp knockout (KO) mice. We observed expression of the human transgene in various tissues and innate immune cells. However, in mouse CAMP transgenic macrophages, TLR activation in the presence of 25(OH)D3 did not induce expression of either CAMP or CYP27B1 as would normally occur in human macrophages, reinforcing important species differences in the actions of vitamin D. Transgenic mice did show increased resistance to colonization by Salmonella typhimurium in the gut. Furthermore, the human CAMP gene restored wound healing in the skin of Camp KO mice. Topical application of 1,25(OH)2vitamin D3 to the skin of CAMP transgenic mice induced CAMP expression and increased killing of Staphylococcus aureus in a wound infection model. Our model can help elucidate the biological importance of the vitamin D-cathelicidin pathway in both pathogenic and non-pathogenic states.
Keywords: cathelicidin, macrophage, innate immunity, vitamin D, TLR, Cyp27b1
1. INTRODUCTION
Vitamin D regulates expression of the cathelicidin antimicrobial peptide (CAMP) gene in both immune and epithelial barrier cells (1–3). We demonstrated conservation of regulation of CAMP by vitamin D in humans and non-human primates, but not other mammals including mice because their cathelicidin genes lacked a vitamin D receptor response element (VDRE) in their promoters (2, 4). The VDRE is located in a primate-specific AluSx short interspersed nuclear element (SINE) (2, 4).
Cathelicidins are synthesized as a prepropeptide consisting of an N-terminal signal peptide, a conserved prosequence (cathelin domain) and a highly variable C-terminal cationic AMP (5). In neutrophils, proteolytic cleavage generates the mature AMP called LL-37 (6, 7). While some mammals express numerous cathelicidins, humans and mice possess a single cathelicidin gene called CAMP or Camp, respectively (8, 9). The protein hCAP18 in humans and CRAMP in mice is synthesized and secreted in significant amounts by those tissues exposed to environmental microbes (10) as well as other tissues (11–14) and fluids (11, 12, 15–17) and is expressed by a wide array of immune cells (18–20). Subsequently, investigators demonstrated in vitro that TLR2/1-signaling by a synthetic 19-kD Mycobacterium tuberculosis–derived lipopeptide enhances the antimicrobial capacity of human monocytes via a vitamin D3-dependent pathway through induction of the CAMP gene and its protein (21–23). Other lines of evidence similarly indicate that hCAP18/CRAMP has important functions in host defense and wound healing in the skin (24–26). Mice deficient in CRAMP are more susceptible to skin infection than WT mice (27), and bacteria induce hCAP18/CRAMP secretion that protects the murine urinary tract against invasive bacterial infection (28). Decreased expression of CAMP in particular human diseases is linked to enhanced susceptibility to infection (29–33). Collectively, these data strongly implicate a key role for CAMP in maintaining adequate host defenses.
In the current model, when a human macrophage or epithelial barrier cell detects a pathogen via TLRs, the signal induces expression of the 1-α-hydroxylase (CYP27B1) gene. This increases the 1-α-hydroxylation of 25(OH)D3 that is taken up from the circulation and increases the local levels of 1,25(OH)2D3 which binds to the VDR. This induces transcription of the CAMP gene, and the resulting protein participates in host defense (21, 34, 35). This mechanism could explain the ability of vitamin D to boost the innate immune response and protect against infection.
In this study, we developed a transgenic C57BL/6J mouse that carries a human genomic DNA fragment containing the CAMP gene to elucidate the biological importance of this pathway in the immune response. We subsequently crossed the transgenic with a Camp KO mouse to generate mice that possesses the human CAMP gene but lack the mouse homolog. We characterized expression of the transgene in various tissues including immune cells. Human CAMP transgenic mice showed increased resistance to colonization by Salmonella typhimurium in the intestinal tract, and the human gene also restored wound healing in the Camp KO mouse. The human CAMP gene was responsive to induction with 1,25(OH)2D3 both in vitro in bone marrow-derived macrophages and in vivo in the skin of transgenic mice. In a wound infection model, topical skin treatment of the CAMP transgenic mouse with 1,25(OH)2D3 lowered the burden of Staphylococcus aureus infection, suggesting a functional role for vitamin D induction of CAMP in skin immune defense.
Interestingly, comparing expression of the human CAMP gene in human and transgenic mouse macrophages substantiated important evolutionary differences in how mice utilize the vitamin D3 pathway in response to immune stimulation via TLR activation. Transgenic mouse macrophages did not induce CAMP following TLR stimulation in the presence of 25(OH)D3, due to a lack of Cyp27b1 expression and induction. We could bypass this problem in transgenic mouse macrophages by inducing CAMP with 1,25(OH)2D3 directly. This novel animal model for an innate immune mechanism specific to humans will help elucidate the biological importance of the vitamin D3-cathelicidin pathway in both pathogenic and non-pathogenic states when using bioactive forms of vitamin D such as 1,25(OH)2D3 and its analogs.
2. MATERIALS AND METHODS
2.1. Generation of human CAMP transgenic mice.
Transgenic mice carrying the human CAMP gene were generated by microinjection of donor eggs from the F2 of C57BL/6J x DBA/2 mice with a 6.02-kb KpnI – EcoRI restriction enzyme fragment (chr3:48221417 – 48227439, UCSC Genome Browser on Human Dec. 2013 (GRCh38/hg38) Assembly). This fragment was isolated from a human genomic λDASH library (36) and contained the gene with 2.09 kb of upstream and 2.02 kb of downstream sequence (Fig. 1). The UCLA/JCC ES Cells & Transgenic Mice Shared Resource (Los Angeles, CA) performed the microinjection of mouse eggs and obtained two founder lines. The two lines were backcrossed nine times with C57BL/6J (The Jackson Laboratory, Bar Harbor, ME). To obtain mice that lacked the murine Camp gene, the human transgenic (Tg+) mice were crossed with Camp-deficient (KO) mice on the C57BL/6J background (27). Mice were screened by standard endpoint and quantitative PCR (protocols available upon request) to identify dizygous human CAMP (Tg+/Tg+) – Camp KO/KO mice. Studies were performed with WT, Tg+/Tg+: WT/WT, Tg+/Tg+: KO/KO and KO/KO mice. In this report, we refer to the dizygous transgenic mice as Tg/WT for CAMP on the WT background, and Tg/KO for CAMP on the Camp KO background. The integration site of the transgene of the line used in this study was mapped by TLA sequencing to mouse chromosome 4: site 140,451,533–140,456,007 resulting in a 4.5kb deletion of genomic DNA at the insertion site (37) (Cergentis, Utrecht, Netherlands). No coding region was impacted directly by the insertion of about 8 Tg-Tg concatemers. All mice used in this research were bred and maintained in the same temperature (23±1°C), humidity (60–70%), and lighting (6:00 am to 6:00 pm) controlled rooms under specific pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at Oregon State University (OSU). Animals were housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the OSU Institutional Animal Care and Use Committee.
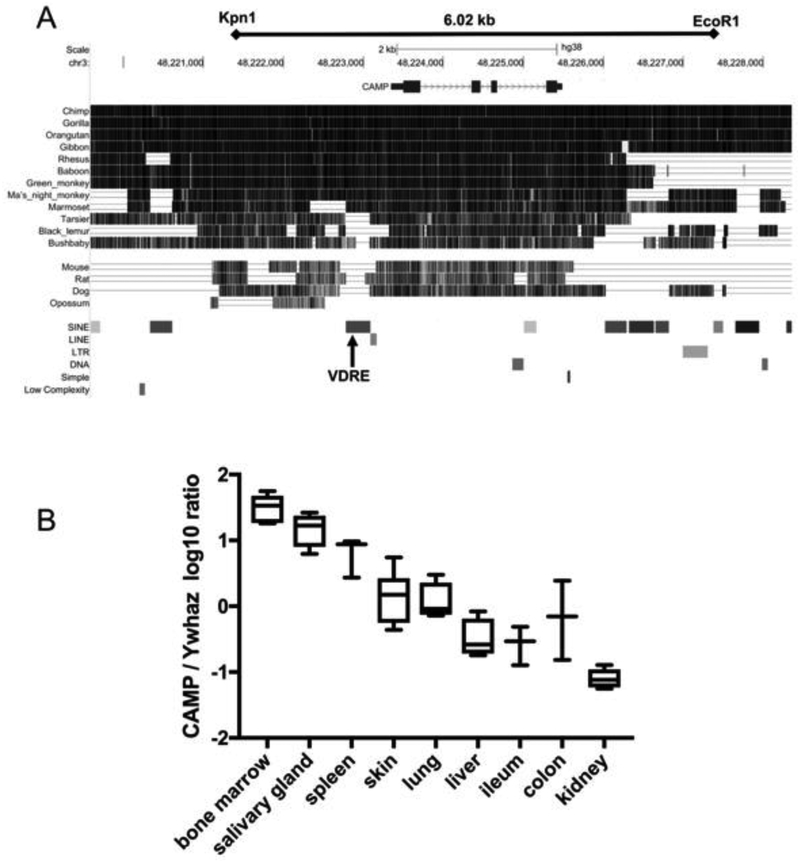
Figure 1. Generation of a CAMP transgenic mouse and expression in different tissues.
(A) This schematic generated from the UCSC Genome Browser December 2013 (GRCh38/hg38) Assembly shows that the human 6.02 kb Kpn1-EcoR1 genomic DNA fragment contains the CAMP gene located on chromosome 3 (base pairs 48,223,347 −48,225,491). It also shows the conserved 5’- and 3’-regions that possess potentially important transcriptional regulatory elements and the VDRE located in a primate-specific AluSx SINE conserved in only apes (chimp, gorilla, orangutan and gibbon), Old World primates (rhesus macaque, baboon and green monkey) and New World primates (Ma’s night monkey and marmoset), but not in prosimians (tarsier, black lemur and bushbaby) or other mammals (mouse, rat, dog and opossum). (B) The human CAMP mRNA is expressed in tissues of Tg mice. Total RNA was isolated from tissues of Tg/WT mice and expression of CAMP mRNA was measured by qRT-PCR. The ratio of CAMP to the reference gene Ywhaz was log10 transformed and graphed as the mean (+S.D.) with the minimum and maximum (Box plots, n=4–6 mice; non-box plots, n=3 mice).
2.2. Analysis of gene expression by qRT-PCR.
Murine tissues were preserved in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was isolated using Trizol (Thermo Fisher Scientific) or Direct™-zol RNA kits (Zymo Research Corp, Irvine, CA, USA) according to the manufacturer’s protocol. Tissues were homogenized using nuclease-free 1.6 mm stainless steel beads in a Bullet Blender (Next Advance, Inc., Averill Park, NY, USA). RNA (0.25–1 μg) was converted to cDNA using iScript reverse transcriptase and random hexamer primers (Bio-Rad Laboratories, Hercules, CA USA) according to the manufacturer’s recommendations. PCR reactions were performed with SsoAdvanced Universal Probes Supermix on a Bio-Rad iCycler iQ5 or CFX-96 QPCR system (Bio-Rad Laboratories). All the threshold cycle (Ct) numbers were normalized to 18S rRNA, -actin or Ywhaz. The probes and primers for the human CAMP and RN18S1 genes were described previously (38). Primers 5’-gtacatggctggggtgtt-3’ 5’-ttctacaatgagctgcgt gt-3’ and probe dCal Gold 540 5’-aggtctcaaacatgatctgggtcatct-3’ BHQ-2 for β-actin were purchased (Thermo Fisher Scientific and Biosearch Technologies, Novato, CA, respectively). Primer-probe mixes for Cyp24A1, Cyp27B1, and Ywhaz were purchased (Integrated DNA Technologies, Coralville, IA, USA).
2.3. Western Blot analysis, ELISA, immunohistochemical staining and immunofluorescence staining.
The primary rabbit polyclonal anti-hCAP18 antibody was described previously (39). Murine monoclonal anti-LL-37 antibody was a kind gift from Dr. Birgitta Agerberth (Karolinska Institute, Stockholm, Sweden). Immunofluorescent staining and flow cytometry were performed as described previously (2, 38, 40). Antibodies used against lineage markers were purchased (Thermo Fisher Scientific). The levels of hCAP18 (ng/ml) in each tissue sample were determined using a non-commercial ELISA with a detection limit of 0.084 ng/ml and an intra- and inter-assay coefficient of variation of ≤6.3% (39). Western blots were performed as described previously (2). Briefly, tissue samples were flash frozen in liquid nitrogen, and were then homogenized in ice-cold RIPA buffer containing cOmplete™ protease inhibitor cocktail (Millipore Sigma, St. Louis, MO USA) and nuclease/protease-free 2.4 mm stainless-steel beads (OMNI International, Kennesaw, GA USA) using a Precellys 24 homogenizer (3 × 30 s cycles). Immunofluorescence staining and microscopy were performed as described previously (41, 42).
2.4. Tissue culture.
For ex vivo experiments, fresh tissues were cultured in DMEM (Mediatech Inc., Manassas, VA, USA) supplemented with 10% (v/v) heat-inactivated FBS, 2 mM L-glutamine, and 1% Pen/Strep (Invitrogen Corporation, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator. Mouse BM-derived macrophages (BMDM) and human PBMC-derived macrophages cells were cultured as described previously (2, 43). Briefly, mouse bone marrow was differentiated with MCSF at 25ng/ml for 7 days, while human PBMC were cultured for 7 days with GM-CSF at 25ng/ml prior to experiments. Whole blood from healthy volunteers was collected under approved IRB guidelines. The stock solution of 1,25(OH)2D3 (1 mM) was prepared in 100% ethanol and subsequently diluted in culture media to a concentration as described in the figure legends.
2.5. Phagocytosis and Superoxide assays.
BMDM were mixed with fluorescently labeled inactivated Escherichia coli bioparticles and processed as described by the manufacturer (pHrodo E. coli, Thermo Fisher Scientific). The samples were analyzed on a BD Facscalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The pHrodo bioparticles increase fluorescence when internalized into acidic phagosomes, thus the percentage of cells internalizing particles and the mean fluorescence intensity were used as measures of phagocytosis.
BMDM or neutrophils were stimulated with PMA (3 μg/ml), incubated at 37°C with a dura-luminol substrate (Thermo Fisher Scientific, Waltham, MA, USA) and monitored for superoxide production using a 96 well plate luminometer (RLU every 2 min for 30 min).
2.6. In vivo gastrointestinal infection with Salmonella typhimurium.
S. typhimurium strain JS135, a generous gift from Dr. James Slauch (University of Illinois, Champaign Urbana, USA), was grown in Luria Bertrani (LB) broth with 25 μg/ml tetracycline overnight (44). The bacteria were pelleted, washed three times in PBS and density adjusted to an OD600 of 1.0 (1.2 ×109 CFU/ml). Infectious doses were calculated and re-suspended in a total of 0.2 ml PBS for oral gavage. Prior to infection, mice were fasted 10 to 12 hours. Post infection, food was returned, and mice monitored for symptoms. Mice were euthanized 3 and 5 days post-infection, spleens and ceca were collected, homogenized and serially diluted for bacterial CFU determination on LB agar plates containing 25 g/ml tetracycline. The inoculum used for infection was also plated to enumerate the starting CFU used for infection.
2.7. In vivo treatment of skin with vitamin D, skin wounding and LPS injection.
A dorsal patch of skin near the shoulder blades was shaved one day prior to treatment. For treatment, 1 nmole of 1,25(OH)2D3 was dissolved in vehicle consisting of a 50% glycerin/ethanol solution. Either 6 l of vehicle or 1,25(OH)2D3 was applied to the skin and spread to facilitate drying. After 24 to 48 h, mice were sacrificed and skin samples were processed for RNA or protein, respectively (42). Skin wounding and analysis of healing was performed as described previously (42). Mice were IP injected with 10 μg/g body weight LPS in PBS or either 0.5 μg/g body weight LPS or with PBS. After 3h or 24 h, mice were euthanized, and tissues were collected and processed for RNA.
2.8. Staphylococcus aureus wound infection model.
Mice were shaved on a dorsal patch of skin as in 2.6 and then a single 5mm skin punch was made using a circular biopsy punch under anesthesia. Mice were injected subcutaneously with Buprenorphine-SR for pain management post wounding (ZooPharm, Fort Collins, CO). Mice were treated 24h later with vehicle or 1,25(OH)2D3 as in part 2.6 directly onto the wounded area. The following day 8×105 CFU of S. aureus Rosenbach strain were applied in a 10μl volume to the wound, and the mice were bandaged with non-stick gauze and Tegaderm™ transparent film dressing (3M™, St. Paul, MN). Bandages were removed 24h post infection and mice were monitored daily. At 2 days post infection, the mice were treated again with either vehicle or 1,25(OH)2D3 at the wound site. At 5 days post infection, mice were euthanized and a 10mm circular biopsy of the skin was taken at the wound site. The skin samples were placed in 1ml PBS and homogenized using steel 2.4mm beads in a Precellys 24 homogenizer. The lysate was then serially diluted and plated on tryptic soy agar plates to enumerate surviving S. aureus bacteria.
2.9. Statistical analysis.
Statistical analysis was performed using GraphPad Prism version 7 software (GraphPad Software, La Jolla, CA, USA). T-test and one-way ANOVA with Tukey’s multiple comparison post-test were used for determination of significance (p<0.05).
3. RESULTS
3.1. Generation of a human CAMP transgenic mouse.
Because the human CAMP gene is regulated by vitamin D but the murine gene is not, in vivo studies with mice would have limited or uncertain relevance to the situation in humans (2, 4). To study the in vivo role of vitamin D-induced human CAMP, we generated a transgenic mouse with a 6.02 kb Kpnl-EcoRl fragment containing all four exons (~2 kb) and flanking sequence (~2 kb 5’of the first exon and ~2 kb 3’ of the last exon). The fragment was isolated from a human placental genomic DNA library (Stratagene, La Jolla, CA) and sub-cloned it into pGEM3Z (Promega Corporation, Madison, WI, USA) (Fig. 1A). This fragment contains the region of highest conservation among all mammals in the current database (Fig. 1A) including the AluSx SINE with the VDRE. The high degree of conservation in the promoter region and 3’-downstream region suggests the presence of important regulatory elements controlling the expression of the gene. Potential transcriptional regulatory elements in the CAMP transgene fragment have been described previously (2, 45–47). The human CAMP promoter (−693 to +14) contains potential binding sites for the CCAATT displacement protein (CDP), STAT3, C/EBP, PU.1, HIF-1 and VDR. DNase I hypersensitivity peak clusters are found immediately upstream of the start ATG and within the 5’-half of the AluSX SINE in the region of the VDRE (UCSC Genome Browser on Human Dec. 2013 (GRCh38/hg38) Assembly). In addition, the Genome Browser shows numerous H3K27Ac sites in the 5’-promoter region which are generally associated with increased transcriptional activation (48). Transfection of the 6.02 kb fragment into murine NIH3T3 cells and treatment with 1,25(OH)2D3 induced human CAMP gene expression from the genomic DNA as detected by qRT-PCR (data not shown). Two founder transgenic lines were subsequently backcrossed nine generations onto the C57BL6/J mouse strain and crossed with the Camp KO mouse on the same background (27). The insertion site of the human CAMP gene into the mouse genome of the line used in this study was mapped using TLA sequencing (37). A single insertion site was identified on mouse chromosome 4, position 140,451,533 to 140,456,007. The insertion of the transgene resulted in a 4.5 kb deletion of mouse genomic DNA that does not contain any known coding genes. The transgene inserted as a concatemer of eight copies per chromosome or 16 copies total in the dizygous mice. Experiments were performed with Tg+/Tg+: WT/WT mice, which we term Tg/WT mice, as well as mice with the human CAMP gene on the mouse Camp KO background (Tg+/Tg+: KO/KO), which we term Tg/KO mice.
3.2. Tissue specific expression of the human CAMP gene in transgenic mice.
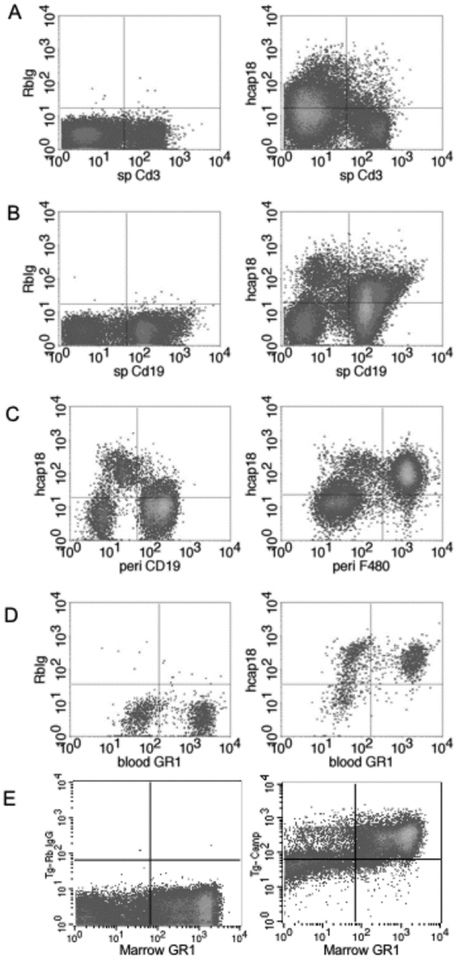
We used qRT-PCR to determine human CAMP gene expression from various tissues in the transgenic mice (Fig. 1B). As expected for hematologic tissues and immune cells, the gene was highly expressed in the BM and spleen, and in non-hematologic organs we observed the highest levels in the salivary glands, followed by skin, lung, liver, ileum, colon and kidney (Fig. 1B). An ELISA for hCAP18 protein expression in tissues and plasma showed that expression generally paralleled the levels of mRNA expression (Table 1). We detected the highest level of hCAP18 in bone marrow, followed by plasma, salivary gland, skin, spleen and lung. To characterize further the expression of hCAP18 in cells of the immune system, FACS staining was performed with splenocytes, peritoneal lavage, and blood and bone marrow cells. We detected little or no expression in CD3+ T-cells from the spleen (Fig. 2A), but significant expression in CD19+ splenic B-cells (Fig. 2B), limited expression in CD19+ positive peritoneal B-cells and robust expression in F4/80+ peritoneal macrophages (Fig. 2C). As expected, Gr-1+ neutrophils from the blood and total bone marrow expressed high levels of hCAP18 (Fig. 2D & E).
Table 1.
Expression of hCAP18 in various tissues from human CAMP Tg/KO mice
| hCAP18 (ng/mg total protein) * | +/− SD | |
|---|---|---|
| Bone Marrow | 10.25 | 1.05 |
| Plasma* | 6.87 | 0.74 |
| Salivary Gland | 4.11 | 2.76 |
| Skin | 0.95 | 0.41 |
| Spleen | 0.40 | 0.11 |
| Lung | 0.21 | 0.07 |
| Ileum | 0.05 | 0.001 |
| Colon | 0.04 | 0.01 |
| Kidney | 0.04 | 0.01 |
| Liver | 0.03 | 0.004 |
hCAP18 protein was measured by ELISA in tissues from Tg/KO transgenic mice (n=3) and expressed as ng/mg total protein or ng/ml for plasma*.
Figure 2. Tg/KO mice express hCAP18 in several lineages of immune cells.
Immune cells from three mice were pooled from spleen, peritoneal cavity, blood, and bone marrow and dual stained with lineage markers and either control rabbit IgG (left panels A, B, D, E) or anti-hCAP18 antibody (left panel C and all right panels) and analyzed by flow cytometry. (A) Splenocytes stained with the T-cell marker CD3. (B) Splenocytes stained with the B-cell marker CD19. (C) Peritoneal cells stained with CD19 (left panel) or F4/80 (right panel). (D) Blood cells stained with the granulocyte marker GR1. (E) Bone marrow cells stained with GR1. Representative plots from three different experiments are shown.
3.3. Phagocytosis and superoxide production are not altered by human CAMP in murine cells.
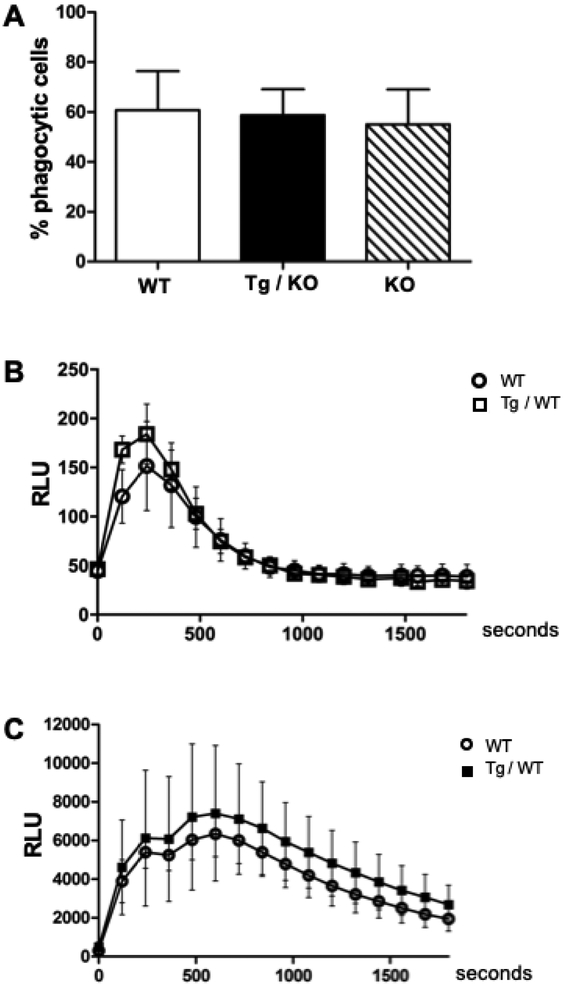
To determine if the human transgene affected phagocytic function, we tested BMDM from WT, Tg/KO and Camp KO macrophages for phagocytosis using labeled E. coli and FACS. The percentage of phagocytic macrophages detected was similar among the three genotypes with no statistically significant differences (Fig. 3A). We also tested superoxide production by WT and Tg/WT macrophages that were stimulated with PMA. We found similar production for each genotype with no statistically significant differences in macrophage superoxide production (Fig. 3B). Neutrophils produce much higher levels of superoxide than macrophages and are the most potent immune cell producers of superoxide. Exogenous LL-37 can induce superoxide production by human neutrophils (49), therefore, we tested whether the transgene in murine neutrophils altered their superoxide production. Bone marrow neutrophil superoxide production was compared between WT and Tg/WT mice, with no statistical differences noted (Fig. 3C). Thus, the human transgene does not alter superoxide production in murine neutrophils.
Figure 3. Human CAMP gene does not alter phagocytosis or superoxide production in murine phagocytes.
(A) BM-derived macrophages from WT, Tg/KO, and KO genotypes were incubated with fluorescent pHrodo E. coli for 60 min and then analyzed by flow cytometry to measure the percentage of phagocytes. Data represent mean (+SD) of four experiments. (B) BM-derived macrophages from WT and Tg/WT mice were incubated with a luminol substrate and stimulated with PMA to induce superoxide as measured by relative light unit (RLU) emission over a time course. Each time point represents the mean (±SD) of three experiments. (C) BM neutrophils from WT and Tg/WT mice were incubated as described in panel B. Each time point represents the mean (±SD) of six biological replicates from two experiments for each genotype. No significant differences were observed between the genotypes.
3.4. The human CAMP Tg decreases S. typhimurium burden in the murine cecum during infection.
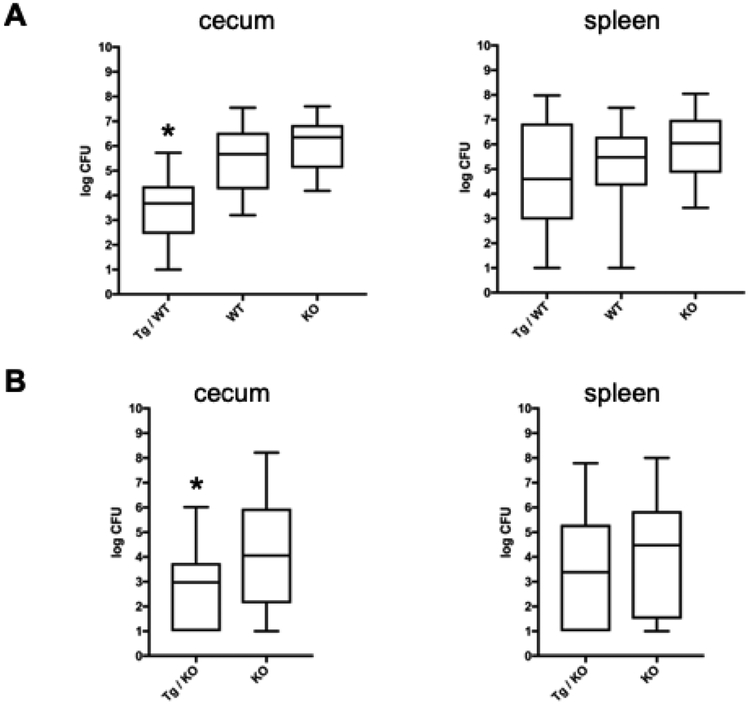
To determine the ability of the human CAMP transgene to protect against infection in the gastrointestinal tract, WT, Camp KO and CAMP Tg/WT were infected by oral gavage with 8 × 108 CFU S. typhimurium. Five days post-infection, mice were euthanized and the burden of infection was determined in both their cecum and spleen. The number of CFUs in the cecum of CAMP Tg/WT mice was significantly lower than the burden in the WT and the Camp KO mice (Fig. 4A, left panel). WT and the Camp KO mice were not significantly different, but the burden of infection in the Camp KO trended higher than in the WT (Fig. 4A, left panel). The splenic burden of infection trended downward in the CAMP Tg/WT and upward in the Camp KO as compared to the WT, but values were not statistically significant (Fig. 4A, right panel). In additional experiments comparing the Camp KO with the human CAMP Tg/KO mice, mice were infected with a 10-fold lower dose (8 × 107 CFU) of S. typhimurium and surviving CFU were measured in the cecum and spleen five days post infection. Human CAMP Tg/KO mice showed a greater resistance to infection than the Camp KO mice as reflected by the statistically significant lower burden in the cecum (Fig. 4B, left panel). The transgenic mice showed a non-significant trend toward lower splenic burden than the knockout (Fig. 4B, right panel). In summary, the CAMP transgene increases protection and lowers CFU burden in the murine cecum, but does not reduce systemic spread once S. typhimurium escapes the intestinal tract.
Figure 4. Human CAMP gene increases resistance to enteric infection in Camp Tg mice.
(A) Mice were infected by oral gavage with 8×108 S. typhimurium. Five days post infection organs were harvested and plated to measure bacterial CFU shown on a log10 scale. The Tg/WT (n=14) group of mice had a significant decrease in cecum burden compared with both the WT (n=13) and KO (n=14) groups (Asterisk indicates p value <.0001, one-way ANOVA,) (B) Tg/KO and Camp KO mice were infected with a lower dose (8×107) of S. typhimurium for 5 days. The CFU burden in Tg/KO mice (n=30) was significantly lower compared with the KO mice (n=33). The asterisk indicates a p value = 0.007 (two-tailed T-test). Data were plotted as the mean CFU (horizontal line) ±SD (box). The bars show the range of the data.
3.4. Induction of the human CAMP gene in murine cells and tissues in vitro.
CAMP expression is induced in human macrophages following treatment with 1,25(OH)2D3. To test the response in Tg/KO macrophages, BMDM were treated with increasing doses of 1,25(OH)2D3 ranging from 1 to 100 nM for 48 h. A dose-responsive increase in hCAP18 protein expression occurred as determined by FACS (Fig. 5A), showing that mouse Tg macrophages responded like human macrophages. Induction of CAMP gene expression was also observed in tissues cultured in vitro after treatment with 100 nM 1,25(OH)2D3 for 24 h (data not shown). Strong induction of CAMP was observed in the kidney with modest increases in liver, ileum, colon and spleen (data not shown). Variability in the level of induction occurred between mice, with some responding more strongly than others. Overall, the results are consistent with expression of the VDR in most tissues.
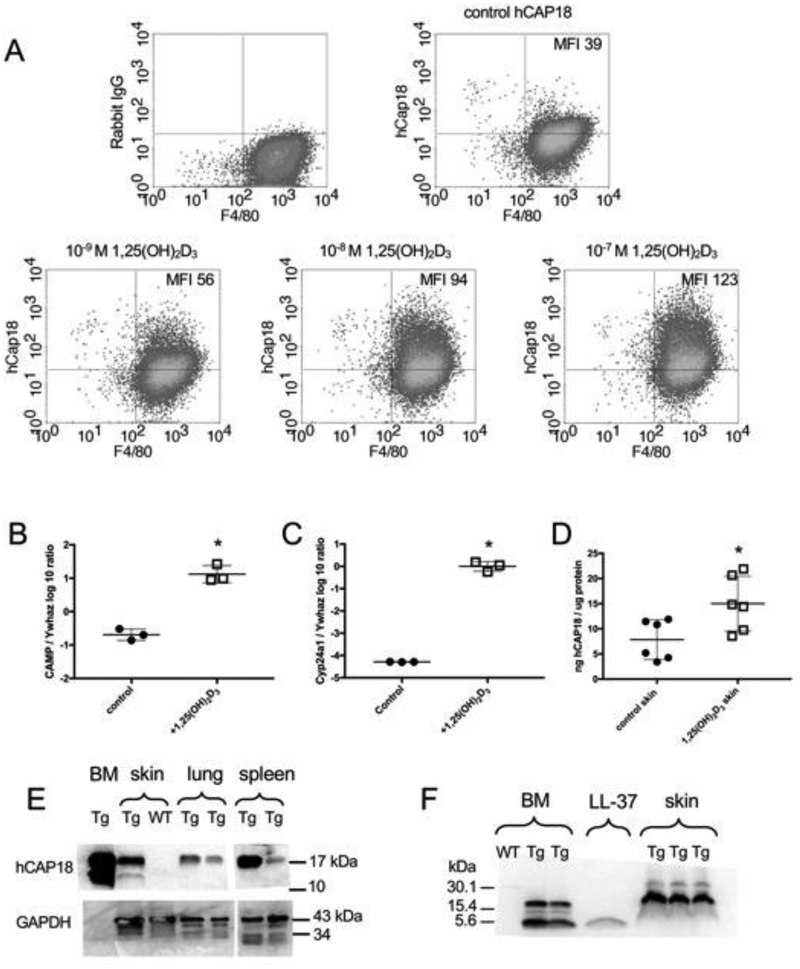
Figure 5. Induction of CAMP gene and hCAP18/LL-37 expression with 1,25(OH)2D3 treatment.
(A) BM-derived macrophages from Tg/KO mice were treated 48 h in vitro with increasing concentrations (10−9, 10−8 and 10−7 M) of 1,25(OH)2D3. hCAP18 and F4/80 staining cells (macrophages) were detected by flow cytometry. Mean fluorescent intensity (MFI) of hCAP18 is noted in the upper right quadrant. Graphs are representative of four experiments. (B-F) Dorsal skin of Tg/KO mice was treated with either vehicle control or 1 nmole 1,25(OH)2D3 for 24 h (mRNA) or 48 h (protein). Expression of CAMP (B) and Cyp24a1 (C) mRNAs (n=3 mice) determined by qRT-PCR was normalized to the housekeeping gene Ywhaz. The ratio was log10 transformed and plotted as the mean (+SD). D) hCAP18 protein levels were determined by ELISA (n=6 mice per treatment) and plotted as the mean (ng hCAP18/mg of total protein, ±SD). (E) hCAP18 expression in tissues from untreated Tg/KO and WT mice was analyzed by Western blot using 50 g protein per lane and an anti-hCAP18 polyclonal antibody. The 18-kDa pro-protein was detected in BM, skin, lung and spleen of the Tg/KO mice. The 14-kDa processed cathelin-domain was detected in the BM and skin. No hCAP18 was detected in the WT skin. GAPDH was used as a loading control, except this particular antibody does not identify GAPDH in bone marrow. (F) BM (untreated) and skin (from treated mice, panel C) samples from WT and Tg/KO mice were analyzed by Western blot with an anti-LL-37 monoclonal antibody. Synthetic LL-37 peptide (4-kDa) was included as a positive control. Both the 18- and 4-kDa forms were detected in the BM. In skin, primarily the 18-kDa was detected with very faint 4-kDa bands. Each Western blot lane represents an individual mouse. Asterisks denote statistical significance (p<0.05) determined by a two-tailed T-test.
3.5. Topical application of 1,25(OH)2D3 on skin of Tg mice induces CAMP mRNA and hCAP18 protein.
Humans and mice express cathelicidin in the skin where this protein functions in barrier defense and wound healing (24, 26). In humans, topical application of 1,25(OH)2D3 and an analog calcipotriol to the skin induces both mRNA and hCAP18 protein (3, 50). To test the induction of the CAMP transgene in the skin of Tg/KO mice, a dorsal patch of skin was shaved and either vehicle or 1 nmole 1,25(OH)2D3 was applied to the area. After 24 h, a significant increase in CAMP mRNA expression was detected by qRT-PCR in the group of mice receiving 1,25(OH)2D3 (Fig. 5B). Strong induction of another VDR target gene Cyp24a1 was also measured in the skin of the treated group (Fig. 5C). To determine the induction of hCAP18 protein, the experiment was repeated with two groups of mice and hCAP18 levels were measured by ELISA after a 48 h treatment. A significant induction of hCAP18 protein was observed in the skin of the 1,25(OH)2D3- treated mice as compared to the controls (Fig. 5D).
While the ELISA quantifies the level of hCAP18 protein expression, it does not discriminate between the various processed forms of the protein. In humans, hCAP18 is processed by proteolytic cleavage to produce a 14-kDa fragment containing the cathelin-like pro-domain and a 4-kDa C-terminal fragment known as LL-37. LL-37 possesses antimicrobial activity and promotes wound healing. We characterized processing of the hCAP18 protein into its 14-kDa and 4-kDa forms in the mouse using western blot analysis. Using a rabbit polyclonal antibody (39), the full length hCAP18 protein was detected and the processed 14-kDa form was found in protein extracts from BM and skin of Tg mice (Fig. 5E). The amount of processing was significantly less in the skin than the BM. The full-length, but not the 14-kDa form was detected in the lung and spleen of the Tg mice, suggesting minimal if any processing of the 18-kDa form (Fig. 5E). The 4-kDa LL-37 fragment was not efficiently detected by the rabbit polyclonal, so a mouse monoclonal antibody specific to LL-37 (51) was used to detect both the hCAP18 full length protein as well as a 4-kDa protein in BM cells from Tg mice that co-migrated with a synthetic LL-37 peptide (Fig. 5F). Skin samples from 1,25(OH)2D3-treated animals were included and a low abundance band was identified that co-migrated with the synthetic 4-kDa LL-37 peptide, consistent with a very low level of processing in the skin (Fig. 5F). Specificity of both antibodies was confirmed using the BM and skin of a WT mouse that were negative for both hCAP18 and LL-37 (Fig. 5E & F). These patterns of detection are consistent with previously published work from human neutrophils and semen (7, 26, 52). Our findings suggest that processing of human hCAP18 protein occurs in the mouse producing both the 14-kDa cathelin-like domain and the 4-kDa peptide in bone marrow and possibly at low levels in unperturbed skin.
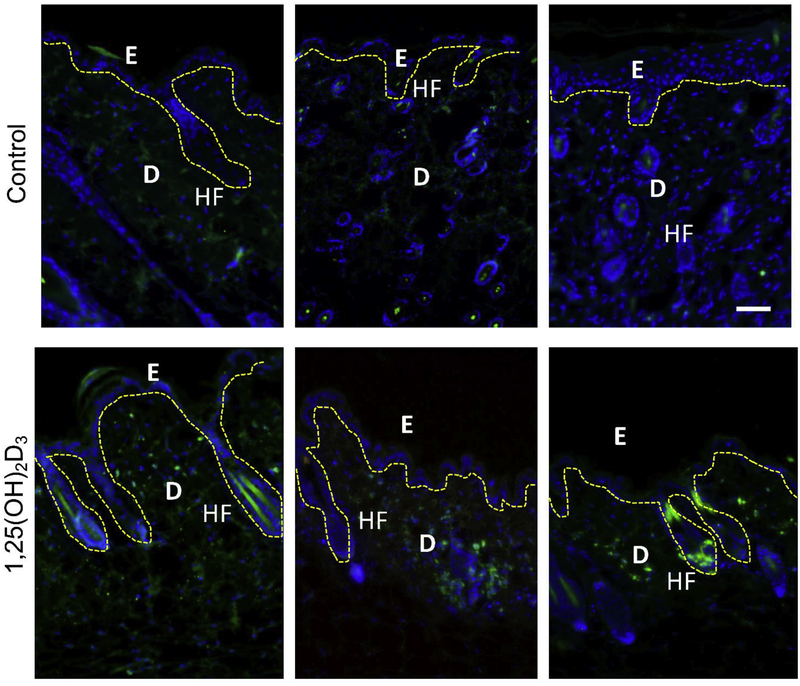
To determine expression of hCAP18 in situ in the skin, immunofluorescence microscopy was performed on skin samples from vehicle and 1,25(OH)2D3-treated mice (Fig. 6). hCAP18 staining was not found in the epidermis of vehicle-treated skin, but was noted to increase in the epidermis in 1,25(OH)2D3-treated skin (Fig. 6, upper vs. lower panels). Higher levels of hCAP18 were detected in the dermis as compared with the epidermis, and again in the 1,25(OH)2D3-treated mice an increase in the staining intensity was observed in the dermis with notable expression near the base of hair follicles (Fig. 6, lower panels).
Figure 6. Topical application of 1,25(OH)2D3 induces hCAP18 in Tg/KO mouse skin.
Tg/KO mice were topically treated for 48 h with either 50% ethanol/glycerin vehicle (control) or 1,25(OH)2D3 in vehicle. Skin samples were fixed and paraffin embedded. Sections were stained with anti-hCAP18 polyclonal antibody and then detected with a secondary antibody conjugated to Alexafluor 488 (green) and counterstained with DAPI (blue). The dashed yellow line indicates the border between the epidermis (E), dermis (D) of the skin, and hair follicles (HF), white scale bar = 50 μm. Top panels are multiple sections from vehicle control mice, while the bottom panels are multiple sections from 1,25(OH)2D3-treated mice.
3.6. Human CAMP transgene restores wound healing in Camp knockout mice.
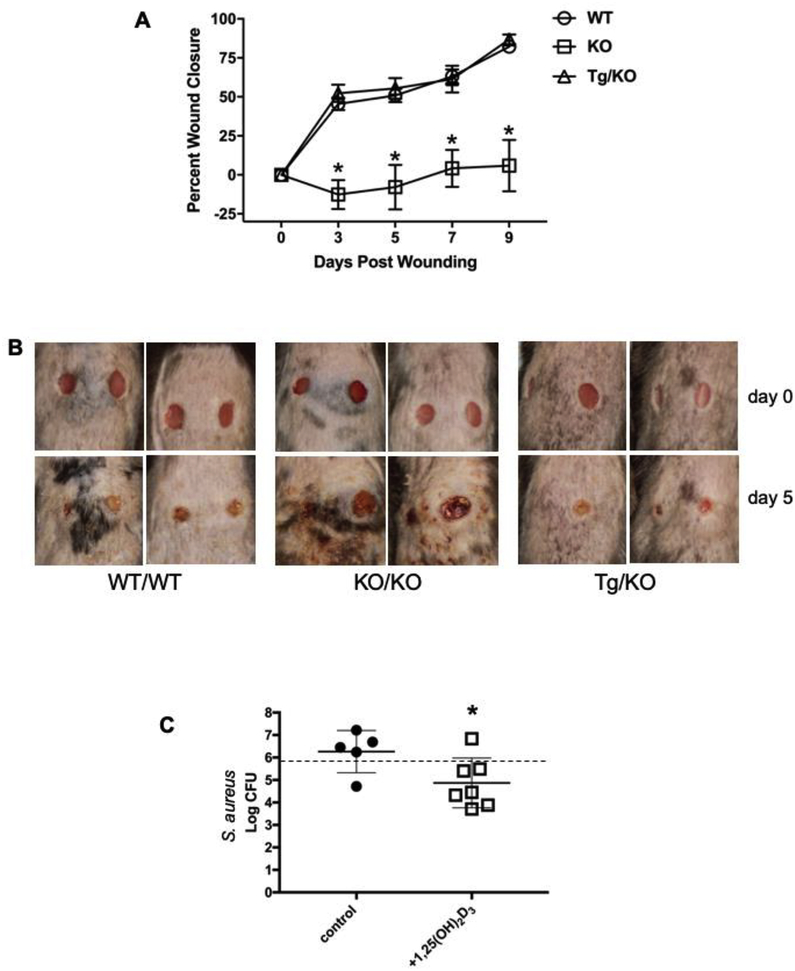
In mice, the Camp gene promotes wound healing and closure in the skin (24). Based on the expression data described above, we hypothesized that the presence of the human CAMP gene would restore defective wound healing observed in the Camp knockout mice. Following a full-thickness wound using a 5 mm skin punch in each flank of the mice, wound closure was monitored over time in Tg/KO, Camp KO and WT mice. The wounds in Camp KO mice showed minimal closure and contraction of the wound over nine days after the initial skin punch (Fig. 7A). The defect observed with the Camp KO mouse was quite striking, in that at five days post wounding no measurable signs of wound healing or closure had occurred, while the WT and Tg/KO mice showed about 50% wound closure and healing (Fig. 7A). The rate of wound closure in the Tg/KO mice was identical to that observed in WT mice throughout the nine days. The Camp KO mice showed additional tissue damage and scab formation that was not observed in the Tg/KO or WT mice (Fig. 7B). Taken together, these data demonstrate that the human CAMP gene restores the healing defect observed in the Camp KO mice.
Figure 7. The human CAMP gene restores wound healing and topical treatment with 1,25(OH)2D3 lowers S. aureus burden in Tg/KO mice.
On Day 0, horizontal dermal wounds were generated on the backs of mice using a 5 mm dermal punch (n=4 per genotype). (A) Percent wound closure was determined by measuring the wound size on days 3, 5, 7, and 9. On Day 5, both WT and Tg/KO mice showed similar wound healing and contraction rates, as measured by total wound area, while a significant lack of wound closure was observed for KO mice (Asterisk indicates p < 0.05, Student’s t-test). (B) Illustrative examples are shown for each genotype at initial wounding day 0 (top panels) and 5 days post wounding (lower panels). (C) Single 5mm dermal wounds were formed on Tg/KO mice and 1 day later mice were treated with either vehicle control (n=5) or 1,25(OH)2D3 (n=7). The following day 8×105 CFU of S. aureus were applied to the wound. At 2 days post infection, the mice were treated again with either vehicle or 1,25(OH)2D3 at the wound site. At 5 days post infection, mice were euthanized and a 10 mm circular biopsy of the wound site was used to enumerate surviving S. aureus bacteria expressed as log10 CFU. The dashed line across the graph indicates the starting infectious CFU, while the center line and brackets indicate the mean and S.D. per group of mice. (Asterisk indicates p < 0.05, two tailed t-test)
3.7. Topical application of 1,25(OH)2D3 on the skin of Tg mice increases killing of S. aureus in a wound infection model.
To test whether application of 1,25(OH)2D3 to the skin of transgenic mice could improve resistance to wound infections, we infected mice with S. aureus and compared bacterial killing in the skin between vehicle control and 1,25(OH)2D3 treated mice. Tg/KO mice were wounded using a 5mm skin punch on a single dorsal site and 24 h later, matched littermates were treated with either vehicle control or 1nmole 1,25(OH)2D3 directly on the wound site. The next day the wound was infected with 8×105 CFU of S. aureus, and then 2 days post infection the wound sites were re-treated with either vehicle or 1,25(OH)2D3. Five days post infection, a 10mm biopsy around the wound site was taken and surviving S. aureus CFU were measured (Fig 7C). In the group of mice treated with the vehicle control, the mean log CFU of S. aureus was 6.2±0.4 (1,840,000 mean CFU) while the 1,25(OH)2D3 treated group of mice had a statistically significant decrease to log 4.8±0.4 CFU (74,400 mean CFU) (Fig 7C). Therefore, in the Tg/KO mice, topical skin treatment with 1,25(OH)2D3 significantly lowered the burden of S. aureus in a wound infection model.
3.8. Absence of Cyp27b1 gene induction upon TLR activation of macrophages in mice compared to humans.
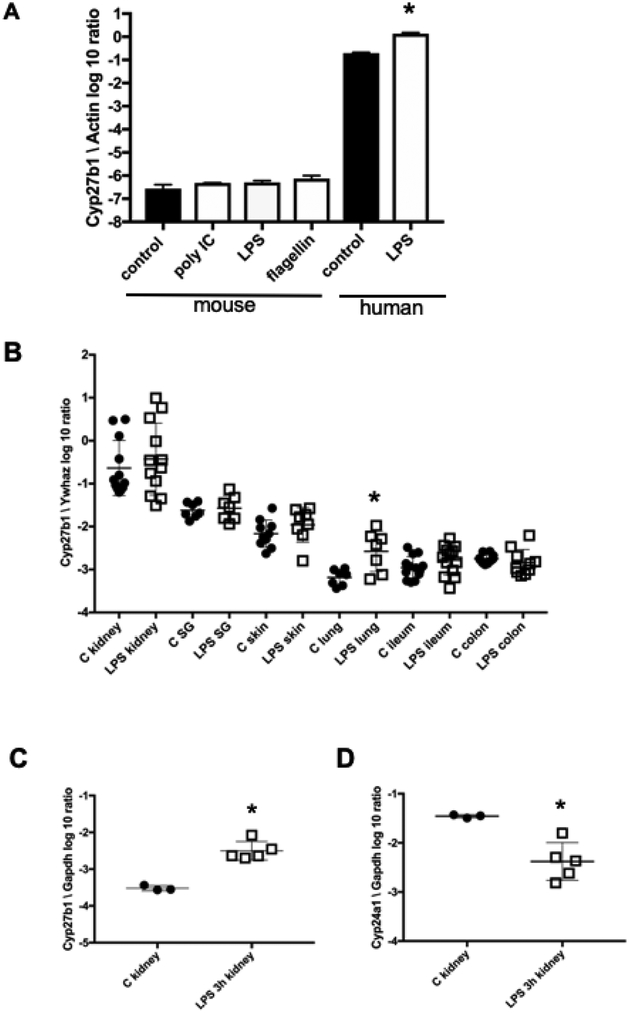
In humans, activation of macrophages with TLR ligands induces CYP27B1 expression and subsequent synthesis of active 1,25(OH)2D3 from 25(OH)D3 which then induces CAMP expression (21). We tested whether activation with TLR ligands in the presence of 25(OH)D3 would induce CAMP expression in Tg mouse bone marrow derived macrophages, but found no induction by LPS, PolyIC, and flagellin (data not shown). One explanation for the lack of CAMP induction in mouse macrophages is that TLR activation does not induce Cyp27b1 gene expression, as has been previously reported for LPS (53, 54). In BMDM from CAMP Tg/WT mice, induction of Cyp27b1 was not observed 24 h post-treatment with either PolyIC, LPS, or flagellin (Fig. 8A). Priming of mouse macrophages with IFN-γ prior to treatment with TLR ligands did not influence the lack of Cyp27b1 induction following TLR stimulation (data not shown). A second vitamin D target gene, Cyp24a1, also showed no induction in Tg/WT macrophages following TLR stimulation (data not shown). On the other hand, basal expression of CYP27B1 in unstimulated human monocyte PBMC-derived macrophages was 5-log higher in unstimulated cells compared with the basal level in unstimulated mouse macrophages (Fig. 8A). Human CYP27B1 was significantly induced 24 h post-treatment with LPS (Fig. 8A). Also, very low basal levels of Cyp27b1 in BM-derived dendritic cells from Tg/WT mice were observed with no significant induction 24 h post-treatment with TLR ligands PolyIC, LPS, flagellin, or CpG DNA (data not shown). In summary, these results demonstrate significant differences between mouse and human macrophages and myeloid dendritic cells with regards to basal and TLR-induced Cyp27b1 expression and explains why human CAMP is not induced in these mouse cells in the presence of 25(OH)D3 and TLR ligands.
Figure 8. Expression of Cyp27b1 in mouse macrophages and tissues after TLR ligand stimulation.
TLR ligands do not induce Cyp27b1 expression in mouse macrophages. (A) BM-derived Tg/WT mouse macrophages and human PBMC-derived macrophages were stimulated with TLR ligands for 24 h and Cyp27b1 gene expression was measured by qRT-PCR, normalized to β-actin and graphed on a log10 scale. Graphs are a single experiment representative of three independent experiments (Asterisks denote significance from control; p<0.05, two-tailed T-test). (B) Tg/WT mice were injected IP with either 10 μg/g body weight LPS or vehicle control for 24 h. Expression of Cyp27b1 was normalized to Ywhaz by qRT-PCR and graphed on a log10 scale. Asterisk denotes a significant increase as compared with the control (p<0.05, T-test with Welch’s correction). (C) WT mice were injected IP with either 0.5 g/g body weight LPS or vehicle control. Kidneys were collected 3 h post injection. Cyp27b1 expression was normalized to Gapdh and graphed on a log10 scale. D) Cyp24a1 expression was normalized to Gapdh and graphed on a log10 scale. Data were graphed as the mean (center line) ±SD (brackets). Each symbol represents an individual mouse for B, C and D. Asterisks denote significance from control (p<0.05, two-tailed T-test).
3.9. Expression and induction of Cyp27b1 in vivo in mice.
The lack of basal Cyp27b1 expression and its induction in mouse macrophages during TLR activation raises the question, what is the source of its activity during the mouse immune response? In humans and mice Cyp27b1 is strongly expressed in the kidney where it serves a primary role in producing 1,25(OH)2D3 that circulates systemically (55–58). In mice, expression of Cyp27b1 in other tissues and cells is not well characterized, but is much lower than in the kidney (54, 59, 60). We hypothesized that TLR activation may induce Cyp27b1 expression in cells from other tissues, thus providing a source of 1,25(OH)2D3 during infection and compensating for the lack of synthesis by macrophages. To test this, CAMP Tg/WT mice were injected IP with either vehicle control or LPS at 20 g/g body weight and the expression of Cyp27b1 was measured 24 h post injection in the kidney, salivary gland, skin, lung, ileum and colon using qRT-PCR (Fig. 8B). As expected, we observed high levels of basal Cyp27b1 expression in the kidney followed by lower levels in the salivary gland and skin (about 1 and 1.5 logs lower, respectively), and even lower expression in the lungs, ileum, and colon (about 2–3 logs lower). LPS did not significantly induce expression of Cyp27b1 in most organs with the exception of the lungs (approximately 6-fold increase) (Fig. 8B). Cyp24a1 expression was also measured in this same experiment by qRT-PCR, but no significant induction was found (data not shown).
A second study was performed in WT mice to determine if a lower dose of LPS at an earlier time point might induce Cyp27b1. Mice were injected IP with LPS at 0.5 g/g body weight. A significant induction of Cyp27b1 mRNA occurred in the kidneys at 3 h post-injection (Fig. 8C), associated with a concurrent decrease in Cyp24a1 expression (Fig. 8D). In all other tissues, no significant increase in Cyp27b1 expression occurred (data not shown). These findings suggest that the kidney may respond to systemic inflammatory challenges with a transient increase of Cyp27b1 at early time points (3 h) that returns to basal levels at a later time point (24 h). Because of the lower expression and a lack of strong induction of Cyp27b1 in most tissues following a systemic inflammatory challenge, the role that extra-renal production of 1,25(OH)2D3 has in the murine immune response is unclear.
4. DISCUSSION
In this report, a human CAMP transgenic mouse was generated and characterized. The BM and immune cells express hCAP18 in a pattern similar to that observed in humans, with neutrophils showing the highest expression, followed by macrophages and lower expression in B-cells (43). Mature murine peritoneal macrophages and BM-derived macrophages expressed levels comparable to that observed in human monocytes and in monocyte-derived macrophages (1, 2). Salivary glands from the transgenic mouse also expressed abundant hCAP18 protein, suggesting a protective function in the oral mucosa. Salivary glands and lingual epithelial cells in mice express CRAMP and in humans, hCAP18/LL-37 is detected in saliva (12). Deficiency of LL-37 in humans due to congenital defects in morbus Kostmann syndrome results in severe periodontal disease, highlighting the importance of LL-37 in protection of the oral cavity (29). We also detected expression of both CAMP mRNA and hCAP18 in the skin of transgenic mice that parallels an important site of expression in humans (10, 26). In both humans and mice, cathelicidin expression is increased following wounding and it is important for wound healing (24, 27). Expression of CRAMP in the skin and neutrophils of mice is also important in defending against group A streptococcal skin infections (27). We detected hCAP18 protein by ELISA in the spleen, lungs, intestinal tract, kidney and liver (Table 1). Using two different antibodies against hCAP18 and LL-37, we observed processing of hCAP18 into both the 14-kDa cathelin pro-domain fragment and the 4-kDa peptide in BM cells of the Tg mouse. While this peptide co-migrated with synthetic LL-37 peptide and was detected by the anti-LL37 antibody, additional studies are needed to determine if it is LL-37.
In the intestinal tract, the human transgene increased resistance to colonization by the enteric pathogen S. typhimurium in the cecum. The presence of the transgene on the mouse WT background increased resistance to colonization compared with the mouse gene alone, suggesting an additive effect of having both the mouse and human genes together in the Tg/WT mice. When the human transgene was present in the Camp KO background, increased resistance to colonization of the cecum occurred as compared to the KO mouse, confirming the functional role of CAMP in the intestinal tract. The role of the endogenous mouse Camp gene in the intestinal tract was previously defined using the KO mouse strain. These mice had an increased susceptibility to enteric infections by Citrobacter rodentium (61).
1,25(OH)2D3 induced the CAMP transgene in a dose-dependent manner in vitro in transgenic mouse BMDM. Organs from transgenic mice cultured in vitro with 1,25(OH)2D3 also showed induction of CAMP mRNA to various degrees depending on tissue type. Topical application of 1,25(OH)2D3 to the skin induced in vivo CAMP expression in the mouse. In humans, application of the vitamin D analog calcipotriol induced hCAP18 protein expression in human skin (3). Calcipotriol is used clinically to treat psoriasis and has several immune modulating functions, including decreasing expression of Th17 pathway cytokines IL-17 and IL-23 (62, 63), while the role of hCAP18 in modulating psoriasis is less clear. The processed form of the protein, LL-37, is increased in psoriatic lesions (26) where it may play a role in enhancing inflammation, yet it also has been described to decrease inflammasome activation in response to cytosolic self-DNA in keratinocytes (64). In contrast to psoriasis, atopic dermatitis patients present with lowered expression of LL-37 and suffer increased susceptibility to skin infections (25, 65). Using immunohistochemistry, we previously observed in our CAMP transgenic mouse model that wounding increased hCAP18 expression in the skin; while in normal skin, expression was lower and not evident in keratinocytes by immunohistochemistry (42). Our findings are consistent with prior studies showing low expression in normal skin, but elevated levels upon wounding and inflammation (10, 24, 26).
When crossed onto the mouse Camp KO background, the CAMP transgene restored wound healing in the skin, demonstrating that the human gene can complement the function of the mouse Camp in vivo. Comparing the wound healing rate between the transgenic CAMP Tg/KO, WT and Camp KO mice showed that both the human and mouse forms of cathelicidin promote healing at similar rates at the time points measured while the KO showed a severe lack of healing. Thus, both the mouse and human cathelicidins appear to play similar roles in wound healing. The similar rates of wound healing also suggest that transgenic hCAP18 is processed into a functional peptide in the mouse. Furthermore, topical treatment of Tg/KO mice with 1,25(OH)2D3 improved resistance to S. aureus growth in a wound infection model. This suggests that induction of human CAMP in the skin of Tg mice by vitamin D has functional significance to protect wound sites from infection. The CAMP transgenic mouse may prove useful as a model to further determine the role of 1,25(OH)2D3-induced expression of CAMP in the skin against infection and other disease states.
The current study also reinforces an important species-specific difference in regulation of CYP27B1 between mouse and human macrophages. The production of the active hormonal form of vitamin D, 1,25(OH)2D3, is controlled by the sequential enzyme activity of CYP2R1 and CYP27B1 (66, 67). In the liver, CYP2R1 activity produces 25(OH)D3 which then circulates and can be converted primarily in the kidney by CYP27B1 to 1,25(OH)2D3, the active hormonal form. Extra-renal CYP27B1 activity has been demonstrated in human skin (68), human placenta (69) and human macrophages and dendritic cells (59, 70–72), where it can promote local effects of vitamin D action, likely by autocrine or paracrine signaling. In human macrophages, TLR signaling results in induction of CYP27B1 expression and subsequent synthesis of 1,25(OH)2D3, which then increases production of CAMP locally (21). We treated mouse WT and transgenic macrophages with multiple TLR ligands to induce Cyp27b1 but did not observe any significant increase at either 3 h or 24 h post stimulation. As a comparison, we also replicated prior findings showing that in human monocyte-derived macrophages following TLR stimulation, a robust expression of CYP27B1 lead to a significant induction of CAMP in the presence of 25(OH)D3. In contrast, transgenic mouse macrophages did not have a significant induction of CAMP following TLR stimulation. This correlated with the lack of constitutive or induced Cyp27b1 expression. This finding is consistent with prior studies showing that LPS stimulation did not induce Cyp27b1 expression in mouse bone marrow derived macrophages (53, 54); however, we also showed that treatment of murine macrophages with additional TLR agonists did not induce Cyp27b1 expression.
The lack of Cyp27b1 expression or induction in murine macrophages raises the question as to how infection may increase the production of 1,25(OH)2D3. Therefore, we examined endogenous Cyp27b1 expression in several murine tissues using qRT-PCR. As expected, the highest basal expression was found in the kidney, followed by the salivary glands and skin. Expression on the order of 100- to 1000-fold less than the kidney was observed for the lungs, ileum, and colon. Acute inflammatory challenge in mice with a high-dose of LPS did not increase Cyp27b1 expression in any of the tested tissues, except the lungs (approximately 6-fold induction 24 h post IP-injection). We also tested a lower dose of LPS with a shorter duration of acute challenge. At 3 h post injection we did observe significant induction of Cyp27b1 in the kidneys, but not in other tissues. These results suggest acute systemic inflammation may briefly upregulate expression of Cyp27b1 in the kidney, but it does not appear to be sustained. A recent study modeling endotoxemia in mice demonstrated increased expression of Cyp27b1 in the kidney that peaked at 4 h post-injection of LPS and resulted in a concomitant increase in circulating 1,25(OH)2D3 (73). Expression of Cyp27b1 mRNA in the kidney and circulating 1,25(OH)2D3 decreased by about 50% at 16 h post-injection (73). Using Cyp27b1-LacZ knock-in mice LPS injection did not increase reporter activity in the kidney when measured at 24 h (54); however, long term chronic inflammatory events caused by DSS induced colitis did increase Cyp27b1reporter activity in the small intestines and colon of about 30% of the mice, suggesting that chronic inflammation may induce Cyp27b1 at extrarenal sites. Induction of Cyp27b1 has been reported in microglia and placenta in mice (74, 75), in both cases, the induction was important for controlling inflammation.
Our findings together with others indicate that certain aspects of immune modulation performed by CYP27B1 in humans are not conserved, particularly in mouse macrophages. Effects may occur locally in tissues through extra-renal synthesis of 1,25(OH)2D3. Future studies are required to determine if other cell types in the mouse contribute to local production. In mouse skin, wounding does increase the vitamin D responsive gene CD14 in WT mice, but not in Cyp27b1 knockout mice, suggesting that inflammatory signals and wound healing may induce Cyp27b1 (34). In human skin, wounding does increase CD14, CYP27B1, CYP24A1 and CAMP expression, which can be blocked by itraconazole, a CYP27B1 inhibitor. This strongly suggests that 1,25(OH)2D3 exerts local effects in the skin (34). TLR responsiveness in cultured human skin keratinocytes is different than in human macrophages, as keratinocytes need a combination of TGF-β1 and 25(OH)D3 to become responsive to TLR2 ligands through induced expression of TLR2 and CYP27B1 (34). Conservation of this pathway in mouse skin needs to be verified. In the CAMP transgenic mouse model, we could bypass the uncertainty of local Cyp27b1 activity by treating topically with 1,25(OH)2D3 to induce CAMP expression. In summary, we have developed a mouse model in which we can test the role of vitamin D3 induction of human cathelicidin during infection and non-pathogenic disease states.
Highlights.
We created a transgenic mouse that expresses the human cathelicidin gene
The human transgene can be regulated by vitamin D3 in the mouse
Expression of human cathelicidin restores wound healing in a mouse knockout model
Human cathelicidin transgenic mice show increased resistance to gut infections
Topical skin treatment with vitamin D3 decreases staph infection in transgenic mice
ACKNOWLEDGEMENTS
We thank Tsuyako Saito and Sandra Uesugi for technical assistance and support (Oregon State University), and Birgitta Agerberth (Karolinska Institute, Stockholm, Sweden) and James Slauch (University of Illinois, Champaign Urbana, USA) for providing reagents. This study was supported by grants from the U.S. National Institutes of Health (NIH) National Cancer Institute 5R01CA26038 (HPK), National Institute of Allergy and Infectious Diseases 1R01AI065604-01A2 (AFG), National Center for Complementary and Integrative Health 5R01AT009168 (AFG) and National Institute of General Medical Sciences 5R01GM123081 (JX). We dedicate this study to Niels Borregaard who provided intellectual support, essential reagents and was a good friend and colleague.
Abbreviations:
- CAMP
cathelicidin antimicrobial peptide
- TLR
Toll-like receptor
- VDRE
vitamin D response element
- VDR
vitamin D receptor
- SINE
short interspersed nuclear element
- BMDM
bone marrow derived macrophage
- BM
bone marrow
- AMP
antimicrobial peptide
- qRT-PCR
quantitative real time polymerase chain reaction
- Tg
transgenic
- KO
knockout
- WT
wildtype
- PBMC
peripheral blood mononuclear cells
- IP
intraperitoneal
- TLA
targeted locus amplification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, and White JH (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173, 2909–2912 [DOI] [PubMed] [Google Scholar]
- 2.Gombart AF, Borregaard N, and Koeffler HP (2005) Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19, 1067–1077 [DOI] [PubMed] [Google Scholar]
- 3.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, and Stahle M (2005) Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol 124, 1080–1082 [DOI] [PubMed] [Google Scholar]
- 4.Gombart AF, Saito T, and Koeffler HP (2009) Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics 10, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanetti M, Gennaro R, and Romeo D (1995) Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 374, 1–5 [DOI] [PubMed] [Google Scholar]
- 6.Panyutich A, Shi J, Boutz PL, Zhao C, and Ganz T (1997) Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun 65, 978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, and Borregaard N (2001) Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 [DOI] [PubMed] [Google Scholar]
- 8.Lehrer RI, and Ganz T (2002) Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol 9, 18–22 [DOI] [PubMed] [Google Scholar]
- 9.Zanetti M, Gennaro R, Scocchi M, and Skerlavaj B (2000) Structure and biology of cathelicidins. Adv Exp Med Biol 479, 203–218 [DOI] [PubMed] [Google Scholar]
- 10.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, and Stahle-Backdahl M (1999) The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun 67, 2561–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bals R, Wang X, Zasloff M, and Wilson JM (1998) The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 95, 9541–9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Ohtake T, Dorschner RA, and Gallo RL (2002) Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res 81, 845–850 [DOI] [PubMed] [Google Scholar]
- 13.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, and Gallo RL (2002) Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol 119, 1090–1095 [DOI] [PubMed] [Google Scholar]
- 14.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, and Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A 92, 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, and Egesten A (2000) The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun 68, 4297–4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammami-Hamza S, Doussau M, Bernard J, Rogier E, Duquenne C, Richard Y, Lefevre A, and Finaz C (2001) Cloning and sequencing of SOB3, a human gene coding for a sperm protein homologous to an antimicrobial protein and potentially involved in zona pellucida binding. Mol Hum Reprod 7, 625–632 [DOI] [PubMed] [Google Scholar]
- 17.Andersson E, Sorensen OE, Frohm B, Borregaard N, Egesten A, and Malm J (2002) Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum Reprod 17, 2529–2534 [DOI] [PubMed] [Google Scholar]
- 18.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, and Gudmundsson GH (2000) The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 [PubMed] [Google Scholar]
- 19.Di Nardo A, Vitiello A, and Gallo RL (2003) Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol 170, 2274–2278 [DOI] [PubMed] [Google Scholar]
- 20.Sorensen O, Arnljots K, Cowland JB, Bainton DF, and Borregaard N (1997) The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90, 2796–2803 [PubMed] [Google Scholar]
- 21.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, and Modlin RL (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Stenger S, Tang DH, and Modlin RL (2007) Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179, 2060–2063 [DOI] [PubMed] [Google Scholar]
- 23.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, and Wilkinson RJ (2007) IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178, 7190–7198 [DOI] [PubMed] [Google Scholar]
- 24.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, and Gallo RL (2001) Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol 117, 91–97 [DOI] [PubMed] [Google Scholar]
- 25.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, and Leung DY (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347, 1151–1160 [DOI] [PubMed] [Google Scholar]
- 26.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, and Gudmundsson GH (1997) The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 272, 15258–15263 [DOI] [PubMed] [Google Scholar]
- 27.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, and Gallo RL (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 [DOI] [PubMed] [Google Scholar]
- 28.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, and Brauner A (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12, 636–641 [DOI] [PubMed] [Google Scholar]
- 29.Putsep K, Carlsson G, Boman HG, and Andersson M (2002) Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 360, 1144–1149 [DOI] [PubMed] [Google Scholar]
- 30.Gombart AF, Hofmann WK, Kawano S, Takeuchi S, Krug U, Kwok SH, Larsen RJ, Asou H, Miller CW, Hoelzer D, and Koeffler HP (2002) Mutations in the gene encoding the transcription factor CCAAT/enhancer binding protein alpha in myelodysplastic syndromes and acute myeloid leukemias. Blood 99, 1332–1340 [DOI] [PubMed] [Google Scholar]
- 31.Yang YH, Zheng GG, Li G, Zhang B, Song YH, and Wu KF (2003) Expression of LL-37/hCAP-18 gene in human leukemia cells. Leuk Res 27, 947–950 [DOI] [PubMed] [Google Scholar]
- 32.An LL, Ma XT, Yang YH, Lin YM, Song YH, and Wu KF (2005) Marked reduction of LL-37/hCAP-18, an antimicrobial peptide, in patients with acute myeloid leukemia. Int J Hematol 81, 45–47 [DOI] [PubMed] [Google Scholar]
- 33.Steinstraesser L, Oezdogan Y, Wang SC, and Steinau HU (2004) Host defense peptides in burns. Burns 30, 619–627 [DOI] [PubMed] [Google Scholar]
- 34.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, and Gallo RL (2007) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117, 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, and Hunninghake GW (2008) Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181, 7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chumakov AM, Grillier I, Chumakova E, Chih D, Slater J, and Koeffler HP (1997) Cloning of the novel human myeloid-cell-specific C/EBP-epsilon transcription factor. Mol Cell Biol 17, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vree PJ, de Wit E, Yilmaz M, van de Heijning M, Klous P, Verstegen MJ, Wan Y, Teunissen H, Krijger PH, Geeven G, Eijk PP, Sie D, Ylstra B, Hulsman LO, van Dooren MF, van Zutven LJ, van den Ouweland A, Verbeek S, van Dijk KW, Cornelissen M, Das AT, Berkhout B, Sikkema-Raddatz B, van den Berg E, van der Vlies P, Weening D, den Dunnen JT, Matusiak M, Lamkanfi M, Ligtenberg MJ, ter Brugge P, Jonkers J, Foekens JA, Martens JW, van der Luijt R, van Amstel HK, van Min M, Splinter E, and de Laat W (2014) Targeted sequencing by proximity ligation for comprehensive variant detection and local haplotyping. Nat Biotechnol 32, 1019–1025 [DOI] [PubMed] [Google Scholar]
- 38.Dong J, Jin G, Wu C, Guo H, Zhou B, Lv J, Lu D, Shi Y, Shu Y, Xu L, Chu M, Wang C, Zhang R, Dai J, Jiang Y, Yu D, Ma H, Zhao X, Yin Z, Yang L, Li Z, Deng Q, Cao S, Qin Z, Gong J, Sun C, Wang J, Wu W, Zhou G, Chen H, Guan P, Chen Y, Liu X, Liu L, Xu P, Han B, Bai C, Zhao Y, Zhang H, Yan Y, Liu J, Amos CI, Chen F, Tan W, Jin L, Wu T, Hu Z, Lin D, and Shen H (2013) Genome-wide association study identifies a novel susceptibility locus at 12q23.1 for lung squamous cell carcinoma in han chinese. PLoS Genet 9, e1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen O, Cowland JB, Askaa J, and Borregaard N (1997) An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 206, 53–59 [DOI] [PubMed] [Google Scholar]
- 40.Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, and Gombart AF Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res 58, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang X, Bhattacharya S, Bajaj G, Guha G, Wang Z, Jang HS, Leid M, Indra AK, and Ganguli-Indra G (2012) Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis. PLoS One 7, e29999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang J, Zhang Y, Indra AK, Ganguli-Indra G, Le MN, Wang H, Hollins RR, Reilly DA, Carlson MA, Gallo RL, Gombart AF, and Xie J (2018) 1alpha,25-dihydroxyvitamin D3-eluting nanofibrous dressings induce endogenous antimicrobial peptide expression. Nanomedicine (Lond) 13, 1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowry MB, Guo C, Borregaard N, and Gombart AF Regulation of the human cathelicidin antimicrobial peptide gene by 1alpha,25-dihydroxyvitamin D in primary immune cells. J Steroid Biochem Mol Biol 143C, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley TL, Ellermeier CD, and Slauch JM (2000) Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar typhimurium survival in Peyer’s patches. J Bacteriol 182, 4406–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larrick JW, Lee J, Ma S, Li X, Francke U, Wright SC, and Balint RF (1996) Structural, functional analysis and localization of the human CAP18 gene. FEBS Lett 398, 74–80 [DOI] [PubMed] [Google Scholar]
- 46.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, and Gudmundsson GH (1996) PR-39, a proline-rich peptide antibiotic from pig, and FALL-39, a tentative human counterpart. Vet Immunol Immunopathol 54, 127–131 [DOI] [PubMed] [Google Scholar]
- 47.Miraglia E, Nylen F, Johansson K, Arner E, Cebula M, Farmand S, Ottosson H, Stromberg R, Gudmundsson GH, Agerberth B, and Bergman P (2016) Entinostat up-regulates the CAMP gene encoding LL-37 via activation of STAT3 and HIF-1alpha transcription factors. Sci Rep 6, 33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross DS, and Garrard WT (1988) Nuclease hypersensitive sites in chromatin. Annu Rev Biochem 57, 159–197 [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, and Ogawa H (2007) Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol 157, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 50.Heilborn JD, Weber G, Gronberg A, Dieterich C, and Stahle M (2010) Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hCAP18/LL-37 during wounding in human skin in vivo. Exp Dermatol 19, 332–338 [DOI] [PubMed] [Google Scholar]
- 51.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, and Agerberth B (2003) Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res 53, 211–216 [DOI] [PubMed] [Google Scholar]
- 52.Sorensen O, Bratt T, Johnsen AH, Madsen MT, and Borregaard N (1999) The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem 274, 22445–22451 [DOI] [PubMed] [Google Scholar]
- 53.Kapetanovic R, Fairbairn L, Beraldi D, Sester DP, Archibald AL, Tuggle CK, and Hume DA (2012) Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J Immunol 188, 3382–3394 [DOI] [PubMed] [Google Scholar]
- 54.Ooi JH, McDaniel KL, Weaver V, and Cantorna MT (2014) Murine CD8+ T cells but not macrophages express the vitamin D 1alpha-hydroxylase. J Nutr Biochem 25, 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araya Z, Norlin M, and Postlind H (1996) A possible role for CYP27 as a major renal mitochondrial 25-hydroxyvitamin D3 1 alpha-hydroxylase. FEBS Lett 390, 10–14 [DOI] [PubMed] [Google Scholar]
- 56.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, and Kato S (1997) 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 277, 1827–1830 [DOI] [PubMed] [Google Scholar]
- 57.Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, and Stewart PM (1999) Expression of 25-hydroxyvitamin D3–1alpha-hydroxylase in the human kidney. J Am Soc Nephrol 10, 2465–2473 [DOI] [PubMed] [Google Scholar]
- 58.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, and Goltzman D (2001) Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 98, 7498–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, and Hewison M (2001) Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86, 888–894 [DOI] [PubMed] [Google Scholar]
- 60.Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, and Deluca HF (2006) Calbindin D(9k) knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci U S A 103, 12377–12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, and Kagnoff MF (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol 174, 4901–4907 [DOI] [PubMed] [Google Scholar]
- 62.Kragballe K (1991) Calcipotriol for psoriasis. Lancet 337, 1229–1230 [DOI] [PubMed] [Google Scholar]
- 63.Kubin ME, Kokkonen N, Palatsi R, Hagg PM, Vayrynen JP, Glumoff V, Haapasaari KM, Hurskainen T, and Tasanen K (2017) Clinical Efficiency of Topical Calcipotriol/Betamethasone Treatment in Psoriasis Relies on Suppression of the Inflammatory TNFalpha - IL-23 - IL-17 Axis. Acta Derm Venereol 97, 449–455 [DOI] [PubMed] [Google Scholar]
- 64.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, and Schauber J (2011) Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Sci Transl Med 3, 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, and Leung DY (2006) Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol 117, 836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, and Russell DW (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 101, 7711–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, and Portale AA (1997) Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol 11, 1961–1970 [DOI] [PubMed] [Google Scholar]
- 68.Bikle DD, Nemanic MK, Gee E, and Elias P (1986) 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest 78, 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gray TK, Lester GE, and Lorenc RS (1979) Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science 204, 1311–1313 [DOI] [PubMed] [Google Scholar]
- 70.Reichel H, Koeffler HP, Barbers R, and Norman AW (1987) Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 65, 1201–1209 [DOI] [PubMed] [Google Scholar]
- 71.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, and Kreutz M (2003) Regulation of 25-hydroxyvitamin D3–1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood 102, 3314–3316 [DOI] [PubMed] [Google Scholar]
- 72.Koeffler HP, Reichel H, Bishop JE, and Norman AW (1985) gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun 127, 596–603 [DOI] [PubMed] [Google Scholar]
- 73.Meurer M, and Hocherl K (2019) Endotoxaemia differentially regulates the expression of renal Ca(2+) transport proteins in mice. Acta Physiol (Oxf) 225, e13175. [DOI] [PubMed] [Google Scholar]
- 74.Boontanrart M, Hall SD, Spanier JA, Hayes CE, and Olson JK (2016) Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol 292, 126–136 [DOI] [PubMed] [Google Scholar]
- 75.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, Equils O, and Hewison M (2011) Vitamin D and the regulation of placental inflammation. J Immunol 186, 5968–5974 [DOI] [PubMed] [Google Scholar]