Abstract
Objective:
The object of this open-label, nonrandomized trial was to evaluate efficacy and safety of bilateral caudate nucleus deep brain stimulation (DBS) for treatment-resistant tinnitus.
Methods:
Six participants underwent DBS implantation. One participant was removed from the study for suicidality unrelated to brain stimulation. Participants underwent a stimulation optimization period that ranged from 5 to 13 months, during which the most promising stimulation parameters for tinnitus reduction for each individual were determined. These individual optimal stimulation parameters were then used during the 24 weeks of continuous caudate stimulation to reach endpoint. The primary outcome for efficacy was the Tinnitus Functional Index (TFI) and executive function safety was a composite z-score from multiple neuropsychological tests (EF-score). The secondary outcome for efficacy was the Tinnitus Handicap Inventory (THI), neuropsychiatric safety was the Frontal Systems Behavior Scale (FrSBe), and hearing safety was pure tone audiometry at 0.5, 1, 2, 3, 4, and 6 kHz and word recognition score (WRS). Other monitored outcomes included surgical and device adverse events. Five participants provided full analyzable data sets. Primary and secondary outcomes were based on differences in measurements between baseline and endpoint.
Results:
The treatment effect size of caudate DBS for tinnitus by TFI mean (SE) = 23.3 (12.4) and THI = 30.8 (10.4), both were statistically significant (Wilcoxon Signed Rank Test, one-tailed, alpha=0.05). Based on clinically significant treatment response categorical analysis, there were 3 responders by TFI (≥ 13 point decrease) and 4 responders by THI (≥ 20 point decrease). Safety outcomes by EF-score, FrSBe, audiometric thresholds, and WRS showed no significant change with chronic caudate stimulation. Surgical and device adverse events were expected, transient, and reversible. There was only one serious adverse event, a suicide attempt unrelated to caudate neuromodulation in a participant who was OFF stimulation for 2 months prior to the event.
Conclusions:
Bilateral caudate nucleus neuromodulation by DBS for severe, refractory tinnitus in this phase I trial showed very encouraging results. Primary and secondary outcomes revealed a highly variable treatment effect size and 60–80% treatment response rate for clinically significant benefit, and no safety concerns. The design of a phase II trial may benefit from targeting refinement for final DBS lead placement to decrease stimulation optimization period duration and to increase treatment effect size uniformity.
Trial registration no.:
Keywords: Caudate nucleus, corticostriatal, deep brain stimulation, executive function, phase I trial, tinnitus
Introduction
Tinnitus is a non-observable, self-reported perceptual disorder where elemental sounds that appear to be emanating from one ear, both ears, or inside the head are without corresponding physical sources. It is a common problem afflicting the general population, with an estimated prevalence of 10–15%37 and incidence of 5.4%.23 Auditory phantoms are often described as ringing, hissing, buzzing, roaring, chirping, or clicking sounds. Occupational noise exposure is the major reason for the onset of constant, chronic tinnitus. Military personnel, Veterans, and civilians in certain professions, such as firefighters and construction workers, are at increased risk for persistent auditory phantoms initiated by hearing loss. While over 80% of tinnitus patients adapt well to their auditory phantoms, still 13 million in the United States and Europe seek medical attention.8
Conventional tinnitus treatment strategies aim to stabilize comorbid stress,3,5,40 depression and anxiety,16,17,38 and sleep disruption 4,10 using pharmacological or behavioral approaches,2,45 and mitigate distress attributed to auditory phantoms deploying acoustical or behavioral or combined acoustical and behavioral therapies.14,22 Nonetheless, between 0.5% and 2% of the adult population or over 1 million in the United States are tinnitus sufferers,24,41 where auditory phantoms intrude on activities of daily living, exacerbate behavioral and emotional problems, and impair mental concentration. For those tinnitus sufferers with auditory phantoms unresponsive or inadequately responsive to conventional therapies, salvage treatment options remain rather limited. Some tinnitus sufferers with intractable symptoms may ultimately choose to participate in invasive, experimental approaches in the hopes of finding meaningful relief. 11,12,34,39
Tinnitus is a distinctly auditory percept,26,29 but nonclassical auditory,27,42 limbic,20,35 and striatal18,36 interactive networks play important roles in its awareness, chronicity, and severity. Targeting the basal ganglia for direct electrical stimulation to effect tinnitus modulation was guided by case reports of impressive tinnitus loudness reduction following caudate nucleus vascular infarction19,21 and a resting-state fMRI study that demonstrated increased corticostriatal connectivity in chronic tinnitus.15 In preliminary studies leading to this phase I clinical trial, caudate nucleus function was directly modulated during deep brain stimulation (DBS) electrode implantation surgery in awake and interactive human subjects with movement disorders (Parkinson’s disease or essential tremor) and comorbid constant, chronic tinnitus. In the first set of experiments, tinnitus loudness modulation was mediated by high frequency striatal stimulation.7 Tinnitus loudness was suppressed to a nadir of level 2 or lower on a 0 to 10 rating scale in 5 subjects where the DBS lead traversed the dorsal striatum, but not in the 1 subject where the DBS lead was positioned outside the caudate nucleus. Depending on the specific parameters of electrical stimulation, tinnitus loudness was temporarily increased in 2 subjects. In the second set of experiments on 6 subjects, also with movement disorders undergoing DBS surgery, 3 with and 3 without comorbid chronic tinnitus, auditory phantom sound quality modulation was mediated by both low and high frequency striatal stimulation.18 Caudate stimulation triggered phantom tones, clicks, and frequency modulated sweeps in 5 subjects, and changed tinnitus baseline sound quality in 1 subject. All manifestations of auditory phantom modulation ceased shortly after stimulation was terminated, confirming reversibility of DBS-related effects.
In this phase I trial of caudate DBS for treatment-resistant tinnitus, there were three main study goals: 1) develop therapy that may have promise to mitigate tinnitus severity meaningfully in sufferers who have exhausted conventional therapies, 2) extend short-term tinnitus loudness reduction benefit reported in preliminary studies to indefinite long-term relief, and 3) exercise critical evaluation of this nonauditory, basal ganglia-centric approach by selecting chronic tinnitus patients without movement disorders to remove the possible confound of nigrostriatal degeneration. Here, we report on primary and secondary efficacy and safety outcomes on a cohort of 5 participants who underwent continuous caudate stimulation for 24 weeks at individualized stimulation parameters and completed all required evaluations to deliver analyzable data sets.
Methods
This was a single institution, open-label, phase I clinical trial to evaluate efficacy and safety of bilateral caudate nucleus neuromodulation by DBS in adults with a big or very big problem25 with chronic tinnitus that has been unresponsive or unsatisfactorily responsive to conventional therapy. The study protocol was approved by the University of California, San Francisco Human Research and San Francisco Veterans Affairs Research & Development Committees (IRB# 13–11641). This study was conducted in accordance with the Declaration of Helsinki and registered with ClinicalTrials.gov with the identifier NCT01988688 prior to study participant recruitment.
Participants
Eligible men and women participants had subjective unilateral or bilateral constant tinnitus longer than 1 year and severity defined by TFI > 50 despite conventional treatment by acoustical or behavioral therapy, Montreal Cognitive Assessment 30 (MoCA) score ≥ 26, and were between 22 and 75 years of age inclusive. Exclusion criteria included: hyperacusis, misophonia, and average air conduction of any 3 consecutive audiometric frequencies (0.5, 1, 2, 4, and 8 kHz) ≥ 56 dB in either ear, and any medical or psychiatric symptoms or conditions that could interfere with study activities or confound interpretation of study results. After completion of endpoint evaluations and prior to study separation, all implanted participants were provided ample opportunities to discuss pros and cons of continuing with stimulation, stopping stimulation but leaving the DBS system in place, and stopping stimulation and removing either part or all of the DBS system. One participant elected complete DBS system removal, which was performed uneventfully, while all other participants chose to continue with chronic caudate stimulation.
Outcome Assessments
Baseline assessments were performed prior to DBS surgery and endpoint assessments were performed after 24 weeks of continuous caudate nucleus stimulation. The primary objectives were to assess tinnitus mitigation efficacy using the Tinnitus Functional Index (TFI),25 and to monitor executive function safety using a composite z-score (EF-score) from select neuropsychological tests (Delis-Kaplan Executive Function System: Design Fluency, Color-Word Interference, Tower Test, Card Sort, and Letter Fluency; Neuropsychological Assessment Battery: Digit Span Backwards) that were administered by a licensed neuropsychologist. The secondary objectives were to assess tinnitus mitigation efficacy using the Tinnitus Handicap Inventory (THI)31 for severity, a 0–10 numeric rating scale (NRS) (0 - no tinnitus, 5 – conversation level, 10 - jet engine)13 for loudness, and a Global Impression of Change (GIC) for qualitative judgment. Other secondary objectives were to assess neuropsychiatric safety using the Frontal Systems Behavior Scale (FrSBe) to measure apathy, disinhibition, and executive dysfunction, and to assess hearing safety by measuring air conduction pure-tone thresholds at 0.5, 1, 2, 3, 4, 6, and 8 kHz and word recognition score (WRS) using the 25-item NU-6 word list for each ear.
Deep Brain Stimulation Surgery
Enrolled study participants underwent stereotactic functional neurosurgery to implant DBS leads (10.5 mm electrode array model 3387, Medtronic, Minneapolis, MN) into both caudate nuclei using a Leksell Frame (Elekta, Stockholm, Sweden) and Framelink software (Medtronic StealthStation, Minneapolis, MN). Participants were awake and interactive for intraoperative interrogation of striatal sites to determine position of final lead placement. The caudate nucleus was targeted by selecting an entry point at or just anterior to the coronal suture. The preliminary target was set in the subthalamic region, 12mm lateral, 3mm posterior and 4mm below the midcommissural point. The entry point was modified to avoid sulci and visible blood vessels, and the trajectory was then shortened in the coronal plane and medialized to position the target at the base of the caudate. The entry point was further modified to place the trajectory along the long axis of the caudate in the coronal plane, avoiding the ventricle. The depth of trajectory was adjusted to center the 10.5 mm length electrode array of a model 3387 DBS electrode (Medtronic, Minneapolis, MN) entirely within the caudate nucleus.
An initial microelectrode recording (MER) pass along the preplanned target trajectory in the first hemisphere captured recordings that marked physiological borders of the caudate nucleus (Alpha Omega, Alpharetta, GA). A DBS lead along the same tract was positioned at the center of the superior and inferior borders in the coronal plane. The electrode configuration was set with distal contact 0 as cathode and proximal contact 3 as anode for bipolar macrostimulation to assess tinnitus modulation relative to baseline, focusing on the following features: loudness (0–10 NRS) in each ear, spatial location (point source localized to one or both ears, sector of the acoustic scene, inside or outside the head), and sound quality (modulation to a higher or lower pitch, addition of distinct sounds, change in intrusiveness, trigger of a new auditory phantom). Stimulation frequency, amplitude and pulse width were varied only one at a time in a stepwise fashion to assess tinnitus modulation (Video 1). A maximum of three MER passes with macrostimulation (original target, 5mm anterior and 5mm posterior along the caudate anteroposterior axis) per hemisphere was carried out. The tract that modulated tinnitus features most strongly determined final positioning of the DBS lead on the first side. Macrostimulation results from the first side were used to inform initial tract exploration on the second side. In 9 of the 12 implanted hemispheres, one of the alternative tracts produced the most convincing tinnitus modulation. In one participant, stereotactic frame deviation discovered during surgery resulted in a lead being placed 2.5mm posterior and medial to the intended target, but still within the caudate; macrostimulation resulted in striking tinnitus modulation at this location, so the lead was not moved.
Postoperative MRI was obtained in all participants and transferred to Framelink to document lead locations. The coronal and sagittal approach angles and lead entry and tip positions in anterior commissure-posterior commissure (AC-PC) coordinates are listed in Table 1, and three-dimensional (3D) plots of the 4 contact locations on each lead in AC-PC space are shown in Figure 1. Trajectories and lead locations were variable due to intraoperative response to macrostimulation and caudate location in 3D space, which varies between participants according to the size of the lateral ventricles.
TABLE 1.
Deep brain stimulation entry and final lead tip positions
| Case No. | Approach Angle in Degrees Coronal / Sagittal |
Entry Position in AC-PC Coordinates [X Y Z] |
Lead Tip Position in AC-PC Coordinates [X Y Z] |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Left | Right | Left | Right | Left | Right | |
|
| ||||||
| U01–02 | 8.3 / 61.5 | 9.1 / 56.2 | [−20.5 36.8 56.4] | [20.5 39.9 54.6] | [−14.3 13.6 13.6] | [14 12.8 14.1] |
| U01–03 | 12.7 / 64.0 | 22.5 / 60.2 | [−21.6 28.2 64.0] | [30.8 27.7 57.9] | [−10.8 4.9 16.3] | [13.4 3.8 15.9] |
| U01–04 | 28.4 / 47.5 | 10.8 / 61.4 | [−35.0 33.9 54.1] | [22.4 30.7 65.4] | [−14.9 −0.1 17] | [13.3 4.9 17.9] |
| U01–10 | 26.1 / 56.2 | 29.6 / 46.9 | [−29.2 25.1 50.2] | [25.3 39.3 49.3] | [−10.6 −0.24 12.4] | [5.3 6.4 14.1] |
| U01–12 | 20.1 / 64.8 | 48.0 / 37.4 | [−28.9 25.8 63.9] | [45.9 36.5 46.0] | [−12.1 4.2 17.9] | [10.8 −4.9 14.3] |
AC-PC – Anterior Commissure-Posterior Commissure.
FIG. 1.
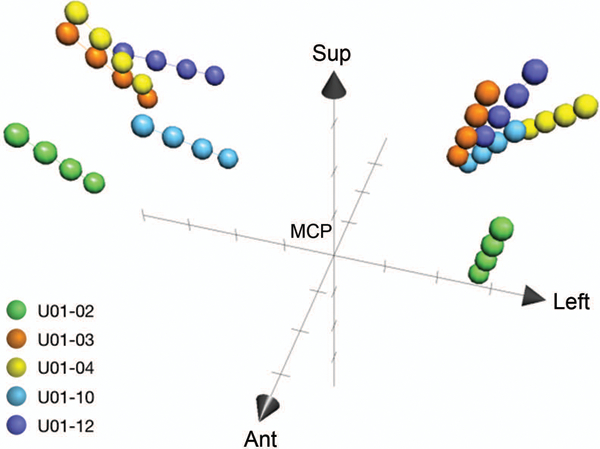
Three-dimensional plots of the four contact locations for each lead in AC-PC space with respect to the midcommissural point. Lead locations varied based on individual caudate anatomy and intraoperative response to macrosimulation. Marks on each axis are at 5mm increments. Sup, superior. Ant, anterior. MCP, midcommissural point.
Stimulation Optimization
After allowing a minimum of 5 weeks to ensure complete wound healing, implanted participants entered into a stimulation optimization period that was variable in duration and required logging of efficacy and safety events. At the conclusion of stimulation optimization, the most promising parameter group was chosen for caudate nucleus continuous stimulation for a fixed period of 24 weeks, allowing for only minor, defined adjustments to parameters to reach endpoint.
At the initial programming session, each of the four contacts was activated in monopolar mode to perform a “monopolar survey,” with a single contact assigned to be the cathode and the internal pulse generator (IPG) as the anode. The pulse width and frequency were fixed at values typically used in movement disorders (pulse width: 90 microseconds, frequency: 150 Hz), and the amplitude was increased by 1 to 2 Volt increments, from 0 to 10 Volts. At each amplitude, the participant was asked to rate his or her tinnitus in each ear independently on the 0–10 NRS loudness scale and report any other modulation of phantom percept features, including spatial location and sound quality. An emphasis was placed on exploring effects of parametric changes to settings that produced acute tinnitus modulation in the operating room, as these settings also produced modulation in most participants in the outpatient clinic. They tended to be bipolar settings (cathode and anode both located on the DBS electrode array) with stimulation distributed over several contacts.
At each programming session, the IPG was configured with 4 different parameter groups. One particular parameter of each group could be varied by the participant using their home controller within limits set by the study team. Initially, stimulation parameter variation focused on amplitude (from 0 to 10 Volts), but over time, it focused on frequency (from 10 to 250 Hz). The patient would cycle through each group as an outpatient, changing a particular parameter incrementally every 2–3 days in a systematic manner and logging tinnitus modulation and adverse effects in their diary. After the range parameter variation in each of the 4 groups was exhausted, the patient would have an in-person visit with the study team for review of the diary to determine the next 4 stimulation groups for home evaluation.
Adverse events interrogation was performed at all visits during stimulation optimization, as well as psychological, neurological, and audiological review of systems. Typically, the one best prior stimulation group was carried over to next 4 groups to provide a reference for comparisons. Over time, groups that provided beneficial tinnitus modulation without side effects were explored in finer detail to identify the most promising stimulation parameters. This lengthy period ranged from 5 to 13 months.
Continuous Stimulation
Once the most promising set of stimulation parameters was agreed upon by the participant and study team, continuous stimulation was carried out for a fixed duration of 24 weeks. Minor adjustments to parameters were allowed for participant U01–04, who strongly believed that switching between defined parameter groups on-demand when benefit was felt to be fluctuating or declining, sustained overall benefit at its highest level. Participants visited with the study team at the beginning, week 8, week 16, and the end of the continuous stimulation period (Video 2). Efficacy and safety outcomes were monitored at intervening weeks 8 and 16, and comprised of brief audiological (TFI and NRS) and neuropsychological (FrSBe and MoCA) assessments.
Sample Size Calculation
The main goal of data analysis from this phase I clinical trial on a limited number of study participants was to estimate treatment effect size and assess safety of chronic caudate stimulation to inform design and analysis of a phase II trial. The sample size of this phase I trial was set by the United States Food & Drug Administration Investigational Device Exemption (G120132) approval letter in 2012 that allowed a maximum of 10 adult (age ≥ 22) participants. Based on the assumption of 10 analyzable data sets, the ability to detect a difference in TFI score for a one-tailed paired t-test with alpha=0.05 and average effect size ≥ 15 TFI points yielded power ≥ 0.86. However, this phase I trial closed with 5 analyzable data sets, necessitating the application of nonparametric statistical analysis. We applied the Wilcoxon Signed Rank Test (one-tailed; alpha=0.05), where H0: The median difference = 0 and H1: The median difference < 0.
Data Analyses
Primary and secondary outcomes were based on differences in assessments between baseline and after 24 weeks of continuous caudate stimulation. The primary outcome for efficacy was difference in the TFI score, using a decline of 13 points as the cutoff for clinically significant improvement,25 and executive function safety was difference in the composite z-score (EF-score). For both primary and secondary outcomes, the descriptive statistics convention of mean (SE) was adopted.
The secondary outcomes for efficacy were difference in the THI score, using a decline of 20 points as the cutoff for clinically significant improvement,31 and difference in NRS (averaged across the two ears), and the qualitative GIC (Very Much Better, Much Better, Better, No Change, Worse, Much Worse, Very Much Worse). The secondary outcome for neuropsychiatric safety was difference in the FrSBe score and hearing safety was difference in air conduction pure-tone average for both low (0.5, 1, and 2 kHz) and high frequency (3, 4, and 6 kHz) bands, and WRS. Criterion for significant threshold shift (hearing loss) was decrease of the low frequency band ≥ 15 dB or the high frequency band ≥ 20 dB, based on recommendation of the American Academy of Otolaryngology – Head and Neck Surgery.1 Criterion for significant WRS change was decrease ≥ 12%, based on the smallest 95% confidence interval of the baseline WRS of the study cohort.6
Adverse Events
This was the first-in-human chronic caudate nucleus stimulation study. Prospective participants were informed about bearing unknown risks to executive, neuropsychiatric, and hearing functions, worsening of baseline tinnitus, and triggering of seizures and other phantom percepts before study enrollment. During the trial, implanted participants kept a log of stimulation-related adverse events and were interviewed in detail during in-person visits with the study team. Implanted participants understood their unassailable right to separate from the study at any time and that the study team could terminate their participation for medical or safety reasons.
Results
One hundred and ninety-five prospective participants were prescreened by reviewing suitability for enrollment based on inclusion and exclusion criteria. Undertreated tinnitus, anxiety and/or depression, misconceptions about invasive DBS surgery, and curiosity to seek information on interim results were some notable reasons for immediate disqualification.
Fourteen prospective participants advanced to screening and underwent comprehensive evaluations that established audiological and neuropsychological baseline data. Of the 9 prospective participants who met criteria for enrollment, 3 declined further engagement. Six enrolled participants were implanted with DBS leads in both caudate nuclei between August 2014 and February 2017. One implanted participant developed serious mood instability while OFF stimulation and attempted suicide 2 months later, culminating in complete DBS system removal without incident. While no analyzable primary outcomes data were captured, serious and other adverse events reported by this participant were included in safety analysis for inclusive reporting. Thus, 5 implanted participants who completed endpoint assessments following 24 weeks of continuous stimulation at their individualized, most promising parameter settings constituted primary and secondary outcomes data for this trial.
Study cohort demographics and stimulation parameters are shown in Table 2. There were 3 male and 2 female participants, and the mean age was 50.6 (4.8) years. The mean tinnitus duration was 14.8 (6.8) years. Tinnitus spatial location was bilateral in 4 of 5 participants and sound quality was tonal in 3 participants and modulated or multiple in 2. The mean stimulation optimization duration was 9.2 (1.0) months, accounting for the majority of time spent in the interval from initial baseline to endpoint evaluations, which had a mean duration of 18.6 (0.8) months (Table 3).
TABLE 2.
Study cohort demographics and stimulation parameters
| Case No. | Age (Years) |
Sex | Tinnitus Duration (Years) |
Prior Treatment |
Tinnitus Description by Ear |
Lead Contacts |
Pulse Width (μs) |
Amplitude (Volts) |
Frequency (Hertz) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| U01–02 | 38 | F | 2 | Hearing aid to Left ear with Maskers | L Hiss with Beeps | [0–1-2+] | 150 | Off | 150 |
|
| |||||||||
| R None | [8–9-10+] | 150 | 6.0 | 150 | |||||
| U01–03 | 58 | M | 2 | Hearing Aids with Maskers | L High Pitched Tone | [C+2–3-] | 60 | 6.5 | 50 |
|
| |||||||||
| R High Pitched Tone | [C+10–11-] | 60 | 6.0 | 50 | |||||
| U01–04 | 58 | M | 40 | Notched Noise Maskers and TRT | L High Pitched Tone | [0–1-2–3+] | 60 or 180 | 3.0 – 5.0 | 150 or 250 |
|
| |||||||||
| R High Pitched Tone | [8–9-10–11+] | 60 or 180 | 3.0 – 5.0 | 150 or 250 | |||||
| U01–10 | 37 | F | 25 | Hearing Aids with Maskers | L Lawn Mower; Table Saw | [C+1–2-] | 90 | 8.0 | 20 |
|
| |||||||||
| R Lawn Mower; Table Saw | [C+8–9-] | 90 | 8.0 | 20 | |||||
| U01–12 | 62 | M | 5 | Hearing Aids and Support Group | L Medium Pitched Tone | [2+3-] | 90 | 6.0 | 10 |
|
| |||||||||
| R Medium Pitched Tone | [10+11-] | 90 | 6.0 | 10 | |||||
Stimulation parameters for 24 weeks of continuous caudate stimulation to endpoint. F – Female. M – Male. TRT – Tinnitus retraining therapy. C – Case of pulse generator. L – Left. R – Right. μs – microsecond.
TABLE 3.
Primary efficacy and safety outcomes
| Case No. | Baseline to Endpoint Interval |
TFI at Baseline |
TFI at Endpoint |
Δ TFI | Significant Improvement |
EF-score at Baseline |
EF-score at Endpoint |
Δ EF-score† |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| U01–02 | 18 Months | 76.8 | 73.2 | −3.6 | No | −1.24 | −1.19 | 0.05 |
| U01–03 | 16 Months | 66.4 | 46 | −20.4 | Yes | 0.10 | −0.35 | −0.45 |
| U01–04 | 21 Months | 61.6 | 42 | −19.6 | Yes | 0.26 | 0.38 | 0.12 |
| U01–10 | 19 Months | 75.6 | 5.2 | −70.4 | Yes | −0.06 | 0.19 | 0.25 |
| U01–12 | 20 Months | 89.2 | 86.8 | −2.4 | No | −0.04 | 0.33 | 0.37 |
TFI – Tinnitus Functional Index. Significant Improvement ≥ 13 point decrease in TFI. EF – Executive Function. Δ = Endpoint – Baseline.
– All differences not significant.
The stimulation parameters chosen for 24 weeks of continuous stimulation leading to endpoint evaluation were heterogeneous, reflecting case-by-case searches of parameter combinations that were most promising to mitigate tinnitus severity. Within individual participants, the stimulation geometry of lead contacts, pulse width, amplitude, and frequency were similar in both hemispheres, save U01–02 where the lead in the left caudate was OFF; this particular participant preferred unilateral stimulation despite the fact that bilateral stimulation was well tolerated. Across all participants, the stimulation geometry of lead contacts varied widely, pulse width ranged from 60 to 180 microseconds, amplitude ranged from 3.0 to 8.0 Volts, and frequency ranged from 10 to 250 Hz. It should be noted that in contrast to movement disorders, where the typical stimulation frequency is high, the best stimulation frequency in chronic tinnitus may be low or high.
Primary Outcomes
The primary outcomes for tinnitus efficacy and executive function safety of chronic caudate DBS are shown in Table 3. All participants were categorized at the top 2 levels of tinnitus severity despite prior conventional treatment using acoustical or combined acoustical and behavioral approaches. The mean baseline TFI score was 73.9 (4.3). The decrease in TFI score had a mean of 23.3 (12.4), ranged from 2.4 to 70.4, and qualified 60% of participants for clinically significant improvement. Application of the nonparametric Wilcoxon Signed Rank Test (one-tailed; alpha=0.05) demonstrated the change between TFI baseline and endpoint scores was statistically significant. The change in executive function safety, measured by the composite EF-score, was not significant in all participants. Participant U01–02 exhibited lower executive function at baseline relative to others, but there was no significant change between baseline and endpoint measurements, consistent with her known history of attention deficit hyperactivity disorder.
Secondary Outcomes
The secondary outcomes for tinnitus efficacy and neuropsychiatric safety of chronic caudate DBS are shown in Table 4. The decrease in THI score had a mean of 30.8 (10.4), ranged from 16 to 72, and qualified 80% of participants for clinically significant improvement. Application of the nonparametric Wilcoxon Signed Rank Test (one-tailed; alpha=0.05) demonstrated the change between THI baseline and endpoint scores was statistically significant. Of the 4 participants with clinically significant improvement by THI, 3 participants reported ‘Much Better’ and 1 participant reported ‘No Change’ on GIC. The decrease in average NRS score for tinnitus loudness had a mean of 2.5 (1.4) and ranged from 0 to 7.8.
TABLE 4.
Secondary treatment efficacy and safety outcomes
| Case No. | THI at Baseline |
THI at Endpoint |
Δ THI | NRS at Baseline |
NRS at Endpoint |
ΔAVG NRS | Global Impression of Change |
FrSBe at Baseline |
FrSBe at Endpoint |
Δ FrSBe† |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| U01–02 | 76 | 56 | −20* | L 8 | 6 | 0 | Much Better | 67 | 70 | 3 |
|
| ||||||||||
| R 0 | 2 | |||||||||
| U01–03 | 54 | 32 | −22* | L 6 | 3 | −2 | Much Better | 53 | 27 | −26 |
|
| ||||||||||
| R 6 | 5 | |||||||||
| U01–04 | 34 | 18 | −16 | L 7 | 5.5 | −1.8 | Minimally Better | 40 | 58 | −18 |
|
| ||||||||||
| R 6 | 4 | |||||||||
| U01–10 | 74 | 2 | −72* | L 9 | 2 | −7.8 | Much Better | 43 | 40 | −3 |
|
| ||||||||||
| R 9 | 0.5 | |||||||||
| U01–12 | 82 | 58 | −24* | L 9 | 8 | −1 | No Change | 41 | 45 | 4 |
|
| ||||||||||
| R 9 | 8 | |||||||||
THI – Tinnitus Handicap Inventory.
– Δ THI decrease by 20 points is the threshold for clinically significant improvement. NRS – Numeric rating scale of tinnitus loudness. ΔAVG NRS – Average NRS change across both ears. L – Left ear. R – Right ear. FrSBe – Frontal Systems Behavior Scale (T < 60 = normal range; T > 65 = clinically elevated). Global Impression of Change (Very Much Better, Much Better, Better, No Change, Worse, Much Worse, Very Much Worse).
– All differences not significant.
There was no significant change in neuropsychiatric safety assessments in any of the participants as measured by the FrSBe score. Participant U01–01, who exhibited lower executive function at baseline, also showed clinically elevated FrSBe scores, but there was no significant change between baseline and endpoint measurements. The Columbia-Suicide Severity Rating Scale (C-SSRS)33 was administered periodically throughout the trial and scores were all negative. Finally, there was no significant change in hearing safety (Table 5), assessed by difference in air conduction pure-tone average for both low and high frequency bands and WRS, in all participants.
TABLE 5.
Hearing safety outcomes
| Case No. | Ear | Audio (SPL) Low Freq |
Audio (SPL) High Freq |
WRS | Audio (SPL) Δ Low Freq† |
Audio (SPL) Δ High Freq† |
Δ WRS† |
|---|---|---|---|---|---|---|---|
|
| |||||||
| U01–02 | Left | 18.3 dB | 23.3 dB | L (96%) | 1.7 dB | 8.3 dB | L (0%) |
|
| |||||||
| Right | 18.3 dB | 15.0 dB | R (96%) | 3.3 dB | 8.3 dB | R (4%) | |
| U01–03 | Left | 18.3 dB | 48.3 dB | L (96%) | 3.3 dB | −1.7 dB | L (4%) |
|
| |||||||
| Right | 10.0 dB | 53.3 dB | R (92%) | 1.7 dB | −3.3 dB | R (0%) | |
| U01–04 | Left | 3.3 dB | 21.7 dB | L (100%) | 1.7 dB | 3.3 dB | L (0%) |
|
| |||||||
| Right | 5.0 dB | 10.0 dB | R (100%) | 0.0 dB | 5.0 dB | R (0%) | |
| U01–10 | Left | 46.7 dB | 48.3 dB | L (84%) | −1.7 dB | −5.0 dB | L (−4%) |
|
| |||||||
| Right | 48.3 dB | 45.0 dB | R (88%) | −1.7 dB | −3.3 dB | R (8%) | |
| U01–12 | Left | 8.3 dB | 31.7 dB | L (100%) | 1.7 dB | 8.3 dB | L (0%) |
|
| |||||||
| Right | 6.7 dB | 41.7 dB | R (96%) | 0.0 dB | 8.3 dB | R (4%) | |
Low Freq = air conduction average of 0.5, 1, and 2 kHz. High Freq = air conduction average of 3, 4, and 6 kHz. Audio – Audiogram. Freq – Frequency. L – Left. R – Right. WRS – Word Recognition Score. SPL – Sound Pressure Level in dB. Δ = Endpoint – Baseline.
– All differences not significant.
Safety
Adverse events (AEs) considered probably or definitely related to surgery or caudate stimulation are summarized in Table 6. Surgery related AEs were transient and expected, such as incisional pain and headache. Stimulation related AEs were also transient and associated with specific stimulation parameters above a certain stimulation amplitude; the effect was immediately reversible by reducing stimulation voltage or changing stimulation parameters. The most common of these stimulation-induced AEs was transient worsening of tinnitus. This was observed at various times in all participants and was an expected outcome as acute caudate stimulation has been shown to both increase and decrease tinnitus loudness, depending on stimulation parameters, in movement disorders patients with comorbid tinnitus undergoing awake DBS surgery.7,18 One participant experienced transient, stimulation-induced visual phantoms at specific stimulation parameters. Separately, this participant welcomed a feeling of increased energy or alertness while on stimulation parameters that provided the best tinnitus benefit.
TABLE 6.
Surgical and device adverse events
| Adverse Event Description |
Number of Participants |
|---|---|
|
| |
| Postop Incisional Pain | 6 |
| Transiently Worsened Tinnitus | 6 |
| Postop Headache | 4 |
| Pulling Sensation at IPG | 3 |
| Facial/Neck Tingling | 2 |
| Lightheadedness/Dizzy | 2 |
| Postop Fatigue | 2 |
| Sleep Disturbance | 2 |
| Worsened Depression | 2 |
| Increased Energy | 1 |
| Postop Nausea | 1 |
| Visual Phantoms | 1 |
| Suicide Ideation/Attempt┼ | 1 |
Adverse events for all 6 implanted participants. All were transient with the exception of elevated electrode impedances in one participant. IPG – Internal pulse generator.
– Serious adverse event.
There were no surgical or stimulation related serious adverse events (SAEs). There was only one SAE in the study, a suicide attempt in an implanted participant that occurred while OFF stimulation. This participant denied suicidality during screening, but later confided to the study team of a several year history of passive suicidal ideation prior to enrollment and subsequent ongoing passive suicidal ideation approximately 5 months after surgery. Outpatient psychiatric care was urgently instituted. The stimulator was deactivated to remove any possible stimulation-related effect. During the 2 months of OFF stimulation leading to attempted suicide, tinnitus severity was unchanged. This participant was removed from the study due to expressed suicidality. Following stabilization of mood, the participant requested removal of the entire DBS system, which was performed without incident.
Discussion
This open-label, first-in-human phase I clinical trial to evaluate efficacy and safety of long-term bilateral caudate nucleus neuromodulation by DBS for severe, treatment-resistant tinnitus in a cohort of 5 adults showed very encouraging results. Primary and secondary outcomes revealed a highly variable effect size and 60–80% treatment response rate for clinically significant benefit, and stable safety profile along the domains of executive function, frontal behaviors, and audiometric thresholds.
A lengthy period of stimulation optimization in all participants to identify individual-specific most promising stimulation parameters for tinnitus mitigation was unanticipated, given the relative ease of DBS to effect tinnitus loudness reduction in the preliminary data cohort of movement disorders patients with comorbid tinnitus.7 There are several nonexclusive explanations: 1) basal ganglia circuits of the phase I trial cohort are relatively more difficult to modulate compared to the preliminary data cohorts with known nigrostriatal degenerative disease, 2) functional connectivity networks in treatment-resistant tinnitus vary in location from individual-to-individual and are not easily modified should stimulation be delivered at a less than ideal treatment target position, and 3) neurological substrates of severe tinnitus refractory to conventional treatments in trial participants are different from comorbid chronic tinnitus in movement disorders patients.
Variations in stimulation frequency among participants to modulate treatment-resistant tinnitus were a surprising finding. Optimal responses were identified at low (20Hz), moderate (50Hz) and high (150+Hz) frequencies. It is possible that different phantoms respond optimally to specific frequencies, although this conjecture cannot be assessed in this small cohort. It is noteworthy that all but one participant required two or three active cathodal contacts, and all but one preferred relatively high stimulation amplitudes (6–8V range) to realize optimal benefit. Such settings reflect a relatively large volume of tissue activation. It is unclear if these stimulation parameters are necessary to achieve modulation of the underlying circuit most directly involved in tinnitus perception, or if the optimal locus of stimulation is farther away from the active contacts than desired. Four of the five participants who completed the study had bilateral tinnitus and all reported a preference for bilateral stimulation to achieve optimal tinnitus modulation. One participant had unilateral tinnitus localized to the left ear and reported preference for right caudate stimulation only, even though bilateral stimulation was well tolerated. This participant was a non-responder, so the significance of her preference for unilateral stimulation is unclear.
Surgical and device AEs were generally expected, always transient, and completely reversible. Notably, changing stimulation parameters dissipated stimulation-induced AEs, typically by reducing amplitude. One interesting AE was stimulation-dependent triggering of visual phantom percepts. This may represent striatal gating of visual phantoms, as connectivity between the caudate nucleus and extrastriate visual cortex has been demonstrated in rhesus monkeys.44 The best known visual phantom variant is Charles Bonnet Syndrome, which is associated with temporary or permanent visual impairment.9 DBS lead trajectories that penetrate the caudate have been associated with greater risk of cognitive decline in Parkinson’s patients, although these findings have been disputed by others.28,43 We did not observe any changes in cognition in our study cohort with direct caudate implantation and stimulation.
One concerning SAE was a suicide attempt by an implanted participant who had been OFF stimulation for 2 months, thus the event was not stimulation-related. This participant sequestered thoughts of passive suicidal ideation, which occurred as often as once a week for several years, prior to entering the study. The SAE highlights fragility of severe tinnitus sufferers, need to treat comorbid mood and related disorders aggressively, and fallibility of even the most rigorous screening procedure for study enrollment. There is not yet an objective diagnostic tool to measure self-reported subjective tinnitus severity and associated comorbidities.
There are two notable study limitations. First, tinnitus is a sensory phantom perceptual disorder and despite our careful selection process for trial enrollment, efficacy outcomes are completely dependent on reliable participant reporting. As this phase I trial was an open-label design, possible biased reporting could have contaminated results. We will consider implementation of randomized stimulation ON and OFF trial blocks with crossovers in association with blinding of study participants and study team members to capture efficacy and safety assessment data in future studies. Second, caudate nucleus target selection for tinnitus modulation was solely based on limited intraoperative macrostimulation interrogation. In contrast to Parkinson’s disease, the most common disorder treated with DBS, tinnitus does not have the benefit of a robust animal model that can be used to explore and evaluate preclinical neuromodulation targets. Moreover, the basal ganglia targets implanted in Parkinson’s disease are small and well characterized. The caudate is an anatomically large structure and methodology for optimal target selection for implantation will require further refinements. Of note, our first report from this patient cohort demonstrated that acute tinnitus loudness reduction was associated with more posterior lead locations in regions of the caudate with stronger functional connectivity to auditory cortex on fMRI.32 We anticipate that a participant-specific, personalized corticostriatal connectivity map15 approach will decrease duration of stimulation optimization and increase treatment effect size in a phase II trial of caudate DBS for treatment-resistant tinnitus.
Conclusions
Bilateral caudate nucleus neuromodulation by DBS for severe, refractory tinnitus in this phase I trial showed very encouraging results. Primary and secondary outcomes revealed a highly variable treatment effect size and 60–80% treatment response rate for clinically significant benefit, and no safety concerns. The design of a phase II trial may benefit from targeting refinement for final DBS lead placement to decrease stimulation optimization period duration and to increase treatment effect size uniformity.
Supplementary Material
Acknowledgments
This study was supported by the NIH-NIDCD (grant no. 5 U01DC013029, S.W.C.), Department of Defense (grant nos. W81XWH-13–1-0494 and W81XWH1810741, S.W.C.), and Coleman Memorial Fund (S.W.C.) and performed under an IDE issued by the FDA (IDE no. G120132, P.S.L).
Financial Support: This study was supported by the NIH-NIDCD (grant no. 5 U01DC013029, S.W.C.), Department of Defense (grant nos. W81XWH-13–1-0494 and W81XWH1810741, S.W.C.), and Coleman Memorial Fund (S.W.C.).
Footnotes
Disclosures
Co-authors Paul S. Larson and Susan Heath have received honoraria from Medtronic, the company that manufactures the deep brain stimulation device used for this study.
References
- 1.AAO-HNS: Otologic Referral Criteria for Occupational Hearing Conservation Programs, in American Academy of Otolaryngology - Head and Neck Surgery Foundation, Alexandria, VA., 1997 [Google Scholar]
- 2.Beebe Palumbo D, Joos K, De Ridder D, Vanneste S: The Management and Outcomes of Pharmacological Treatments for Tinnitus. Curr Neuropharmacol 13:692–700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz LT, Muhlberger A, Langguth B, Schecklmann M: Stress Reactivity in Chronic Tinnitus. Sci Rep 7:41521, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt JM, Bhattacharyya N, Lin HW: Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 127:466–469, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canlon B, Theorell T, Hasson D: Associations between stress and hearing problems in humans. Hear Res 295:9–15, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Carney E, Schlauch RS: Critical difference table for word recognition testing derived using computer simulation. J Speech Lang Hear Res 50:1203–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cheung SW, Larson PS: Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169:1768–1778, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Coles RR: Epidemiology of tinnitus: (1) prevalence. J Laryngol Otol Suppl 9:7–15, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Coltheart M: Charles Bonnet Syndrome: Cortical Hyperexcitability and Visual Hallucination. Curr Biol 28:R1253–R1254, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Cronlein T, Langguth B, Pregler M, Kreuzer PM, Wetter TC, Schecklmann M: Insomnia in patients with chronic tinnitus: Cognitive and emotional distress as moderator variables. J Psychosom Res 83:65–68, 2016 [DOI] [PubMed] [Google Scholar]
- 11.De Ridder D, Joos K, Vanneste S: Anterior cingulate implants for tinnitus: report of 2 cases. J Neurosurg 124:893–901, 2016 [DOI] [PubMed] [Google Scholar]
- 12.De Ridder D, Vanneste S, Plazier M, Menovsky T, van de Heyning P, Kovacs S, et al. : Dorsolateral prefrontal cortex transcranial magnetic stimulation and electrode implant for intractable tinnitus. World Neurosurg 77:778–784, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Dewyer NA, Kiringoda R, Kram YA, Chang JL, Chang CY, Cheung SW: Stapedectomy Effects on Tinnitus: Relationship of Change in Loudness to Change in Severity. Otolaryngol Head Neck Surg 153:1019–1023, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Henry JA, Schechter MA, Zaugg TL, Griest S, Jastreboff PJ, Vernon JA, et al. : Outcomes of clinical trial: tinnitus masking versus tinnitus retraining therapy. J Am Acad Audiol 17:104–132, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hinkley LB, Mizuiri D, Hong O, Nagarajan SS, Cheung SW: Increased striatal functional connectivity with auditory cortex in tinnitus. Front Hum Neurosci 9:568, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehrle HM, Sampaio AL, Granjeiro RC, de Oliveira TS, Oliveira CA: Tinnitus Annoyance in Normal-Hearing Individuals: Correlation With Depression and Anxiety. Ann Otol Rhinol Laryngol 125:185–194, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G: Tinnitus and depression. World J Biol Psychiatry 12:489–500, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Larson PS, Cheung SW: Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery 70:398–405; discussion 405–396, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Larson PS, Cheung SW: A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J Neurosurg 118:192–194, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP: Dysregulation of limbic and auditory networks in tinnitus. Neuron 69:33–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry LD, Eisenman LM, Saunders JC: An absence of tinnitus. Otol Neurotol 25:474–478, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A: Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev:Cd005233, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Martinez C, Wallenhorst C, McFerran D, Hall DA: Incidence rates of clinically significant tinnitus: 10-year trend from a cohort study in England. Ear Hear 36:e69–75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFadden D: Tinnitus: facts, theories, and treatments. Washington, D.C.: National Academy Press, 1982 [PubMed] [Google Scholar]
- 25.Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. : The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 33:153–176, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Melcher JR, Sigalovsky IS, Guinan JJ, Jr., Levine RA: Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83:1058–1072, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Mirz F, Gjedde A, Ishizu K, Pedersen CB: Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl 543:241–243, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Morishita T, Okun MS, Jones JD, Foote KD, Bowers D: Cognitive declines after deep brain stimulation are likely to be attributable to more than caudate penetration and lead location. Brain 137:e274, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Muhlnickel W, Elbert T, Taub E, Flor H: Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95:10340–10343, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Newman CW, Sandridge SA, Jacobson GP: Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J Am Acad Audiol 9:153–160, 1998 [PubMed] [Google Scholar]
- 32.Perez PL, Wang SS, Heath S, Henderson-Sabes J, Mizuiri D, Hinkley LB, et al. : Human caudate nucleus subdivisions in tinnitus modulation. J Neurosurg:1–7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. : The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rammo R, Ali R, Pabaney A, Seidman M, Schwalb J: Surgical Neuromodulation of Tinnitus: A Review of Current Therapies and Future Applications. Neuromodulation, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Rauschecker JP, Leaver AM, Muhlau M: Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66:819–826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauschecker JP, May ES, Maudoux A, Ploner M: Frontostriatal Gating of Tinnitus and Chronic Pain. Trends Cogn Sci 19:567–578, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shargorodsky J, Curhan GC, Farwell WR: Prevalence and characteristics of tinnitus among US adults. Am J Med 123:711–718, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Trevis KJ, McLachlan NM, Wilson SJ: Psychological mediators of chronic tinnitus: The critical role of depression. J Affect Disord 204:234–240, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, et al. : Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci Rep 7:11960, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, De Ridder D: The neural correlates of tinnitus-related distress. Neuroimage 52:470–480, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Vio MM, Holme RH: Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today 10:1263–1265, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T: Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med 2:e153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T, et al. : Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain 136:2109–2119, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Yeterian EH, Pandya DN: Corticostriatal connections of extrastriate visual areas in rhesus monkeys. J Comp Neurol 352:436–457, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Zenner HP, Delb W, Kroner-Herwig B, Jager B, Peroz I, Hesse G, et al. : A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur Arch Otorhinolaryngol 274:2079–2091, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


