Abstract
Objective:
Understanding lung cancer screening behaviour is crucial to identifying potentially modifiable factors for future intervention. Qualititative work has explored attitudes and beliefs about lung cancer screening from the perspective of the participant, but the theoretically grounded factors that influence screening-eligible individuals to screen are unknown. We tested an explanatory framework for lung cancer screening participation from the individual’s perspective.
Methods:
Data were collected as part of a sequential explanatory mixed methods study, the quantitative component of which is reported here. A national purposive sample of 515 screening-eligible participants in the United States was recruited using Facebook-targeted advertisement. Participants completed surveys assessing constructs of the Conceptual Model for Lung Cancer Screening Participation. Path analysis was used to assess the relationships between variables.
Results:
Path analyses revealed that a clinician recommendation to screen, higher self-efficacy scores, and lower mistrust scores were directly associated with screening participation (p < 0.05). However, the link between screening behaviour and self-efficacy appeared to be fully mediated by fatalism, lung cancer fear, lung cancer family history, knowledge of lung cancer risk and screening, income, clinician recommendation, and social influence (p < 0.05).
Conclusions:
This study found that medical mistrust, self-efficacy, and clinician recommendation were significant in the decision of whether to screen for lung cancer. These findings offer insight into potentially modifiable targets most appropriate on which to intervene. This understanding is critical to design meaningful clinician- and patient-focused interventions.
Keywords: Lung cancer screening, health behaviour, path analysis, conceptual model, health beliefs
Introduction
Lung cancer screening could avert approximately 11,000 lung cancer deaths annually in the United States and has been recommended by the United States Preventive Services Task Force (USPSTF) since 2013 for long-term current and former smokers.1 In a recent study, only 3.9% of eligible individuals reported undergoing lung cancer screening in the past year.2 Low uptake of lung cancer screening is the result of multiple patient, provider, and system-level factors, and a greater understanding of the impact of these variables on lung cancer screening behaviour is needed.3,4 The identification of variables that influence screening behaviour would provide foundational information to develop interventions to enhance decision-making and follow-through when the decision is to screen. Smokers are a unique population, different from those targeted for other types of cancer screening because they battle nicotine addiction, and experience stigma and blame from others, who consider tobacco-related diseases to be self-inflicted. Based on our preliminary research, as well as research by others, psychological variables such as perceived stigma, medical mistrust, cancer fatalism, fear, and worry seem to be relevant to lung cancer screening.5–9
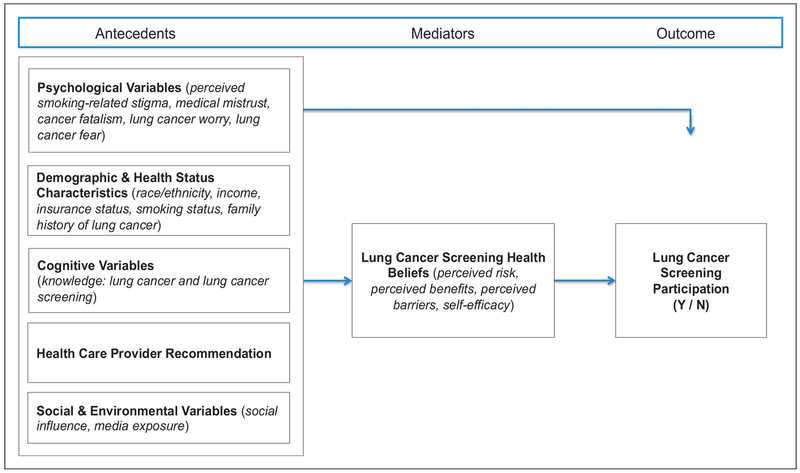
We developed a conceptual model to explain lung cancer screening behaviour from the perspective of the individual making the decision to screen or not (Figure 1).10 The Conceptual Model for Lung Cancer Screening Participation is based on: (i) empirically supported theoretical linkages from a comprehensive literature review; (ii) screening-eligible individuals’ perspectives of key Health Belief Model (HBM) constructs; and (iii) our preliminary focus group findings.5,10 Our proposed framework depicts psychological variables as key factors in lung cancer screening behaviour, and links these and other factors to traditional HBM constructs that have predicted participation in screening for other cancers.10 Linking the uniquely important psychological variables with traditional HBM constructs has the potential to offer new foundational knowledge needed to tailor future interventions for this high-risk population.
Figure 1.
Conceptual model for lung cancer screening participation.
This paper reports the results from the path analysis component of a sequential explanatory mixed methods study that tested our proposed framework for lung cancer screening behaviour. This study complements other efforts that are directed at provider- and healthcare system levels, by examining factors that influence lung cancer screening participation from the perspective of the individual considering screening. The study objective was to test the relationships among the antecedent, mediator, and outcome variables depicted in the conceptual model (Figure 1), using structural equation modelling in a sample of long-term current and former smokers, who were eligible for lung cancer screening. We hypothesized that (i) psychological variables, demographic and health status characteristics, cognitive variables, healthcare provider recommendation, social, and environmental variables (antecedents) would be significantly associated with lung cancer screening health beliefs (mediators); (ii) psychological variables, demographic and health status characteristics, cognitive variables, healthcare provider recommendation, and social and environmental variables (antecedents), and lung cancer screening health beliefs (mediators) would have significant direct associations with lung cancer screening participation (outcome); (iii) psychological variables, demographic and health status characteristics, cognitive variables, healthcare provider recommendation, social and environmental variables (antecedents) would have significant indirect associations with lung cancer screening participation (outcome) through lung cancer screening health beliefs (mediators); and (iv) the overall model would demonstrate goodness of fit by well-established thresholds.
Methods
After institutional review board approval was obtained from Indiana University, a cross-sectional study was conducted nationwide from May to July 2017 using survey methodology. A national purposive sample of 515 participants was recruited. Sample size was determined based on the overall aims of the study. For the purpose of this specific paper, Monte Carlo simulations (using Mplus software) with 1000 simulated datasets were conducted to determine the power for estimating the coefficients for all effects in the proposed path model. Assuming an alpha level of 0.05, a sample size of 500 provides adequate power (⩾80%) to detect significance for each path coefficient in the model when the direct effect sizes are 0.53 or larger (with an indirect effect size of 0.20 or larger, indicating a smaller effect size through mediation). Eligibility criteria mirrored the USPSTF recommendation for individuals eligible for lung cancer screening1 and included individuals (1) aged 55 to 80, (2) minimum 30 pack-year tobacco smoking history, (3) current smoker or former smoker who had quit within the past 15 years, and (4) never been diagnosed with lung cancer.
To recruit 515 participants, we used Facebook-targeted advertisement.11 Facebook has the ability to target an advertisement by demographics and keywords listed in users’ profiles or interest lists, which allowed us to purposively sample people who were aged 55 or older and who indicated smoking as an interest. Data were collected via a single web-based survey developed using the REDCap (Research Electronic Data Capture) system, a secure web-based application for building and managing online surveys and databases. REDCap provides audit trails for tracking data manipulation and user activity as well as automated export procedures for secure data downloads to common statistical packages. A screening questionnaire was used to determine eligibility. Eligible participants received a message on screen inviting them to participate in the study, with an embedded informed consent form for their review. A telephone number to the research office and study email address were provided for those who had questions, needed technical assistance, or additional information. Participants completed the 25-min survey and received a $15 gift card at completion.
Data were collected via self-report using a compilation of items and scales to assess the outcome, antecedent, and mediator variables via a web-based survey.
The outcome variable Lung Cancer Screening Behaviour was measured using a stage of adoption algorithm guided by the Precaution Adoption Process Model.12 Those in stages 1 (unaware), 2 (unengaged), 3 (undecided), and 4 (decided not to act) were considered not to have participated, and those in stages 5 through 7 (i.e. stage 5, those who indicated they decided to screen for lung cancer; stage 6, had recently completed lung cancer screening; or stage 7, were currently screening annually) were considered participants for lung cancer screening (for algorithm see Table 1).
Table 1.
Stage of adoption: lung cancer screening.a
| Stage classification | |
|---|---|
| 1. Have you ever heard of lung cancer screening with a lung scan (also commonly called a low-dose CAT scan)? | |
| No | 1 = Unaware |
| Yes (proceed to next question) | |
| 2. Have you ever thought about having a lung scan (low-dose CAT scan) to screen for lung cancer? | |
| No | 2 = Unengaged |
| Yes (proceed to next question) | |
| 3. Do you plan to have a lung scan to screen for lung cancer? | |
| I don’t know | 3 = Undecided |
| No | 4 = Decided not to act |
| Yes | 5 = Decided to act (and proceed to next question) |
| 4. Have you made an appointment to have a lung scan? | |
| No | 5 = Decided to act |
| Yes | 6 = Action |
| 5. Are you currently having lung scans on a yearly basis? | |
| No | 6 = Action |
| Yes | 7 = Maintenance |
Based on the Precaution Adoption Process Model.
Among the antecedent variables, Perceived Smoking-Related Stigma was measured using the five-item smoking-related stigma subscale of the Cataldo Lung Cancer Stigma Scale,13 with response options ranging from 1 = strongly disagree to 4 = strongly agree. Medical Mistrust was measured using the Patient Trust in the Medical Profession Scale.14 The five-point Likert responses measured the extent to which patients perceive their provider to be honest, thorough, careful, and trusted, versus caring more about convenience. Cancer Fatalism was measured using the Revised Powe Fatalism Inventory,15 which assesses the degree to which an individual equates cancer with death. The inventory uses a dichotomous response for 11 belief statements that assess cancer fear, pessimism, predetermination, and inevitability of death. Lung Cancer Worry was measured using items adapted from the Lerman Breast Cancer Worry Scale,16 which consists of three Likert-style response items. Lung Cancer Fear was measured using items adapted from the Champion Breast Cancer Fear Scale.17 Demographic and Health Status Characteristics were assessed with questions addressing race/ethnicity, income level, insurance status, smoking status, and family history of lung cancer. Knowledge: Lung Cancer and Lung Cancer Screening was assessed with an eight-item multidimensional scale used in our preliminary studies, adapted from literature specific to lung cancer.18,19 Investigator-developed items were used to assess Healthcare Provider Recommendation, Social Influence, and Media Exposure. Specifically, healthcare provider recommendation was assessed with the dichotomous item: “Has a doctor or nurse practitioner ever recommended that you have a lung scan to screen for lung cancer?” Social influence was assessed with four items adapted from the social influence scale by McQueen et al. to assess the importance of other individuals, such as family members, other people their own age, friends, and their doctor, thinking it is important for the participant to screen for lung cancer.20 Media exposure was assessed with three dichotomous items to assess seeing or hearing a newspaper/magazine, radio, or television advertisement about lung cancer screening in the past 30 days.
For Mediator Variables, the Lung Cancer Screening Health Belief Scales assessed perceived risk of lung cancer, perceived benefits of, perceived barriers to, and self-efficacy for lung cancer screening.18 Table 2 details all survey measures and their internal consistency reliability where appropriate for this study.
Table 2.
Measures.
| Variable/construct | Scale | # of items | Cronbach’s alpha |
|---|---|---|---|
| Outcome variable | |||
| Lung cancer screening participation | Precaution Adoption Process Model Algorithm | 5 | – |
| Antecedent variables | |||
| Psychological variables | |||
| Perceived smoking-related stigma | Cataldo Lung Cancer Stigma Scale – Smoking Subscale | 5 | 0.72 |
| Medical mistrust | Patient Trust in the Medical Profession Scale | 5 | 0.76 |
| Cancer fatalism | Revised Powe Fatalism Inventory | 11 | 0.88 |
| Lung cancer worry | Adaptation of the Lerman Breast Cancer Worry Scale | 3 | 0.79 |
| Lung cancer fear | Champion Breast Cancer Fear Scale | 8 | 0.93 |
| Cognitive variables | |||
| Knowledge: Lung cancer and lung cancer screening | Multidimensional Knowledge of Lung Cancer & Screening Questionnaire | 8 | – |
| Mediator variables | |||
| Lung cancer screening health beliefs | |||
| Perceived risk of lung cancer | Lung Cancer Screening Health Belief Scales – Perceived Risk Subscale | 3 | 0.86 |
| Perceived benefits of lung cancer screening | Lung Cancer Screening Health Belief Scales – Perceived Benefits Subscale | 6 | 0.86 |
| Perceived barriers to lung cancer screening | Lung Cancer Screening Health Belief Scales – Perceived Barriers Subscale | 17 | 0.91 |
| Self-efficacy for lung cancer screening | Lung Cancer Screening Health Belief Scales – Self-Efficacy Subscale | 9 | 0.91 |
Mediation analyses were performed with path analysis models using Mplus v 7.31. Direct paths were specified from each of the antecedent variables to all of the mediating variables, from antecedent variables to the dichotomous screening participation variable, and from the mediating variables to the screening participation variable. Indirect paths were specified from the antecedents to the screening participation variable through the mediating variables. Rather than using the four-part piecewise mediation method as proposed by Baron and Kenny,21 this path analysis utilized structural equation modelling to model every path simultaneously, accounting for the variance of each association. Model modification indices were inspected to determine if additional paths should be included between antecedents or between mediators, or to account for correlations between mediators, and to improve model fit. All analytic assumptions were verified. The theta parameterization was used, due to the binary nature of the screening participation outcome. The logit link was specified for paths with a binary-dependent variable, and the linear link for paths pointing toward a continuous variable. All antecedent variables in the conceptual model (see Figure 1) were included in the path model because they were carefully selected and hypothesized based on theoretical rationale and prior literature. Therefore, the path analysis was a confirmatory analysis of a hypothesized model. All path coefficients for the conceptual model were tested simultaneously. All tests were two-sided. Alpha of 0.05 was used to interpret significance for all tests. Because multiple testing could introduce the possibility of inflated Type I error, we have not reported bivariate relationships, and instead focus on results from the multivariable path model for which the effects of predictors are adjusted for each other in a single model.
Results
Participant sociodemographic and health status characteristics are shown in Table 3. Participants (n = 515) ranged in age from 55 to 80 (mean, 61.4 (SD 5.4)) with a greater number being female (64.9%; n = 334) and white (84.5%; n = 435). Participants were diverse in education and annual income levels, and more than half were current smokers (63.3%; n = 326). The average number of years smoked among all participants was 38.3 (SD 9.9), and the mean pack years smoked was 56.6 (26.8).
Table 3.
Sociodemographic and health status characteristics.
| Characteristics (categorical) | Total sample (n = 515) | n % |
|---|---|---|
| Gender | ||
| Female | 334 | 64.9 |
| Male | 179 | 34.8 |
| Other | 2 | 0.4 |
| Race | ||
| White | 435 | 84.5 |
| Black | 53 | 10.3 |
| Asian/Asian-American | 1 | 0.2 |
| American Indian/Alaskan Indian | 4 | 0.8 |
| Hawaiian/Pacific Islander | 1 | 0.2 |
| Other | 8 | 1.6 |
| Multi | 13 | 2.5 |
| Hispanic | 29 | 5.6 |
| Education | ||
| Less than high school | 39 | 7.6 |
| High school graduate | 157 | 30.5 |
| Some college | 184 | 35.7 |
| College degree or higher | 135 | 26.2 |
| Marital status | ||
| Married | 198 | 38.5 |
| Divorced | 135 | 26.3 |
| Widowed | 64 | 12.5 |
| Separated | 25 | 4.9 |
| Never married | 57 | 11.1 |
| Living with partner | 33 | 6.4 |
| Don’t know | 2 | 0.4 |
| Working status | ||
| Full time | 132 | 25.7 |
| Part time | 83 | 16.2 |
| No | 299 | 58.2 |
| Annual income | ||
| <$25,000/year | 223 | 43.3 |
| $25,000–$50,000/year | 175 | 34.0 |
| >$50,000/year | 117 | 22.7 |
| Financial security | ||
| After paying bills, there is enough to buy special things | 102 | 20.0 |
| After paying bills, there is little extra | 205 | 40.1 |
| Enough for bills, if I cut back on things | 119 | 23.3 |
| Difficulty paying bills | 85 | 16.6 |
| Had health insurance | 463 | 89.9 |
| Current smoker | 326 | 63.3 |
| Family history of lung cancer | 130 | 25.2 |
| Stage of adoption n (%) | n | % |
| 1 (unaware) | 227 | 44.1 |
| 2 (unengaged) | 56 | 10.9 |
| 3 (undecided) | 56 | 10.9 |
| 4 (decided not to act) | 4 | 0.8 |
| 5 (decided to act) | 47 | 9.1 |
| 6 (acting) | 70 | 13.6 |
| 7 (maintenance) | 55 | 10.7 |
| Characteristics (continuous) | Mean (SD); median (range) | |
| Age (years) | 61.41 (5.41); 61.0 (55–80) | |
| Pack years | 56.61 (26.84); 50.0 (30–247.5) | |
| Current packs per day (n=324) | 1.99 (3.13); 1.0 (0–30) | |
| Years smoked in total (n=324) | 38.28 (9.87); 40.0 (15–68) | |
| Packs smoked when used to smoke (n=150)) | 2.21 (4.05); 1.5 (1–30) | |
| Years smoked former smokers (n=148) | 37.20 (9.84); 38.5 (15–65) | |
After the initial path analysis was performed, it was determined that significant correlations existed between mediating variables, namely, between self-efficacy for and benefits of lung cancer screening, and self-efficacy for and barriers to lung cancer screening. When these additional correlation paths were added to the model, the path analysis model’s goodness of fit indices indicated good fit (chi-square = 3.195, p = 0.526; RMSEA = 0.000 (90% CI = 0.000, 0.061), CFI = 1.0, and WRMR = 0.188, Table 4). Because of the chi-square p-value >0.05, the null hypothesis of good fit was not rejected. Prior methodology studies established that the following thresholds indicate good fit of the model to the data: RMSEA <0.0622; CFI >0.9522; and WRMR < 1.0.23 Goodness of fit indices are given to evaluate whether the conceptual model accurately captures the relationships among the variables. Our fit indices exceeded (i.e. satisfied) these published thresholds.22,23
Table 4.
Path analysis: standardized coefficients from antecedents (A), mediators (M) and outcome (O).
| M = Perceived risk |
M = Perceived benefits |
M = Perceived barriers |
M = Self-efficacy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Antecedent (A) | A to M (direct) | A to M to O (indirect) | A to M (direct) | A to M to O (indirect) | A to M (direct) | A to M to O (indirect) | A to M (direct) | A to M to O (indirect) | A to O (direct) |
| Perceived stigma | −0.06 | 0.00 | −0.03 | 0.00 | −0.08 | 0.00 | −0.05 | −0.01 | −0.03 |
| Medical mistrust | 0.00 | 0.00 | 0.04 | 0.00 | −0.22 | 0.01 | 0.06 | 0.01 | −0.13 |
| Cancer fatalism | 0.13 | 0.00 | −0.12 | 0.00 | 0.22 | −0.01 | −0.14 | −0.03 | 0.08 |
| Lung cancer worry | 0.45 | −0.01 | 0.11 | 0.00 | 0.01 | 0.00 | −0.03 | −0.01 | 0.03 |
| Lung cancer fear | −0.11 | 0.00 | 0.06 | 0.00 | 0.23 | −0.01 | −0.17 | −0.04 | 0.00 |
| Race (White) | 0.08 | 0.00 | 0.02 | 0.00 | −0.13 | 0.01 | 0.06 | 0.01 | 0.03 |
| Annual income | 0.03 | 0.00 | 0.09 | 0.00 | −0.07 | 0.00 | 0.12 | 0.03 | 0.03 |
| Health insurance | −0.03 | 0.00 | 0.00 | 0.00 | −0.09 | 0.00 | 0.07 | 0.02 | −0.01 |
| Current smoker | 0.14 | 0.00 | 0.00 | 0.00 | 0.13 | −0.01 | −0.02 | −0.01 | 0.06 |
| Family history of lung cancer | 0.07 | 0.00 | 0.02 | 0.00 | −0.16 | 0.01 | 0.10 | 0.02 | 0.06 |
| Knowledge of lung cancer and screening | 0.06 | 0.00 | 0.08 | 0.00 | −0.04 | 0.00 | 0.15 | 0.03 | −0.06 |
| Provider recommendation | 0.09 | 0.00 | −0.07 | 0.00 | −0.07 | 0.00 | 0.13 | 0.03 | 0.47 |
| Social influence | 0.03 | 0.00 | 0.39 | 0.01 | −0.19 | 0.01 | 0.40 | 0.09 | −0.04 |
| Media exposure | 0.00 | 0.00 | 0.02 | 0.00 | −0.02 | 0.00 | 0.04 | 0.01 | 0.06 |
| Overall model fit (N = 509) |
|||||||||
| Mediators (M) | M to O (direct) | Goodness of fit test: Chi-Square = 3.195, p = 0.526 (i.e. failed to reject Ho of good fit). | |||||||
| Perceived risk of lung cancer | −0.03 | Fit Indices | Criteria for good fit | ||||||
| Perceived benefit of lung cancer screening | −0.01 | RMSEA = 0.000 (90% CI: (0.000, 0.061)) | <0.06 | ||||||
| Perceived barriers to lung cancer screening | −0.04 | CFI = 1.000 | >0.95 | ||||||
| Self-efficacy for lung cancer screening | 0.23 | WRMR = 0.188 | <1.00 | ||||||
Note: Bolded standardized coefficients are significant (p < 0.05). Parameter estimates are from MPLUS using Weighted least square mean and variance estimation and Standardized Estimate of y with X.
There were several significant direct-effect paths from antecedents to mediators. Table 4 shows all standardized coefficients from the path analysis model. We highlight the significant findings here. Variables positively associated with perceived risk of lung cancer were: cancer fatalism (standardized path coefficient β = 0.13), lung cancer worry (β = 0.45), smoking status (β = 0.14), and healthcare provider recommendation (β = 0.09). Only fear was negatively associated with perceived risk (β = −0.11). Variables positively associated with perceived benefits of lung cancer screening were lung cancer worry (β = 0.11), income (β = 0.09), knowledge of lung cancer and screening (β = 0.08), and social influence (β = 0.39). Only cancer fatalism was negatively associated with perceived benefits (β = −0.12). Variables positively associated with perceived barriers to lung cancer screening were current smoking status (β = 0.13), cancer fatalism (β = 0.22), and lung cancer fear (β = 0.23). Variables negatively associated were smoking-related stigma (β = −0.08), race (white β = −0.13), insurance status (β = −0.09), family history of lung cancer (β = −0.16), and social influence (β = −0.19). Variables positively associated with self-efficacy for lung cancer screening were income (β = 0.12), family history of lung cancer (β = 0.10), knowledge of lung cancer and screening (β = 0.15), healthcare provider recommendation (β = 0.13), and social influence (β = 0.40). Cancer fatalism (β = −.14) and lung cancer fear (β = −.17) were negatively associated with self-efficacy.
Medical mistrust was negatively (β = −0.13), and healthcare provider recommendation was positively (β = 0.47), associated with screening participation as direct effects. The only mediator with a significantly direct effect with screening participation was self-efficacy for lung cancer screening (β = 0.23).
In the tests of mediation, the indirect effects between all antecedents (including medical mistrust and healthcare provider recommendation) and screening participation were non-significant for perceived risk of lung cancer, perceived benefits of, and perceived barriers to lung cancer screening. However, there were significant indirect effects from the following antecedents through self-efficacy to lung cancer screening, although the effects were of small magnitude: cancer fatalism (β = −0.03), lung cancer fear (β = −0.04), family history of lung cancer (β = 0.02), knowledge of lung cancer and screening (β = 0.03), healthcare provider recommendation (β = 0.03), and social influence (β = 0.09).
Discussion
In testing the Conceptual Model for Lung Cancer Screening Participation,5 and consistent with other types of cancer screening,24–26 receiving a healthcare provider recommendation to screen is associated with lung cancer screening behaviour. Inversely, higher levels of medical mistrust were associated with individuals who indicated that they had not, or would not, screen for lung cancer. Although the other antecedent variables in the conceptual model were not statistically significant in this sample, many had significant direct effects with the mediators (perceived risk of lung cancer, perceived benefits of, perceived barriers to, and self-efficacy for lung cancer screening), and significant indirect effects with the mediating variable, self-efficacy for lung cancer screening. Further qualitative exploration of screening behaviour from the perspective of the individual making the decision on whether to screen for lung cancer can assist in understanding lung cancer screening behaviour beyond quantitative survey scores, and may provide a more robust elucidation of screening behaviour and the scientific utility of retaining or removing a variable from the current conceptual model on lung cancer screening participation.
Our efforts to understand which people are more likely to undergo lung cancer screening showed that people who perceived their risk of getting lung cancer to be higher were current smokers, had higher levels of cancer fatalism and lung cancer worry, and lower levels of lung cancer fear. Those who perceived higher benefits of lung cancer screening had higher levels of lung cancer worry, greater knowledge about lung cancer and screening, were highly influenced by their social circle, had higher incomes, and reported lower levels of cancer fatalism. People who perceived greater barriers to lung cancer screening were current smokers, African American, reported higher levels of cancer fatalism and lung cancer fear, were not readily influenced by their social circle, and had no family history of lung cancer. Those with higher levels of self-efficacy for lung cancer screening had higher incomes, a family history of lung cancer, lower levels of cancer fatalism and lung cancer fear, were highly influenced by their social circle, and had received a provider recommendation to screen. An unanticipated finding was that stigma was negatively associated with perceived barriers to lung cancer screening (p < 0.05). Exploration of these two variables qualitatively may explain this unexpected finding.
Although health beliefs have historically predicted participation in other types of cancer screening, self-efficacy seems to have direct implications both as a predictor and mediator of lung cancer screening behaviour. Not only does self-efficacy play an important role in lung cancer screening participation, it is also a factor amenable to modification. As clinician-targeted interventions are developed to support the shared decision-making process about this recent screening recommendation, targeting educational efforts to include salient mediators has the potential to enhance the shared decision-making process and patient behaviour change. By identifying the antecedents associated with self-efficacy, clinician educational interventions can increase awareness of which factors might warrant consideration when discussing screening with eligible patients. For example, social influence is associated with self-efficacy, suggesting that clinicians may find value in engaging their patients in a discussion about who in their lives may have suggested they screen, or not, for lung cancer. In addition, the self-efficacy items address confidence to complete a lung scan from a practical and logistical standpoint, but also from the perspective of the confidence to complete a lung scan despite worries or anxiety about the process or the results. Therefore, if a clinician recommends lung cancer screening, and the individual makes the decision to screen after engaging in a shared decision-making discussion with their clinician, but then does not follow through, the clinician would know it is probably attributable to one of these two confidence issues. The clinican could therefore tailor their conversation with the individual on a subsequent visit to one that is most meaningful and has the potential for impact.
While cross-sectional survey data preclude causal inference, the results suggest which psychological variables are associated with individual lung cancer screening health beliefs, and also the potential direction of those associations. In addition to extending prior qualitative research on individual attitudes and beliefs about lung cancer screening,7,8,27–29 this study extends our previous work by supporting the potentially modifiable intervention targets on which to tailor decision support tools and educational materials, from the perspective of the individual considering screening. In addition, because the standardized coefficients we report are adjusted for standard deviations of the variables, these coefficients serve as effect sizes, and allow valid assessment of the relative strength of those relationships for variables with different scales of measurement.
The population targeted for lung cancer screening engages in a behaviour (i.e. smoking) that is often stigmatized. It is important that future interventions support patient-clinician discussions, are theoretically grounded, and include a variety of intervention components, to improve understanding of what drives behaviour change in lung cancer screening. Understanding this unique population more robustly not only has potential positive educational outreach implications for those considering lung cancer screening but also for tobacco treatment interventions in this high-risk population.
This study is not without limitations that must be considered when interpreting the results. Generalizability may be limited by use of a cross-sectional self-reported survey design, as well as characteristics specific to individuals with computer or mobile device access. However, a national sample was recruited using the most common social media platform. While recruiting Facebook users increased the representativeness of participants from multiple demographic backgrounds, it may also limit generalizability to people who use this particular social media platform. However, the Pew Research Center reported that 84% of United States adults use the Internet, 74% of online adults use social networking sites, and 71% of online adults use Facebook.30 There is also potential for self-selection bias from Facebook recruitment, but this is true for any study recruiting volunteers. Furthermore, our preliminary study demonstrated that demographics did not vary substantially between those recruited via Facebook versus more traditional methods.11 In addition, due to the low numbers of screening-eligible individuals who have been screened, those who indicated they intended to screen were categorized as screening participants. Although used as a proxy for screening participation, intention may not always lead to behaviour. As the number of those screened in the population increases, future research should include categorical analysis across the Precaution Adoption Process Model stage continuum, to understand screening behaviour more robustly.
Conclusions
Results from this study fill a critical gap in knowledge by identifying the most salient factors associated with lung cancer screening participation in long-term smokers. In addition to identifying potential predictor variables, mediators were also identified, including the relative weights of the psychological variables in relation to the Health Belief Model variables and other antecedents. This study fills the current gap in the science by informing the development of effective patient-focused interventions to support screening decision-making and increase lung cancer screening uptake among high-risk long-term smokers.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.United States Preventive Services Task Force. United States Preventive Services Task Force Lung Cancer Screening Guidelines, www.uspreventiveservicestaskforce.org/ (2013, accessed 12 March 2019).
- 2.Jemal A and Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol 2017; 3: 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans’ Health Administration. JAMA Intern Med 2017; 177: 399–406. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Chung S, Wei EK, et al. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv Res 2018; 18: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter-Harris L, Ceppa DP, Hanna N, et al. Lung cancer screening: what do long-term smokers know and believe? Health Expect 2017; 20: 59–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter-Harris L, Brandzel S, Wernli KJ, et al. A qualitative study exploring why individuals opt out of lung cancer screening. Fam Pract 2017; 34: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaife SL, Vrinten C, Ruparel M, et al. Smokers’ interest in a lung cancer screening programme: a national survey in England. BMC Cancer 2018; 497: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanodra N, Pope C, Halbert C, et al. Primary care provider and patient perspectives on lung cancer screening: a qualitative study. Ann Am Thorac Soc 2016; 13: 1977–1982. [DOI] [PubMed] [Google Scholar]
- 9.Ali N, Lifford KJ, Carter B, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: A mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015; 5: e008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter-Harris L, Davis LL and Rawl SM. Lung cancer screening participation: developing a conceptual model to guide research. Res Theory Nurs Pract 2016; 30: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter-Harris L, Ellis RB, Warrick A, et al. Beyond traditional newspaper advertisement: leveraging Facebook-targeted advertisement to recruit long-term smokers for research. J Med Internet Res.2016; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein ND, Sandman PM and Blalock SJ. The precaution adoption process model In: Glanz K and Rimer BK (eds) Human behavior and health education. 4th ed. San Francisco: Jossey-Bass, 2008. pp.123–147 [Google Scholar]
- 13.Cataldo JK, Slaughter R, Jahan TM, et al. Measuring stigma in people with lung cancer: psychometric testing of the cataldo lung cancer stigma scale. Oncol Nurs Forum 2011; 38: E46–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugan E, Trachtenberg F and Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. BMC Health Serv Res 2005; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo RM, Ureda JR and Parker VG. Importance of fatalism in understanding mammography screening in rural elderly women. J Women Aging 2001; 13: 57–72. [DOI] [PubMed] [Google Scholar]
- 16.Lerman C, Trock B, Rimer BK, et al. Psychological side effects of breast cancer screening. Health Psychol 1991; 10: 259–267. [DOI] [PubMed] [Google Scholar]
- 17.Champion VL, Skinner CS, Menon U, et al. A breast cancer fear scale: psychometric development. J Health Psychol 2004; 9: 753–762. [DOI] [PubMed] [Google Scholar]
- 18.Carter-Harris L, Slaven JE 2nd, et al. Development and psychometric evaluation of the lung cancer screening health belief scales. Cancer Nurs 2017; 40: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter-Harris L, Slaven JE Jr, Monahan PO, et al. Understanding lung cancer screening behavior: racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep 2018; 10: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQueen A, Tiro JA and Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiol Biomarkers Prev 2008; 17: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron RM and Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 22.Hu L and Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Eq Model 1999; 6: 1–55. [Google Scholar]
- 23.DiStefano C, Liu J, Jiang N, et al. Examination of the weighted root mean square residual: Evidence for trustworthiness? Struct Eq Model 2018; 25: 453–466. [Google Scholar]
- 24.Dominick KL, Skinner CS, Bastian LA, et al. Provider characteristics and mammography recommendation among women in their 40s and 50s. J Womens Health (Larchmt) 2003; 12: 61–71. [DOI] [PubMed] [Google Scholar]
- 25.Ramdass P, Petraro P, Via C, et al. Providers role in colonoscopy screening for colorectal cancer. Am J Health Behav 2014; 38: 234–244. [DOI] [PubMed] [Google Scholar]
- 26.Ye J, Xu Z and Aladesanmi O. Provider recommendation for colorectal cancer screening: examining the role of patients’ socioeconomic status and health insurance. Cancer Epidemiol 2009; 33: 207–211. [DOI] [PubMed] [Google Scholar]
- 27.Bergamo C, Lin JJ, Smith C, et al. Evaluating beliefs associated with late-stage lung cancer presentation in minorities. J Thorac Oncol 2013; 8: 12–18. [DOI] [PubMed] [Google Scholar]
- 28.Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer 2012; 77: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel D, Akporobaro A, Chinyanganya N, et al. Attitudes to participation in a lung cancer screening trial: a qualitative study. Thorax 2012; 67: 418–425. [DOI] [PubMed] [Google Scholar]
- 30.Duggan M, Ellison NB, Lampe C, et al. Pew Internet & American Life Project: Demographics of key social networking platforms, www.pewinternet.org/2015/01/09/demographics-of-key-social-networking-platforms-2/ (2014, accessed 13 March 2019).