Abstract
Physical activity (PA) combats the effects of multimorbidity and antiretroviral therapy in people living with HIV (PLWH), but PLWH often don’t meet recommended PA guidelines. The purpose of our review was to investigate whether supervised PA improved functional capacity in PLWH. Preferred Reporting Items for Systematic Reviews and Meta-Analyses were followed. Five databases were searched for randomized controlled trials in English, with participants ages 18 years and older, and a supervised PA intervention. A database search yielded 8,267 articles, with 15 eligible for review inclusion. We found a low risk of bias within and across studies. Combined aerobic/progressive resistance training (PRT) improved strength, cardiovascular, and flexibility outcomes; aerobic interventions alone showed no significant improvements; PRT improved strength outcomes; yoga or yoga/meditation showed no outcome differences; and t’ai chi showed cardiovascular and flexibility improvements. We found that supervised PA increased functional capacity in PLWH and that self-report was not a reliable assessment.
Keywords: functional capacity, HIV, physical activity, supervision
There are currently 1.1 million people living with HIV (PLWH) in the United States (Centers for Disease Control and Prevention [CDC], 2017). Due to advances in antiretroviral therapy (ART), PLWH are living longer (Antiretroviral Therapy Cohort Collaboration, 2008, Boyd, 2009) and HIV is now managed as a chronic disease in much of the developed world (Deeks, Lewin, & Havlir, 2013). Despite the steady increase in life expectancy (Samji et al., 2013), non-HIV-related mortality, specifically, cardiovascular disease, has eclipsed HIV-related mortality as the major cause of death among PLWH (Farahani, Mulinder, Farahani, & Marlink, 2017).
Evidence has suggested that physical activity (PA) is safe and beneficial in promoting the health and medical stability of PLWH (Hand et al., 2009, Jaggers, 2018, Nixon et al., 2005, O’Brien et al., 2016, Yahiaoui et al., 2012). Specifically, PA can effectively mitigate the effects of cardiovascular disease (Hand et al., 2009, Kamitani et al., 2017, O’Brien et al., 2016), the leading cause of death among PLWH (Farahani et al., 2017), and symptoms of long-term HIV exposure and long-term ART (O’Brien et al., 2016, Webel et al., 2015, Yahiaoui et al., 2012). For example, PLWH who participate in PA have a reduced risk for heart disease, increased energy, improved regulation of bowel function, improved sleep, and lower stress (Hand et al., 2009, U.S. Department of Veterans Affairs, 2015, Yahiaoui et al., 2012). PA is also an important strategy for improving aerobic capacity, muscle strength, and flexibility in this population (Haskell et al., 2007, Jones and Carter, 2000, O’Brien et al., 2016, Poton et al., 2016).
The CDC (2015) defined PA as any expenditure of energy by skeletal muscles to produce any bodily movement. PA is a broader concept than exercise, as exercise, a subset of PA, is defined as a planned, structured, and repetitive activity (Caspersen, Powell, & Christenson, 1985). Recommended PA for adult PLWH includes 20 to 40 minutes of combined aerobic and resistance exercise at least three times per week (O’Brien et al., 2016, Yahiaoui et al., 2012). These recommendations were shown to reduce the severity of comorbidities (Dirajlal-Fargo et al., 2016, Kamitani et al., 2017, Yahiaoui et al., 2012) and reduce inflammation associated with long-term HIV and long-term ART (d’Ettorre et al., 2014, Hand et al., 2009, Webel et al., 2015). Given the benefits of PA, regular participation for PLWH is essential; however, their PA participation has remained below recommended levels (Montoya et al., 2015, Simonik et al., 2016, Vancampfort et al., 2016, Webel et al., 2015). Weekly PA recommendations make no mention of supervision, despite being based on studies of both supervised and unsupervised PA interventions (O’Brien et al., 2016, Yahiaoui et al., 2012).
Supervised PA has the potential to encourage PLWH to participate in PA regularly. Studies of adults with other chronic conditions (e.g., arthritis, obesity, kidney failure patients on dialysis, heart failure, diabetes, and cancer) were shown to have increased PA participation after involvement in a PA intervention supervised by a clinician or allied health professional (Akbaba et al., 2016, Casla et al., 2014, Colak et al., 2017, Daul et al., 2004, Klempfner et al., 2015, Negri et al., 2010, Nicolai et al., 2009). In some cases, regular participation in PA continued well beyond the end of the study (Azad et al., 2012, Casla et al., 2014, Trinh et al., 2014).
Additionally, when outcomes were compared between supervised and unsupervised PA interventions, more pronounced improvements were seen as a result of supervised PA. To detail, participants of supervised PA interventions showed significant improvement in cardiovascular fitness, muscle strength, and significantly decreased fat mass, body mass index (BMI), and body weight, compared with controls (Akbaba et al., 2016, Boshuizen et al., 2005, Dalager et al., 2015, Nicolai et al., 2009, Rossomanno et al., 2012). However, the evidence to support the benefits of supervised PA as the main intervention for PLWH is missing from the current body of literature. Past systematic reviews have examined the effects of both supervised and unsupervised PA interventions for PLWH and reported aggregate results (Gomes-Neto et al., 2015, Gomes-Neto et al., 2013b, Jaggers, 2018, O’Brien et al., 2016, Poton et al., 2016, Yahiaoui et al., 2012). None have segregated supervised from unsupervised interventions in their reviews or analyzed the outcomes of these respective interventions separately. The purpose of our review was to investigate the impact of supervised PA interventions on functional capacity among adults living with HIV.
Methods
Search Strategy
Our review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). A total of five databases were systematically searched for all relevant literature, including PubMed, Cumulative Index to Nursing and Allied Heath Literature (CINAHL), PsycINFO, Embase, and Physical Education Index. First, search terms were created based on the population and interventions of interest in PubMed. Second, comparable search terms were identified in each subsequent database. For example, a combination of MeSH terms, MeSH headings, keywords and phrases, and Boolean operators were used to create the search terms used in PubMed. The resultant search terms in PubMed were as follows: (“HIV”[Mesh] OR HIV [Text Word] OR “HIV Long-Term Survivors”[Mesh] OR “HIV Long-Term Survivors” OR “HIV Infections”[Mesh] OR “HIV Infections” OR “HIV Seropositivity”[Mesh] OR “HIV Seropositivity” OR “HIV Seroprevalence”[Mesh] OR “HIV Seroprevalence”) AND (“Physical Activity”[Mesh] OR “Physical Activity” OR “Exercise”[Mesh] OR “Exercise” OR “Physical Exercise”[Mesh] OR “Physical Exercise” OR “Aerobic Exercise”[Mesh] OR “Aerobic Exercise” OR “Exercise Therapy”[Mesh] OR “Exercise Therapy” OR “Exercise Training”[Mesh] OR “Exercise Training” OR “Motor Activity” [Mesh] OR “Motor Activity”). All possible combinations of MeSH terms, MeSH headings, keywords, and phrases were used to identify articles for inclusion.
Eligibility Criteria
Study inclusion criteria included studies that (a) were randomized controlled trials that evaluated the effect of a supervised PA intervention as compared with usual care, (b) examined functional capacity as an outcome, (c) included adults ages 18 years and older living with HIV, and (d) were available as full text articles in English. Supervised PA was defined in our review as PA interventions that were supervised by a health care or allied health professional. Functional capacity was defined as per the American Heart Association definition, as the integrated efforts and health of an individual’s pulmonary, cardiovascular, and skeletal muscles to perform activities of daily living (Arena et al., 2007). Functional capacity outcomes in our review included strength measures, cardiovascular fitness measures, flexibility measures, and self-reported measures of functional capacity. We excluded poster sessions, presentations, protocols, letters, comments, editorials, correspondence, or gray literature (e.g., blogs, newsletters, videos).
Study Selection and Data Extraction
We used Covidence (Veritas Health Innovation, 2018), a web-based program designed to support the systematic review process, to facilitate screening at the title/abstract and full text level, as well as for data extraction, adjudication of disagreements, and confirmation of data. A hand search was conducted for additional studies meeting inclusion criteria. Two authors independently assessed eligibility, and a third consulted in instances of uncertainty/disagreement. The following information was extracted from the final studies: name of the first author, publication year, locations of the studies, study sample size, type of supervised PA interventions, frequency/duration of interventions, and duration of the studies. No publication date or restrictions were used in the search.
Quality Assessment
The quality appraisal was conducted using the Cochrane Collaboration Risk of Bias Tool (CCRBT; Higgins et al., 2011). The CCRBT describes six domains to assess possible avenues of bias: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and, other sources of bias. Each domain is evaluated as yes (low risk of bias), no (high risk of bias), or unclear (uncertain risk of bias). Each study is then assigned an overall grade of high, moderate, or low risk of bias. Two authors independently appraised the quality of the studies, and discrepancies were reviewed until consensus was reached.
Results
Study Selection
The database literature search yielded 8,267 articles; 126 articles were identified from the hand search. One hundred ninety-five articles remained for full text screening after duplicates were removed and title and abstract screening was completed; 180 articles were excluded based on the full text review. Of the 180 excluded articles, full text for 28 abstracts were not found despite independent searches, the assistance of university librarians, and requests from other institutions via interlibrary loan. Fifteen studies remained and were included in this systematic review. Figure 1 summarizes the results of the search and study selection.
Figure 1.
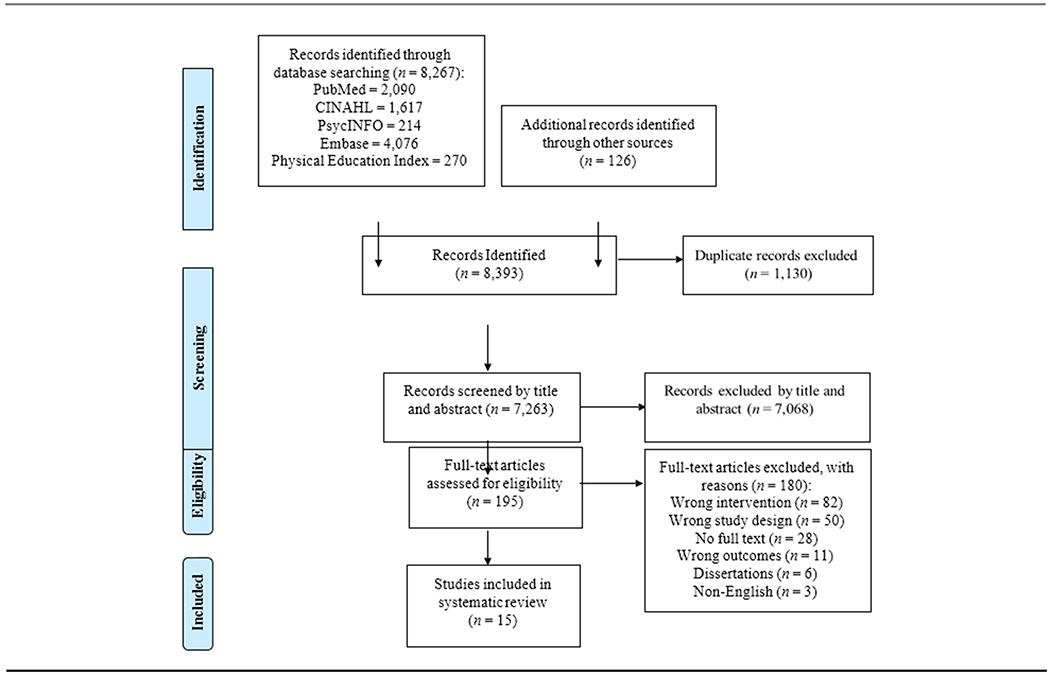
Flow diagram of study selection.
Study Characteristics
The combined studies had 537 participants whose ages ranged between 18 and 73 years. The supervisors of the PA interventions included certified yoga instructors, exercise physiologists, nurse trainers, physical therapists, exercise specialists, physiotherapists, cardiologists, and personal trainers. Table 1 summarizes the characteristics of the included studies. The duration of the PA interventions ranged between 31 minutes and 2 hours, and the intensity ranged from low to vigorous. The length of the PA interventions ranged from 6 weeks to 6 months across studies.
Table 1.
Characteristics of the Physical Activity Interventions of Included Studies (n = 15)
| Study and Sample Size | Country | Intervention | Frequency (per Week) | Session Duration | Study Length (Weeks) |
|---|---|---|---|---|---|
|
Agarwal et al., 2015 (n = 24) |
USA | Yoga & meditation | 2 | 60 min | 8 |
|
Agin et al., 2001 (n = 30) |
USA | PRT | 3 | 3 sets, 8-10 repetitions per muscle group | 14 |
|
Baigis et al., 2002 (n = 99) |
USA | Aerobics | 3 | 40 min | 15 |
|
Cade et al., 2010 (n = 50) |
USA | Yoga | 2-3 | 60 min | 20 |
|
Dolan et al., 2006 (n = 38) |
USA | Aerobics & PRT | 3 | 120 min | 16 |
|
Driscoll et al., 2004 (n = 25) |
USA | Aerobics & PRT | 3 | Weeks 1-2: 20 min aerobics + PRT Weeks 3-12: 30 min aerobics + PRT PRT: 3 sets, 10 repetitions per muscle group |
12 |
|
Dudgeon et al., 2012 (n = 26) |
USA | Aerobics & PRT | 2 | Aerobics: 30 min PRT: 1 set, 12 repetitions per muscle group |
6 |
|
Farinatti et al., 2010 (n = 27) |
Brazil | Aerobics & PRT | 3 | 90 min | 12 |
|
Fillipas et al., 2006 (n = 35) |
Australia | Aerobics & PRT | 2 | 60 min | 24 |
|
Galantino et al., 2005 (n = 38) |
USA | Aerobics & T’ai Chi | 4 | 60 min | 8 |
|
McDermott et al., 2016 (n = 11) |
Ireland | Aerobics | 3 | 31-52 min | 16 |
|
Ogalha et al., 2011 (n = 63) |
Brazil | Aerobics & PRT | 3 | 60 min | 24 |
|
Perez-Moreno et al., 2007 (n = 19) |
Spain | Aerobics & PRT | 3 | 90 min | 16 |
|
Strawford et al., 1999 (n = 22) |
USA | PRT | 3 | 60 min | 8 |
|
Terry et al., 2006 (n = 30) |
Brazil | Aerobics | 3 | 60 min | 12 |
Note. PRT = progressive resistance training.
Study Findings
Supervised PA interventions were classified into the following categories: (a) aerobics, (b) progressive resistance training (PRT), (c) combined aerobics and PRT, (d) yoga and meditation, and (e) t’ai chi. Each intervention in the review was assessed as a change in one or a combination of four functional capacity outcomes of interest: cardiovascular (e.g., VO2max [maximal aerobic capacity]; BMI; the 6-Minute Walk Test), strength (e.g., the one-repetition maximum, 6-repetition maximum), flexibility and balance (i.e., Sit and Reach and Forward Reach tests), and self-report (e.g., SF-36 Physical Functioning and Role Limitations due to Physical Health subscale scores, exit interview questions, health diary). Table 2 describes the functional capacity outcomes of each study.
Table 2.
Functional Capacity Outcomes of Intervention Groups in the Included Studies (n = 15)
| Type of Supervised PA | Study (Year) | Functional Capacity Measure | Functional Capacity Result |
|---|---|---|---|
| Aerobics | Baigis (2002) | VO2max Diary, DASI, MOS-HIV, SIP |
Ø Ø |
| Galantino (2005) | Sit-up Stair climbing |
↑ ↑ |
|
| McDermott (2016) | VO2max, treadmill time, light/moderate/vigorous PA | Ø | |
| Terry (2006) | Peak HR, BMI, BP | Ø | |
| Progressive Resistance Training | Agin (2001) | 1-RM SF-36 |
↑ Ø |
| Strawford (1999) | 1-RM | ↑ | |
| Aerobics + Progressive Resistance Training | Dolan (2006) | VO2max, Bike time, 6MWT Change in strength Exit interview questions |
↑ ↑ ↑ |
| Driscoll (2004) | BP, Exercise time Change in strength 1& 2 |
↑ ↑, Ø |
|
| Dudgeon (2012) | Change in strength | ↑ | |
| Farinatti (2010) | BMI Heart rate, 12-RM, Sit and reach |
Ø ↑ |
|
| Fillipas (2006) | Heart rate HRQoL |
↑ Ø |
|
| Ogalha (2011) | BMI, Resting HR VO2max, MET Change in muscle mass, SF-36 |
Ø, ↑ ↑ ↑ |
|
| Perez-Moreno (2007) | Workload, Peak HR, Decline HR 6-RM |
↑ ↑ |
|
| Yoga + Meditation | Agarwal (2015) | SF-36 | Ø |
| Cade (2010) | SF-36 | Ø | |
| T’ai Chi | Galantino (2005) | Forward reach Sit and reach | ↑ |
Note. Ø = no significant change in functional capacity; ↑ = significantly improved functional capacity; VO2max = maximal aerobic capacity; PA = physical activity; BMI = body mass index; DASI = Duke Activity Status Index; MOS-HIV = Medical Outcomes Study, HIV Health Survey; SIP = Sickness Impact Profile; HR = heart rate; BP = blood pressure; 1-RM = 1 repetition maximum; 6-RM = 6 repetition maximum; 12-RM = 12 repetition maximum; SF-36 = Medical Outcomes Study, 36-item Short Form Health Survey; 6MWT = 6-Minute Walk Test; Change in strength 1: leg curl, leg extension, lateral pull down, arm curl, chest press; Change in strength 2: leg press; HRQoL = Health Related Quality of Life; MET = metabolic equivalent.
Aerobics interventions (n = 4)
Four studies employed aerobic interventions in their respective study samples (Baigis et al., 2002, Galantino et al., 2005, McDermott et al., 2016, Terry et al., 2006). Three out of four studies reported no significant differences between intervention and control group participants in cardiovascular and self-reported outcomes (Baigis et al., 2002, McDermott et al., 2016, Terry et al., 2006). However, the participants in the Galantino and colleagues (2005) study showed improvements in both cardiovascular (p < .05) and flexibility outcomes (p < .01).
Progressive resistance training interventions (n = 2)
Agin and colleagues (2001) and Strawford and colleagues (1999) administered PRT as the intervention in their studies. Both studies showed significant improvements in strength outcomes in the intervention group compared to those in the control group (p < .001 and p < .05, respectively).
Combined aerobic and PRT interventions (n = 7)
Seven studies employed combined aerobic and PRT interventions (Dolan et al., 2006, Driscoll et al., 2004, Dudgeon et al., 2012, Farinatti et al., 2010, Fillipas et al., 2006, Ogalha et al., 2011, Perez-Moreno et al., 2007). All strength, cardiovascular, and flexibility outcome measures associated with these interventions demonstrated a significant improvement (p ≤ .05) in the intervention group as compared to control group participants. However, BMI in the Farinatti and colleagues (2010) and Ogalha and colleagues (2011) studies and health-related quality of life in the Fillipas and colleagues (2006) study demonstrated no significant difference between intervention and control groups (p > .05).
Yoga and meditation interventions (n = 2)
One study administered a combined yoga and meditation intervention (Agarwal, Kumar, & Lewis, 2015) and another employed yoga alone (Cade et al., 2010). Both studies measured change in functional capacity via self-reported measures, the SF-36 Physical Functioning and Physical Limitations subscale scores. In both studies, changes in functional capacity were not significantly different from the control group at study end.
T’ai chi intervention (n = 1)
The t’ai chi group showed significant improvements in both flexibility (p < .01) and cardiovascular (p < .05) outcomes (Galantino et al., 2005).
Study Quality
Seven of 15 studies had a low risk of bias (Agin et al., 2001, Baigis et al., 2002, Dolan et al., 2006, Fillipas et al., 2006, Galantino et al., 2005, Perez-Moreno et al., 2007, Strawford et al., 1999). The remaining eight studies had an unclear risk (Agarwal et al., 2015, Cade et al., 2010, Farinatti et al., 2010, McDermott et al., 2016, Terry et al., 2006) or high risk of bias (Driscoll et al., 2004, Dudgeon et al., 2012, Ogalha et al., 2011). For sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, the risk of bias was low or unclear risk for all studies. Most of the studies demonstrated low risk of bias for incomplete outcome data. The results of the assessment of study quality using CCRBT are reported in Figure 2, and the Cochrane risk of bias summary is reported in Figure 3.
Figure 2.
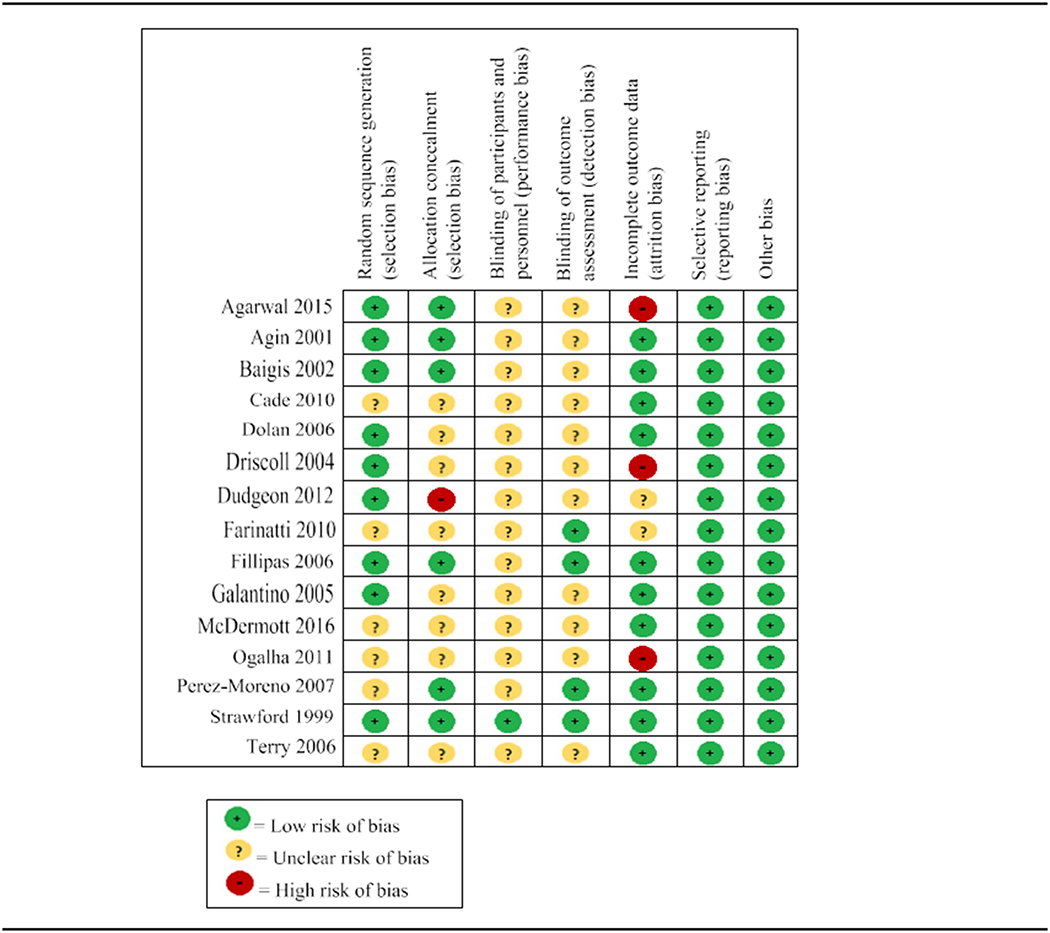
Risk of bias within studies.
Figure 3.

Risk of bias across studies.
Discussion
We examined the impact of supervised PA interventions on the functional capacity of adult PLWH. Overall, our findings supported supervised PA interventions as an effective intervention for increasing functional capacity among adult PLWH. Supervised combined aerobics and PRT interventions improved functional capacity outcomes across all of the following outcomes: cardiovascular, strength, flexibility and balance, and self-report. However, studies that assessed functional capacity via self-reported measures alone revealed inconsistent results.
We found that supervised combined aerobics and PRT was the most effective intervention, followed by supervised t’ai chi, PRT, aerobics, yoga, and combined yoga and meditation to increase functional capacity in this population. The participants in all seven studies involving supervised combined aerobics and PRT (Dolan et al., 2006, Driscoll et al., 2004, Dudgeon et al., 2012, Farinatti et al., 2010, Fillipas et al., 2006, Ogalha et al., 2011, Perez-Moreno et al., 2007) showed statistically significant improvements in cardiovascular, strength, and flexibility and balance outcomes. Improvements in cardiovascular health, strength, and flexibility have been associated with reduced cholesterol and risk of heart attack and stroke, increased muscle mass and strengthening of bones, and reduced stress in both PLWH and those without HIV infection (Lachman et al., 2018, U.S. Department of Veterans Affairs, 2015, Yahiaoui et al., 2012). These health benefits are of particular import to PLWH, as the incidence of heart attack, lipodystrophy, and wasting syndrome are high in this population (Deeks et al., 2013b, Farahani et al., 2017, Nguyen et al., 2016, Palmeira dos Santos et al., 2017, Schouten et al., 2014). While yoga is considered a form of PA and has been shown to effectively improve health-related outcomes in both healthy and diseased populations (Govindaraj et al., 2016, Ross and Thomas, 2010), in our review, yoga failed to demonstrate an improvement in functional capacity for PLWH.
Supervised combined aerobics and PRT interventions showed improvements in strength, cardiovascular, and flexibility and balance outcomes; however, cardiovascular outcomes associated with supervised aerobics alone showed no significant changes between groups in three out of four studies (Baigis et al., 2002, McDermott et al., 2016, Terry et al., 2006). In our review, when PRT was included with aerobics, cardiovascular outcomes demonstrated significant improvement in functional capacity. Improved cardiovascular outcomes in the Galantino and colleagues (2005) study may be attributed to three possible reasons: (a) the sample of male-only subjects, whereas the subjects in the other aerobics studies were comprised of male and female subjects; (b) the investigator was not blinded to the participants in the Galantino and colleagues (2005) study, and therefore, introduced a source of bias in the data collection or analysis; and (c) the cardiovascular outcome measures in the Galantino and colleagues (2005) study involved the simplest interactions with equipment, and thus, a reduced likelihood of equipment malfunction or failure, unlike the measures in the Baigis and colleagues (2002), McDermott and colleagues (2016), and Terry and colleagues (2006) studies. For instance, the latter studies used VO2max, peak heart rate, and blood pressure as cardiovascular outcomes among others. Measuring VO2max involves a mouth guard, nose clip, and treadmill. Peak heart rate measurements require electrode sensors and an electrocardiogram machine. Blood pressure was measured with an automated sphygmomanometer. Sit-up and stair-climbing tests in the Galantino and colleagues (2005) study involved counts of each within a specified time frame.
Of the studies that employed self-reported measures, (Agarwal et al., 2015, Agin et al., 2001, Baigis et al., 2002, Cade et al., 2010, Dolan et al., 2006, Fillipas et al., 2006, Ogalha et al., 2011), the findings were inconclusive. Self-reported improvement of functional capacity was more likely to be significant when supervised PRT was included in the PA intervention. This finding is supported by evidence that subjective assessments of PA are beneficial only to ranking participants as more or less physically active, but not quantifying PA in terms of time spent in each level of PA (Masse & de Niet, 2012). In other words, reporting bias may have affected participant recall for time spent in PA.
Our findings support evidence that PA interventions are effective at improving cardiovascular fitness and muscular strength in PLWH (Gomes-Neto et al., 2013a, O’Brien et al., 2016, O’Brien et al., 2017, Poton et al., 2016). While other systematic reviews focused on overall PA levels in PLWH (Schuelter-Trevisol et al., 2012, Vancampfort et al., 2016), benefits of PA and PA recommendations (Gomes-Neto et al., 2015, Gomes-Neto et al., 2013a, Gomes-Neto et al., 2013b, Kamitani et al., 2017, O’Brien et al., 2016), or facilitators and barriers of PA (Vancampfort et al., 2017a, Vancampfort et al., 2017b), our review stands apart because of the focus on the supervisory aspect of PA interventions and its potential to improve functional capacity for PLWH. Essentially, our review showed that supervision of PA increases functional capacity in adult PLWH and may be an enabling factor to support regular PA among PLWH.
HIV is now managed as a chronic illness for many PLWH in the United States and the developed world due to the success of ART (Deeks et al., 2013a, Samji et al., 2013). Research has shown that chronically ill persons are less likely to engage in PA (Mansfield et al., 2018, Miravitlles et al., 2014, Volaklis et al., 2018). Studies of supervised PA interventions in HIV-uninfected, chronically ill populations have suggested that incorporation of formal, structured programs may facilitate increased PA participation (Allen and Morey, 2010, Bousquet-Dion et al., 2018), and supervision may play a role in retaining participants in PA interventions (Akbaba et al., 2016, Dalager et al., 2015, Rossomanno et al., 2012). Increases in PA may be due, in part, to the reported improved morale and confidence to engage in PA when a clinician is supervising the activity (Bauman et al., 2012, Colak et al., 2017, Tully et al., 2010). Studies of people living with diabetes, heart failure, kidney failure, cancer, arthritis, and obesity have demonstrated empirical improvement in PA participation where supervised PA was the intervention under inquiry (Akbaba et al., 2016, Azad et al., 2012, Casla et al., 2014, Colak et al., 2017, Daul et al., 2004, Klempfner et al., 2015, Negri et al., 2010, Nicolai et al., 2009, Tully et al., 2010). Therefore, supervised PA interventions can serve as formal, structured programs to increase and maintain regular PA among PLWH.
Recommendations and Implications
Recommendations
The therapeutic alliance between a health professional and patient, an important component in patient engagement (Higgins, Larson, & Schnall, 2017), may increase regular PA in PLWH. For example, the benefit of the therapeutic alliance during a PA intervention was demonstrated in the Galantino and colleagues (2005) study. Those participants reported a newly formed “brotherhood,” working harder during the PA, and that the added presence of a leader facilitated their participation in the PA, where they would not have participated if a leader was not present. A recent systematic review and meta-analysis investigating dropout from PA interventions with PLWH revealed reduced dropout rates involving supervised PA interventions (p < .001) and interventions using qualified professionals (p < .001; Vancampfort et al., 2017a, Vancampfort et al., 2017b). Therefore, supervised PA interventions modeled after the general medicine therapeutic alliance, with the presence of a health care or allied health professional, may increase regular PA participation and ultimately sustained improvement in functional capacity in PLWH.
Nursing implications
Findings from our study present a unique opportunity for nurses who care for PLWH. Because supervised PA has been shown to improve outcomes in PLWH and nurses are the largest sector of health professionals, this provides an opportunity for nurses to supervise PA interventions for PLWH. Moreover, the presence of a health care professional, such as a nurse, during PA may encourage PLWH to participate in and work harder during the PA interventions, improve long-term adherence to PA, and improve motivation and self-efficacy to reach PA goals (Peddle-McIntyre et al., 2013, Trinh et al., 2014, Vancampfort et al., 2017a, Vancampfort et al., 2017b). Nurses can fill that gap by becoming the PA facilitator.
Additionally, our findings present an opportunity for nurse scientists to help fill gaps in the literature. Specifically, the current literature is comprised of studies with small sample sizes and high variability of intervention components (e.g., frequency, session duration, and length) and outcome measures. This variability makes comparison via meta-analysis and drawing succinct conclusions difficult. Further research by nurse scientists with more rigorous and robust research designs will enhance knowledge of the ability of PA interventions to improve health outcomes in PLWH.
Limitations
Inclusion of only English language studies was a limitation because there may be relevant studies published in African languages, given that the burden of the HIV epidemic persists in Africa (World Health Organization, 2017). In our review, none of the included studies took place in an African country. Therefore, the external validity of our findings is limited to the countries represented: Australia, Brazil, Ireland, Spain, and the United States. Additionally, 9 of the 15 studies had samples of 30 or less and all had fewer than 100 participants. Selection bias may be present as the study participants may represent PLWH who were already motivated to take ownership of their health and participate in PA. The small sample sizes in the individual studies also presented the potential for a type 1 error. Inclusion of randomized controlled trials and the inherent randomization, however, elevated the level of evidence presented here and countered the potential for selection bias and type 1 error. The number of articles included in this review may also be a limitation, as 15 articles may be considered a small number of articles to comprise a systematic review. However, PRISMA guidelines were followed and the search strategy and selection criteria were developed a priori. The resulting articles included for review reflected the dearth of studies on this topic. Our systematic review will, nonetheless, make an important contribution to the literature considering increased longevity in PLWH and the benefits of supervised PA interventions on health outcomes in this population.
The heterogeneity of studies, including the length of the interventions, duration of the study, follow-up assessment period, assessment instruments, and assessment modality, precluded a meta-analysis. Like other systematic reviews, our review demonstrated the need for consistent protocols and outcome measures across studies (Gomes-Neto et al., 2013a, Schuelter-Trevisol et al., 2012, Vancampfort et al., 2017a, Vancampfort et al., 2017b). At the individual study level, clinical significance may have been present where statistical significance was absent. For instance, at the end of 16 weeks in the McDermott and colleagues (2016) study, there was a trend toward improvement in cardiovascular fitness that may have revealed statistically significant improvements with a longer follow-up period.
Conclusions
The results of our review indicate that supervised PA interventions are associated with improved functional capacity in adult PLWH and may motivate PLWH to regularly participate in PA. Of all the interventions, the supervised aerobics and PRT interventions demonstrated improvements in strength, cardiovascular, and flexibility and balance outcomes. The inclusion of PRT with aerobics interventions demonstrated improved cardiovascular outcomes compared to aerobics alone. Furthermore, functional capacity assessed by self-report rather than objective measures may not reflect improvements in functional capacity, except where PRT is included in the intervention.
Future studies should consider PA interventions with and without supervision to quantify the benefit of these interventions in this population. A special focus should be placed on the long-term impact and outcomes of supervised PA for elderly PLWH as the average age of PLWH continues to rise.
Supplementary Material
Acknowledgments
The authors would like to thank Alexandra Medline and Alexandra Sepolen for their assistance in the development of this manuscript. Natalie Voigt is supported by the Robert Wood Johnson Foundation Future of Nursing Scholars Program, Columbia University School of Nursing, and Northwell Health. Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR015737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
Natalie Voigt, School of Nursing, Columbia University, New York, New York.
Hwayoung Cho, School of Nursing, Columbia University, New York, New York.
Rebecca Schnall, School of Nursing, Columbia University, New York, New York, USA.
References
- Agarwal RP, Kumar A, Lewis JE (2015). A pilot feasibility and acceptability study of yoga/meditation on the quality of life and markers of stress in persons living with HIV who also use crack cocaine. The Journal of Alternative and Complementary Medicine, 21(3), 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agin D, Gallagher D, Wang J, Heymsfield SB, Pierson RN Jr., Kotler DP (2001). Effects of whey protein and resistance exercise on body cell mass, muscle strength, and quality of life in women with HIV. AIDS, 15(18), 2431–2440. [DOI] [PubMed] [Google Scholar]
- Akbaba YA, Yeldan I, Guney N, Ozdincler AR (2016). Intensive supervision of rehabilitation programme improves balance and functionality in the short term after bilateral total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy, 24(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Allen K, Morey MC (2010). Physical activity and adherence In Bosworth H (Ed.), Improving Patient Treatment Adherence (1st ed) (pp. 9–38). New York, NY: Springer-Verlag [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration. (2008). Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet, 372(9635), 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ: American Heart Association Council on Cardiovascular Nursing (2007) Assessment of functional capacity in clinical and research settings: A scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation, 116(3), 329–343. [DOI] [PubMed] [Google Scholar]
- Azad NA, Bouchard K, Mayhew A, Carter M, Molnar FJ (2012). Safety and predictors of adherence of a new rehabilitation program for older women with congestive heart failure. Journal of Geriatric Cardiology, 9(3), 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigis J, Korniewicz DM, Chase G, Butz A, Jacobson D, Wu AW (2002). Effectiveness of a home-based exercise intervention for HIV-infected adults: A randomized trial. Journal of the Association of Nurses in AIDS Care, 13(2), 33–45. [DOI] [PubMed] [Google Scholar]
- Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW: Lancet Physical Activity Series Working Group. (2012). Correlates of physical activity: Why are some people physically active and others not? Lancet, 380(9838), 258–271. [DOI] [PubMed] [Google Scholar]
- Boshuizen HC, Stemmerik L, Westhoff MH, Hopman- Rock M (2005). The effects of physical therapists’ guidance on improvement in a strength-training program for the frail elderly. Journal of Aging and Physical Activity, 13(1), 5–22. [DOI] [PubMed] [Google Scholar]
- Bousquet-Dion G, Awasthi R, Loiselle SE, Minnella EM, Agnihotram RV, Bergdahl A, Scheede-Bergdahl C (2018). Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncologica 1–11. [DOI] [PubMed] [Google Scholar]
- Boyd MA (2009). Improvements in antiretroviral therapy outcomes over calendar time. Current Opinion in HIV and AIDS, 4(3), 194–199. [DOI] [PubMed] [Google Scholar]
- Cade WT, Reeds DN, Mondy KE, Overton ET, Grassino J, Tucker S, Yarasheski KE (2010). Yoga life-style intervention reduces blood pressure in HIV-infected adults with cardiovascular disease risk factors. HIV Medicine, 11(6), 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casla S, Hojman P, Cubedo R, Calvo I, Sampedro J, Barakat R (2014). Integrative exercise and lifestyle intervention increases leisure-time activity in breast cancer patients. Integrative Cancer Therapies, 13(6), 493–501. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM (1985). Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports, 100(2), 126–131. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2015). Glossary of terms. Retrieved from https://www.cdc.gov/physicalactivity/basics/glossary/index.htm
- Centers for Disease Control and Prevention (CDC). (2017). HIV continuum of care, U.S., 2014, overall and by age, race/ethnicity, transmission route and sex. Retrieved from https://www.cdc.gov/nchhstp/newsroom/2017/HIV-Continuum-of-Care.html
- Colak TK, Kavlak B, Aydogdu O, Sahin E, Acar G, Demirbuken I, … Polat MG (2017). The effects of therapeutic exercises on pain, muscle strength, functional capacity, balance and hemodynamic parameters in knee osteoarthritis patients: A randomized controlled study of supervised versus home exercises. Rheumatology International, 37(3), 399–407. [DOI] [PubMed] [Google Scholar]
- Dalager T, Bredahl TG, Pedersen MT, Boyle E, Andersen LL, Sjogaard G (2015). Does training frequency and supervision affect compliance, performance and muscular health? A cluster randomized controlled trial. Manual Therapy, 20(5), 657–665. [DOI] [PubMed] [Google Scholar]
- Daul AE, Schafers RF, Daul K, Philipp T (2004). Exercise during hemodialysis. Clinical Nephrology, 61(Suppl. 1), S26–S30. [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV (2013a). The end of AIDS: HIV infection as a chronic disease. Lancet, 382(9903), 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, Douek DC (2013b). Systemic effects of inflammation on health during chronic HIV infection. Immunity, 39(4), 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ettorre G, Ceccarelli G, Giustini N, Mastroianni CM, Silvestri G, Vullo V (2014). Taming HIV-related inflammation with physical activity: A matter of timing. AIDS Research and Human Retroviruses, 30(10), 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirajlal-Fargo S, Webel AR, Longenecker CT, Kinley B, Labbato D, Sattar A, McComsey GA (2016). The effect of physical activity on cardiometabolic health and inflammation in treated HIV infection. Antiviral Therapy, 21(3), 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, Dorman R, … Grinspoon S (2006). Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: A randomized trial. Archives of Internal Medicine, 166(11), 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll SD, Meininger GE, Lareau MT, Dolan SE, Killilea KM, Hadigan CM, … Grinspoon SK (2004). Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS, 18(3), 465–473. [DOI] [PubMed] [Google Scholar]
- Dudgeon WD, Jaggers JR, Phillips KD, Durstine JL, Burgess SE, Lyerly GW, … Hand GA (2012). Moderate-intensity exercise improves body composition and improves physiological markers of stress in HIV-infected men. ISRN AIDS, 2012, 145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani M, Mulinder H, Farahani A, Marlink R (2017). Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: A systematic review and meta-analysis. International Journal of STD and AIDS, 28(7), 636–650. [DOI] [PubMed] [Google Scholar]
- Farinatti PT, Borges JP, Gomes RD, Lima D, Fleck SJ (2010). Effects of a supervised exercise program on the physical fitness and immunological function of HIV-infected patients. Journal of Sports Medicine and Physical Fitness, 50(4), 511–518. [PubMed] [Google Scholar]
- Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL (2006). A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immuno-deficiency virus: A randomised controlled trial. Australian Journal of Physiotherapy, 52(3), 185–190. [DOI] [PubMed] [Google Scholar]
- Galantino ML, Shepard K, Krafft L, Laperriere A, Ducette J, Sorbello A, … Farrar JT (2005). The effect of group aerobic exercise and t’ai chi on functional outcomes and quality of life for persons living with acquired immunodeficiency syndrome. Journal of Alternative and Complementary Medicine, 11(6), 1085–1092. [DOI] [PubMed] [Google Scholar]
- Gomes-Neto M, Conceicao CS, Carvalho VO, Brites C (2013a). A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo), 68(8), 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Neto M, Conceicao CS, Carvalho VO, Brites C (2015). Effects of combined aerobic and resistance exercise on exercise capacity, muscle strength and quality of life in HIV-infected patients: a systematic review and meta-analysis. PLoS One, 10(9), e0138066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Neto M, Ogalha C, Andrade AM, Brites C (2013b). A systematic review of effects of concurrent strength and endurance training on the health-related quality of life and cardiopulmonary status in patients with HIV/AIDS. Biomed Research International, 2013, 319524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraj R, Karmani S, Varambally S, Gangadhar BN (2016). Yoga and physical exercise – review and comparison. International Review of Psychiatry, 28(3), 242–253. [DOI] [PubMed] [Google Scholar]
- Hand GA, Lyerly GW, Jaggers JR, Dudgeon WD (2009). Impact of aerobic and resistance exercise on the health of HIV-infected persons. American Journal of Lifestyle Medicine, 3(6), 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, … Bauman A (2007). Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise, 39(8), 1423–1434. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD.: Cochrane Statistical Methods Group. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. British Medical Journal, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T, Larson E, Schnall R (2017). Unraveling the meaning of patient engagement: A concept analysis. Patient Education and Counseling, 100(1), 30–36. [DOI] [PubMed] [Google Scholar]
- Jaggers JR (2018). Exercise and positive living in human immunodeficiency virus/AIDS. Nursing Clinics of North America, 53(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Jones AM, Carter H (2000). The effect of endurance training on parameters of aerobic fitness. Sports Medicine, 29(6), 373–386. [DOI] [PubMed] [Google Scholar]
- Kamitani E, Sipe TA, Higa DH, Mullins MM, Soares J: CDC HIV/AIDS Prevention Research Synthesis (PRS) Project. (2017). Evaluating the effectiveness of physical exercise interventions in persons living with HIV: overview of systematic reviews. AIDS Education and Prevention, 29(4), 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempfner R, Kamerman T, Schwammenthal E, Nahshon A, Hay I, Goldenberg I, … Arad M (2015). Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: Results of a structured exercise training program in a cardiac rehabilitation center. European Journal of Preventative Cardiolgy, 22(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Lachman S, Boekholdt SM, Luben RN, Sharp SJ, Brage S, Khaw KT, Wareham NJ (2018). Impact of physical activity on the risk of cardiovascular disease in middle-aged and older adults: EPIC Norfolk prospective population study. European Journal of Preventative Cardiology, 25(2), 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield M, Thacker M, Spahr N, Smith T (2018). Factors associated with physical activity participation in adults with chronic cervical spine pain: a systematic review. Physiotherapy, 104(1), 54–60. [DOI] [PubMed] [Google Scholar]
- Masse LC, de Niet JE (2012). Sources of validity evidence needed with self-report measures of physical activity. Journal of Physical Activity and Health, 9(Suppl. 1), S44–S55. [DOI] [PubMed] [Google Scholar]
- McDermott A, Zaporojan L, McNamara P, Doherty CP, Redmond J, Forde C, Bergin C (2016). The effects of a 16-week aerobic exercise programme on cognitive function in people living with HIV. AIDS Care 1–8. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Cantoni J, Naberan K (2014). Factors associated with a low level of physical activity in patients with chronic obstructive pulmonary disease. Lung, 192(2), 259–265. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. British Medical Journal, 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Wing D, Knight A, Moore DJ, Henry BL (2015). Development of an mHealth Intervention (iSTEP) to promote physical activity among people living with HIV. Journal of the International Association of Providers in AIDS Care, 14(6), 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri C, Bacchi E, Morgante S, Soave D, Marques A, Menghini E, … Moghetti P (2010). Supervised walking groups to increase physical activity in type 2 diabetic patients. Diabetes Care, 33(11), 2333–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KA, Peer N, Mills EJ, Kengne AP (2016). A meta- analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One, 11(3), e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai SP, Kruidenier LM, Leffers P, Hardeman R, Hidding A, Teijink JA (2009). Supervised exercise versus non-supervised exercise for reducing weight in obese adults. Journal of Sports Medicine and Physical Fitness, 49(1), 85–90. [PubMed] [Google Scholar]
- Nixon S, O’Brien K, Glazier RH, Tynan AM (2005). Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev (2), CD001796. [DOI] [PubMed] [Google Scholar]
- O’Brien KK, Tynan AM, Nixon SA, Glazier RH (2016). Effectiveness of aerobic exercise for adults living with HIV: Systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infectious Diseases, 16, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KK, Tynan AM, Nixon SA, Glazier RH (2017). Effectiveness of Progressive Resistive Exercise (PRE) in the context of HIV: Systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infectious Diseases, 17(1), 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogalha C, Luz E, Sampaio E, Souza R, Zarife A, Neto MG, … Brites C (2011). A randomized, clinical trial to evaluate the impact of regular physical activity on the quality of life, body morphology and metabolic parameters of patients with AIDS in Salvador, Brazil. Journal of Acquired Immune Deficiency Syndromes, 57(Suppl. 3), S179–S185. [DOI] [PubMed] [Google Scholar]
- Palmeira dos Santos TM, Barros da Silva D, Monteiro-Franco T, Ribeiro dos Santos V, DeMendonca J, Dos Santos Junior JA, Da Costa D (2017). Lipodystrophy and the relationship with cardiovascular risk factors and metabolic syndrome in HIV-infected patients. Nutrcion Clinica y Dietetica Hospitalaria, 37(2), 12–20. [Google Scholar]
- Peddle-McIntyre CJ, Bell G, Fenton D, McCargar L, Courneya KS (2013). Changes in motivational outcomes after a supervised resistance exercise training intervention in lung cancer survivors. Cancer Nursing, 36(1), E27–E35. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno F, Camara-Sanchez M, Tremblay JF, Riera- Rubio VJ, Gil-Paisan L, Lucia A (2007). Benefits of exercise training in Spanish prison inmates. International Journal of Sports Medicine, 28(12), 1046–1052. [DOI] [PubMed] [Google Scholar]
- Poton R, Polito M, Farinatti P (2016). Effects of resistance training in HIV-infected patients: A meta-analysis of randomised controlled trials. Journal of Sports Sciences, 35(24), 2380–2389. [DOI] [PubMed] [Google Scholar]
- Ross A, Thomas S (2010). The health benefits of yoga and exercise: A review of comparison studies. Journal of Alternative and Complementary Medicine, 16(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Rossomanno CI, Herrick JE, Kirk SM, Kirk EP (2012). A 6-month supervised employer-based minimal exercise program for police officers improves fitness. Journal of Strength and Conditioning Research, 26(9), 2338–2344. [DOI] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, … North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. (2013). Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 8(12), e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J, Wit FW, Stolte IG, Kootstra NA, Van Der Valk M, Geerlings SE, Reiss P (2014). Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between hiv-infected and uninfected individuals: The age HIV cohort study. Clinical Infectious Diseases, 59(12), 1787–1797. [DOI] [PubMed] [Google Scholar]
- Schuelter-Trevisol F, Wolff FH, Alencastro PR, Grigoletti S, Ikeda ML, Brandao AB, … Fuchs SC (2012). Physical activity: Do patients infected with HIV practice? How much? A systematic review. Current HIV Research, 10(6), 487–497. [DOI] [PubMed] [Google Scholar]
- Simonik A, Vader K, Ellis D, Kesbian D, Leung P, Jachyra P, O’Brien KK (2016). Are you ready? Exploring readiness to engage in exercise among people living with HIV and multimorbidity in Toronto, Canada: A qualitative study. BMJ Open, 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawford A, Barbieri T, Van Loan M, Parks E, Catlin D, Barton N, Hellerstein MK (1999). Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV-related weight loss: A randomized controlled trial. Journal of the American Medical Association, 281(14), 1282–1290. [DOI] [PubMed] [Google Scholar]
- Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP (2006). Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Medicine and Science in Sports and Exercise, 38(3), 411–417. [DOI] [PubMed] [Google Scholar]
- Trinh L, Mutrie N, Campbell AM, Crawford JJ, Courneya KS (2014). Effects of supervised exercise on motivational outcomes in breast cancer survivors at 5-year follow-up. European Journal of Oncology Nursing, 18(6), 557–563. [DOI] [PubMed] [Google Scholar]
- Tully NE, Morgan KM, Burke HM, McGee HM (2010). Patient experiences of structured heart failure programmes. Rehabilitation Research and Practice, 2010, 157939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs. (2015). Exercise and HIV: Entire lesson. Retrieved from https://www.hiv.va.gov/patient/daily/exercise/single-page.asp
- Vancampfort D, Mugisha J, De Hert M, Probst M, Firth J, Gorczynski P, Stubbs B (2016). Global physical activity levels among people living with HIV: A systematic review and meta-analysis. Disability and Rehabilitation, 40(4), 388–397. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Mugisha J, Richards J, De Hert M, Lazzarotto AR, Schuch FB, … Stubbs B (2017a). Dropout from physical activity interventions in people living with HIV: A systematic review and meta-analysis. AIDS Care, 29(5), 636–643. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Mugisha J, Richards J, De Hert M, Probst M, Stubbs B (2017b). Physical activity correlates in people living with HIV/AIDS: A systematic review of 45 studies. Disability and Rehabilitation, 40(14), 1618–1629. [DOI] [PubMed] [Google Scholar]
- Veritas Health Innovation. (2018). Covidence: Accelerate your systematic review. Retrieved from https://www.covidence.org
- Volaklis KA, Thorand B, Peters A, Halle M, Heier M, Strasser B, … Meisinger C (2018). Physical activity, muscular strength, and polypharmacy among older multimorbid persons: Results from the KORA-Age study. Scandinavian Journal of Medicine and Science in Sports, 28(2), 604–612. [DOI] [PubMed] [Google Scholar]
- Webel AR, Barkley J, Longenecker CT, Mittelsteadt A, Gripshover B, Salata RA (2015). A cross-sectional description of age and gender differences in exercise patterns in adults living with HIV. Journal of the Association of Nurses in AIDS Care, 26(2), 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017). HIV/AIDS. Retrieved from http://who.int/mediacentre/factsheets/fs360/en/
- Yahiaoui A, McGough EL, Voss JG (2012). Development of evidence-based exercise recommendations for older HIV-infected patients. Journal of the Association of Nurses in AIDS Care, 23(3), 204–219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


