Abstract
Introduction
Glycogenic acanthosis (GA) is a benign, polypoid lesion frequently seen in upper endoscopy with little known aetiology. Information about how it occurs and its clinical significance is lacking.
Aim
In this study, the relationship between GA and reflux symptoms was investigated in patients who underwent endoscopy due to reflux symptoms.
Material and methods
Sixty patients undergoing endoscopy for reflux symptoms and 60 controls without reflux symptoms were included in the study. Among the patients with reflux symptoms, two groups were formed: GA group 1 (n = 30) and non-GA group 2 (n = 30).
Results
The mean age of all patients participating in the study was 44.65 ±15.54 years; in group 1 it was 52.56 ±10.90 years and in group 2 it was 39.40 ±13.87 years. The mean age of group 1 patients was statistically significantly higher than that of group 2 patients (p < 0.05). The incidence of GA was higher in group 1 than in the control group (p = 0.001). In the reflux group, group 1 and group 2 were compared in terms of oesophagitis; group 1 had a higher incidence of oesophagitis (p < 0.05). In the reflux group, in those with GA, the risk of oesophagitis was 6.6 times higher than among those without GA (OR = 6.571; 95% CI: 2.109–20.479).
Conclusions
We think that GA is associated with advanced age, reflux disease, and oesophagitis in our study.
Keywords: gastroesophageal reflux, glycogenic acanthosis, esophagitis
Introduction
Gastroesophageal reflux disease (GERD) is a common disease worldwide as well as in our country. Above all, it considerably decreases quality of life due to the problems it causes in the oesophagus and outside the oesophagus. Diagnosis of the disease is usually made clinically, and its endoscopic diagnostic criteria are limited [1]. In addition to the clinical signs, the definite diagnosis is made based on esophagitis signs seen in endoscopy, histopathology, pH measurement, radiologic studies, and manometric studies. Because GERD is the basis for Barrett’s oesophagus, which is considered precancerous, correct diagnosis and follow-up of the disease is important [2]. The tests required for the correct diagnosis are partly laborious and not easy to find in each center. On the other hand, the rate of endoscopically demonstrating oesophagitis is not very high in GERD. When patients found to have an abnormal gastroesophageal reflux with a pH-meter were evaluated endoscopically, oesophagitis was found in 25% to 60% [3]. Therefore, in daily practice, new endoscopic clues are needed for the diagnosis of GERD.
Glycogenic acanthosis (GA) is a benign lesion frequently seen in upper endoscopy but with unknown clinical significance. Glycogenic acanthosis is reported as an incidental finding in 3.5% of endoscopies and is a diffuse benign oesophageal lesion easily recognised by experienced endoscopists. Although GA, which is seen as oval, slightly raised, whitish mucosal plaques of 0.2 to 1.5 cm, is occasionally associated with GERD in the literature, data are insufficient and its place in the diagnosis of GERD has remained uncertain [4, 5]. Histologically, it is characterised by multifocal plaques containing hyperplasia of the squamous epithelium and intracellular glycogen deposits [4]. In contrast to its name, it was supposed that GA has no relation with glucose metabolism disorders like diabetes or with skin disorders like acanthosis nigricans [6]. However, a recent study has suggested that GA may be associated with insulin resistance and metabolic syndrome [7].
Aim
The aim of this study is to identify the relationship between glycogenic acanthosis and reflux symptoms in patients undergoing endoscopy because of reflux symptoms.
Material and methods
Patients presenting to Gaziantep University Medical Faculty Gastroenterology Department Outpatient Unit with reflux symptoms were questioned about their symptoms, and these symptoms were recorded according to the history of the patient. Patients with complaints of at least two pyroses and regurgitation for the last 6 months were included in the study.
In our study, tests such as pH measurement and manometry were not applied to diagnosis GERD, only clinical reflux findings were compared with GA symptoms, which was a shortcoming of our study.
Patients were asked about the presence of pyrosis and regurgitation, and those answering “yes” to both were regarded as “positive” for having reflux symptoms. All patients underwent endoscopy. A fellow gastroenterologist inquired about the symptoms and another performed the endoscopy procedure.
Emergency cases, patients under 18 years of age, smokers, pregnant women, patients suffering from chronic diseases, patients taking some specific medications (such as calcium channel blockers, drugs affecting glucose metabolism, aspirin, nonsteroidal anti-inflammatory drugs, diuretics, oral contraceptives, anti-acid therapy drugs) were excluded from the study.
The control group was made up of patients undergoing endoscopy in the absence of reflux symptoms and usually with the purpose of screening for malignancy.
The education level of patients was also questioned due to the inadequate number of studies showing the relationship between education level and reflux symptoms alongside demographic characteristics such as age and gender.
Primary school and junior high school graduates were recorded as having received middle school education (8-year education), and high school and university graduates as higher education (> 12 years).
Patients with reflux symptoms (n = 60) and control patients without reflux symptoms (n = 60) were included in the study. Patients with reflux symptoms were then divided into two groups: those with GA (group 1; n = 30) and those without GA (group 2; n = 30).
The oesophagus was endoscopically evaluated in two parts as lower and upper oesophagus. Starting from the front teeth, the part below the 28th cm was defined as the lower oesophagus and the part above the 28th cm as the upper oesophagus.
Glycogenic acanthosis was defined as the presence of oval, whitish plaques of size 0.2 to 1.5 cm slightly elevated from normal oesophageal mucosa in endoscopy (Figure 1). A biopsy was taken from every patient for the diagnosis of GA. The diagnosis of GA was confirmed in cases with increased cellular glycogen and hyperplasia in the lesions noted in pathology [4]. Oesophagitis was evaluated without taking biopsies from mucosal breaks on the “Z” line in endoscopy, and the presence and severity of oesophagitis was determined by using the Los Angeles classification (Table I).
Figure 1.
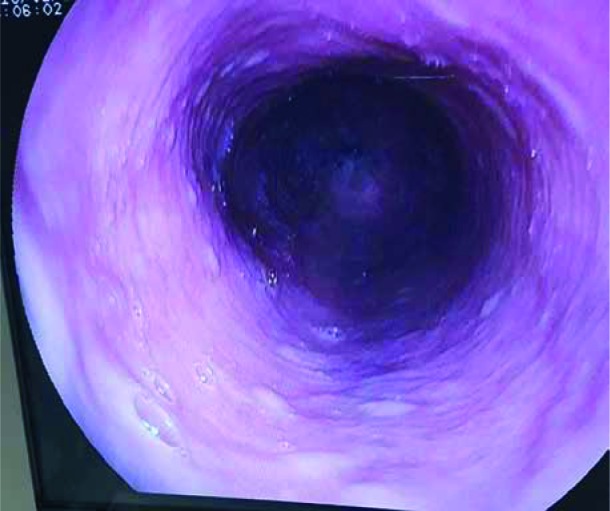
Endoscopic appearance of glycogenic acanthosis lesions
Table I.
Current endoscopic staging for oesophagitis
|
Los Angeles classification Stage A: non-confluent lesions less than 5 mm in length Stage B: non-confluent lesions more than 5 mm in length Stage C: confluent lesions over less than 75% of the lumen Stage D: confluent lesions over more than 75% of the oesophageal lumen |
Endoscopies were performed by a single fellow, who had carried out at least 1000 upper endoscopies before. The same model (Olympus GIF240) of gastroscope was used for endoscopy.
The study was conducted in accordance with the principles of the Helsinki Declaration of 1975 as revised in 2008 and with the approval numbered 02.11.2010/19 of the University of Gaziantep Faculty of Medicine Clinical Research Ethics Committee. All patients were informed about the study protocol, and written consent was obtained from each subject.
Statistical analysis
Normalcy of distribution of continuous variables was evaluated with the Kolmogorov-Smirnov test, and they were found to be normally distributed. When two independent groups were compared, the significance test of the difference between two mean values for continuous variables expressed in measures (independent samples t-test) was used, and mean and standard deviation values were given as descriptive statistics. The χ2 test or Yates’s corrected χ2 test was used for the group comparisons of qualitative variables, and frequency and percentage values were given as descriptive statistics. Power analysis could not have been conducted in this study due to the lack of clear data about the true frequency of GA in the population. In addition, the odds ratio was calculated to determine the risk of a factor and was given together with the 95% confidence interval. SPSS 23.0 software was used for all the analyses. Values of p < 0.05 were considered statistically significant.
Results
The mean age of the patients included in the study was 44.65 ±15.54 years; in group 1 it was 52.56 ±10.90 years, and in group 2 it was 39.40 ±13.87 years. There was a significant difference between the ages of the two groups. The mean age of the patients in group 1 was significantly higher than that in group 2 (p < 0.05). Of all the patients, 40 (66.7%) were female: 22 (73.3%) patients in group 1 and 40 (66.7%) patients in group 2. There was no statistically significant difference between the two groups in terms of gender (p > 0.05). When the education status of the groups was compared, the frequency of reflux and GA was higher in individuals with middle school education than in those with higher education (p < 0.05). There was a statistically significant difference between the control group and group 1 regarding the incidence of GA. GA was more frequent in the patients in group 1 compared with the control group (p = 0.001) (Table II). There was no difference in the levels of GA in group I patients. In the group with reflux, the frequency of esophagitis was higher in group 1 when groups 1 and 2 were compared in terms of the presence of oesophagitis (p < 0.05) (Table III). In the group with reflux, risk of oesophagitis was 6.6-fold higher in those with GA than in those without (OR = 6.571; 95% CI: 2.109–20.479).
Table II.
Demographic characteristics of the control group and patients with reflux symptoms included in the study
| Parameters | Control (n = 60) | Reflux group (n = 60) | P-value |
|---|---|---|---|
| Age | 39.73 ±12.28 | 44.65 ±15.54 | NS |
| Gender, n (%): | |||
| Female | 33 (45.0) | 40 (66.7) | NS |
| Education, n (%): | |||
| Middle | 26 (43.3) | 46 (76.7) | 0.001 |
| Higher | 34 (56) | 14 (23.3) | 0.001 |
| GA, n (%): | |||
| Present | 9 (15) | 30 (50) | 0.001 |
| GA localizations, n (%): | |||
| Upper oesophagus | 4 | 14 (46.7) | NS |
| Lower oesophagus | 5 | 16 (53.3) | NS |
| Esophagitis, n (%): | |||
| Present | 2 (3.3) | 33 (55) |
GA – glycogenic acanthosis, NS – not significant, p < 0.05.
Table III.
Demographic characteristics of patients with reflux symptoms included in the study
| Parameter | Reflux group | P-value | |
|---|---|---|---|
| Group 1 (n = 30) | Group 2 (n = 30) | ||
| Age | 52.56 ±10.90 | 39.40 ±13.87 | 0.001 |
| Gender, n (%): | |||
| Female | 22 (73.3) | 18 (60) | NS |
| Education, n (%): | |||
| Middle | 26 (86.7) | 20 (66.7) | NS |
| Higher | 4 (13.3) | 10 (33.3) | NS |
| Esophagitis present, n (%) | 23 (76.7) | 10 (33.3) | 0.01 |
NS – not significant, p < 0.001.
Discussion
Gastroesophageal reflux disease, which occurs with both industrial nutrition and increased consumption of toxic substances e.g. smoking and alcohol, has recently become one of the most common diseases. While educational publications issued by governments partly renders the population aware of this subject, GERD remains a serious problem. In a study showing that low education level was a significant risk factor for GERD, it was seen that GERD is more common among individuals whose highest education level is elementary school, as opposed to people who received a university education [8]. In our study the education level of patients presenting with reflux symptoms was lower than that of the control group, suggesting that GERD can be associated with intellectual status.
Articles found in Anglo-Saxon literature about GA do not currently lead us to a clinical conclusion, and most of them are considered a variation of the norm. Nevertheless, in some studies, GA has been described as the presence of polypoid lesions occurring secondarily to the reflux. 24-hour pH monitorisation was performed in patients pathologically diagnosed with glycogenic acanthosis, and pathologic gastroesophageal acid reflux was found at a rate of 83%; pH was < 4.0 in 37.3% of cases. In this study showing the correlation between reflux and GA, symptoms resolved with anti-reflux treatment, but GA lesions did not vanish. It was emphasised in the same study that although oesophagitis is not seen endoscopically, the presence of GA directly justifies reflux treatment [5]. In our study, there was a statistically significant correlation between the control group and reflux patients in terms of incidence of GA. The GA was more frequent in reflux patients than in the control group. Our results were similar to those in the literature [9, 10]. Oesophagitis was more frequent in those having GA in the reflux group (p < 0.05), and risk of oesophagitis was found to be 6.6-fold higher in those with GA. In a study by Jaskiewicz et al. similar results were obtained to ours, except for H. pylori, for which we did not test [11].
The aetiology of GA presenting with polypoid, local, benign thickenings in the oesophageal mucosa is not clear. It is usually considered a harmless lesion without risk of carcinoma [12, 13]. Because it is seen in the fifth and sixth decades of life and becomes larger and more numerous with increasing age, this condition was thought to be an age-related degenerative process [4]. Although its aetiology and pathogenesis are not yet known, it has been emphasised that GA is a benign lesion increasing with age, but it can be confounding and this appearance should be differentiated from others with more significant prognostic values [14]. In addition, in a study by Nazligül et al., it was emphasised that GA is mainly an age-related disease and can be associated with reflux and hiatal hernia [15]. In our study, the mean age of reflux patients with GA was significantly greater. Thus, it can be assumed that GA is a benign lesion directly related with age and not with reflux. However, the risk of endoscopic oesophagitis was found to be 6.6-fold higher in the group with reflux symptoms accompanying GA. This supports the hypothesis that GA cannot be associated with age only and can be a sign accompanying GERD.
Gastroesophageal reflux is a physiological event that can occur many times during the day without any symptoms or mucosal damage. A kind of oesophageal clearance is obtained with this short-term physiological reflux that is usually seen after meals [16]. This condition is not felt in healthy individuals with full integrity of oesophageal mucosa. However, this becomes noticeable in GERD, which is evaluated as a disease [17]. The prevalence of GERD and its complications increases with age [18]. Although it is physiological, an increase of GA with age can arise from exposure to gastric acid and probably from the cumulative effect of the acid damage in the oesophagus over time. Another factor that plays a role in oesophageal clearance is salivary secretion. It is known that swallowing is increased in order to neutralise the material refluxed into the oesophagus with saliva [19]. Because all other secretions including saliva decrease with aging, acid clearance of the oesophagus also decreases, and exposure to the acid increases, thus explaining the increase of GA with age. In our study there was no difference between the groups in terms of the localisations of GA. This result suggests that GA can occur at every site in contact with the acid in the oesophagus.
In contrast to studies showing a potential correlation between GA and reflux oesophagitis [9, 10], there are also studies showing that there is no such correlation [14]. In a study finding 24 GAs in 160 patients, no relationship was found between GA and reflux or other GIS diseases [14]. Similarly, GA was seen in only 23% of patients despite reflux symptoms in our study and signs of oesophagitis were not seen. These patients were already considered clinically GERD. Therefore, even though oesophagitis was not seen in these patients, we think that the presence of GA should be considered an endoscopic sign of GERD, just like seeing oesophagitis.
In the literature, there are many reflux case patients who have presented with oesophagitis [20]. However, there are also cases in which symptoms persist even after oesophagitis has improved endoscopically following anti-reflux therapy [3]. In another study, the presence of GA suggests that oesophagitis could be more than 6.6-fold higher. In this regard, it is potentially a scar lesion secondary to reflux.
Our study had some limitations. In our study, tests such as pH measurement and manometry were not applied to diagnose GERH, and only clinical reflux findings were compared with GA symptoms, which was a shortcoming of our study. Although the symptoms of reflux are quite common in the general population, relatively few patients were included in the study. Resulting from gagging or an inability to provide adequate distention inside the oesophagus, sometimes GA has not been detected and has even been mentioned as an insignificant lesion in reports. For this reason, there are no clear data about the true frequency of GA in the population, so power analysis could not have been conducted in this study. Although our study shows a close relationship between GA and gastric reflux, further studies are needed to investigate the presence of an intense accumulation of glycogen in the esophageal epithelium after exposure to acid.
Conclusions
We suggest that the presence of GA in our study can be an equivalent of oesophagitis and can be used as an auxiliary sign in upper endoscopy performed for the diagnosis of GERD.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Bor S, Vardar R, Vardar E, et al.; GORHEN Study Group . Endoscopic findings of gastroesophageal reflux disease in Turkey: Multicenter prospective study (GORHEN). Gastroenterology 2008; 134 (Suppl 1): T 2014. [Google Scholar]
- 2.Rösch T. Reports 2003 Orlando. Reflux disease and Barrett’s esophagus. Endoscopy 2003; 35: 809-15. [DOI] [PubMed] [Google Scholar]
- 3.Richter JE. Severe reflux esophagitis. Gastrintest Endosc Clin North Am 1994; 4: 677-98. [PubMed] [Google Scholar]
- 4.Ghahremani GG, Rushovich AM. Glycogenic acanthosis of the esophagus: radiographic and pathologic features. Gastrointest Radiol 1984; 9: 93-8. [DOI] [PubMed] [Google Scholar]
- 5.Vadva MG, Triadafilopoulos G. Glycogenic acanthosis of the esophagus and gastroesophageal reflux. J Clin Gastroenterol 1993; 17: 79-83. [DOI] [PubMed] [Google Scholar]
- 6.Rose D, Furth EE, Rubesin SE. Glycogenic acanthosis. AJR Am J Roentgenol 1995; 164: 96. [DOI] [PubMed] [Google Scholar]
- 7.Tahaacı M, Demirezer Bolat A, Tayfur Yürekli Ö, et al. . Is glycogenic acanthosis a predictor of insulin resistance and metabolic syndrome? Turk J Gastroenterol 2017; 28: 337-41. [DOI] [PubMed] [Google Scholar]
- 8.Dore MP, Maragkoudakis E, Fraley K, et al. . Diet, lifestyle and gender in gastro-esophageal reflux disease. Dig Dis Sci 2008; 53: 2027-32. [DOI] [PubMed] [Google Scholar]
- 9.Berliner L, Redmont P, Horowitz L, et al. . Glycogen plaques (glycogenic acanthosis) of the esophagus. Radiology 1981; 141: 607-10. [DOI] [PubMed] [Google Scholar]
- 10.Clemencon G, Gıoor F. Bening epithelial hyperplasia of the esophagus: glycogenic acanthosis. Endoscopy 1974; 6: 214-7. [Google Scholar]
- 11.Jaskiewicz K, Louwrens HD, Woodroof CW, et al. . The association of Campylobacter pylori with mucosal pathological changes in a population at risk for gastric cancer. S Afr Med J 1989; 75: 417-9. [PubMed] [Google Scholar]
- 12.Rywlin AM, Ortega R. Glycogenic acanthosis of the esophagus. Arch Pathol 1970; 90: 439-43. [PubMed] [Google Scholar]
- 13.Bender MD, Allison J, Cuartas F, et al. . Glycogenic acanthosis of the esophagus: a form of bening epithelial hyperplasia. Gastroenterology 1973; 65: 373-80. [PubMed] [Google Scholar]
- 14.Stern Z, Sharon P, Ligumsky M, et al. . Glycogenic acanthosis of the esophagus: a bening but confusing endoscopic lesion. Am J Gastroenterol 1980; 74: 261-3. [PubMed] [Google Scholar]
- 15.Nazlıgül Y, Aslan M, Esen R, et al. . Benign glycogenic acanthosis lesions of the esophagus. Turk J Gastroenterol 2012; 23: 199-202. [DOI] [PubMed] [Google Scholar]
- 16.Orlando RC, Dobrucali A. Gastroesophageal reflux disease. In: Atlas of Esophageal Diseases. 2nd edn Feldman M, Orlando RC (eds). Current Science Group, Philadelphia: 2002; 91-116. [Google Scholar]
- 17.Vakil N, Van Zanten S, Kahrilas P, et al. . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900-920. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DA, Fenerty MB. Heartburn severity underestimates erosive esophagitis severity in elderly patientswith gastroesophageal reflux disease. Gastroenterology 2004; 126: 660-4. [DOI] [PubMed] [Google Scholar]
- 19.Hemmink GJ, Weusten BL, Bredenoord AJ, et al. . Increased swallowing frequency in GORD is likely to be caused by perception of reflux episodes. Neurogastroenterol Motil 2009; 21: 143-8. [DOI] [PubMed] [Google Scholar]
- 20.Moraes-Filho JP. Gastroesophageal reflux disease: prevelans and management in Brazil. Best Pract Res Clin Gastroenterol 2004; 18: 23-6. [DOI] [PubMed] [Google Scholar]


