Abstract
Background: This study compared the effects of pre-transplantation measurable residual disease (pre-MRD) on outcomes in Philadelphia chromosome (Ph)-positive ALL patients who underwent human leukocyte antigen-matched sibling donor transplantation (MSDT) or who received unmanipulated haploidentical SCT (haplo-SCT).
Methods: A retrospective study (n = 202) was performed. MRD was detected by RT-PCR and multiparameter flow cytometry.
Results: In the total patient group, patients with positive pre-MRD had a higher 4-year cumulative incidence of relapse (CIR) than that in patients with negative pre-MRD (26.1% vs. 12.1%, P = 0.009); however, the cumulative incidence of non-relapse mortality (NRM) (7.4% vs. 15.9%, P = 0.148), probability of leukemia-free survival (LFS) (66.3% vs. 71.4%, P = 0.480), and overall survival (OS) (68.8% vs. 76.5%, P = 0.322) were comparable. In the MSDT group, patients with positive pre-MRD had increased 4-year CIR (56.4% vs. 13.8%, P < 0.001) and decreased 4-year LFS (35.9% vs. 71.0%, P = 0.024) and OS (35.9% vs. 77.6%, P = 0.011) compared with those with negative pre-MRD. In haplo-SCT settings, the 4-year CIR (14.8% vs. 10.7%, P = 0.297), NRM (7.3% vs. 16.3%, P = 0.187) and the 4-year probability of OS (77.7% vs. 72.3%, P = 0.804) and LFS (80.5% vs. 75.7%, P = 0.660) were comparable between pre-MRD positive and negative groups. In subgroup patients with positive pre-MRD, haplo-SCT had a lower 4-year CIR (14.8% vs. 56.4%, P = 0.021) and a higher 4-year LFS (77.7% vs. 35.9%, P = 0.036) and OS (80.5% vs. 35.9%, P = 0.027) than those of MSDT. Multivariate analysis showed that haplo-SCT was associated with lower CIR (HR, 0.288; P = 0.031), superior LFS (HR, 0.283; P = 0.019) and OS (HR, 0.252; P = 0.013) in cases with a positive pre-MRD subgroup.
Conclusions: Our results indicate that the effects of positive pre-MRD on the outcomes of patients with Ph-positive ALL are different according to transplant modality. For Ph-positive cases with positive pre-MRD, haplo-SCT might have strong graft-vs.-leukemia (GVL) effects.
Keywords: haploidentical allografts, Philadelphia-chromosome positive, acute lymphoblastic leukemia, HLA-matched sibling donor transplantation, measurable residual disease
Introduction
Philadelphia chromosome (Ph) positivity is one of the most unfavorable cytogenetic prognostic factors in acute lymphoblastic leukemia (ALL), comprising 3–5% children (1), 5–15% adolescents (2) and 25–40% adults (2). The probability of 5-year overall survival (OS) of this subgroup of cases is approximately 30–45% (3–7), although the outcomes have been remarkably improved with the combination of tyrosine kinase inhibitors (TKI) and multiagent chemotherapy. Currently, allogeneic stem cell transplantation (allo-SCT) is a curable therapy for patients with Ph-positive ALL. However, hematological relapse remains one of the major causes of death after allo-SCT (8). Thus, prediction and intervention before leukemia hematological relapse are important in reducing the cumulative incidence of relapse (CIR) and improving transplant outcomes.
Many studies suggest that measurable residual disease (MRD) is an independent prognostic factor in ALL patients who were treated with chemotherapy alone or allo-SCT, making detection of MRD a tool to predict relapse and criteria of risk stratification (9–28). Cazzaniga et al. (29) indicated that Ph-positive ALL patients with negative MRD after consolidation had a lower risk of relapse compared to those with positive MRD. Mizuta et al. (20) demonstrated that negative pre-transplantation MRD (pre-MRD) status, as detected by real-time quantitative polymerase chain reactions (RT-PCR), is associated with significantly lower incidences of relapse in Ph-positive ALL patients who underwent allo-SCT in CR1. Similar results were observed by Ruggeri et al. (21) in pediatric patients with ALL who underwent umbilical cord blood transplantation (UCBT). Zhao et al. (30) indicates that in patients with Ph-positive ALL, MRD detected at early stages after allo-SCT is an important predictor of patient outcomes. Nevertheless, these studies mainly focused on human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT), HLA-matched unrelated donor transplantation (MUDT) and UCBT.
In the past 10 years, the routine use of haploidentical SCT (haplo-SCT) has allowed almost all patients to undergo allo-SCT (31). Our previous study showed that treating ALL patients with haplo-SCT could achieve outcomes comparable to those of MSDT (32). A study on behalf of the Acute Leukemia Working Party of the Europe Bone Marrow Transplantation (EBMT) (33) suggests that unmanipulated haploidentical allografts could be considered an alternative option for adult patients with high-risk ALL who lack HLA-identical donors, preferably in early disease status. Currently, few data concentrate on the effects of pre-transplantation MRD (pre-MRD) on transplant outcomes in patients with ALL who underwent haplo-SCT (34). Therefore, in this study, we aimed to evaluate the effects of pre-MRD determined by MFC on clinical outcomes in patients with Ph-positive ALL who underwent haplo-SCT. We also investigated whether there were differences in the impacts of pre-MRD on outcomes between Ph-positive ALL patients who underwent haplo-SCT and those of patients who received MSDT.
Patients and Methods
Study Design
Two hundred and two Ph-positive ALL patients including children (n = 36) and adults (n = 166) who underwent MSDT (n = 61) and haplo-SCT (n = 141) were retrospectively enrolled in this study between March 2011 and December 2016. All of the included subjects provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Peking University.
Chemotherapy Before Transplantation
The induction chemotherapy regimen included daunorubicin, cyclophosphamide (Cy), vincristine, prednisone (VDCP), and L-asparaginase or Cy, daunorubicin, vindesine, prednisone (CODP). Consolidation chemotherapy regimen included hyper-CVAD (B) (methotrexate and cytosine arabinoside), high-dose methotrexate with/without L-asparaginase, and the VDCP or CODP regimen, which were given in turn. Prophylaxis for central nervous system leukemia was given to every enrolled patient, which consisted of intrathecal chemotherapy with methotrexate, cytosine arabinoside, and dexamethasone for at least four doses during induction and consolidation chemotherapy (35, 36).
Transplant Protocol
Unmanipulated haplo-SCT and MSDT were performed according to the protocols reported previously by our group (8, 32).
Tyrosine Kinase Inhibitors (TKI) Treatment Before and After Transplantation
All Ph-positive ALL patients were treated with a TKI, mainly imatinib, as induction and/or consolidation therapy before transplantation (37). A TKI, usually imatinib, was administered depending on the blood cell counts or the molecular level of the BCR-ABL fusion gene 1, 2. Treatment with imatinib was initiated (1) if patient peripheral blood absolute neutrophil counts were >1.0 × 109/L without granulocyte colony-stimulating factor administration, and the platelet count was >50.0 × 109/L, regardless of the level of BCR-ABL transcript; or (2) if the level of BCR-ABL transcript in the bone marrow was detectable and transcript levels increased for two consecutive tests, or if the BCR-ABL transcript level was ≥10−2 after the initial engraftment, although patients' absolute neutrophil counts or platelet count were below the above values. Other criteria for initiation of treatment included that patients could tolerate oral imatinib without gut graft-vs.-host disease (GVHD) or life-threatening infection. Imatinib treatment was scheduled for 3–12 months after hematopoietic cell transplantation, until BCR-ABL transcript levels were negative at least for three consecutive tests or complete molecular remission was sustained for at least 3 months. The initial dose of imatinib was 400 mg/day for adults (age > 17 years) and 260 mg/m2/day for children (age < 17 years) 1, 2, 16. The daily dose of imatinib was adjusted according to the National Comprehensive Cancer Network practice guidelines regarding the management of imatinib toxicity (2005 version).
Donor Lymphocyte Infusion (DLI)
The indications for DLI included hematological leukemia relapse, receiving chemotherapy followed by DLI, molecular test results that provided evidence of persistent leukemia or recurrence in subjects without graft-vs.-host disease (GVHD), and graft failure (GF). The DLI protocol was applied according to our previous study (38–40). For relapse treatment, induction chemotherapy followed by DLI and GVHD prophylaxis was given. For relapse prophylaxis or GF, only DLI and GVHD prevention were used.
MRD Detection
The BCR-ABL transcript levels in the bone marrow of patients were detected through RT-PCR with ABL as the control gene. Five milliliters of fresh bone marrow (BM) was collected. Samples obtained in EDTA were treated within 2 h of collection to lyse the red blood cells. RNA extraction, complementary DNA synthesis, and RT-PCR analysis were performed as previously described in the literature (41). ABL was selected as a control gene to compensate for variations in the quality and quantity of RNA and cDNA. BCR-ABL primers and probes that amplified both b3a2 and b2a2 junctions were designed using Primer Express software version 2.0. There were similar primers and probes described in Europe against Cancer Program report (41). The primer and probe sequences of BCR-ABL mRNA have been described previously (42).
ABL primers and probes were referred to in the report of the Europe Against Cancer Program (43). The copy numbers of all ABL samples were more than 3 × 104. The reproducible sensitivity of PCR was five copies. All experiments were performed in duplicate. If BCR-ABL mRNA was detected, the sample was considered positive, and the number of transcripts was calculated as BCR-ABL/ABL %. If BCR-ABL mRNA was undetected, the sample was regarded as negative, and BCR-ABL/ABL% was equal to zero. The molecular responses in PB and BM samples were defined as the log-reductions of BCR-ABL mRNA level from the baseline value of PB and BM, respectively, which were the median levels from newly diagnosed CP CML patients. Major molecular response (MMR) in PB and BM samples were defined as ≥3 log-reductions of BCR-ABL mRNA level from the baseline value of PB and BM, respectively. MRD negative and MRD positive were defined as not detectable and detectable as previous report by Yanada et al. (44) The threshold for quantification was 50 copies/μg RNA, which corresponded to a sensitivity of 10−5.
The MRD was also determined by multiparameter flow cytometry (MFC) according to previous publication (30). A lower limit of detection (LOD) of 0.001% was targeted.
MRD detection was performed in all patients as a routine clinical test on bone marrow aspirate samples that were obtained at 1 month before SCT as well as at days 30, 60, 90, 120, and 180 after transplantation (37).
In this study, positive pre-MRD was defined using a cutoff value of 0.001% determined by MFC according to our publication (34). In our previous study, we showed that for ALL patients who underwent haplo-SCT, cases with positive pre-MRD (≧ 0.001%) detected by MFC had a significantly higher CIR than that of cases with negative one (<0.001%) (34).
Outcomes
The primary study endpoint was the CIR. The secondary endpoints were the cumulative incidence of non-relapse mortality (NRM), the probability of leukemia-free survival (LFS) and overall survival (OS). Engraftment, infection, NRM, relapse, LFS, OS, acute GVHD, and chronic GVHD were defined as previously described (38, 45, 46).
Propensity Score Matched Analysis
Propensity score matched analysis was performed by attempting to match each patient who underwent MSDT with those who underwent haplo-SCT (a 1:1 match). Using the nearest-neighbor-matching method, propensity score matching was performed using the following parameters: sex, age, pre-MRD. A match occurred when the difference in logits of propensity score was <0.2 times the standard deviation of scores.
Statistical Analysis
Patient characteristics were compared between the positive pre-MRD and negative using χ2 statistic categorical variables and the Mann-Whitney test for continuous variables. The probability of relapse, non-relapse mortality (NRM), LFS and OS were estimated with the Kaplan-Meier method. NRM was defined as death without relapse and was treated as a competing risk for relapse. However, relapse was considered a competing risk for NRM. MRD status pre-transplantation and all variables in Table 1 were included in the univariate analysis. Only variables with P < 0.1 were included in a Cox proportional hazards model with time-dependent variables. Unless otherwise specified, P-values were based on two-sided hypothesis tests. Alpha was set at 0.05. Most analyses were performed with SPSS 16.0 (Mathsoft, Seattle, WA, USA).
Table 1.
Patient and donor characteristics (n = 202).
| Characteristic | All patients | MSDT | Haplo-SCT | ||||
|---|---|---|---|---|---|---|---|
| MRD neg | MRD pos | P-value | MRD neg | MRD pos | P-value | ||
| Number of patients | 202 | 48 | 13 | 100 | 41 | ||
| Median age (range), years | 32 (4–63) | 40 (7–63) | 38 (8–60) | 0.828 | 27 (4–57) | 32 (11–53) | 0.368 |
| Male, n (%) | 117 (57.9%) | 23 (47.9%) | 6 (46.2%) | 0.910 | 60 (60.0%) | 28 (68.3%) | 0.356 |
| Disease status, n (%) | 0.006 | 0.003 | |||||
| CR1 | 188 (93.1%) | 47 (97.9%) | 9 (69.2%) | 98 (98.0%) | 34 (82.9%) | ||
| CR2 | 13 (6.9%) | 1 (2.1%) | 4 (30.8%) | 2 (2.0%) | 6 (14.7%) | ||
| CR > 2 | 1 (0.5%) | 0 | 0 | 0 | 1 (2.4%) | ||
| IKZF, n (%) | 0.409 | 0.890 | |||||
| Positive | 49 (24.3%) | 9 (18.8%) | 4 (30.8%) | 20 (20.0%) | 16 (39.0%) | ||
| Negative | 57 (28.2%) | 9 (18.8%) | 3 (23.1%) | 35 (35.0%) | 10 (24.4%) | ||
| Unknown | 96 (47.5%) | 30 (62.5%) | 6 (46.2%) | 45 (45.0%) | 15 (36.6%) | ||
| Cytogenetic subgroup of ALL, n (%) | 1.000 | 0.770 | |||||
| t(9;22) | 136 (67.3%) | 35 (72.9%) | 9 (69.2%) | 66 (66.0%) | 26 (63.4%) | ||
| t(9;22) with other karyotypic abnormalities | 66 (32.7%) | 13 (27.1%) | 4 (30.8%) | 34 (34.0%) | 15 (36.6%) | ||
| WBC at diagnosis, n (%) | 0.850 | 0.254 | |||||
| High | 98 (48.5%) | 16 (33.3%) | 5 (38.4%) | 53 (53.0%) | 24 (58.5%) | ||
| Normal | 79 (39.1%) | 28 (58.3%) | 7 (53.9%) | 30 (30.0%) | 14 (34.1%) | ||
| Unknown | 25 (12.4%) | 4 (8.4%) | 1 (7.7%) | 17 (17.0%) | 3 (7.3%) | ||
| Levels of pre–transplant MRD, median (range) | 0.03% (0.001–2.210%) | 0.03% (0.001–0.760%) | 0.03% (0.001–2.210%) | 0.670 | |||
| Donor-recipient sex-matched grafts, n (%) | 0.731 | 0.320 | |||||
| Male-male | 47 (23.3%) | 12 (25.0%) | 2 (15.4%) | 20 (20.0%) | 13 (31.7%) | ||
| Male-female | 35 (17.3%) | 13 (27.1%) | 5 (38.5%) | 13 (13.0%) | 4 (9.8%) | ||
| Female-male | 71 (35.1%) | 12 (25.0%) | 4 (30.8%) | 40 (40.0%) | 15 (36.6%) | ||
| Female-female | 49 (24.3%) | 11 (22.9%) | 2 (15.4%) | 27 (27.0%) | 9 (22.0%) | ||
| Donor-recipient relationship, n (%) | 0.600 | 0.649 | |||||
| Father-child | 47 (23.3%) | 0 | 0 | 35 (35.0%) | 12 (29.3%) | ||
| Mother-child | 15 (7.4%) | 0 | 0 | 10 (10.0%) | 5 (12.2%) | ||
| Sibling-sibling | 105 (52.0%) | 47 (97.9%) | 13 (100%) | 34 (34.0%) | 11 (26.8%) | ||
| Child-parent | 30 (14.9%) | 0 | 0 | 19 (19.0%) | 11 (26.8%) | ||
| Other | 5 (2.5%) | 1 (2.1%) | 0 | 2 (2.0%) | 2 (4.9%) | ||
| ABO matched graft, n (%) | 0.657 | 0.153 | |||||
| Matched | 117 (57.9%) | 31 (64.6%) | 10 (76.9%) | 57 (57.0%) | 19 (46.3%) | ||
| Major mismatch | 45 (22.3%) | 8 (16.7%) | 2 (15.4%) | 20 (20.0%) | 15 (36.6%) | ||
| Minor mismatch | 30 (14.9%) | 7 (14.6%) | 1 (7.7%) | 18 (18.0%) | 4 (9.8%) | ||
| Bi-directional mismatch | 10 (5.0%) | 2 (4.2%) | 0 | 5 (5.0%) | 3 (7.3%) | ||
| Cell compositions in allografts | |||||||
| Infused nuclear cells, (range) 108/kg | 8.02 (2.53–13.14) | 7.57 (2.53–13.14) | 7.99 (5.75–11.41) | 0.467 | 8.22 (4.92–12.06) | 8.12 (5.89–11.94) | 0.404 |
| Infused CD34+ cells, (range) 106/kg | 2.82 (0.59–8.51) | 2.77 (0.59–8.51) | 2.54 (0.59–8.51) | 0.664 | 2.82 (0.62–6.67) | 2.96 (0.84–7.20) | 0.108 |
| DLI after transplant, n (%) | 1.000 | 0.205 | |||||
| For relapse prophylaxis and intervention | 7 (31.8%) | 1 (20.0%) | 2 (40.0%) | 4 (57.1%) | 0 | ||
| For relapse treatment | 15 (68.2%) | 4 (80.0%) | 3 (60.0%) | 3 (42.9%) | 5 (100%) | ||
| Pre-transplantation TKI | 0.012 | 0.138 | |||||
| Imatinib | 163 (80.7%) | 44 (91.7%) | 8 (61.5%) | 82 (82.0%) | 29 (70.7%) | ||
| Others | 39 (19.3%) | 4 (8.3%) | 5 (38.5%) | 18 (18.0%) | 12 (29.3%) | ||
| Post-transplantation TKI | 0.004 | 0.069 | |||||
| Imatinib | 178 (88.1%) | 44 (91.7%) | 7 (53.8%) | 93 (93.0%) | 34 (82.9%) | ||
| Others | 24 (11.9%) | 4 (8.3%) | 6 (46.2%) | 7 (7.0%) | 7 (17.1%) | ||
| Median courses of chemotherapy | 4 (1–16) | 3 (2–9) | 5 (2–16) | 0.055 | 4 (1–11) | 4 (2–15) | 0.480 |
HLA, human leukocyte antigen; MSDT, vHLA-matched sibling donor transplantation; Haplo-SCT, unmanipulated haploidentical stem cell transplantation; MRD, minimal residual disease; neg, negative, pos, positive; CR, complete remission; DLI, donor lymphocyte infusions; TKI, tyrosine kinase inhibitor.
Results
Patient Characteristics
All 202 patients had <5% bone marrow blasts and met the morphological criteria for a leukemia-free state and CR. The median time from diagnosis to SCT was 6 months (2.5–25.0 months). All patients (n = 202) achieved sustained, full-donor chimerism and stable neutrophil engraftment. The characteristics of these patients are summarized in Table 1. Both in the MSDT group and the haplo-SCT group, the percentages of cases with ≥CR2 were significantly higher in patients with positive pre-MRD than those of subjects with negative MRD (P < 0.05 for all, Table 1). The cumulative incidence of grade II–IV acute GVHD was 21.5%. After a median follow-up of 1,001 days (range, 24–2,575 days), the 4-year cumulative incidence of chronic GVHD was 47.7%. The 4-year CIR and TRM were 15.7 and 13.7%, respectively. The 4-year LFS and OS were 70.2 and 74.5%, respectively.
Correlation of Pre-MRD With Outcomes in Total Cases who Received allo-SCT
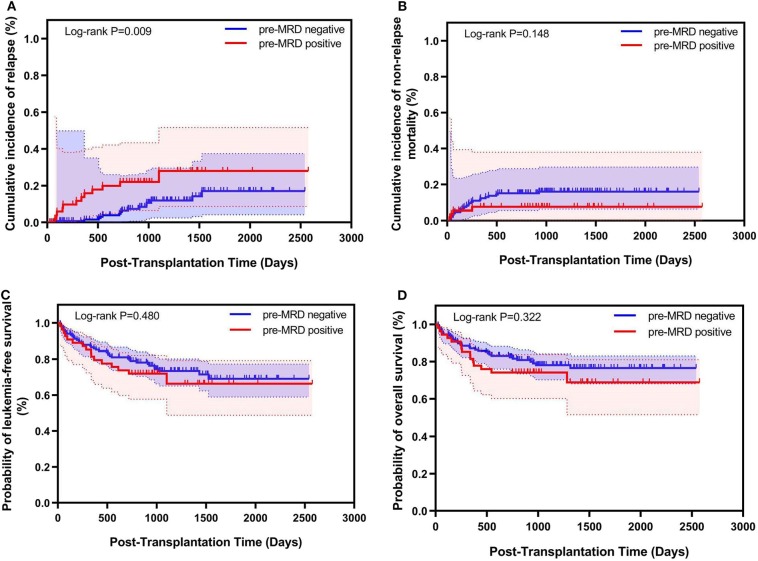
Among all 202 Ph-positive ALL patients, 54 (26.7%) had positive pre-MRD. Kaplan-Meier analysis showed that patients with positive pre-MRD had a higher 4-year CIR (26.1% vs. 12.1%, P = 0.009) compared to those with negative pre-MRD. The 4-year NRM, OS and LFS were comparable between patients with positive pre-MRD and those with negative pre-MRD (NRM 7.4% vs. 15.9%, P = 0.148; OS 68.8% vs. 76.5%, P = 0.322; LFS 66.3% vs. 71.4%, P = 0.480) (Figure 1). Multivariate analysis showed an association of disease status with CIR (HR, 4.079; P = 0.001) (Table 2).
Figure 1.
Relationship between pre-transplantation MRD and transplant outcomes for Ph-positive ALL patients who underwent allo-SCT (n = 202). Kaplan–Meier estimates of (A) cumulative incidence of relapse mortality, (B) cumulative incidence of non-relapse, (C) leukemia-free survival, and (D) overall survival.
Table 2.
Multivariate analysis of factors associated with outcomes of patients with Ph positive ALL who underwent allo-SCT (n = 202).
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Relapse | ||||||
| Disease status* | 2.953 | 1.500–5.815 | 0.002 | 4.079 | 1.821–9.137 | 0.001 |
| Pre-transplantation MRD (positive vs. negative) | 2.584 | 1.206–5.539 | 0.015 | |||
| Donor-recipient sex-matched graft | ||||||
| Female-male | 0.928 | 0.232–3.710 | 0.916 | 0.518 | 0.112–2.392 | 0.399 |
| Female-female | 3.817 | 1.196–12.181 | 0.024 | 3.248 | 1.006–10.484 | 0.049 |
| Male-male | 1.655 | 0.509–5.378 | 0.402 | 1.464 | 0.448–4.789 | 0.528 |
| Male-female | 1 | 1 | ||||
| Transplant–related mortality | ||||||
| Platelet engraftment (yes vs. no) | 0.047 | 0.020–0.107 | <0.001 | 0.047 | 0.020–0.107 | <0.001 |
| Leukemia-free survival | ||||||
| Donor-recipient sex-matched graft | ||||||
| Female-male | 0.774 | 0.306–1.962 | 0.590 | 1.344 | 0.499–3.568 | 0.566 |
| Female-female | 2.363 | 1.072–5.209 | 0.033 | 3.166 | 1.414–7.086 | 0.005 |
| Male-male | 1.505 | 0.708–3.196 | 0.288 | 2.426 | 1.088–5.408 | 0.030 |
| Male-female | 1 | 1 | ||||
| Platelet engraftment (yes vs. no) | 0.067 | 0.033–0.133 | <0.001 | 0.054 | 0.025–0.115 | <0.001 |
| Overall survival | ||||||
| Disease status | 1.956 | 0.980–3.905 | 0.057 | |||
| Donor-recipient sex-matched graft | ||||||
| Female-male | 0.492 | 0.168–1.439 | 0.195 | 0.898 | 0.290–2.780 | 0.851 |
| Female-female | 1.823 | 0.799–4.158 | 0.154 | 2.471 | 1.067–5.723 | 0.035 |
| Male-male | 1.352 | 0.629–2.909 | 0.440 | 2.290 | 1.006–5.215 | 0.048 |
| Male-female | 1 | 1 | ||||
| Platelet engraftment (yes vs. no) | 0.059 | 0.029–0.119 | <0.001 | 0.050 | 0.023–0.110 | <0.001 |
allo-SCT, allogeneic stem cell transplantation; MRD, measurable residual disease; HR, hazard ratio; CI, confidence interval.
All variables were first included in the univariate analysis, including sex, age, donor-recipient sex-matched grafts, donor-recipient relationship, ABO matched graft, pre-transplantation MRD status, disease status and hematopoietic engraftments; only variables with P < 0.1 were included in the Cox proportional hazards model with time-dependent variables.
Correlation Between Pre-MRD and Clinical Outcomes After MSDT
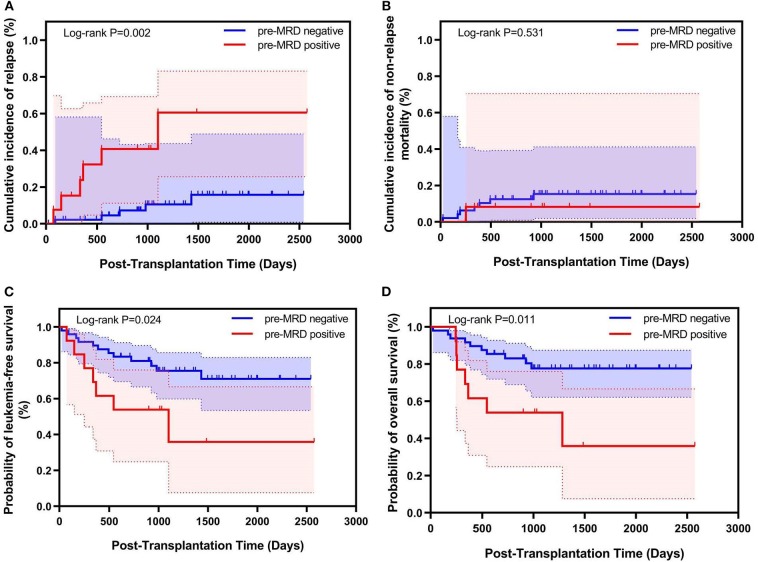
In 61 patients who were treated with MSDT. The cumulative 100-day incidence of grade II-IV acute GVHD (19.1% vs. 23.8%, P = 0.760) and 4-year cumulative incidence of chronic GVHD (56.5% vs. 38.3%, P = 0.643) were comparable between the pre-MRD negative group and the pre-MRD positive group. Patients with negative pre-MRD experienced a significantly lower 4-year CIR (13.8% vs. 56.4%, P < 0.001) as well as higher 4-year LFS (71.0% vs. 35.9%, P = 0.024) and OS (77.6% vs. 35.9%, P = 0.011). The 4-year NRM was similar in the pre-MRD negative and pre-MRD positive groups (15.2% vs. 7.7%, P = 0.654) (Figure 2 and Table 3). Multivariate analysis indicated that positive pre-MRD status was associated with higher CIR (HR, 6.049; P = 0.003) and lower LFS (HR, 2.797; P = 0.031) and OS (HR, 3.256; P = 0.017) (Table 4). In addition, subgroup analysis of Ph-positive patients in CR1 showed that patients with positive pre-MRD had a higher 4-year CIR compared to those with negative pre-MRD in the MSDT subset (33.3% vs. 14.0%, P = 0.055) (Table S1). Multivariate analysis showed that positive pre-MRD status was related to higher CIR (HR, 4.006; P = 0.058) in cases who receiving MSDT (n = 56).
Figure 2.
Relationship between pre-transplantation MRD and transplant outcomes for Ph-positive ALL patients who underwent MSDT (n = 61). Kaplan–Meier estimates of (A) cumulative incidence of relapse mortality, (B) cumulative incidence of non-relapse, (C) leukemia-free survival, and (D) overall survival.
Table 3.
Transplant outcomes for patients that underwent allogeneic stem cell transplantation (n = 202).
| Neutrophil engraftment | Platelet engraftment | Grades 2–4 acute GVHD | Chronic GVHD | Relapse at 4 years | NRM at 4 years | LFS at 4 years | OS at 4 years | ||
|---|---|---|---|---|---|---|---|---|---|
| MSDT (n = 61) |
Pre-MRD neg (Group1, n = 48) |
100% (95%CI, 100%) |
97.9% (95%CI, 93.8–100%) |
19.1% (95%CI, 7.9–30.3%) |
56.5% (95%CI, 40.4–72.6%) |
13.8% (95%CI, 1.6–26.0%)a |
15.2% (95%CI, 4.6–25.8%) |
71.0% (95%CI, 56.3–85.7%)b |
77.6% (95%CI, 65.3–89.9%)c |
| Pre–MRD pos (Group2, n = 13) |
100% (95%CI, 100%) |
100% (95%CI, 100%) |
23.8% (95%CI, 0.1–47.5%) |
38.3% (95%CI, 7.7–68.9%) |
56.4% (95%CI, 15.8–97.0%) |
7.7% (95%CI, 0–23.0%) |
35.9% (95%CI, 2.0–69.8%) |
35.9% (95%CI, 2.0–69.8%) |
|
| Haplo-HSCT (n = 141) |
Pre-MRD neg (Group3, n = 100) |
100% (95%CI, 100%) |
90.1% (95%CI, 83.8–96.4%) |
22.1% (95%CI, 13.9–30.3%) |
44.9% (95%CI, 33.5–56.3%) |
10.7% (95%CI, 3.8–17.6%)a |
16.3% (95%CI, 9.0–23.6%) |
72.3% (95%CI, 63.1–81.5%)d |
75.7% (95%CI, 66.3–85.1%)e |
| Pre-MRD pos (Group4, n = 41) |
100% (95%CI, 100%) |
94.9% (95%CI, 87.8–100%) |
22.0% (95%CI, 9.3–34.7%) |
38.5% (95%CI, 21.4–55.6%) |
14.8% (95%CI, 3.6–26.0%)f |
7.3% (95%CI, 0–15.3%) |
77.7% (95%CI, 64.8–90.6%)g |
80.5% (95%CI, 68.3–92.7%)h |
P < 0.001 compared with the Pre-MRDpos MSDT group.
P = 0.024 compared with the Pre-MRDpos MSDT group.
P = 0.011 compared with the Pre-MRDpos MSDT group.
P = 0.043 compared with the Pre-MRDpos MSDT group.
P = 0.020 compared with the Pre-MRDpos MSDT group.
P = 0.021 compared with the Pre-MRDpos MSDT group.
P = 0.036 compared with the Pre-MRDpos MSDT group.
P = 0.027 compared with the Pre-MRDpos MSDT group.
MSDT, human leukocyte antigen matched sibling donor transplantation; haplo-HSCT, haploidentical stem cell transplantation; MRD, minimal residual disease; Pre-MRD pos, positive MRD status before transplantation; Pre-MRD neg, negative MRD status before transplantation; GVHD, graft-vs.-host disease; NRM= non-relapse mortality.
Table 4.
Multivariate analysis of factors associated with outcomes of patients with Ph positive ALL who underwent MSDT (n = 61).
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Relapse | ||||||
| Disease status (CR > 1 vs. CR1)* | 5.672 | 1.455–22.117 | 0.012 | |||
| Pre-transplantation MRD (positive vs. negative) | 6.049 | 1.829–20.007 | 0.003 | 6.049 | 1.829–20.007 | 0.003 |
| Leukemia-free survival | ||||||
| Pre-transplantation MRD (positive vs. negative) | 2.797 | 1.096–7.140 | 0.031 | 2.797 | 1.096–7.140 | 0.031 |
| Overall survival | ||||||
| Pre-transplantation MRD (positive vs. negative) | 3.256 | 1.234–8.594 | 0.017 | 3.256 | 1.234–8.594 | 0.017 |
| Sex (male vs. female) | 0.430 | 0.159–1.164 | 0.097 | |||
MSDT, human leukocyte antigen-matched sibling donor transplantation; HR, hazard ratio; CI, confidence interval.
All variables were first included in the univariate analysis, including sex, age, donor-recipient sex-matched grafts, donor-recipient relationship, ABO matched graft, pre-transplantation MRD status, disease status and engraftments; only variables with P < 0.1 were included in the Cox proportional hazards model with time-dependent variables.
Correlation Between Pre-MRD and Clinical Outcomes After haplo-SCT
The cumulative 100-day incidence of grade II-IV acute GVHD (22.0% vs. 22.1%, P = 0.971) and 4-year cumulative incidence of chronic GVHD (38.5% vs. 44.9%, P = 0.687) were comparable. Patients with positive pre-MRD had similar transplant outcomes compared to those without positive pre-MRD (4-year CIR 14.8% vs. 10.7%, P = 0.297; 4-year NRM 7.3% vs. 16.3%, P = 0.187; 4-year LFS 77.7% vs. 72.3%, P = 0.660; 4-year OS 80.5% vs. 75.7%, P = 0.804) (Table 3). Multivariate analysis showed that there was no association between pre-MRD positive status and relapse, NRM, LFS, or OS (data not shown). Only disease status (≥CR2 vs. CR1) was associated with higher CIR (HR, 2.604; 95% CI, 1.096–6.183, P = 0.030).
Association of Transplant Modality With Outcomes in Pre-MRD Positive Subgroup
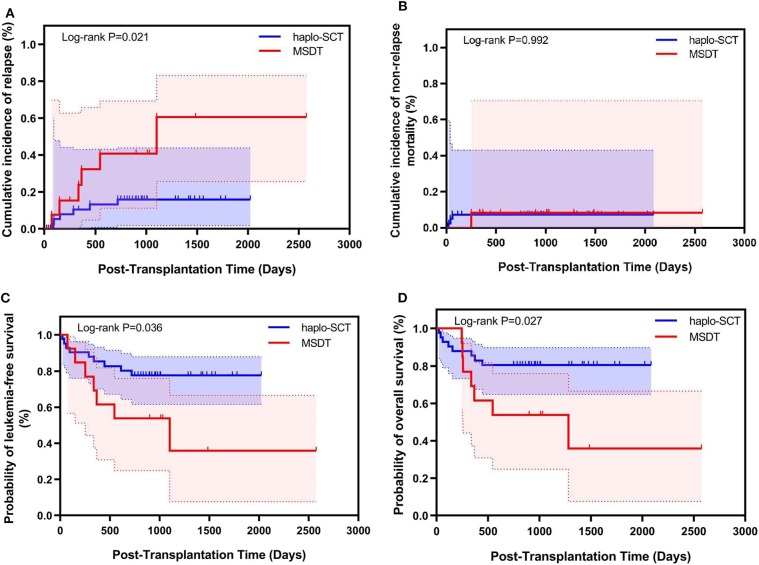
Our previous study has shown that compared to MSDT, treating acute myeloid leukemia (AML) patients with positive pre-MRD with haplo-SCT could achieve lower CIR (31). In this study, fifty-four patients had positive pre-MRD, the median level of MRD was 0.03% (0.001–2.210%). There were no difference in the level of pre-MRD between patients who underwent haplo-SCT and those received MSDT (Table 1). However, compared with patients in the haplo-SCT group, more cases in MSDT group received post-transplantation TKIs other than imatinib (P = 0.033). In comparison to those with positive pre-MRD undergoing MSDT, patients who were treated with haplo-SCT had a lower 4-year CIR (14.8% vs. 56.4%, P = 0.021) and higher 4-year LFS (77.7% vs. 35.9%, P = 0.036), OS (80.5% vs. 35.9%, P = 0.027). The 4-year NRM was comparable in the MSDT and haplo-SCT groups (7.3% vs. 7.7%, P = 0.992) (Figure 3). Multivariate analysis revealed that haplo-SCT was associated with lower CIR (HR, 0.288; P = 0.031) and high probability of LFS (HR, 0.283; P = 0.019) and OS (HR, 0.252; P = 0.013) (Table 5). In Ph-positive patients in CR1 with positive pre-MRD, cases underwent haplo-SCT had a lower 4-year CIR compared to those cases underwent MSDT (9.0% vs. 33.3%, P = 0.057). Multivariate analysis showed that haplo-SCT was related to lower CIR (HR, 0.235; P = 0.077) (Tables S1, S2). We did not find differences in kinetics of the BCR/ABL levels before day 180 after transplantation between patients with positive pre-MRD who underwent haplo-SCT and those who received MDST. This could be related to the results that, in the current study, 27 patients relapsed, 21 of them relapsed after 180 days post transplantation (Table S3).
Figure 3.
Relationship between transplant modality and transplant outcomes for Ph-positive ALL patients with pre-transplantation MRD who underwent allo-SCT (n = 54). Kaplan–Meier estimates of (A) cumulative incidence of relapse mortality, (B) cumulative incidence of non-relapse, (C) leukemia-free survival, and (D) overall survival.
Table 5.
Multivariate analysis of factors associated with outcomes of Ph positive ALL patients with positive pre-transplantation MRD who underwent allo-SCT (n = 54).
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Relapse | ||||||
| Transplant modality (haplo-SCT vs. MSDT)* | 0.288 | 0.093–0.895 | 0.031 | 0.288 | 0.093–0.895 | 0.031 |
| Disease status (CR > 1 vs. CR1) | 2.304 | 0.989–5.366 | 0.053 | |||
| Transplant-related mortality | ||||||
| Platelet engraftment (yes vs. no) | 0.072 | 0.007–0.707 | 0.024 | 0.072 | 0.007–0.707 | 0.024 |
| Leukemia-free survival | ||||||
| Transplant modality (haplo-SCT vs. MSDT) | 0.363 | 0.135–0.977 | 0.045 | 0.283 | 0.099–0.810 | 0.019 |
| Platelet engraftment (yes vs. no) | 0.090 | 0.019–0.428 | 0.002 | 0.056 | 0.011–0.293 | 0.001 |
| Overall survival | ||||||
| Transplant modality (haplo-SCT vs. MSDT) | 0.334 | 0.121–0.924 | 0.035 | 0.252 | 0.084–0.752 | 0.013 |
| Platelet engraftment (yes vs. no) | 0.090 | 0.019–0.425 | 0.002 | 0.052 | 0.010–0.276 | 0.001 |
MSDT, human leukocyte antigen-matched sibling donor transplantation; halo-SCT, haploidentical stem cell transplantation; HR, hazard ratio; CI, confidence interval.
All variables were first included in the univariate analysis, including sex, age, donor-recipient sex-matched grafts, donor-recipient relationship, ABO matched graft, transplantation modality, disease status and engraftments; only variables with P < 0.1 were included in the Cox proportional hazards model with time-dependent variable.
Correlation Between Pre-MRD and Clinical Outcomes in a Propensity Score Matched Analysis
Sixty-one patients who underwent MSDT and 61 patients who received haplo-SCT were enrolled in the propensity score matched analysis. For patients who were treated with MSDT, cases with positive pre-MRD had a significantly higher 4-year CIR (56.4% vs. 13.8%, P = 0.008) and lower 4-year LFS (35.9% vs. 71.0%, P = 0.024) and OS (35.9% vs. 77.6%, P = 0.011) compared to those with negative pre-MRD. However, in haplo-SCT subgroup, patients with positive pre-MRD had similar transplant outcomes compared to those with negative pre-MRD (CIR 15.4% vs. 13.5%, P = 0.683; NRM 15.4% vs. 18.8%, P = 0.843; OS 84.6% vs. 69.9%, P = 0.468; LFS 69.2% vs. 67.8%, P = 0.880). Univariate analysis showed that, in the pre-MRD positive subgroup, patients who were treated with haplo-SCT had a lower 4-year CIR than that of cases received MSDT (15.4% vs. 56.4%, P = 0.002) (Table S4). Multivariate analysis was not performed considering there were 26 patients with positive pre-MRD.
Discussion
In agreement with previous reports (20, 26), the results of our study indicated that positive pre-MRD, detected by MFC, was associated with higher CIR in Ph-positive ALL patients who underwent allo-SCT. In the MSDT group, positive pre-MRD was not only associated with higher CIR but also related to lower survival rates. Surprisingly, we observed no negative effects of positive pre-MRD on outcomes in haplo-SCT treatment cases. Subgroup analysis of pre-MRD positive cases showed that, compared to MSDT, haplo-SCT was associated with lower CIR and superior survival. Overall, our data not only showed that there are different effects of positive pre-MRD on outcomes according to transplant modality but also suggested that haplo-SCT might have stronger graft-vs.-leukemia (GVL) effects compared to MSDT, as previously reported by others (47) and us (48, 49), although controversy remains (50).
A number of previous studies confirm a negative effect of positive pre-MRD in patients undergoing allo-SCT (9–27). In a recent meta-analysis of 21 studies, Shen et al. (51) found that in HLA-matched allo-SCT settings, positive pre-MRD was related to higher CIR (HR, 3.26; P < 0.05) as well as lower relapse-free survival (HR, 2.53; P < 0.05), LFS (HR, 4.77; P < 0.05), and OS (HR, 1.98; P < 0.05). In the current study, we found that Ph-positive ALL cases with positive pre-MRD in the MSDT group experienced higher CIR and lower OS and LFS. Therefore, the results reported by others (15, 27) and us suggest that in HLA-matched allograft modalities, positive pre-MRD is related to inferior survival regardless of the condition regimen, GVHD prophylaxis, and sources of stem cells, such as sibling donors, unrelated donors, and cord blood. Fortunately, the efficacies of blinatumomab and chimeric antigen receptor T-cells (CAR-T) in relapsed, refractory, or MRD-positive ALL have been confirmed by several studies (52, 53), which provide novel strategies for resolving a positive pre-MRD status to a negative one to improve transplant outcomes.
Previous studies by others (47, 54) and us (48, 49, 55) showed that, given AML patients with positive pre-MRD or subjects with Hodgkin's disease who relapsed after autologous SCT, patients receiving haplo-SCT experienced lower CIR and superior survival compared to patients receiving MDST, although controversy remains (50). Interestingly, we found that treatment based on haplo-SCT could overcome the negative effects of a positive pre-MRD diagnosis on relapse in patients with Ph-positive ALL and lead to better survival (Figure 2). This result was not replicated in patients treated instead with MDST, as found via subgroup analysis in positive pre-MRD Ph-positive ALL cases. The results of our study add further evidence to previous studies suggesting that, compared to MDST, haplo-SCT might have stronger GVL effects. Several reasons may account for the stronger GVL effects of haplo-SCT: First, Dabas et al. (56) demonstrated that anti-thymocyte globulin (ATG) at clinically relevant concentrations kills leukemic blasts. In this study, ATG was only used in the haplo-SCT setting. Second, the stronger GVL effects of haplo-SCT might be ascribed to the large number of alloreactive T-cell targets encoded by the fully mismatched haplotype and/or HLA disparity (54). Third, alloreactive natural killer (NK) cells may also play an important role in anti-leukemia activity in haplo-SCT settings (47).
Presently, some researchers demonstrated that 3-year OS was 83% in patients who did not undergo SCT in first remission (ASH 2019), however, the current recommendation is the pursue allo-SCT for Ph+ ALL (57–59). A consensus of North American experts also indicates that allo-SCT is an alternative method either for Ph+ ALL with negative pre-transplant MRD or for cases with positive pre-transplant MRD (7). Of course, the administration of TKIs in our study may contribute to improved survival according to previous study (60).
There are still limitations in our study. First, this study is a retrospective study and was conducted at a single center. Second, the haplo-SCT protocols are based on the utility of granulocyte colony-stimulating factor and ATG. Third, the detection of post-transplantation MRD is based on RT-PCR or MFC only. It would be more precise to evaluate the pre-MRD level by combining MFC with the utility of RQ-PCR. A multicenter prospective study is needed to confirm our findings in haplo-SCT modalities, including haplo-SCT with post-cyclophosphamide.
In conclusion, our results indicate that the effects of positive pre-MRD on outcomes are different according to transplant modality. For Ph-positive ALL patients with positive pre-MRD, haplo-SCT was related to lower incidences of relapse and a higher probability of survival. This suggests that haplo-SCT has a stronger GVL effect based on this study and previous reports (48, 49). This study provides novel evidence supporting the claim that, for Ph-positive ALL patients with positive pre-MRD, haplo-SCT is a better option than MSDT, especially for patients without HLA-identical sibling donors.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Peking University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Y-JC designed the study. S-QL, Q-ZF, and Y-JC collected the data. S-QL, Q-ZF, Y-JC, and X-JH analyzed the data and drafted the manuscript. All authors contributed to the data interpretation, manuscript preparation, and approval of the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the faculty members who participated in these studies.
Footnotes
Funding. This work was supported (in part) by The National Key Research and Development Program of China. Grant Number: 2017YFA0104500, Beijing Municipal Science and Technology Commission. Grant Number: Z181110009618032, the National Natural Science Foundation of China (Grant No. 81470342), and the Key Program of National Natural Science Foundation of China (Grant No. 81230013).
Equal Contribution
This study will be partly presented as an oral presentation at the 24h APBMT&ICBMT Annual Meeting and Exposition (August 30, 2019) in Busan, Korea.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00320/full#supplementary-material
References
- 1.Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. New Eng J Med. (2000) 342:998–1006. 10.1056/NEJM200004063421402 [DOI] [PubMed] [Google Scholar]
- 2.Eckert C, Henze G, Seeger K, Hagedorn N, Mann G, Panzer-Grumayer R, et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol Offi J Am Soc Clin Oncol. (2013) 31:2736–42. 10.1200/JCO.2012.48.5680 [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. (2005) 19:1509–16. 10.1038/sj.leu.2403886 [DOI] [PubMed] [Google Scholar]
- 4.Rousselot P, Coude MM, Gokbuget N, Gambacorti Passerini C, Hayette S, Cayuela JM, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive all. Blood. (2016) 128:774–82. 10.1182/blood-2016-02-700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. (2015) 100:653–61. 10.3324/haematol.2014.118588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravandi F, O'Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. (2015) 121:4158–64. 10.1002/cncr.29646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. (2015) 125:3711–9. 10.1182/blood-2015-02-627935 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Zhao XY, et al. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome-positive acute lymphobla stic leukemia. J Hematol Oncol. (2012) 5:29. 10.1186/1756-8722-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachanova V, Burke MJ, Yohe S, Cao Q, Sandhu K, Singleton TP, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant. (2012) 18:963–8. 10.1016/j.bbmt.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with all. Leukemia. (2002) 16:1668–72. 10.1038/sj.leu.2402552 [DOI] [PubMed] [Google Scholar]
- 11.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the all-REZ BFM Study Group. J Clin Oncol. (2009) 27:377–84. 10.1200/JCO.2008.17.6065 [DOI] [PubMed] [Google Scholar]
- 12.Balduzzi A, Di Maio L, Silvestri D, Songia S, Bonanomi S, Rovelli A, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol. (2014) 164:396–408. 10.1111/bjh.12639 [DOI] [PubMed] [Google Scholar]
- 13.Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. (2014) 2014:421723. 10.1155/2014/421723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doney K, Gooley TA, Deeg HJ, Flowers ME, Storb R, Appelbaum FR. Allogeneic hematopoietic cell transplantation with full-intensity conditioning for adult acute lymphoblastic leukemia: results from a single center, 1998-2006. Biol Blood Marrow Transplant. (2011) 17:1187–95. 10.1016/j.bbmt.2010.12.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elorza I, Palacio C, Dapena JL, Gallur L, Sanchez de Toledo J, Diaz de Heredia C. Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica. (2010) 95:936–41. 10.3324/haematol.2009.010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandemer V, Pochon C, Oger E, Dalle JH, Michel G, Schmitt C, et al. Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol. (2014) 165:392–401. 10.1111/bjh.12749 [DOI] [PubMed] [Google Scholar]
- 17.Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris EL, Garland RJ, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. (1998) 92:4072–9. 10.1182/blood.V92.11.4072 [DOI] [PubMed] [Google Scholar]
- 18.Lankester AC, Bierings MB, van Wering ER, Wijkhuijs AJ, de Weger RA, Wijnen JT, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. (2010) 24:1462–9. 10.1038/leu.2010.133 [DOI] [PubMed] [Google Scholar]
- 19.Logan AC, Vashi N, Faham M, Carlton V, Kong K, Buno I, et al. Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant. (2014) 20:1307–13. 10.1016/j.bbmt.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuta S, Matsuo K, Maeda T, Yujiri T, Hatta Y, Kimura Y, et al. Prognostic factors influencing clinical outcome of allogeneic hematopoietic stem cell transplantation following imatinib-based therapy in BCR-ABL-positive all. Blood cancer J. (2012) 2:e72. 10.1038/bcj.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri A, Michel G, Dalle JH, Caniglia M, Locatelli F, Campos A, et al. Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia. (2012) 26:2455–61. 10.1038/leu.2012.123 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Garcia J, Serrano J, Serrano-Lopez J, Gomez-Garcia P, Martinez F, Garcia-Castellano JM, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in all. Bone Marrow Transplant. (2013) 48:396–402. 10.1038/bmt.2012.147 [DOI] [PubMed] [Google Scholar]
- 23.Spinelli O, Peruta B, Tosi M, Guerini V, Salvi A, Zanotti MC, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. (2007) 92:612–8. 10.3324/haematol.10965 [DOI] [PubMed] [Google Scholar]
- 24.Sramkova L, Muzikova K, Fronkova E, Krejci O, Sedlacek P, Formankova R, et al. Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. (2007) 48:93–100. 10.1002/pbc.20794 [DOI] [PubMed] [Google Scholar]
- 25.Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. (2015) 168:395–404. 10.1111/bjh.13142 [DOI] [PubMed] [Google Scholar]
- 26.Tucunduva L, Ruggeri A, Sanz G, Furst S, Cornelissen J, Linkesch W, et al. Impact of minimal residual disease on outcomes after umbilical cord blood transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia: an analysis on behalf of eurocord, cord blood committee and the acute leukaemia working party of the european group for blood and marrow transplantation. Br J Haematol. (2014) 166:749–57. 10.1111/bjh.12970 [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Slack R, Jorgensen JL, Wang SA, Rondon G, de Lima M, et al. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma, Myeloma Leuk. (2014) 14:319–26. 10.1016/j.clml.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarnas JC, Brown PA, Aoun P, Ballen KK, Bellam N, Blum W, et al. Acute lymphoblastic leukemia. J Natl Compr Canc Netw JNCCN. (2012) 10:858–914. 10.6004/jnccn.2012.0089 [DOI] [PubMed] [Google Scholar]
- 29.Cazzaniga G, De Lorenzo P, Alten J, Rottgers S, Hancock J, Saha V, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica. (2018) 103:107–15. 10.3324/haematol.2017.176917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Zhao X, Chen H, Qin Y, Xu L, Zhang X, et al. Comparative analysis of flow cytometry and RQ-PCR for the detection of minimal residual disease in philadelphia chromosome-positive acute lymphoblastic leukemia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24:1936–43. 10.1016/j.bbmt.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Chang YJ, Wang Y, Huang XJ. Haploidentical stem cell transplantation for the treatment of leukemia: current status. Expert Rev Hematol. (2014) 7:635–47. 10.1586/17474086.2014.954543 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res. (2016) 22:3467–76. 10.1158/1078-0432.CCR-15-2335 [DOI] [PubMed] [Google Scholar]
- 33.Santoro N, Ruggeri A, Labopin M, Bacigalupo A, Ciceri F, Gulbas Z, et al. Unmanipulated haploidentical stem cell transplantation in adults with acute lymphoblastic leukemia: a study on behalf of the acute leukemia working party of the EBMT. J Hematol Oncol. (2017) 10:113. 10.1186/s13045-017-0480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao XS, Liu YR, Xu LP, Wang Y, Zhang XH, Chen H, et al. Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with all receiving unmanipulated haploidentical allografts. Am J Hematol. (2019) 94:512–21. 10.1002/ajh.25417 [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Liu KY, Xu LP, Chen YH, Han W, Zhang XH, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2015) 21:1110–6. 10.1016/j.bbmt.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 36.Subspecialty Group of Hematology, the Society of Pediatrics, Chinese Medical Association, Editorial Board, Chinese Journal of Pediatrics, Subspecialty Group of Hematology the Society of Pediatrics Chinese Medical Association et al. [Guidelines for the diagnosis and treatment of childhood acute lymphoblastic leukemia]. Zhonghua er ke za zhi. (2014) 52:641–4. 10.3760/cma.j.issn.0578-1310.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 37.Hematology Oncology Committee, Chinese Anti- Cancer Association, Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association [Chinese guidelines for diagnosis and treatment of acute lymphoblastic leukemia (2016)]. Zhonghua xue ye xue za zhi. (2016) 37:837–45. 10.3760/cma.j.issn.0253-2727.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol. (2016) 34:1855–63. 10.1200/JCO.2015.63.8817 [DOI] [PubMed] [Google Scholar]
- 39.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. (2007) 92:414–7. 10.3324/haematol.10570 [DOI] [PubMed] [Google Scholar]
- 40.Yan CH, Wang Y, Wang JZ, Chen YH, Chen Y, Wang FR, et al. Minimal residual disease- and graft-vs.-host disease-guided multiple consolidation chemotherapy and donor lymphocyte infusion prevent second acute leukemia relapse after allotransplant. J Hematol Oncol. (2016) 9:87. 10.1186/s13045-016-0319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin YZ, Liu YR, Zhu HH, Li JL, Ruan GR, Zhang Y, et al. Different kinetic patterns of BCR-ABL transcript levels in imatinib-treated chronic myeloid leukemia patients after achieving complete cytogenetic response. Int J Lab Hematol. (2008) 30:317–23. 10.1111/j.1751-553X.2007.00962.x [DOI] [PubMed] [Google Scholar]
- 42.Jiang Q, Zhao XY, Qin YZ, Liu YR, Lai YY, Jiang B, et al. The differences and correlations of BCR-ABL transcripts between peripheral blood and bone marrow assays are associated with the molecular responses in the bone marrow for chronic myelogenous leukemia. Am J Hematol. (2012) 87:1065–9. 10.1002/ajh.23321 [DOI] [PubMed] [Google Scholar]
- 43.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of 'real-time' quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. (2003) 17:2318–57. 10.1038/sj.leu.2403135 [DOI] [PubMed] [Google Scholar]
- 44.Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. (2006) 24:460–6. 10.1200/JCO.2005.03.2177 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant. (2014) 49:426–33. 10.1038/bmt.2013.191 [DOI] [PubMed] [Google Scholar]
- 46.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. (2005) 11:945–56. 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. (2002) 295:2097–100. 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 48.Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. (2017) 10:134. 10.1186/s13045-017-0502-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Wang Z, Ruan G, Liu Y, Wang Y, Zhang X, et al. Impact of pre-transplantation minimal residual disease determined by multiparameter flow cytometry on the outcome of AML patients with FLT3-ITD after allogeneic stem cell transplantation. Ann Hematol. (2018) 97:967–75. 10.1007/s00277-018-3265-1 [DOI] [PubMed] [Google Scholar]
- 50.Ringden O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. (2016) 30:447–55. 10.1038/leu.2015.232 [DOI] [PubMed] [Google Scholar]
- 51.Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z, et al. Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC cancer. (2018) 18:755. 10.1186/s12885-018-4670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gokbuget N, Topp MS, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive b-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. (2017) 35:1795–802. 10.1200/JCO.2016.69.3531 [DOI] [PubMed] [Google Scholar]
- 53.Liu D, Zhao J, Song Y, Luo X, Yang T, Scheuring UJ, et al. Clinical trial update on bispecific antibodies, antibody-drug conjugates, and antibody-containing regimens for acute lymphoblastic leukemia early minimal residual disease (MRD) analysis during treatment of Philadelphia chromosome/Bcr-Abl-positive acute lymphoblastic leukemia with the Abl-tyrosine kinase inhibitor imatinib (STI571). J Hematol Oncol. (2019) 12:15 10.1186/s13045-019-0703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariotti J, Devillier R, Bramanti S, Sarina B, Furst S, Granata A, et al. T cell-replete haploidentical transplantation with post-transplantation cyclophosphamide for hodgkin lymphoma relapsed after autologous transplantation: reduced incidence of relapse and of chronic graft-versus-host disease compared with HLA-Identical related donors. Biol Blood Marrow Transplant. (2018) 24:627–32. 10.1016/j.bbmt.2017.11.030 [DOI] [PubMed] [Google Scholar]
- 55.Chang YJ, Zhao XS, Wang Y, Liu YR, Xu LP, Zhang XH, et al. Effects of pre- and post-transplantation minimal residual disease on outcomes in pediatric patients with acute myeloid leukemia receiving human leukocyte antigen-matched or mismatched related donor allografts. Am J Hematol. (2017) 92:E659–61. 10.1002/ajh.24910 [DOI] [PubMed] [Google Scholar]
- 56.Dabas R, Lee R, Servito MT, Dharmani-Khan P, Modi M, van Slyke T, et al. Antithymocyte globulin at clinically relevant concentrations kills leukemic blasts. Biol Blood Marrow Transplant. (2016) 22:815–24. 10.1016/j.bbmt.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 57.DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the american society for transplantation and cellular therapy. Biol Blood Marrow Transplant. (2019) 25:2113–23. 10.1016/j.bbmt.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 58.Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. (2019) 54:1525–52. 10.1038/s41409-019-0516-2 [DOI] [PubMed] [Google Scholar]
- 59.Short NJ, Jabbour E, Albitar M, de Lima M, Gore L, Jorgensen J, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. (2019) 94:257–65. 10.1002/ajh.25338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brissot E, Labopin M, Beckers MM, Socie G, Rambaldi A, Volin L, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. (2015) 100:392–9. 10.3324/haematol.2014.116954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.