Abstract
Viruses are the most abundant biological entities in marine environments, however, despite its potential ecological implications, little is known about virus removal by ambient non-host organisms. Here, we examined the effects of a variety of non-host organisms on the removal of viruses. The marine algal virus PgV-07T (infective to Phaeocystis globosa) can be discriminated from bacteriophages using flow cytometry, facilitating its use as a representative model system. Of all the non-host organisms tested, anemones, polychaete larvae, sea squirts, crabs, cockles, oysters and sponges significantly reduced viral abundance. The latter four species reduced viral abundance the most, by 90, 43, 12 and 98% over 24 h, respectively. Breadcrumb sponges instantly removed viruses at high rates (176 mL h−1 g tissue dry wt−1) which continued over an extended period of time. The variety of non-host organisms capable of reducing viral abundance highlights that viral loss by ambient organisms is an overlooked avenue of viral ecology. Moreover, our finding that temperate sponges have the huge potential for constant and effective removal of viruses from the water column demonstrates that natural viral loss has, thus far, been underestimated.
Subject terms: Parasitology, Viral transmission, Microbial ecology, Marine biology
Introduction
Viruses are the most numerically abundant entities in the oceans with an estimated abundance up to 108 mL−1 1. Via infection and mortality of their microorganism hosts, viruses have the ability to regulate not only host population dynamics but also to drive biogeochemical cycling and carbon sequestration within marine systems2–6. As lytic virus infections of microbial hosts inevitably result in the death of the host cell, any decay of infectious virus particles is likely to have important repercussions for hosts as it results in lower encounter rates and thus reduced infection levels. Despite marine viruses influencing fundamental biological processes2,3, research investigating virus decay (e.g. loss of infectivity) and particle loss has primarily focused on abiotic factors such as UV radiation and temperature (loss of infectivity) and adhesion to clay particles and aggregates (loss of viral particles)7–11. However, biological factors resulting in removal of virus particles from the water column is still understudied (Fig. 1). Marine heterotrophic nanoflagellates have been reported to graze on viruses, albeit at a relatively low rate of 0.1% of the viral population day −1, or a clearance rate of around 4%12,13. Furthermore, the Red Sea sponge Negombata magnifica was reported to filter viruses with an average efficiency of 23%14. And recently the appendicularian stage of the pelagic Oikopleura dioica tunicate was reported to remove viruses at significant rates15.
Figure 1.
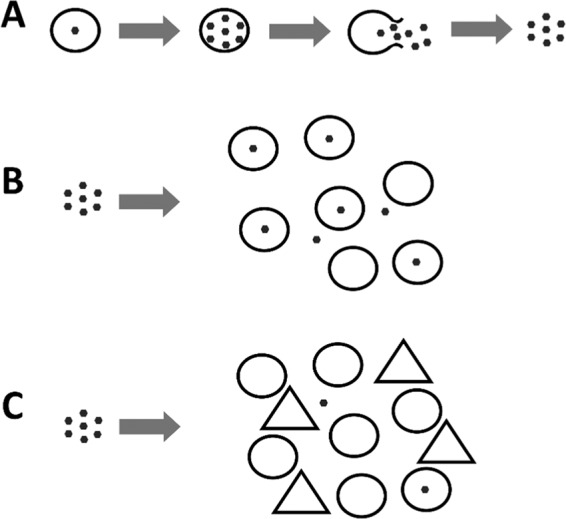
This conceptual diagram shows lytic viruses (⬣) infecting and replicating inside their susceptible host (○). After the host lyses, virus progeny are released into the surrounding environment where they have the potential to proceed and infect a new host (A). In a simple system, these newly produced viruses are available to infect the available succeeding hosts (B). In complex systems, typically found in nature, viruses may be lost due to interactions with non-host organisms (△), resulting in reduced encounter rates and disease prevalence within the ecosystem (C).
While such reductive effects of non-host organisms on marine viral abundance are still understudied, similar removal effects have been well demonstrated in other aquatic host-pathogen systems. For instance, infective stages of helminths are removed by a wide range of non-host organisms via predation and other mechanisms16–20, with reductions in free-living infective stages reducing infection levels in downstream hosts14. The variety of non-host organisms causing the reductions is not only limited to active predators but also includes passive predators such as filter feeders and organisms creating physical barriers between the parasite and its host16,21. Thus, non-host organisms are known to affect parasite transmission in macro parasite-host systems, a phenomenon known as transmission interference17,22–24. In this study, we used various ecologically relevant non-host organisms to assess their effects on transmission interference on a microparasite-host system. Specifically, we investigated the potential of a variety of pelagic and benthic marine non-host species, including bivalve filter feeders and decapod predators, to remove a marine algal virus from the surrounding seawater. We used the marine algal virus PgV, which is host specific and known to infect the bloom forming algae, Phaeocystis globosa25, and a selection of non-host organisms that are found in coastal areas where the algal host,virus, and non-host organisms coincide. In addition, the organism most proficient in reducing viral abundance was studied in more detail to determine if efficient removal of viruses could be sustained for a prolonged time. Understanding which organisms and to what extent non-host organisms regulate marine viral abundances is unreservedly important for more accurate predictions of the ecological impact viruses have on host population dynamics in the seas and oceans.
Materials and methods
Viruses and non-host organisms
The marine phytoplankton virus PgV-07T (NIOZ culture collection25) was chosen as a model system because it can be clearly discriminated (and enumerated) from co-occurring bacteriophages using flow cytometry, based on fluorescence after staining with the nucleic acid-specific dye SYBR Green I26. Phaeocystis globosa strain G (A) (culture collection of the University of Groningen, the Netherlands) is the host of the double stranded DNA virus PgV-07T (hereafter known as PgV; around 150 nm diameter and 470 nm kbp genome size25) and is an ecologically important primary producer with a wide distribution in temperate (coastal) seas during spring and summer9,27–31. Algal cultures were grown in Mix-TX medium32 at 15 °C and a light:dark cycle of 16:8 h. Cultures were transferred weekly to keep the cells growing exponentially. The virus PgV was produced by infecting exponentially growing hosts and allowing complete lysis to occur during the following week. Prior to experimental use, the lysate was cleared from most of the lysed P. globosa cell debris by centrifugation (Eppendorf 5810 R, Hamburg, Germany) at 2450 × g for 30 min at 15 °C. The supernatant containing the PgV was carefully pipetted off and stored at 15 °C until experimental use (max. 2 days). Filtration of the supernatant resulted in reduced PgV abundances in the filtrate and hence we chose to use unfiltered supernatant for our experiments. The infectivity of the PgV batches used for the experiments was determined by most probable number (MPN) endpoint dilution33. Percentage infectious viruses (obtained by dividing the MPN abundance by the total abundance from flow cytometric analysis26) was near 100%, ensuring that potential removal of PgV by non-host organisms was not a selective process for either infectious or non-infectious virus particles.
PgV was enumerated according to the protocol by Brussaard et al. (2004)26 with modification as described by Mojica et al. 201434). In short, stored samples collected during the experiment were thawed and diluted in sterile 0.2 µm filtered TE buffer (10:1 Tris-EDTA, pH 8.2; Minisart high flow Syringe Filter, Sartorius A.G., Göttingen, Germany), stained with the nucleic acid-specific green fluorescent dye SYBR Green I (Invitrogen-Molecular Probes) for 10 min in the dark at 80 °C, after which PgV was enumerated using a BD FACSCanto™ flow cytometer (BD Biosciences, USA). The trigger was set on the green fluorescence for the detection of stained PgVs. Data were processed using FCS Express 4 software (De Novo Software).
Non-host organisms tested for their ability to reduce viral abundance were chosen based on their geographic distribution coinciding with that of the algal host-virus model system and included organisms of varying feeding mechanisms (filter feeders, predators, etc.) as well as range in size (small copepods to large oysters). Bivalves such as oysters, mussels and cockles are found in intertidal-subtidal areas and are considered bioengineers, altering substrate type, filtering vast amounts of water and removing particles <250 µm in size35. Organisms such sponges, anemones and sea squirts are found on hard surfaces such as harbor walls and are typically subtidal but can also be found in some intertidal areas. Both sponges and sea squirts are capable of filtering large volumes of water (300 L h−1 and 200 mL min−1 respectively36,37) and retaining nano-sized particles14,38–40. Polychaete larvae and copepods are pelagic, feeding on prey <53 µm in size41. Copepods live in coastal and upwelling regions and switch prey between algae and ciliates under 60 µm in size with clearance rates of <86 m1 d−l)42,43. The species used, i.e. anemones (Actinia equina), barnacles (Semibalanus balanoides, attached to one valve of an empty mussel shell), cockles (Cerastoderma edule), crabs (Carcinus maenas), mussels (Mytilus edulis), oysters (Magallana gigas), sea squirts (Styela clava) and adult copepods (Acartia tonsa, >125 µm) and polychaete larvae (a mix of species, >125 µm) were all collected between spring and summer from the coastal area along the island of Texel (the Netherlands). Breadcrumb sponges (Halichondria panicea) for the first experiment (Exp. 1) were collected along the same coast, but to prevent impacting the local sponge community too much the sponges for subsequent tests were collected from the Oosterschelde (southern Netherlands) and transported to the NIOZ in cool boxes. After collection, all organisms were gently cleaned to remove any visible epibionts. Before being transferred to sterile 100 mL polystyrene pots for the experiments, the cleaned organisms were starved for 24 h in flow-through aquaria (80 × 40 × 40 cm) at 15 °C with a light:dark cycle of 8:16 h.
Experimental set-up
Experiment 1: Removal of viruses when in the presence of non-host organisms
Experiment 1 (Exp. 1) assessed a variety of organisms for their ability to interfere with a marine virus-host transmission pathway by removing infectious virus particles from the water. Sterilized 100 mL polystyrene pots with screw cap (VWR International, Leuven, Belgium) were used during the experiment as aquaria. Prior to the experiment the pots were sterilized using 6 M HCl for 1 h, followed by rinsing in deionized water and then finally washed in 90 °C deionized water to remove any traces of HCl. The experiment consisted of two types of treatment: the first with the non-host organism (typically 1 individual per pot, except for barnacles which were 10 on an empty mussel valve, and copepods and polychaete larvae which had 16 individuals per pot) in 80 mL of the PgV lysate (around 1 × 106 mL−1), and the second treatment with only the lysate to assess for adherence to the pots. In addition, an extra control with only the test organism in culture media was used to assess for the introduction of viruses by the test organism. As these controls indicated that no viral particles in the same size range as PgV were introduced, they were left out of further analysis. Each of the two regular treatments was replicated six times. All trials took place in a single climate room kept at 15 °C. During the experiment, samples (1 mL) were taken using sterile pipettes (one for each replicate) and placed into 1.5 mL Eppendorf tubes containing 20 µL 25% glutaraldehyde (0.5% final concentration, EM-grade, Sigma-Aldrich, St. Louis, USA) for 30 min at 4 °C, flash-frozen in liquid nitrogen and stored at −80 °C until further analysis (Brussaard et al. 2004). Samples were taken before the test organisms were added (pre-test or PT), 15 min after the animal were adapted to the pot (T0), after a period of 3 h (T3) and then after 24 h (T24), with the exception of crab, cockle, barnacle and mussel treatments where samples were taken before the test organisms were added (pre-test or PT) and after a period of 3 h (T3).
Experiment 2: Continuous clearance of virus by breadcrumb sponge
Breadcrumb sponges were shown to significantly decrease PgV abundance (Exp. 1) and so Experiment 2 (Exp. 2) was designed to assess the ability of the sponges to continuously remove PgV over a longer time period. The experimental set-up was similar to Exp. 1, but PgV (approx. 2 × 106 mL−1) was added (‘spiked’) at 20 min intervals for 6 h to avoid depletion of viruses by the sponges. Samples (1 mL) were taken using individual sterile pipettes prior to and immediately after adding viruses to the system to allow for the calculation of clearance rates. Treatments consisted of test organism spiked with PgV, and two controls (sponge spiked with growth medium to test for disturbance effect due to spiking, and PgV, spiked with PgV to obtain the ultimate PgV abundance without the sponge present). All treatments were replicated four times but due to the mortality of one sponge during the experiment, only three replicates were used for statistical analysis. Samples were processed and stored as in Exp. 1.
Statistics
All statistical tests were carried out using R (R Core Team 2014). For Exp. 1, the effect of the presence of non-host organisms on changes in PgV abundance over time, was statistically tested by comparing changes in PgV abundance from one sampling time to the next, between the treatment where a non-host organism was present and the control treatment. A univariate analysis of variance (ANOVA) was used when samples were only taken at two time points (i.e., when there was only a single change observed between the start and end of the trial), whereas a multivariate analysis of variance (MANOVA) was used when samples were taken at more than two occasions (e.g. at the start, after 15 min, 3 h and then again after 24 h). The univariate test statistic is the F-value, the multivariate test statistic is Pillai’s trace. Significances indicate that viral abundances over time differed among treatments. As there are only two treatments (with and without non-host organisms), the tests are in fact equivalent to the t-test and to Hotelling’s T2 test.
For Exp. 2, sponge clearance rates were calculated for 20 min intervals using the viral abundances directly before spiking and the viral abundance immediately after the aquaria were spiked with viruses. Sponge clearance rates were determined using the following formula:
whereby Cl is volume of water cleared of suspended particles per unit of time; V is the volume, K is the number of individual sponges and N0 and Nt are the virus cell abundance at time 0 and time t, respectively44. In this study one piece of sponge (i.e. one individual of 52 mm Ø in size; 1.76 ± 0.99 g dry weight; 0.42 ± 0.16 g ash free dry weight) was used per treatment. Clearance rates were calculated for the period after clearance had stabilized, i.e. from 1–5.5 h into the experiment, unless otherwise stated. Linear regressions were subsequently used to test for density dependency by comparing the viral abundances directly before spiking against clearance rates for that specific 20 min period.
Results and Discussion
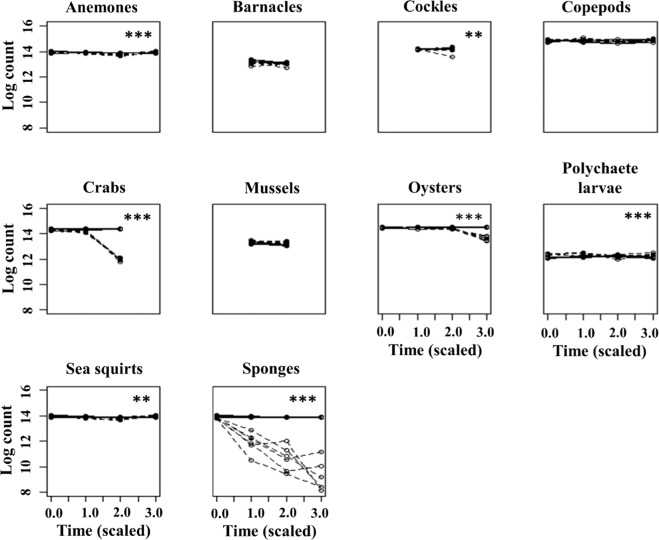
Experiment 1: Removal of viruses by non-host organisms
Ten marine non-host species were tested to assess their ability to reduce abundances of the model virus PgV. As expected, the control treatments containing only PgV showed no significant decline in PgVs over time and controls containing only the non-host organisms did not display virus enrichment. All non-host organisms, except barnacles, copepods and mussels, significantly affected PgV abundances (Table 1, Fig. 2), showing that the interference of virus transmission by non-host organism is a common process. Whilst anemones, polychaete larvae and sea squirts tested as significant, the rate of change was very small so that they did not result in an ecologically relevant reduction in viruses by the end of the experiment (Supplement Fig. S1). Conversely, another sessile marine tunicate or ‘sea squirt’ has been reported to remove up to 7 × 105 Emiliania huxleyi viruses mL−1 (by 0.4 animals mL−1), with a clearance rate of 50 mL ind−1 day−1 15 suggesting that removal of viruses by tunicates may be species-specific. The presence of oysters and crabs resulted in significant reduction of PgV abundances over the 24 h experimental period, i.e. <12% and 90% respectively within 3 h. In other studies, after exposure to water containing human enteric viruses, bivalves and crabs showed internal accumulation of the viruses, with recovery of viruses from tissues such as the digestive tract45–49. This indicates that both decapods and bivalves have the potential to take up viruses from their surrounding environment and, collectively, significantly contribute to the reduction in viral abundances. Moreover, studies have shown that after uptake by bivalve species viral particles released via fecal matter are inactive and thus suggesting that the digestion process renders the particles non-infective50,51. In other marine macroparasite-host systems crabs and oysters have been shown to effectively reduce the number of free-living trematode parasites16,18. While the infective cercarial stages of trematode parasites are considerably larger (body length of several hundred µm52) than virus particles, our study demonstrates the extent of their interactions and their potential to alter a wide range of infectious diseases within a system. Besides the existing studies focusing on the concentration of viruses and their impact on the human food chain we are, to our knowledge, the first to report the removal of non-human viruses by marine shellfish.
Table 1.
Uni- (ANOVA) and multivariate (MANOVA) analyses results testing for the effect of the presence of a non-host organism on changes in PgV abundance over time, compared to the control treatment where no non-host organisms were present.
| Test organism | Pillai trace | - F | P | Significance |
|---|---|---|---|---|
| Anemones | 0.84 | — | <0.001 | *** |
| Barnacles | — | 0.0 | 0.970 | |
| Cockles | — | 0.0 | 0.997 | |
| Copepods | 0.16 | — | 0.457 | |
| Crabs | 0.99 | — | <0.001 | *** |
| Mussels | — | 4.0 | 0.073 | (*) |
| Oysters | 0.93 | — | <0.001 | *** |
| Polychaete larvae | 0.79 | — | <0.001 | *** |
| Sea squirts | 0.71 | — | 0.004 | ** |
| Sponges | 0.87 | — | <0.001 | *** |
Significance levels indicate whether there was a significant change in viral abundance (over time) between the control and treatments.
ANOVA tests used F values and were conducted when samples were only taken at two time points. MANOVA tests, however, were used when samples were taken at more than two time points and used Pillai’s trace test.
Significance codes are as follows: *** =0.001; ** =0.01; * =0.05; and (*) =0.1.
Figure 2.
Viral abundance (log PgV mL) over time in the presence (dashed lines) and absence (control; black lines) of a non-host organisms. Asterisks identify non-host organism which had a significant effect on changes in viral abundance (‘***’ P < 0.001 ‘**’P < 0.01 ‘*’P < 0.05). Each line represents one replicate. Note that time axis is not continuous, but scaled to four observation times, start of experiment (time 0), approximately 15 min (time 1), 3 h (time 2), and 24 h (time 3) after the start. The y-axis shows the Log of PgV counts, that is the natural log base of the exponential of the virus counts. All virus counts were in the range of x106.
The strongest removal of PgV was caused by the sponges, not only by removing the most viruses but also because removal of viruses from the system began instantly (Fig. 2, Supplement Fig. 1). The Breadcrumb sponge reduced PgV abundance by 94% in the first 3 h and a minimum of 14-fold by the end of the 24 h experimental period, with an end reduction of 98%. Sponges are known to be effective at filtering out small particles such as algal and bacterial cells53–56, as well as dissolved organic matter (DOM)57. According to the standard practical classification and operational definition, particles <0.2 µm are included in the DOM pool, i.e. the DOM particle size range also includes the nanometer range of virus particles. In this study we used only algal virus PgV-07T but, given the size range of prey sponges can take up, we would expect comparable removal rates of other viruses (algal viruses and bacteriophages). Natural seawater viruses, dominated by typically smaller-sized bacteriophages (20–60 nm range)58, have been reported to be removed by a tropical sponge14. Furthermore, even the very small influenza viruses can be effectively removed by bivalves51. While not the focus of this study, the overall removal of virus particles is likely to have selected consequences for nutrient cycling via the direct loss of viruses (containing and average of 41 C and 16 N and 4.2 P atoms per virus capsid head; potentially accounting for <0.01 to 50% of the total marine DOP, <7% DON depending on the viral group and other physical parameters59) and the indirect alterations in biogeochemical cycling resulting from host cell lysis2,3. The effective removal of ecologically relevant virus concentrations by the temperate breadcrumb sponge highlights that the filtration activity and the removal of viruses from the surrounding water by non-host organisms is compelling. The virus removal rates shown here are much higher than previously reported for other sponge species. For example the tropical sponge Negombata magnifica reduced viruses with an efficiency of 23 ± 3%14. Discrepancies in sponge efficiency between their and the present study may be a result of anatomical differences between the sponges used; here we used a temperate sponge from the order Halichondrida, whereas Hadas and coworkers (2006) used a tropical sponge from the order ‘Poecilosclerida’. Furthermore, the sponge species used in our study has fluctuating removal efficiencies depending on the season. This study was conducted during the period when the sponges exhibit their highest energy demand (April and August60). Importantly, the differences between the studies illustrate that the contribution of sponges in controlling viral abundance in marine systems is thus far substantially underestimated. Such high reductions in viral abundance are likely to have ecological consequences with knock-on effects for the algal host and virus-host contact rates.
Experiment 2: Continuous clearance of viruses by breadcrumb sponge
To test the sponges’ ability to consistently remove viruses over time, we spiked replicates with PgV every 20 min for up to 6 h with approx. 2.5 × 106 PgV mL−1 to avoid complete depletion in the surrounding water (Fig. 3). Removal rates of the 3 sponges (one sponge died during the experiment) in the first 3 h and over 24 h were comparable to exp. 1 with a 91% and 94% reduction in viruses. After 1 h the removal of PgV stabilized and the sponge continued to clear PgV at a rate of around 5 mL h−1 (Fig. S2). During the stabilized period (1 to 5.5 h into the experiment) the sponges removed a total of about 9.3 × 107 PgVs (Fig. 3). Initial PgV abundances varied between the two experiments but all initial abundances were within the natural range found in marine environments61. Our results thus demonstrate a constant and very effective virus removal by the breadcrumb sponge. Clearance rates of the viruses by the breadcrumb sponges stabilized after the start of the experiment and remained constant until the end of the testing period. Sponges cleared viruses at a rate of 176 mL h−1 g tissue dry wt−1 (based on initial 15 min). Given that virus clearance rates reported thus far (e.g. Hadas et al. 200614; 648 mL h−1 g tissue wet weight converted to 38 mL h−1 g tissue dry wt−1 as outlined in Frost 197862) are lower than in our study, the ecological importance of virus loss by sponge activity is most likely extremely underestimated. Previous studies on viral and DOM loss by sponges have primarily used tropical sponge species55,63. To our knowledge, we are the first to show that a temperate sponge species is efficient at removing viruses and thus it is likely that other temperate sponge species are also able to reduce viral abundance with equal or better efficiency.
Figure 3.
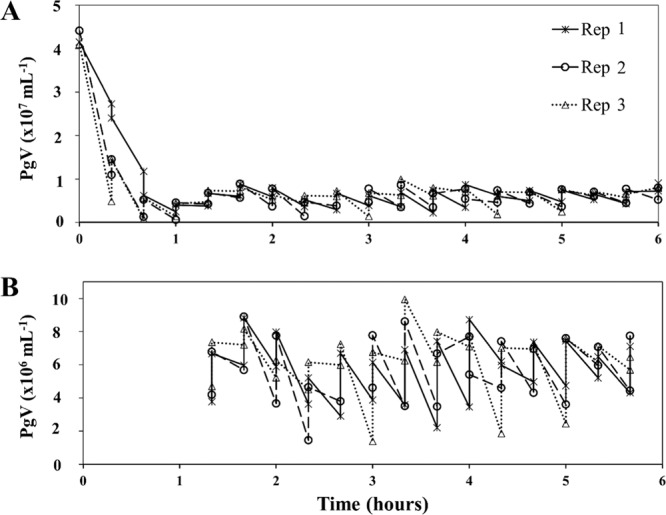
The removal of PgV by breadcrumb sponges over the entire incubation period (A) and a close up of the stabilized period from 1 to 5.5 h into the experiment when PgV was added at 20 min intervals (B). Experiment was performed in triplicate (Rep 1–3).
Theoretically there might have been the possibility that sponges filtered the suspension volume (80 mL) more than once, however given that the mean clearance rate was 176 mL h−1 g sponge dry weight and the average sponge dry weight per replicate was 1.76 ± 0.99 g, the average clearance rate was 77 mL (range between 34 and 121 mL) within the 15 min sampling period. Although a higher time resolution is recommended it was logistically constrained by the sample processing time required immediately after taking the samples. By utilizing a controlled experimental design and setup such as the one used in this study we are able to show that the loss of viruses was a direct effect of the sponge and not an artefact due to interactions caused by the presence of other organisms within the system. Furthermore, unlike Hadas et al. 200614, by using a controlled experiment and filtered water we can also conclude that there was no other potential ‘food’ in the water, potentially supporting previous suggestions that viruses act as a nutrient source and a part of the sponge pump57,64.
Reductions in natural virus abundance by such high degree as shown in our study, have the potential to impact local microbial host population dynamics as the virus-host contact rate drops due to the decline in virus abundance. For PgV specifically, viruses infecting P. globosa have been shown to contribute considerably to bloom demise and, depending on the environmental conditions, viral activity may even prevent bloom formation8,65,66. Thus any reduction in contact rate between PgV and its P. globosa host results in reduced virally induced mortality of the algal host, subsequently promoting a longer blooming period or shifting the share of loss factors from viral lysis to grazing67,68. Sponges have a crude ability to remove particles based on size69–73 and it is well documented that bacteria and phytoplankton are a major part of sponge prey70,74,75. This could influence host population dynamics directly (removal of host algae) and indirectly (removal of viruses). This complex form of transmission interference may thus reduce contact rates between viruses and their hosts even further (due to the dual reduction of host and virus). Therefore, the presence of a non-host organism may be a ‘double edged sword’ affecting the abundance of both viruses and hosts. We conducted a pilot experiment to compare simultaneous removal of PgV, P. globosa and bacteria, and, indeed, all three types of particles were efficiently cleared (within 24 h) by the breadcrumb sponge. The bacterial clearance rate of 535 mL h−1 g tissue dry wt−1 falls within the rates published for other sponges (10–5000 mL h−1 g tissue dry wt−1)55,62,63,69,76. The P. globosa clearance rate (68.2 mL h−1 g tissue dry wt−1) was in the middle range of published rates for phytoplankton76. Substantial variation in clearance rates for both phytoplankton and bacteria do occur which may be caused by factors such as initial particle concentration, time of year, temperature, species and individual sponge behavior63,76,77. Regardless of these differences, our study demonstrates that the virus clearance rates are within the range of the acknowledged natural food particles, bacterial and algal cells.
Given that sponges can be found in high densities in coastal regions, such as harbors78, as well as in tropical79 and deep sea reefs80,81, collectively they are highly likely to continuously interact with the viruses in the water column creating strong localized differences in viral abundance. For example, the overall effects of sponges on viruses here in the Netherlands is likely to have less of an impact than in other regions such as coral reefs or harbors where the majority of surfaces comprise of hard substrate. In such locations sponge coverage can be high, e.g. ranging from 45–70 m−2 (Indonesia82) to>1400 individuals m−2 (Ireland83), therefore, in locations such as relatively enclosed bays, the effect of sponges on virus removal is probably vastly underestimated. For example, in a hypothetical hard-substrate bay measuring 10 km × 10 km × 0.01 km, an average sponge density of 1,400 sponges m−2, and a sponge clearance rate of viruses of 5 mL h−1 (Exp 2 this study), an estimated 7 × 109 L h−1 is cleared of viruses by the sponges. That equates to 1.7% of the bays water volume every hour. Given stable conditions (e.g. stagnant water), all the viruses in the bay could, hypothetically, be cleared within 3 days. Although such estimates should be approached with caution, the above estimation underlines the enormous potential of sponges as virus loss factor whereby the ultimate ecological impact depends on the local conditions such as tide phase, current strength, local sponge cover, other non-host organisms potentially removing viruses from the water column (e.g. bivalves), as well as host presence and abundance.
In conclusion, our results stress the notion that a wide range of non-host organisms and in particular sponges, have the potential to reduce nano-sized pathogen abundance via transmission interference. It is very likely that there are many more species capable of removing viruses from the water column. It is also likely that there are a variety of factors which facilitate or impede the removal of viruses, such as stratification or mixing of the water column altering contact rates, ambient temperatures affecting non-host organism feeding rates, free-floating clays and aggregates to which viruses may adhere8,84–86 all of which should be tested for their effects on transmission interference. An interesting follow up experiment would be to expand on the experiments investigating the effect of sponges on the contact rates of host and virus when both are present. Considering that even temperate sponges effectively remove viruses from the water column (this study), the global ecological consequences are expected to be considerable and hence, there is a need for further investigations to determine how, when and to what extent non-host organisms affect virus-host dynamics.
Supplementary information
Acknowledgements
We wish to thank our colleagues for their valued contribution to the research: Anna Noordeloos and Kirsten Kooijman for laboratory assistance, and Douwe Maat, Kristina Mojica and Tristan Biggs for their time given to discussing the research. We also thank the reviewers for their time and constructive comments.
Author contributions
Experimental planning and design: C.B., D.T., J.V. and J.W. Preparation and running of experiments: J.W., P.S. and K.R. Flow cytometry: P.S., K.R. and J.W. Data analysis: J.V., P.S., C.B. and J.W. Writing of manuscript: J.W. Critical comments and revisions: C.B., J.V. and D.T.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author and are available via the 4TU data repository [10.4121/uuid:9dbe319e-c42e-4b47-a4ce-5d2e33243c1c].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61691-y.
References
- 1.Wigington CH, et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016;1:15024. doi: 10.1038/nmicrobiol.2015.24. [DOI] [PubMed] [Google Scholar]
- 2.Suttle CA. Marine viruses — major players in the global ecosystem. Nat. Rev. Microbiol. 2007;5:801–812. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 3.Brussaard CPD, et al. Global-scale processes with a nanoscale drive: the role of marine viruses. ISME J. 2008;2:575–578. doi: 10.1038/ismej.2008.31. [DOI] [PubMed] [Google Scholar]
- 4.Danovaro R, et al. Marine viruses and global climate change. FEMS Microbiol. Rev. 2011;35:993–1034. doi: 10.1111/j.1574-6976.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J, Wilhelm S. Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 2012;4:2–9. doi: 10.3410/B4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mojica KDA, Huisman J, Wilhelm SW, Brussaard CPD. Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean. ISME J. 2015;10:500–514. doi: 10.1038/ismej.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000;64:69–114. doi: 10.1128/MMBR.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojica KDA, Brussaard CPD. Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 2014;89:495–515. doi: 10.1111/1574-6941.12343. [DOI] [PubMed] [Google Scholar]
- 9.Brussaard CPD, Kuipers B, Veldhuis MJW. A mesocosm study of Phaeocystis globosa population dynamics: I. Regulatory role of viruses in bloom control. Harmful Algae. 2005;4:859–874. doi: 10.1016/j.hal.2004.12.015. [DOI] [Google Scholar]
- 10.Syngouna VI, Chrysikopoulos CV. Interaction between viruses and clays in static and dynamic batch systems. Environ. Sci. Technol. 2010;44:4539–44. doi: 10.1021/es100107a. [DOI] [PubMed] [Google Scholar]
- 11.Lipson Steven M., Stotzky G. Specificity of virus adsorption to clay minerals. Canadian Journal of Microbiology. 1985;31(1):50–53. doi: 10.1139/m85-011. [DOI] [PubMed] [Google Scholar]
- 12.Suttle CA, Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl. Environ. Microbiol. 1992;58:3721–3729. doi: 10.1128/AEM.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González J, Suttle CA. Grazing by marine nanoflagellates on virus-sized particles: ingestion and digestion. Mar. Ecol. Prog. Ser. 1993;94:1–10. doi: 10.3354/meps094001. [DOI] [Google Scholar]
- 14.Hadas E, Marie D, Shpigel M, Ilan M. Virus predation by sponges is a new nutrient-flow pathway in coral reef food webs. Limnol. Oceanogr. 2006;51:1548–1550. doi: 10.4319/lo.2006.51.3.1548. [DOI] [Google Scholar]
- 15.Lawrence J, et al. Viruses on the menu: The appendicularian Oikopleura dioica efficiently removes viruses from seawater. Limnol. Oceanogr. 2017;63:S244–S253. doi: 10.1002/lno.10734. [DOI] [Google Scholar]
- 16.Welsh JE, van der Meer J, Brussaard CPD, Thieltges DW. Inventory of organisms interfering with transmission of a marine trematode. J. Mar. Biol. Assoc. United Kingdom. 2014;94:697–702. doi: 10.1017/S0025315414000034. [DOI] [Google Scholar]
- 17.Thieltges DW, Bordalo MD, Caballero Hernandez A, Prinz K, Jensen KT. Ambient fauna impairs parasite transmission in a marine parasite-host system. Parasitology. 2008;135:1111–1116. doi: 10.1017/S0031182008004526. [DOI] [PubMed] [Google Scholar]
- 18.Thieltges DW, Reise K, Prinz K, Jensen KT. Invaders interfere with native parasite–host interactions. Biol. Invasions. 2009;11:1421–1429. doi: 10.1007/s10530-008-9350-y. [DOI] [Google Scholar]
- 19.Prinz K, Kelly TC, Riordan RMO, Culloty SC. Non-host organisms affect transmission processes in two common trematode parasites of rocky shores. Mar. Biol. 2009;156:2303–2311. doi: 10.1007/s00227-009-1258-2. [DOI] [Google Scholar]
- 20.Kaplan AT, Rebhal S, Lafferty KD, Kuris AM. Small estuarine fishes feed on large trematode cercariae: lab and field investigations. J. Parasitol. 2009;95:477–480. doi: 10.1645/GE-1737.1. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PTJ, Lund PJ, Hartson RB, Yoshino TP. Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc. Biol. Sci. 2009;276:1657–63. doi: 10.1098/rspb.2008.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 23.Welsh JE, Liddell C, van der Meer J, Thieltges DW. Parasites as prey: the effect of cercarial density and alternative prey on consumption of cercariae by four non-host species. Parasitology. 2017;144:1775–1782. doi: 10.1017/S0031182017001056. [DOI] [PubMed] [Google Scholar]
- 24.Goedknegt, M. A. Pacific oysters and parasites: Species invasions and their impact on parasite-host interactions. (VU University Amsterdam, The Netherlands., 2017).
- 25.Baudoux AC, Brussaard CPD. Characterization of different viruses infecting the marine harmful algal bloom species Phaeocystis globosa. Virology. 2005;341:80–90. doi: 10.1016/j.virol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Brussaard CPD. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 2004;70:1506–1513. doi: 10.1128/AEM.70.3.1506-1513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancelot C, Billen G. Activity of heterotrophic bacteria and its coupling to primary production during the spring phytoplankton bloom in the southern bight of the North Sea. Limnol. Oceanogr. 1984;29:721–730. doi: 10.4319/lo.1984.29.4.0721. [DOI] [Google Scholar]
- 28.Cadée GC, Hegeman J. Seasonal and annual variation in phaeocystis pouchetii (haptophyceae) in the westernmost inlet of the Wadden Sea during the 1973 to 1985 period. Netherlands J. Sea Res. 1986;20:29–36. doi: 10.1016/0077-7579(86)90058-X. [DOI] [Google Scholar]
- 29.Cadée GC, Hegeman J. Phytoplankton in the Marsdiep at the end of the 20th century; 30 years monitoring biomass, primary production, and Phaeocystis blooms. J. Sea Res. 2002;48:97–110. doi: 10.1016/S1385-1101(02)00161-2. [DOI] [Google Scholar]
- 30.Baudoux A, Noordeloos A, Veldhuis M, Brussaard C. Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat. Microb. Ecol. 2006;44:207–217. doi: 10.3354/ame044207. [DOI] [Google Scholar]
- 31.Ruardij P, Veldhuis M, Brussaard C. Modeling the bloom dynamics of the polymorphic phytoplankter: impact of grazers and viruses. Harmful Algae. 2005;4:941–963. doi: 10.1016/j.hal.2004.12.011. [DOI] [Google Scholar]
- 32.Maat DS, Brussaard CPD. Both phosphorus- and nitrogen limitation constrain viral proliferation in marine phytoplankton. Aquat. Microb. Ecol. 2016;77:87–97. doi: 10.3354/ame01791. [DOI] [Google Scholar]
- 33.Suttle, C. A. Handbook of methods in aquatic microbial ecology. (CRC Press, 1993).
- 34.Mojica KDA, Evans C, Brussaard CPD. Flow cytometric enumeration of marine viral populations at low abundances. Aquat. Microb. Ecol. 2014;71:203–209. doi: 10.3354/ame01672. [DOI] [Google Scholar]
- 35.Gosling, E. Bivalve Molluscs: Biology, Ecology and Culture. (John Wiley & Sons, 2003).
- 36.Petersen JK, Riisgard HU. Filtration capacity of the ascidian Ciona intestinalis and its grazing impact in a shallow fjord. Mar. Ecol. Prog. Ser. 1992;88:9–17. doi: 10.3354/meps088009. [DOI] [Google Scholar]
- 37.Cebrian E, Agell G, Martí R, Uriz MJ. Response of the Mediterranean sponge Chondrosia reniformis Nardo to copper pollution. Environ. Pollut. 2006;141:452–458. doi: 10.1016/j.envpol.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado M, et al. Selective feeding by sponges on pathogenic microbes: a reassessment of potential for abatement of microbial pollution. Mar. Ecol. Prog. Ser. 2010;403:75–89. doi: 10.3354/meps08411. [DOI] [Google Scholar]
- 39.Petersen JK. Ascidian suspension feeding. J. Exp. Mar. Bio. Ecol. 2007;342:127–137. doi: 10.1016/j.jembe.2006.10.023. [DOI] [Google Scholar]
- 40.Petersen JK, Riisgard HU. Filtration capacity of the ascidian Ciona intestinalis and its grazing impact in a shallow fjord. Marine Ecology Progress Series. 1992;88:9–17. doi: 10.3354/meps088009. [DOI] [Google Scholar]
- 41.Hansen BW, et al. Swimming behavior and prey retention of the polychaete larvae Polydora ciliate (Johnston) J. Exp. Biol. 2010;213:3237–3246. doi: 10.1242/jeb.038810. [DOI] [PubMed] [Google Scholar]
- 42.Kiørboe T, Saiz E, Viitasalo M. Prey switching behaviour in the planktonic copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 1996;143:65–75. doi: 10.3354/meps143065. [DOI] [Google Scholar]
- 43.Jonsson P, Tiselius P. Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar. Ecol. Prog. Ser. 1990;60:35–44. doi: 10.3354/meps060035. [DOI] [Google Scholar]
- 44.Riisgård H. The stony road to reliable filtration rate measurements in bivalves: a reply. Mar. Ecol. Prog. Ser. 2001;215:307–310. doi: 10.3354/meps215307. [DOI] [Google Scholar]
- 45.Hejkal TW, Gerba CP. Uptake and survial of enteric viruses in the blue crab, Callinectes sapidus. Appl. Environ. Microbiol. 1981;41:207–211. doi: 10.1128/AEM.41.1.207-211.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerba CP, Goyal SM. Detection and occurrence of enteric viruses in shellfish: a review. J. Food Prot. 1978;41:743–754. doi: 10.4315/0362-028X-41.9.743. [DOI] [PubMed] [Google Scholar]
- 47.Bookelaar BE, Reilly AJO, Lynch SA, Culloty SC. Role of the intertidal predatory shore crab Carcinus maenas in transmission dynamics of ostreid herpesvirus-1 microvariant. Dis. Aquat. Organ. 2018;130:221–233. doi: 10.3354/dao03264. [DOI] [PubMed] [Google Scholar]
- 48.DiGirolamo R, Wiczynski L, Daley M, Miranda F, Viehweger C. Uptake of bacteriophage and their subsequent survival in edible west coast crabs after processing. Appl. Microbiol. 1972;23:1073–1076. doi: 10.1128/AEM.23.6.1073-1076.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Bella G, et al. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy) Food Environ. Virol. 2017;9:179–186. doi: 10.1007/s12560-016-9273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLeod C, Hay B, Grant C, Greening G, Day D. Inactivation and elimination of human enteric viruses by Pacific oysters. J. Appl. Microbiol. 2009;107:1809–1818. doi: 10.1111/j.1365-2672.2009.04373.x. [DOI] [PubMed] [Google Scholar]
- 51.Faust C, Stallknecht D, Swayne D, Brown J. Filter-feeding bivalves can remove avian influenza viruses from water and reduce infectivity. Proc. R. Soc. B Biol. Sci. 2009;276:3727–35. doi: 10.1098/rspb.2009.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galaktionov, K. V. & Dobrovolskij, A. A. The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmission, and Evolution of Digenetic Trematodes. (Springer Science & Business Media, 2003), 10.1007/978-94-017-3247-5.
- 53.Reiswig HM. Bacteria as food for temperate-water marine sponges. Can. J. Zool. 1975;53:582–589. doi: 10.1139/z75-072. [DOI] [Google Scholar]
- 54.Sidri, M. Chondrilla nucula (Porifera, Demonspongiae): an example of successful plasticity. Ecological and morphological aspects. (Biologisches Institut der Universität Stuttgart, 2004).
- 55.Peterson BJ, Chester CM, Jochem FJ, Fourqurean JW. Potential role of sponge communities in controlling phytoplankton blooms in Florida Bay. Mar. Ecol. Prog. Ser. 2006;328:93–103. doi: 10.3354/meps328093. [DOI] [Google Scholar]
- 56.Ledda FD, Pronzato R, Manconi R. Mariculture for bacterial and organic waste removal: A field study of sponge filtering activity in experimental farming. Aquac. Res. 2014;45:1389–1401. doi: 10.1111/are.12084. [DOI] [Google Scholar]
- 57.De Goeij JM, V D Berg H, Van Oostveen MM, Epping EHG, Van Duyl FC. Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar. Ecol. Prog. Ser. 2008;357:139–151. doi: 10.3354/meps07403. [DOI] [Google Scholar]
- 58.Torrella F, Morita RY. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, Oregon: Ecological and taxonomical implications. Appl. Environ. Microbiol. 1979;37:774–778. doi: 10.1128/AEM.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jover LF, Effler TC, Buchan A, Wilhelm SW, Weitz JS. The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 2014;12:519–528. doi: 10.1038/nrmicro3289. [DOI] [PubMed] [Google Scholar]
- 60.Barthel D. On the ecophysiology of the sponge Halichondria panicea in Kiel Bight. II. Biomass, production, energy budget and integration in environmental processes.” Marine ecology progress series. Mar. Ecol. 1988;43:87–93. doi: 10.3354/meps043087. [DOI] [Google Scholar]
- 61.Baudoux, A.-C. C. The role of viruses in marine phytoplankton mortality. (University of Groningen, the Netherlands, 2007).
- 62.Frost TM. In situ measurements of clearance rates for the freshwater sponge Spongilla lucustris. Limnol. Oceanogr. 1978;23:1034–1039. doi: 10.4319/lo.1978.23.5.1034. [DOI] [Google Scholar]
- 63.Stuart V, Klumpp DW. Evidence for food-resource partitioning by kelp-bed filter feeders. Mar. Ecol. Prog. Ser. 1984;16:27–37. doi: 10.3354/meps016027. [DOI] [Google Scholar]
- 64.De Goeij JM, et al. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science (80-.). 2013;342:108–110. doi: 10.1126/science.1241981. [DOI] [PubMed] [Google Scholar]
- 65.Brussaard CPD. Viral control of phytoplankton populations - a review. J. Eukaryot. Microbiol. 2004;51:125–138. doi: 10.1111/j.1550-7408.2004.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 66.Brussaard CPD, Bratbak G, Baudoux A-C, Ruardij P. Phaeocystis and its interaction with viruses. Biogeochemistry. 2007;83:201–215. doi: 10.1007/s10533-007-9096-0. [DOI] [Google Scholar]
- 67.Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. doi: 10.2216/i0031-8884-32-2-79.1. [DOI] [Google Scholar]
- 68.Cloern J, Dufford R. Phytoplankton community ecology: principles applied in San Francisco Bay. Mar. Ecol. Prog. Ser. 2005;285:11–28. doi: 10.3354/meps285011. [DOI] [Google Scholar]
- 69.Ribes M, Coma R, Gili J. Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar. Ecol. Prog. Ser. 1999;176:179–190. doi: 10.3354/meps176179. [DOI] [Google Scholar]
- 70.Hanson CE, McLaughlin MJ, Hyndes GA, Strzelecki J. Selective uptake of prokaryotic picoplankton by a marine sponge (Callyspongia sp.) within an oligotrophic coastal system. Estuar. Coast. Shelf Sci. 2009;84:289–297. doi: 10.1016/j.ecss.2009.05.019. [DOI] [Google Scholar]
- 71.McMurray SE, Johnson ZI, Hunt DE, Pawlik JR, Finelli CM. Selective feeding by the giant barrel sponge enhances foraging efficiency. Limnol. Oceanogr. 2016;61:1271–1286. doi: 10.1002/lno.10287. [DOI] [Google Scholar]
- 72.Yahel G, Eerkes-Medrano DI, Leys SP. Size independent selective filtration of ultraplankton by hexactinellid glass sponges. Aquat. Microb. Ecol. 2006;45:181–194. doi: 10.3354/ame045181. [DOI] [Google Scholar]
- 73.Turon X, Galera J, Uriz MJ. Clearance rates and aquiferous systems in two sponges with contrasting life-history strategies. J. Exp. Zool. 1997;278:22–36. doi: 10.1002/(SICI)1097-010X(19970501)278:1<22::AID-JEZ3>3.0.CO;2-8. [DOI] [Google Scholar]
- 74.Riisgård HU, Larsen PS. Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 2010;418:255–293. doi: 10.3354/meps08755. [DOI] [Google Scholar]
- 75.Yahel G, et al. In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate operated fjord. Limnol. Oceanogr. 2007;52:428–440. doi: 10.4319/lo.2007.52.1.0428. [DOI] [Google Scholar]
- 76.Riisgård HU, Thomassen S, Jakobsen H, Weeks JM, Larsen PS. Suspention feeding in marine sponges Halichondria panicea and Halichondria urceolus: effects of temperature on filtration rate and energy cost of pumping. Mar. Ecol. Prog. Ser. 1993;96:177–188. doi: 10.3354/meps096177. [DOI] [Google Scholar]
- 77.Larsen PS, Riisgåd HU. The sponge pump. J. Theor. Biol. 1994;168:53–63. doi: 10.1006/jtbi.1994.1087. [DOI] [Google Scholar]
- 78.Connell SD, Glasby TM. Do urban structures influence local abundance and diversity of subtidal epibiota? A case study from Sydney Harbour, Australia. Mar. Environ. Res. 1999;47:373–387. doi: 10.1016/S0141-1136(98)00126-3. [DOI] [Google Scholar]
- 79.Diaz C, Rützler K. Sponges: An essential component of Caribbean coral reefs. Bull. Mar. Sci. 2001;69:535–546. [Google Scholar]
- 80.Hogg, M. M. et al. Deep-sea Sponge Grounds: Reservoirs of Biodiversity. UNEP-WCMC Biodiversity Series No. 32. UNEP-WCMC, Cambridge, UK. (2010).
- 81.Beazley LI, Kenchington EL, Murillo FJ, del Mar Sacau M. Deep-sea sponge grounds enhance diversity and abundance of epibenthic megafauna in the Northwest Atlantic. ICES J. Mar. Sci. 2013;70:1471–1490. doi: 10.1093/icesjms/fst124. [DOI] [Google Scholar]
- 82.Bell JJ, Smith DP. Ecology of sponges (Porifera) in the Wakatobi region, south-eastern Sulawesi, Indonesia: richness and abundance. J. Mar. Biol. Assoc. United Kingdom. 2004;84:581–591. doi: 10.1017/S0025315404009580h. [DOI] [Google Scholar]
- 83.Bell JJ. The sponge community in a semi-submerged temperate sea cave: Density, diversity and richness. Mar. Ecol. 2002;23:297–311. doi: 10.1046/j.1439-0485.2002.02784.x. [DOI] [Google Scholar]
- 84.Brussaard CPD, Mari X, Van Bleijswijk JDL, Veldhuis MJW. A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics: II. Significance for the microbial community. Harmful Algae. 2005;4:875–893. doi: 10.1016/j.hal.2004.12.012. [DOI] [Google Scholar]
- 85.Gerba CP. Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 1984;30:133–168. doi: 10.1016/S0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 86.Lipson SM, Stotzky G. Effect of proteins on reovirus adsorption to clay minerals. Appl. Environ. Microbiol. 1984;48:525–530. doi: 10.1128/AEM.48.3.525-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author and are available via the 4TU data repository [10.4121/uuid:9dbe319e-c42e-4b47-a4ce-5d2e33243c1c].