Abstract
Cognitive aging creates major individual and societal burden, motivating search for treatment and preventive care strategies. Behavioural interventions can improve cognitive performance in older age, but effects are small. Basic research has implicated dopaminergic signalling in plasticity. We investigated whether supplementation with the dopamine-precursor L-dopa improves effects of cognitive training on performance. Sixty-three participants for this randomised, parallel-group, double-blind, placebo-controlled trial were recruited via newspaper advertisements. Inclusion criteria were: age of 65–75 years, Mini-Mental State Examination score >25, absence of serious medical conditions. Eligible subjects were randomly allocated to either receive 100/25 mg L-dopa/benserazide (n = 32) or placebo (n = 31) prior to each of twenty cognitive training sessions administered during a four-week period. Participants and staff were blinded to group assignment. Primary outcomes were latent variables of spatial and verbal fluid intelligence. Compared to the placebo group, subjects receiving L-dopa improved less in spatial intelligence (−0.267 SDs; 95%CI [−0.498, −0.036]; p = 0.024). Change in verbal intelligence did not significantly differ between the groups (−0.081 SDs, 95%CI [−0.242, 0.080]; p = 0.323). Subjects receiving L-dopa also progressed slower through the training and the groups displayed differential volumetric changes in the midbrain. No statistically significant differences were found for the secondary cognitive outcomes. Adverse events occurred for 10 (31%) and 7 (23%) participants in the active and control groups, correspondingly. The results speak against early pharmacological interventions in older healthy adults to improve broader cognitive functions by targeting the dopaminergic system and provide no support for learning-enhancing properties of L-dopa supplements in the healthy elderly. The findings warrant closer investigation about the cognitive effects of early dopamine-replacement therapy in neurological disorders. This trial was preregistered at the European Clinical Trial Registry, EudraCT#2016-000891-54 (2016-10-05).
Subject terms: Working memory, Randomized controlled trials, Ageing, Magnetic resonance imaging, Chromatography
Introduction
Age-related cognitive decline and dementia are serious public health problems with devastating impact on the quality of life of individuals, their caregivers, but also on healthcare in general. The current total worldwide cost of dementia is about a trillion US dollars a year and is expected to double by 20301. Whilst pharmacological treatment approaches have been unsuccessful, recent studies have demonstrated that combining cognitive training with exercise and a healthy diet can affect cognitive functioning in at-risk older people2. Both exercise and dietary nutrients have been put forward as enhancers of neurobiological plasticity3,4, and may therefore increase effectiveness of cognitive training. One of the mechanisms through which exercise and diet are thought to modulate brain plasticity is through their action on dopaminergic neurotransmission5,6. Indeed, several independent lines of basic research have demonstrated the involvement of dopamine signalling in learning7–11 and neurobiological plasticity12,13. There is also a substantial body of evidence indicating a role for the dopamine system in modulating training-related gains in human cognitive ability10,14,15. The dopaminergic system is also negatively affected in aging16,17, potentially explaining the commonly observed reduction in learning and training gains for older adults18. Several lines of evidence thus point to a value of improving dopaminergic signalling in older adults undertaking cognitive training.
Temporary augmentation of dopamine release can be accomplished in clinical19 and healthy populations8,20 by administering the catecholamine precursor L-dopa, with some studies reporting beneficial effects of L-dopa supplementation on cognitive performance and learning in patients’ with Parkinson’s disease, but also in healthy adults7,8,21–23. We therefore hypothesised that administering L-dopa during cognitive training would increase the effects of cognitive training on general cognitive performance in healthy older adults. We designed the present randomised placebo-controlled clinical trial centred on transfer effects of working memory training to fluid intelligence24,25, which was selected as a primary outcome that may pick up task-independent training effects on working memory ability due to the important role that this ability plays in solving fluid intelligence tasks26–28. The strong involvement of the dopaminergic system in working memory functioning14,15,29 further motivated this focus.
Results
Between January 1st 2017 and October 10th 2017, we screened 235 subjects, 64 of whom entered the study. Out of 64 recruited participants, one dropped already before randomization and pretest assessment (because he/she found the tasks too difficult), and one dropped out during the cognitive training intervention (private commitments). Thus, 62 completed the study. A total sample of n = 63 was used in the analysis of primary outcomes. MRI scans were collected for 57 of them (Supplement S1). The scans were not obtained for five subjects because of psychological or physical discomfort that interfered with scanning sessions (neck problems, large head, claustrophobic reaction). Table 1 summarises the background characteristics as a function of group. As expected, demographic data and baseline cognitive scores were similar in the two groups. Both groups completed equivalent number of training sessions (Placebo: 18.43 ± 1.25, range = 15–20, L-dopa: 18.19 ± 1.69, range = 13–20; two-sample t-test, t(57) = 0.65, p = 0.52).
Table 1.
Baseline characteristics of the experimental groups.
| Placebo | L-dopa | |||
|---|---|---|---|---|
| (n = 31) | (n = 32) | |||
| Mean | SD | Mean | SD | |
| Age, years | 69.65 | 3.27 | 69.47 | 2.03 |
| Sex, f/m | 17/14 | 19/13 | ||
| MMSE score; median (range) | 30 (28–30) | 30 (26–30) | ||
| Education*; median (range) | 3 (0–10) | 3.5 (0–8.5) | ||
| BMI | 25.22 | 3.06 | 25.43 | 3.99 |
| SBP, mmHg | 139.87 | 11.65 | 137.13 | 12.05 |
| DBP, mmHg | 79.27 | 7.29 | 77.65 | 7.17 |
| Raven’s Progressive Matrices score | 6.84 | 2.96 | 6.87 | 2.50 |
| Session, morning/afternoon | 16/15 | 18/14 | ||
| Drop-out, n subjects | 1 | 0 | ||
| MRI data collected, n (%) | 28 (90.32%) | 29 (90.63%) | ||
| 6-month follow-up available, n (%) | 24 (77.42%) | 27 (84.37%) | ||
*Years after high-school;
f/m – female/male ratio; BMI – body-mass index; SBP/DBP – systolic/diastolic blood pressure;
MMSE – Mini Mental State Examination.
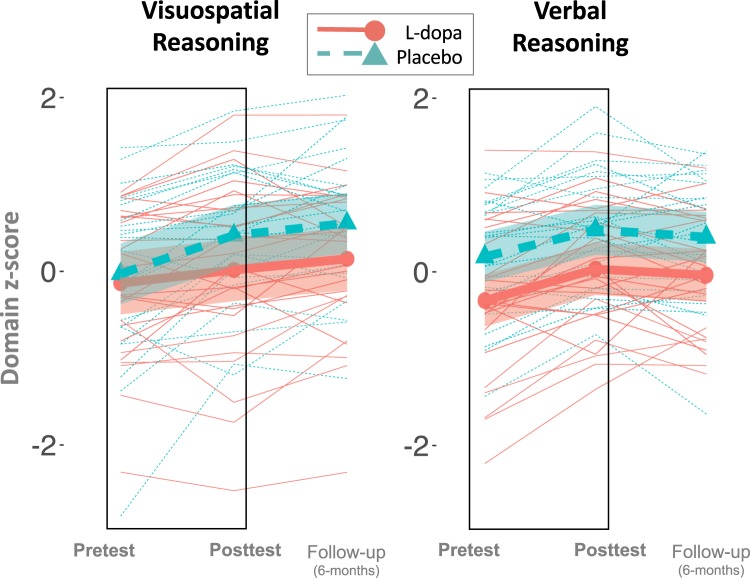
Analyses of primary outcomes using structural equation modelling revealed that change of spatial fluid intelligence differed significantly between the groups, with the L-dopa group improving less compared to the placebo group between pretest and posttest (Group × Time: standardised effect size −0.267 SDs, 95% CI [−0.498, −0.036]; p = 0.024; and Fig. 1). Change of verbal fluid intelligence scores did not significantly differ between groups (Group × Time: standardised effect size, −0.081 SDs, 95% CI [−0.242, 0.080]; p = 0.323). Of note, traditional linear mixed analyses on unit-weighted composites of the primary outcomes showed essentially the same results as those we report here: spatial fluid intelligence, t(60) = 2.16, p = 0.03, and verbal fluid intelligence, t(60) = 0.11, p = 0.91.
Figure 1.
Performance on the primary outcomes as a function of time (pretest, posttest, and 6-month follow up) and experimental group (L-dopa, red; Placebo, green). Performance is a standardized (z-score, mean of 0 and SD of 1) composite of three measures of the respective ability (spatial and verbal reasoning) administred at pretest, posttest, and follow up (off L-dopa). Thin lines represent individual subjects, thick lines represent means, and shading represent 95% CI around the mean. The boxes represent the preregistered timeline for the main analysis that compare differences in changes from pretest to posttest between the experimental groups. Compared to the placebo group, subjects receiving L-dopa before the cognitive training sessions during a four-week working memory training program improved less in spatial reasoning domain.
Six-month follow-up data collected for a subset of 51 subjects revealed that the observed between-group differences in the spatial fluid intelligence improvements were still present 6 months after the intervention (standardised effect estimate: −0.371, 95% CI [−0.62, −0.122], p = 0.004). No statistically significant difference was found for verbal fluid intelligence.
No statistically significant between-group difference was found for change in any of the secondary cognitive outcomes (See Supplement S2), but the effects sizes were all in the direction of smaller improvement for the group receiving L-dopa. Individual test scores (means and standard deviations) are available in the Supplement S3.
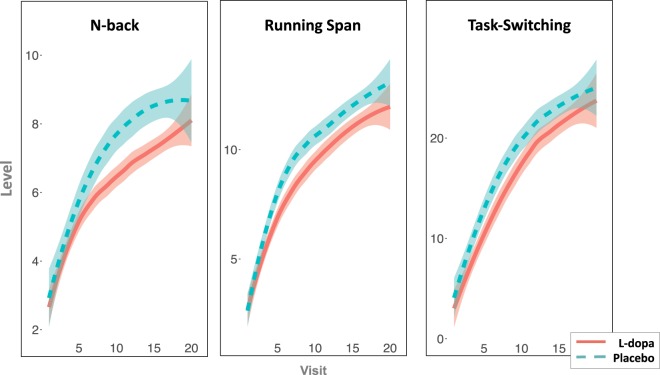
Between-group differences in training progress over the course of the intervention supported the main findings with the control group reaching higher difficulty levels across all three trained tasks (t(60) = 1.99, p = 0.05, Fig. 2).
Figure 2.
Mean difficulty levels of the training tasks as a function training session (visit 1–20) and experimental group (L-dopa, red; Placebo, green). Compared to the placebo group, subjects receiving L-dopa before each of the cognitive training sessions during the four-week working memory training program reached a lower difficulty level in all tasks, suggesting slower learning during L-dopa supplementation. The lines are fitted with locally weighted scatterplot smoothing and shaded areas represent 95% CI. The wider CIs towards the end of the training period are caused by fewer subjects in these session (i.e., not all subjects completed all 20 sessions; the mean was 18).
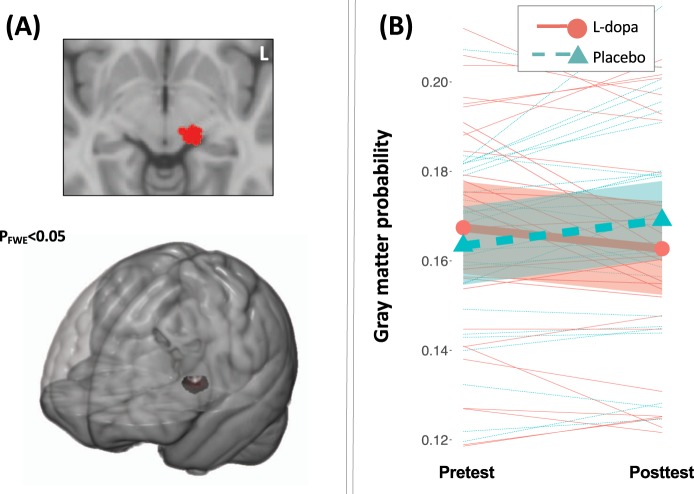
Estimation of Group × Time effects on brain morphometry yielded a single significant cluster located in the midbrain (PFWE < 0.05; MNI coordinates of the peak: -12 -24 -6 mm; see Fig. 3; for uncorrected map see Supplement S4). Matching it with the normalised high-resolution delineations of the midbrain revealed an overlap with the substantia nigra (Supplement S5). When analysed separately, the structural changes in this region were significant in both groups. Specifically, control subjects showed increases in grey matter probability t(26) = 3.02, p = 0.006, whereas the L-dopa group exhibited reductions t(27) = −2.26, p = 0.032.
Figure 3.
Changes in brain structure (grey matter probability) measured with T1-weighted MR images analysed with voxel-based morphometry. (A) The cluster of voxels in a midbrain region displaying statistically significant between-group differences in change of grey matter probability (Group × Time F-contrast; PFWE < 0.05). (B) Extracted grey matter probability from the cluster of voxels in the midbrain displaying statistically significant between-group differences in change of grey matter probability, displayed as a function of time (pretest, posttest) and group (L-dopa, red; Placebo, green). Thin lines represent individual subjects, thick lines represent means, and shading represent 95% CI around the mean.
Concentrations of L-dopa and HVA in plasma were within the expected ranges (L-dopa: 2–1000 ng/ml; HVA: 1–500 ng/ml). As expected, Log10-transformed concentrations of L-dopa and homovanillic acid were higher in the L-dopa than in the placebo group: t(60) = 15.01, p < 0.001 and t(60) = 9.96, p < 0.001, correspondingly (See Supplement S6 for more details). In addition, a significant 1.04 SDs increase in L-dopa concentrations was observed at the last training/intake day in the active group compared to the first administration, t(59) = 15.11, p < 0.001).
In the active group, a negative relationship was found between plasma levels of the drug and improvements in visuospatial reasoning (i.e., those who had larger effective concentrations of L-dopa tended to improve less in the primary outcome of spatial reasoning; t(30) = 2.06, p = 0.048, Fig. 4). It is also worth noting that the moderating effect of the body-mass index (BMI) on improvements in visuospatial reasoning was non-significant in the active group (Time × BMI interaction: t(29) = 0.25, p = 0.8) presenting no evidence for overdosing. It is also worth mentioning that BMI range in our sample was 19.8–37.9, without any extremely under- (BMI < 16) or overweight (BMI > 40) subjects.
Figure 4.
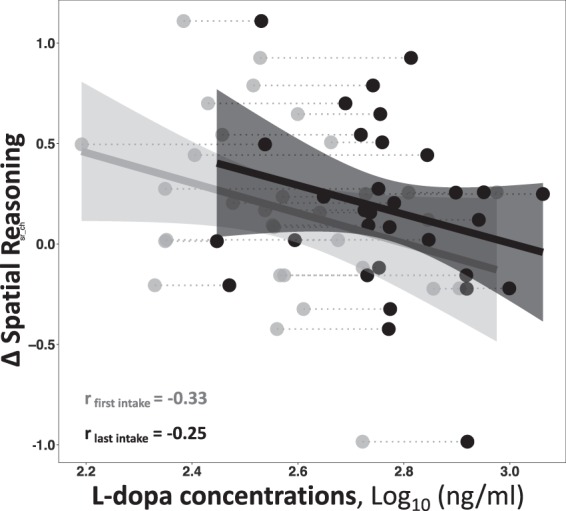
Scatterplot of the correlation between plasma levels of L-dopa and changes (posttest – pretest) in visuospatial reasoning (a z-scored composite of three measures) in the group receiving L-dopa before the cognitive training sessions. The individual points represent subjects, with the grey points being the l-dopa concentration at the first training visit and the black point representing concentrations at the subjects’ last training visit. The plot shows that subjects who had larger plasma levels of the drug tended to improve less in spatial reasoning performance compared to those who had lower effective concentrations of L-dopa.
Thirty-one adverse events (AEs, 11 in the placebo and 21 in the L-dopa group) occurred in 17 subjects over the course of training. Typical AEs were common cold and related conditions (11), mild pain (7), mild vertigo/nausea (4). Total number of subjects with AEs did not significantly differ between the groups (Placebo: 7 subjects, L-dopa: 10 subjects, χ2 = 2.27, df = 4, p = 0.68); same was true for the drug-related AEs (Placebo: 1 subject, L-dopa: 2 subjects, χ2 = 0.98, df = 2, p = 0.61). Subjects’ quality of sleep was evaluated over the course of the study with the Karolinska Sleep Questionnaire and did not yield significant between-group differences for any of the subscales. Similarly, no significant between-group difference was found for mood and motivation over the course of training. Post-hoc evaluation of masking success revealed chance-level proportion of correct guesses in L-dopa (55%) and placebo (40%) groups.
Discussion
We did not find evidence for beneficial effects of L-dopa supplementation on cognitive performance and learning in healthy older adults. On the contrary, subjects receiving L-dopa improved less on visuospatial fluid intelligence, a primary outcome of the training intervention, after four weeks of working memory training compared to those who received placebo treatment. Subjects receiving L-dopa also progressed worse during training when compared to placebo subjects. The observed between-group differences in visuospatial fluid intelligence were still statistically significant 6 months after the intervention. The groups also demonstrated opposite direction of structural changes in a midbrain region, overlapping with the template location of substantia nigra, which is a key regulatory region rich in dopamine neurons involved in learning and plasticity30,31. Specifically, the control group exhibited increases of grey matter volume in this region whereas the group that received L-dopa showed reductions.
Our study rationale was based on animal studies demonstrating effects of L-dopa on electrophysiological markers of synaptic plasticity12, human studies showing effects of the drug on (nigrostriatal) dopamine release8,20, and especially on a few previous studies showing positive effects of L-dopa on learning in younger adults7,21. Our negative results are strikingly different from these previous studies. Although post-hoc and speculative, we interpret our results to suggest that the exogenous administration of the dopamine precursor may have perturbed a balanced dopaminergic system that as previous studies show, is to be involved in training-related effects on performance17. This was also supported by negative correlation between training-related improvements in performance and plasma concentrations of the drug observed in the active group. The differential volumetric changes further support this interpretation. Thus, whilst our results are in line with basic science that clearly implicates dopamine in learning and plasticity10,12,13, they indicate that exogenous administration the dopamine precursor is rather negative than beneficial for the healthy aging brain. Here we however hasten to clarify that exact interpretations of the study remain speculative and limited because we have no direct measure of dopaminergic neurotransmission. That is, we can link our results to L-dopa supplementation, but the biological pathways of the observed effects remain unknown, and it is possible that the findings are mediated by effects on other neurotransmitters or on glia cells.
In this context, it is also worth mentioning that the vast majority, if not all, of the studies showing plasticity- and learning-enhancing properties of L-dopa and its impact on dopamine levels consistently report larger effects in cases when dopaminergic structures are either lesioned or degenerated8,22,32. This may also point to the possibility that the negative effects observed in our study of healthy adults could be mediated by biological effects independent of dopaminergic neurotransmission. On the other hand, with this background, it is difficult to argue that the fact that we examined older adults explains why our results are in conflict with the previous ones showing positive effects of L-dopa on younger adults’ learning7,21. That is, if anything, one would expect even more positive effects of L-dopa supplementation on learning in older populations compared to the ones reported in healthy younger individuals, since age-related degeneration of dopaminergic circuits is a very well established observation16,17,33. An explanation for the mixed results could instead be that L-dopa may exhibit differential effects on learning in different cognitive domains. In the previous two longitudinal studies of repeated L-dopa administration the positive effects were demonstrated on world learning, which rather pertains to episodic memory domain, whereas the main domains studied in the present study was working memory and executive functions. This interpretation may also be in line with the findings from a recent study showing detrimental effects of L-dopa on reward reversal learning in younger and older healthy adults, which were of the same magnitude irrespective of initial cognitive performance and expected baseline dopamine levels34.
Further studies are needed to clarify under what circumstances and for which cognitive domains pro-dopaminergic pharmacological interventions may have differential effects, especially in the light of recent findings showing clear regional heterogeneity of age-related decline in dopamine receptor availability35. The exact biological mechanisms behind these effects also need to be traced down. We here speculate that the negative response to pro-dopaminergic medication observed in the present and some past studies already suggests that cognitive decline in healthy aging is a complex and multifactorial process, a process that appears to result in balanced physiological states, which, whilst being associated with reduced overall self-regulatory capacity36, still preserve mechanisms of homeostatic plasticity37–39, which implies that activity within the dopaminergic circuit is regulated by intrinsic feedback loops to maintain optimally balanced levels of the neurotransmitter thereby preventing excessive increases in excitatory activity. The negative effect observed in the present study appears to be the opposite of what is known as “dopamine supersensitivity” typically present in dopamine-deficient conditions40–42. Translating this finding further to clinical and cognitive neuroscience, it is worth noting that although the dopamine system is affected in several ways in aging17,23,35,43, acute pro-dopaminergic supplementation appears to perturb a balanced system, with negative behavioural consequences. This is fully in line with the results from positron emission tomography studies showing that despite apparent negative effects of age on dopamine transporters and receptor density in the healthy individuals, its synthesis capacity remains relatively unaffected43. It is also consistent with recent evidence indicating that the balance between dopamine release and receptor density is critical for cognitive performance44. In age-related neurodegenerative diseases, on the other hand, disruption of the homeostasis has reliably been observed and may even be the core pathophysiological abnormality triggering development and deterioration of cognitive functions45. Thus, it appears clear that aging should not be approached as a disease per se, but rather as a physiological process associated with a gradual decline in many interconnected biological and behavioural capacities. Maintenance of the aging organism may therefore be best achieved by early investments in healthy lifestyle and multi-modal interventions affecting it1,2.
Our results warrant more studies about the effects of L-dopa on brain and cognition in clinical populations, which often involve substantially higher dosages and longer time periods. Indeed, even though dopamine replacement therapy has been shown to be successful for counteracting motor impairment associated with Parkinson’s disease, studies that explore its effects on cognition have yielded mixed results22,46. The negative effects of L-dopa supplementation on learning and midbrain structures observed in our study present an urgent need to carefully investigate the longitudinal course of brain changes in de-novo Parkinson’s patients, for which early dopamine replacement therapy is currently a subject of debates47,48.
Some important study limitations need to be addressed. The most important one is the absence of additional control groups not receiving any cognitive training interventions. This limits interpretation of the results, as with only two groups we cannot unambiguously infer that the between-group differences are driven by the interaction of L-dopa with cognitive training and not by direct and prolonged effects of the drug on cognitive abilities or an interaction with re-test effects (which would still entail effects on learning but of a different kind). However, elimination of L-dopa is fast (blood concentrations were expected to be negligible when the subjects were leaving the training facility), and all subjects had minimum 24-hour washout period and were medication-free on pre- and post-testing days. This, however, does not completely rule out a possibility of a cumulative effect on the brain concentrations, which, in turn, may drive the observed detrimental effects on performance. In this context, it is also worth mentioning that L-dopa concentrations were significantly higher in the active group at the last compared to the first intake. We interpret this finding as due to accelerated gut absorption of the drug previously reported in animal studies with repeated L-dopa administration49. Nevertheless, similar to the main results, the placebo group reached higher difficulty levels over the course of training in all tasks. We think that these learning-related effects are the most likely explanation of the performance differences at post test, which may also explain why they were still present at long-term follow-up conducted 6 months after the intervention (at a point when differential L-dopa concentration between the groups are unlikely). Some caution in interpreting the results is also warranted because not all of the cognitive measures showed statistically significant effects. It is however worth noting that the observed direction of effect (i.e. placebo group improving more compared to L-dopa group) was generally consistent across all tasks. There may also be several reasons for why statistical significance is reached only for the primary outcome “spatial reasoning”. For example, the reasoning tasks have higher reliability as compared to the other tests, which, in turn, increases precision when estimating difference in change (i.e. larger signal-to-noise ratio of the measured domain). Another explanation may be that the primary outcomes were always the first to be collected during extensive evaluation weeks of psychometric testing. One such week of testing can be considered as a cognitive training activity in itself and measures collected later in the weeks may therefore show less room for improvements. We of course also selected spatial reasoning as a primary outcome because it is centrally important in the context of this study. The matrix measures of spatial intelligence have a very substantial working memory component26–28 and may therefore pick up task-independent training effects on working memory. In turn, a large literature of both human and animal studies links dopamine to several aspects of working memory.
It is also important to acknowledge limitations of the MRI-derived measures of grey matter probability. The differential changes in the midbrain were observed in measures that are derived from T1-weighted images, which are known to be highly sensitive to pharmacological manipulations50 and levels of functional activity51. Thus, we cannot determine whether the observed changes are due to true volumetric changes or whether they are a result of relatively transient changes in for example blood flow52. At the same time, it must be noted that the increases observed for the control group in the present study are consistent with previous reports of changes in the dopaminergic system induced by cognitive training10. In line with this background, we also hypothesise that the observed effects in the L-dopa group may reflect reactive changes in the dopamine system in response to repeated administration of an exogenous precursor of the neurotransmitter. It is, however, possible that these effects on brain structure index other processes than changes in dopaminergic neurons, such as for example effects of the drug on the glia cells in the midbrain. These changes may also be independent of the effect observed on the cognitive measures. Indeed, correlations between these changes were of small magnitudes and not statistically significant in the group receiving L-dopa.
In addition, even though our literature review indicated that the selected dosage was appropriate to induce the effects of interest with minimal side-effects, we cannot completely rule out the possibility that other drug amounts may lead to different results. However, a negative and linear correlation between plasma concentrations of the drug and improvements in visuospatial reasoning observed in the active group, as well as absence of any moderating effects of the body-mass index on the aforementioned improvements make this possibility unlikely. Finally, despite the fact that our study is well-powered and the largest of its kind7,21 an additional caution must be advised for making direct generalisations to broader populations, especially to patient groups discussed above.
We conclude that daily L-dopa supplementation does not enhance cognitive performance and learning during cognitive training in healthy older adults and may in fact have disadvantageous effects. Our findings raise serious concerns about usefulness of novel L-dopa-containing supplements that claim to have neuroprotective and learning-enhancing properties and suggest that caution is needed with regard to early dopamine replacement treatment interventions in neurological disorders, encouraging more rigorous evaluation of their effects on the brain and cognition in populations that often receive the drug in larger doses over long periods.
Methods
Study design and participants
For the present randomised, parallel-group, double-blind, placebo-controlled trial conducted at the Karolinska University Hospital in Huddinge, Stockholm, Sweden, healthy older individuals aged 65–75 years were recruited via daily newspaper advertisement. Eligibility criteria were initially assessed via a telephone screening and later during introduction meetings. Mini-Mental State Examination was used to screen out those with suspected dementia cases. A cut-off score of >25 was used as recommended by Kukull et al.53. Other inclusion criteria were absence of any serious medical or psychiatric conditions, no history of brain injuries or serious head traumas, no metal implants hindering Magnetic Resonance Imaging (MRI), no previous participation in studies employing cognitive training, right-handedness, and absence of colour-blindness. All subjects were also carefully screened for the use of medications that may interact with L-dopa, presence of which was an exclusion criterion (see 9.6 “Non-permitted medications” in the EduraCT protocol).
All experimental protocols (see “Main documents” at https://osf.io/nwwx8/) were approved by the regional ethics review board in Stockholm (Etikprövningsmyndigheten, 2016-1897-31/1, approval date: 2016–11–17) and the Swedish medical product agency (Läkemedelsverket, 20016-000891-54, approval date: 2016–11–28). The study was conducted in accordance with the revised declaration of Helsinki (2013), the International Good Clinical Practice guidelines. Participants provided written informed consent before enrolment.
Randomisation and blinding
After a medical screening and baseline cognitive assessments (pretest) conducted by experienced physicians, research assistants, and study nurses, eligible participants were randomised (1:1) to either cognitive training and L-dopa administration or cognitive training and placebo. Age, sex, and the score on Raven’s Progressive Matrices were used as stratifiers. The randomisation was conducted using label shuffling with post-hoc non-parametric tests for the stratifiers and was run separately for each of the five waves using an R-script available at https://github.com/alex-lebedev (“RBTII” repository). All participants and all staff involved in administering the drug, cognitive training, and outcome assessments were masked to group assignment. To achieve masked drug administration, orange juice was mixed with L-dopa or administered as placebo. This was prepared and labelled by a nurse who was not involved in any other aspect of the study.
Intervention procedures
Between pretest and posttest assessments, the study implemented an intervention period of four weeks, with five visits (visit 1-visit 20) each week (~2.5 hours per visit, Monday-Friday), resulting in a maximum total of 20 intervention visits (see Supplement S7 for a figure of the timeline of assessments).
All procedures were identical for the intervention group and the control group. Upon arrival, participants were given orange juice (with or without 100/25 mg of L-dopa/benserazide, trade name: Madopark Quick mite. Roche, Basel, Switzerland) by a nurse who was masked to the group specification. After 45 minutes, during which time questionnaires evaluating mood, motivation, alertness and sleep were completed, participants commenced the cognitive training, which lasted for approximately 60 minutes. All participants then remained at the clinic for an additional 45 minutes for observation. The time window between the administration of the orange juice and the start of the cognitive training (45 min) was aligned with expected peaks in drug concentrations and effects. Similarly, total visit duration (2.5 hours) was motivated by drug elimination curves, according to which plasma concentrations of the drug are negligible 2.5 hours after the administration of L-dopa in combination with peripheral decarboxylase inhibitor54. Dose selection was motivated by the results from two longitudinal studies completed in the healthy younger population that demonstrated positive effects of the drug on learning and good tolerability7,21. The cognitive training was designed according to the current recommendations in that it was adaptive in nature, targeted more than one construct (updating and switching), and promoted process-based as opposed to strategy-based improvements by including several training tasks and varying stimuli sets55. The protocol incorporated three exercises: one focused on the ability to flexibly switch between different tasks (task-switching) and two others on the ability to continuously maintain and update mental representations (running span and n-back).
Outcomes assessed at pretest and posttest
Study outcomes included cognitive measures and Magnetic Resonance Imaging (MRI) data collected in five behavioural and one MRI session in the week before (pretest) and the week after the intervention period (posttest).
The primary cognitive outcomes were: (1) spatial fluid intelligence, as a latent variable (see Statistical Analysis) measured by the Raven’s Progressive Matrices, the Wechsler Abbreviated Scale of Intelligence, and the BETA-III matrix reasoning test; and (2) verbal fluid intelligence, as a latent variable measured with the Analogies Task from Berlin Intelligence Structure Test, Syllogisms, and the Verbal Inference Test from the ETS Kit (See Supplement S8 for complete list). The testing battery is described in details in one of our previous publications55. Selection of the primary outcomes was motivated by previous literature suggesting a possibility that working memory training can produce improvements in measures of fluid intelligence24,56. Secondary cognitive outcomes were measures of working memory, episodic memory, and task switching ability.
Structural brain imaging data were collected as a further secondary outcome. MRI scanning session (MRI Center, Huddinge Hospital) on a 3 Tesla scanner Siemens MAGNETOM Prisma equipped with a 24-channel research head coil was performed at the end of pretest and posttest weeks. The procedure employed a standardised GRAPPA MPRAGE acquisition protocol according to Alzheimer’s Disease Neuroimaging Initiative standards (ADNI-3). TR/TE = 2300/2.95 ms, Base resolution = 256, FoV read = 270 mm, Voxel size = 1.1 × 1.1 × 1.3 mm.
Six months later, participants were invited back to complete the cognitive assessment once more. Subjects were not unblinded until after the last follow-up visits.
Adverse events were assessed according to the most recent guidelines for Good Clinical Practice (European Medicines Agency, December 1st 2016) and entailed comprehensive evaluation of their severity and possible connection with the drug. For detailed description, see study protocol at https://osf.io/89bcw/.
In order to quantify effective concentrations of the investigated drug, subjects’ blood samples were collected in the ethylenediaminetetraacetic acid-treated tubes at the first and last cognitive training visit, approximately 30–40 minutes after completing the training, to evaluate plasma levels of L-dopa and homovanillic acid. Plasma separation was performed within 4–5 hours via a 30-minute centrifugation at 3000 × g. Plasma samples were stored in 1 ml aliquots at −80 °C. The chemical analysis of L-dopa and homovanillic acid was performed with high-performance liquid chromatography analysis (Nexera-i HPLC system, Hertogenbosch, Netherlands; Antec electrochemical detection system, Leiden, Netherlands) and was blinded to the study groups. See Supplement S6 for more detailed description.
Statistical analysis
Approximate power analysis was performed prior to the study launch with G*power 3, estimating the required sample size for detecting a group by time interaction (mixed ANOVA, F-test) on the primary outcomes, assuming a net standardised effect size (improvement for active group – improvement for control group) of 0.3 standard deviations, a test-retest stability coefficient of 0.70, and an alpha level (threshold for statistical significance) of 0.05. With these assumptions, we estimated a sample of 56 subjects as sufficient for detecting a true group × time interaction with a statistical power of 0.80. Considering a Bonferroni-corrected threshold for statistical significance (0.05/2 primary outcomes = 0.025), we aimed for a total of 64 subjects, which results, under the premises described above, in a statistical power of 0.786.
The main analysis used an implementation of structural equation modeling with latent change score modeling57 to test the effect of L-dopa versus placebo on the outcomes of cognitive training (see Supplement S2 and S9). The analysis was implemented in the ‘lavaan’ package58 within the R programming language environment, version 3.3.2 (2016-10-31). Latent variables were formed similarly for pre- and post-test assessments based on shared variance from multiple tests measuring each specific construct, and a latent change score, which represented the difference between the assessments, was estimated. Estimating intervention-related changes in this way, by forming a latent variable out of several tests, has the advantage of reducing the influence of measurement error and task-specific variance on the outcome measure, and hence also biases (e.g., regression to mean) that may affect raw change scores. The change factor was regressed on the group predictor (L-dopa/Placebo; dummy coded 1 vs. 0). The regression effect indicates the effect of experimental group on latent change from pretest to posttest (i.e., a time by group interaction). Prior to estimation, we z-standardised all variables, such that the size of this effect corresponds to the difference in gains over time between the two groups expressed in standard deviations. A separate model was estimated for each of the considered primary outcomes (spatial and verbal fluid intelligence) and a Bonferroni-corrected threshold for statistical significance of 0.025 (0.05/2 primary outcomes) were applied. The secondary cognitive outcomes (see Supplement S2 and S8 for a complete list) were analysed in the same way, also applying a Bonferroni-corrected threshold for statistical significance (0.05/6 outcomes = 0.008). The same analytic strategy was employed to exploratively analyse the 6-month follow-up data.
Before model estimation, we cleaned and screened the data for outliers using the outlier labeling rule multiplying the interquartile range by a factor of 2.2. Detected outliers were deleted using pairwise deletion and the resulting missing values were accommodated under the missing-at-random assumption using full information maximum likelihood (FIML) estimation. Non-normally distributed variables were transformed employing applicable transforms until normality assumptions were met. We included all available data. Prior to hypothesis testing, measurement invariance assumptions were evaluated to ensure that the same latent variables are represented on each measurement occasion59. Both models that incorporated the primary outcomes, spatial and verbal intelligence, met criteria for strict invariance (see Supplement S2 for all outcomes).
Training progress was analysed employing linear modelling that compared average level reached over the course of training (nested in tasks) between the groups, applying a threshold for statistical significance of p < 0.05.
Plasma concentration of L-dopa and homovanillic acid (HVA) were analysed adhering to standard protocols of high-performance liquid chromatography (HPLC) and electrochemical detection (See Supplement S6 for detailed description). Statistical analyses were conducted employing linear modelling (Group, Group × Visit effects on L-dopa/HVA levels, within-subject, random intercepts), applying a threshold for statistical significance of p < 0 0.05.
Structural MRI images underwent standardised steps for bias-field correction, segmentation, spatial normalisation and smoothing (FWHM of 8 mm) as implemented in the CAT12 (http://www.neuro.uni-jena.de), an SPM12 (http://www.fil.ion.ucl.ac.uk) toolbox installed in the MATLAB 2016 environment. See Supplement S5 for more detailed description. Normalised and modulated grey matter probability maps were analysed employing mass-univariate within-subject ANOVA estimating group × time as a primary effect-of-interest. Yielded statistical parametric maps were adjusted for multiple tests employing a family-wise error-correction procedure. This was accomplished by testing the data against an empirical null distribution of maximum cluster size across 10,000 Gaussian noise simulations with an initial cluster-forming threshold of p < 0.005. Clusters with expected false positive rate of <5% of (PFWE < 0.05) were considered statistically significant.
Significance
The results put constraints on the hypothesis of a key role of the deteriorated dopaminergic system in age-related decline of learning abilities, and speak against early pharmacological interventions in older healthy adults to improve cognitive functions by targeting the dopaminergic system. Our findings also raise concerns about usefulness of novel L-dopa-containing supplements that claim to have neuroprotective and learning-enhancing properties. There is need for careful investigation of how cognitive abilities are affected by early L-dopa medication in clinical populations often receiving substantially larger doses of the drug.
Supplementary information
Acknowledgements
First and foremost, we thank all participants of the REBOOT-II project. We also thank Marie Helsing for help with administrative work, recruitment and organisation. Thanks also to Aleksandra Lebedeva, Erika Bereczki, Marc Guitart-Masip, Lars Bäckman, Patrik Fazio, and Yvonne Brehmer for the valuable discussions and comments on the study plan and results. European Research Council (ERC Grant agreement #617280–REBOOT), Wallenberg Clinical Scholars, and Stiftelse Stockholms Sjukhem. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.Open access funding provided by Karolinska Institute.
Author contributions
M.L. conceived the study. M.L., A.V.L., J.N. and M.K. designed the trial. A.V.L., M.L. and M.K. coordinated the trial. A.V.L. and M.L. analysed the behavioural and MRI data. D.v.d.B. and E.C.M.d.L. analysed the blood samples. A.L., M.L., J.N. interpreted the results. G.S., P.A., U.A., J.L., W.F. and C.H. collected the data. M.L. obtained funding. All authors revised the paper for important intellectual content.
Data availability
All of the analysis steps are documented in R and MATLAB scripts at https://github.com/alex-lebedev (“RBTII” repository). This trial was preregistered at the European Clinical Trial Registry on 2016-10-05, EudraCT # 2016-000891-54, and The Open Science Framework Registry, DOI 10.17605/OSF.IO/AAM9U. According to Swedish law the whole dataset and biological materials cannot be freely accessible, but can be requested from the authors for specific research projects. This requires a data transfer agreement, which effectively transfers the confidentiality obligations of the institution at which the original research was conducted to the institution of the recipient of the data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62172-y.
References
- 1.Alzheimer’s Disease International (ADI). The World Alzheimer Report 2018: the state of the art of dementia research. London, September 2018.
- 2.Ngandu T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cognit. Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuckovic MG, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [(1)(8)F]fallypride. Mov. Disord. 2010;25:2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J. lipid Res. 2002;43:1209–1219. doi: 10.1194/jlr.M200132-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Knecht S, et al. Levodopa: faster and better word learning in normal humans. Ann. Neurol. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- 8.Floel A, et al. Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiol. Aging. 2008;29:267–279. doi: 10.1016/j.neurobiolaging.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backman L, et al. Effects of working-memory training on striatal dopamine release. Science. 2011;333:718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire N, et al. Effects of dopamine depletion on LFP oscillations in striatum are task- and learning-dependent and selectively reversed by L-DOPA. Proc. Natl Acad. Sci. USA. 2012;109:18126–18131. doi: 10.1073/pnas.1216403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusuki T, Imahori Y, Ueda S, Inokuchi K. Dopaminergic modulation of LTP induction in the dentate gyrus of intact brain. Neuroreport. 1997;8:2037–2040. doi: 10.1097/00001756-199705260-00046. [DOI] [PubMed] [Google Scholar]
- 13.Pendolino V, et al. l-DOPA reverses the impairment of Dentate Gyrus LTD in experimental parkinsonism via beta-adrenergic receptors. Exp. Neurol. 2014;261:377–385. doi: 10.1016/j.expneurol.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Brehmer Y, et al. Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett. 2009;467:117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Soderqvist S, et al. Dopamine, working memory, and training induced plasticity: implications for developmental research. Dev. Psychol. 2012;48:836–843. doi: 10.1037/a0026179. [DOI] [PubMed] [Google Scholar]
- 16.Lovden M, Backman L, Lindenberger U, Schaefer S, Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychological Bull. 2010;136:659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- 17.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci. Biobehav. Rev. 2010;34:670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Schmiedek, F., Lovden, M. & Lindenberger, U. Hundred Days of Cognitive Training Enhance Broad Cognitive Abilities in Adulthood: Findings from the COGITO Study. Frontiers in aging neuroscience2, 10.3389/fnagi.2010.00027 (2010). [DOI] [PMC free article] [PubMed]
- 19.Pavese N, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67:1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- 20.Black KJ, et al. Levodopa effects on [(11)C]raclopride binding in the resting human brain. F1000Research. 2015;4:23. doi: 10.12688/f1000research.5672.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shellshear, L. et al. Levodopa enhances explicit new-word learning in healthy adults: a preliminary study. Human psychopharmacology, 10.1002/hup.2480 (2015). [DOI] [PubMed]
- 22.Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson’s disease: a review. Ther. Adv. Psychopharmacol. 2013;3:101–113. doi: 10.1177/2045125312470130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury R, et al. Dopamine restores reward prediction errors in old age. Nat. Neurosci. 2013;16:648–653. doi: 10.1038/nn.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc. Natl Acad. Sci. USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au J, et al. Improving fluid intelligence with training on working memory: a meta-analysis. Psychonomic Bull. Rev. 2015;22:366–377. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- 26.Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14(4):389–433. doi: 10.1016/S0160-2896(05)80012-1. [DOI] [Google Scholar]
- 27.Carpenter PA, Just MA, Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychol. Rev. 1990;97:404–431. doi: 10.1037/0033-295X.97.3.404. [DOI] [PubMed] [Google Scholar]
- 28.Kane MJ, et al. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J. Exp. Psychol. Gen. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- 29.Backman L, et al. Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol. Aging. 2011;32:1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc. Natl Acad. Sci. USA. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Berker AO, Rutledge RB. A role for the human substantia nigra in reinforcement learning. J. Neurosci. 2014;34:12947–12949. doi: 10.1523/JNEUROSCI.2854-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez M, et al. Different levodopa actions on the extracellular dopamine pools in the rat striatum. Synapse. 2007;61:61–71. doi: 10.1002/syn.20342. [DOI] [PubMed] [Google Scholar]
- 33.Berry AS, et al. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016;36:12559–12569. doi: 10.1523/JNEUROSCI.0626-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vo A, Seergobin KN, MacDonald PA. Independent effects of age and levodopa on reversal learning in healthy volunteers. Neurobiol. Aging. 2018;69:129–139. doi: 10.1016/j.neurobiolaging.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Seaman, K. L. et al. Differential regional decline in dopamine receptor availability across adulthood: Linear and nonlinear effects of age. Hum Brain Mapp, 10.1002/hbm.24585 (2019). [DOI] [PMC free article] [PubMed]
- 36.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. pharmacology. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc. Natl Acad. Sci. USA. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fieblinger T, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungerstedt U, Ljungberg T, Schultz W. Dopamine receptor mechanisms: behavioral and electrophysiological studies. Adv. Biochem. Psychopharmacol. 1978;19:311–321. [PubMed] [Google Scholar]
- 41.Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J. Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson S, Smith DM, Mizumori SJ, Palmiter RD. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc. Natl Acad. Sci. USA. 2004;101:13329–13334. doi: 10.1073/pnas.0405084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol. Aging. 2017;57:36–46. doi: 10.1016/j.neurobiolaging.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papenberg, G. et al. Balance between transmitter availability and dopamine D2 receptors in prefrontal cortex influences memory functioning. Cerebral Cortex (in press) (2019). [DOI] [PubMed]
- 45.Styr B, Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. 2018;21:463–473. doi: 10.1038/s41593-018-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda M, Kataoka H, Ueno S. Can levodopa prevent cognitive decline in patients with Parkinson’s disease? Am. J. Neurodegener. Dis. 2017;6:9–14. [PMC free article] [PubMed] [Google Scholar]
- 47.Fox SH, Lang AE. ‘Don’t delay, start today’: delaying levodopa does not delay motor complications. Brain. 2014;137:2628–2630. doi: 10.1093/brain/awu212. [DOI] [PubMed] [Google Scholar]
- 48.Group PDMC, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384:1196–1205. doi: 10.1016/S0140-6736(14)60683-8. [DOI] [PubMed] [Google Scholar]
- 49.Murata M, Kanazawa I. Repeated L-dopa administration reduces the ability of dopamine storage and abolishes the supersensitivity of dopamine receptors in the striatum of intact rat. Neurosci. Res. 1993;16:15–23. doi: 10.1016/0168-0102(93)90004-A. [DOI] [PubMed] [Google Scholar]
- 50.Franklin TR, et al. A VBM study demonstrating ‘apparent’ effects of a single dose of medication on T1-weighted MRIs. Brain structure Funct. 2013;218:97–104. doi: 10.1007/s00429-012-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansson KNT, et al. Viewing Pictures Triggers Rapid Morphological Enlargement in the Human Visual Cortex. Cereb. Cortex. 2019 doi: 10.1093/cercor/bhz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge Q, et al. Short-term apparent brain tissue changes are contributed by cerebral blood flow alterations. PLoS One. 2017;12:e0182182. doi: 10.1371/journal.pone.0182182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kukull WA, et al. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J. Clin. Epidemiol. 1994;47:1061–1067. doi: 10.1016/0895-4356(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 54.Contin M, Martinelli P. Pharmacokinetics of levodopa. J. Neurol. 2010;257:S253–261. doi: 10.1007/s00415-010-5728-8. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson J, Lebedev AV, Rydstrom A, Lovden M. Direct-Current Stimulation Does Little to Improve the Outcome of Working Memory Training in Older Adults. Psychological Sci. 2017;28:907–920. doi: 10.1177/0956797617698139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karbach J, Verhaeghen P. Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychological Sci. 2014;25:2027–2037. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McArdle, J. J. & Nesselroade, J. R. In The West Virginia University conferences on life-span developmental psychology. Life-span developmental psychology: Methodological contributions. (eds. Cohen, S. H. & Reese, H. W.) 223–267 (Lawrence Erlbaum Associates, Inc., 1994).
- 58.Rosseel Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012;48(2):1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 59.Kievit, R. A. et al. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Developmental cognitive neuroscience, 10.1016/j.dcn.2017.11.007 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the analysis steps are documented in R and MATLAB scripts at https://github.com/alex-lebedev (“RBTII” repository). This trial was preregistered at the European Clinical Trial Registry on 2016-10-05, EudraCT # 2016-000891-54, and The Open Science Framework Registry, DOI 10.17605/OSF.IO/AAM9U. According to Swedish law the whole dataset and biological materials cannot be freely accessible, but can be requested from the authors for specific research projects. This requires a data transfer agreement, which effectively transfers the confidentiality obligations of the institution at which the original research was conducted to the institution of the recipient of the data.