Abstract
The development of CDK 4/6 inhibitors has dramatically changed the therapeutic management of hormone receptor-positive (HR+) and HER2 negative metastatic breast cancer (MBC). In combination with fulvestrant, palbociclib, ribociclib and abemaciclib have each been approved for HR+/HER2- MBC following the results of randomized Phase III studies (PALOMA-3, MONALEESA-3, MONARCH-2) and shown a significant advantage in PFS. Data from clinical trials support the combination with aromatase inhibitors in the first line setting and with fulvestrant in the second line. Each agent is well tolerated, and most of the toxicities observed with this class of drugs are generally easily manageable and free from particular complications. The latest evidence from MONARCH-2 and MONALEESA-3 trials shows benefits in terms of overall survival (OS), suggesting an option of using fulvestrant in combination with CDK 4/6 inhibitors in the first line setting. Additional research is needed to determine optimal treatment sequencing, understand the mechanisms of resistance, and develop novel therapeutic strategies to overcome clinical resistance and further improve the outcomes of patients with HR+/HER- MBC. Key questions in the field include the further impact on progression-free survival, overall survival, and the role of continuing CDK 4/6 blockade beyond progression. The purpose of this review is to describe the clinical relevance of fulvestrant in combination with CDK 4/6 inhibitors in HR+/HER2- MBC patients, as well as to discuss the current controversies and evolving research areas.
Keywords: metastatic breast cancer, cyclin-dependent kinases 4 and 6, fulvestrant, clinical trials, resistance mechanisms, palbociclib, ribociclib, abemaciclib
Introduction
According to major international guidelines, the treatment of hormone-receptor positive (HR+), human epidermal growth factor 2 negative (HER2 -) advanced breast cancer (ABC) is mostly palliative and mainly based on the administration of endocrine therapy (ET), excluding for those patients with life-threatening presentation of the disease or with visceral crisis.1 The main goals of endocrine treatment for advanced diseases are to prolong survival, improve or maintain the quality of life, and possibly delay the initiation of chemotherapy. The choice is primarily based on the extent of the disease, previous response to adjuvant endocrine therapy and patients’ clinical status and preferences.
Fulvestrant is a highly selective estrogen receptor downregulator, which is able to bind, block, and accelerate estrogen receptor (ER) degradation.2 In postmenopausal HR+/HER2- advanced breast cancer patients, the latest evidence has validated the role of fulvestrant monotherapy, both in completely endocrine-naive patients3,4 and in those who progressed while receiving, or shortly after the completion of, endocrine adjuvant therapy.5,6
Nevertheless, the landscape of the treatment of HR+/HER2- advanced breast cancer is changing rapidly. In the last few years, increasing understanding of the underlying biological mechanisms of endocrine resistance for metastatic breast cancer (MBC) has prompted several clinical trials of fulvestrant in combination with a targeted agent, in particular cyclin-dependent kinase 4/6 (CDK4/6) inhibitors.7
CDK4/6 inhibitors are orally bioavailable drugs, which have been investigated as anti-cancer agents in the last decade. These agents directly block the activity of the cyclin D–CDK4/6 holoenzyme, and act to limit the proliferation of sensitive tumor cells. They specifically prevent cell cycle progression from the G1 to the S phase of the cell cycle. In sensitive cells, CDK4/6 inhibition normally induces a phenotype reminiscent of cellular senescence,8 consistent with the critical role of the retinoblastoma (RB) tumor suppressor in mediating senescence.9
ER+ breast cancers are the subtype for which CDK4/6 inhibition has the strongest rationale, because they typically retain RB function at presentation, meaning that the principal pathway upon which the agents act is intact.10
Additionally, the encoding cyclin D1 (CCND1) is a direct target gene of the estrogen receptor, and consequently it is often highly expressed in ER+ breast cancers. In the pre-clinical setting, when CDK4/6 inhibitors have been added to standard anti-estrogen therapies a strong synergy has been reported.9 As a result, extensive randomized clinical trials have confirmed that the addition of CDK4/6 inhibitors to hormonal therapy is a valuable clinical approach.11–14
The Food and Drug Administration (FDA) has approved palbociclib, ribociclib and abemaciclib for HR+/HER2-metastatic breast cancer in combination with specific endocrine therapies.
The purpose of this review is to summarize the background and latest evidence for the use of fulvestrant in combination with CDK4/6 inhibitors in breast cancer, and to discuss some of the unanswered and emerging questions about the use of these agents in clinical practice.
The Backbone: Fulvestrant
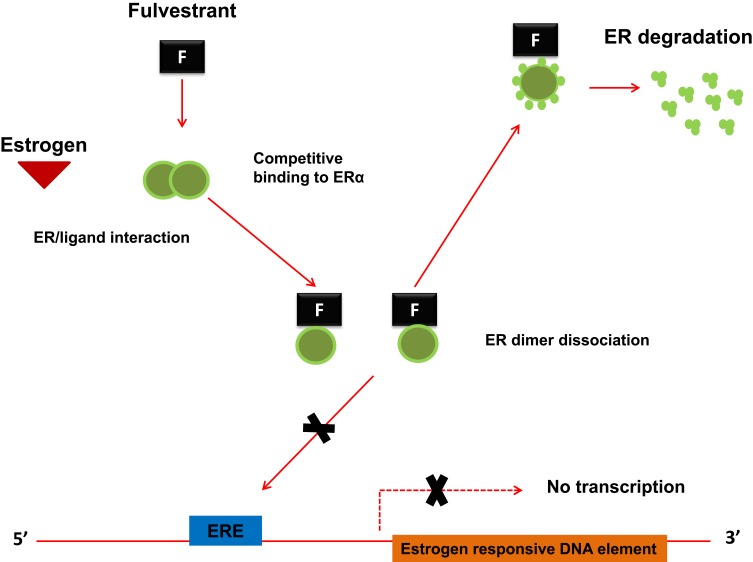
Fulvestrant acts through a selective downregulation of the estrogen receptor, which is competitively bound. The binding established between fulvestrant and ER inhibits the dimerization of the estrogen receptor and blocks the nuclear localization of the receptor itself15,16 as shown in Figure 1. The binding affinity of fulvestrant is 100 times greater than that of the other class of endocrine drugs, which includes tamoxifen.17,18 The binding of fulvestrant with ER also leads to a rapid degradation of the fulvestrant-ER complex and makes the receptor unavailable to estrogens, so the ability of ER to promote gene transcription is attenuated.19
Figure 1.
The molecular mechanism of action for Fulvestrant. Fulvestrant, being a pure steroidal ERα antagonist, inhibits the dimerization of the estrogen receptor and blocks the nuclear localization of the receptor itself. The binding of fulvestrant with ER also leads to a rapid degradation of the fulvestrant-ER complex and makes the receptor unavailable to estrogens, so the ability of ER to promote gene transcription is attenuated.
Endocrine therapy is known to be successful in treating most patients with advanced HR +/HER2− breast cancer; however, in many cases there is a relapse and the disease becomes refractory to such approaches.20 There are many reasons for this resistance, but the factors involved include activation of mutations in the ESR1 gene encoding for ER; an increase in CDK4/6 activity; and upregulation of signaling pathways, such as phosphoinositide-3-kinase (PI3K)/AKT/mTOR and activated protein HER2/mitogenic kinase (MAPK).21–24
Targeting these potential molecular and genomic alterations involved in endocrine resistance has resulted in the development of targeted therapies, which have changed the landscape of HR+/HER2− advanced breast cancer treatment.
The reccomended dose for Fulvestrant in combination with CDK 4/6 inhibitors is 500 mg/monthly based on the results of the Phase 3 clinical trial CONFIRM completed in 736 postmenopausal women with advanced breast cancer who had disease recurrence on or after adjuvant endocrine therapy or progression following endocrine therapy for advanced disease. Overall survival data from the time of final analysis showed a median time to death of 26.4 months for fulvestrant 500 mg versus 22.3 months for fulvestrant 250 mg (HR (95% CI) 0.81 (0.69, 0.96), p-value 0.016).20
CDK 4/6 Inhibitors Plus Fulvestrant: Different Populations
The increased inhibiting activity of CDK4/6 in HR+ breast cancer validates a new therapeutic strategy to enhance the efficacy of fulvestrant therapy and also to potentially reverse fulvestrant resistance.7 The three selective CDK4/6 inhibitors recently introduced into clinical practice after demonstrating their activity in clinical trials in advanced HR+/HER2- breast cancer are Ribociclib (Kisqali®; LEE011, Novartis); palbociclib (Ibrance®; PD-0332991; Pfizer); and abemaciclib (Verzenios®; LY-2835219; Eli Lilly) and the Phase III studies leading to FDA drug approvals for these agents in combination with fulvestrant are summarized in Table 1.
Table 1.
Studies of CDK 4/6 Inhibitors with Fulvestrant in the ABC HR+/HER2- Population
| Study | Population | Phase | n | Setting | Treatment | Most Recent ET | Prior CT | Postmenop. at Study Entry | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| De novo | ADJ/neo | ABC | |||||||||
| Palbociclib | |||||||||||
| PALOMA-3 | Pre- and Postmenopausal women, progressed on/or ≤ 12 months from prior adj therapy with AI/TAM | III | 521 | 2nd-line or greater | Fulvestranta plus palbociclib or placebo | – | 114 (22%) | 406 (78%) | 34% (1 prior line) | 413 (79%) | PFS 11.2 vs 4.6 mos HR 0.50; p < 0.0001 |
| Ribociclib | |||||||||||
| MONALEESA-3 | Men and postmenopausal women in first-line treatment (treatment naive for ABC) or in second line + early relapsers | III | 726 | ≤ 1st line of prior ET for ABC | Fulvestrant plus Ribociclib or placebo | 139 (20%) | 431 (60%) | 150 (20%) | No | 726 (100%) | PFS 20.6 vs 12.8 mos HR 0.60; p < 0.001 OS NR vs 40 mos HR 0.724; p=0.00455 |
| Abemaciclib | |||||||||||
| MONARCH 2 | Pre – and Postmenopausal women relapsed on neoajuvant or on/within 12 months of ET or progressed on first-line therapy | III | 669 | Failed ET in localized or 1st-line for ABC | Fulvestranta plus abemaciclib or placebo | – | 396* (60%) | 256* (38%) | No | 552 (82%) | PFS 16.4 vs 9.3 mos HR 0.55; p < 0.001 OS 46.7 vs 37.3 mos HR 0.757; p=0.01 |
Notes: aGoserelin (luteinizing hormone-releasing hormone analog) is coadministered with fulvestrant to premenopausal women in PALOMA-3, MONARCH-2. *ET history was not available for 17 patients.
Abbreviations: ABC, advanced breast cancer; HER2, human epidermal growth factor receptor 2; HR+, hormone receptor-positive; PFS, progression-free survival; OS, overall survival; NR, not reached; ET, endocrine treatment; ADJ adjuvant setting; AI aromatase inhibitor; TAM tamoxifen.
All these combination studies have shown a significant improvement in PFS, but the study populations are fairly different in each of the phase III trials. The PALOMA-3 trial (fulvestrant with palbociclib/placebo) included women with HR+/HER2- MBC, ones who were pre-menopausal (goserelin was added for these women) or post-menopausal and who had relapsed or progressed during prior endocrine treatment (78% of the entire population). In this trial, there was no limit to previous endocrine therapies (2nd line or more)25,26 and 34% of the patients had received prior chemotherapy for ABC. The addition of palbociclib resulted in an improvement in PFS from 4.6 months to 11.2 months (HR 0.50; p < 0.0001). Although the results of the analysis of overall survival did not meet the pre-specified threshold for statistical significance, the addition of palbociclib to fulvestrant resulted in an absolute prolongation of overall survival of 6.9 months among patients with HR+/HER2- advanced breast cancer, who suffered disease progression after previous endocrine therapy.27
The MONARCH-2 trial (fulvestrant with abemaciclib/placebo) included women with HR+/HER2- MBC of any menopausal status (LHRH agonist added for premenopausal women) who had progressed during prior endocrine therapy, but by no more than one line (2nd line),28 and only 38% were pretreated for advanced disaese. The addition of abemaciclib resulted in an improvement in PFS from 9.3 to 16.4 months (HR 0.55; P < 0.001). At the pre-specified interim analysis, 338 deaths (77% of the planned 441 in the final analysis) were observed in the intent-to-treat population, with a median OS of 46.7 months for abemaciclib plus fulvestrant and 37.3 months for placebo plus fulvestrant (HR 0.757; 95% CI,0.606–0.945; P = 0.01). Improvement in OS was consistent across all stratification factors.29
The MONALEESA-3 trial explored fulvestrant with or without ribociclib in postmenopausal women and men with HR+/HER2- MBC who had received 0–1 lines of endocrine therapy for advanced disease, and thus included both first-line and second-line patients.30 In particular, in this trial 80% of the patients were not pretreated for ABC. Overall, the addition of ribociclib resulted in an improvement in PFS from 12.8 to 20.5 months (HR 0.60; P < 0.001). MONALEESA-3 demonstrated a statistically significant OS benefit with ribociclib plus fulvestrant vs placebo plus fulvestrant, with a 28% reduction in the relative risk of death (HR: 0.724; 95% CI, 0.568–0.924; P = 0.00455). OS benefit was consistent across patient subgroups.31
Toxicities and Drug-Drug Interactions
A detailed toxicity assessment was available for each of the CDK4/6 inhibitors in the pivotal clinical trials, including PALOMA with palbociclib, MONALEESA with ribociclib, MONARCH with abemaciclib and in post-marketing reports.7,12–14,26,28 During the clinical trials adverse events from the CDK4/6 inhibitors were easily managed through dose modification and established supportive care measures, although in general they were well tolerated. There was some toxicity overlap for each CDK4/6 inhibitor; nonetheless, each drug showed certain unique characteristics.
CDK4/6 inhibitors are commonly associated with hematological toxicities, primarily neutropenia. However, such toxicities are not usually complicated and are manageable with dose interruption or reduction. It is rare to encounter serious events, such as febrile neutropenia. Cytopenia is an on-target effect of CDK4/6 inhibitors due to the role of CDK6 in the proliferation of hematologic precursors.32
The main action of CDK4/6 inhibitors that leads to toxicity is in the form of a cytostatic effect on neutrophil precursors; the neutropenia induced by the agents can thus be rapidly reversed by withdrawing the drug. For this reason, myeloid growth factors (G-CSF) are generally not indicated.33 On the other hand, chemotherapy usually destroys progenitor cells, resulting in a more persistent and severe neutropenia.
Palbociclib and ribociclib are administered in intermittent doses to allow hematologic cells to recover. Abemaciclib is different because it shows higher selectivity for CDK. Consequently, lower rates of hematologic toxicities have been reported, and for this reason it can be dosed continuously.34
Across the class of CDK4/6 inhibitors, common non-hematologic toxicities include fatigue, nausea, vomiting, stomatitis, alopecia, rash, diarrhea, decreased appetite and infections. For most patients, such effects are mild, and therapy is not usually affected.
More frequently than other CDK 4/6 inhibitors, ribociclib may induce hepatotoxicity. Therefore, liver function test (LFT) surveillance at baseline and throughout therapy is necessary. The median time to onset of severe hepatotoxicity (Grade ≥ 3) is 85 days and that to resolution to Grade ≤ 2 is 22 days when used in combination with fulvestrant.30 Hepatotoxicty may call for the dose of ribociclib to be reduced, or indeed, for its interruption or discontinuation altogether.
Another toxicity typically associated with ribociclib is the reversible, concentration-dependent prolongation of the QT interval.30 Nevertheless, no cases of torsades de pointes have been described in the clinical investigations of ribociclib. QT interval monitoring should be conducted upon initiation of and during therapy. Ribociclib is to be avoided in patients who have QT prolongation or who are at significant risk of developing it (e.g. those with long QT syndrome, uncontrolled/significant cardiac disease, electrolyte abnormalities, or are concomitantly taking medications with QT-prolonging potential). Electrolytes should also be monitored in these patients.
In the MONARCH trials, abemaciclib showed increased rates of fatigue and diarrhea, especially during the first month of therapy, in comparison with the other two agents in the class perhaps because of its greater affinity for CDK4.28,29 The median time to diarrhea onset was approximately 7 days, while the median duration of Grade 2–3 diarrhea was 6–11 days. Diarrhea may warrant abemaciclib dose interruption or reduction.
Patients should commence taking antidiarrheals, such as loperamide, at the onset of loose stools and oral fluid intake should be increased. Prophylactic loperamide has been employed in some clinical trials of abemaciclib. In MONARCH-3, the median time to onset of severe hepatotoxicity (Grade ≥ 3) was approximately 60 days and median time to resolution to < Grade 3 approximately 14 days; therefore, LFT monitoring is recommended at baseline and during therapy.
Furthermore, MONARCH-2 and -3 reported a higher proportion of venous thromboembolic events (VTE) in patients treated with abemaciclib than those receiving a placebo (5% vs 0.9% in MONARCH-2, and 5% vs 0.6% in MONARCH-3). It is essential that patients receiving abemaciclib be fully informed regarding the risk and signs/symptoms of VTE. Treating physicians should monitor patients for such signs and symptoms and of pulmonary embolism and treat them as medically appropriate. An increased level of serum creatinine (SCr) is common with abemaciclib, since there are several tubular secretion transporters which become inhibited. Glomerular function remains unaffected, nor is it reflective of renal damage.33
CDK4/6 inhibitors interact with other drugs primarily mediated by modification of the cytochrome P450 (CYP) pathway, since palbociclib, ribociclib and abemaciclib are all major substrates of the CYP3A4 enzyme. Therefore, one must avoid concomitant use of potent CYP3A inhibitors/inducers with CDK4/6 inhibitors whenever possible, and consider alternative therapies.
If it is decided to administer a potent CYP3A inhibitor in addition to CDK4/6 inhibitors, then the dose must be reduced. Caution should also be paid to administering moderate CYP3A inhibitors/inducers and dose modifications need to be considered. Grapefruit and grapefruit juice are to be avoided in patients on any CDK4/6 inhibitor because of the potential for increased drug exposure.
Palbociclib is a weak CYP3A4 inhibitor, whereas ribociclib is a moderate one. Therefore, concomitant use of CYP3A substrates with a narrow therapeutic index (eg, cyclosporine, everolimus, fentanyl and tacrolimus) should be made cautiously. Dose reductions of the CYP3A substrate may be warranted. Drugs with QT interval-prolonging potential (such as amiodarone, haloperidol, methadone, moxifloxacin, ondansetron and sotalol) are not to be used with ribociclib. Drug information resources should be studied when assessing QT prolongation potential with specific drugs and the appropriateness of concomitant ribociclib administration.
Abemaciclib inhibits P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Although the clinical impact of abemaciclib on P-gp or BCRP substrates remains unknown, caution should be exercised in cases where sensitive substrates, such as digoxin, are concomitantly administered.
Toxicity profiles may differ between the combinations of cyclin inhibitors with fulvestrant or with letrozole. Table 2 summarizes these differences.
Table 2.
Toxicity Profile of Cycline Inhibitors with Fulvestrant or with Letrozole
| Palbociclib | Ribociclib | Abemaciclib | ||||||
|---|---|---|---|---|---|---|---|---|
| Monotherapy42 | +letrozole PALOMA-214 | +fulvestrant PALOMA-36 | Monotherapy43 | +letrozole MONALEESA-211 | +fulvestrant MONALEESA-328 | Monotherapy MONARCH-133 | +fulvestrant MONARCH-226 | +letrozole/anastrozole MONARCH-312 |
| Adverse events any grade | ||||||||
| Neutropenia (92%) Leukopenia (100%) Thrombocytopenia (76%) Anemia (70%) Lymphopenia (65%) |
Neutropenia (80%) Leukopenia (40%) Nausea (35%) Fatigue (35%) Arthralgia (33%) Alopecia (33%) |
Neutropenia (80%) Leukopenia (50%) Infections (42%) Fatigue (40%) Nausea (32%) |
Neutropenia (46%) Leukopenia (43%) Thrombocytopenia (30%) Fatigue (45%) Nausea (42%) |
Neutropenia (75%) Leukopenia (33%) Nausea (50%) Infections (50%) Fatigue (37%) Diarrhea (35%) Alopecia (33%) |
Neutropenia (70%) Nausea (45%) Fatigue (32%) Diarrhea (29%) Leukopenia (28%) Vomiting (27%) Constipation (25%) Arthralgia (24%) Cough (22%) Headache (22%) Alopecia (19%) Rash (18%) Anemia (17%) |
Neutropenia (85%) Leukopenia (90%) Thrombocytopenia (76%) Anemia (69%) Diarrhea (90%) Fatigue (65%) Nausea (65%) Decreased appetite (45%) Abdominal pain (40%) Vomiting (35%) |
Neutropenia (46%) Diarrhea (86%) Nausea (45%) Fatigue (40%) Abdominal pain (35%) |
Neutropenia (41%) Diarrhea (81%) Nausea (39%) Fatigue (40%) Infections (39%) |
| Adverse events grade 3–4 | ||||||||
| Neutropenia (54%) Leukopenia (51%) Lymphopenia (30%) |
Neutropenia (66%) Leukopenia (25%) |
Neutropenia (65%) Leukopenia (28%) |
Neutropenia (27%) Leukopenia (17%) Fatigue (2%) Nausea (2%) |
Neutropenia (59%) Leukopenia (21%) |
Neutropenia (53%) Leukopenia (14%) Anemia (3%) Fatigue (2%) Back Pain (2%) Nausea (1%) Constipation (1%) Headache (1%) |
Neutropenia (27%) Leukopenia (28%) Diarrhea (20%) |
Neutropenia (24%) | Neutropenia (21%) |
Discussion
Fulvestrant in Combination with a CDK 4/6 Inhibitor: Which Line?
Following the approval of CDK4/6 inhibitors with endocrine therapy as the treatment of choice for women with HR+/HER2- MBC, data from clinical trials supports the combination with aromatase inhibitors as first line and fulvestrant as second line.
The pivotal phase III trials of CDK 4/6 inhibitors in combination with aromatase inhibitors were conducted in the first-line setting. However, the MONALEESA-3 trial is the first study of a CDK4/6 inhibitor plus fulvestrant in de novo HR+/HER2– ABC, or following relapse of at least 12 months or more after prior endocrine therapy, with no subsequent treatment for advanced disease. First- line patients showed significantly higher benefit compared to second-line ones, with a median PFS of 33.6 months in the ribociclib arm, versus 19.2 months in the placebo arm (HR 95% CI 0.54). In the second-line setting, the median PFS was 14.6 months in the combination arm, versus 9.1 months in the placebo arm.31
Moreover, MONALEESA-3 demonstrated a statistically significant OS benefit with ribociclib plus fulvestrant vs placebo plus fulvestrant, with a 28% reduction in the relative risk of death (HR: 0.724; 95% CI, 0.568–0.924; P = 0.00455). OS benefit was consistent across patient subgroups.31
Interestingly, the remarkable results of the phase III FALCON trial for endocrine therapy-naïve HR+ MBC patients, comparing upfront anastrozole with upfront fulvestrant, showed a PFS advantage of fulvestrant (16.6 vs 13.8 months, HR 0.80, P = 0.049), with the most benefit seen in patients without visceral disease (22.3 vs 13.8 months, HR 0.59).3
Therefore, the FALCON and MONALEESA-3 trial results are encouraging and fulvestrant plus a CDK 4/6 inhibitor may represent a reasonable option for patients with de novo HR+/HER2- MBC. In addition, the updated results of the MONALEESA-2 study, after 26.4 months of follow-up, showed a benefit from ribociclib plus letrozole versus placebo plus letrozole, with a 9.3-month improvement in median PFS with the addition of ribociclib. However, the OS data remained immature at the time of the secondary interim analysis, and median OS was not reached in the ribociclib plus letrozole arm compared with the 33.0 months among patients treated with the placebo plus letrozole.35
There are differences in study design that may lead to misinterpretation of the results, especially when direct comparisons of efficacy outcomes are made across trials. Fulvestrant could be the preferred endocrine backbone, but it is currently unclear whether this is the best option for all patients.
In clinical trials, the tumor tissue biomarkers associated with sensitivity and/or resistance to CDK 4/6 inhibitors have been evaluated. In the PALOMA-3 trial, baseline tumor ESR1 and PIK3CA mutation rates were lower among long-term responders in both arms. In addition, ribociclib prolonged PFS, irrespective of PIK3CA or TP53 mutation status.35 Patients with wild-type PIK3CA and TP53 had a numerically longer PFS versus those harboring altered PIK3CA or TP53, irrespective of treatment.36 According to the available evidence, the determination of tumor tissue biomarkers such as PI3CKA mutations should be considered before starting treatment with CDK4/6 inhibitors in order to plan an optimal sequence.
All three phase III global registration trials included patients progressing within 12 months of completion of adjuvant endocrine therapy (early relapse) or while on prior therapy for advanced/metastatic disease (second-line treatment). All the trials demonstrated a PFS advantage for this population (PALOMA-3 median PFS 11.2 vs 4.6 months HR 0.50, 95% CI 0.40–0.62; MONALEESA-3 median PFS 14.6 vs 9.1 months HR 0.571, 95% CI, 0.443–0.737; MONARCH-2 median PFS 16.9 vs 9.3 months HR, 0.553, 95% CI, 0.449–0.681).
Although the populations included in the MONARCH-2 and MONALEESA-3 trials were different, the exploratory endpoints for both trials included time to second disease progression (PFS2) and time to first chemotherapy (TTC). In the MONARCH-2 trial, median PFS2 was 23.1 months in the abemaciclib-treated arm vs 20.6 months in the placebo arm (HR, 0.675; 95% CI, 0.558–0.816). Median TTC (censoring patients who died prior to receiving chemotherapy) was 50.2 months in the abemaciclib arm vs 22.1 months in the placebo arm (HR, 0.625; 95% CI, 0.501–0.779). In the MONALEESA-3 trial, median PFS2 was 39.8 months in the ribociclib arm vs 29.4 months in the placebo arm (HR, 0.670; 95% CI, 0.542–0.830). Median TTC was not reached in the ribociclib arm vs 29.5 months in the placebo arm (HR, 0.696; 95% CI, 0.551–0.879).
These results suggest that CDK 4/6 inhibitor should be included in first-line treatment to obtain the greatest benefit and gives rise to the hypothesis that these drugs can have a carry-over effect.
Is There Overall Survival Improvement with Fulvestrant and CDK 4/6 Inhibitors?
In HR+/HER2- MBC, it is generally challenging to demonstrate an overall survival benefit.
New and updated data from the two studies reported at the European Society for Medical Oncology (ESMO) meeting in 2019 showed that treatment with a CDK4/6 inhibitor plus fulvestrant improves OS in women with HR+/HER2- (MONALEESA-3 median OS 40.2 vs 32.5 months, HR 0.730 95% CI, 0.530–1.004; MONARCH-2 median OS 46.7 vs 37.3 months HR 0.757 95% CI, 0.606–0.945). The two studies included different patient populations, as well as different CDK4/6 inhibitors and different lines of therapy. MONARCH-2 evaluated abemaciclib plus fulvestrant in patients with advanced breast cancer after failure of endocrine therapy, regardless of the menopausal status, while MONALEESA-3 investigated ribociclib plus fulvestrant as first- or second-line only in postmenopausal patients.
The PALOMA-3 trial of fulvestrant with palbociclib/placebo in the second line and beyond did not demonstrate an overall OS benefit (34.9 months vs 28 months, HR 0.81, 95% CI 0.64–1.03, P=0.09), but the palbociclib group was favored with an absolute improvement of 6.9 months.26 This advantage was reported, although 16% of the patients in the placebo-fulvestrant group received CDK 4/6 inhibitor treatment post-randomization. The patients with sensitivity to previous endocrine therapy were those who had a significant OS.27
An explanation for the different results could be related to the different populations, in particular a greater number of pretreated patients, and if a previous line of chemotherapy had been permitted.27
In the MONARCH-2 study, patients receiving abemaciclib plus fulvestrant and with primary resistance to endocrine therapy showed a better OS in the overall survival subgroup analysis.31 The separation curves between the abemaciclib arm and the placebo arm occurred early, in the first year of treatment. In the PALOMA-3 study with palbociclib and fulvestrant, the OS data are different27
The mechanism potentially responsible for the effects on OS in populations with visceral disease and with disease primarily resistant to endocrine therapy is unknown. However, the factors that could be involved in the mechanism include the fact that abemaciclib can be administered continuously, and its greater potency for CDK4 over CDK6, as demonstrated in enzymatic tests.28,36
It is recommended that further studies confirm these observations prospectively.
In MONARCH-2, a total of 17% of patients in the placebo arm received a CDK4/6 inhibitor as post-discontinuation therapy. This “crossover” might have attenuated an even more significant OS benefit for the abemaciclib arm.
These results give the treating physician the full spectrum of CDK4/6 inhibitor choices for each individual patient, although the three CDK4/6 inhibitors have slightly different management requirements and toxicity profiles.
All three CDK4/6 inhibitors were tested in studies powered for progression-free survival and not for overall survival, but taken together the data are strong enough to support endocrine-based therapy plus a CDK4/6 inhibitor instead of endocrine therapy alone in the first/second- line setting of HR+/HER2- MBC.
A meta-analysis would be helpful to assess the OS question, given the limited power of each individual trial.
Are CDK 4/6 Inhibitors All the Same?
Retrospective comparison of the subgroup analyses between the different studies with each CDK 4/6 inhibitor can be difficult, as each study had a different design, different patient populations, and unintentional patient biases. However, all three CDK 4/6 inhibitors have shown benefits in every single clinical subset, from liver metastases to multiple sites of metastases and to short disease-free intervals, suggesting that the three agents are likely have similar efficacy.
Despite comparable results in terms of clinical efficacy, the three CDK4/6-inhibitors present substantial pharmacological differences.
Palbociclib and ribociclib inhibit both CDK4 and 6, and with cumulative dosing lead to neutropenia because they inhibit CDK6, which can cause some bone marrow suppression and neutropenia. Abemaciclib is 14 times more potent against CDK4 than it is against CDK6, and it results in less neutropenia and bone marrow suppression (50% lower neutropenia rate compared to palbociclib and ribociclib) because CDK6 plays a critical role in hematopoetic stem cell differentiation.
As a result, abemaciclib administration is continuous. Concerning target activity, palbociclib and ribociclib are only able to inhibit CDK4 and CDK6, whereas abemaciclib has additional activity against CDK9.37,38 This activity against CDK9 could in part explain the clinical efficacy of the abemaciclib monotherapy shown in the MONARCH-1 trial34 and the specific gastrointestinal toxicity that is less pronounced with ribociclib and palbociclib.39
The three CDK4/6 inhibitors, have few, but consistent, differences in terms of toxicity profile. Palbociclib and ribociclib have predominantly bone marrow toxicity, while abemaciclib administration has been correlated with gastrointestinal symptoms and less pronounced hematologic toxicity.
Another important factor to consider for all three agents is dose reduction, which in pivotal studies did not appear to compromise the effectiveness of the treatment.40 Dose reduction is easier with ribociclib, as it is sufficient to reduce the number of tablets, rather than having to call back the patient to write a new prescription. In fact, it is important to consider both the cost and the discomfort related to multiple hospital visits by patients, as well as the experience of the doctors. Once all CDK 4/6 inhibitors are available, it will be increasingly important to conduct patient-centered decision-making and effective discussion which takes into account the pros and cons of each agent.
Should CDK 4/6 Inhibitors Be Used Beyond Progression?
Based on the emerging data from clinical trials, we have no evidence to support the continuation of a CDK 4/6 inhibitor beyond progression after previous CDK 4/6 therapy. In particular, we have no data on either switching to another CDK 4/6 inhibitor or switching to another endocrine therapy and continuing with the same CDK 4/6 inhibitor. However, the concept of switching is appealing, particularly if we consider that this approach has demonstrated activity in other disease settings (for example, continuation of anti-HER2 therapy in HER2+ MBC); nevertheless, toxicity and costs need to be considered.
Both MONALEESA-3 and MONARCH-2 trials showed improvements in PFS2 and time to chemotherapy, suggesting that the benefit of CDK 4/6 inhibitors may extend beyond the study treatment.
A number of trials have explored the continued use of CDK 4/6 inhibitors post-progression.
An international, multicenter, randomized, open-label, Phase II clinical trial is ongoing to evaluate the efficacy and safety of continuation of palbociclib in combination with second-line endocrine therapy in HR+/HER2- ABC patients who have achieved clinical benefit during first-line palbociclib-based treatment (PALMIRA) (NCT03809988). Another trial with a similar design is the MAINTAIN trial (NCT02632045). This is a randomized trial for patients with metastatic HR+/HER2- breast cancer who have progressed on an aromatase inhibitor plus a CDK4/6 inhibitor (either palbociclib or ribociclib) to either fulvestrant alone or fulvestrant with ribociclib. The purpose of the trial is to determine whether there is continued benefit for patients to remain on a CDK4/6 inhibitor at the time of switching anti-estrogen therapy.
Conclusion
The approval of CDK 4/6 inhibitors has permanently changed the treatment paradigm of HR +/HER2- metastatic breast cancer. Palbociclib, ribociclib and abemaciclib have all been approved in combination with an aromatase inhibitor or fulvestrant. Each agent is well tolerated and most of the toxicities observed with this class of drugs are generally easily manageable and free of particular complications. These toxicities often disappear with a simple reduction in dosage.
Fulvestrant, with its unique mechanism of action, has demonstrated efficacy in treating patients with HR+/HER2− advanced breast cancer, either given alone or in combination with targeted therapies. In particular, such a combination is a valuable therapeutic choice because it is well‐tolerated, also offers significant efficacy, is safe, and respects the quality of life.
Until the recent emerging evidence, fulvestrant monotherapy showed superior efficacy as a first‐line treatment option, especially in endocrine‐naïve cases, while combining fulvestrant with a CDK4/6 inhibitor was the preferred treatment option in patients with prior exposure to an AI.
Finally, although well‐defined indications for fulvestrant in the therapeutic algorithm of HR+/HER2- ABC exist, the optimal position has yet to be clearly defined, and the latest data showing the OS advantage suggest that fulvestrant in association with a CDK 4/6 inhibitor may be included in first-line treatment.
With regard to the future, the next-generation selective oral ER degraders (SERDs) currently in clinical development have been shown to be more potent than fulvestrant, with specific activity in endocrine resistance and ESR1 mutations.41 Furthermore, being orally bioavailable, they may increase patient convenience. One such example is elacestrant (RAD1901), which has enhanced the efficacy of both palbociclib and abemaciclib in vitro.42 Other oral SERDs that are currently being evaluated in clinical trials in combination with CDK4/6 inhibitors include LSZ102 in combination with ribociclib (NCT02734615), and GDC 9545 in combination with palbociclib (NCT03332797).
The next steps consist of identifying biomarkers beyond the estrogen receptor to predict response; determining whether to continue CDK4/6 inhibitors after disease progression; combining these agents with other therapies; and expanding their use into settings other than HR+/HER2− advanced breast cancer.43 Currently, numerous studies are under way with the aim of exploring CDK 4/6 inhibitors in various disease contexts for breast cancer, including adjuvant and neoadjuvant settings, and the combination with other targeted agents and with immunotherapy in advanced disease, as well as in the post-progression phase. Moreover, observational real-world studies will be able to provide new insights into the implementation of these drugs in clinical practice.
Disclosure
EM has received an honorarium as consultant/advisor from Pierre Fabre, Genomic Health, Eisai. Dr Manuelita Mazza reports personal fees from Novartis, personal fees from Gentili, personal fees from Pfizer, personal fees from Lilly, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 2.Deeks ED. Fulvestrant: a review in advanced breast cancer not previously treated with endocrine therapy. Drugs. 2018;78(1):131–137. doi: 10.1007/s40265-017-0855-5 [DOI] [PubMed] [Google Scholar]
- 3.Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. doi: 10.1016/S0140-6736(16)32389-3 [DOI] [PubMed] [Google Scholar]
- 4.Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–4535. doi: 10.1200/JCO.2008.21.1136 [DOI] [PubMed] [Google Scholar]
- 5.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–998. doi: 10.1016/S1470-2045(13)70322-X [DOI] [PubMed] [Google Scholar]
- 6.Perey L, Paridaens R, Hawle H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00). Ann Oncol. 2007;18(1):64–69. doi: 10.1093/annonc/mdl341 [DOI] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2- metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 8.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- 9.Narita M, Nunez S, Heard E, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Crown JP, Lang I, et al. The cyclindependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptorpositive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised Phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 12.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 13.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 15.Robertson JF, Nicholson RI, Bundred NJ, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61:6739–6746. [PubMed] [Google Scholar]
- 16.Robertson J, Harrison M. Fulvestrant: pharmacokinetics and pharmacology. Br J Cancer. 2004;90(Suppl 1):S7–S10. doi: 10.1038/sj.bjc.6601630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol. 1987;112:R7–R10. doi: 10.1677/joe.0.112R007 [DOI] [PubMed] [Google Scholar]
- 18.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 19.Zhang X, Diaz MR, Yee D. Fulvestrant regulates epidermal growth factor (EGF) family ligands to activate EGF receptor (EGFR) signaling in breast cancer cells. Breast Cancer Res Treat. 2013;139:351–360. doi: 10.1007/s10549-013-2541-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337. doi: 10.1093/jnci/djt337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: american Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 22.Poggio F, Lambertini M, Blondeaux E, et al. Role of fulvestrant in the treatment of postmenopausal metastatic breast cancer patients. Expert Rev Clin Pharmacol. 2016;9:1153–1161. doi: 10.1080/17512433.2016.1215243 [DOI] [PubMed] [Google Scholar]
- 23.Pritchard KI, Chia SK, Simmons C, et al. Enhancing endocrine therapy combination strategies for the treatment of postmenopausal HR+/HER2− advanced breast cancer. Oncologist. 2017;22:12–24. doi: 10.1634/theoncologist.2016-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Wang Z, Shao Z. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: a review. Cancer Med. 2019;8(5):1943–1957. doi: 10.1002/cam4.2019.8.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spring LM, Wander SA, Zangardi M, Bardia A. CDK 4/6 inhibitors in breast cancer: current controversies and future directions. Curr Oncol Rep. 2019;21(3):25. doi: 10.1007/s11912-019-0769-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner NC, Ro J, André F, et al. Palbociclib in hormone- receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 27.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 28.Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 29.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 31.Slamon DJ, Neven P, Chia S, et al. Overall survival results from the phase III MONALEESA-3 study of fulvestrant ± ribociclib in postmenopausal patients with HR+/HER2− advanced breast cancer. Ann Oncol. 2019;30:v856–v857. doi: 10.1093/annonc/mdz394.007 [DOI] [Google Scholar]
- 32.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malumbres M, Sotillo R, Santamaría D, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 34.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle- dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 36.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A phase II study of Abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR +/HER2 − metastatic breast cancer. Clin Cancer Res. 2017;23:5218–5224. doi: 10.1158/1078-0432.CCR-17-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garriga J, Grana X. CDK9 inhibition strategy defines distinct sets of target genes. BMC Res Notes. 2014;7:301. doi: 10.1186/1756-0500-7-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marra A, Curigliano G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer. 2019;29(5):27. doi: 10.1038/s41523-019-0121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corona SP, Generali D. Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer. Drug Des Devel Ther. 2018;12:321–330. doi: 10.2147/DDDT.S137783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2- Negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, Phase III study (PALOMA-3). Oncologist. 2016;21(10):1165–1175. doi: 10.1634/theoncologist.2016-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonnell DP, Wardell SE, Norris JD. Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J Med Chem. 2015;58:4883–4887. doi: 10.1021/acs.jmedchem.5b00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin LA, Pancholi S, Simigdala N, et al. Abstract P4-04-09: new oral SERD elacestrant (RAD1901) shows efficacy in breast cancer models harbouring ESR1 mutations and enhances the antiproliferative activity of mTORC1 and CDK4/6 inhibitors. Cancer Res. 2018;78:P4-04–09. [Google Scholar]
- 43.DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258 [DOI] [PubMed] [Google Scholar]