Abstract
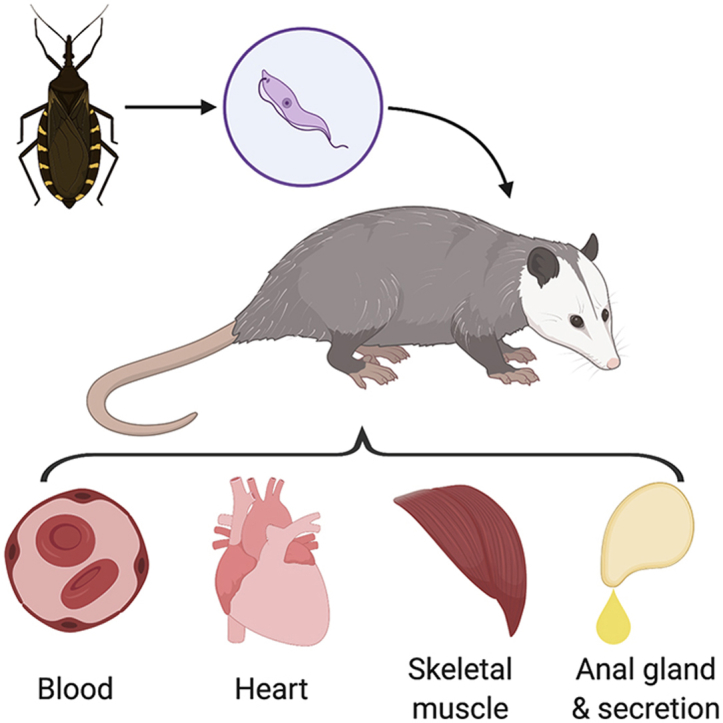
Trypanosoma cruzi, a zoonotic protozoan parasite, infects a wide range of mammals. The southern United States has endemic sylvatic transmission cycles maintained by several species of wildlife and domestic dogs. We hypothesized that urban-dwelling opossums (Didelphis virginiana) in South Texas are infected with T. cruzi, and that tissue pathology would be associated with infection. In 2017, we collected blood, heart tissue and anal gland secretions from 100 wild opossums across three seasons that were trapped by animal control in South Texas. In addition, anal gland tissue and intercostal muscle were collected from 43 of the 100 opossums for which time allowed the extra tissue collection. All blood, tissue, and secretion samples were screened for T. cruzi DNA using qPCR with confirmation of positive status achieved through one or more additional PCR assays, including a qPCR to determine the parasite discrete typing unit (DTU). T. cruzi DNA was detected in at least one tissue of 15% of the opossums sampled: blood clot (9%), heart tissue (10%), anal gland secretions (12%), intercostal muscle (16.3%), and anal gland tissue (11.6%). Infection was detected in two or more different tissue types in nine of the opossums. The 35 tissues for which parasite DTU was determined were exclusively ‘Tcl’- a DTU previously associated with locally-acquired human disease in the United States. T. cruzi-positive opossums were nearly 14 times more likely to exhibit significant heart lesions on histopathology (lympoplasmacytic inflammation±fibrosis) when compared to negative opossums (OR = 13.56, CI = 1.23–751.28, p-value = 0.03). Three triatomines were opportunistically collected from the study site, of which two were infected (66.7%), and bloodmeal analysis revealed canine, opossum, and human bloodmeals. Given the presence of parasite in opossum blood, unique potential for shedding of parasite in anal glad secretions, and evidence of vectors feeding on opossums, it is likely that opossums serve as wild reservoirs around urban dwellings in South Texas.
Keywords: Chagas disease, Texas, Kissing bugs, Triatomine, Urban wildlife
Graphical abstract
Highlights
-
•
Trypanosoma cruzi type ‘TcI’ DNA was detected in 15% of opossums from South Texas.
-
•
Infected tissues included blood, heart, skeletal muscle, and anal glands and secretions.
-
•
Heart pathology occurred more frequently in opossums with infected tissues.
-
•
Local triatomines were found to feed on opossum, dog, and human.
-
•
Urban opossums likely serve as T. cruzi reservoirs in the US.
1. Introduction
Triatomines are hematophagous insects that transmit a hemoflagellate parasite, Trypanosoma cruzi, in their feces. With a variety of common names based on geographic location, including kissing bug, triatomines are distributed across the Americas, including the southern United States. Although transmission of T. cruzi is primarily vector borne, other forms of transmission exist, including oral and congenital transmission. Oral transmission has caused outbreaks in humans via contaminated food (Alarcon de Noya and Noya González, 2015) and is thought to be a major transmission route for animals (Coura and Junqueira, 2015). Infected humans and other mammals could potentially develop a deadly disease known as Chagas disease. The disease affects an estimated 8 million people worldwide (Montgomery et al., 2014). This parasite may cause cardiac abnormalities but may affect other organ systems as well. Six genetic variats, discrete typing units (DTUs), of the parasite exist- T. cruzi I-VI (Tc1-TcVI) (Zingales et al., 2009). A unique bat DTU (TcBat) has been described as well (Pinto et al., 2012). DTUs may be exclusive to certain geographical areas and often overlap (Zingales et al., 2012). However, TcI and TcIV are the most common DTUs found in the United States (Curtis-Robles et al., 2018a). Infections of T. cruzi have been documented in animal hosts from different orders, including Chiroptera, Carnivora, Rodentia, Pilosa and others (Santos et al., 2019), with variation in the degree to which certain wild or domestic species serve as parasite reservoirs in different epidemiological settings. For example, in Argentina and Mexico, domestic cats and dogs have been identified as key reservoir hosts that maintain the domestic transmission cycle (Cardinal et al., 2007; Gurtler et al., 2007; Jimenez-Coello et al., 2010). In the southern United States , T. cruzi is maintained in sylvatic cycles by several wildlife species, including raccoons (Procyon lotor) and skunks (Memphitis memphitis) (Hodo and Hamer, 2017), with dogs as key hosts in peridomestic cycles (Curtis-Robles et al., 2017).
In Latin America, peridomestic and urban wildlife like Didelphis sp. opossums have long been recognized as T. cruzi reservoirs where they bridge transmission cycles to domestic animals and humans (Herrera and Urdaneta-Morales, 1992; Roque et al., 2008). Furthermore, the lineages of T. cruzi discrete typing unit (DTU) TcI are thought to have originated and evolved in opossums, and are consistently isolated from opossums (Yeo et al., 2005). Opossums have been identified as important reservoir hosts in some parts of the southern United States (Herrera and Urdaneta-Morales, 1992; Houk et al., 2010) but the pathological consequence of infection on animal health has not been thoroughly investigated. The Virginia opossum (Didelphis virginiana) has a wide geographic range spanning from Central to North America and is the only marsupial found in the United States (McManus, 1974). Opossums are primarily nocturnal animals that thrive in urban environments and peridomestic environments. They are opportunistic omnivores and insectivores, and could have a high risk of infection if they consume infected triatomines. Opossums and other peridomestic animals can attract triatomines to peridomestic environments thus increasing the risk of transmission to humans and other domestic animals (Ruiz-Pina and Cruz-Reyes, 2002). Being natural hosts of T. cruzi, opossums living in urban areas may pose a public health threat in areas where risk factors are already present (Yeo et al., 2005).
Texas is a hostpot of Chagas transmission, and autochthonous human, domestic animal, and wildlife cases have been reported (Curtis-Robles et al., 2016; Hodo et al., 2019; Nolan et al., 2018). The Rio Grande Valley (RGV) region of south Texas, along the United States-Mexico border, has at least four triatomine species and a higher than expected density of Triatoma gerstaeckeri, a species with >50% infection prevalence (Curtis-Robles et al., 2018a, 2018b). The first documented autochthonous case of Chagas disease in the United States was in 1955 in a child in Corpus Christi, Texas, approximately 275 km north of the United States-Mexico border and the RGV. (Woody and Woody, 1955). Interestingly, the case was speculated to be attributed to a triatomine infected by opossums around the home of the victim, as the authors of the original report stated "Many opossums have been seen in the area at night. Some time before the child's illness the carcass of a dead opossum was seen being fed upon by triatomid bugs. These insects, locally called blood-suckers, have been a source of trouble in the family home for several months, often biting the occupants during sleep” (Woody and Woody, 1955). Despite historical suggestion of opossums contributing to the transmission cycle in the region, the role they play in the ecology of T. cruzi within the RGV is unknown.
The objectives of this study were to assess the epidemiology of T. cruzi in Virginia opossums (Didelphis virginiana) in a transmission hotspot of the southern United States and determine if infection is associated with pathology that may impact animal health. Further, we wished to determine if the anal glad secretions in T. cruzi-infected Virginia opossums harbored parasite DNA, thereby creating the potential for transmission via exposure to these secretions (Roellig et al., 2009).
2. Methods
2.1. Study site
A large animal shelter located in the RGV of South Texas intakes over 30,000 animals a year including an average of 6750 opossums annually (from 2016 to 2018). Animals come to the shelter from multiple animal control agencies. Animal control agencies respond to resident complaints about nuisance opossums and trap or pick up trapped opossums which are then deposited at the animal shelter.
2.2. Opossum necropsy and tissue sampling
We conducted a repeated cross-sectional study of opossums from a South Texas shelter in the winter, spring, and summer of 2017. The opossums (n = 100) used in this study were euthanized for reasons unrelated to our study. City of animal origin (location) and season (winter, spring, summer) of collection were noted. Age was determined by shelter staff through weight approximation (<2.2 kg = juvenile, >2.2 kg = adult); based on age determination by weight (Petrides, 1949), only adults were used in this study. Necropsies were performed 20–60 min post-euthanasia. Heart tissue, blood, and anal gland secretions were collected from all 100 animals; in addition, anal gland tissue and intercostal muscle were collected from 43 animals for which time allowed the extra tissue collection. At the time of necropsy, anal gland secretions were collected by manual compression of the anal glands into a sterile vial and were maintained on ice. Using standard necropsy procedures, the heart, anal glands, and approximately 2–3 anterior ribs with attached muscle were completely excised from the body and placed into separate specimen bags. All cutting tools and forceps used were sterilized between subjects using a 50% Clorox® bleach solution, then rinsed with 70% ethanol, and then followed by flame sterilization using a handheld butane torch (Pro Chef's Torch, BonJour). Approximately 8–12 ml of blood were collected from the thoracic cavity with a syringe and placed into sterile vials with no additives that were maintained on ice. Blood was centrifuged at 5488 RCF for 8 min to recover the blood clot. All samples were saved in −20 °C until processing.
After thawing, an incision was made along the coronary sulcus of the heart to expose the interior of the right ventricle followed by an incision towards the right atrium. An incision was then made from the apex to the left auricle to expose the interior of the left ventricle and atrium. A 1.5–2 cm section of tissue from each chamber was collected with half preserved in 10% neutral-buffered formalin for histopathology and half placed into a nuclease free tube with no additives for prompt molecular analysis. Anal glands and intercostal muscle excised from a rib section were evaluated with separate sections preserved in formalin and prepared for molecular analysis. Extra tissues were saved and stored in −20 °C.
2.3. Detection of parasite DNA in blood clot, anal gland secretions, and tissues
DNA was extracted from the blood clot and tissues using the E.Z.N.A. kit (Omega Bio- Tek, Norcross, GA). Prior to extraction, tissues were macerated and a 30 mg volume was used. For heart samples, all four chambers were prepared and macerated together to represent all parts of the heart. All extraction steps were conducted as per manufacturer instructions with the addition of a longer tissue lysis duration (18–24 h). To detect T. cruzi DNA in samples, the two-step process started with a multiplex Real-Time PCR to amplify a 166-bp segment of the T. cruzi 195-bp repetitive satellite DNA (Duffy et al., 2013). Next, any sample that screened positive (Ct value less than 40 with a sigmoidal amplification curve) was then subjected to discrete typing unit (DTU) determination using a second multiplex Real-Time PCR to amplify the spliced leader intergenic region (SL-IR) (Cura et al., 2015). Finally, any sample that screened positive but was negative on the SL-IR assay was then subjected to a third PCR using the T. cruzi 121/122 primers to amplify a 330bp region of kinetoplast DNA (Curtis-Robles et al., 2016; Virreira et al., 2003; Wincker et al., 1994). The proportion of samples testing positive on each PCR is presented, and samples that had positive results on two independent PCRs were considered to be infected. This requirement of positivity on multiple PCRs that amplify independent genetic regions was used to reduce the chance that positive samples may have resulted from amplicon contamination.
2.4. Histopathology
Histopathology evaluation was performed by a board-certified veterinary pathologist (CLH) on twelve opossums that were PCR-positive on at least one tissue type and on twelve randomly-selected opossums that were negative on all PCR tests. The formalin fixed tissues from these animals were routinely processed for histopathology and stained with hematoxylin and eosin. Inflammation was semiquantitatively scored (Inflammation Score = IS) for each heart chamber, intercostal muscle, and anal glands on a numeric scale as normal (0), minimal (1), mild (2), moderate (3), or severe/marked (4). Additionally, the presence of fibrosis, cardiomyocyte degeneration or necrosis, and the distribution (focal, multifocal, focally extensive) and location (interstitial, myocardial, epicardial) of lesions were recorded. An overall heart inflammation score for each animal was calculated by adding the inflammation scores from the left and right sides of the heart. For analysis, animals were dichotomized by heart pathology status (significant lesions present or absent), in which significant was defined as an overall heart inflammation score (≥3). As previously described, we chose the inflammation cutoff of three because it represented at least mild inflammation in one section and minimal in another, or at least moderate inflammation in any one heart chamber (Hodo et al., 2020).
2.5. Epidemiological and statistical analysis
Molecular prevalence in blood was calculated as the total number of opossums that had PCR-positive blood clot, over the total number of opossums enrolled in the study. Molecular prevalence in tissues and anal gland secretions was calculated as the total number of opossums with a PCR-positive tissue type, over the total number of opossums with that specific tissue type collected. This metric may be useful in estimating the prevalence of animals that have parasite localized in specific tissue; tissue tropism of the parasite could vary by parasite genetic strain (Vera-Cruz et al., 2003). Overall infection prevalence was calculated as the total number of opossums that were PCR-positive on any blood clot, anal gland secretion, or tissue type, over the total number of opossums enrolled in the study.
Statistical analysis was performed in RStudio version 1.1.423. (R Development Core Team, 2008). Bivariate analysis using Fisher's exact test was used to evaluate the relationship between risk factors (sex, season, location) and PCR status (positive on at least one sample vs negative on all samples) and between presence of significant cardiac inflammation and PCR status. Risk factors were further investigated using a generalized linear model if P ≤ 0.25 in the bivariate analysis (Ranganathan et al., 2017), followed by calculating odds ratios and 95% confidence intervals.
2.6. Opportunistic triatomine vector collection and processing
Ongoing education of shelter staff and distribution of educational materials were conducted to raise awareness of triatomine vectors and Chagas disease. Passive vector surveillance was conducted by engaging shelter staff in the safe collection of triatomines on the premises using methods detailed through our Texas A&M University Kissing Bug Citizen Science Program (Curtis-Robles et al., 2018a). Triatomines were identified morphologically to species, sexed, surface-sterilized, and dissected (Curtis-Robles et al., 2015; Lent and Wygodzinsky, 1979). At the time of insect dissection, the presence of host blood in the hindgut was noted and scored from 1 (no blood observed) to 5 (fully engorged bug). DNA was extracted (KingFisher Cell and Tissue DNA kit, Thermo Fisher Scientific, Waltham, MA) from the vector hindguts and tested for infection and DTU of T. cruzi using the molecular methods described above. In order to determine the vertebrate hosts upon which triatomines previously fed, a molecular bloodmeal analysis (BMA) was conducted using PCR amplification of host cytochrome B sequences using ‘herp’ and ‘BM’ primers and Sanger sequencing (Eton Bioscience Inc., San Diego, CA, USA) as previously detailed (Curtis-Robles et al., 2018c). Samples were considered positive for a human blood meal only when yielding human DNA sequences on two independent assays (Curtis-Robles et al., 2018c).
3. Results
3.1. Demographic results
Of the 100 opossums sampled, 48 were female and 52 were male; exclusively adults were sampled. The number of opossums collected by season are as follows: winter (n = 42), spring (n = 27), summer (n = 31). The majority of the opossums originated from McAllen, TX (92%) while the rest originated from Alamo, TX (6%) and Palmview, TX (2%). None of these factors (sex: p-value = 1, season: p-value = 0.44, location: p-value = 1) were associated with T. cruzi infection.
3.2. Molecular results and overall infection prevalence
Of 100 opossums, 15 (15%) had at least one sample that met the criteria for being called positive (positive on two independent PCR tests), including 9 animals that had two or more PCR positive tissues of which five were positive in all samples tested (Table 1). Based on the criterion of being positive on two independent PCR tests, the molecular prevalence in blood was 9%, molecular prevalence in heart tissue was 10%, and molecular prevalence in anal gland secretion was 12%. There were three additional samples that screened positive (CT value less than 40 in the initial PCR) yet were negative on all subsequent assays and were therefore considered negative in the prevalence estimates. These samples comprised two intercostal muscle (from animals with no other positive samples) and one anal gland tissue (from an animal that also had positive heart tissue), and in all cases had relatively high CT values (36.1, 37.9, and 38.7, respectively). If these samples were instead interpreted as positive, then the overall infection prevalence in opossums would be increased from 15% to 17% (17 of 100 animals). The DTU for all PCR positive blood clots, heart tissue samples, and five of 12 anal glad secretions was determined, and in all cases it was TcI. The rest of the anal gland secretions (n = 7) did not amplify on the SL-IR PCR that is used for DTU determination, yet were confirmed positive on the third PCR assay to amplify kinetoplast DNA. Intercostal muscle and anal glands were sampled from 43 opossums. The molecular prevalence was 16.3% (n = 7) in intercostal muscle and 9.3% (n = 4) in anal glands; all positive samples were infected with T. cruzi DTU TcI. All of the samples in which DTU was determined (n = 35) were exclusively TcI.
Table 1.
Molecular and histopathology results (inflammation scores; IS) in Virginia opossums (Didelphis vifginiana) of South Texas that tested PCR-positive on at least one tissue. All PCR-positive tissues were determined to be DTU TcI unless notes in footnote.
| ID | Sex | Blood Clot | Anal Gland Secretion | Heart Tissue |
IS |
Significant Heart Inflammation |
Intercostal Muscle | IS | Anal Gland Tissue | IS | Number of PCR Positive Tissues | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | |||||||||||
| OP03 | F | Negative | Positivea | Negative | b | b | b | N/A | N/A | N/A | N/A | 1/3 |
| OP05 | F | Negative | Positivea | Negative | b | b | b | N/A | N/A | N/A | N/A | 1/3 |
| OP06 | F | Negative | Positivea | Negative | b | b | b | N/A | N/A | N/A | N/A | 2/3 |
| OP07 | M | Positive | Positive | Positive | 3 | 2 | Yes | N/A | N/A | N/A | N/A | 3/3 |
| OP09 | F | Negative | Positive | Negative | 0 | 1 | No | N/A | N/A | N/A | N/A | 1/3 |
| OP17 | F | Positive | Positivea | Positive | 2 | 2 | Yes | N/A | N/A | N/A | N/A | 3/3 |
| OP34 | M | Negative | Positive | Negative | 1 | 0 | No | N/A | N/A | N/A | N/A | 1/3 |
| OP63 | F | Positive | Negative | Positive | 0 | 2 | No | Positive | 0 | Negative | 2 | 3/5 |
| OP69 | F | Positive | Positive | Positive | 2 | 2 | Yes | Positive | 0 | Positive | 3 | 5/5 |
| OP72 | M | Positive | Positivea | Positive | 2 | 3d | Yes | Positive | 0c | Positive | 2 | 5/5 |
| OP81 | M | Positive | Positive | Positive | 2 | 2 | Yes | Positive | 0c | Positive | 1 | 5/5 |
| OP82 | M | Negative | Negative | Positive | 1 | 1 | No | Negative | 0c | Negative | 2 | 1/5 |
| OP84 | M | Positive | Positivea | Positive | 2 | 3 | Yes | Positive | 0 | Negative | 0 | 4/5 |
| OP91 | M | Positive | Negative | Positive | 2 | 2 | Yes | Positive | 2 | Positive | 1 | 4/5 |
| OP94 | M | Positive | Positivea | Positive | 1 | 0 | No | Positive | 1c | Negative | 0 | 4/5 |
N/A = Not available.
No DTU detected, confirmed positive using 121/122 PCR
No histology performed.
Sarcocysts present.
Eosinophils present.
3.3. Histology
Heart inflammation was evaluated via histopathology on twelve opossums that were positive on at least one tissue type (positive group) and twelve randomly-selected opossums that were negative on all PCR tests (negative group). Of the 12 hearts from the positive group, all 12 showed some level of inflammation (Table 1), including eight (66.7%) with bilateral lymphoplasmacytic inflammation and four with unilateral lymphoplasmacytic inflammation. In contrast, of the 12 hearts from the negative group, eight showed some level of inflammation in which bilateral inflammation was present in only a single animal (8.3%) (Table 2). Overall, seven (58.3%) animals from the PCR-positive group had inflammation categorized as significant (overall heart inflammation score ≥ 3). In contrast, only one (8.3%) of the hearts from the negative group had significant inflammation (Table 1). Opossums from the positive group were nearly 14 times more likely to exhibit significant heart lesions when compared to opossums in the negative group (OR = 13.56, CI = 1.23–751.28, p-value = 0.03).
Table 2.
Histopathology results (inflammation scores; IS) in Virginia opossums (Didelphis virginiana) of South Texas that tested PCR negative.
| ID | Sex | Heart IS |
Significant Heart Inflammation |
Intercostal Muscle IS | Anal Gland Tissue IS | |
|---|---|---|---|---|---|---|
| L | R | |||||
| OP01 | M | 0 | 1 | No | N/A | N/A |
| OP11 | F | 0 | 0 | No | N/A | N/A |
| OP23 | M | 0 | 1 | No | N/A | N/A |
| OP38 | M | 0 | 0 | No | N/A | N/A |
| OP58 | M | 0 | 1 | No | 1 | 1 |
| OP60 | F | 3a | 1 | Yes | 1 | 3 |
| OP66 | F | 0 | 1 | No | 0 | 2 |
| OP74 | M | 2 | 0 | No | 0 | 2 |
| OP88 | M | 1 | 0 | No | 0 | 2 |
| OP95 | M | 0 | 2a | No | 1 | 2 |
| OP99 | M | 0 | 0 | No | 0 | 2 |
| OP100 | M | 0 | 0 | No | 1 | 1 |
N/A = Not available.
Eosinophils present.
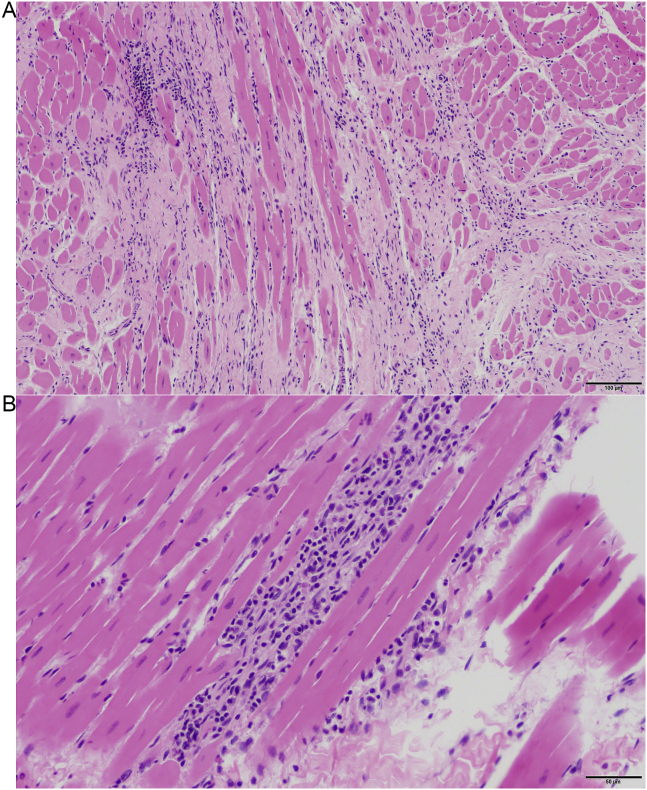
The section of heart from one of the PCR-positive opossum that had moderate inflammation in the left chamber also had myocardial fiber loss with replacement fibrosis (Fig. 1). Few eosinophils were observed in one of the right chambers of a heart from a positive opossum (OP72). The heart from the negative opossum that had left sided moderate inflammation and the heart from the negative opossum that had right sided mild inflammation had numerous eosinophils, which are more commonly associated with other etiologies and not T. cruzi (Barr et al., 1991).
Fig. 1.
Example of histopathology in T. cruzi-infected opossum (Opossum 07) Hematoxylin and eosin stain.: A Left Ventricle 10X: There is marked myocardial fiber loss with replacement fibrosis. B Left Ventricle 20X: Myocardial inflammation is characterized by lymphocytes and plasma cells.
Minimal to mild inflammation was observed in intercostal muscles from two animals in the positive group and four animals in the negative group. Sarcocystis sp. cysts (sarcocysts) were observed in the intercostal muscle of four positive animals, in one case associated with mild inflammation. Minimal to moderate inflammation was observed in 75% of the anal gland tissues from the positive group (Table 1) and all anal gland tissues of the negative group (Table 2).
3.4. Triatomine vector results
A total of 3 triatomines were collected by shelter staff, including one prior to the start of the study and two across the study period, and all were identified as adult female Triatoma gerstaeckeri (Table 3). All three bugs showed engorgement: two were assigned a blood meal score (BMS) of 4 and one was assigned a BMS of 5, indicative of recent meals. Two (66.7%) triatomines were PCR positive for T. cruzi; both were infected with DTU TcI. Bloodmeal analysis of one PCR-positive triatomine showed the presence of domestic canine (Canis lupus familiaris) DNA, and the other PCR-positive bug showed opossum (Didelphis virginiana) DNA. Blood meal analysis in the PCR-negative bug revealed human DNA.
Table 3.
Triatomines found alive in an outdoor kennel of a South Texas animal shelter where opossums were sampled.
| Triatomine ID | Date Captured | Life stage | Species | Sex | BMS | PCR | DTU | BMA |
|---|---|---|---|---|---|---|---|---|
| PS0126 | 6/2013 | Adult | T. gerstaeckeri | F | 4 | + | TcI | opossum |
| PS3031 | 4/2017 | Adult | T. gerstaeckeri | F | 5 | + | Tc1 | canine |
| PS3035 | 5/2017 | Adult | T. gerstaeckeri | F | 4 | – | n/a | human |
BMS = blood meal score; DTU = parasite discrete typing unit; BMA = bloodmeal analysis.
4. Discussion
Opossums play diverse roles in the ecology of zoonotic disease. For example, studies of opossums in the Lyme disease system concluded that opossums serve as ‘ecological traps’ because they groom off and consume attached ticks, thereby reducing the risk of Lyme disease (Keesing et al., 2009). In contrast, opossums in Galveston, TX carry fleas infected with Rickettsia typhi that are thought to contribute to recent cases of murine typhus in humans (Blanton et al., 2016). In this study, we found T. cruzi in 15–17% of opossums across multiple biologic samples from a Chagas-endemic region along the Texas-Mexico border. Given that opossum populations thrive in urban environments, there is potential for a large number of T. cruzi infected opossums to occur in the southern United States in close proximity to human populations. There was no association between infection and location of opossum capture, season of collection, or sex of the opossum. Our study found similar results to a T. cruzi study in Didelphis virginiana of Mexico where there was no association between sex and infection status (Ruiz-Piña and Cruz-Reyes, 2002).
Although PCR positivity in blood does not demonstrate the presence of viable parasites, the 10% of opossums with PCR-positive blood clots indicates a population that may be parasitemic and could potentially be infective to vectors. While the biological significance of the findings of PCR positive anal gland secretions is yet to be determined, parasites in the secretions may be important in transmission. In experimental studies, all laboratory mice inoculated with opossum anal gland secretions containing T. cruzi metacyclic trypomastigoes died (intraperitoneal, oral, and ocular inoculation), allowing the authors to conclude that infections acquired from anal glands are highly virulent (Urdaneta-Morales and Nironi, 1996). Opossums frequently use their anal gland secretions as a territory marker, release secretions when frightened, release secretions to deter predators, and release secretions at defecation (Steindel et al., 1988; Urdaneta-Morales and Nironi, 1996; Francq, 1969; McManus, 1970). One study suggests that opossum feces may become contaminated with infected anal gland secretions (Lenzi et al., 1984), suggesting there could be concern for oral transmission to domestic animals in contact with the parasite-contaminated opossum feces. Dogs are known to roll in and eat feces of other animals, which is a behavior suggested to increase the risk of zoonotic transmission for parasites like Toxoplasma (Frenkel and Parker, 1996) and could similarly play a role in T. cruzi transmission given that opossums and dogs frequently share peridomestic environments.
All infections for which the parasite DTU was ascertained were DTU TcI. Despite DTUs TcI and TcIV being detected in near equal abundance in triatomines (Curtis-Robles et al., 2018a) and dogs of the region (Hodo et al., 2019), TcI has been associated with locally-acquired human disease whereas TcIV has not (Garcia et al., 2017). Our data, and that of others (Hodo et al., 2018; Roellig et al., 2009) support a strong host association between TcI and Virginia opossums.
Through histopathologic examination, we observed significant lymphoplasmacytic myocardial inflammation in 58.3% of the hearts from the positive group and only 8.33% in the hearts from the negative group, indicating that cardiac inflammation is more commonly found in T. cruzi-infected opossums. In skeletal muscle (intercostal muscle), we observed only minimal or mild inflammation in 25% of opossums in the positive group compared to 50% of opossums in the negative group. Different strains of T. cruzi show differential tissue tropism; for example, TcI is commonly found in heart, skeletal muscles, and intestine, and less commonly in the brain (Cruz et al., 2015). In previous studies on Didelphis marsupialis infected with various strains of T. cruzi (Carreira et al., 1996), mild inflammation and few amastigotes were observed in skeletal muscle, indicating variation in T. cruzi strains and coinfection with multiple strains may impact pathology outcomes (Roellig et al., 2009).
Almost all of the anal glands in the positive and negative group showed lymphoplasmacytic inflammation with few eosinophils. The high incidence of inflammation in anal gland tissue could be due to T. cruzi or could be normal or incidental for opossums (Brown, 1972). Lymphoplasmacytic inflammation could be a result of other pathogens like Sarcocystis sp. and Besnoitia darlingi (Barr et al., 1991). T. cruzi is often multifocally distributed in tissues which may affect results if the regions sampled for PCR versus histology differ. Additionally, parasite DNA may not be detectable due to limitations that occur during the DNA extraction process, such as issues at the enzyme digestion step due to excess tissue (James et al., 2002). Although measures were taken to prevent fecal contamination, it possible that there may have been PCR inhibitors (Monteiro et al., 1997) from feces around the anal glands affecting the detection of parasite DNA.
Three triatomines were opportunistically collected in outdoor kennels at the shelter, including two T. cruzi-infected bugs which harbored TcI - the same DTU that infected the opossums at the shelter. The three bugs had bloodmeals from an opossum, dog, and human. At the shelter, dogs and opossums are housed in outdoor environments. Although the bloodmeal analysis procedure cannot inform where the insects fed on these hosts, their high levels of engorgement suggest the bloodmeals were recently acquired and may have been obtained at the shelter. Alternatively, but less likely, insects may have fed in the surrounding environment before dispersing to the shelter. Although vectors were found on site at the shelter, the infected opossums in our study likely acquired infection prior to arrival at shelter because opossums do not remain on site at the shelter for long, and triatomines are widespread in the RGV (Curtis-Robles et al., 2018b).
We show opossums are infected with T. cruzi, sometimes associated with pathology in multiple organs, in the RGV where ongoing human and canine transmission occurs (Curtis-Robles et al., 2017; Nolan et al., 2018). The high prevalence of T. cruzi infection in anal gland secretions of infected animals may signal a potential source of infection for humans and animals. Future research will determine the relative importance of opossums as reservoirs of T. cruzi in the United States and the threat they pose to domestic animals and public health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Bailee Ethridge and Keswick Killets for assistance in the field and with tissue preparation. We thank shelter veterinary medical personnel and staff for assistance. The shelter staff and veterinary medical personnel gained no financial benefits for their participation. The Texas A&M AgriLife /Texas A&M Veterinary Medical Diagnostic Laboratory Seed Grant Program and the Texas A&M AgriLife Insect Vector Grant provided financial support. The Texas A&M CVM Diversity Fellowship (Zecca) and Boehringer Ingelheim Veterinary Scholars Program (Slack) provided student support. Graphical abstract was created with BioRender.com.
References
- Alarcon de Noya A., Noya González O. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop. 2015;151:94–102. doi: 10.1016/j.actatropica.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Barr S., Brown C.C., Dennis V.A., Klei T.R. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. J. Parasitol. 1991;77:624–627. [PubMed] [Google Scholar]
- Blanton L.S., Idowu B.M., Tatsch T.N., Henderson J.M., Bouyer D.H., Walker D.H. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am. J. Trop. Med. Hyg. 2016;95:457–461. doi: 10.4269/ajtmh.16-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. 1972. Morphology of the Anal Sac Glands of the Opossum (Didelphis marsupialis Virginiana) (Doctoral Dissertation) [Google Scholar]
- Cardinal M., Lauricella M., Marcet P., Orozco M., Kitron U., Gürtler R. Impact of community-based vector control on house infestation and Trypanosoma cruzi infection in Triatoma infestans, dogs and cats in the Argentine Chaco. Acta Trop. 2007;103:201–211. doi: 10.1016/j.actatropica.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira J.C.A., Jansen A.M., Deane M.P., Lenzi H.L. Histopathological study of experimental and natural infections by Trypanosoma cruzi in Didelphis marsupialis. Mem. Inst. Oswaldo Cruz. 1996;91:609–618. doi: 10.1590/s0074-02761996000500012. [DOI] [PubMed] [Google Scholar]
- Coura J.R., Junqueira A.C.V. Ecological diversity of Trypanosoma cruzi transmission in the Amazon basin. The main scenarioes in the Brazilian Amazon. Acta Trop. 2015;151:51–57. doi: 10.1016/j.actatropica.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Cruz L., Vivas A., Montilla M., Hernández C., Flórez C., Parra E., Ramírez J.D. Comparative study of the biological properties of Trypanosoma cruzi I genotypes in a murine experimental model. Infect. Genet. Evol. 2015;29:110–117. doi: 10.1016/j.meegid.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Cura C.I., Duffy T., Lucero R.H., Bisio M., Péneau J., Jimenez-Coello M., Calabuig E., Gimenez M.J., Valencia Ayala E., Kjos S.A., Santalla J., Mahaney S.M., Cayo N.M., Nagel C., Barcán L., Málaga Machaca E.S., Acosta Viana K.Y., Brutus L., Ocampo S.B., Aznar C., Cuba Cuba C.A., Gürtler R.E., Ramsey J.M., Ribeiro I., VandeBerg J.L., Yadon Z.E., Osuna A., Schijman A.G. Multiplex real-time PCR assay using TaqMan probes for the identification of Trypanosoma cruzi DTUs in biological and clinical samples. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R., Aukland L.D., Snowden K.F., Hamer G.L., Hamer S.A. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect. Genet. Evol. 2018;58:171–180. doi: 10.1016/j.meegid.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Curtis-Robles R., Hamer S.A., Lane S., Levy M.Z., Hamer G.L. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other Southern States, USA. Am. J. Trop. Med. Hyg. 2018;98:113–121. doi: 10.4269/ajtmh.17-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R., Lewis B.C., Hamer S.A. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. Int. J. Parasitol. Parasites Wildl. 2016;5:117–123. doi: 10.1016/j.ijppaw.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R., Meyers A.C., Auckland L.D., Zecca I.B., Skiles R., Hamer S.A. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop. 2018;188:225–233. doi: 10.1016/j.actatropica.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Curtis-Robles R., Wozniak E.J., Auckland L.D., Hamer G.L., Hamer S.A. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R., Zecca I.B., Roman-Cruz V., Carbajal E.S., Auckland L.D., Flores I., Millard A.V., Hamer S.A. Trypanosoma cruzi (agent of chagas disease) in sympatric human and dog populations in “colonias” of the Lower Rio Grande Valley of Texas. Am. J. Trop. Med. Hyg. 2017;96:805–814. doi: 10.4269/ajtmh.16-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy T., Cura C.I., Ramirez J.C., Abate T., Cayo N.M., Parrado R., Bello Z.D., Velazquez E., Muñoz-Calderon A., Juiz N.A., Basile J., Garcia L., Riarte A., Nasser J.R., Ocampo S.B., Yadon Z.E., Torrico F., de Noya B.A., Ribeiro I., Schijman A.G. Analytical performance of a multiplex Real-Time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francq E.N. Behavioral aspects of feigned death in the opossum Didelphis marsupialis. Am. Midl. Nat. 1969:556–568. [Google Scholar]
- Frenkel J.K., Parker B.B. An apparent role of dogs in the transmission of Toxoplasma gondii. the probable importance of xenosmophilia. Ann. N. Y. Acad. Sci. 1996;791:402–407. doi: 10.1111/j.1749-6632.1996.tb53546.x. [DOI] [PubMed] [Google Scholar]
- Garcia M.N., Burroughs H., Gorchakov R., Gunter S.M., Dumonteil E., Murray K.O., Herrera C.P. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect. Genet. Evol. 2017;49:151–156. doi: 10.1016/j.meegid.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Gurtler R., Cecere M.C., Lauricella M.A., Cardinal M.V., Kitron U., Cohen J.E. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera L., Urdaneta-Morales S. Didelphis marsupialis: a primary reservoir of Trypanosoma cruzi in urban areas of Caracas, Venezuela. Ann. Trop. Med. Parasitol. 1992;86:607–612. doi: 10.1080/00034983.1992.11812716. [DOI] [PubMed] [Google Scholar]
- Hodo C.L., Ba R.M., Edwards E.E., Wozniak E.J., Sarah A. Pathology and discrete typing unit associations of Trypanosoma cruzi infection in coyotes (Canis latrans) and raccoons (Procyon lotor) of Texas, USA. J. Wildl. Dis. 2020;56 doi: 10.7589/2019-03-071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodo C.L., Hamer S.A. Toward an ecological framework for assessing reservoirs of vector-borne pathogens: wildlife reservoirs of Trypanosoma cruzi across the southern United States. ILAR J. 2017;58:379–392. doi: 10.1093/ilar/ilx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodo C.L., Rodriguez J.Y., Curtis-Robles R., Zecca I.B., Snowden K.F., Cummings K.J., Hamer S.A. Repeated cross-sectional study of Trypanosoma cruzi in shelter dogs in Texas, in the context of Dirofilaria immitis and tick-borne pathogen prevalence. J. Vet. Intern. Med. 2019;33:158–166. doi: 10.1111/jvim.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodo C.L., Wilkerson G.K., Birkner E.C., Gray S.B., Hamer S.A. Trypanosoma cruzi transmission among captive nonhuman primates, wildlife, and vectors. EcoHealth. 2018;15:426–436. doi: 10.1007/s10393-018-1318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk A.E., Goodwin D.G., Zajac A.M., Barr S.C., Dubey J.P., Lindsay D.S. Prevalence of antibodies to Trypanosoma cruzi, Toxoplasma gondii, Encephalitozoon cuniculi, Sarcocystis neurona, Besnoitia darlingi, and Neospora caninum in north American opossums, Didelphis virginiana, from southern Louisiana. J. Parasitol. 2010;96:1119–1122. doi: 10.1645/GE-2515.1. [DOI] [PubMed] [Google Scholar]
- James M.J., Yabsley M.J., Pung O.J., Grijalva M.J. Amplification of Trypanosoma cruzi-specific DNA sequences in formalin-fixed raccoon tissues using polymerase chain reaction. J. Parasitol. 2002;88:989–993. doi: 10.1645/0022-3395(2002)088[0989:AOTCSD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jimenez-Coello M., Ortega-Pacheco A., Guzman-Marin E., Guiris-Andrade D.M., Martinez-Figueroa L., Acosta-Viana K.Y. Stray dogs as reservoirs of the zoonotic agents Leptospira interrogans, Trypanosoma cruzi, and Aspergillus spp. in an urban area of Chiapas in Southern Mexico. Vector Borne Zoonotic Dis. 2010;10:135–141. doi: 10.1089/vbz.2008.0170. [DOI] [PubMed] [Google Scholar]
- Keesing F., Brunner J., Duerr S., Killilea M., LoGiudice K., Schmidt K., Vuong H., Ostfeld R.S. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B Biol. Sci. 2009;276:3911–3919. doi: 10.1098/rspb.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent H., Wygodzinsky P.W. Revision of the triatominae (Hemiptera, Reduviidae), and their significance as vectors of chagas' disease. Bull. Am. Mus. Nat. Hist. 1979;163:123–520. [Google Scholar]
- Lenzi H.L., Jansen A.M., Deane M.P. 1984. The Recent Discovery of what Might Be a Primordial Escape Mechanism for Trypanosoma cruzi. [Google Scholar]
- McManus J.J. American Midland Naturalist; 1970. Behavior of Captive Opossums, Didelphis marsupialis Virginiana; pp. 144–169. [Google Scholar]
- McManus J.J. Didelphis virginiana. Mamm. Species. 1974;40:1–6. [Google Scholar]
- Monteiro L., Bonnemaison D., Vekris A., Petry K.G., Bonnet J., Vidal R., Cabrita J., Mégraud F. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 1997;35(4):995–998. doi: 10.1128/jcm.35.4.995-998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.P., Starr M.C., Cantey P.T., Edwards M.S., Meymandi S.K., Cantey P.T., Meymandi S.K., Montgomery S.P. Neglected parasitic infections in the United States: chagas disease. Am. J. Trop. Med. Hyg. 2014;90:814–818. doi: 10.4269/ajtmh.13-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M.S., Aguilar D., Brown E.L., Gunter S.M., Ronca E., Hanis C.L., Murray K.O. Continuing evidence of Chagas disease along the Texas-Mexico border. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides G.A. Sex and age determination in the opossum. J. Mammal. 1949;30(4):364–378. [PubMed] [Google Scholar]
- Pinto C.M., Kalko E.K.V., Cottontail I., Wellinghausen N., Cottontail V.M. TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal Zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infect. Genet. Evol. 2012;12:1328–1332. doi: 10.1016/j.meegid.2012.04.013. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . 2008. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ranganathan P., Pramesh C.S., Aggarwal R. Common pitfalls in statistical analysis: logistic regression. Perspect. Clin. Res. 2017;8(3):148. doi: 10.4103/picr.PICR_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roellig D.M., Ellis A.E., Yabsley M.J. Genetically different isolates of Trypanosoma cruzi elicit different infection dynamics in raccoons (Procyon lotor) and Virginia opossums (Didelphis virginiana) Int. J. Parasitol. 2009;39:1603–1610. doi: 10.1016/j.ijpara.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A.L.R., Xavier S.C.C., Da Rocha M.G., Duarte A.C.M., D'Andrea P.S., Jansen A.M. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008;79:742–749. [PubMed] [Google Scholar]
- Ruiz-Pina H.A., Cruz-Reyes A. The opossum Didelphis virginiana as a synanthropic reservoir of Trypanosoma cruzi in Dzidzilche, Yucatan, Mexico. Mem. Inst. Oswaldo Cruz. 2002;97:613–620. doi: 10.1590/s0074-02762002000500003. [DOI] [PubMed] [Google Scholar]
- Santos F.M., Barreto W.T.G., de Macedo G.C., da Silva Barros J.H., das Chagas Xavier S.C., Garcia C.M., Mourão G., de Oliveira J., Rimoldi A.R., de Oliveira Porfírio G.E., de Andrade G.B. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large neotropical wetland. Acta Trop. 2019;199:105098. doi: 10.1016/j.actatropica.2019.105098. [DOI] [PubMed] [Google Scholar]
- Steindel M., Scholz A.F., Toma H.K., Schlemper B.R. Presence of Trypanosoma cruzi in the anal glands of naturally infected opossum (Didelphis marsupialis) in the state of Santa Catarina, Brazil. Mem. Inst. Oswaldo Cruz. 1988;83:135–137. doi: 10.1590/s0074-02761988000100017. [DOI] [PubMed] [Google Scholar]
- Urdaneta-Morales S., Nironi I. Trypanosoma cruzi in the anal glands of urban opossums. I-isolation and experimental infections. Mem. Inst. Oswaldo Cruz. 1996;91:399–403. doi: 10.1590/s0074-02761996000400002. [DOI] [PubMed] [Google Scholar]
- Vera-Cruz J.M., Magallon-Gastelum E., Grijalva G., Rincon A.R., Ramos-Garcia C., Armendariz-Borunda J. Molecular diagnosis of Chagas' disease and use of an animal model to study parasite tropism. Parasitol. Res. 2003;89:480–486. doi: 10.1007/s00436-002-0787-0. [DOI] [PubMed] [Google Scholar]
- Virreira M., Torrico F., Truyens C., Alonso-Vega C., Solano M., Carlier Y., Svoboda M. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 2003;68:574–582. doi: 10.4269/ajtmh.2003.68.574. [DOI] [PubMed] [Google Scholar]
- Wincker P., Britto C., Pereira J.B., Cardoso M.A., Oelemann W., Morel C.M. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg. 1994;51:771–777. doi: 10.4269/ajtmh.1994.51.771. [DOI] [PubMed] [Google Scholar]
- Woody N.C., Woody H.B. American trypanosomiasis (chagas' disease): first indigenous case in the United States. J. Am. Med. Assoc. 1955;159:676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]
- Yeo M., Acosta N., Llewellyn M., Sánchez H., Adamson S., Miles G. a J., López E., González N., Patterson J.S., Gaunt M.W., de Arias A.R., Miles M. a. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int. J. Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Zingales B., Andrade S., Briones M., Campbell D., Chiari E., Fernandes O., Guhl F., Lages-Silva E., Macedo A., Machado C., Miles M., Romanha A., Sturm N., Tibayrenc M., Schijman A. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M.G.G., Schijman A.G., Llewellyn M.S., Lages-Silva E., Machado C.R., Andrade S.G., Sturm N.R. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]