Abstract
Here we present a case of a 41-year old immunocompetent female from central Maryland, who presented with new onset seizures. Magnetic resonance imaging of brain revealed a solitary ring-enhancing lesion. Stereotactic brain biopsy confirmed Blastomyces dermatitidis brain abscess. Patient's clinical course was complicated by voriconazole-induced pancytopenia that prompted surgical resection and amphothericin-induced severe hypokalemia necessitating change to high dose fluconazole. Four months after surgical resection, patient remains in radiographic and clinical remission.
Keywords: Blastomyces dermatitidis, Fungal brain abscess, Dimorphic fungi, Endemic mycoses, Drug induced, Pancytopenia, Drug induced hypokalemia
1. Introduction
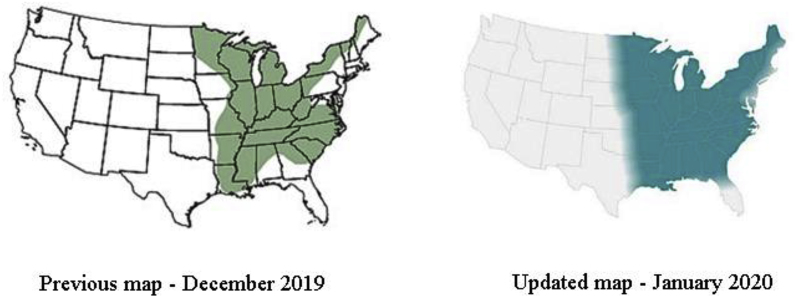
Blastomyces dermatitidis is a dimorphic fungus presenting as a broad-based budding yeast at 37 °C that converts to hyphae at 22–25 °C. It is endemic to North America along the Great Lakes and Ohio, Mississippi, and Saint Lawrence River valley. Blastomyces spp. Can infect both humans and animals. The fungus inhabits moist soils, decaying organic material or rotting woods [1]. Established risk factors include living in endemic areas and immunosuppression. Fig. 1 shows previous and recently updated endemic range maps for blastomycosis provided by CDC [2]. Diabetes, cardiovascular diseases, previous history of pneumonia and collagen vascular disease are weakly associated risk factors [3].
Fig. 1.
Adaptive CDC maps showing previous and updated endemic ranges for blastomycosis in the U.S.
Primary transmission occurs through inhalation of fungal conidia. In rare circumstances it can be also acquired via direct cutaneous inoculation. The incubation period is usually between 3 and 14 weeks. Clinical manifestations of blastomycosis vary from asymptomatic infection to life-threatening pneumonia that can be complicated by acute respiratory distress syndrome. Since inhalation is the primary route of acquisition, more than 79% of patients present with pulmonary involvement [1]. Rarely does blastomycosis disseminate to other sites such as musculoskeletal system, genitourinary tract and central nervous system (CNS) [1]. Blastomycosis is a potentially fatal infection with mortality ranging from 4% to 22% worldwide and is responsible for 0.21 deaths in the United States per million person-years [4]. Diagnosis is made based on clinical presentation, serology, cultures, histopathology, and antigen detection [5]. In mild to moderate severity cases, patients can be successfully treated with oral antifungal therapies. These therapies include itraconazole, fluconazole or voriconazole. In severe cases liposomal amphothericin B is recommended. Posaconasole is also a theoretical therapeutic option although limited data has been published for its efficacy [6]. Here we report a case of a solitary Blastomyces dermatitidis brain abscess in an immunocompetent patient without any epidemiological risk factors and who also developed voriconazole induced pancytopenia.
2. Case
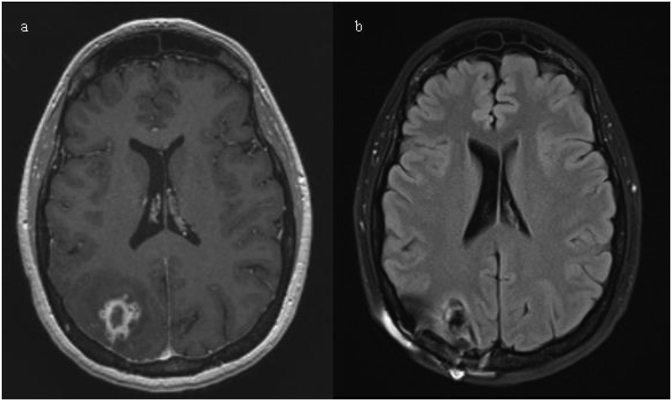
A 41 year-old female, with a past medical history of Hashimoto thyroiditis and recurrent chronic sinusitis presented to local community emergency room with new onset tonic-clonic seizures (day 0). She had been in her usual state of health preceding the seizures. Physical exam was significant for tachycardia and mild decreased strength in all four extremities equally. Complete blood count (CBC) and complete metabolic panel (CMP) were unremarkable. Computer tomography (CT) of the head without contrast revealed vasogenic edema in the right parietal and occipital lobes. Subsequent, magnetic resonance imaging (MRI) of the brain, showed a 1.7× 1.3 × 1.5cm rim-enhancing mass in the right hemisphere at the junction of the parietal and occipital lobes with significant vasogenic edema (Fig. 2). Initial concern was for neoplastic process and the patient underwent CT chest, abdomen and pelvis with contrast which were all unremarkable. Lumbar puncture was preformed and cerebrospinal fluid (CSF) analysis was unremarkable. Both blood and CSF cultures were negative after 5 days. Transthoracic echocardiogram showed no abnormalities. Given concern for primary CNS neoplastic process, the patient was discharged on levetiracetam and dexamethasone and scheduled to undergo a stereotactic brain biopsy at the University of Maryland Medical Center. 5 weeks later, while still on dexamethasone, seizures recurred that resulted in the admission to the University of Maryland Medical Center (day 35).
Fig. 2.
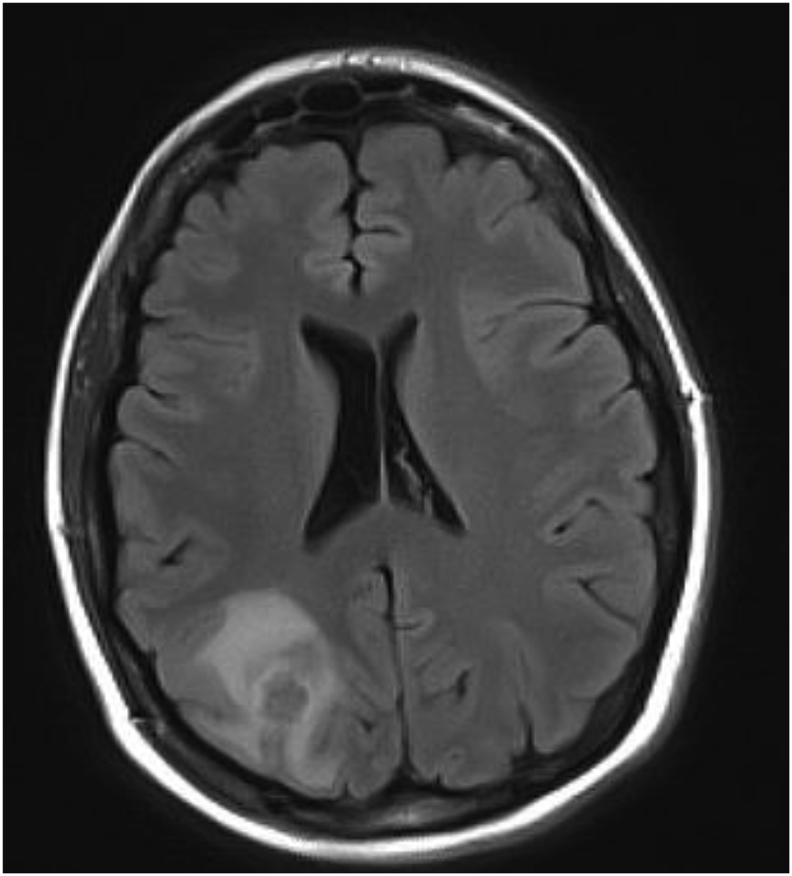
MRI of the brain with contrast from day 0, showing a 1.7 × 1.3 × 1.5cm rim-enhancing mass in the right hemisphere at the junction of the parietal and occipital lobes. Surrounded by vasogenic edema.
Admitting medications included levothyroxine, levetiracetam, dexamethasone and cetirizine. Epidemiological interview revealed that she spent her whole life in central Maryland and denied any hiking, camping, gardening, fishing or swimming in lakes or rivers. She had 2 cats and worked in an office setting. No significant travel history except to New Jersey. She admitted to using Neti Pots, but last use was 7 months prior to the initial presentation (prior to day 0). She denied any smoking, alcohol or illicit drugs. Family history was significant for melanoma and neuroblastoma.
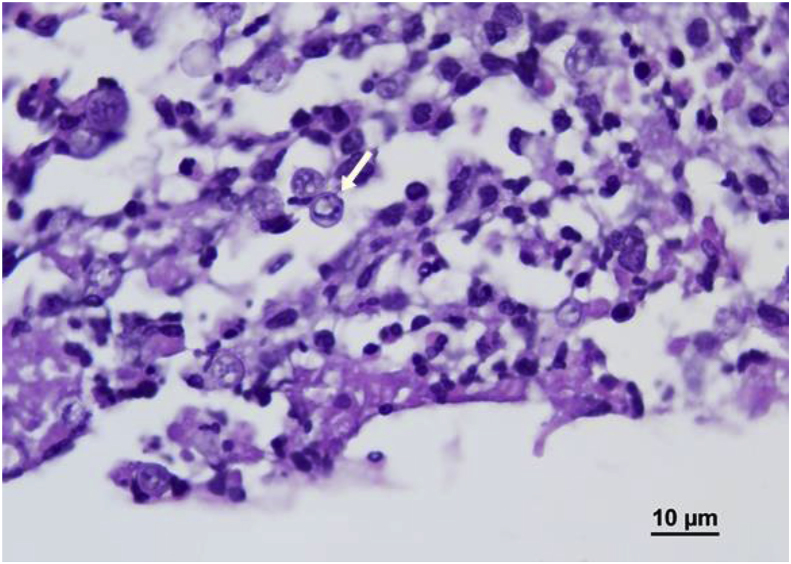
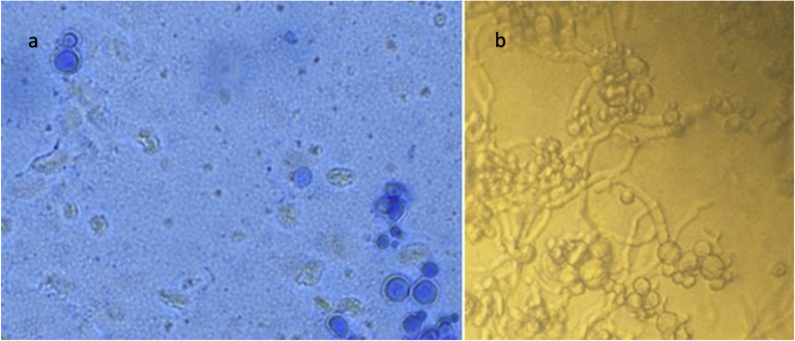
Physical exam was unremarkable as were CBC and CMP. MRI of the brain showed a complex lesion with peripheral enhancement and central necrosis in the right parietal lobe. Extending from this central lesion were smaller peripheral enhancing lesions which when included made the heterogenic mass 2.3 × 2x1.8 cm in size (Fig. 3a). Stereotactic brain biopsy was conducted on the day of admission (day 35). Preliminary pathology report was concerning for free-living amoebic infection (Fig. 4). Coverage for presumptive granulomatous amoebic encephalitis (GAE) was started with miltefosine, fluconazole, flucytosine, thorazine, azithromycin and trimetoprim/sulfametaxazole. Pathology images were sent to the Center for Disease Control and Prevention's (CDC) Division of Parasitic Diseases who were unable to identify a pathogen (day 38). As a result, fluconazole was changed to oral voriconazole 300 mg q 12 hours to broaden fungal coverage while still covering for GAE. Urine Histoplasma antigen, Blastomyces serum antibodies, Cryptococcus serum antigen, 1, 3-Beta-D-glucan, HIV and toxoplasmosis serologies were all negative. A week later (day 42), a broad-based budding yeast was visualized on Lactophenol cotton blue stain from a single colony growing on Sheep Blood Agar (Fig. 5). This was subsequently identified as Blastomyces dermatitidis by mass spectrometry and next generation sequencing. Potato dextrose agar confirmed dimorphic character of the fungus (Fig. 5). Repeat MRI showed decrease in size of the abscess (day 43). Given radiographic improvement, while on voriconazole therapy, the patient was discharged on oral voriconazole 300 mg twice daily (day 44). Voriconazole level of 3.5 mcg/mL (reference range: 1.0–6.0 mcg/mL) proved appropriate dosing. Unfortunately, after 3 weeks of oral voriconazole therapy she developed pancytopenia, including moderate neutropenia that resulted in progression of her brain abscess seen on MRI (day 62). This prompted readmission and surgical resection of the abscess on day 65. After resection the patient was started on intravenous liposomal amphotericin B that was continued upon discharge 4 days later (day 69). Unfortunately she developed severe electrolyte imbalances with persistent hypokalemia. Even despite aggressive oral potassium replacement therapy with 120 meq a day, her potassium level remained at 2.7 mEq. Given home administration, it was deemed unsafe and amphotericin was changed to high dose oral fluconazole 800mg daily just after 1 week of therapy (day 72). Patient was weaned off dexamethasone 1 week later (day 79). MRI of the brain 1 month and 2 months (Fig. 3b) after surgical resection showed no recurrence (day 93 and 125 respectively). The patient is back to her normal state of health with no recurrence of seizures and no permanent neurological deficits. Work up for underlying immunodeficiency (CD4, CD8, complement, IgA, IgG including subclasses, IgM, IgE, myeloperoxidase level, neutrophil function assay and leukocyte adhesion deficiency panel) did not reveal any abnormalities. She is scheduled to continue high dose fluconazole therapy for 12 months from time of surgical resection with serial clinical monitoring.
Fig 3.
3a) MRI of the brain with contrast on day 35 showing a complex lesion with peripheral enhancement and central necrosis in the right parietal lobe. Together with surrounding sister lesions measuring 2.3 × 2x1.8 cm in size. 3b) Follow up MRI 2 months after resection with almost complete resolution of vasogenic edema and post-surgical changes in right parietal surgical bed.
Fig. 4.
Hematoxylin and Eosin stain of brain biopsy at right parietal lobe lesion,
Fig. 5.
a) Lactophenol cotton blue stain from colony in the yeast form (on the left). b) Potato dextrose agar showing conversion of yeast to mold form at room temperature (on the right).
3. Discussion
In our case, the initial histology suggested a free-living amoebic infection (Fig. 4). Naegleria fowleri was unlikely given that primary amoebic meningoencephalitis usually results in death within 2 weeks. Balamuthia and Acanthamoeba cause GAE, which has a more indolent course. Due to high mortality associated with GAE, we initiated an empiric GAE therapeutic regimen. Unfortunately, no standard regimen exists and miltefosine, fluconazole, flucytosine, thorazine, azithromycin and trimetoprim/sulfametaxazole were chosen based on adequate blood-brain barrier penetration and historical in vitro sensitivities [[7], [8], [9]]. Potential other pathogens: Toxoplasma, Nocardia, Mycobacterium, Rhodococcus, Taeniasolium, Cryptococcus, Candida, Aspergillus, Mucor or dimorphic fungi were unlikely given immunocompetent status, lack of exposure, benign CSF and negative serologies.
The final diagnosis of Blastomyces dermatiditis was unexpected. The patient had spent her whole life in central Maryland and did not participate in any outdoor activities that could have exposed her to blastomycosis. The CDC recently updated maps displaying endemic ranges of blastomycosis. Fig. 1 shows the previous and updated maps. This case reinforces the new endemic blastomycosis ranges. A multitude of factors likely have led to blastomycosis having a much wider endemic range than was previously thought [2,10]. Factors, such as climate change, continue to change our environment and therefore endemic ranges of blastomycosis and other endemic mycoses will likely continue to evolve.
It remains unclear how our patient acquired her blastomycosis infection and why she presented with only a solitary CNS abscess. She never had any skin lesions or pneumonia and CT of the chest, abdomen and pelvis were unremarkable. Inhalation of spores commonly causes infections in lungs, skin, bones and genitourinary system. Only 5–10% of cases involve CNS and usually hosts are severely immunocompromised [11]. In one study, 74% of patients infected with this dimorphic fungus had some immunodeficiency [12]. In addition, typically up to 80% of patients have positive blastomycosis serology testing and most have positive Histoplasma urine antigen, given the cross-reactivity of this test with Blastomyces [5,13]. Our patient had no immunodeficiency history and her blastomycosis serologies and urine antigen testing were negative. Therefore the diagnosis of blastomycosis was quite arduous. Testing for 1,3-beta-D-glucan is expected to be negative for Blastomyces as it was in our patient [1,5]. 1, 3-beta-D-glucan is a cell wall polysaccharide in most fungi. Three clinically relevant fungi that usually have negative tests include: cryptococci, the zygomycetes, and Blastomyces dermatitidis. These 3 fungi either lack 1,3-beta-D-glucan in their cell walls or produce it at very minimal levels. With respect to Blastomyces, the yeast form produces little if any 1,3-beta-D-glucan [14].
Once the diagnosis of blastomycosis was made, our patient had already been on azole therapy for 7 days given this was part of our GAE treatment. Repeat MRI showed regression of brain abscess that encouraged us to continue voriconazole therapy. The IDSA guidelines for treatment of CNS blastomycosis recommend an induction phase with parenteral liposomal amphotericin B for 4–6weeks followed by 12 months with one of the azoles which include fluconazole, itraconazole or voriconazole [6]. However case reports of cerebral blastomycosis have shown successful treatment with monotherapy with either voriconazole or fluconazole without surgical intervention [15,16]. Given improvement on MRI, our patient elected to be treated with oral voriconazole therapy instead of amphotericin due to better toxicity profile and close clinical monitoring as outpatient. It should be noted that voriconazole has theoretical better CNS penetration compared to amphotericin while also having equal in vitro activity against Blastomyces [9,17]. Unfortunately, after 3 weeks on voriconazole, she developed drug-induced pancytopenia. Her WBC decreased from 11.2 to 2.7 K/mcl with moderate neutropenia of 0.86 Kmcl. Voriconazole induced pancytopenia and more specifically neutropenia is an uncommon adverse event [18]. Of note her voriconazole level was therapeutic at 3.5 mcg/mL (reference range: 1.0–6.0 mcg/mL). After discontinuation of voriconazole her pancytopenia and moderate neutropenia resolved. Coinciding with her neutropenia, a repeat MRI showed progression of the brain abscess that prompted surgical resection. While this cannot be proven it is believed that her abscess expansion was related to her neutropenia and not from failure of voriconazole. Surgical resection was successful and the entire abscess was removed en bloc without any neurological deficits.
Unfortunately, her amphotercin course was also complicated by severe hypokalemia refractory to aggressive oral supplementation. Amphotericin reduces potassium levels by directly damaging renal tubules. The incidence of hypokalemia with amphotericin use ranges from 36% to 54% in several studies [19]. Several techniques have been implemented to prevent hypokalemia. These range from early aggressive supplementation to use of potassium sparing diuretics. It is well established that potassium supplementation is needed prior to development of significant hypokalemia [19]. While our patient was aggressively supplemented prior to development of hypokalemia, potassium sparing diuretics may have allowed for a prolonged course of amphotericin in our patient. Once changing to high dose fluconazole, hypokalemia normalized within 2 days.
It is highly unusual for a healthy middle aged female to have a solitary fungal brain abscess. Therefore our patient underwent evaluation for a potential underlying immunodeficiency, but testing was unremarkable. However it should be noted that detecting underlying deficiencies can have important clinical ramifications. For instance, depending on the deficiency, certain therapies may need to be prolonged or in some instances continued indefinitely at lower doses to prevent relapse. Therefore testing for an underlying immunodeficiency should be considered when patients either have unusual presentations of rare infections or with recurrent infections.
In conclusion, this is a rare case of an immunocompetent female without any risk factors who developed a blastomycosis brain abscess followed by an uncommon adverse reaction to voriconazole. Also unique to this case were negative blastomycosis serologies and negative histoplasma urine antigen testing. This case reinforces the recent changes made to endemic blastomycosis ranges provided by the CDC. It remains to be proven what the most advantageous treatment of blastomycosis brain abscesses is. Unfortunately, given the rarity of the condition, prospective studies will need immense longitudinal accrual periods. Therefore likely limiting decisions on best treatments for this rare condition to retrospective studies and case series.
Declaration of competing interest
None.
Acknowledgements
Grateful for the assistance provided by the University of Maryland microbiology laboratory director's Dr. Jennifer Johnson and Dr. Paul Luethy.
References
- 1.McBride J.A., Gauthier G.M., Klein B.S. Clinical manifestations and treatment of Blastomycosis. Clin. Chest Med. 2017;38:435–449. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC, Center for Disease Control and Prevention CDC 24/7: saving lives, protecting people. Sources of Blastomycosis. https://www.cdc.gov/fungal/diseases/blastomycosis/causes.html
- 3.Choptiany M., Wiebe L., Limerick B. Risk factors for acquisition of endemic blastomycosis. Can. J. Infect Dis. Med. Microbiol. 2009;20:117–121. doi: 10.1155/2009/824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuu D., Shafir S., Bristow B., Sorvillo F. Blastomycosis mortality rates, United States, 1990-2010. Emerg. Infect. Dis. 2014;20(11):1789–1794. doi: 10.3201/eid2011.131175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saccente M., Woods G.L. Clinical and laboratory update on blastomycosis. Clin. Microbiol. Rev. 2010;23(2):367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman S.W., Dismukes W.E., Proia L.A. Clinical practice guidelines for the management of Blastomycosis: 2008 update by the infectious diseases society of America. Clin. Infect. Dis. 2008;46(12):1801–1812. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 7.Parija S.C., Dinoop K., Venugopal H. Management of granulomatous amebic encephalitis: laboratory diagnosis and treatment. Tropenmed. Parasitol. 2015;5(1):23–28. doi: 10.4103/2229-5070.149889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva R.A.E., Araújo S.D.A., Avellar I.F.D.F.E., Pittella J.E.H., Oliveira J.T.D., Christo P.P. Granulomatous amoebic meningoencephalitis in an immunocompetent patient. Arch. Neurol. 2010;67(12):1516–1520. doi: 10.1001/archneurol.2010.309. [DOI] [PubMed] [Google Scholar]
- 9.Nau R., Sörgel F., Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seitz A.E., Younes N., Steiner C.A., Prevots D.R. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bariola J.R., Perry P., Pappas P.G. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin. Infect. Dis. 2010;50(6):797–804. doi: 10.1086/650579. [DOI] [PubMed] [Google Scholar]
- 12.Rupcich Ch, Reddy V., Gattuso P. 146 Blastomycosis in immunocompromised patients: a clinicopathologic review. Am. J. Clin. Pathol. 2018;149(1):S62. [Google Scholar]
- 13.Wheat J., Wheat H., Connolly P. Cross-reactivity in histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 1997;24(6):1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 14.Pickering J.W., Sant H.W., Bowles C.A., Roberts W.L., Woods G.L. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005;43(12):5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakleh M., Aksamit A.J., Tleyjeh I.M., Marshall W.F. Successful treatment of cerebral Blastomycosis with voriconazole. Clin. Infect. Dis. 2005;40(9):e69–e71. doi: 10.1086/429319. [DOI] [PubMed] [Google Scholar]
- 16.Brick K.E., Agger W.A. Successful treatment of brainstem blastomycosis with fluconazole. Clin. Med. Res. 2012;10(2):72–74. doi: 10.3121/cmr.2011.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R.K., Ciblak M.A., Nordoff N., Pasarell L., Warnock D.W., McGinnis M.R. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 2000;44(6):1734–1736. doi: 10.1128/aac.44.6.1734-1736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiden C., Peyrière H., Cociglio M. Adverse effects of voriconazole: analysis of the French pharmacovigilance database. Ann. Pharmacother. 2007;41(5):755–763. doi: 10.1345/aph.1H671. [DOI] [PubMed] [Google Scholar]
- 19.Usami E., Kimura M., Kanamatsu T. Evaluation of hypokalemia and potassium supplementation during administration of liposomal-amphotericin B. Exp. Therm. Med. 2014;7(4):941–946. doi: 10.3892/etm.2014.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]