Abstract
Pathogenic Leptospira species are the causative agents of leptospirosis, a world-spreading zoonotic infectious disease. The pathogens possess a powerful invasiveness by invading human body through mucosal/skin barriers, rapid entry into bloodstream to cause septicemia, diffusion from bloodstream into internal organs and tissues to cause aggravation of disease, and discharge from urine through renal tubules to form natural infectious sources. Leptospirosis patients present severe inflammatory symptoms such as high fever, myalgia and lymphadenectasis. Hemorrhage and jaundice are the pathological features of this disease. Previous studies revealed that some outer membrane proteins of Leptospira interrogans, the most important pathogenic Leptospira species, acted as adherence factors to binding to receptor molecules (fibronectin, laminin and collagens) in extracellular matrix of host cells. Collagenase, metallopeptidases and endoflagellum contributed to the invasiveness of L. interrogans. Except for lipopolysaccharide, multiple hemolysins of L. interrogans displayed a powerful ability to induce pro-inflammatory cytokines and hepatocyte apoptosis. vWA and platelet activating factor acetylhydrolase-like proteins from L. interrogans could induce severe pulmonary hemorrhage in mice. L. interrogans utilized cellular endocytic recycling and vesicular transport systems for intracellular migration and transcellular transport. All the research achievements are helpful for further understanding the virulence of pathogenic Leptospira species and pathogenesis of leptospirosis.
Keywords: Leptospira, Virulence, Pathogenic mechanism, Leptospirosis, Pathogenesis
Leptospirosis caused by pathogenic Leptospira species is a zoonotic infectious disease of global importance [1]. Every year, there are approximate one million new patients and ten-thousand fatal cases of leptospitrosis in the world [2,3]. This disease is endemic in Asia, Oceania and South America [[4], [5], [6]], but in recent years it is frequently reported in Europe, North America and Africa [[7], [8], [9], [10]]. Therefore, leptospirosis has been considered as an emerging or re-emerging infectious disease in many areas of the world [11].
Leptospira is classified as pathogenic and saprophytic species [12]. Furthermore, according to the diversity of molecular genetics and pathogenic ability, all leptospiral strains are divided into pathogenic, intermediate and saprophytic types [[13], [14], [15], [16], [17]]. The pathogenic type contains Leptospira alexanderi, Leptospira alstonii, Leptospira borgpetersenii, Leptospira interrogans, Leptospira kirschneri, Leptospira kmetyi, L. mayottensis, L. noguchii, Leptospira santarosai and Leptospira weilii genospecies, in which L. interrogans is the most prevalent genospecies in the world but the pathogenicity of L. kmetyi has been disputed. The intermediate type contains Leptospira broomii, Leptospira fainei, Leptospira inadai, Leptospira licerasiae and Leptospira wolffii genospecies that occasionally cause disease in human and animals. The saprophytic type contains Leptospira biflexa, L. meyeri, Leptospira vanthielii, L. wolbachii and Leptospira yanagawae genospecies that are living in natural water and never cause disease.
Approximate 200 animals including livestock and dogs have been confirmed as the hosts of pathogenic Leptospira species [12]. The infected animals present mild or no symptoms but can persistently discharge leptospires from urine to contaminate environments. Animal kidneys are the preferential organs in residence as a reservoir of pathogenic Leptospira species [18]. Human individuals are infected by contact with the Leptospira-contaminated natural water or wet soil [19]. However, nearly all of the infected individuals suffer from serious leptospirosis [19,20]. Pathogenic Leptospira species are able to rapidly invade into human body through mucosal and skin barriers and fast enter bloodstream to cause a septicemia and all the patients present severe inflammatory symptoms such as high fever, myalgia and superficial lymphadenectasis [20,21]. In many cases, the pathogens are diffused from bloodstream into lungs, liver, kidneys and cerebrospinal fluid to cause lethal pulmonary diffuse hemorrhage, severe jaundice-induced renal failure and meningoencephalitis [[19], [20], [21]]. In the course of leptospirosis, jaundice and hemorrhage are served as the most important clinical features [12,20,22]. In addition, partial leptospirosis patients also present a short period of leptospiral discharge from urine at convalescence stage and many pathogen-unknown patients with chronic kidney diseases were found due to infection of pathogenic Leptospira species [23,24]. Recently, leptospirosis has been considered as a systemic inflammatory response syndrome (SIRS) due to the storm of cytokines in the patients [[25], [26], [27]]. However, until now, the molecular basis of pathogenic Leptospira remains limitedly understood [28].
Leptospira has a cell wall similar to that of Gram-negative bacteria. However, many previous studies revealed that lipopolysaccharide (LPS) of Leptospira has a lower endotoxic activity than that of enteric bacilli such as Escherichia coli LPS [12,[29], [30], [31]]. Except of expression of many hemolysins, no any typical exotoxin-encoding genes can be found in genomes of pathogenic Leptospira species [[32], [33], [34]]. It is well known that pathogenic ability of prokaryotic microbes is dependent on invasiveness and toxins that decide the infected state formation and tissue injury. In this review, we summarize the recent achievements in the virulence factors and their effective mechanisms of pathogenic Leptospira species.
Adherence factors
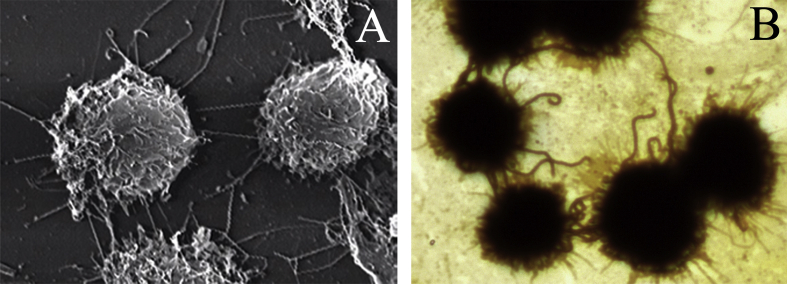
Adherence is a process of pathogenic microbes attaching to surface of host cells by binding of microbial ligands to cellular receptors and it is considered as the first step for further colonization or invasion into hosts of microbial pathogens during infection. Adherence factors are the surface molecules or components of microbes such as teichoic acids of Gram-positive bacteria and pili of Gram-negative bacteria. In previous view, the cellular receptors binding to microbial adherence factors should be located in surface of host cells. However, many recent studies revealed that the molecules in extracellular matrix (ECM) of host cells, such as fibronectin (FN), laminin (LN) and different types of collagens (COLs), act as the major receptors of adherence factors from many bacteria including spirochetes [[35], [36], [37]]. L. interrogans was able to adhere to the surface of mouse macrophages and monkey renal fibroblasts [38]. We found that L. interrogans can adhere to the surface of cells with one or two terminals of leptospiral body (Fig. 1). However, the adherence mechanism of L. interrogans has not been revealed.
Fig. 1.
Adherence of L. interrogans to mouse J774A.1 macrophages, observed by scanning electron microscopy (A) and ordinary microscopy after silver-staining (B).
Leptospira has a Gram-negative cell wall with inner and outer membranes but never been found to produce pilus. Therefore, some outer membrane proteins (OMPs) of pathogenic Leptospira species have been considered as the potential adherence factors. Endostatin-like OMPs of L. interrogans were the first identified adherence factors binding to the FN and LN in ECM of host cells [39]. Subsequently, a series of L. interrogans OMPs and outer membrane lipoproteins (OMLPs), such as Lsa21/32/63 and LipL32/53, were reported to be involved in the leptospiral adherence to cellular ECM and binding to FN, LN and/or COL4 [[40], [41], [42], [43], [44]]. In particular, LigA and LigB of L. interrogans, the two members of bacterial immunoglobulin superfamily, were also confirmed to bind to ECM proteins in attachment of host cells [45]. Interestingly, the OMPs and OMLPs of Leptospira santorosai have been shown to increase the accumulation of ECM through TGF-β/Smad signaling pathway and the expression of FN through Toll-like receptor 2 (TLR2) pathway [46,47].
However, these ECM protein-binding proteins of pathogenic Leptospira species need a further determination about their function as adherence factors using more reliable and accurate methods such as surface plasmon resonance (BiaCore) and atomic force microscopy because nearly all of the studies only ELISA was used to detect the combination of the leptospiral OMPs and OMLPs with cellular ECM proteins. Moreover, the ECM proteins expressed by different host cells have a large diversity. For example, FN is the unique ECM protein expressed by human macrophages while human vascular endothelial cells express FN, LN and COL3/4 [48].
Invasive enzymes
Except of adherence factors, invasive enzymes also play important roles in invasiveness of bacteria during infection. Pathogenic Leptospira species s possess a powerful invasive ability, for example, rapid invading into human body and bloodstream, diffusion from bloodstream into internal organs and tissues, and discharge from urine.
Collagenase has been confirmed to play an important role in invasiveness of many pathogenic bacteria during infection. A recent study demonstrated that L. interrogans serovar Lai strain Lai produce a collagenase named as ColA that hydrolyzed COL1/3/4 with a high hydrolytic ability in vitro [49]. When the spirochete was co-incubated with human umbilical vein endothelial cells (HUVEC) and renal epithelial cells (HEK293), the expression and secretion of ColA collagenase were significantly increased. Compared to the wild-type strain, the colA gene-knockout mutant presented a remarkably attenuated transcytosis through the monolayers of HUVEC and HEK293 cells as well as a notably decreased leptospire-loading in tissues and discharge from urine in hamsters.
Metalloprotease/metallopeptidase (MP) from eukaryotes and prokaryotes is a large group of Zn2+-dependent protein/peptide hydrolases that are classified into at least 60 types. The extracellular MP has been reported to contribute to the invasiveness of bacterial pathogens [50]. In the genome of L. interrogans, there are numerous MP-encoding genes [33]. We found that L. interrogans serovar Lai strain Lai expressed three M16-type MPs that could hydrolyze FN, LN and COL1/3/4. The deletion of the M16-MPs-encoding genes caused a significant decrease of the leptospiral loading in lungs, liver and kidneys as well as a significantly attenuated virulence in hamsters. Besides, a mammalian cell entry (Mce) protein from L. interrogans was reported to mediate the leptospiral internalization into mouse macrophages by its RGD motif binding to α5β1/αVβ3 integrin pathway and the endoflagellum of L. interrogans was also demonstrated to contribute to in leptospiral invasiveness [51,52].
Toxins
Endotoxin and exotoxin are the two major types of toxins produced by bacterial pathogens. Pathogenic Leptospira species have no any typical exotoxin-encoding genes but possess a complete set of LPS (i.e. endotoxin) synthesis genes in their genomes [33,34]. Phagocytosis plays a crucial role in innate and adaptive anti-infection immunity to kill and eliminate invaded microbes in hosts [53]. Therefore, anti-phagocytosis has been considered as an important agent in virulence of microbial pathogens. However, an earlier study firstly found that L. interrogans was able to induce apoptosis of mouse macrophages [54]. L. interrogans was then found to cause apoptosis of human and mouse macrophages through cytomembrane Fas/FasL-triggered caspase-8/3-dependent and ROS/p53-triggered caspase-independent mitochondrial AIF/EndoG pathways [[55], [56], [57]]. However, until now, the toxins of pathogenic Leptospira species have not been completely characterized yet.
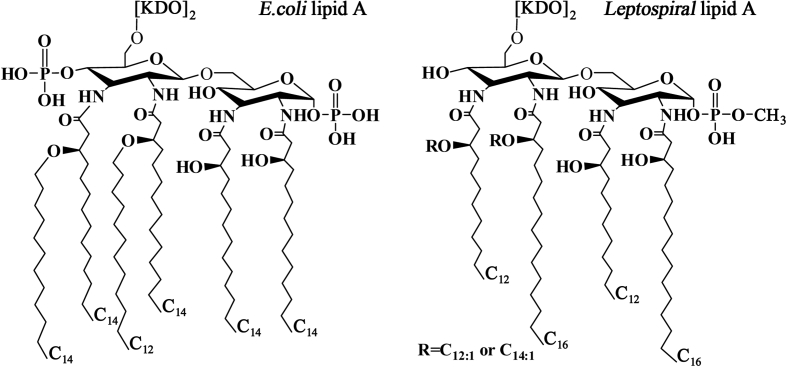
1. LPS LPS is also called endotoxin probably due to it is a structural component in cell wall of Gram-negative bacteria and has an extensive toxicity to mammalian cells. LPS is a biomacromolecule composed of lipid A, core polysaccharide and O-antigenic polysaccharide, in which lipid A decides the toxicity while O-specific polysaccharide determines antigenicity [58]. In many pathogenic Gram-negative bacteria, such as E. coli, Shigella and Salmonella spp, LPS is the major toxin to cause pathological changes [59]. In 1986, LPS from L. interrogans serovar Copenhageni was first identified [60]. The basic structure of lipid A in LPS from L. interrogans serovar Pomona was similar to that from E. coli but presented some significant differences [61]. The lipid A of E. coli contains two phosphate groups at C1 and C4 sites of diaminoglucose backbone linked by one lauric acid (C12) and five myristic acids (C14), while that of the spirochete displayed a lack of the phosphate group at C4 site and a methylation of the phosphate group at C1 site as well as its diaminoglucose backbone is linked by two lauric acids (C12) and one lauric olefinic acid (C12:1), one myristic olefinic acid (C14:1) and two palmitic acids (C16) [61,62]. The structure of leptospiral lipid A and its comparison with lipid A of E. coli were summarized in Fig. 2. Previous studies confirmed that the phosphate groups in lipid A, especially that at C1 site, are closely associated with endotoxic activities such as pyrogenicity, Shwartzman reaction and limulus amebocyte lysate solidification as well as the fatty acids in lipid A play an important role in toxicity in which myristic acid is more toxic than other fatty acids [[63], [64], [65]]. Although the clinical symptoms and pathological changes in leptosirosis patients are similar to endotoxicosis such as endotoxin-like inflammatory reaction, capillary endothelial injury, microthrombosis and decreased blood coagulation [12,20,22], previous studies still demonstrated that the general endotoxic activities of L. interrogans LPS were lower than those from enteric bacilli such as E. coli LPS [12,[29], [30], [31]].
Fig. 2.
Comparison between lipid A structures from L. inetrrogans and E. coli (summarized from Que-Gewirth NLS et al. J Biol Chem 2004; 279:25420–9 and Hinckley MB et al. J Biol Chem 2005; 280:30214–24).
Differing from E. coli LPS recognized by TLR4, LPS of L. interrogans activated mouse macrophages through TLR2-dependent mechanism [66]. In particular, a recent study found that LPS of L. interrogans induced the expression and cytomembrane translocation of Fas/FasL proteins in human and mouse macrophages through JNK/p38MAPK signaling pathways to promote the macrophage apoptosis [67]. LPS from L. interrogans presented a higher molecular weight and contained additional fucose, two different types of dideoxy N-acetyl-hexosamines and extended O-antigenic polysaccharide compared to that from L. licerasiae, an intermediate type of Leptospira [68].
2. Hemolysins Some of bacterial pathogens produce several hemolysins that can be classified as sphingomyelinases and non-sphingomyelinases [69,70]. However, in the genomes of several L. interrogans serovars, there are at least nine hemolysin-encoding genes, in which sph1-4 and sphH were annotated to encode sphingomyelinase-type hemolysins while hlpA, hlyC, hlyX and tlyA were predicted to encode non-sphingomyelinase hemolysins [33,34]. The first reported leptospiral hemolysin, SphH, was confirmed as a pore-forming toxin on human pulmonary epithelial and monkey renal epithelial cells [71]. Another leptospiral hemolysin, Sph2, was identified as a Mg2+-dependent sphingomyelinase and was increased in the expression during infection of cells [72,73]. The Sph1-3, HlpA and TlyA of L. interrogans were secreted and displayed a hemolytic activity in vitro. Importantly, these five secreted leptospiral hemolysins induced the strong production of IL-1β, IL-6 and TNF-α of human and mouse macrophages through TLR2/4-JNK/NF-кB signaling pathways [32]. In particular, a recent study reported that Sph2 of Leptospira intertrogans damaged cytomembrane of human blood vessel endothelial, lung epithelial and liver cells, invaded into the cells through clathrin-mediated endocytosis and translocated onto mitochondria to induce the increase of intracellular reactive oxygen species (ROS) and decrease of the mitochondrial membrane potential (MMP). The high ROS and low MMP levels caused the apoptosis of the three types of cells [74]. Except for erythrocatalysis, these hemolysins of L. interrogans seem to induce inflammatory reaction, cytomembrane injury and cell apoptosis.
3. Hemorrhage inducers Leptospirosis was initially called Weil's disease with the clinical features such as jaundice, hemorrhage, conjunctival congestion and renal failure [8]. Therefore, hemorrhage is one of the important pathological changes of leptospirosis. For example, pulmonary diffuse hemorrhage (PDH), a severe type of leptospirosis, usually causes the death of over 50% of the patients [12]. LPS from bacteria including Leptospira can cause blood vascular engorgement and permeability increase but nearly has no ability to directly induce hemorrhage in tissues [60].
Blood coagulation system has an important physiological function against hemorrhage [75]. In the blood coagulation process, platelet aggregation plays a crucial role by providing a platform for interaction and activation of blood coagulation factors while von Willebrand factor (vWF) initiates the platelet aggregation through its region-A binding to GPIbα receptor of platelets [76]. Platelet activating factor (PAF) promotes platelet aggregation by induction of free Ca2+ increase and coagulation factor-III secretion but it is inactivated by PAF acetylhydrolase (PAF-AH) [77]. L. interrogans vwa-I and vwa-II genes contain region-A domains and their products (vWA) caused pulmonary hemorrhage in mice by competitive binding to platelet GPIbα receptor competed with vWF but no ability to activate platelet aggregation-dependent PI3K/AKT-ERK and PLC/PKC signaling pathways [78]. In addition, the product of L. interrogans LA_2144 gene was proved to have PAF-AH activity in vitro [79]. Our recent experiments showed that the LA_2144 gene product caused the decrease of serum PAF and coagulation factor-III, coagulation time extension of peripheral blood and pulmonary and renal hemorrhage in mice.
4. Jaundice inducers Jaundice is another clinical feature of leptospirosis but its mechanism remains unexplored. Excess cholerythrin mainly due to low function of hepatocytes is a common causative agent of jaundice. An earlier study found that the hepatocytes of L. interrogans-infected guinea pigs were apoptotic [80]. LPS has an extensive toxicity to different types of mammalian cells. Although no experimental evidences have been obtained, LPS of Leptospira causing hepatocyte injury is reasonable presumption. For example, a recent study showed that Sph2, a hemolysin secreted from L. intertrogans, induced the apoptosis of human liver cells in vitro [74].
5. Others A recent study reported that OMP047 of L interrogans displayed a high affinity to bind to human and mouse Fas proteins to induce apoptosis of human and mouse macrophages in vitro [67]. Toxin-antitoxin (TA) systems/modules as stress-response elements have been found in many bacteria [81]. The toxins in TA modules cause bacterial cycle growth arrest and programmed death while the antitoxins neutralize the toxins. In the TA modules of E. coli, MazEF and ChpIK, the toxic proteins acted as endoribonucleases and interferases to inhibit bacterial protein synthesis and cause bacterial death [82,83]. The MazF and ChpK, the toxins in MazEF and ChpIK TA modules of L. interrogans, were over-expressed and externally secreted during infection of human macrophages that caused the decreased viability and necrosis of macrophages [84]. Among the two leptospiral toxins, MazF was confirmed as a RNA lysase (RNase).
Inflammatory reaction in leptospirosis
Inflammation is an essential component of innate and adaptive immune responses in hosts against microbial pathogens. Leptospirosis patients present a strong inflammatory reaction and excessive inflammatory reaction can cause extensive tissue injury and multiple organ failure [25,26]. Monocyte-derived macrophages and neutrophils are the two major infiltrating phagocytes to kill invaded microbes and produce inflammatory cytokines during infection. However, macrophages but not neutrophils acted as the main infiltrating and leptospiral phagocytes in leptospirosis patients and Lepospira-infected mice [85]. In addition, the macrophage chemokines, MCPs, MIP-δ/MIP-1αs and RANTES, but not the neutrophil chemokines, IL-8 and KC, in the sera of patients and Lepospira-infected mice were significantly increased by detection of cytokine microarray.
1. Inflammatory cytokines in leptospirosis Many pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-8 and IL-12, can be detectable in the sera from leptospirosis patients but TNF-α and IL-6 have been considered to be closely associated with the severity and mortality of this disease [[86], [87], [88], [89]]. However, IL-1β, IL-6 and TNF-α were reported as the main pro-inflammatory cytokines in the sera of both leptospirosis patients and L. interrogans-infected mice detected by cytokine microarray [32].
2. Pro-inflammatory cytokine inducers LPS is known as a strong inducer of pro-inflammatory cytokines. However, some proteins from pathogenic Leptospira species have been reported as pro-inflammatory cytokine inducers. The OMPs, especially LipL32, from L. santarosai were found to induce the expression of pro-inflammatory cytokines from different renal cells and caused mouse nephritis through TLR2/p38MAPK signaling pathway [[90], [91], [92], [93]]. As shown above, the secreted hemolysins of L. interrogans had a powerful ability to induce the IL-1β, IL-6 and TNF-α of macrophages through TLR2/4-JNK/NF-кB signaling pathways [32]. In particular, our recent experiments showed that the PAF-AH of L. interrogans displayed a low phospholipase A2 (PLA2) activity to cause the significant increase of serum prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) in mice, the two common lipid inflammatory factors.
Leptospiral diffusion in leptospirosis
In the course of leptospirosis, pathogenic Leptospira species are able to migrate from bloodstream into lungs, liver, kidneys and cerebrospinal fluid to cause aggravation of disease [[20], [21], [22]]. As described above, the animal hosts of pathogenic Leptospira species can persistently discharge leptospires from urine to form natural infectious source [19]. However, leptospirosis patients and infected animal hosts usually present one time of septicemia at early stage during infection, implying that the spirochetes should propagate in kidneys. Therefore, migration of pathogenic Leptospira species through small blood vessels and renal tubules is important for aggravation of leptospirosis in patients and transmission of the spirochetes from animals to humans.
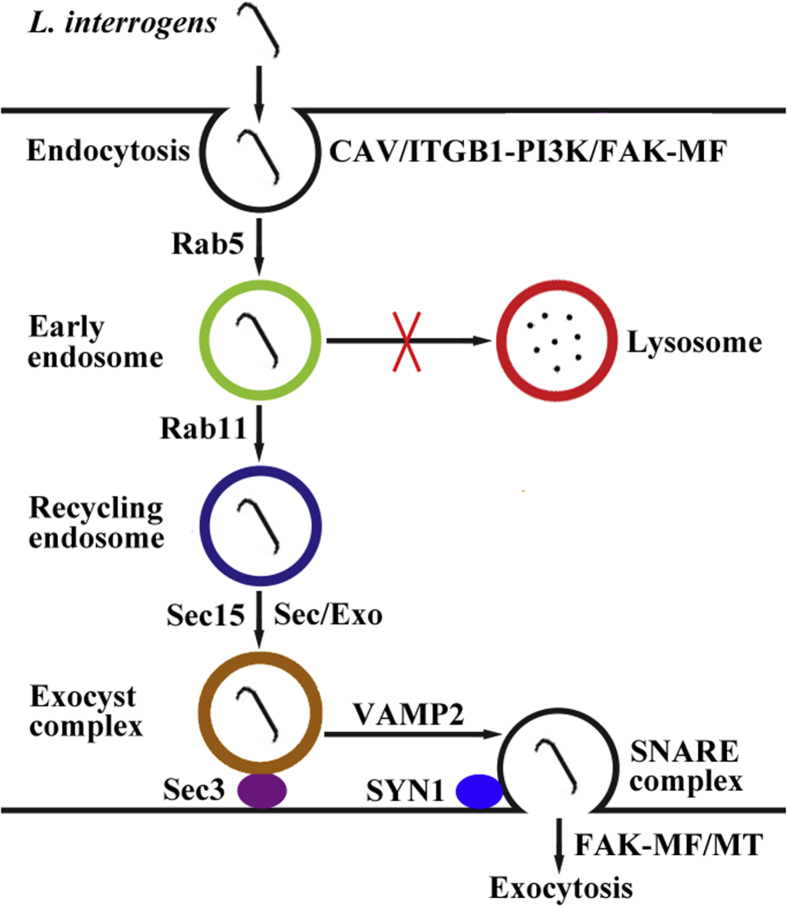
A recent study revealed that L. interrogans entered human and mouse blood vessel endothelial and renal tubule epithelial cells and fibroblasts through caveolae/integrin-β1-PI3K/FAK-microfilament endocytosis pathways to form leptospiral vesicles that avoided fusion with lysosomes [94]. The leptospiral vesicles recruited Rab5/Rab11 and Sec/Exo-SNARE proteins in endocytic recycling and vesicular transport systems for intracellular migration and then release from the cells through a SNARE-complex-mediated FAK-microfilament/microtubule exocytosis pathway (Fig. 3). In the process of leptospiral vesicle transport, L. interrogans was found to propagate in mouse fibroblasts alone. Endocytosis is divided into clathrin-, caveolae- or lipid raft-dependent types and macropinocytosis. The filipin, a caveolae-dependent endocytosis inhibitor, blocked the endocytosis of L. interrogans into all the cells [94]. However, chlorpromazine, a clathrin-dependent endocytosis inhibitor, blocked the internalization of L. interrogans Sph2 hemolysin into human blood vessel endothelial, lung epithelial and liver cells [74].
Fig. 3.
Schematic diagram of L. interrogans transcytosis through blood vessel endothelial and renal tubule epithelial cells and fibroblasts (cited from Li Y et al. eLife 2019; 8:e44594-622).
Conclusion
The course of leptospirosis seem to be a process of continuous migration and transcytosis of pathogenic Leptospira species through mucosal and skin barriers to invade into hosts, blood vessel wall to enter or exit from bloodstream and renal tubule epithelium to discharge in urine. In the process, pathogenic Leptospira species produce many virulence factors to cause pathological changes in patients. Although leptospiral LPS possesses a lower endotoxic activity than typical bacterial LPS, so many secreted leptospiral hemolysins and their powerful pro-inflammatory cytokine-inducing ability play important roles in inflammatory clinical manifestation and tissue inflammatory injury in leptospirosis pateints. However, until now, the mechanisms about the migration of bacteria including Leptospira in vivo have been rarely reported. Endocytosis is the first step of leptospiral transcytosis and it is initiated by binding of leptospiral adherence factors to ECM molecule receptors. However, pathogenic Leptospira possesses many different adherence factors and ECM molecules expressed by different cells are obviously various. Furthermore, S100A10, a Ca2+-binding protein in S100 family, and annexin A2 (AnxA2), a membrane phospholipid-binding protein, were reported to form S100A10-AnxA2 complex that induced endocytosis of Salmonella typhimurium by stimulation of cytoskeleton rearrangement [95]. Summarily, human leptospirosis can be considered as an invasive infectious and systemic inflammatory disease and the mechanisms of pathogenic Leptospira species migration in hosts need to be further studied.
Conflicts of Interest
The authors declare they have no conflicts of interest.
Acknowledgments
We would like to thank the National Natural Science Foundation of China, PR China to support this work (Grant No.: 81671974).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2011. Report of the second meeting of the leptospirosis burden epidemiology reference group; p. 37. [Google Scholar]
- 3.Costa F., Hagan J.E., Calcagno J., Kane M., Torgerson P., Martinez-Silveira M.S. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898–e0003917. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu W.L., Lin X.A., Yan J. Leptospira and leptospirosis in China. Curr Opin Infect Dis. 2014;27:432–436. doi: 10.1097/QCO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.K., Young M.M., Wilson K.L., Craig S.B. Leptospirosis following a major flood in Central Queensland, Australia. Epidemiol Infect. 2013;141:585–590. doi: 10.1017/S0950268812001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miraglia F., Matsuo M., Morais Z.M., Dellagostin O.A., Seixas F.K., Freitas J.C. Molecular characterization, serotyping, and antibiotic susceptibility profile of Leptospira interrogans serovar Copenhageni isolates from Brazil. Diagn Microbiol Infect Dis. 2013;77:195–199. doi: 10.1016/j.diagmicrobio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Goris M.G., Boer K.R., Duarte T.A., Kliffen S.J., Hartskeerl R.A. Human leptospirosis trends, The Netherlands, 1925-2008. Emerg Infect Dis. 2013;19:371–378. doi: 10.3201/eid1903.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes A.E., Zochowski W.J., Dubrey S.W., Sivaprakasam V. Leptospirosis and Weil's disease in the UK. Q J Med. 2012;105:1151–1162. doi: 10.1093/qjmed/hcs145. [DOI] [PubMed] [Google Scholar]
- 9.Traxler R.M., Callinan L.S., Holman R.C., Steiner C., Guerra M.A. Leptospirosis-associated hospitalizations, United States, 1998-2009. Emerg Infect Dis. 2014;20:1273–1279. doi: 10.3201/eid2008.130450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries S.G., Visser B.J., Nagel I.M., Goris M.G., Hartskeerl R.A., Grobusch M.P. Leptospirosis in sub-saharan Africa: a systematic review. Int J Infect Dis. 2014;28:47–64. doi: 10.1016/j.ijid.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Hartskeerl R.A., Collares-Pereira M., Ellis W.A. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 12.Adler B. 1st ed. vol. 387. Springer; Berlin, Heidelberg: 2015. Leptospira and leptospirosis. (Current topics in microbiology and immunology (eBook)). 11-42,65-98, 139-186. [Google Scholar]
- 13.Brenner D.J., Kaufmann A.F., Sulzer K.R., Steigerwalt A.G., Rogers F.C., Weyant R.S. Further determination of DNA relatedness between serogroups and serovars in the family Leptospiraceae with a proposal for Leptospira alexanderi sp. nov. and four new Leptospira genomospecies. Int J Syst Bacteriol. 1999;49:839–858. doi: 10.1099/00207713-49-2-839. [DOI] [PubMed] [Google Scholar]
- 14.Smythe L., Adler B., Hartskeerl R.A., Galloway R.L., Turenne C.Y., Levett P.N. Classification of Leptospira genomospecies 1, 3, 4 and 5 as Leptospira alstonii sp. nov., Leptospira vanthielii sp. nov., Leptospira terpstrae sp. nov. and Leptospira yanagawae sp. nov., respectively. Int J Syst Bacteriol. 2013;63:1859–1862. doi: 10.1099/ijs.0.047324-0. [DOI] [PubMed] [Google Scholar]
- 15.Fouts D.E., Matthias M.A., Adhikarla H., Adler B., Amorim-Santos L., Berg D.E. What makes a bacterial species pathogenic?: comparative genomic analysis of the genus Leptospira. PLoS Negl Trop Dis. 2016;10:e0004403–e0004459. doi: 10.1371/journal.pntd.0004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Zhu Y., Wang Y., Chang Y.F., Zhang Y., Jiang X. Whole genome sequencing revealed host adaptation-focused genomic plasticity of pathogenic Leptospira. Sci Rep. 2016;6:20020–20030. doi: 10.1038/srep20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagadec E., Gomard Y., Minter G., Cordonin C., Cardinale E., Ramasindrazana B. Identification of Tenrec ecaudatus, a wild mammal introduced to Mayotte Island, as a reservoir of the newly identified human pathogenic Leptospira mayottensis. PLoS Negl Trop Dis. 2016;10:e0004933–e0004944. doi: 10.1371/journal.pntd.0004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou L.F., Chen T.W., Yang H.Y., Chang M.Y., Hsu S.H., Tsai C.Y. Murine renal transcriptome profiles upon leptospiral infection: implications for chronic kidney diseases. J Infect Dis. 2018;218:1411–1423. doi: 10.1093/infdis/jiy339. [DOI] [PubMed] [Google Scholar]
- 19.Adler B., Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Haake D.A., Levett P.N. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride A.J., Athanazio D.A., Reis M.G., Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 22.Palaniappan R.U., Ramanujam S., Chang Y.F. Leptospirosis: pathogenesis, immunity, and diagnosis. Curr Opin Infect Dis. 2007;20:284–292. doi: 10.1097/QCO.0b013e32814a5729. [DOI] [PubMed] [Google Scholar]
- 23.Yang H.Y., Hung C.C., Liu S.H., Guo Y.G., Chen Y.C., Ko Y.C. Overlooked risk for chronic kidney disease after leptospiral infection: a population-based survey and epidemiological cohort evidence. PLoS Negl Trop Dis. 2015;9:e0004105–e0004119. doi: 10.1371/journal.pntd.0004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C.W. Leptospirosis renal Disease: emerging culprit of chronic kidney disease unknown etiology. Nephron. 2018;138:129–136. doi: 10.1159/000480691. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz H., Turhan V., Yasar K.K., Hatipoglu M., Sunbul M., Leblebicioglu H. Characteristics of leptospirosis with systemic inflammatory response syndrome: a multicenter study. Ann Clin Microbiol Antimicrob. 2015;14:54–58. doi: 10.1186/s12941-015-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cagliero J., Villanueva S.Y.A.M., Matsui M. Leptospirosis pathophysiology: into the storm of cytokines. Front Cell Infect Microbiol. 2018;8:204–211. doi: 10.3389/fcimb.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirathaworn C., Supputtamongkol Y., Lertmaharit S., Poovorawan Y. Cytokine levels as biomarkers for leptospirosis patients. Cytokine. 2016;85:80–82. doi: 10.1016/j.cyto.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita. Nat Rev Microbiol. 2017;15:297–307. doi: 10.1038/nrmicro.2017.5. [DOI] [PubMed] [Google Scholar]
- 29.Vinh, Shi M.H., Alder B., Faine S. Characterization and taxonomic significance of lipopolysaccharide of Leptospira interrogans serovar hardjo. J Gen Microbiol. 1989;135:2663–2673. doi: 10.1099/00221287-135-10-2663. [DOI] [PubMed] [Google Scholar]
- 30.Isogai E., Isogai H., Kurebayashi Y., Ito N. Biological activities of leptospiral lipopolysaccharide. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;261:53–64. doi: 10.1016/s0176-6724(86)80062-1. [DOI] [PubMed] [Google Scholar]
- 31.Isogai E., Kitagawa H., Isogai H., Matsuzawa T., Shimizu T., Yanagihara Y. Effects of leptospiral lipopolysaccharide on rabbit platelets. Zentralblatt Bakteriol. 1989;271:186–196. doi: 10.1016/s0934-8840(89)80072-6. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Wu Y.F., Ojcius D.M., Yang X.F., Zhang C.L., Ding S.B. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2- and 4-mediated JNK and NF-κB signaling pathways. PLoS One. 2012;7:e42266–e42280. doi: 10.1371/journal.pone.0042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren S.X., Fu G., Jiang X.G., Zeng R., Miao Y.G., Xu H. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422:888–893. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 34.Nascimento A.L., Ko A.I., Martins E.A., Monteiro-Vitorello C.B., Ho P.L., Haake D.A. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004;186:2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanein D., Horwitz R. The structure of cell-matrix adhesions: the new frontier. Curr Opin Cell Biol. 2012;24:134–140. doi: 10.1016/j.ceb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron C.E., Brouwer N.L., Tisch L.M., Kuroiwa J.M. Defining the interaction of the Treponema pallidum adhesion Tp0751 with laminin. Infect Immun. 2005;73:7485–7494. doi: 10.1128/IAI.73.11.7485-7494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma A., Brissette C.A., Bowman A., Stevenson B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun. 2009;77:4940–4946. doi: 10.1128/IAI.01420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y.Y., Zheng W., Li L.W., Mao Y.F., Yan J. Pathogenesis of leptospirosis: interaction of Leptospira interrogans with in vitro cultured mammalian cells. Med Microbiol Immunol. 2007;196:233–239. doi: 10.1007/s00430-007-0047-0. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson B., Choy H.A., Pinne M., Rotondi M.L., Miller M.C., Demoll E. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS One. 2007;2:e1188–e1198. doi: 10.1371/journal.pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atzingen M.V., Barbosa A.S., De Brito T., Vasconcellos S.A., de Morais Z.M., Lima D.M. Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection. BMC Microbiol. 2008;8:70–85. doi: 10.1186/1471-2180-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira M.L., de Morais Z.M., Goncales A.P., Romero E.C., Vasconcellos S.A., Nascimento A.L. Lsa63, a newly identified surface protein of Leptospira interrogans binds laminin and collagen IV. J Infect. 2010;60:52–64. doi: 10.1016/j.jinf.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 42.Domingos R.F., Fernandes L.G., Romero E.C., de Morais Z.M., Vasconcellos S.A., Nascimento A.L. Novel Leptospira interrogans protein Lsa32 is expressed during infection and binds laminin and plasminogen. Microbiology. 2015;161:851–864. doi: 10.1099/mic.0.000041. [DOI] [PubMed] [Google Scholar]
- 43.Hauk P., Macedo F., Romero E.C., Vasconcellos S.A., de Morais Z.M., Barbosa A.S. LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect Immun. 2008;76:2642–2650. doi: 10.1128/IAI.01639-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira T.R., Longhi M.T., Goncales A.P., de Morais Z.M., Vasconcellos S.A., Nascimento A.L. LipL53, a temperature regulated protein from Leptospira interrogans that binds to extracellular matrix molecules. Microb Infect. 2010;12:207–217. doi: 10.1016/j.micinf.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Choy H.A., Kelley M.M., Chen T.L., Moller A.K., Matsunaga J., Haake D.A. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun. 2007;75:2441–2450. doi: 10.1128/IAI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian Y.C., Chen Y.C., Hung C.C., Li Y.J., Chang M.Y., Wu M.S. Leptospiral outer membrane protein induces extracellular matrix accumulation through a TGF-β1/Smad-dependent pathway. J Am Soc Nephrol. 2006;17:2792–2798. doi: 10.1681/ASN.2006020159. [DOI] [PubMed] [Google Scholar]
- 47.Tian Y.C., Hung C.C., Li Y.J., Chen Y.C., Chang M.Y., Yen T.H. Leptospira santorosai serovar Shermani detergent extract induces an increase in fibronectin production through a Toll-like receptor 2-mediated pathway. Infect Immun. 2011;79:1134–1142. doi: 10.1128/IAI.01287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao X., Sun A.H., Ge Y.M., Wang H., Yan Jie. Leptospira interrogans enters different host cells by binding to distinct molecules in the extracellular matrix. Arch Biol Sci. 2015;67:31–39. [Google Scholar]
- 49.Kassegne K., Hu W.L., Ojcius D.M., Sun D., Ge Y.M., Zhao J.F. Identification of collagenase as a critical virulence factor for invasiveness and transmission of pathogenic Leptospira species. J Infect Dis. 2014;209:1105–1115. doi: 10.1093/infdis/jit659. [DOI] [PubMed] [Google Scholar]
- 50.Cafardi V., Biagini M., Martinelli M., Leuzzi R., Rubino J.T., Cantini F. Identification of a novel zinc metalloprotease through a global analysis of Clostridium difficile extracellular proteins. PLoS One. 2013;8:e81306–e81319. doi: 10.1371/journal.pone.0081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao S.M., Sun A.H., Ojcius D.M., Wu S.L., Zhao J.F., Yan J. Inactivation of the fliY gene encoding a flagellar motor switch protein attenuates mobility and virulence of Leptospira interrogans strain Lai. BMC Microbiol. 2009;9:253–263. doi: 10.1186/1471-2180-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L., Zhang C.L., Ojcius D.M., Sun D., Zhao J.F., Lin X.A. The mammalian cell entry (Mce) protein of pathogenic Leptospira species is responsible for RGD motif-dependent infection of cells and animals. Mol Microbiol. 2012;83:1006–1023. doi: 10.1111/j.1365-2958.2012.07985.x. [DOI] [PubMed] [Google Scholar]
- 53.Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Merien F., Baranton G., Perolat P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect Immun. 1997;65:729–738. doi: 10.1128/iai.65.2.729-738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin D.D., Ojcius D.M., Sun D., Dong H.Y., Luo Y.H., Mao Y.F. Leptospira interrogans induces apoptosis in macrophages via caspase-8- and caspase-3-dependent pathways. Infect Immun. 2009;77:799–809. doi: 10.1128/IAI.00914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu W.L., Ge Y.M., Ojcius D.M., Sun D., Dong H.Y., Yang X.F. p53-signaling controls cell cycle arrest and caspase-independent apoptosis in macrophages infected with pathogenic Leptospira species. Cell Microbiol. 2013;15:1624–1659. doi: 10.1111/cmi.12141. [DOI] [PubMed] [Google Scholar]
- 57.Hu W.L., Dong H.Y., Li Y., Ojcius D.M., Li S.J., Yan J. Bid-induced release of AIF/EndoG from mitochondria causes apoptosis of macrophages during infection with Leptospira interrogans. Front Cell Infect Microbiol. 2017;7:471–483. doi: 10.3389/fcimb.2017.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Racth C.R.H. Biochemistry of endotoxin. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 59.Brooks G.F., Butel J.S., Morse S.A. 22th ed. McGraw-Hill; New York: 2001. Medical microbiology; pp. 217–228. [Google Scholar]
- 60.Vinh T., Alder B., Faine S. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar Copenhageni. J Gen Microbiol. 1986;132:103–109. doi: 10.1099/00221287-132-1-103. [DOI] [PubMed] [Google Scholar]
- 61.Que-Gewirth N.L.S., Ribeiro A.A., Kalb S.R., Cotter R.J., Bulach D.M., Adler B. A methylacted phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A. J Biol Chem. 2004;279:25420–25429. doi: 10.1074/jbc.M400598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinckley M.B., Reynods C.M., Ribeiro A.A., McGrath S.C., Cotter R.J., Lauw F.N. A Leptospira interrogans enzyme with similarity to yeast Ste14P that methylates the 1-phosphate group of lipid A. J Biol Chem. 2005;280:30214–30224. doi: 10.1074/jbc.M506103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowotny A. Molecular aspects of endotoxic reactions. Bacteriol Rev. 1969;33:72–98. doi: 10.1128/br.33.1.72-98.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galanos C., Hanscnhagge T., Lehmann V., Lüderitz O. Comparison of the capacity of lipid A precursor molecules to express the local Shwartzman phenomenon. Infect Immun. 1985;48:355–358. doi: 10.1128/iai.48.2.355-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takayama K., Qureshi N., Raetz C.R.H., Ribi E., Peterson J., Cantrell J.L. Influence of fine structure of lipid A on limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984;45:350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Werts C., Tapping R.I., Mathison J.C., Chuang T.H., Kravchenko V., Saint Girons I. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 67.Du P., Li S.J., Ojcius D.M., Li K.X., Hu W.L., Lin X.A. A novel Fas-binding outer memebrane protein and lipopolysaccharide of Leptospira interrogans induce macrophage apoptosis through Fas/FasL-caspase-8/-3 pathway. Emerg Microb Infect. 2018;7:135–151. doi: 10.1038/s41426-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Patra K.P., Choudhury B., Matthias M.M., Baga S., Bandyopadhya K., Vinetz J.M. Comparative analysis of lipopolysaccharides of pathogenic and intermediately pathogenic Leptospria species. BMC Microbiol. 2015;15:244–254. doi: 10.1186/s12866-015-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gleason T.G., Houlgrave C.W., May A.K., Crabtree T.D., Sawyer R.G., Denham W. Hemolytically active alpha-hemolysin elicits interleukin-1β but augments the lethality of Escherichia coli by an IL-1- and tumor necrosis factor-independent mechanism. Infect Immun. 1998;66:4215–4221. doi: 10.1128/iai.66.9.4215-4221.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braun J.S., Novak R., Gao G., Murray P.J., Shenep J.L. Pneumolysin, a protein toxin of Streptocccus pneumoniae, induce nitric oxide production from macrophages. Infect Immun. 1999;67:3750–3756. doi: 10.1128/iai.67.8.3750-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S.H., Kim S., Park S.C., Kim M.J. Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells. Infect Immun. 2002;70:315–322. doi: 10.1128/IAI.70.1.315-322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narayanavari S.A., Kishore N.M., Sritharan M. Structural analysis of the leptospiral sphingomyelinases: in silico and experimental evaluation of Sph2 as an Mg++-dependent sphingomyelinase. J Mol Microbiol Biotechnol. 2012;22:24–34. doi: 10.1159/000337013. [DOI] [PubMed] [Google Scholar]
- 73.Narayanavari S.A., Lourdault K., Sritharan M., Haake D.A., Matsunaga J. Role of sph2 gene regulation in hemolytic and sphingomyelinase activities produced by Leptospira interrogans. PLoS Negl Trop Dis. 2015;9:e0003952–e0003974. doi: 10.1371/journal.pntd.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Che R.B., Ding S.B., Zhang Q.C., Yang W.Q., Yan J., Lin X.A. Haemolysin Sph2 of Leptospira interrogans induces cell apoptosis via intracellular reactive oxygen species elevation and mitochondrial membrane injury. Cell Microbiol. 2019;21:e12959–e12972. doi: 10.1111/cmi.12959. [DOI] [PubMed] [Google Scholar]
- 75.Dahlbäck B. Blood coagulation. Lancet. 2000;355:1627–1632. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 76.Lenting P.J., Christophe O.D., Denis C.V. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- 77.Nomikos T., Fragopoulou E., Antonopoulou S., Panagiotakos D.B. Mediterranean diet and platelet-activating factor: a systematic review. Clin Biochem. 2018;60:1–10. doi: 10.1016/j.clinbiochem.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Fang J.Q., Imran M., Hu W.L., Ojcius D.M., Li Y., Ge Y.M. vWA proteins of Leptospira interrogans induce hemorrhage in leptospirosis by competitive inhibition of vWF/GPIb-mediated platelet aggregation. eBioMedicine. 2018;37:428–441. doi: 10.1016/j.ebiom.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J.W., Zhang Y.X., Xu J., Geng Y., Chen X.Y., Yang H.L. Serum activity of platelet-activating factor acetylhydrolase is a potential clinical marker for leptospirosis pulmonary hemorrhage. PLoS One. 2009;4:e4181–e4192. doi: 10.1371/journal.pone.0004181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merien F., Truccolo J., Rougier Y., Baranton G., Perolat P. In vivo apoptosis of hepatocytes in Guinea pigs infected with Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol Lett. 1998;169:95–102. doi: 10.1111/j.1574-6968.1998.tb13304.x. [DOI] [PubMed] [Google Scholar]
- 81.Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science. 2003;301:1496–1499. doi: 10.1126/science.1088157. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y.L., Zhang J.J., Hoeflich K.P., Ikura M., Qing G.L., Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12:913–923. doi: 10.1016/s1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y.L., Zhu L., Zhang J.J., Inouye M. Characterization of ChpBK, an mRNA interferase from Escherichia coli. J Biol Chem. 2005;280:26080–26088. doi: 10.1074/jbc.M502050200. [DOI] [PubMed] [Google Scholar]
- 84.Komi K.K., Ge Y.M., Xin X.Y., Ojcius D.M., Sun D., Hu W.L. ChpK and MazF of the toxin-antitoxin modules are involved in the virulence of Leptospira interrogans during infection. Microb Infect. 2015;17:34–47. doi: 10.1016/j.micinf.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Tajiki H., Salomão R. Association of plasma levels of tumor necrosis factor alpha with severity of disease and mortality among patients with leptospirosis. Clin Infect Dis. 1996;3:1177–1178. doi: 10.1093/clinids/23.5.1177. [DOI] [PubMed] [Google Scholar]
- 86.de Fost M., Hartskeerl R.A., Groenendijk M.R., van der Poll T. Interleukin 12 in part regulates gamma interferon release in human whole blood stimulated with Leptospira interrogans. Clin Diagn Lab Immunol. 2003;10:332–335. doi: 10.1128/CDLI.10.2.332-335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vernel-Pauillac F., Goarant C. Differential cytokine gene expression according to outcome in a hamster model of leptospirosis. PLoS Negl Trop Dis. 2010;4:e582–e590. doi: 10.1371/journal.pntd.0000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papa A., Kotrotsiou T. Cytokines in human leptospirosis. Trans R Soc Trop Med Hyg. 2015;109:749–754. doi: 10.1093/trstmh/trv095. [DOI] [PubMed] [Google Scholar]
- 89.Chen X., Li S.J., Ojcius D.M., Sun A.H., Hu W.L., Lin X.A. Mononuclear-macrophages but not neutrophils act as major infiltrating anti-leptospiral phagocytes during leptospirosis. PLoS One. 2017;12:e0181014–e0181038. doi: 10.1371/journal.pone.0181014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang C.W., Wu M.S., Pan M.J., Hsieh W.J., Vandewalle A., Huang C.C. The Leptospira outer membrane protein LipL32 induces tubulointerstitial nephritis-mediated gene expression in mouse proximal tubule cells. J Am Soc Nephrol. 2002;13:2037–2045. doi: 10.1097/01.asn.0000022007.91733.62. [DOI] [PubMed] [Google Scholar]
- 91.Yang C.W., Hung C.C., Wu M.S., Tian Y.C., Chang C.T., Pan M.J. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 2006;69:815–822. doi: 10.1038/sj.ki.5000119. [DOI] [PubMed] [Google Scholar]
- 92.Hung C.C., Chang C.T., Tian Y.C., Wu M.S., Yu C.C., Pan M.J. Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through Toll-like receptor 2 and p38 mitogen activated protein kinase. Nephrol Dial Transplant. 2006;21:898–910. doi: 10.1093/ndt/gfi316. [DOI] [PubMed] [Google Scholar]
- 93.Chang M.Y., Cheng Y.C., Hsu S.H., Ma T.L., Chou L.F., Hsu H.H. Leptospiral outer membrane protein LipL32 induces inflammation and kidney injury in zebrafish larvae. Sci Rep. 2016;6:27838–27849. doi: 10.1038/srep27838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y., Li K.X., Hu W.L., Ojcius D.M., Fang J.Q., Li S.J. Endocytic recycling and vesicular transport systems mediate transcytosis of Leptospira interrogans across endothelial and epithelial cell monolayers. eLife. 2019;8:e44594–e44622. doi: 10.7554/eLife.44594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Jolly C., Winfree S., Hansen B., Steele-Mortimer O. The Annexin A2/p11 complex is required for efficient invasion of Salmonella typhimurium in epithelial cells. Cell Microbiol. 2014;16:64–77. doi: 10.1111/cmi.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]