1. Introduction
Randomised controlled trials (RCTs) have demonstrated that HPV-based cervical cancer screening offers higher protection against cervical precancer and cancer compared to cytology-based screening [1,2]. An additional advantage of screening using HPV assays is that the test can be performed on a self-sample collected by the woman herself. Recent systematic reviews have shown that an HPV test on a vaginal self-sample is as accurate to detect cervical intra-epithelial neoplasia of grade 2 or worse (CIN2+) as an HPV test applied on a clinician-taken cervical sample, if a clinically validated PCR-based assay is used [3,4].
A particular interesting property of self-sampling is that it can be used as a strategy to reach women who do not participate in regular screening programmes [[5], [6], [7], [8], [9], [10], [11], [12]]. Taking a vaginal self-sample is an attractive alternative method to obtain a specimen for cervical cancer screening, both for non-attending women as well as for women who do participate in preventive programmes [13,14]. A systematic review of RCTs revealed that sending a self-sampling kit to women who were not or irregularly screened was more effective in generating a response than sending routine invitations or reminders to have a Pap smear taken by a clinician [12]. However, the gain in response is highly variable among studies and settings. Therefore, no universal recommendations can be formulated. Local pilot studies should be set up to identify a format of intervention that generates satisfactory results before the general roll-out of strategies that include offering self-sampling kits.
This study reports the participation rates of a randomised controlled trial conducted in a general practitioner (GP) practice in Belgium. The current screening policy in Belgium is 3-yearly screening using Pap smears taken by a GP or gynaecologist. However, differences exist regarding the organisation of screening between the three Belgian Regions (Flemish, Brussels-Capital and Walloon region). Since 2013 there is an organized programme targeting women between 25 and 64 years old in the Flemish region which includes sending routine invitations to women who do not have a Pap smear registered less than three years ago. In the Brussels-Capital and Walloon region screening is offered opportunistically, meaning that participation depends on the initiative of the woman herself or the clinician. In 2006, the 3-yearly screening coverage was around 60% in Belgium [15].
With the current study we want to investigate how the non-participants (defined as women who do not have a Pap smear taken in the last three years) can be reached through the support of a GP. This was addressed by comparing the response of non-participants between two study arms: a) self-sampling arm where a self-sampling kit was directly offered by a GP and b) control arm where a GP gave a recommendation to have a Pap smear taken by a clinician (GP or gynaecologist).
2. Methods
2.1. Study design
Women were recruited in a GP practice with five GPs in Schepdaal, a Flemish municipality located at the border of Brussels between November 2014 and April 2015. The study population comprised women between 25 and 64 years old who did not have a Pap smear taken in the last three years at the day of her consultation. Every day during the study period, the first fifteen eligible women (three women per GP) who came to a consultation – for whatever reason – were informed about the study and asked whether they wanted to participate. If they gave their consent, they were allocated according to a computer generated randomisation list in a 1:1 ratio without blinding to one of the two study arms. The study obtained ethical approval from the medical ethics committee of the University Hospital of Antwerp on September 1, 2014.
2.2. Self-sampling arm
In the experimental arm, women were given a self-sampling kit containing a Evalyn® Brush (Rovers Medical Devices, B.V., Oss, Netherlands) together with instructions on how to use it. Self-samples were taken at home and stored dry at room temperature [16]. Women could send the used self-sampling device to the laboratory with a prepaid envelope or they could bring it back to the GP practice where it was picked up by the courier service of the laboratory AML (Sonic Healthcare Benelux, Antwerp, Belgium). At AML, the samples were transferred into a vial with liquid PreservCyt medium (Hologic Inc., Bedford, MA, USA) and stored for other investigations (not reported in this study).
2.3. Control arm
In the control arm, women were encouraged by the GP to make an appointment to have a Pap smear taken by a GP of the practice or a gynaecologist of choice. Participation was defined as having a Pap smear taken before the end of the study period. Pap smears were collected with a Cervex-Brush® (Rovers Medical Devices, B.V., Oss, Netherlands) or with the combination of an extended tip spatula and an endo-cervical brush according to European guidelines [17]. Subsequently the scraped cervical cells were transferred into a vial with liquid PreservCyt medium and stored for other investigations (not reported in this study).
2.4. Statistics
The primary endpoint was participation in screening which was defined in the control arm as the proportion of women who had a Pap smear taken by a GP or gynaecologist and in the self-sampling arm as the proportion of women of which AML had received a self-sampling kit before April 1, 2015. The hypothesis to be addressed was that the difference in response between the self-sampling and control arm would be 30% with an expected response in the control arm of 50%, accepting a confidence interval (CI) of 95% and a power of 80%. The required sample size in each arm was 39, which was increased to 45 when taking into account possible dropout and loss of specimens.
Pearson's test was used to assess differences in participation between categorical variables (i.e. age category, level of education, time interval since last Pap smear, physician who took last Pap smear), if the expected counts for each cell of the 2 × 2 contingency tables were larger than or equal to five. Otherwise, Fisher's exact p test was used [18]. The relative participation rate (self/control) and absolute participation difference (self-control) together with their 95% CIs were computed with epitab in Stata. A logistic regression was performed with participation as outcome and study arm as predictor variable. The above mentioned covariates were included in the model as potential confounders. Crude and adjusted odds ratios (ORs), together with their 95% CIs, were calculated for participation in the self-sampling versus control arm.
All statistical analyses were performed using Stata version 14 (StataCorp LLC, College Station, TX, USA). The p-value for statistical significance was defined at ≤0.05.
3. Results
3.1. Descriptive statistics
Eighty-eight women (43 and 45 in the control and self-sampling arm, respectively) were enrolled in the study (Table 1). Most women (42%, 37/88) were between 55 and 64 years old, 28% (25/88) was between 45 and 54 years, 20% (18/88) was between 35 and 44 years old and 9% (8/88) was between 25 and 34 years. The distribution of highest reached level of education was 15% (13/88) for primary school, 41% (36/88) for secondary school and 44% (39/88) for higher education. For 82% (72/88) of women with known screening history their last Pap smear was taken four years ago or more, for 18% (16/88) the last pap smear was taken three years ago. The last Pap smear was taken in 57% (50/88) and 38% (33/88) of the women by a GP or gynaecologist, respectively.
Table 1.
Study characteristics of the women enrolled in the randomised trial comparing a routine recommendation from the GP to have a Pap smear taken by a physician (control arm) vs the offer of a self-sampling kit (self-sampling arm).
| Study arm |
Control arm (n = 43) |
Self-sampling arm (n = 45) |
Total (n = 88) |
|||
|---|---|---|---|---|---|---|
| Covariate | n | % | n | % | n | % |
| Age category (years) | ||||||
| 25–34 | 4 | 9 | 4 | 9 | 8 | 9 |
| 35–44 | 7 | 16 | 11 | 24 | 18 | 20 |
| 45–54 | 10 | 23 | 15 | 33 | 25 | 28 |
| 55–64 | 22 | 51 | 15 | 33 | 37 | 42 |
| Education level | ||||||
| Primary school | 7 | 16 | 6 | 13 | 13 | 15 |
| Secondary school | 21 | 49 | 15 | 33 | 36 | 41 |
| Higher education | 15 | 35 | 24 | 53 | 39 | 44 |
| Time interval since last Pap smear (years)a | ||||||
| 3 | 10 | 23 | 6 | 13 | 16 | 18 |
| ≥4 | 33 | 77 | 39 | 87 | 72 | 82 |
| Pap smear-taker (of previous Pap smear) | ||||||
| GP | 24 | 56 | 26 | 58 | 50 | 57 |
| Gynaecologist | 17 | 40 | 16 | 36 | 33 | 38 |
| Never | 1 | 2 | 2 | 4 | 3 | 3 |
| Unknown | 1 | 2 | 1 | 2 | 2 | 2 |
Note: GP = general practitioner.
Interval was calculated from the year in which the last pap smear was taken until the study year, i.e. 2015.
3.2. Participation
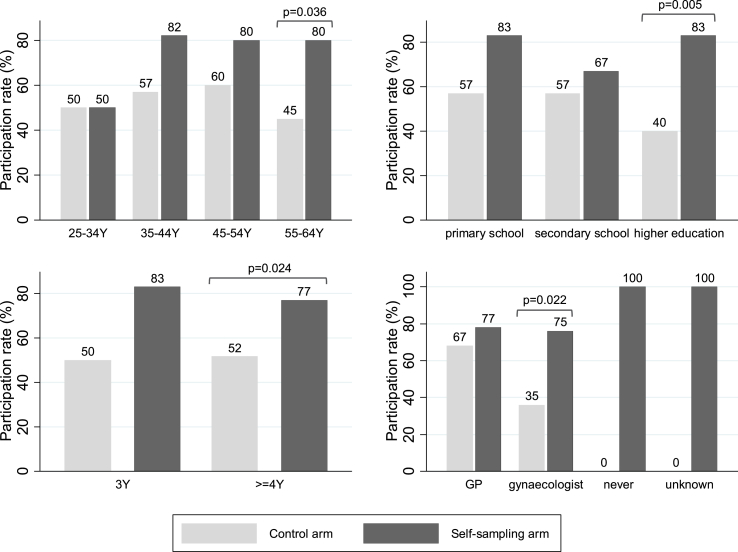
The participation rate in the self-sampling arm was 78% (35/45) which was significantly different from the participation rate in the control arm 51% (22/43) ( p = 0.009). Women in the self-sampling arm were 1.52 times more likely (95% CI [1.09–2.12]) to participate than women in the control arm. The absolute participation difference was 27% (95% CI [7–46%]). The crude OR for participation was 3.34 (95% CI [1.33–8.41], p-value = 0.010) and remained significant after adjusting for covariates (adjusted OR = 3.41, 95% CI [1.31–8.87], p-value = 0.012). None of the covariates played a significant role in the logistic regression. Fig. 1 shows that the participation rates were higher in the self-sampling arm (black boxes) than in the control arm (grey boxes) for all the study characteristics subgroups. However, this was only significant when women were between 55 and 64 years old ( p-value = 0.036), women obtained a degree in higher education ( p-value = 0.005), women's last Pap smear was taken four years ago or more ( p-value = 0.024) or a gynaecologist was the sample taker ( p-value = 0.022).
Fig. 1.
Participation rate (=proportion of women having a cervical cancer screening test) per study arm, stratified by four covariates: a) age-group in years [Y] (top left); b) highest education level reached by the woman (top right); c) number of years since previous screening [Y] (bottom left) and; d) clinician who took a previous Pap smear (bottom right).
4. Discussion
4.1. Main findings
This small trial found a high response rate of 78% when GPs directly offered a self-sampling kit to women who were not screened since at least three years. This was substantially higher than observed in a recent meta-analysis which included large population-based participation trials where the efficacy of strategies including mailing of self-samplers to the woman's home address was compared with sending routine invitation or reminder letters [12]. The pooled response in the self-sampling arms of 21 trials was 19.2% (95% CI [15.7–23.0%]) [12]. The participation difference in the Brussels trial was 27% which contrasts with the pooled participation difference of 7.3% (95% CI [4.1–10.6%]) in the mail-to-all scenario [12]. Nevertheless, a separate meta-analysis with four studies where the self-sampling device was directly offered to women by community health workers during door-to-door visits, showed a very high pooled participation rate of 94.2% (95% CI [80.2–100.0%]) and a similar participation rate of 53.3% (95% CI [10.2–93.2%]) in the control arm [[19], [20], [21], [22]]. These latter findings and our results show that the involvement of community health workers or GPs, either through directly offering self-sampling kits or encouraging women to have a Pap smear taken, enhances response. However, the four studies from the meta-analysis were conducted in low-to-middle income countries with limited infrastructure, human resources and restricted access to cervical cancer screening [19,21,23,24]. Therefore, in settings with an established cervical cancer screening programme, we suggest to further enlarge the role of the GP by encouraging patients to participate and providing them with necessary information on how to use the self-sampling kit.
4.2. Screening in Belgium
The role of the GP in cervical cancer screening is currently limited in Belgium as they perform only 11% of the Pap smears which varied also between the three regions (14–26% in Flemish region, 7–10% in Brussels-Capital and 2–5% in Walloon region) [15]. To assist GPs in this role, risk flags can be integrated in electronic patient records which can alert the GP when a patient has not been screened for more than three years [25]. Therefore, a linkage with the records from the Belgian Cancer Registry, that manages a comprehensive cervical cyto-histopathology registry from all laboratories for pathological anatomy enriched with health insurance data, through the national social security number is needed to collect screening data. Efforts are currently being undertaken to have such information directly available in the patient GP files through secure linkage with the Cancer Registry.
4.3. Limitations
In this study the role of covariates could not be assessed precisely because of the small sample size of the trial. The role of behavioural and demographic factors that might affect women's adherence to the screening programme, like their risk perception, the role of embarrassment, socioeconomic status and knowledge of HPV and screening should be addressed in a larger trial [26,27]. Our trial did not include the offer of taking a Pap smear immediately by the GP in the control arm. Contrary to Northern Europe, in Belgium and also in several other countries of West, South and Central Europe, Pap smears are taken mainly by gynaecologists. GPs often consider secondary prevention of cervical cancer as a task of gynaecologists [15]. In Belgium, on average 11% of cervical specimen collected in 2006 were taken by GPs and this proportion varied from 14% in the Flemish region to 2% in the Walloon region [15]. If the control arm of our trial would have included sending invitation letters to the eligible women (who are consulting for any reason) to have a Pap smear taken immediately by the GP, uptake might have been low. Therefore, this action was not foreseen in the trial. Moreover, the control intervention was intended to reflect current routine practice.
Another important issue that was not addressed in this study is the follow-up of women with a HPV-positive self-sample who also require an additional appointment to have a Pap smear taken for triage purposes. This might compromise the gain in response rate for the self-sampling arm. However, a recent meta-analysis showed that the adherence to follow-up in the self-sampling arm was 9% lower compared to the control arm, but it was not statistically significant [12]. The availability of triage methods like genotyping and methylation that are applicable on the self-sample itself would limit this problem.
Our findings must be interpreted with caution because the estimated effect in this study might be positively biased as it was not feasible to blind the patient. Patients were aware of the study arm assignment which might have led to a higher willingness to participate in the self-sampling arm [28]. The time interval since last Pap smear was obtained by anamnesis or from the patient file. Therefore, misclassification of the screening status could have occurred. More reliable information could be obtained from the Belgian Cancer Registry. However, at the time of the study, GPs did not have access to this source of information.
In addition, this trial took place in one particular location and therefore the general success of the intervention cannot be claimed for the whole region or country.
The accuracy of HPV testing on self-samples taken with particular self-collection devices as well as women's concerns, attitudes and preferences was beyond the scope of this study [29]. These aspects are currently being evaluated in the VALHUDES study [30].
5. Conclusion
The results of this study support previous findings that underscreened women are more likely to participate in cervical cancer screening when they are offered self-sampling kits compared to a recommendation to have a Pap smear taken by a clinician. Moreover, the study shows that the response in both arms was positively enhanced through the direct involvement of the GP which differs from the current screening strategy in Belgium and many other high-income countries where women are reached through sending invitation letters. Larger trials are needed to verify whether the impressive findings of this small trial are reproducible at population scale.
Funding
Financial support was received from: (1) the COHEAHR Network (grant No. 603019) and the RISCC Network, funded by the 7th Framework Programme and the Horizon 2020 programme, respectively of DG Research and Innovation, European Commission (Brussels, Belgium).
CRediT authorship contribution statement
E. Peeters: Formal analysis, Writing - original draft. K. Cornet: Conceptualization, Methodology, Formal analysis, Resources, Investigation. D. Devroey: Writing - review & editing. M. Arbyn: Formal analysis, Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2020.100194.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ronco G., Dillner J., Elfstrom K.M. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M., Ronco G., Anttila A. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M., Verdoodt F., Snijders P.J.F. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M., Castle P. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical cancer screening program. Canc. Epidemiol. Biomarkers Prev. 2015;24(5):769–772. doi: 10.1158/1055-9965.EPI-14-1417. [DOI] [PubMed] [Google Scholar]
- 5.Giorgi-Rossi P., Marsili L.M., Camilloni L. The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600) Br. J. Canc. 2011;104(2):248–254. doi: 10.1038/sj.bjc.6606040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giorgi-Rossi P., Fortunato C., Barbarino P. Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br. J. Canc. 2015 Jan 29;112(4):667–675. doi: 10.1038/bjc.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikstrom I., Lindell M., Sanner K., Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br. J. Canc. 2011 Jul 26;105(3):337–339. doi: 10.1038/bjc.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultana F., English D.R., Simpson J.A. Home-based HPV self-sampling improves participation by never- and under-screened women: results from a large randomised trial (iPap) in Australia. Int. J. Canc. 2016 Feb 6;139(2):281–290. doi: 10.1002/ijc.30031. [DOI] [PubMed] [Google Scholar]
- 9.Kellen E., Benoy I., Vanden Broeck D., Martens P., Bogers Annemie H.J., Van L.E. A randomized, controlled trial of two strategies of offering the home-based HPV self-sampling test to non- participants in the Flemish cervical cancer screening program. Int. J. Canc. 2018 Mar 23;143(4):861–868. doi: 10.1002/ijc.31391. [DOI] [PubMed] [Google Scholar]
- 10.Lam J.U., Rebolj M., Ejegod D.M. Human papillomavirus self-sampling for screening non-attenders: opt-in pilot implementation with electronic communication platforms. Int. J. Canc. 2017 Feb 13;140(10):2212–2219. doi: 10.1002/ijc.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haguenoer K., Sengchanh S., Gaudy-Graffin C. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br. J. Canc. 2014 Sep 23;111:2187–2196. doi: 10.1038/bjc.2014.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbyn M., Smith S.B., Temin S., Sultana F., Castle P.E. The Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson E.J., Maynard B.R., Loux T., Fatla J., Gordon R., Arnold L.D. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex. Transm. Infect. 2017;93(1):56–61. doi: 10.1136/sextrans-2016-052609. [DOI] [PubMed] [Google Scholar]
- 14.Polman N.J., Snijders P.J.F., Kenter G.G., Berkhof J., Meijer C.J.L.M. HPV-based cervical screening: rationale, expectations and future perspectives of the new Dutch screening programme. Prev. Med. 2019 Feb;119:108–117. doi: 10.1016/j.ypmed.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M., Fabri V., Temmerman M., Simoens C. Attendance at cervical cancer screening and use of diagnostic and therapeutic procedures on the uterine cervix assessed from individual health insurance data (Belgium, 2002-2006) PloS One. 2014;9(4) doi: 10.1371/journal.pone.0092615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Baars R., Bosgraaf R.P., ter Harmsel B.W., Melchers W.J.G., Quint W.G., Bekkers R.L. Dry storage and transport of a cervicovaginal self-sample using the Evalyn Brush(R): reliable HPV detection combined with women's comfort. J. Clin. Microbiol. 2012 Sep 26;50:3937–3943. doi: 10.1128/JCM.01506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbyn M., Herbert A., Schenck U. European guidelines for quality assurance in cervical cancer screening: recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology. 2007;18(3):133–139. doi: 10.1111/j.1365-2303.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 18.Lydersen S., Fagerland M.W., Laake P. Recommended tests for association in 2 x 2 tables. Stat. Med. 2009 Mar 30;28(7):1159–1175. doi: 10.1002/sim.3531. [DOI] [PubMed] [Google Scholar]
- 19.Lazcano-Ponce E., Lorincz A.T., Cruz-Valdez A. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011 Nov 26;378(9806):1868–1873. doi: 10.1016/S0140-6736(11)61522-5. [DOI] [PubMed] [Google Scholar]
- 20.Arrossi S., Thouyaret L., Herrero R. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015 Feb;3(2):e85–e94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 21.Moses E., Pedersen H.N., Mitchell S.M. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop. Med. Int. Health. 2015 May 29;20(10):1355–1367. doi: 10.1111/tmi.12549. [DOI] [PubMed] [Google Scholar]
- 22.Modibbo F., Iregbu K.C., Okuma J. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect. Agents Canc. 2017;12(1):11. doi: 10.1186/s13027-017-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny L., de S.S., Mutebi M. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet. 2017 Feb 25;389(10071):861–870. doi: 10.1016/S0140-6736(16)31795-0. [DOI] [PubMed] [Google Scholar]
- 24.Steben M., Jeronimo J., Wittet S. Upgrading public health programs for human papillomavirus prevention and control is possible in low- and middle-income countries. Vaccine. 2012 Nov 20;30(Suppl 5):F183–F191. doi: 10.1016/j.vaccine.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Almario C.V., Chey W.D., Iriana S. Computer versus physician identification of gastrointestinal alarm features. Int. J. Med. Inf. 2015;84(12):1111–1117. doi: 10.1016/j.ijmedinf.2015.07.006. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng F.F., Mitchell S.M., Sekikubo M. Understanding the role of embarrassment in gynaecological screening: a qualitative study from the ASPIRE cervical cancer screening project in Uganda. BMJ Open. 2014;4(4) doi: 10.1136/bmjopen-2014-004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IARC . vol 10. IARCPress; Lyon: 2005. (Cervix Cancer Screening. IARC Handbooks of Cancer Prevention). [Google Scholar]
- 28.Wood L., Egger M., Gluud L.L. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008 Mar 15;336(7644):601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sultana F., Mullins R., Murphy M. Women’s views on human papillomavirus self-sampling: focus groups to assess acceptability, invitation letters and a test kit in the Australian setting. Sex. Health. 2015 Jun 1;12(4):279–286. doi: 10.1071/SH14236. [DOI] [PubMed] [Google Scholar]
- 30.Arbyn M., Peeters E., Benoy I. VALHUDES: a protocol for VALidation of HUman papillomavirus assays and collection DEvices for HPV testing on Self-samples and urine samples. J. Clin. Virol. 2018;117:52–56. doi: 10.1016/j.jcv.2018.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.