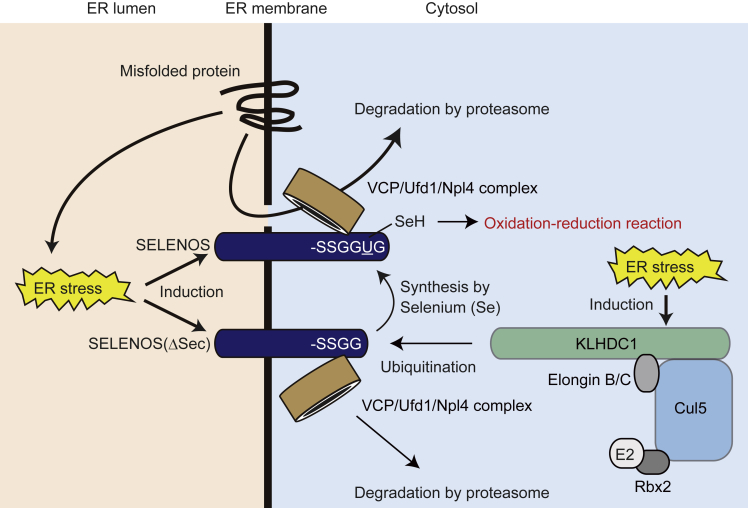
Summary
The UGA codon signals protein translation termination, but it can also be translated into selenocysteine (Sec, U) to produce selenocysteine-containing proteins (selenoproteins) by dedicated machinery. As Sec incorporation can fail, Sec-containing longer and Sec-lacking shorter proteins co-exist. Cul2-type ubiquitin ligases were recently shown to destabilize such truncated proteins; however, which ubiquitin ligase targets truncated proteins for degradation remained unclear. We report that the Cul5-type ubiquitin ligase KLHDC1 targets truncated SELENOS, a selenoprotein, for proteasomal degradation. SELENOS is involved in endoplasmic reticulum (ER)-associated degradation, which is linked to reactive oxygen species (ROS) production, and the knockdown of KLHDC1 in U2OS cells decreased ER stress-induced cell death. Knockdown of SELENOS increased the cell population with lower ROS levels. Our findings reveal that, in addition to Cul2-type ubiquitin ligases, KLHDC1 is involved in the elimination of truncated oxidoreductase-inactive SELENOS, which would be crucial for maintaining ROS levels and preventing cancer development.
Subject Areas: Molecular Biology, Cell Biology, Cancer
Graphical Abstract
Highlights
-
•
KLHDC1 is a Cul5-type ubiquitin ligase
-
•
KLHDC1 targets immature SELENOS for proteasomal degradation
-
•
KLHDC1 knockdown in U2OS cells decreases ER stress-induced cell death
Molecular Biology; Cell Biology; Cancer
Introduction
Protein sequences generally consist of 20 common amino acids. The UGA mRNA codon signals protein translation termination, but it can also be translated into selenocysteine (Sec, U), the 21st amino acid, when a Sec insertion sequence (SECIS) element in the mRNA 3′ untranslated region (3′ UTR), Sec tRNA, Sec-specific elongation factor, and the SECIS-binding protein SBP2 are present (Papp et al., 2007, Vindry et al., 2018). At least 25 selenoproteins, including glutathione peroxidase, thioredoxin reductase, and selenoprotein S (SELENOS, formerly known as valosin-containing protein [VCP]-interacting membrane protein) have been identified (Davis et al., 2012, Gladyshev et al., 2016, Kryukov et al., 2003, Ye et al., 2004). Selenoproteins are largely involved in oxidation-reduction reactions (Davis et al., 2012). In vitro, SELENOS possesses both reductase and peroxidase activities, but only when Sec is incorporated and not when Sec is mutated to Cys (Liu et al., 2013, Liu and Rozovsky, 2013). These findings indicate the importance of Sec for the oxidoreductase activity of SELENOS.
UGA redefinition is regulated by selenium availability (Howard et al., 2013) and involves a risk of premature translation termination. Indeed, Sec-containing mature and Sec-lacking truncated selenoproteins have been detected in human embryonic kidney (HEK)293 cells (Lin et al., 2015). Human SELENOS consists of 189 amino acids, and Sec is the 188th amino acid. Therefore, it is difficult to distinguish mature and truncated SELENOS, and thus, it remains unknown how efficiently mature SELENOS is translated. SELENOS is a single transmembrane protein located at the ER membrane that eliminates misfolded proteins from the ER via retro-translocation in collaboration with VCP (Christensen et al., 2012, Ye et al., 2004). SELENOS expression is upregulated upon treatment with the ER stress-inducer tunicamycin (TM), which prevents N-linked protein glycosylation, and the knockdown of SELENOS inhibits membrane translocation of VCP and enhances ER stress-induced apoptosis (Kim et al., 2007, Lee et al., 2014). Importantly, Pro178 and Pro183, but not Sec188, are important for interactions with VCP and ER-associated protein degradation (ERAD) (Lee et al., 2014).
The two major functions of SELENOS, oxidoreductase activity and ERAD-related functions, are dependent or independent on Sec, respectively, indicating the importance of regulating the expression levels of mature and truncated SELENOS. Cullin 2 (Cul2)-containing ubiquitin ligase has been shown to target truncated but not mature SELENOS (Lin et al., 2015). Cullin really interesting new gene (RING) ligases (CRLs) function in the ubiquitin proteasome system, and a protein complex consisting of Cul2, elongins B and C, von Hippel–Lindau (VHL) box protein, and RING-box protein 1 (RBX1) belongs to the CRL superfamily (Kamura et al., 2004a, Lipkowitz and Weissman, 2011, Okumura et al., 2012). Proteins containing the VHL box target their substrate for ubiquitination and proteasomal degradation. Among VHL box proteins, Kelch domain-containing (KLHDC) 2 and 3 have been shown to destabilize truncated SELENOS directly or indirectly (Lin et al., 2015). Five to seven Kelch repeats form a β-propeller structure that is involved in protein-protein interactions (Adams et al., 2000). Recent research has revealed a “C-end rule,” which implies that the amino acid sequence at the C terminus of a protein acts as a degron, a specific, short peptide motif of a substrate protein recognized by ubiquitin ligase (Koren et al., 2018, Lucas and Ciulli, 2017, Varshavsky, 1991). KLHDC2 recognizes the C-terminal -Gly-Gly as a degron (Koren et al., 2018, Lin et al., 2018), whereas KLHDC3 recognizes the C-terminal -Arg-(any amino acid, Xaa)n-Arg-Gly, -Arg-(Xaa)n-Lys-Gly, and -Arg-(Xaa)n-Gln-Gly sequences (Koren et al., 2018, Lin et al., 2018). As the C terminus of mature SELENOS is -Gly-Gly-Sec-Gly, it is not recognized by KLHDC2/3. However, truncated SELENOS ends with -Gly-Gly, and thus, it is targeted by KLHDC2 for ubiquitination and proteasomal degradation. KLHDC2 is the best-studied Cul2-type ubiquitin ligase that targets truncated but not mature SELENOS.
KLHDC1, 2, and 3 belong to the same protein family; their identity and similarity, respectively, are as follows: 40.8% and 54.9% between KLHDC1 and KLHDC2, 23.1% and 34.2% between KLHDC1 and KLHDC3, and 23.5% and 36.7% between KLHDC2 and KLHDC3 (based on pairwise alignments using EMBOSS Needle, https://www.ebi.ac.uk/Tools/psa/emboss_needle/). Nevertheless, ectopic KLHDC1 and KLHDC2 predominantly localize to the cytoplasm and the nucleus, respectively (Chin et al., 2007), suggesting that they have different biological roles. It has not clear whether KLHDC1 also targets truncated SELENOS, as does KLHDC2, and whether KLHDC1 also interacts with Cul2. The aim of the current study was to identify the substrate protein of KLHDC1. We purified and identified KLHDC1-interacting proteins in HEK293T cells by mass spectrometry. To study the hitherto unknown biological function of KLHDC1, we established KLHDC1-knockdown U2OS cell lines and examined ER stress-dependent cell death and reactive oxygen species (ROS) levels.
Results
KLHDC1 Interacts with SELENOS and Cul5
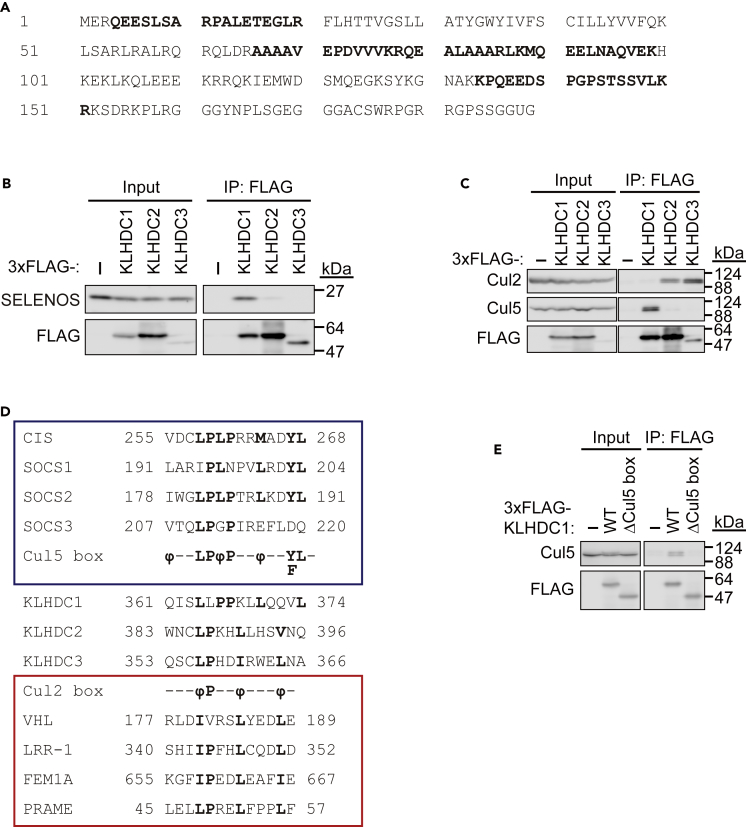
To identify the potential KLHDC1-interacting proteins by mass spectrometry, 3×FLAG-tagged KLHDC1 (3×FLAG-KLHDC1) was purified from HEK293T cell lysates. Cul5, but not Cul2, and SELENOS were identified as KLHDC1-interacting candidates. The peptides of SELENOS identified by mass spectrometry are shown in Figure 1A. To confirm this, 3×FLAG-KLHDC1, 3×FLAG-KLHDC2, and 3×FLAG-KLHDC3 were expressed in HEK293T cells, cell lysates were subjected to immunoprecipitation (IP) with an anti-FLAG antibody, and the immunoprecipitates were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting (IB) with an anti-SELENOS or anti-FLAG antibody. The interaction between 3×FLAG-KLHDC1 and endogenous SELENOS was confirmed (Figure 1B). We also detected weak interaction between KLHDC2 and SELENOS. Given that the anti-SELENOS antibody recognizes both mature and truncated SELENOS, it was not clear which form is detected by KLHDC1. Next, 3×FLAG-KLHDC1, 3×FLAG-KLHDC2, and 3×FLAG-KLHDC3 were expressed in HEK293T cells, cell lysates were subjected to IP with an anti-FLAG antibody, and the immunoprecipitates were subjected to SDS-PAGE and IB with an anti-Cul2, anti-Cul5, or anti-FLAG antibody. In line with previously reported findings, KLHDC2 and KLHDC3 interacted with Cul2 (Koren et al., 2018, Lin et al., 2015, Lin et al., 2018) (Figure 1C). In contrast, KLHDC1 interacted only with Cul5, suggesting the existence of a Cul5 box, but not a Cul2 box, in this protein (Kamura et al., 2004b, Mahrour et al., 2008, Okumura et al., 2012). The amino acid sequences of KLHDC1, KLHDC2, and KLHDC3 were aligned with those of well-known Cul2 box-containing proteins including VHL tumor suppressor (VHL), leucine-rich repeat protein (LRR)-1, feminization 1 (FEM1)A, and preferentially expressed antigen of melanoma (PRAME), as well as Cul5 box-containing proteins including cytokine-inducible Src homology 2 domain-containing protein (CIS), suppressor of cytokine signaling (SOCS)1, SOCS2, and SOCS3 (Figure 1D). This revealed that KLHDC1 contains a consensus Cul5 box sequence ϕxxLPϕPxxϕxxYL, whereas KLHDC2 and KLHDC3 contain the consensus Cul2 box sequence ϕPxxϕxxxϕ, where ϕ represents hydrophobic amino acids and x represents any amino acid. Next, we deleted the Cul5 box (amino acids 340–406) from KLHDC1 (KLHDC1(ΔCul5 box)), which resulted in a loss of interaction with Cul5 (Figure 1E). These findings indicated that KLHDC1 is a Cul5-interacting protein that recognizes SELENOS.
Figure 1.
KLHDC1 Interacts with SELENOS and Cul5
(A) Amino acid sequence of human SELENOS. Identified peptides by mass spectrometry are shown in bold font. U, selenocysteine.
(B) SELENOS interacts with KLHDC1 and KLHDC2. 3×FLAG-KLHDC1, KLHDC2, or KLHDC3 was expressed in HEK293T cells, and cell lysates were subjected to immunoprecipitation (IP) with an anti-FLAG antibody and immunoblotted with an anti-FLAG or anti-SELENOS antibody.
(C) KLHDC1 is a Cul5-type ubiquitin ligase. 3×FLAG-KLHDC1, KLHDC2, or KLHDC3 was expressed in HEK293T cells, and cell lysates were subjected to IP with an anti-FLAG antibody and immunoblotted with an anti-Cul2, anti-Cul5, or anti-FLAG antibody.
(D) Amino acid sequence alignment of Cul2 box- or Cul5 box-containing human proteins. The numbers indicate the amino acid position in each protein. Conserved Cul5 box and Cul2 box sequences are shown at the bottom or the top of the box, respectively, in bold font. ϕ, hydrophobic residue.
(E) Cul5 box-dependent interaction between KLHDC1 and Cul5. 3×FLAG-KLHDC1 (wild-type, WT) or a Cul5 box-deletion mutant KLHDC1(ΔCul5 box) was expressed in HEK293T cells, and cell lysates were subjected to IP with an anti-FLAG antibody and immunoblotted with an anti-Cul5 or anti-FLAG antibody.
KLHDC1 Destabilizes Truncated SELENOS (SELENOS(ΔSec))
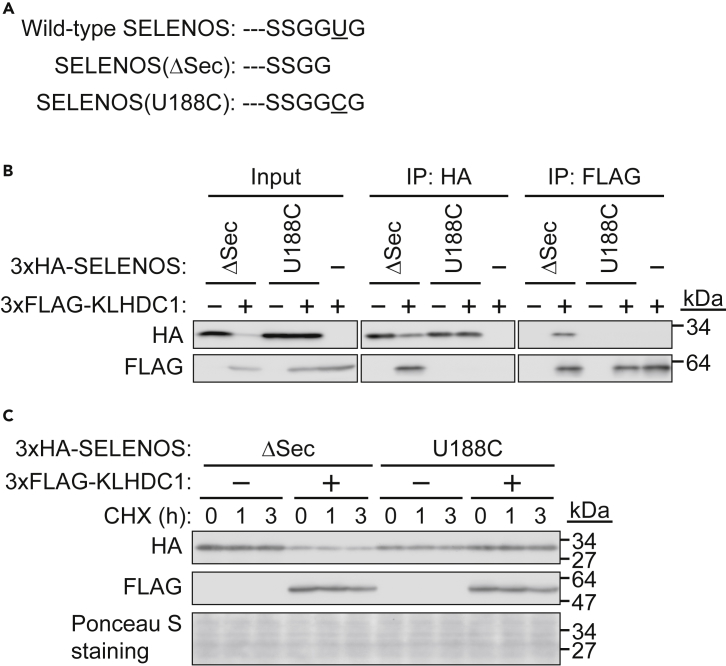
KLHDC2 targets truncated but not mature SELENOS (Koren et al., 2018, Lin et al., 2018). Therefore, we examined whether KLHDC1, which strongly interacts with SELENOS rather than KLHDC2 (Figure 1B), also destabilizes truncated SELENOS. A SELENOS(U188C) mutant is generally utilized as full-length native SELENOS in experiments because the plasmid construct used to express full-length Sec-containing SELENOS with the SECIS element in the 3′ UTR possibly produces both full-length and truncated SELENOS (Lin et al., 2015), making it difficult to determine whether only full-length (or even, only truncated) SELENOS is expressed and how efficiently it is expressed. In this study, we also utilized SELENOS(U188C) as the full-length protein and compared it with SELENOS(ΔSec) in terms of its interaction with KLHDC1 (Figures 2A and 2B). Here, 3×FLAG-KLHDC1 and 3×hemagglutinin (HA)-tagged SELENOS(ΔSec or U188C) were expressed in HEK293T cells, cell lysates were subjected to IP with an anti-FLAG or anti-HA antibody, and the immunoprecipitates were subjected to SDS-PAGE and western blotting with an anti-HA or anti-FLAG antibody. 3×FLAG-KLHDC1 interacted with 3×HA-SELENOS(ΔSec) but not SELENOS(U188C) (Figure 2B). As KLHDC1 overexpression led to downregulation of the expression of SELENOS(ΔSec) but not SELENOS(U188C), we examined the protein stability of SELENOS(ΔSec) and SELENOS(U188C) in the presence or absence of KLHDC1 overexpression. HEK293T cells were transfected with expression vectors for 3×FLAG KLHDC1 and 3×HA SELENOS(ΔSec or U188C) and treated with the protein translation inhibitor cycloheximide, after which protein levels were determined. As shown in Figure 2C, both SELENOS(ΔSec) and SELENOS(U188C) were stable for at least 3 h in the absence of ectopic KLHDC1 expression. In contrast, SELENOS(ΔSec) expression was lower than that of SELENOS(U188C) with ectopic KLHDC1 expression. Nevertheless, SELENOS(ΔSec) was still stable for up to 3 h. Since SELENOS was transiently expressed in cells, the initial expression levels were slightly different, which was caused by different transfection efficiencies. For example, the expression of SELENOS(U188C) was slightly lower in the absence of 3×FLAG KLHDC1 compared with levels in the presence of 3×FLAG KLHDC1. These findings suggested that KLHDC1 binds to SELENOS(ΔSec) but not SELENOS(U188C) and destabilizes SELENOS(ΔSec) after more than 3 h under the conditions used in this study.
Figure 2.
KLHDC1 Destabilizes Truncated SELENOS (SELENOS(ΔSec))
(A) Alignment of the C-terminal amino acid sequence of human SELENOS and mutants. Wild-type SELENOS contains selenocysteine (U) before the last glycine (G). SELENOS(ΔSec) is produced by failed U incorporation during protein translation and results in (A) -GG end. SELENOS(U188C) is generally utilized as wild-type SELENOS.
(B) KLHDC1 recognizes SELENOS(ΔSec) but not SELENOS(U188C). 3×HA-SELENOS(ΔSec) or SELENOS(U188C) was expressed in HEK293T cells, and cell lysates were subjected to immunoprecipitation (IP) with an anti-HA or anti-FLAG antibody and immunoblotted with an anti-HA or anti-FLAG antibody.
(C) SELENOS(ΔSec) is weakly expressed in the presence of KLHDC1. 3×HA-SELENOS(ΔSec) or SELENOS(U188C) was expressed in HEK293T cells with or without 3×FLAG-KLHDC1. The cells were exposed to cycloheximide (25 μg/mL) for 1 or 3 h, and cell lysates were subjected to immunoblotting (IB) with an anti-HA or anti-FLAG antibody. Ponceau S staining rather than a particular protein, such as tubulin or actin, was used as a loading control.
Ubiquitination and Proteasome-Dependent Destabilization of SELENOS(ΔSec) by KLHDC1
As KLHDC1 was found to interact with Cul5 (Figure 1) and KLHDC2 and KLHDC3 act as Cul2-type ubiquitin ligases (Koren et al., 2018, Lin et al., 2015, Lin et al., 2018), KLHDC1 was considered a potential Cul5-type ubiquitin ligase. Therefore, we examined whether downregulation of SELENOS(ΔSec) expression upon KLHDC1 overexpression depends on proteasome activity. 3×FLAG-KLHDC1 and 3×HA-SELENOS(ΔSec or U188C) were expressed in HEK293T cells that were then treated with the proteasome inhibitor MG132 (2 μM for 15 h), and cell lysates were subjected to SDS-PAGE and IB with an anti-HA, anti-FLAG, or anti-polyubiquitin antibody (FK2) (Figure 3A). SELENOS(ΔSec or U188C) did not accumulate upon MG132 treatment in the absence of 3×FLAG-KLHDC1. In contrast, SELENOS(ΔSec) but not SELENOS(U188C) accumulated upon MG132 treatment in the presence of 3×FLAG-KLHDC1, indicating that the downregulation of SELENOS(ΔSec) by 3×FLAG-KLHDC1 was achieved through proteasomal degradation. 3×FLAG-KLHDC1 was also degraded by the proteasome, and IB with an anti-polyubiquitin antibody showed that proteasome inhibition was the same in all samples. Next, polyubiquitination of SELENOS was examined in the presence or absence of 3×FLAG-KLHDC1 by utilizing His6-ubiquitin (His6-Ub) (Figure 3B). 3×FLAG-KLHDC1 and 3×HA-SELENOS(ΔSec or U188C) were expressed in HEK293T cells that were then treated with MG132 and lysed with 8 M urea-containing lysis buffer to dissociate any protein-protein interactions. His6-Ub and covalently His6-Ub-modified proteins were pulled down using Ni-agarose and subjected to SDS-PAGE and IB with an anti-HA, anti-FLAG, or anti-His6 antibody (Figure 3B). Results showed that 3×HA-SELENOS(ΔSec) and 3×HA-SELENOS(U188C) were polyubiquitinated. Importantly, the polyubiquitination of 3×HA-SELENOS(ΔSec) but not 3×HA-SELENOS(U188C) was increased with the co-expression of 3×FLAG-KLHDC1, verifying that KLHDC1 targets SELENOS(ΔSec). In addition, 3×FLAG-KLHDC1 was also shown to be polyubiquitinated under these experimental conditions. These findings indicated that KLHDC1 destabilizes SELENOS(ΔSec) but not SELENOS(U188C) via polyubiquitination and proteasomal degradation.
Figure 3.
Ubiquitination and Proteasome-Dependent Destabilization of SELENOS(ΔSec) by KLHDC1
(A) 3×HA-SELENOS(ΔSec) or SELENOS(U188C) was expressed in HEK293T cells, which were cultured in the presence or absence of MG132 (2 μM for 15 h). Cell lysates were subjected to immunoblotting (IB) with an anti-HA, anti-FLAG, or anti-polyubiquitin antibody. Ponceau S staining was used as a loading control.
(B) KLHDC1-dependent ubiquitination of SELENOS(ΔSec). 3×HA-SELENOS(ΔSec) or SELENOS(U188C) was expressed in HEK293T cells with or without 3×FLAG-KLHDC1 and His6-ubiquitin (Ub). The cells were cultured in the presence of MG132 (2 μM for 15 h). Cell lysates containing 8 M urea, which prevents protein-protein interactions, were subjected to Ni-NTA agarose pull down to purify proteins modified by His6-Ub, followed by IB analysis with an anti-HA, anti-FLAG, or anti-His6 antibody.
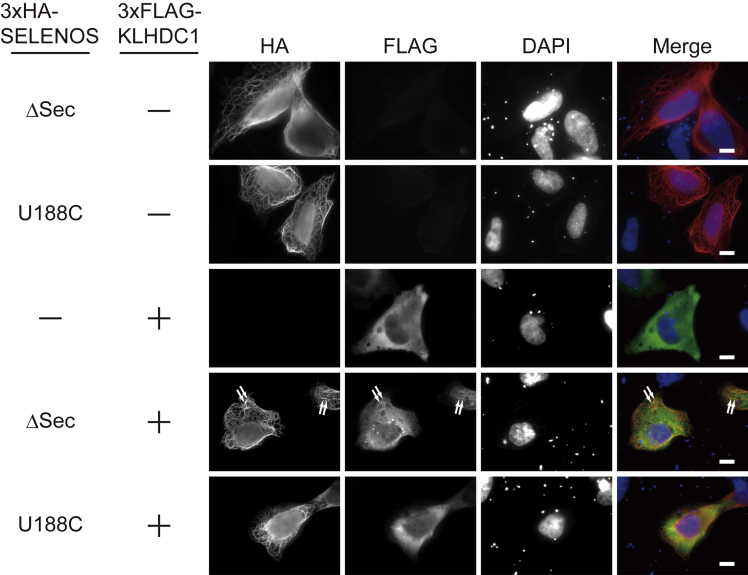
SELENOS(ΔSec) Co-localizes with KLHDC1
Microtubules (MTs) interact with ER-residing proteins and play important roles in ER membrane organization and dynamics in mammalian cells (Goyal and Blackstone, 2013, Vedrenne and Hauri, 2006). In previous studies, the overexpression of SELENOS(ΔSec) was found to change ER morphology to filamentous structures by interacting with MTs and the rough ER protein cytoskeleton-linking membrane protein (CLIMP)-63 but not the smooth ER protein receptor expression-enhancing protein 1 (Noda et al., 2014, Ye et al., 2004). The knockdown of SELENOS resulted in the migration of the ER membrane proteins Sec61 and CLIMP-63 to the cell periphery, suggesting the involvement of SELENOS in ER structural organization (Noda et al., 2014). In line with these previous findings, the overexpression of SELENOS(ΔSec) resulted in filamentous ER-MT bundles (Figure 4). It remained unclear whether mature SELENOS has similar activity; however, given that the overexpression of SELENOS(U188C) also resulted in filamentous ER-MT bundles, mature SELENOS might function in ER structural organization. Therefore, the quality control of SELENOS mediated by KLHDC1 is probably not important for its function in ER organization. Nevertheless, the localization of KLHDC1 was examined (Figure 4). Although ectopic KLHDC1 localized mainly to the cytosol upon co-overexpression with SELENOS(ΔSec), a very small fraction of KLHDC1 re-localized to the filamentous structures and co-localized with SELENOS(ΔSec), supporting the idea that KLHDC1 interacts with SELENOS(ΔSec) (Figure 2). In contrast, co-overexpression of SELENOS(U188C) did not lead to the re-localization of KLHDC1 to filamentous structures (Figure 4). These findings suggested that both mature and truncated SELENOS are involved in ER organization in collaboration with MTs and some ER-residing proteins and that the quality control of SELENOS mediated by KLHDC1 (i.e., degradation of truncated SELENOS) is not important for ER organization.
Figure 4.
SELENOS(ΔSec) Co-localizes with KLHDC1
U2OS cells expressing 3×HA-SELENOS(ΔSec) or SELENOS(U188C) with or without 3×FLAG-KLHDC1 were immunostained with anti-HA and anti-FLAG antibodies. Nuclei were stained with DAPI. Scale bar, 10 μm. Representative co-localization is indicated by white arrows.
KLHDC1 Enhances ER Stress-Induced U2OS Cell Death
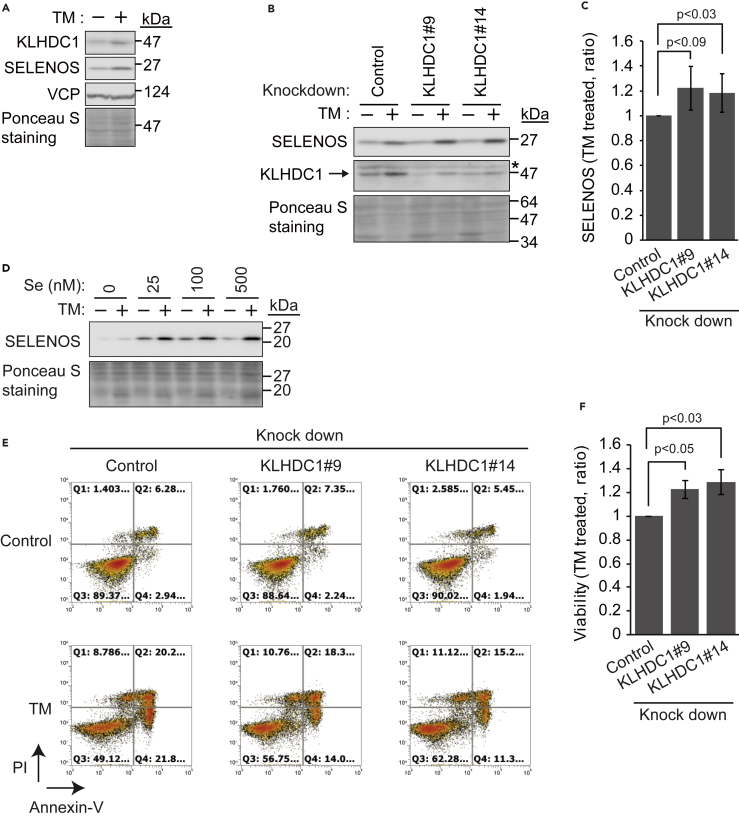
We further examined whether KLHDC1 expression is induced by ER stress and if KLHDC1 affects ER stress-induced apoptosis. We utilized U2OS osteosarcoma cells, which express wild-type p53. U2OS cells were cultured in the presence of TM (1 μg/mL) for 24 h, and the expression of KLHDC1, SELENOS (mature and truncated forms), and VCP was analyzed (Figure 5A). KLHDC1 and SELENOS, but not VCP, were induced by TM treatment. If KLHDC1 targets SELENOS(ΔSec) but not mature SELENOS for proteasomal degradation, the downregulation of KLHDC1 should result in SELENOS(ΔSec) accumulation and total SELENOS should increase. Thus, we established two independent KLHDC1-knockdown cell lines (#9 and #14) and compared SELENOS expression (mature and truncated forms) in the presence or absence of TM treatment (1 μg/mL for 24 h; Figures 5B and 5C). Total SELENOS was slightly increased upon KLHDC1 knockdown only in the presence of TM. This result prompted us to examine selenium-dependent stabilization of SELENOS. U2OS cells were cultured without serum, which contains selenium at approximately 15 nM (Touat-Hamici et al., 2014) but might vary depending on the calf, for 15 h. Then, medium was changed, and cells were incubated for 1 day with or without TM (1 μg/mL) and different concentrations of selenium (0–500 nM) in the absence of serum. SELENOS was expressed and upregulated by TM treatment without selenium supplement, but expression was weaker than that in the selenium-supplemented condition (Figure 5D). Furthermore, 25 nM selenium was sufficient to upregulate SELENOS expression with or without TM treatment, implying that selenium is incorporated into SELENOS to produce full-length SELENOS, which is not recognized by KLHDC1 for proteasomal degradation. Nevertheless, it was suggested that the slight increase in total SELENOS was the result of SELENOS(ΔSec) accumulation (Figures 5B and 5C), that SELENOS(ΔSec) represents a minor fraction of these forms, and/or that another ubiquitin ligase such as KLHDC2 is also involved in the proteasomal degradation of SELENOS(ΔSec) as suggested previously (Lin et al., 2015). The role of KLHDC1 in ER stress-induced apoptosis was examined by fluorescence-activated cell sorting (FACS; Figures 5E and 5F). Control and KLHDC1-knockdown U2OS cells (#9 and #14) were treated with TM and stained with propidium iodide (PI) and Annexin V. PI is a fluorescent intercalating agent, which is not membrane permeable, and therefore, it is useful to differentiate dead and healthy cells based on membrane integrity. Phosphatidylserine is located on the inner surface of the cell membrane but is exposed on the outside during apoptosis (Wlodkowic et al., 2012). Annexin V is not membrane permeable and binds phosphatidylserine, thus recognizing apoptotic cells. The Annexin V-positive and PI-negative early-apoptotic cell populations (Q4 fraction in Figure 5E) and Annexin V and PI double-positive late-apoptotic cell populations (Q2 fraction in Figure 5E) were partly decreased by KLHDC1 knockdown. Q1 and Q3 fractions indicate non-apoptotic dead cell populations and viable cell populations, respectively. In other words, the loss of cell viability induced by TM treatment (from 89% to 49% of control cell) was partly rescued by KLHDC1 knockdown (from 89% to 57% of KLHDC1#9 knockdown cells and from 90% to 62% of KLHDC1#14 knockdown cells). Given that Pro178 and Pro183, but not Sec188, are important for interactions with VCP and ERAD (Lee et al., 2014), SELENOS(ΔSec) accumulation upon KLHDC1 knockdown would contribute to ERAD and overcome ER stress-dependent cell death. Together, these findings suggested that ER stress-induced KLHDC1 targets SELENOS(ΔSec), which binds VCP and supports the degradation of undesirable proteins in the ER. Therefore, the downregulation of KLHDC1 led to a partial increase of total SELENOS and cell viability under ER stress and SELENOS(ΔSec) was suggested to be a minor fraction compared with mature SELENOS in the current experimental conditions.
Figure 5.
KLHDC1 Enhances ER Stress-Induced U2OS Cell Death
(A) KLHDC1 is induced by tunicamycin (TM) treatment. U2OS cells were cultured in the presence or absence of TM (1 μg/mL) for 24 h, and cell lysates were subjected to immunoblotting (IB) with an anti-KLHDC1, anti-SELENOS, or anti-VCP antibody. Ponceau S staining was used as a loading control.
(B) Control or KLHDC1-knockdown (#9 or #14) U2OS cells were treated with TM (1 μg/mL) for 24 h, and cell lysates were subjected to IB with an anti-KLHDC1 or anti-SELENOS antibody. Ponceau S staining was used as a loading control.
(C) Quantification of SELENOS expression in (B). SELENOS signals were normalized to those of ponceau S staining. Expression in control samples with TM treatment was set as 1. Data represent the mean ± SD of three independent experiments.
(D) U2OS cells were cultured without serum for 15 h. Then, medium was changed, and cells were incubated for 1 day with or without TM (1 μg/mL) and different concentrations of selenium in the absence of serum. Cell lysates were subjected to IB with an anti-SELENOS antibody. Ponceau S staining was used as a loading control.
(E) KLHDC1 knockdown partly prevents cell death induced by TM treatment. Control or KLHDC1-knockdown (#9 or #14) U2OS cells were treated with TM (1 μg/mL) for 24 h, and the cells were stained with propidium iodide (PI) and Annexin V and subjected to FACS analysis. PI-negative and Annexin V-negative populations represent viable cells. Cell numbers in Q1–4 indicate the cell population in each fraction. Representative data from three independent experiments are shown.
(F) Cell viability of control and KLHDC1-knockdown (#9 or #14) U2OS cells after TM treatment. The viability of control knockdown cells with TM treatment was set as 1. Data represent the mean ± SD of three independent experiments. Statistical significance of differences was not considered, as described in the methods section.
ROS Production Is Decreased by Downregulating SELENOS in U2OS Cells
The oxidoreductase activity of SELENOS in vivo and the underlying molecular mechanisms and substrate have not been well studied. Therefore, we examined global cellular ROS levels by staining cells with the superoxide indicator dihydroethidium (DHE) (Gomes et al., 2005, Wardman, 2007, Wojtala et al., 2014, Zhao et al., 2003). DHE is a cell-permeable blue fluorescent dye that upon reaction with superoxide anion forms a red fluorescent product, 2-hydroxyethidium, which intercalates DNA (Wojtala et al., 2014). DHE is also oxidized by peroxynitrite (ONOO−), hydroxyl radical (⋅OH), and cytochrome c into ethidium (Wojtala et al., 2014). The fluorescence spectra of 2-hydroxyethidium and ethidium are similar, and these oxidization products are generally both taken into account. We established two independent SELENOS-knockdown U2OS cell lines (#210 and #247) as reported previously (Noda et al., 2014) (Figure S1A). SELENOS-knockdown and control cells, as well as KLHDC1-knockdown cells, were cultured with or without TM (1 μg/mL) for 24 h, incubated with DHE (2 μM) for 30 min, and subjected to FACS (Figure S1B). Cells were conveniently divided into two groups, comprising DHE staining positive and negative. KLHDC1 knockdown did not affect the ROS level in the presence or absence of TM (approximately 50%–60% of cells were DHE staining negative) compared with that in control cells (approximately 50% of cells were DHE staining negative). In contrast, SELENOS knockdown decreased ROS levels (approximately 70%–80% of cells were DHE staining negative) regardless of TM treatment (Figure S1B). These findings suggested that SELENOS enhanced ROS production or decreased ROS removal activity. SELENOS is an oxidoreductase, and it is not clear whether fluctuations in ROS levels are dependent on SELENOS activity directly or indirectly. To confirm the importance of Sec in SELENOS, SELENOS-knockdown cells were reconstituted with SELENOS(ΔSec) or SELENOS(U188C) (Figure S2A). SELENOS(ΔSec) was weakly expressed in control vector-transduced cells, probably because of KLHDC1 and KLHDC2-dependent proteasomal degradation. The expression of SELENOS(U188C) was stronger than that of SELENOS(ΔSec) but was downregulated upon TM treatment. SELENOS-knockdown and control cells were cultured with or without TM (1 μg/mL) for 24 h, incubated with DHE (2 μM) for 30 min, and analyzed by FACS (Figure S2B). Cells were conveniently divided into two groups, comprising DHE staining positive and negative. Given that SELENOS(ΔSec) was weakly expressed, it was not clear whether it was involved in ROS production. SELENOS(U188C) did not affect the ROS level in the absence of TM treatment compared with that with control treatment (approximately 90% of both cell lines were DHE staining negative), indicating that the Sec residue of SELENOS is crucial for ROS production. Consistent herewith, Sec was shown to be required for SELENOS oxidoreductase activity (Liu et al., 2013). TM did not affect ROS production in all conditions examined. These findings suggested that SELENOS promotes ROS production either directly or indirectly, although in vitro, SELENOS has reductase and peroxidase activities (Liu et al., 2013, Liu and Rozovsky, 2013). Finally, we examined the potential role of SELENOS in cell growth (Figure S2C). SELENOS-knockdown cell lines grew faster than control cells, and SELENOS(U188C) expression did not affect cell growth. As ROS can initiate and prevent cancer development (Mitra et al., 2019), there might be a relationship between the increased U2OS cell growth and the reduced ROS level. Together, these findings indicated the importance of mature SELENOS for ROS production and cell growth.
Discussion
Here, we propose that KLHDC1, like KLHDC2, recognizes the -Gly-Gly degron at the C terminus of SELENOS(ΔSec) and induces protein destabilization. Interestingly, unlike KLHDC2, which is a Cul2-type ubiquitin ligase, KLHDC1 is a Cul5-type ubiquitin ligase. Both Cul2- and Cul5-containing ubiquitin ligases contain elongins B and C, which are adaptor proteins connecting Cul2 or Cul5 to Cul2 or Cul5 box-containing proteins (Kamura et al., 2004b, Mahrour et al., 2008, Okumura et al., 2012). Elongin BC complexes containing Cul2-type and Cul5-type ubiquitin ligases are considered two distinct protein assemblies (Kamura et al., 2004b, Mahrour et al., 2008). The fact that both KLHDC1 and KLHDC2 target SELENOS(ΔSec) suggests that these ubiquitin ligases might act in different circumstances and/or locations. The affinity of KLHDC2 for a SELENOS 8-aa degron was previously determined in vitro to be 21 nM (Rusnac et al., 2018), but it might be different in vivo. KLHDC1 and KLHDC2 predominantly localize to the cytoplasm and nucleus, respectively (Chin et al., 2007), which might reflect the high affinity of KLHDC1 for SELENOS. It might also be possible that a domain other than the SELENOS 8-aa degron might affect the affinity of KLHDC2 because full-length SELENOS was utilized in Figure 1B. KLHDC2 recognizes the -Gly-Gly degron of SELENOK(ΔSec), SELENOS(ΔSec), and the N-terminal proteolytic product of the deubiquitinating enzyme USP1 with different affinities, with SELENOK(ΔSec) showing the highest affinity (Rusnac et al., 2018), suggesting that KLHDC2 recognizes not only the -Gly-Gly degron but also other parts of the substrate protein. Therefore, although both KLHDC1 and KLHDC2 recognize the -Gly-Gly degron of SELENOS(ΔSec), the affinities might be different because of the different amino acid sequences of KLHDC1 and KLHDC2, which recognize sequences other than the degron.
MTs play important roles in ER membrane organization and dynamics via dynamic and static interactions with ER-residing proteins (Goyal and Blackstone, 2013, Vedrenne and Hauri, 2006). ER and Golgi apparatus-residing syntaxin 5 long form (Syn5L) contributes to ER structural organization by interacting with CLIMP-63 and MTs (Hui et al., 1997, Miyazaki et al., 2012). SELENOS interacts with MTs through CLIMP-63 and Syn5L regardless of the presence of Sec at position 188; however, deletion of the C-terminus of SELENOS in SELENOS(1–146) results in the loss of these interactions and consequently decreased ER-MT-bundling activity (Noda et al., 2014). Thus, mature SELENOS and SELENOS(ΔSec) seem to have similar roles in ER structural maintenance. In fact, SELENOS depletion causes migration of the ER to the cell periphery (Noda et al., 2014), suggesting that the quality control of SELENOS mediated by KLHDC1 and KLHDC2 in part prevents irregular ER-MT bundling by downregulating the total amount of SELENOS.
Given that SELENOS is localized to the ER membrane and interacts with VCP (Ye et al., 2004), its role in ERAD has been studied (Kim and Kim, 2013, Kim et al., 2007, Lee et al., 2014). These studies revealed that SELENOS aids in the removal of unfolded proteins from the ER membrane in collaboration with VCP and reduces ER stress in the mouse macrophage cell line RAW 264.7, mouse neuroblastoma cell line Neuro-2a, and mouse preadipocyte cell line 3T3 L1. In contrast, SELENOS does not protect intestinal epithelial cells from oxidative cell death, the unfolded protein response, and ER stress (Speckmann et al., 2014). In accordance herewith, we observed that SELENOS knockdown did not enhance TM-induced cell death (data not shown), although the increase in SELENOS induced by KLHDC1 knockdown partly protected U2OS cells from TM-induced cell death. These findings suggest that SELENOS depletion might activate other pathways to eliminate unexpected proteins from the ER membrane or that the degree of SELENOS contribution to ERAD might be cell type dependent. Nevertheless, it is suggested that SELENOS can support the removal of unwanted proteins.
The quality control of SELENOS mediated by KLHDC1 and KLHDC2 is expected to be important for maintaining oxidoreductase activity. It is assumed that a high level of Sec-containing mature SELENOS contributes to cell health by attenuating ER stress and maintaining the oxidoreductase system. KLHDC1 knockdown led to a slight increase in SELENOS(ΔSec), which supposedly is inactive, and as expected, we did not detect a difference in the ROS level in these cells. SELENOS knockdown decreased the ROS levels in cells, suggesting that SELENOS directly or indirectly produces ROS. The reduced glutathione content is slightly increased in SELENOS−/− mice compared with that in wild-type littermates (Addinsall et al., 2018), indicating the role of SELENOS in the oxidoreductase system. In fact, the mRNA expression of thioredoxin inhibitor protein and thioredoxin-1 is reduced in fast-twitch extensor digitorum longus (EDL) muscles but increased in soleus muscles in SELENOS−/− mice when compared with that in wild-type littermates (Addinsall et al., 2018). SELENOS knockout has no significant effect on mRNA expression of the ER stress chaperone glucose-regulated protein (Grp)78/binding immunoglobulin protein in EDL or soleus muscles (Addinsall et al., 2018, Wang et al., 2009). However, the mRNA expression of Grp94, which is also an indicator of ER stress and encodes an HSP90-like chaperone in the ER lumen (Lee, 1981, Marzec et al., 2012), and CCAAT-enhancer-binding protein homologous protein were found to be reduced in SELENOS−/− EDL muscles, whereas their expression was not affected in soleus muscles (Addinsall et al., 2018). These findings indicated that SELENOS has distinct effects on ER stress and the oxidoreductase system in different muscle types. Increased ER stress induces the expression of a number of genes, leading to activation of nuclear factor-κB, which then activates the transcription of genes such as those encoding proinflammatory cytokines (Pahl and Baeuerle, 1997). In fact, SELENOS suppresses inflammation, as indicated by the fact that SELENOS knockdown increases the production of interleukin-6 and tumor necrosis factor alpha in RAW264.7 macrophage cells (Curran et al., 2005). Given that Sec is necessary for both the reductase and peroxidase activities of SELENOS, at least in vitro (Liu et al., 2013, Liu and Rozovsky, 2013), SELENOS(ΔSec) might have a dominant negative effect on Sec-containing mature SELENOS during oxidoreductase reactions. Therefore, the quality control of SELENOS, that is, the degradation of SELENOS(ΔSec) by KLHDC1 and KLHDC2, is important to maintain the oxidoreductase system.
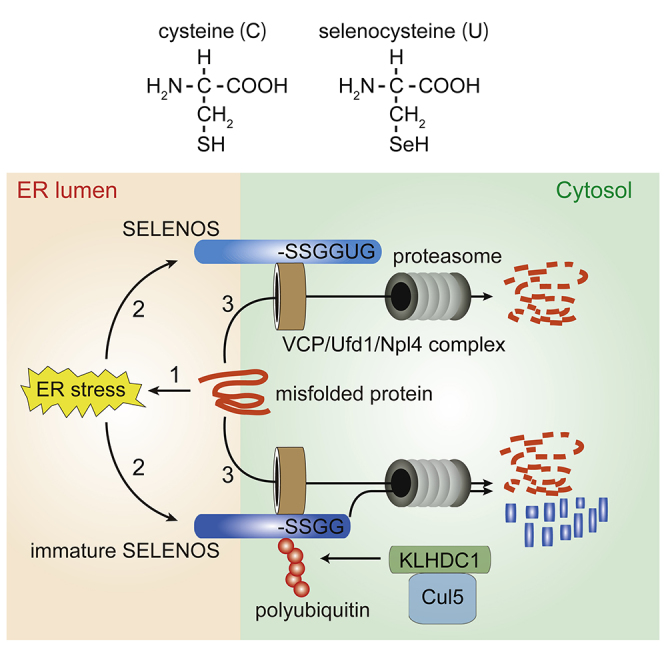
KLHDC1 was found to act as a Cul5-type ubiquitin ligase that recognizes the -Gly-Gly degron of SELENOS(ΔSec), flagging it for proteasomal degradation (Figure 6). Both mature SELENOS and SELENOS(ΔSec) can interact with the VCP/Ufd1/Npl4 complex (Buchberger et al., 2015, Lee et al., 2014) and degrade misfolded ER membrane-residing proteins to reduce ER stress. Interestingly, SELENOS is induced by ER stress and thus effectively contributes to ERAD. KLHDC1 is also induced by ER stress and degrades SELENOS(ΔSec), indicating that ERAD is partly prevented by a decrease in total SELENOS. This seems to be disadvantageous for ER stress reduction, but SELENOS also functions as an oxidoreductase. From this point of view, Sec is necessary for this activity, and SELENOS(ΔSec) might act as a dominant-negative enzyme by sequestering its physiological binding partner from mature SELENOS, thus suppressing the oxidoreductase system. The failed incorporation of selenium during protein translation and the lack of selenium intake led to a decrease in mature SELENOS and an increase in SELENOS(ΔSec). Therefore, KLHDC1-dependent degradation of SELENOS(ΔSec) would be important for maintenance of the oxidoreductase system.
Figure 6.
Putative Model of KLHDC1 and SELENOS-Dependent Regulation of Endoplasmic Reticulum (ER)-Associated Protein Degradation (ERAD) and Oxidation-Reduction Reactions
Misfolded proteins on ER membranes induce ER stress responses and SELENOS expression. Both selenocysteine (Sec)-lacking SELENOS(ΔSec) and mature SELENOS interact with the VCP/Ufd1/Npl4 complex and contribute to the proteasomal degradation of misfolded proteins. Sec is involved in oxidation-reduction reactions, although the exact molecular mechanism has not been elucidated, and is expected to regulate reactive oxygen species (ROS) levels in the cell. The Cul5-type ubiquitin ligase KLHDC1 is also induced by ER stress and targets only SELENOS(ΔSec), not mature SELENOS, for ubiquitination and proteasomal degradation.
Selenoproteins such as glutathione peroxidase, thioredoxin reductase, and SELENOS are largely involved in oxidation-reduction reactions (Davis et al., 2012), and selenium deficiency induces ER stress mainly by preventing native protein folding (Wang et al., 2013, Xu et al., 2018, Yao et al., 2015). Therefore, the quality control of SELENOS by ER stress-induced KLHDC1 is crucial. Given that KLHDC2 targets -Gly-Gly-ended proteins, KLHDC1 might also target -Gly-Gly-ended proteins other than SELENOS(ΔSec) and contribute to C-end role-dependent eukaryotic proteome shaping as recently suggested (Koren et al., 2018).
Limitations of the Study
Unfortunately, a SELENOS(ΔSec)-specific antibody that can distinguish SELENOS(ΔSec) from mature SELENOS is currently not available, and mass-spectrometric quantification of both forms has remained unsuccessful. Therefore, it was impossible to separately quantify mature SELENOS and SELENOS(ΔSec), as well as the half-life and ubiquitination of these proteins.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 17H03652 (to F.O., K.N., and T.K.) and 18K06213 (to F.O.), the Uehara Memorial Foundation (to F.O.), the Inamori Foundation (to F.O.), the Sumitomo Foundation (to F.O.), the Takeda Science Foundation (to F.O.), and Fukuoka Women’s University (to F.O.).
Author Contributions
Conceptualization, F.O. and T.K.; Methodology, Validation, Formal Analysis, Investigation, F.O., Y.F., N.O., and K.O.; Data Curation, F.O., A.N. Y.F., and T.K.; Writing – Original Draft, F.O.; Writing – Review & Editing, F.O., K.N., and T.K.; Supervision, F.O. and T.K.; Project Administration, F.O.; Funding Acquisition, F.O., K.N., and T.K.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100970.
Contributor Information
Fumihiko Okumura, Email: okumura@fwu.ac.jp.
Takumi Kamura, Email: z47617a@nucc.cc.nagoya-u.ac.jp.
Supplemental Information
References
- Adams J., Kelso R., Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- Addinsall A.B., Wright C.R., Shaw C.S., McRae N.L., Forgan L.G., Weng C.H., Conlan X.A., Francis P.S., Smith Z.M., Andrikopoulos S. Deficiency of selenoprotein S, an endoplasmic reticulum resident oxidoreductase, impairs the contractile function of fast-twitch hindlimb muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R380–R396. doi: 10.1152/ajpregu.00244.2017. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Schindelin H., Hanzelmann P. Control of p97 function by cofactor binding. FEBS Lett. 2015;589:2578–2589. doi: 10.1016/j.febslet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Chin K.T., Xu H.T., Ching Y.P., Jin D.Y. Differential subcellular localization and activity of kelch repeat proteins KLHDC1 and KLHDC2. Mol. Cell Biochem. 2007;296:109–119. doi: 10.1007/s11010-006-9304-6. [DOI] [PubMed] [Google Scholar]
- Christensen L.C., Jensen N.W., Vala A., Kamarauskaite J., Johansson L., Winther J.R., Hofmann K., Teilum K., Ellgaard L. The human selenoprotein VCP-interacting membrane protein (VIMP) is non-globular and harbors a reductase function in an intrinsically disordered region. J. Biol. Chem. 2012;287:26388–26399. doi: 10.1074/jbc.M112.346775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J.E., Jowett J.B., Elliott K.S., Gao Y., Gluschenko K., Wang J., Abel Azim D.M., Cai G., Mahaney M.C., Comuzzie A.G. Genetic variation in selenoprotein S influences inflammatory response. Nat. Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- Davis C.D., Tsuji P.A., Milner J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012;32:73–95. doi: 10.1146/annurev-nutr-071811-150740. [DOI] [PubMed] [Google Scholar]
- Gladyshev V.N., Arner E.S., Berry M.J., Brigelius-Flohe R., Bruford E.A., Burk R.F., Carlson B.A., Castellano S., Chavatte L., Conrad M. Selenoprotein gene nomenclature. J. Biol. Chem. 2016;291:24036–24040. doi: 10.1074/jbc.M116.756155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A., Fernandes E., Lima J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Goyal U., Blackstone C. Untangling the web: mechanisms underlying ER network formation. Biochim. Biophys. Acta. 2013;1833:2492–2498. doi: 10.1016/j.bbamcr.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M.T., Carlson B.A., Anderson C.B., Hatfield D.L. Translational redefinition of UGA codons is regulated by selenium availability. J. Biol. Chem. 2013;288:19401–19413. doi: 10.1074/jbc.M113.481051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N., Nakamura N., Sonnichsen B., Shima D.T., Nilsson T., Warren G. An isoform of the Golgi t-SNARE, syntaxin 5, with an endoplasmic reticulum retrieval signal. Mol. Biol. Cell. 1997;8:1777–1787. doi: 10.1091/mbc.8.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T., Hara T., Matsumoto M., Ishida N., Okumura F., Hatakeyama S., Yoshida M., Nakayama K., Nakayama K.I. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat. Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- Kamura T., Maenaka K., Kotoshiba S., Matsumoto M., Kohda D., Conaway R.C., Conaway J.W., Nakayama K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.Y., Kim K.H. Dexamethasone-induced selenoprotein S degradation is required for adipogenesis. J. Lipid Res. 2013;54:2069–2082. doi: 10.1194/jlr.M034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Gao Y., Walder K., Collier G.R., Skelton J., Kissebah A.H. SEPS1 protects RAW264.7 cells from pharmacological ER stress agent-induced apoptosis. Biochem. Biophysical Res. Commun. 2007;354:127–132. doi: 10.1016/j.bbrc.2006.12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren I., Timms R.T., Kula T., Xu Q., Li M.Z., Elledge S.J. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell. 2018;173:1622–1635 e1614. doi: 10.1016/j.cell.2018.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigo R., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Lee A.S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J. Cell Physiol. 1981;106:119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Kwon J.H., Jeon Y.H., Ko K.Y., Lee S.R., Kim I.Y. Pro178 and Pro183 of selenoprotein S are essential residues for interaction with p97(VCP) during endoplasmic reticulum-associated degradation. J. Biol. Chem. 2014;289:13758–13768. doi: 10.1074/jbc.M113.534529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.C., Ho S.C., Chen Y.Y., Khoo K.H., Hsu P.H., Yen H.C. SELENOPROTEINS. CRL2 aids elimination of truncated selenoproteins produced by failed UGA/Sec decoding. Science. 2015;349:91–95. doi: 10.1126/science.aab0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.C., Yeh C.W., Chen Y.F., Lee T.T., Hsieh P.Y., Rusnac D.V., Lin S.Y., Elledge S.J., Zheng N., Yen H.S. C-terminal end-directed protein elimination by CRL2 ubiquitin ligases. Mol. Cell. 2018;70:602–613 e603. doi: 10.1016/j.molcel.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz S., Weissman A.M. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer. 2011;11:629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li F., Rozovsky S. The intrinsically disordered membrane protein selenoprotein S is a reductase in vitro. Biochemistry. 2013;52:3051–3061. doi: 10.1021/bi4001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rozovsky S. Contribution of selenocysteine to the peroxidase activity of selenoprotein S. Biochemistry. 2013;52:5514–5516. doi: 10.1021/bi400741c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas X., Ciulli A. Recognition of substrate degrons by E3 ubiquitin ligases and modulation by small-molecule mimicry strategies. Curr. Opin. Struct. Biol. 2017;44:101–110. doi: 10.1016/j.sbi.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Mahrour N., Redwine W.B., Florens L., Swanson S.K., Martin-Brown S., Bradford W.D., Staehling-Hampton K., Washburn M.P., Conaway R.C., Conaway J.W. Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J. Biol. Chem. 2008;283:8005–8013. doi: 10.1074/jbc.M706987200. [DOI] [PubMed] [Google Scholar]
- Marzec M., Eletto D., Argon Y. GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Nguyen L.N., Akter M., Park G., Choi E.H., Kaushik N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers. 2019;11:1–31. doi: 10.3390/cancers11071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Wakana Y., Noda C., Arasaki K., Furuno A., Tagaya M. Contribution of the long form of syntaxin 5 to the organization of the endoplasmic reticulum. J. Cell Sci. 2012;125:5658–5666. doi: 10.1242/jcs.105304. [DOI] [PubMed] [Google Scholar]
- Noda C., Kimura H., Arasaki K., Matsushita M., Yamamoto A., Wakana Y., Inoue H., Tagaya M. Valosin-containing protein-interacting membrane protein (VIMP) links the endoplasmic reticulum with microtubules in concert with cytoskeleton-linking membrane protein (CLIMP)-63. J. Biol. Chem. 2014;289:24304–24313. doi: 10.1074/jbc.M114.571372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura F., Matsuzaki M., Nakatsukasa K., Kamura T. The role of elongin BC-containing ubiquitin ligases. Front. Oncol. 2012;2:10. doi: 10.3389/fonc.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H.L., Baeuerle P.A. The ER-overload response: activation of NF-kappa B. Trends Biochem. Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- Papp L.V., Lu J., Holmgren A., Khanna K.K. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Rusnac D.V., Lin H.C., Canzani D., Tien K.X., Hinds T.R., Tsue A.F., Bush M.F., Yen H.S., Zheng N. Recognition of the diglycine C-end degron by CRL2(KLHDC2) ubiquitin ligase. Mol. Cell. 2018;72:813–822 e814. doi: 10.1016/j.molcel.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckmann B., Gerloff K., Simms L., Oancea I., Shi W., McGuckin M.A., Radford-Smith G., Khanna K.K. Selenoprotein S is a marker but not a regulator of endoplasmic reticulum stress in intestinal epithelial cells. Free Radic. Biol. Med. 2014;67:265–277. doi: 10.1016/j.freeradbiomed.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Touat-Hamici Z., Legrain Y., Bulteau A.L., Chavatte L. Selective up-regulation of human selenoproteins in response to oxidative stress. J. Biol. Chem. 2014;289:14750–14761. doi: 10.1074/jbc.M114.551994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. Naming a targeting signal. Cell. 1991;64:13–15. doi: 10.1016/0092-8674(91)90202-a. [DOI] [PubMed] [Google Scholar]
- Vedrenne C., Hauri H.P. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vindry C., Ohlmann T., Chavatte L. Translation regulation of mammalian selenoproteins. Biochim. Biophys. Acta Gen. Subj. 2018 doi: 10.1016/j.bbagen.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Q., Niu X.L., Liu Z.W., Zhu Y.H., Gao D.F. Selenium deficiency is associated with endoplasmic reticulum stress in a rat model of cardiac malfunction. Biol. Trace Elem. Res. 2013;156:196–201. doi: 10.1007/s12011-013-9834-1. [DOI] [PubMed] [Google Scholar]
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Wlodkowic D., Skommer J., Darzynkiewicz Z. Cytometry of apoptosis. Historical perspective and new advances. Exp. Oncol. 2012;34:255–262. [PMC free article] [PubMed] [Google Scholar]
- Wojtala A., Bonora M., Malinska D., Pinton P., Duszynski J., Wieckowski M.R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014;542:243–262. doi: 10.1016/B978-0-12-416618-9.00013-3. [DOI] [PubMed] [Google Scholar]
- Xu J., Pan S., Gan F., Hao S., Liu D., Xu H., Huang K. Selenium deficiency aggravates T-2 toxin-induced injury of primary neonatal rat cardiomyocytes through ER stress. Chem. Biol. Interact. 2018;285:96–105. doi: 10.1016/j.cbi.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Yao L., Du Q., Yao H., Chen X., Zhang Z., Xu S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals. 2015;28:255–265. doi: 10.1007/s10534-014-9819-3. [DOI] [PubMed] [Google Scholar]
- Ye Y., Shibata Y., Yun C., Ron D., Rapoport T.A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vasquez-Vivar J., Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.