Abstract
A green, direct and cost-effective fabrication method is proposed for Eco-environmentally silver nanoparticles (AgNPs) through leaf extraction of Carya illinoinensis from Iran. Formation of Ag NPs was confirmed through different characterization techniques such as UV–Vis Spectroscopy, X-ray Diffraction (XRD), Scanning Electron Microscope (SEM) and Transmission Electron Microscopy (TEM). UV-Visible spectrophotometer showed absorbance peak at 440 nm due to the Surface Plasmon Resonance (SPR). Based on XRD results and SEM and TEM analysis, AgNPs were crystalline with face-centered cubic geometry and in different sizes ranged 12–30 nm. Furthermore, FTIR Spectroscopy was utilized to recognize the specific functional groups responsible for reducing ion silver to silver nanoparticles and the capping agents available in the leaf extract. In addition, the antibacterial effect of Eco-friendly synthesized nanoparticles and also leaf extract, were evaluated on four pathogens by implementing minimum inhibitory concentration test (MIC) and agar diffusion assay. The MIC results exhibits more inhibiting activity against gram-negative microorganisms (Escherichia coli and Pseudomonas aeruginosa) rather than gram-positive microorganisms (Staphylococcus aureus and Listeria monocytogenes). Compared to leaf extract, nanoparticles have better antimicrobial activity against both Gram-positive and Gram-negative bacteria.
Keywords: Natural product chemistry, Green synthesis, Silver nanoparticles, Antibacterial activities, Pecan, Carya illinoinensis
Natural product chemistry, Green synthesis; Silver nanoparticles; Antibacterial activities; Pecan; Carya illinoinensis.
1. Introduction
Noble metal nanoparticle synthesis, Ag, Pt, Au, and Pd in particular, are extensively studied in the last decade, due to their peculiar characteristics for applications in catalysis, photothermal therapy, water purification, pharmaceutical formulation, electronics, optics, environmental, drug delivery, and biotechnology [1, 2, 3, 4, 5, 6, 7].
Most recently, green synthesis methods using plants and microorganisms including bacteria, fungi, algae, and the like have attracted tremendous attention. By using plant extracts (PE), metal nanoparticles have the most immense implications for synthesizing nanoparticles with big scale and without additional impurities in order to minimize hazardous solvents, and reduce agents and stabilizers additionally to develop environmentally benign technologies in material synthesis [8, 9, 10, 11]. Accessibility and safety which can contribute to the reduction of silver ions are regarded as the main advantages of extracting plant for synthesizing silver nanoparticles [12, 13, 14, 15, 16].
Over the last decade, silver nanoparticles have long been recognized for their antibacterial [17, 18, 19] properties in the medical industry and inhibitory effects on microbes, such as topical ointments to prevent from infecting against burned and open wounds [20, 21, 22].
Carya illinoinensis, which is recognized as pecan, is common in North America and Mexico and is related to the Juglandaceae family [23, 24, 25] (Figure 1). In folk medicine, the leaves of pecan are usually utilized for treating smoking as a hypoglycemic, cleansing, astringent, keratolytic, antioxidant, antimicrobial, as well as carminative agent [26, 27]. Unlike pecan nut and kernel, few studies have been conducted regarding the phytochemicals and antioxidant properties of pecan leaves [28]. According to previous works, pecan is of a great importance among the foods due to having the highest bioactive molecules such as sterols, tocopherols, and a high content of total phenolic compounds with a variety of health-beneficial properties. However, there is few report on phytochemical compositions of pecan's leaves in literature, it seems the phenolic acids, flavonoids and tannins in the leaves of C. illinoensis, revealed by HPLC, might be responsible for the antimicrobial activity the of pecan's leaf [29, 30, 31]. To the best of our knowledge, no information is available for the synthesis of Ag NPs using pecan and kernel as well.
Figure 1.

Image of Carya illinoinensis leaf.
The present study aimed to use the Carya illinoinensis extract in a green synthesis of silver nanoparticles, and evaluate the antibacterial activities related to extracted leaves and synthesized AgNPs.
2. Materials and methods
Methanol (CH3OH, 99.9%), AgNO3 (99.98%), nutrient agar and Mueller-Hinton agar (MHA) were purchased from Merck (Germany). Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Listeria monocytogenes (ATCC 7644) were obatained from Iranian Research Organization for Science and Technology (IROST).
2.1. Plant material and preparation of the extract
Mature leaves of Carya illinoinensis were collected in May 2019 from Golestan agricultural resources research and education center, Gorgan, (center of Golestan province, Iran). The leave surface was completely cleaned with distilled water, and was dried in the shade at room temperature. In order to prepare of pecan leaf extract, 30 g of the dried and powdered plant materials was extracted with 100 ml methanol (ratio 1:10 w/v) by implementing percolation method at the room temperature for 24 h. Subsequently, the extract was filtered with Whatman filter paper No. 1 (Maidstone, UK) and the solvent was completely removed under vacuum at 40 °C using a rotary evaporator (Heidolph, Laborota 4000, Schwabach, Germany). The extract was kept in the dark at 4 °C with a maximum period of one week.
2.2. Synthesis of silver nanoparticles (AgNPs)
90 ml of AgNO3 (0.01 M) was added to 10 ml of extract (10.0 mg mL−1/water) in a vessel [32]. The reaction was conducted under a stirring rate of 700 rpm at 25 °C for 24 h. After completing, AgNPs were concentrated and purified by centrifugal ultrafiltration (Hermle, Labortechnik, Z 36 HK, Ger) for 15 min at 12000 rpm and rinsed with distilled water, acetone, and ethanol in order to eliminate the remaining soluble ions on the particle surface. Then, the pellet was re-dispersed using ultrasound in the sterile distilled water in order to ensure a better separation, and accordingly it was recentrifuged at 12000 rpm for 15 min. Finally, the purified pellets were placed on a petri plate and dried at 70 °C for 5 h.
2.3. Characterization
UV–Vis Spectroscopy was conducted using a Unico S-2150 in 300–600 nm range. The configuration of the AgNPs was evaluated by measuring X-ray Diffraction (XRD) by using a powder X-ray diffractometer instrument (Panalytical X’PERT PRO) with a Ka1 Cu target and a graphite monochromator operating at the voltage of 35 kV and the current of 30 mA radiation. In addition, the morphology and size of the product were evaluated through implementing Scanning Electron Microscope (FEI ESEM QUANTA 200) and transmission electron microscopy (TEM), along with a Zeiss EM900 electron microscope, respectively. Further, the distributions of the particle size were determined by utilizing IMAGEJ2 (ImageJDev) software inspired by NIH image [33]. Fourier transform infrared (FT-IR) spectra for Carya illinoinensis leaf extract powder and silver nanoparticles were recorded within the range of 4,000–400 cm−1 by using an IR-VERTEX-70 Bruker FT-IR spectrophotometer, by KBr pellet.
2.4. Antibacterial activity
2.4.1. Agar well diffusion method
The agar well diffusion method was applied to investigate antibacterial activities of methanol, extracts [34] against 4 bacteria. For this purpose of concentrations of 40, 20, 10, and 5 μg/mL of the plant extracts were used to study the bacteria strains in disk diffusion. Besides, the concentrations of 20, 10, 5, and 2.5 μg/mL were employed to evaluate the antibacterial activities of AgNPs well as. First, 0.5 McFarland standard of bacteria was prepared. Then, Mueller-Hinton agar plates were seeded with a lawn of bacteria. By using of a cup-borer, well was prepared in the plates and 0.1 mL of 106 cells per mL suspension of the tested microorganisms was pipetted in the well. The plates were placed at room temperature for 1 h in order to penetrate of extract or AgNPs into the agar. The Petri dishes were put in the refrigerator for 3 h and then incubated at 37 °C for 24 h. Gentamicin (16 μg/ml) and DMSO (%5) were prepared as positive and negative controls, respectively. These processes were repeated in triplicate and the inhibition zone diameter was measured in term of mm.
2.4.2. The minimum inhibitory concentration (MIC)
The microdilution method was used for evaluating the minimum inhibitory concentration (MIC) [35] of Carya illinoinensis leaf extract and synthesized AgNps on both Gram-negative and Gram-positive bacteria. All tests were conducted in nutrient broth, followed by mixing of extracs in 5% dimethylsulphoxide (DMSO). In addition, a serial doubling dilution of extracts and nanoparticles was prepared in a 96- well micro titer plate over the range of 7.8–1000 μg mL-1. In a typical experiement, the 96-well plates were made by dispensing 95 μL of nutrient broth and inocula into each well. In the next procedure, a 100 μL of aliquot was separately prepared from the stock solutions of the extracts and AgNPs at the concentration of 1000 μg mL-1 and were added into the first wells. Then, 100 μL from their serial dilutions was moved into seven consecutive wells. The last well included 195 μL of nutrient broth without any compound. Distilled water and 5% dimethylsulphoxide (DMSO) were used for controlling both positively and negatively, respectively. A sterile plate sealer was used for covering the plate. Further, the contents were mixed well on a plate shaker at 200 rpm for 40 s, and then incubated at suitable temperatures for 24 h. Bacterial growth was evaluated by absorbing at 620 nm and proved by plating 5 μL samples on nutrient agar medium from clear wells. Finally, the MIC was described as the lowest concentration of the extracts or NPs where the bacteria do not represent visible growth.
3. Results and discussion
3.1. UV-visible spectra of AgNPs
There are different methods to confirm the preparation of nanoparticles, among which the color change of the colorless solution of AgNO3 to brown suspension of silver nanoparticles, as demonstrated in Figure 2.
Figure 2.
Synthesis of AgNPs by using extract of Carya illinoinensis: a) Precursor solution of AgNO3, b) Extract of Carya illinoinensis, and c) Extract of Carya illinoinensis treated with AgNO3.
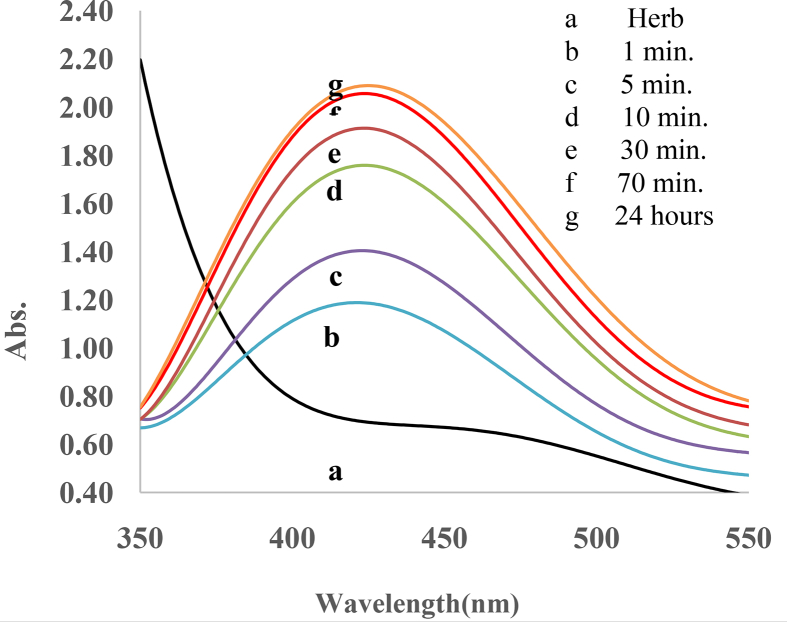
The ultraviolet-visible (UV-Vis) spectra of the prepared solution were captured at different time intervals from 1 min up to 24 h, where distilled water was used as a blank. As illustrated in Figure 3, the absorbance peak was observed at 440 nm, which sharpened over time, starting a few minutes after reaction begin (Figure 3). Increase in the intensity is attributed to the excitation of surface plasmon resonance (SPR) in the AgNPs, in agreement with previous reports [36, 37], suggesting the reduction of silver nitrate into silver nanoparticles. Since the SPR absorbance relies heavily on the size, shape, and environment by which the nanoparticles are formed [38], the plasmon bands are broad with an absorption tail in the longer wavelengths by increasing the concentration of AgNO3, indicating an enhancement in particle size distribution of the synthesized nanoparticles.
Figure 3.
UV–visible spectra of AgNps at different time intervals.
3.2. FTIR spectroscopy of AgNPs
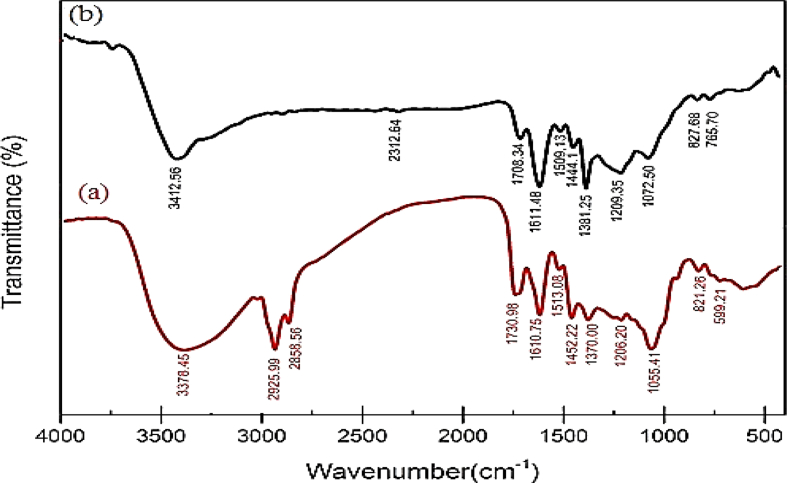
FTIR analysis was utilized for determining the organic functional groups like OH or C=O linked to the surface of nanoparticles. Figure 4 displays the FTIR spectrum of Carya illinoinensis leaf extract and Ag NPs. The FTIR spectra of extracting Carya illinoinensis in Figure 4(a) demonstrates an absorption peak in 3378 cm−1, which is assigned as –OH stretching in alcohols and phenolic compounds with strong hydrogen bonds or resulted from stretching the –NH band of amino groups [39]. The absorption peaks at about 2858 cm−1 and 2925 cm−1 can be related to aldehyde –CH stretching vibrations and the band at 1730 cm−1 can prove the presence of carbonyl group of an aldehyde. Further, the FTIR spectra of the extract indicated the bands of 1610, 1513, and 1452 cm−1 are recognized as a carbonyl (C=O) group of amide I and amide II [40]. Furthermore, the absorption bands in the range of 1370-1055 cm−1 are related to C–O, C–N, and C–C stretching vibrations of phenolic compounds, aliphatic amines, and alkanes, respectively.
Figure 4.
(a) FTIR spectrum of Carya illinoinensis extract, (b) FTIR spectrum of AgNPs.
The FTIR of synthesized AgNO3 in Figure 4(b) demonstrated a slight change in position and intensity in the peaks. The displacement at 3412 cm-1 is related to the breakdown of the hydrogen bond, which plays a role in decreasing silver ions into silver nanoparticles. The nanoparticles consist of the compounds in the extract existing in a layer around the nanoparticles due to the similarity of spectral pattern for plant extract and nanoparticles [41].
3.3. X-ray diffraction of AgNPs
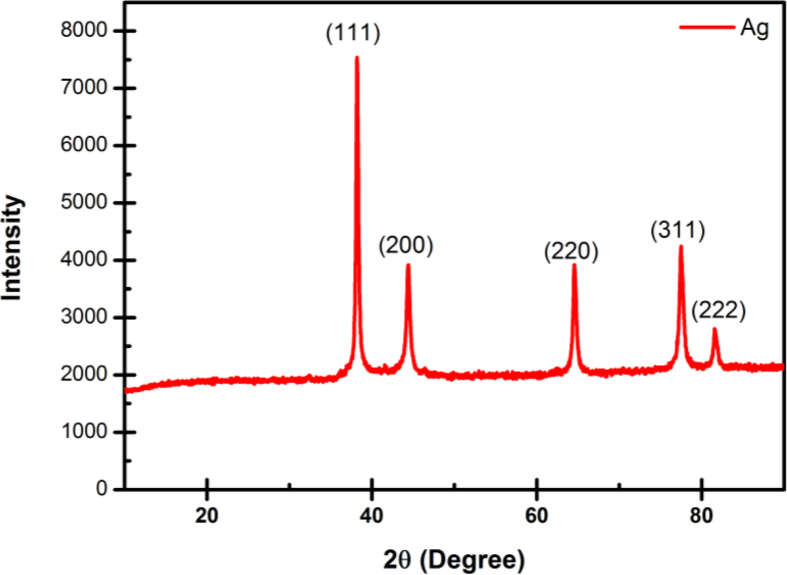
The prepared AgNPs were determined by X-ray diffraction (XRD) in order to evaluate its crystalline structure, size, and purity. Figure 5 displays XRD patterns for AgNPs synthesized by Carya illinoinensis leaf extract. The diffraction peaks of 2θ = 38.4, 44.5, 64.8, 77.7, and 81.7 are related to 111, 200, 220, 311, and 222 crystallographic planes of face-centered cubic (fcc) structure for the silver powder sample, in agreement with (JCPDS file No. 04-0783) [42]. The characteristic peak of the silver structure was reported by indicating the purity of synthesized silver nanoparticles without any additional diffraction peaks.
Figure 5.
XRD pattern of AgNPs obtained using extract of Carya illinoinensis.
3.4. SEM and TEM analysis
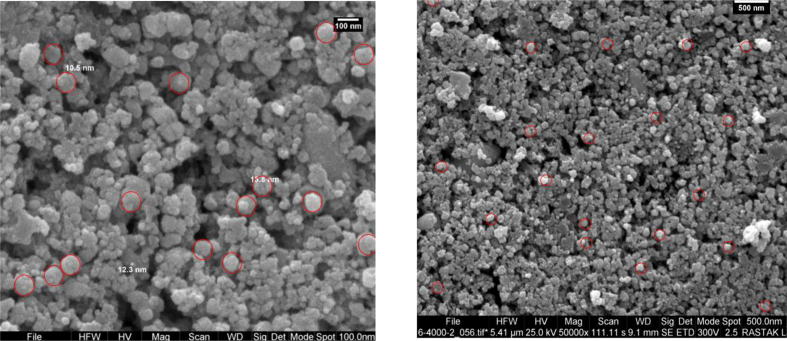
The morphology of green synthesized AgNPs was evaluated by SEM. Based on the SEM image in Figure 6, the metal particles were well-dispersed with a spherical shape. Thus, the optical and electronic features of metal nanoparticles are mostly influenced by the shape of nanoparticles [43].
Figure 6.
SEM micrograph images of AgNPs synthesized using Leaf extract of Carya illinoinensis with different magnifications (left 100 nm) and (right 500 nm).
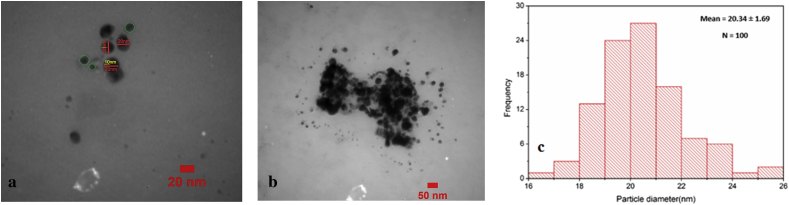
In addition to SEM analysis, TEM technique was implemented to demonstrate the size and shape related to AgNPs created by plant. Figure 7 illustrates the typical TEM image and size distributions, along with the calculated histogram of AgNPs. Based on the results of the TEM image and the size distributions, the mean diameters and standard deviation of AgNPs were 20.34 ± 1.69 nm. The number of AgNPs in the TEM image was around 100 nm. Further, the synthesized silver nanoparticles were measured in different sizes ranged 12–30 nm. According to TEM image taken at 20 nm, It is observed that nanoparticles were spherical along with the layer and contain two separate parts in nature and different in color. The dark part of the color represents the nanoparticles and the lighter part (less darkness) forms the attached or sticking layer. Based on the scale inserted the figure, It was found out that the nanoparticles thickness which covered by an extract layer is 20 nm in diameter. The other pale spots around them are extracted not nanoparticles which are scattered in the matrix (green circles) and they should not be consider for estimating the size of nanoparticles containing a layer of extract. Therefore, according to yellow lines in the image, the average thickness of the adhesive layers on the nanoparticles is about 10 nm. Finally, based in the TEM images, the particles are rather fine and spherically shaped.
Figure 7.
TEM images (a, b), the thickness (a) and size distributions (c) of AgNPs synthesized using Leaf extract of Carya illinoinensis.
3.5. Antimicrobial activities of AgNPs and extract
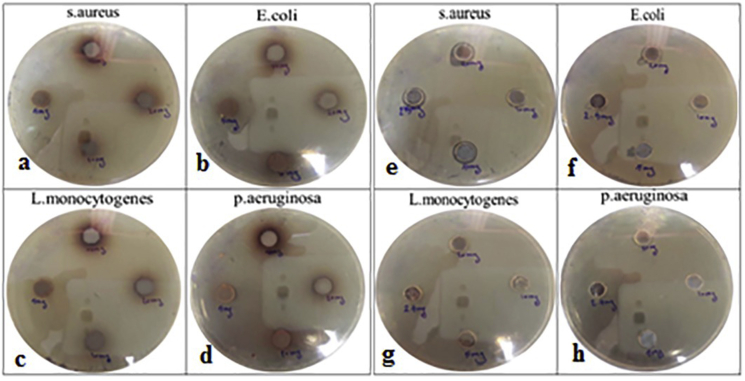
The antibacterial activity of Carya illinoinensis leaves extract and Ag nanoparticles produced from this plant were evaluated against Gram-positive bacteria namely, S. aureus (ATCC 25923) and L. monocytogenes (ATCC 7644), and Gram-negative bacteria E. coli (ATCC 25922) and P. aeruginosa (ATCC 27853) using agar well diffusion (Figure 8) and minimum inhibitory concentration and the results were shown in Table 1 and Table 2, respectively. As shown in Tables, synthesized AgNps are more effective against negatively charged bacterial cell, compared to the positively charged bacterial cell in both methods. These results were the agreement with previous reports [28, 44, 45, 46], while there were some works shown that the formed Ag NPs using plant extract were more toxic against positive pathogens compared with negative one [47].
Figure 8.
Antibacterial activity of silver nanoparticles against S. aureus, E. coli, L. monocytogenes, and P. aeruginosa, in different concentrations, a, b, c, and d (AgNPs), e, f, g, and h (plant extract).
Table 1.
Inhibition zone diameter (IZD) of AgNPs and plant extract in four concentrations.
| IZD of Extract (DD)a | IZD of AgNPs (DD)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/ml) | 40 | 20 | 10 | 5 | 20 | 10 | 5 | 2.5 |
| staphylococcus aureus (ATCC 25923) |
6 | - | - | - | 11 | 10 | 9 | 7 |
| Listeria monocytogenes (ATCC 7644) | 5 | - | - | - | 12 | 11 | 10 | 8 |
| Escherichia coli (ATCC 25922) |
9 | - | - | - | 15 | 13 | 12 | 10 |
| Pseudomonas aeruginosa (ATCC 27853) | 7 | - | - | - | 13 | 11 | 10 | 9 |
A dash (–) indicate no antibacterial activity.
Diameter of inhibition zone including disc diameter of 6 (mm).
Table 2.
MIC of AgNPs and plant extract.
| Bacteria | MIC AgNPs (μg/ml) | MIC leaf extract (μg/ml) |
|---|---|---|
| Staphylococcus aureus (ATCC 25923) | 128 | 875 |
| Listeria monocytogenes (ATCC 7644) | 64 | 3500 |
| Escherichia coli (ATCC 25922) | 16 | 218 |
| Pseudomonas aeruginosa (ATCC 27853) | 32 | 1750 |
According to Table 1, by increasing in concentration of AgNPs and plant extract, the inhibition zone diameter also increased. In case of plant extract, by decreasing in concentrations, no inhibitory was observed. It might be that the AgNPs had better penetration through the agar and consequently into cell bacteria than the plant extract due to the small size of nano particles [48]. The highest inhibition zone diameter (IZD) was achieved for E. coli (9 mm and 15 mm) for extract and AgNPs, respectively.
The lowest MIC value (16 μg mL-1) was achieved for E. coli, demonstrating that the AgNPs has the maximum toxicity to this microorganism. P. aeruginosa is another sensitive Gram-negative microorganism against the silver nanoparticles which its MIC value was 32 μg mL-1. However, two Gram-positive bacteria, L. monocytogenes and S. aureus, were treated with a higher dose of AgNps for MIC (64 μg mL-1 and 128 μg mL-1).
Although, the mechanism of the bactericidal effect of silver and AgNPs is not well documented, several studies suggested that AgNPs may be attached to the surface of the cell wall and membrane of the bacterial cell, and can inhibit the respiratory enzymes of bacterial [49, 50].
Regarding E. coli, the activity improvement of nano silver particles may be related to the inhibition of phosphate absorption, as well as penetrating nanoparticles into the cell wall of bacteria leading to the structural changes to cell death. Silver particles have high tendency to sulfur and phosphorus which are founded enormously in the cell membrane of bacteria [51, 52, 53]. Further, S. aureus and L. monocytogenes prevented from penetrating silver ions in the cytoplasm due to a cell wall including thicker peptidoglycan. Therefore, higher concentration of AgNps was essential for growth inhibition of S. aureus and L. monocytogenes compared to E. coli and P. aeruginosa [54]. Smaller AgNPs can give more bactericidal effect than the larger AgNPs due to the large surface area available for interaction and easier absorption [55].
In addition to synthesized AgNPs, the antibacterial activity of leaf extract was tested against four mentioned microorganisms. The results indicated that silver nanoparticles have considerably more antibacterial effect compared to leaf extract of the plant. According to results, the lowest MIC values of plant extract obtained for E. coli (128 μg mL-1) and S. aureus (875 μg mL-1), exhibited they were generally more sensitive against leaf extract while the data demonstrated that two pathogens, Listeria monocytogenes and Pseudomonas aeruginosa, were more resistant strains to leaf extract (3500 μg mL-1 and 1750 μg mL-1) respectively. Based on the literature, different values in MIC of leaf extracts may relate to variation in their chemical compositions and the components which have volatile nature that lead to various mechanisms for antimicrobial activities of leaf extracts on the pathogenic microorganisms [56].
4. Conclusion
The present study proposes an eco-friendly, fast and convenient green route for synthesizing AgNPs through implementing Carya illinoinensis leaf extract at ambient temperature. Initially, the observation of changing in color of Ag + solution form colorless to dark brown confirmed the formation of AgNPs. The SPR peak was observed at 440 nm in the UV-Vis spectrum. The morphology and chemical composition of the silver nanoparticles were determined by different techniques such as UV-Vis, FTIR, XRD, SEM, and TEM. In addition, the antibacterial activities of both leaf extract and nanoparticles synthesized by this plant were evaluated against four microorganisms by using agar well diffusion and the MIC methods. The experimental results indicated that the silver nanoparticles had considerably more antibacterial effects in comparison to leaf extract although their antibacterial actions were not recognized well. Further, the results suggesting the synthesized nano silvers are more effective to gram-negative than gram-positive and they have antibacterial activities at low concentrations. Thus, the use of a rapid and green synthetic method for extracting plants in this study may potentially suggest the use of such nanoparticles in the future drug development and food industry.
Declarations
Author contribution statement
Sahar Javan bakht Daliru: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hooreih Jahanbani: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Farzaneh Nabati: Performed the experiments; Analyzed and interpreted the data.
Malak Hekmati: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ghorbani H.R., Safekordi A.A., Attar H., Sorkhabadi S.M.R. Biological and non-biological methods for silver nanoparticles synthesis. Chem. Biochem. Eng. Q. 2011;25:317–326. [Google Scholar]
- 2.Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Bar H., Bhui D.K., Sahoo G.P., Sarkar P., Pyne S., Misra A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloid. Surface. Physicochem. Eng. Aspect. 2009;348:212–216. [Google Scholar]
- 4.Wang Y., Toshima N. Preparation of Pd-Pt bimetallic colloids with controllable core/shell structures. J. Phys. Chem. B. 1997;101:5301–5306. [Google Scholar]
- 5.Rahimi-Nasrabadi M., Pourmortazavi S.M., Shandiz S.A.S., Ahmadi F., Batooli H. Green synthesis of silver nanoparticles using Eucalyptus leucoxylon leaves extract and evaluating the antioxidant activities of extract. Nat. Prod. Res. 2014;28:1964–1969. doi: 10.1080/14786419.2014.918124. [DOI] [PubMed] [Google Scholar]
- 6.Miri A., Sarani M., Rezazade Bazaz M., Darroudi M. Plant-mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;141:287–291. doi: 10.1016/j.saa.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Vilchis-Nestor A.R., Sánchez-Mendieta V., Camacho-López M.A., Gómez-Espinosa R.M., Camacho-López M.A., Arenas-Alatorre J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008;62:3103–3105. [Google Scholar]
- 8.Li S., Shen Y., Xie A., Yu X., Qiu L., Zhang L., Zhang Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852–858. [Google Scholar]
- 9.Pourmortazavi S.M., Taghdiri M., Makari V., Rahimi-Nasrabadi M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;136:1249–1254. doi: 10.1016/j.saa.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Ghaedi M., Yousefinejad M., Safarpoor M., Khafri H.Z., Purkait M.K. Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J. Ind. Eng. Chem. 2015;31:167–172. [Google Scholar]
- 11.Sadeghi B., Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;134:310–315. doi: 10.1016/j.saa.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Baghizadeh A., Ranjbar S., Gupta V.K., Asif M., Pourseyedi S., Karimi M.J., Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015;207:159–163. [Google Scholar]
- 13.Loo Y.Y., Chieng B.W., Nishibuchi M., Radu S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia Sinensis. Int. J. Nanomed. 2012;7:4263–4267. doi: 10.2147/IJN.S33344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amooaghaie R., Saeri M.R., Azizi M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015;120:400–408. doi: 10.1016/j.ecoenv.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Ghaffari-Moghaddam M., Hadi-Dabanlou R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014;20:739–744. [Google Scholar]
- 16.Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009;5:382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Ghiassi S., Sedaghat S., Mokhtary M., Kefayati H. Plant-mediated bio-synthesis of silver–montmorillonite nanocomposite and antibacterial effects on gram-positive and -negative bacteria. J. Nanostruct. Chem. 2018;8:353–357. [Google Scholar]
- 18.Masurkar S.A., Chaudhari P.R., Shidore V.B., Kamble S.P. Rapid biosynthesis of silver nanoparticles using cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011;3:189–194. [Google Scholar]
- 19.Shu M., He F., Li Z., Zhu X., Ma Y., Zhou Z., Yang Z., Gao F., Zeng M. Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res. Lett. 2020;15:14. doi: 10.1186/s11671-019-3244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A.A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. 2007;42:919–923. [Google Scholar]
- 21.Atiyeh B.S., Costagliola M., Hayek S.N., Dibo S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Do Prado A.C.P., Aragão A.M., Fett R., Block J.M. Antioxidant properties of Pecan nut [Carya illinoinensis (Wangenh.) C. Koch] shell infusion. Grasas Aceites. 2009;60:330–335. [Google Scholar]
- 23.De La Rosa L.A., Alvarez-Parrilla E., Shahidi F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis) J. Agric. Food Chem. 2011;59:152–162. doi: 10.1021/jf1034306. [DOI] [PubMed] [Google Scholar]
- 24.Villarreal-Lozoya J.E., Lombardini L., Cisneros-Zevallos L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007;102:1241–1249. doi: 10.1021/jf901719s. [DOI] [PubMed] [Google Scholar]
- 25.Abu Taha Nael. Utility and importance of walnut, Juglans regia Linn: a review. Afr. J. Microbiol. Res. 2011;5:5796–5805. [Google Scholar]
- 26.Eitenmiller R., Pegg R. CRC Press; 2008. Compositional Characteristics and Health Effects of Pecan [Carya Illinoinensis (Wangenh.) K. Koch] pp. 273–298. [Google Scholar]
- 27.Bottari N.B., Lopes L.Q.S., Pizzuti K., Filippi dos Santos Alves C., Corrêa M.S., Bolzan L.P., Zago A., de Almeida Vaucher R., Boligon A.A., Giongo J.L., Baldissera M.D., Santos R.C.V. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb. Pathog. 2017;104:190–195. doi: 10.1016/j.micpath.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Atanasov A.G., Sabharanjak S.M., Zengin G., Mollica A., Szostak A., Simirgiotis M., Huminiecki Ł., Horbanczuk O.K., Nabavi S.M., Mocan A. Pecan nuts: a review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018;71:246–257. [Google Scholar]
- 29.Caxambú S., Biondo E., Kolchinski E.M., Padilha R.L., Brandelli A., Sant’Anna V. Evaluation of the antimicrobial activity of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell aqueous extract on minimally processed lettuce leaves. Food Sci. Technol. 2016;36:42–45. [Google Scholar]
- 30.do Prado A.C.P., da Silva H.S., da Silveira S.M., Barreto P.L.M., Vieira C.R.W., Maraschin M., Ferreira S.R.S., Block J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crop. Prod. 2014;52:552–561. [Google Scholar]
- 31.Adebayo-Tayo B., Salaam A., Ajibade A. Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasrollahzadeh M., Sajadi M.M., Babaei F., Maham M. Euphorbia helioscopia Linn as a green source for synthesis of silver nanoparticles and their optical and catalytic properties. J. Colloid Interface Sci. 2015;450:374–380. doi: 10.1016/j.jcis.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Perez C., Pauli M., Bazerque P. An antibiotic assay by agar well diffusion method. Acta Biol. Med. Exp. 1990;15:113–115. [Google Scholar]
- 34.Rahimi-Nasrabadi M., Nazarian S., Farahani H., Fallah Koohbijari G.R., Ahmadi F., Batooli H. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell. Int. J. Food Prop. 2013;16:369–381. [Google Scholar]
- 35.Djahaniani H., Rahimi-Nasrabadi M., Saiedpour M., Nazarian S., Ganjali M., Batooli H. Facile synthesis of silver nanoparticles using Tribulus longipetalus extract and their antioxidant and antibacterial activities. Int. J. Food Prop. 2017;20:922–930. [Google Scholar]
- 36.Wei H., Xu H. Nanowire-based plasmonic waveguides and devices for integrated nanophotonic circuits. Nanophotonics. 2012;1:155–169. [Google Scholar]
- 37.Femi-Adepoju A.G., Dada A.O., Otun K.O., Adepoju A.O., Fatoba O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): characterization and antimicrobial studies. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dada A.O., Adekola F.A., Dada F.E., Adelani-Akande A.T., Bello M.O., Okonkwo C.R., Inyinbor A.A., Oluyori A.P., Olayanju A., Ajanaku K.O., Adetunji C.O. Silver nanoparticle synthesis by Acalypha wilkesiana extract: phytochemical screening, characterization, influence of operational parameters, and preliminary antibacterial testing. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagtap U.B., Bapat V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013;46:132–137. [Google Scholar]
- 40.Dada A.O., Inyinbor A.A., Idu E.I., Bello O.M., Oluyori A.P., Adelani-Akande T.A., Okunola A.A., Dada O. Effect of operational parameters, characterization and antibacterial studies of green synthesis of silver nanoparticles using Tithonia diversifolia. PeerJ. 2018;6 doi: 10.7717/peerj.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanje A.S., Sharma S.J., Pode R.B. Synthesis of silver nanoparticles: a safer alternative to conventional antimicrobial and antibacterial agents. J. Chem. Pharmaceut. Res. 2010;2:478–483. [Google Scholar]
- 42.Yamanaka M., Hara K., Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benakashani F., Allafchian A.R., Jalali S.A.H. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int. J. Mod. Sci. 2016;2:251–258. [Google Scholar]
- 44.Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A., Saleh N.M., Das R., Landis R.F., Bigdeli A., Motamedchaboki K., Campos A.R., Pomeroy K., Mahmoudi M., Rotello V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futur. 2017;1 [Google Scholar]
- 46.Awwad A.M., Salem N.M., Aqarbeh M.M., Abdulaziz F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2020;6:42–48. [Google Scholar]
- 47.Lee J.H., Lim J.M., Velmurugan P., Park Y.J., Park Y.J., Bang K.S., Oh B.T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B Biol. 2016;162:93–99. doi: 10.1016/j.jphotobiol.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 48.Dong Y., Zhu H., Shen Y., Zhang W., Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PloS One. 2019;14 doi: 10.1371/journal.pone.0222322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kvítek L., Panáček A., Soukupová J., Kolář M., Večeřová R., Prucek R., Holecová M., Zbořil R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs) J. Phys. Chem. C. 2008;112:5825–5834. [Google Scholar]
- 50.Tamboli D.P., Lee D.S. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J. Hazard Mater. 2013;260:878–884. doi: 10.1016/j.jhazmat.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Chaloupka K., Malam Y., Seifalian A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Venkatpurwar V., Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Mater. Lett. 2011;65:999–1002. [Google Scholar]
- 53.Behravan M., Hossein Panahi A., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 54.Malanovic N., Lohner K. Gram-positive bacterial cell envelopes: the impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2016;1858:936–946. doi: 10.1016/j.bbamem.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Jin R., Cao Y., Mirkin C.A., Kelly K.L., Schatz G.C., Zheng J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science. 2001;294:1901–1903. doi: 10.1126/science.1066541. (80-) [DOI] [PubMed] [Google Scholar]
- 56.Gonelimali F.D., Lin J., Miao W., Xuan J., Charles F., Chen M., Hatab S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]