Highlights
-
•
Intra-abdominal desmoid tumor is rare with no particular imaging features, so preoperative diagnosis is quite difficult.
-
•
R0 resection is essential for treatment but often requires extensive surgical trauma, which can be a risk for recurrence.
-
•
A laparoscopic approach for this tumor was effective, with the resulting diagnosis and resection being less traumatic.
-
•
The first report of successful laparoscopic complete resection and reconstructive procedures were demonstrated.
Keywords: Intra-abdominal desmoid tumor, Surgical trauma, Laparoscopic complete resection, Minimal invasiveness
1. Introduction
Intra-abdominal desmoid tumor is a slow-growing benign tumor; however, the potential for recurrence is high due to its characteristics as a locally infiltrating neoplasm. The occurrence of Intra-abdominal desmoid tumor is rare, and there are no specific imaging features, making preoperative diagnosis extremely difficult. Complete resection with a negative surgical margin is the gold standard for treatment, but the tumor often requires combined resection, resulting in enormous surgical trauma, known to be a major risk factor for recurrence (Lazar et al., 2008). Laparoscopic resection is effective for intra-abdominal desmoid tumors, and because of its minimal invasiveness, it is a diagnostic and resection strategy that can reduce the risk of recurrence. Complete resection often requires reconstruction of the adjacent organs, and a skillful intracorporeal suturing is required to accomplish the reconstruction procedure laparoscopically.
In this case report, we describe the case of a total laparoscopic complete resection of an intra-abdominal desmoid tumor that had infiltrated the patient’s rectum and ureter.
2. Case summary
The patient was a 47-year-old woman who presented with a complaint of slight left back pain. She had undergone a hysterectomy and bilateral salpingectomy for carcinoma in situ of the uterine cervix 2 years ago. Upon presentation, abdominal ultrasonography was performed, and left hydronephrosis was revealed. Abdominal contrast-enhanced computed tomography (CT) was then performed, and a strongly enhanced mass, approximately 3 cm in diameter, was discovered at the left obturator fossa and attached to the left ureter (Fig. 1a). It appeared as a hypointense mass upon abdominal magnetic resonance (MR) T1-weighted imaging and a slightly hyperintense mass upon T2-weighted imaging; hence, a metastatic lymph node was suspected. Subsequent fluorodeoxyglucose-positron emission tomography (FDG-PET) revealed what appeared to be a metastatic left obturator node. However, the FDG uptake was not intense (SUVmax = 3.00), and an abnormal FDG uptake was not seen elsewhere (Fig. 1b). Results of blood tests, including those for renal function, were unremarkable, and tumor markers (SCC, CA125, CA19-9, CEA) were not elevated. The results of these examinations led us to suspect that a metastatic left obturator node was responsible for the left hydronephrosis. However, no primary lesion was detected, and we were unable to diagnose the mass precisely.
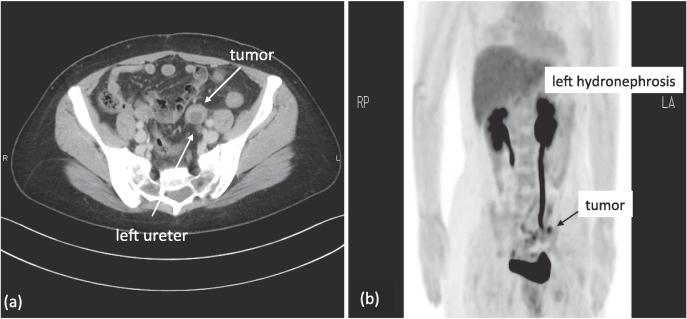
Fig. 1.
Preoperative CT/FDG-PET, (a) Contrast-enhanced computed tomography imaging demonstrated a well-circumscribed 3 cm mass, which was attached to the left ureter. (b) Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging suggested a metastatic left obturator node, but FDG uptake was not intense (SUVmax = 3.0), and no other abnormal uptake was seen.
We aimed to treat the patient’s back pain by placing a stent in the left ureter, but severe stricture prevented this. Laparoscopic surgery was thus indicated for both a histologic diagnosis of the mass and treatment of the left hydronephrosis.
Laparoscopic inspection of the abdominal cavity revealed a marked adherence of the tumor to the rectum and left ureter but no dissemination or metastasis. Abdominal washing cytology was negative for cancer. The tumor was located at the left obturator fossa, involved the rectum and left ureter, and was fixed to the left pelvic sidewall due to infiltration to the left parametrium. To achieve complete resection with negative surgical margins, resection of the left adnexa, left parametrium, and combined resection of the left ureter and rectum were necessary. We performed these surgical procedures laparoscopically, and detached the tumor from the pelvic structures with clear surgical margins (Fig. 2a).
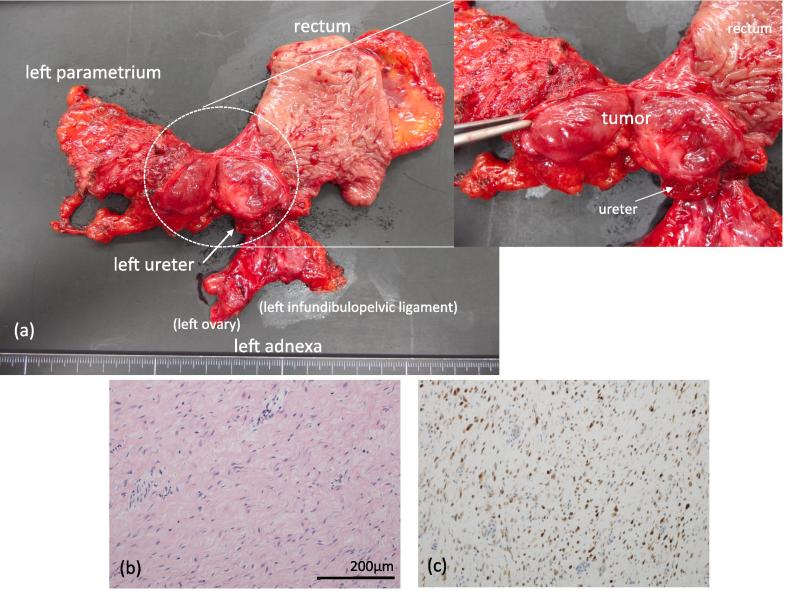
Fig. 2.
Photograph of the surgical specimen and pathology of the tumor, (a) Complete resection with negative surgical margins was achieved. (b) Microscopic findings showed slightly atypical spindle cells (Hematoxylin and eosin stain). (c) Immunohistochemical staining showed nuclear accumulation of β-catenin.
Upon intraoperative pathological assessment, the mass was diagnosed as an intra-abdominal desmoid tumor (Fig. 2b and c). The left ureter and rectum were reconstructed laparoscopically to minimize surgical trauma. The left ureter had been transected at the pelvic brim to secure a negative surgical margin, and the ensuing ureteral defect was approximately 9 cm. This long defect required ureteral re-implantation with a combined Boari flap and psoas hitch procedure. The combined Boari flap and psoas hitch reconstruction were performed safely as a total laparoscopic procedure, as was rectal reconstruction by the double stapling technique (Fig. 3). Only two 12-mm and two 5-mm skin incisions were necessary, and there were no intraoperative complications. The operation time was 393 mins, and the blood loss volume was 50 mL. There was no need for a blood transfusion.
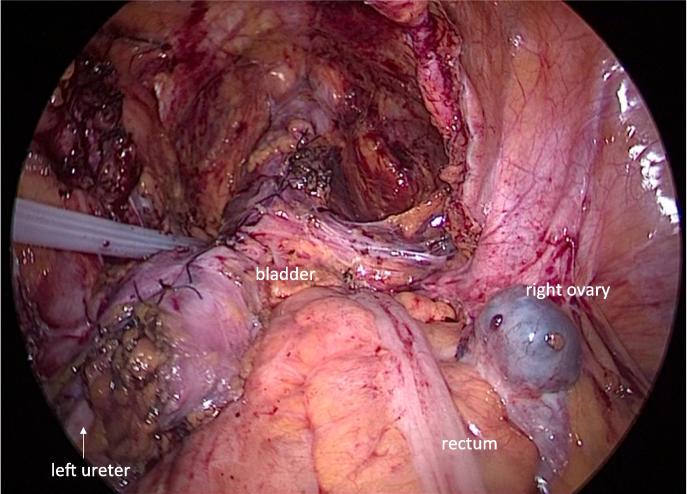
Fig. 3.
Intraoperative view, Total laparoscopic combined Boari flap and psoas hitch ureteral reconstruction was performed safely along with rectal reconstruction using the double stapling technique.
The patient was discharged 7 days after the surgery without any complications. Two years and 3 months have passed since the surgery, and there has been no evidence of tumor recurrence.
3. Discussion
Desmoid tumors are rare, with an annual incidence of 2–4 cases per million people. They are slow-growing fibrous soft tissue tumors and are classified histologically as benign. However, they are locally aggressive and can infiltrate adjacent organs without distant metastasis; therefore, the potential for local recurrence is high (Lazar et al., 2008).
The exact etiology of desmoid tumors is unknown. However, abnormalities affecting the genes governing tissue repair have been suggested to be the primary underlying cause of desmoid tumor development. Desmoid tumors are thought to develop when stimulating factors are expressed together with these gene abnormalities. The stimulating factors include trauma, such as laparotomy or abdominal injury, adenomatous polyposis coli gene abnormalities such as those causing familial adenomatous polyposis and Gardner syndrome, and hormonal factors (Reitamo et al., 1986). Belliveau et al. reported that 45% of their patients with an intra-abdominal desmoid tumor had undergone laparotomy, and 22% had Gardner syndrome (Beliveau and Graham, 1984). Thus, laparotomy appears to be a major risk factor for the development of an intra-abdominal desmoid tumor. Notably, our patient had undergone a laparotomy 2 years before the detection of the intra-abdominal desmoid tumor.
As noted above, the potential for local recurrence of a desmoid tumor after surgical resection is high (19–77%); this is because of the infiltrating character of the tumor. Therefore, complete resection with negative surgical margins (R0 resection) is essential (Shinagare et al., 2011). In many cases, accomplishing R0 resection of a desmoid tumor means that surrounding organs should also be resected, and such surgery often means severe surgical trauma. However, the very nature of the desmoid tumor makes operative trauma a major risk factor for the development of such a tumor (Vitellaro et al., 2011).
Laparoscopic surgery is minimally invasive, as only 4 ports are used; however, a considerably longer surgical time is needed than laparotomy to repair the colon and GU systems. Sinha et al. (Sinha et al., 2018) stated that as the small bowel and its mesentery are exposed to cooling, drying, and retraction outside the abdomen during laparotomy, it may increase the risk of developing desmoid tumors. They reviewed 112 patients with familial adenomatous polyposis, who underwent colectomy and ileorectal anastomosis; the approaches were open and laparoscopic in 43 and 69 cases, respectively. Fewer patients developed desmoid tumors after laparoscopic ileorectal anastomosis (p = 0.043). We speculate that the surgical technique, and not the duration of operation determines the risk of developing desmoid tumors. Therefore, laparoscopic surgery was considered for the diagnostic, therapeutic, and reconstructive procedures in our patient.
Gari et al. (2012) reported that although intra-abdominal desmoid tumors appear to be well-circumscribed on CT and MR images, they are often infiltrative. In our case, preoperative CT depicted a well-circumscribed tumor. Nevertheless, it had infiltrated the left ureter and the rectum, making combined resection and reconstructive surgery of the rectum and left ureter necessary. The left ureter was transected at the pelvic brim to secure negative surgical margins, and, as noted above, the ureteral defect was approximately 9 cm. Therefore, ureteral re-implantation with a combined Boari flap-psoas hitch was necessary. Laparoscopic performance of the Boari flap-psoas hitch procedure is technically challenging; extensive intracorporeal suturing experience is required. To date, only 42 cases of laparoscopic ureteral re-implantation with a Boari flap have been reported, and all by urologists (Bansal et al., 2017). To our knowledge, ours is the first reported case of successful total laparoscopic Boari flap-psoas hitch reconstruction of the ureter plus double stapling reconstruction of the rectum.
Desmoid tumors can be self-limiting; growth arrest occurs in some cases and spontaneous regression in others, most often if the tumor is primary (Lewis et al., 1999). Therefore, simple observation is a justified approach when the diagnosis is confirmed. However, desmoid tumors have no characteristic features (Mizuno et al., 2017), and thus preoperative diagnosis is extremely difficult. We were not able to diagnose our patient’s tumor preoperatively, and it was symptomatic; thus, diagnostic-therapeutic surgery was indicated.
To the best of our knowledge, this report is the first, of a successful total laparoscopic complete resection of an intra-abdominal desmoid tumor, infiltrating the rectum and ureter. We found this approach to be quite effective, with the resulting diagnosis and resection being less invasive and traumatic than those performed under open surgery, which possibly reduced the risk of recurrence. We realize that our conclusion rests on our experience in a single case with only a short follow-up period, and we acknowledge that an accumulation of cases and long-term follow up data are needed to clarify the oncologic outcomes of such a minimally invasive approach to intra-abdominal desmoid tumors.
4. Conclusion
In conclusion, our report indicates that laparoscopic diagnosis and resection of an intra-abdominal desmoid tumor proved to be less invasive and traumatic than open surgery; this may have reduced the risk of recurrence.
Author contribution
Conceptualization: H.K., M.O.; Data curation: H.K., M.O.; Formal analysis: H.K.; Funding acquisition: n/a; Investigation: Y.A.; Methodology: H.K.; Project administration: Y.A.; Resources: S.O.; Software: H.K.; Supervision: N.T.; Validation: N.T.; Visualization: H.K.; Writing - original draft: M.O; Writing - review & editing: all.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bansal A., Sinha R.J., Jhanwar A. Laparoscopic ureteral reimplantation with Boari flap for the management of long- segment ureteral defect: A case series with review of the literature. Turk. J. Urol. 2017;43:313–318. doi: 10.5152/tud.2017.44520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau P., Graham A.M. Mesenteric desmoid tumor in Gardner’s syndrome treated by sulindac. Dis. Colon. Rectum. 1984;27:53–54. doi: 10.1007/BF02554079. [DOI] [PubMed] [Google Scholar]
- Gari M.K., Guraya S.Y., Hussein A.M. Giant mesenteric fibromatosis: report of a case and review of the literature. World. J. Gastrointest. Surg. 2012;4:79–82. doi: 10.4240/wjgs.v4.i3.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A.J., Tuvin D., Hajibashi S. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am. J. Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J.J., Boland, P.J., Leung, D.H., et al., 1999. The enigma of desmoid tumors. Ann. Surg. 229, 866–872, discussion 872–873. https://doi.org/10.1097/00000658-199906000-00014. [DOI] [PMC free article] [PubMed]
- Mizuno M., Kawaguchi Y., Kawanishi A. An intra-abdominal desmoid tumor, embedded in the pancreas, preoperatively diagnosed as an extragastric growing gastrointestinal stromal tumor. Case. Rep. Oncol. 2017;10:301–307. doi: 10.1159/000468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitamo J.J., Scheinin T.M., Hayry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am. J. Surg. 1986;151:230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Shinagare A.B., Ramaiya N.H., Jagannathan J.P. A to Z of desmoid tumors. Am. J. Roentgenol. 2011;197:W1008–W1014. doi: 10.2214/AJR.11.6657. [DOI] [PubMed] [Google Scholar]
- Sinha A., Burns E.M., Latchford A. Risk of desmoid formation after laparoscopic versus open colectomy and ileorectal anastomosis for familial adenomatous polyposis. Br. J. Surg. 2018;2:452–455. doi: 10.1002/bjs5.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitellaro M., Bonfanti G., Sala P. Laparoscopic colectomy and restorative proctocolectomy for familial adenomatous polyposis. Surg. Endosc. 2011;25:1866–1875. doi: 10.1007/s00464-010-1478-z. [DOI] [PubMed] [Google Scholar]