Highlights
-
•
High theta and low gamma activity in the dynamic pain connectome is linked to chronic pain.
-
•
High alpha-band activity is present when neuropathic pain is likely.
-
•
Spectral frequency band strength distinguish neuropathic from non-neuropathic pain.
Keywords: Chronic pain, Neuropathic pain, Magnetoencephalography, Default mode network, Salience network, Somatosensory cortex
Abstract
We previously identified alpha frequency slowing and beta attenuation in the dynamic pain connectome related to pain severity and interference in patients with multiple sclerosis-related neuropathic pain (NP). Here, we determined whether these abnormalities, are markers of aberrant temporal dynamics in non-neuropathic inflammatory pain (non-NP) or when NP is also suspected. We measured resting-state magnetoencephalography (MEG) spectral density in 45 people (17 females, 28 males) with chronic back pain due to ankylosing spondylitis (AS) and 38 age/sex matched healthy controls. We used painDETECT scores to divide the chronic pain group into those with only non-NP (NNP) and those who likely also had a component of NP in addition to their inflammatory pain. We also assessed pain severity, pain interference, and disease activity with the Brief Pain Inventory and Bath AS Disease Activity Index (BASDAI). We examined spectral power in the dynamic pain connectome, including nodes of the ascending nociceptive pathway (ANP), default mode (DMN), and salience networks (SN). Compared to the healthy controls, the AS patients exhibited increased theta power in the DMN and decreased low-gamma power in the DMN and ANP, but did not exhibit beta-band attenuation or peak-alpha slowing. The NNP patients were not different from HCs. Compared to both healthy controls and NNP, NP patients had increased alpha power in the ANP. Increased alpha power within the ANP was associated with reduced BASDAI in the NNP group, and increased pain in the mixed-NP group within the DMN, SN, and ANP. Thus, high theta and low gamma activity may be markers of chronic pain but high alpha-band activity may relate to particular features of neuropathic chronic pain.
1. Introduction
People who have chronic pain exhibit functional abnormalities in brain areas that are associated with attention, salience, and pain processing and modulation (Baliki et al., 2008; Davis and Moayedi, 2013; Hemington et al., 2016; Kaplan et al., 2019; Kim et al., 2019; Porreca et al., 2002). These areas are collectively known as the dynamic pain connectome (DPC) and include nodes in the ascending (ANP) and descending nociceptive pathways, the default mode (DMN) and salience (SN) networks (Kucyi and Davis, 2015, 2017). Findings of abnormal activity in the DPC in chronic pain is mostly based on data acquired using slow, hemodynamic-based fMRI that has a temporal resolution of seconds and so cannot capture faster occurring brain activity (Kucyi and Davis, 2015, 2017). However, aberrant activity within the DPC that occurs in the frequency range of 1–100 Hz that is associated with chronic pain can be examined using techniques that have a temporal resolution of milliseconds, such as EEG or magnetoencephalography (MEG). Using these methods, slower resting peak alpha frequency (PAF) in healthy individuals predicted greater pain sensitivity during pain (Furman et al., 2018). Beta and gamma power has also been shown to be related to the intensity of ongoing chronic pain (May et al., 2019) . Furthermore some studies have reported that patients with chronic pain exhibit PAF slowing (Boord et al., 2008; Vries et al., 2013; Lim et al., 2016; Sarnthein et al., 2005; Stern et al., 2006; Wydenkeller et al., 2009) and increased alpha and theta power oscillations (Pietro et al., 2018; Kim et al., 2019; Lim et al., 2016; Meneses et al., 2016; Pinheiro et al., 2016; Sarnthein et al., 2005; Stern et al., 2006; Broeke et al., 2013; Walton et al., 2010) that can normalize following treatment (Sarnthein et al., 2005; Stern et al., 2006). Reduced beta (Kim et al., 2019) and increased gamma (Lim et al., 2016) brain activity have also been found in chronic pain conditions and in some cases increased band power was linked to pain (Pietro et al., 2018; Kim et al., 2019; Lim et al., 2016; Schmidt et al., 2012).
Despite the evidence that there can be aberrant brain activity in patients with chronic pain, it is unclear whether these dysfunctions are ubiquitous across pain conditions. Notably, one EEG study failed to identify any temporal abnormalities in chronic pain (Schmidt et al., 2012) and in another study, abnormalities were only seen in patients with neuropathic pain (NP) (Vuckovic et al., 2014). Our recent MEG study of multiple sclerosis (MS) (Kim et al., 2019) also showed that abnormalities in activity differed between the patients who had non-NP and those with mixed-NP. As a chronic pain group, these MS patients had increased alpha power in nodes of the ANP and the SN. However, the subgroup of patients with mixed-NP had slowing of the PAF in various DPC nodes and reduced beta-band power in nodes of the ANP and these abnormalities were associated with pain interference. Thus, temporal abnormalities seem to be more evident when NP is likely (Boord et al., 2008; Pietro et al., 2018; Sarnthein et al., 2005; Stern et al., 2006; Walton et al., 2010; Wydenkeller et al., 2009), which occurs in up to 20% of the general population (Bouhassira, 2019; Bouhassira et al., 2008; Fayaz et al., 2016; Harifi et al., 2013; Toth et al., 2009). These findings raise the question of how aberrant neural dynamics relate to disease and pain measures and whether these relationships are a general marker of chronic pain or if they are specific to certain attributes such as NP.
Thus, the aim of this study was to determine whether abnormalities in resting state MEG spectral density and their association with disease activity and clinical pain differ for those with only inflammatory pain (non-NP, NNP) and those who are also likely to have a NP component. We hypothesized that abnormalities would be specific to the ankylosing spondylitis (AS) patients who have a component of NP. Towards this goal, we focussed on patients with AS because this patient population has relatively few co-morbidities and affects adults much younger than most other types of chronic pain (Braun and Sieper, 2007). These patients suffer from spondyloarthritis that mostly affects the spine and causes chronic pain that can be mixed neuropathic and inflammatory pains (Pathan and Inman, 2017; Wu et al., 2013), which allowed us to examine the effect of a NP component.
2. Methods
2.1. Participants
Participants with AS were recruited from Toronto Western Hospital Spondylitis clinic in collaboration with staff rheumatologists. Age/sex-matched healthy control (HCs) participants were recruited through advertisements that were posted at the University Health Network and through word of mouth. The study was approved by the Research Ethics Board of the University Health Network and all participants signed an informed consent form. Participants were asked to refrain from caffeine and alcohol for at least 1 and 8 h, respectively, before testing.
The inclusion criteria for participants with AS was a diagnosis of AS based on the modified New York criteria (Van Der Linden et al., 1984), that includes low back pain that improves with exercise, impaired mobility of the spine and sacroiliitis finding at radiography. This was verified in the Spondylitis clinic at the Toronto Western Hospital. Exclusion criteria for all participants were: (1) the presence of acute pain or a history of chronic pain (other than AS in the patient group), (2) any diagnosis of neurological or psychiatric disorder, (3) any diagnosis of a major health issue (e.g. diabetes), and (4) taking medications on a regular basis, except for the AS-related treatments.
There were 83 (45 AS, 38 HCs) participants in this study that were included in different analyses: The main analysis of the AS vs HCs groups comprised 38 AS and 38 age/sex-matched HCs. However, we also performed analyses on several subgroups. We selected participants to ensure age/sex-matching between groups and for subgroup analyses (see Section 2.2) that resulted in: (1) 12 AS and 12 age/sex-matched HC (for the comparison of mixed-NP vs HCs groups), (2) 26 AS and 26 age/sex-matched HCs (for the comparison of NNP vs HCs groups), and (3) 12 NP and 12 age/sex-matched NNPs (for the comparison of mixed-NP vs NNP groups).
2.2. Clinical assessment and questionnaires
Key AS symptoms were evaluated by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (Garrett et al., 1994), obtained at the most recent visit of the patient to the AS clinic. The BASDAI index includes 6 items that assess fatigue, joint swelling and pain, local tenderness and morning stiffness. The composite BASDAI score ranges from 0 to 10, with 10 indicative of high disease activity. Patients were also asked to verbally rate their current pain (typically localized to the lower back/buttock area) and the average general pain in the last 4 weeks, on a scale that ranged from 0 (“none”) to 10 (“max”) using the painDETECT questionnaire that also asses pain severity (Freynhagen et al., 2006). AS can include a NP component (Wu et al., 2013), and to assess this we used the painDETECT questionnaire (Freynhagen et al., 2006). The painDETECT scores range from 0–38 and patients rate how much they suffer from different neuropathic components (e.g. burning, tingling, electric shocks). Scores of 12 and under indicate that NP is unlikely; patients with these scores were deemed to have inflammatory non-neuropathic pain (NNP). Scores of 19 and greater indicate a high likelihood of NP. A score of 13–18 indicates that there is a likelihood of some NP and so patients with these scores were categorized as having mixed inflammatory-NP. In this study we considered patients with a score of 13 or higher as having mixed inflammatory-NP (NP) to be consistent with our previous studies (Bosma et al., 2018; Cheng et al., 2018; Kim et al., 2019; Wu et al., 2013). All participants also completed the Beck Depression Inventory (BDI) questionnaire. The BDI evaluates the cognitive and affective symptoms of depression in the past week and consists of 21 questions, each rated from 0 to 3. The total score thus ranges from 0 to 63; scores of 0–9 classified as none to minimal depression, and scores of 10–18, 19–29, and 30–63 considered as mild-moderate, moderate-severe, and severe depression, respectively (Beck et al., 1988).
2.3. Magnetoencephalography and MRI acquisition
All participants underwent a 5 min resting state MEG scan acquired with a 306 channel Elekta Neuromag TRIUX system, with a sampling rate of 1000 Hz and a DC bandpass of 330 Hz. Prior to the MEG scan, the nasion and bilateral pre-auricular positions were marked for each participant. These locations were used as fiducial reference points that later served for motion correction and registration to the MRI anatomical scan (Velmurugan et al., 2014). Participants were scanned sitting in an upright position with their eyes open and fixated on a cross on a screen in front of them with the room lights off. Participants were instructed to avoid structured thinking and to let their mind wander. The position of the participant's head was monitored continuously through head position indicator coils affixed to the participant's head. The spatiotemporal signal space separation (tSSS) algorithm (Gonzalez-Moreno et al., 2014; Taulu and Simola, 2006), implemented in the MaxFilter program, was used for artifact and head movement correction. Following the MEG scan, a 3T MRI (GE) high resolution T1 anatomical image was obtained with the following acquisition parameters: voxel size = 1 mm3, matrix = 256 × 256, 180 axial slices, TR = 7.8 s, TE = 3 ms, inversion time = 450 ms.
2.4. Magnetoencephalography data preprocessing, region of interest and beamforming
We preprocessed the MEG data using the same approach we previously described (Kim et al., 2019). Briefly, to analyze the resting state data we used the FieldTrip toolbox, run on MATLAB software (ver R2015b). The first and last 10 s of the recorded data were removed, leaving 280 s of resting state data for each participant. Data were bandpass-filtered between 1 and 150 Hz and a notch filter was applied at 60 Hz. Down sampling to 300 Hz was then performed and Independent component analysis (ICA) used (“runica” function) to remove artifacts associated with cardiac artifacts, eye blinks, breathing and muscle activity. This was done by visually inspecting the different components and removing components that appear to be noise and periodic signals. The procedure for visual ICA removal varied for each subject as some subjects had more noise than others. Thus there was a range of components that were removed but it was not more than 15 out of 204 components in each subject. To register the resting state MEG data to the participant's anatomical image, the fiducial points of the nasion and bilateral pre-auricular were first identified on the anatomical image and these were used to co-register the MEG data to the anatomical image. After each person's MEG data is registered to their own MRIs by using fiducial points previously defined before the MEG scan, each individuals preprocessed data is then warped into a template brain. The anatomical image was then segmented using statistical parametric mapping (SPM), resulting in a geometrical representation of the brain which was then used in a single-shell forward model.
We used a linearly constrained minimum variance beamformer (Veen et al., 1997) to extract a continuous time series for nodes of the dynamic pain connectome. The beamformer technique assumes that there is no long term perfect correlation in the local field potentials of different sources and uses spatial filtering to maximize the signal in an area of interest while suppressing it in sources of no interest (Hillebrand and Barnes, 2005; Hillebrand et al., 2005). This is accomplished by applying a weighted vector of linear combination of sensor outputs that yield a separate time course for each source that can then be used for further analysis (Dymond et al., 2014; Engels et al., 2016; Hillebrand and Barnes, 2005; Kim et al., 2019). Thus, 14 regions of interest (ROI) were defined and used as “virtual sensors”. These ROIs coordinates (x, y, z) in MNI space, were defined based on previous publications (Hemington et al., 2016; Kim et al., 2019; Kucyi et al., 2013; Rogachov et al., 2016) and included areas of the ANP: bilateral thalamus (+/−12, −18, 8), left (−34, −30, 54) and right (34, −28, 54) primary somatosensory cortex (S1), left (−60, −30, 20) and right (60, −22, 18) secondary somatosensory cortex (S2), bilateral (+/−34, −20, 18) posterior insula (pINS); SN: right temporoparietal junction (TPJ) (50, −32, 28), right anterior insula (aINS) (34, 18, 4), mid-cingulate cortex (2, 12, 34, MCC) and the right dorsolateral prefrontal cortex (34, 46, 22, DLPFC); DMN: posterior cingulate cortex (−2, −46, 28, PCC) and medial prefrontal cortex (−2, 50, 2, mPFC). The SN included only regions within the right hemisphere because the SN is a right-lateralized network and these area show higher activity when participants attend to pain (Kucyi and Davis, 2015).
2.5. Power spectra analysis
The output time series from beamforming analysis was normalized (z-score) within subject and as previously described (Kim et al., 2019). Specifically, after obtaining each individual's resting state time-series from beamforming (a “virtual electrode”), the power spectrum density (PSD) was obtained using Welch's Power Density. The PSD values were then normalized because each individual will have different raw magnitude of relative power spectra thus it must be normalized before group level inferences could be made. Thus z-scoring was used to normalize each individual's resting state timer-series before calculating the PSD used for group level statistics. The Welch power spectral density (pwelch function in MATLAB R2015b) method was used to estimate the power spectral density for each frequency point in each of the 14 ROIs (i.e. sources). The Welch method divides the time series into 8 or less overlapping segments (50% overlap), computes the power spectral density of each segment using a fast Fourier transform with a Hamming window and then averages the power spectral density of all segments (Welch, 1967). This process reduces the variance of the estimated power spectral density and resulted in a power spectral density value for each frequency in the 1–150 Hz range, for each of the ROIs.
2.6. Statistical analysis
Statistical analysis was performed in GraphPad Prism (version 7.03 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com). Our analyses focused on the theta (4–7 Hz), alpha (8–13 Hz), beta (14–30 Hz) and low gamma (31–59 Hz) range. For each frequency point, within each ROI, we calculated the spectral power mean and standard deviation (SD) for HCs, the entire group of AS patients and also the NP and NNP sub-groups. Because of a previous report of a shift in the PAF in chronic neuropathic pain patients (Kim et al., 2019), we also calculated the mean and SD of the individual PAF within each group by identifying the maximal power value within the alpha band for each participant.
2.6.1. Demographic characteristics
Differences in age, BDI score and sex between HCs and AS patients were tested using Student t-tests (age and BDI) and Chi-square (sex) tests. Because of the small number of NP patients, nonparametric Mann–Whitney U test was used to examine differences between NP and NNP subgroups in age, BDI, the number of years in pain, average pain and BASDAI. Sex differences were tested using Chi-square.
2.6.2. Spectral power
To examine group differences that were related to chronic pain, we first compared the entire group of AS patients with the group of HCs. Thereafter, to examine abnormalities that were specific to the likelihood of a neuropathic pain component, we compared NP and NNP subgroups to age/sex-matched HCs in a separate analysis. As NP patients were significantly older than NNP patients, we compared our NP patients with a subgroup of the NNP patients, who were age/sex-matched. As the spectral power was normalized these group comparisons were examined using parametric tests as follows: These were performed for the power spectra of each frequency point in the range 4–60 Hz as well as the individual PAF. We used multiple student t-tests for group comparisons and corrected for the number of ROIs with a false discovery rate (FDR, P < 0.05) using the Benjamini Hochberg method (Benjamini and Hochberg, 1995). We repeated the analysis for regions with significant results, correcting for the multiple frequencies with a false discovery rate (FDR, P < 0.05) using the Benjamini Hochberg method. The effect size for statistically significant differences is reported using Cohen's d, as calculated using the effect size calculator at: https://www.uccs.edu/lbecker/. It should be noted that although we examined individual frequency bins, we also divided them into bands in order to interpret the results with the existing knowledge (see Results and Discussion). We then performed Spearman's correlation analysis between AS clinical measures (BASDAI, average pain in the last 4 weeks – i.e. trait-pain) and the power spectra of areas and frequencies that significantly differed between NP and NNP subgroups. We chose the BASDAI and trait-pain as two measures that reflect different aspects of the AS disease. The BASDAI includes multiple factors other than pain (e.g., fatigue, swelling and stiffness) and the trait-pain reflect the overall experience of pain, not limited to the current moment (Davis and Cheng, 2019). As this analysis was exploratory and based on the results of the previous step, it was not corrected for multiple comparisons and was considered statistically significant for p < 0.05.
3. Results
3.1. Demographics
Our main analysis compared the data from 38 AS patients to 38 age/sex-matched HCs (23 M, 15F in each group). In most cases, patients were matched within 3 years to a HC. However, given the difficulty in recruiting older HCs, in some instances we accepted a difference of 4 years (5 patients) and 5 years (1 patient). Demographic characteristics of both HCs and chronic pain patients and the patients’ disease characteristics (AS patients, NP and NNP subgroups) are provided in Table 1. Individual PainDETECT scores are presented in Fig. 1. The AS group included 12 inflammatory pain patients with a likelihood of NP (PainDETECT score ≥ 13) and 26 inflammatory pain patients with NNP. There were no significant differences in age or sex between HCs and AS patients (age and sex: p > 0.917), nor between the HCs matched to each AS subgroup (age and sex: p > 0.831). Between NP and NNP subgroups, there were no significant differences in sex (Chi-square: p = 0.367), the number of years in pain (Mann–Whitney U: p = 0.097), current (Mann–Whitney U: p = 0.231) and average (Mann–Whitney U: p = 0.311) pain, or BASDAI scores (Mann–Whitney U: p = 0.224). However, since the NP group was significantly older than the NNP group (Mann–Whitney U: p = 0.002), we also examined group differences between NP patients and 12 age/sex-matched NNP patients (i.e. restricted group). This analysis included an additional 7 patients with NNP that were not included in the main analysis between patients with AS and HCs. Of note, these 7 NNP patients were not included in the group comparison to HCs because we could not age/sex match them to HCs. There were no differences between the NP and the restricted group of NNP patients in age, current and average pain, BASDAI or years with pain (Mann–Whitney U: age p = 0.966; current pain p = 0.877; average pain p = 0.984; BASDAI p = 0.247; years with pain p = 0.139). The BDI scores were higher in the AS group (t-test: p < 0.001) and the NP subgroup (Mann–Whitney U: p = 0.009) but not in the NNP subgroup (the 26 NNP that were compared to HCs) (Mann–Whitney U: p = 0.082) compared to the HCs group. There was no significant difference in BDI scores between the NP and NNP (Mann–Whitney U: p = 0.085) nor between the NP and the restricted NNP subgroup (Mann–Whitney U: p = 0.155).
Table 1.
Demographic and disease characteristics .
| HC Group | AS Chronic pain group | ||||
|---|---|---|---|---|---|
| All | NP | NNP | NNP age/sex-matched to NP | ||
| N | 38 | 38 | 12 | 26 | 12 |
| Age* (years) | 31.5 ± 9.8 | 31.7 ± 9.9 | 39.0 ± 9.1a | 28.4 ± 8.5b | 38.8 ± 10.1 |
| Sex | 23 M,15F | 23 M,15F | 6 M,6F | 17 M,9F | 6 M,6F |
| BASDAI | n/a | 3.4 ± 2.3 | 4.3 ± 2.4 | 2.9 ± 2.2 | 3.3 ± 2.9 |
| PainDetect | n/a | 9.0 ± 5.4 | 15.3 ± 2.5 | 6.1 ± 3.6 | 5.9 ± 3.2 |
| Current pain | n/a | 2.7 ± 2.4 | 3.3 ± 2.4 | 2.4 ± 2.4 | 3.2 ± 2.6 |
| Average pain (0–10 NPS) | n/a | 3.6 ± 2.4 | 4.0 ± 2.0 | 3.4 ± 2.6 | 4.3 ± 2.8 |
| Years with pain | n/a | 13.7 ± 9.4 | 17.2 ± 8.8 | 12.2 ± 9.4 | 22.8 ± 10.1 |
| BDI* | 3.7 ± 3.3a | 8.2 ± 7.1b | 11.3 ± 7.2b | 6.7 ± 6.7 | 7.4 ± 8.2 |
Group data are shown as mean +/- standard deviation.
Attributes that have significant group differences are indicated by *, with the specific groups that are significantly different from one another indicated by a,b. The Bath AS Disease Activity Index (BASDAI) and average pain scores were missing for 3 NNP and 1 NNP patients, respectively. NPS-numerical pain scale.
Fig 1.
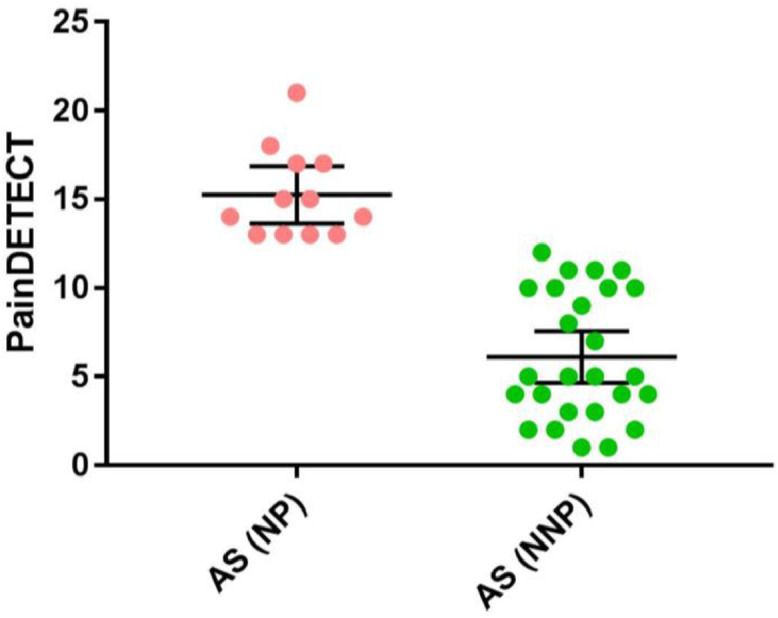
PainDETECT scores in the inflammatory non-neuropathic (NNP) and mixed inflammatory and neuropathic (NP) pain subgroups – The NP subgroup had a PainDETECT score of 13 or higher and the NNP subgroup had a PainDETECT score of 12 or less. The graph also indicates the mean and 95% confidence interval for each subgroup.
3.2. Increased theta and decreased gamma resting state power in chronic pain
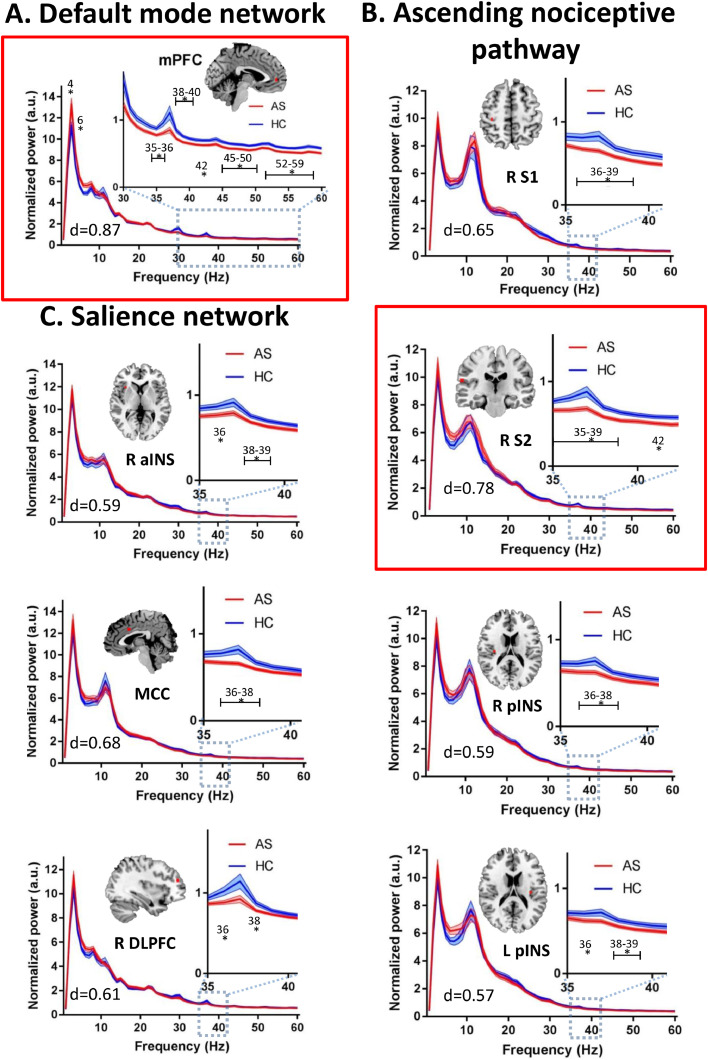
The whole group analysis indicated that AS chronic pain patients’ exhibit abnormal resting state activity in the theta and gamma bands within several nodes of the DMN, ANP, and SN of the DPC (Fig. 2), but there were no significant group differences in the PAF for any of the ROIs (p ≥ 0.878). Specifically, the AS chronic pain group exhibited significantly increased resting state activity power in the theta (4, 6 Hz; max effect size: d = 0.74) band but decreased gamma band power (max effect size: d = 0.87) within the mPFC of the DMN (FDR corrected at p < 0.05) compared to the HC group (Fig. 2A). Furthermore, compared to HCs, the AS chronic pain group had decreased resting state gamma power within various nodes of the ANP (right S1, right S2, right and left pINS; max effect size: d = 0.77) (Fig. 2B) and the SN (right aINS, MCC, right DLPFC; max effect size: d = 0.68) (Fig. 2C). For more details on the significance and effect sizes see Supplemental Table 1. When we repeated the analysis with a more conservative threshold, corrected for the multiple frequency points, the NP patients still exhibited increased theta activity in the DMN (mPFC) and decreased gamma activity in the ANP (right S2) and the DMN (mPFC) (Fig. 2A and B).
Fig 2.
AS chronic pain patients have increased theta power and decreased gamma power in the dynamic pain connectome. Average resting state MEG activity in the A. default mode network (left medial prefrontal cortex (mPFC)), B. the salience network (right anterior insula (aINS), mid-cingulate cortex (MCC), dorsolateral prefrontal cortex (DLPFC)), and the C. ascending nociceptive pathway (right primary somatosensory cortex (S1), secondary somatosensory cortex (S2), bilateral posterior insula (pINS)) is shown for the ankylosing spondylitis (AS) chronic pain group (red) and healthy control (HC) group (blue). Increased power in the theta (4−7 Hz) band and decreased power in the low gamma (31–60 Hz) band in the AS compared to HC group is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Regions that survived correction for the multiple frequencies are marked in a red frame. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.3. Increased theta and alpha power and decreased gamma power specific to neuropathic pain
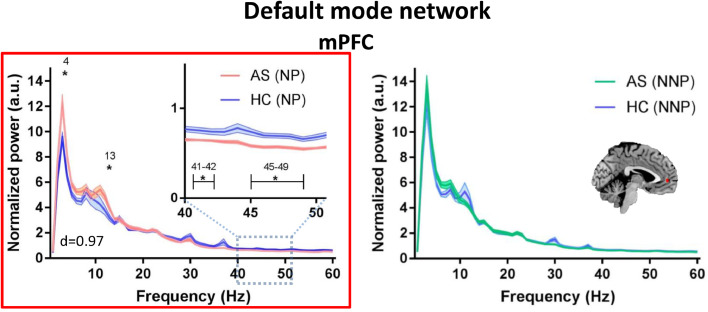
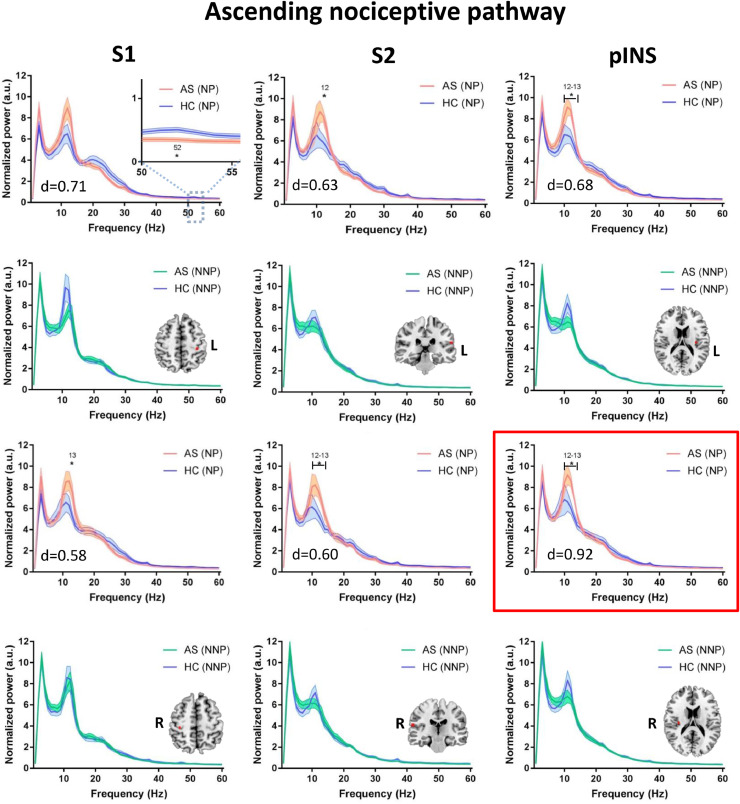
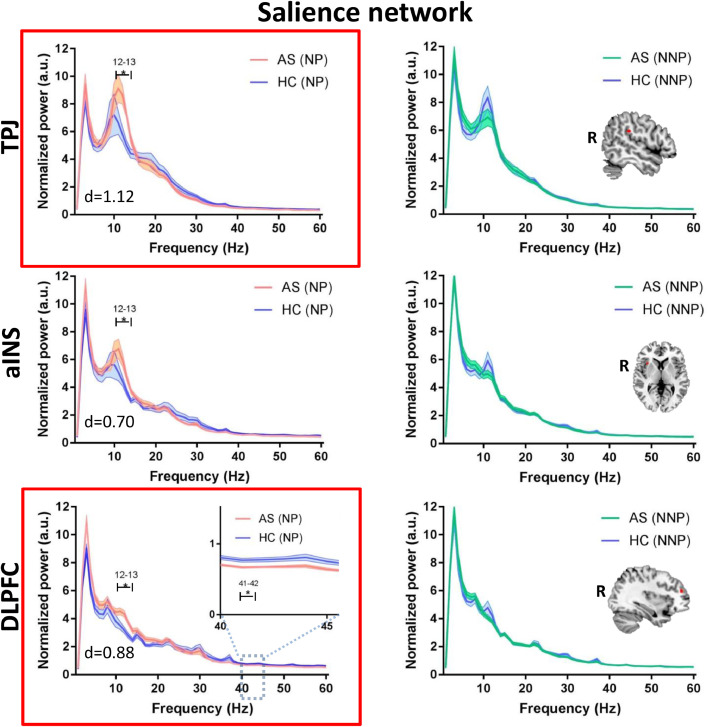
We evaluated whether the abnormalities identified for the entire AS group are related to chronic pain per se or specifically to the likelihood of a neuropathic pain component. To do this, we compared the power spectra of the subgroups of NP and NNP patients to age/sex-matched HCs (see Fig 3, Fig 4, Fig 5). Compared to HCs, the NP but not the NNP patients showed significantly increased theta (4 Hz; max effect size: d = 0.84) power in the mPFC of the DMN (Fig. 3), as well as increased alpha (12–13 Hz) power in various areas of the DPC including the mPFC (max effect size: d = 0.57) of the DMN (Fig. 3), the ANP (right and left thalamus, right S1, right and left S2, right and left pINS; max effect size: d = 0.92) (Fig. 4) and the SN (right aINS, right TPJ, and right DLPFC; max effect size: d = 1.12) (Fig. 5). The NP but not the NNP subgroup also showed decreased gamma power in the mPFC (max effect size: d = 0.97) of the DMN (Fig. 3), in the left S1 (effect size: d = 0.71) of the ANP (Fig. 4), and in the right DLPFC (max effect size: d = 0.77) of the SN (Fig. 5). Details on the significance and effect size of these findings are provided in Supplemental Table 2. When we repeated the analysis correcting for the multiple frequency points, NP patients continued to exhibit increased alpha activity in the ANP (right pINS) and SN (right DLPFC and right TPJ) and decreased gamma activity in the DMN (mPFC) (Fig. 3–5). There were no significant differences between HCs and NNP in any of the regions of interested tested for any of the frequencies (p > 0.05) (See Fig 3, Fig 4, Fig 5). There were also no differences between HCs and NP or NNP patients in the PAF (p ≥ 0.4).
Fig 3.
NP patients have increased theta and alpha power and decreased gamma in the default mode network. Average resting state MEG activity in the left medial prefrontal cortex (mPFC) of the default mode network is shown for the mixed inflammatory and neuropathic pain (NP) subgroup (pink), inflammatory none-neuropathic pain (NNP) subgroup (green) and healthy control (HC) subgroups (blue). Increased power in the theta (4–7 Hz) and alpha (8–13 Hz) band and decreased power in the low gamma (31–60 Hz) band in the NP compared to HC group is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. This region also survived correction for the multiple frequencies, marked in a red frame. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Fig 4.
NP patients have increased alpha power and decreased gamma power in the ascending nociceptive pathway. Average resting state MEG activity in the bilateral primary somatosensory cortex (S1), secondary somatosensory cortex (S2), and posterior insula (pINS) of the ascending nociceptive pathway is shown for the mixed inflammatory and neuropathic pain (NP) subgroup (pink), inflammatory none-neuropathic pain (NNP) subgroup (green) and healthy control (HC) subgroups (blue). Increased power in the alpha (8–13 Hz) band and decreased power in the low gamma (31–60 Hz) band in the NP compared to HC group is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Regions that survived correction for the multiple frequencies are marked in a red frame. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Fig 5.
NP patients have increased alpha power and decreased gamma power in the salience network. Average resting state MEG activity in the right temporoparietal junction (TPJ), anterior insula (aINS), and dorsolateral prefrontal cortex (DLPFC) of the salience network is shown for the mixed inflammatory and neuropathic pain (NP) subgroup (pink), inflammatory none-neuropathic pain (NNP) subgroup (green) and healthy control (HC) subgroups (blue). Increased power in the alpha (8–13 Hz) band and decreased power in the low gamma (31–60 Hz) band in the NP compared to HC group is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Regions that survived correction for the multiple frequencies are marked in a red frame. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
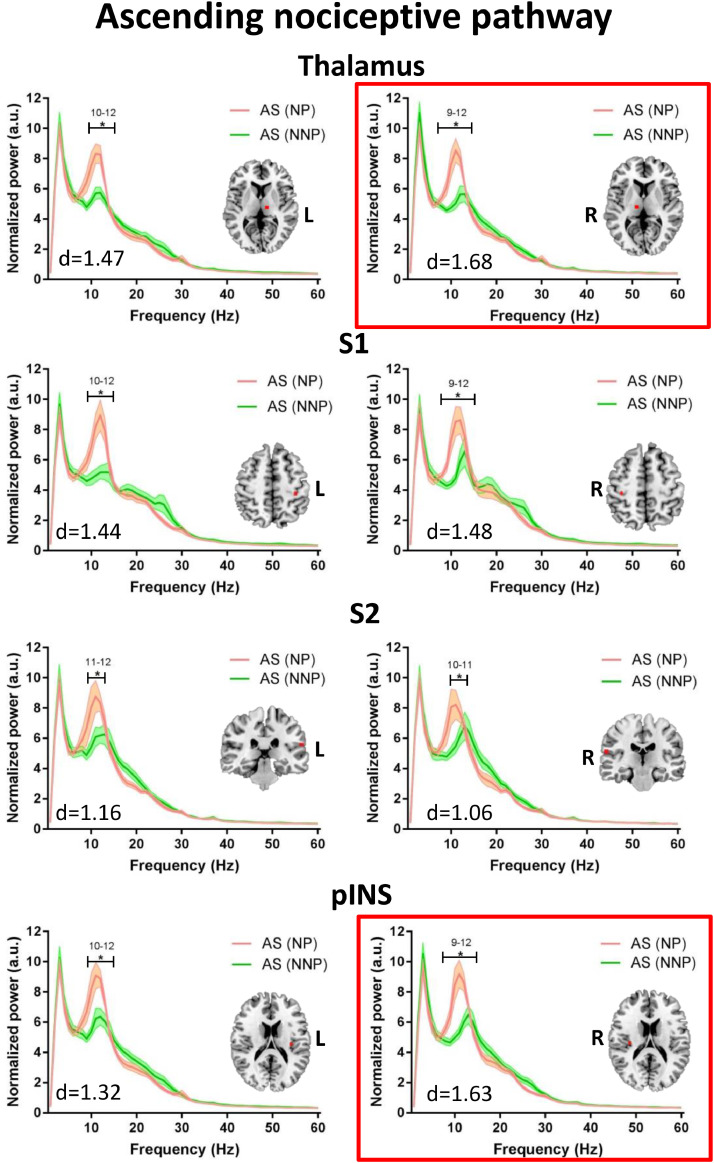
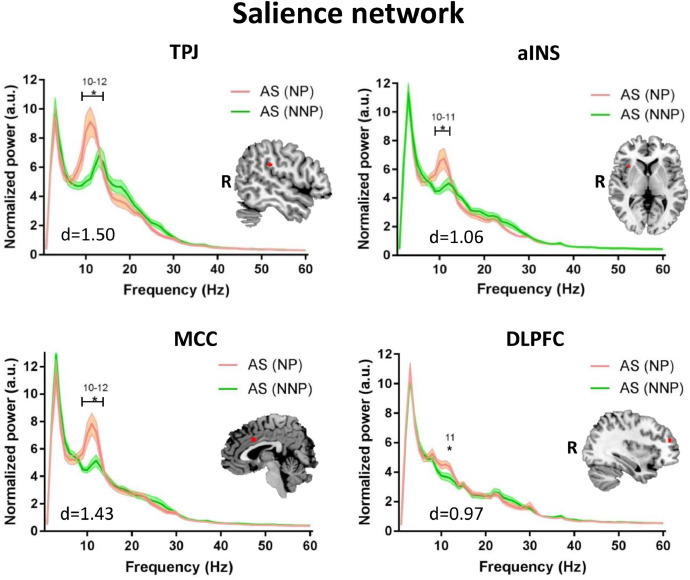
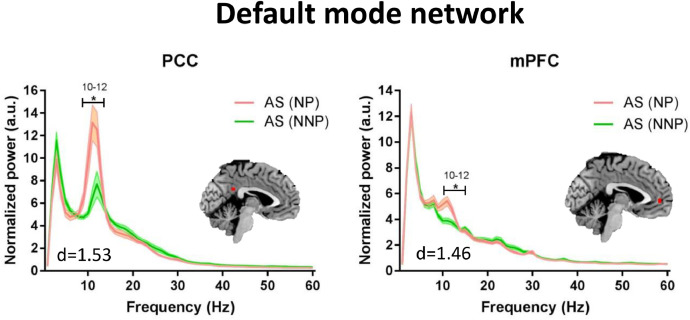
Because the patients with NP were significantly older than those with NNP, we conducted a secondary analysis using selected 12 age/sex-matched NNP. Compared to the patients with NNP, those with NP showed higher alpha power (9–12 Hz) within multiple nodes of the DPC: in the ANP (left and right thalamus, left and right S1, left and right S2, left and right pINS; max effect size: d = 1.68) (Fig. 6), the SN (right TPJ, right aINS, MCC, right DLPFC; max effect size: d = 1.50) (Fig. 7); and the DMN (PCC, mPFC; max effect size: d = 1.53) (Fig. 8). For more details on the significance and effect size see Supplemental Table 3. When we repeated the analysis correcting for the multiple frequency points, NP patients continued to exhibit increased alpha activity in the ANP (right pINS and right thalamus) (Fig. 6). There were no significant subgroup differences in the theta, beta or lower gamma bands, nor in the PAF (p > 0.8).
Fig 6.
Compared to NNP, NP patients have increased alpha power in the ascending nociceptive pathway. Average resting state MEG activity in the bilateral thalamus, primary somatosensory cortex (S1), secondary somatosensory cortex (S2), and posterior insula (pINS) of the ascending nociceptive pathway is shown for the mix inflammatory and neuropathic pain (NP) subgroup (pink) and inflammatory none-neuropathic pain (NNP) subgroup (green). Increased power in the alpha (8–13 Hz) band in the NP compared to NNP subgroup is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Regions that survived correction for the multiple frequencies are marked in a red frame. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Fig 7.
Compared to NNP, NP patients have increased alpha power in the salience network. Average resting state MEG activity in the right temporoparietal junction (TPJ), anterior insula (aINS), mid-cingulate cortex (MCC) and dorsolateral prefrontal cortex (DLPFC) of the salience network is shown for the mixed inflammatory and neuropathic pain (NP) subgroup (pink) and inflammatory none-neuropathic pain (NNP) subgroup (green). Increased power in the alpha (8–13 Hz) band in the NP compared to NNP subgroup is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Fig 8.
Compared to NNP, NP patients have increased alpha power in the default mode network. Average resting state MEG activity in the posterior cingulate cortex (PCC) and the medial prefrontal cortex (mPFC) of the default mode network is shown for the mixed inflammatory and neuropathic pain (NP) subgroup (pink) and inflammatory none-neuropathic pain (NNP) subgroup (green). Increased power in the alpha (8–13 Hz) band in the NP compared to NNP subgroup is shown. Lines indicate mean ± SE. Significant group differences (indicated by *) was set using false discovery rate (FDR, p < 0.05) corrected for the number of regions. Brain images are displayed using the radiological convention. d – the maximal effect size of significant differences within the region. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.4. Associations with clinical pain and disease activity
The power spectra in areas and frequencies that showed significant differences between the NP and NNP subgroups were further examined to identify Spearman correlations with disease activity (BASDAI score) and clinical trait-pain (average pain in the last 4 weeks). The BASDAI score was missing for 2 NNP patients so that the analysis for association with the BASDAI score was done for 10 NNP and 12 NP patients. For all significant correlations in this restricted group of NNP patients, we also examined the association in the larger group of 33 NNP (i.e. full NNP subgroup) patients to determine whether the finding was present across a broader age span. As the BASDAI score was missing for 3 NNP patients, this analysis was done for 30 NNP patients.
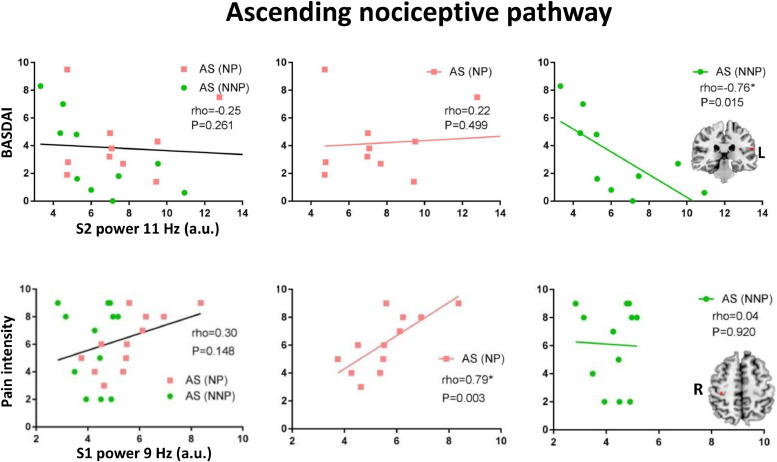
We found that the alpha band power spectra in nodes of the ANP were negatively correlated with the BASDAI score in the restricted group of NNP patients but not in the NP patients. These associations were identified in the right S1 and bilaterally in the thalamus, and pINS. These associations remained significant in the full NNP subgroup in the left thalamus, S2 and pINS (see Fig. 9 and Supplemental Table 4).
Fig 9.
Increased alpha power in the ascending nociceptive pathway is associated with lower disease activity in NNP patients and with higher clinical pain in NP patients. Spearman correlation between the alpha power and disease activity (i.e., BASDAI) is shown for the left secondary somatosensory cortex (S2) of the ascending nociceptive pathway. Spearman correlation between the alpha power and trait clinical pain intensity (average pain over the last 4 weeks) is shown for the right primary somatosensory cortex (S1) of the ascending nociceptive pathway. Associations are shown in the entire ankylosing spondylitis (AS) chronic pain group (left), in the mixed inflammatory and neuropathic pain (NP) subgroup (pink, middle), and in the matched inflammatory none-neuropathic pain (NNP, right). Significant correlation is indicated by *. The lines are only meant to visually show treadlines to demonstrate the direction of the Spearman relationship and do not indicate a linear relationship. Brain images are displayed using the radiological convention. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
We also identified significant positive correlations between clinical pain in NP, but not in NNP, with the alpha (9–10 Hz) power in nodes of the ANP, SN and DMN. Specifically, this relationship was found in the right thalamus, S1, and pINS of the ANP, the right aINS of the SN, and in the mPFC of the DMN (see Fig. 9, Supplemental Table 5).
4. Discussion
A brain signature of chronic pain that is consistently present across different types of pain has been elusive, partly due to the complexities and heterogeneity of chronic pain conditions. Identifying these abnormalities can help guide the development of new treatments for pain relief such as neurofeedback (Mayaud et al., 2019; Vuckovic et al., 2019). A promising approach towards identifying chronic pain markers is to use a technique such as MEG that is capable of both identifying the location and temporal characteristics of such a brain signature. In this MEG study, our aim was to determine whether aberrant temporal dynamics in the DPC and their association with disease activity and clinical pain is a marker of chronic pain in general, or whether these abnormalities are only present when there may be a component of NP. We found that the AS patients who had a mix of inflammatory and neuropathic pains did not exhibit PAF slowing or beta attenuation compared to NNP patients. However, detailed analyses of the NP and NNP subgroups revealed several prominent findings that indicate particular abnormalities in the DPC's networks and pathways: (1) Patients with AS chronic pain had increased theta (4–7 Hz) power in the DMN and decreased gamma (31–60 Hz) power in the ANP and DMN compared to healthy controls. (2) A subgroup analysis revealed that the theta and gamma band abnormalities along with increased alpha (8–13 Hz) power are specific to patients who are likely to have NP. (3) Increased alpha power throughout the DPC was associated with increased trait-pain in the NP group. Interestingly, alpha power was negatively associated with AS disease activity in the NNP group.
EEG has identified abnormalities in chronic pain (Pinheiro et al., 2016); usually increased theta and alpha power but there is variability across studies and pain conditions. For example, increased theta power was reported in orofacial NP, chronic neurogenic pain, complex regional pain syndrome (CRPS) and central NP (Pietro et al., 2018; Sarnthein et al., 2005; Stern et al., 2006; Vuckovic et al., 2014; Walton et al., 2010). Increased alpha power was found in orofacial NP, rheumatoid arthritis, post cancer persistent pain and central NP (Pietro et al., 2018; Meneses et al., 2016; Broeke et al., 2013; Vuckovic et al., 2014). Decreased alpha power was also reported for central NP (Sarnthein et al., 2005; Stern et al., 2006). Decreased beta power was reported for neurogenic pain and MS-related NP (Kim et al., 2019; Sarnthein et al., 2005; Stern et al., 2006), while increased beta and gamma was reported in fibromyalgia (Lim et al., 2016). PAF slowing was reported for central NP, NP following spinal cord injury (SCI), chronic pancreatitis, fibromyalgia and MS-related NP (Boord et al., 2008; Vries et al., 2013; Kim et al., 2019; Lim et al., 2016; Sarnthein et al., 2005; Vuckovic et al., 2014; Wydenkeller et al., 2009). However, no abnormalities were found for low back pain patients (Schmidt et al., 2012). Given both the similarities and discrepancies across studies, the Ploner group (Dinh et al., 2019) sought to identify an EEG marker across multiple pain conditions but did not find striking differences in PAF or power (Dinh et al., 2019). Another large cohort study of NP patients found increased theta, beta and gamma and decreased alpha power (Vanneste et al., 2018). However, most of these studies examined a small number of patients (Boord et al., 2008; Vries et al., 2013; Kim et al., 2019; Lim et al., 2016; Sarnthein et al., 2005; Stern et al., 2006; Broeke et al., 2013; Vanneste et al., 2018; Walton et al., 2010) or a large group of patients from multiple pain conditions (Dinh et al., 2019; Vanneste et al., 2018). This likely led to the variability in findings across studies and suggests that there is no consistent brain activity marker of chronic pain per se. However, temporal abnormalities in brain activity are reported more often in NP than in NNP and these may also be different for peripheral versus central NP.
Different frequency bands of resting state brain activity are associated with multiple and distinct functions (Buzsaki, 2006). Understanding these associations can provide insight into the temporal abnormalities that are often reported in chronic pain. In healthy individuals, alpha and beta band activity is high at rest and decreases when a sensory stimulus is presented, whereas gamma and theta activity show the opposite pattern (Hanslmayr et al., 2011).
Our finding of increased alpha and theta power and decreased gamma power in NP could be attributed to several factors related to the temporal features and location of the aberrant activity. In our study, compared to HCs, patients who were also likely to have a NP component showed increased theta power. As suggested by others (Pietro et al., 2018; Llinas et al., 1999), increased cortical theta power in chronic pain may be due to thalamocortical dysrhythmia, resulting from increased thalamic theta firing activity. As the thalamic activity was not elevated in our cohort, this explanation is less likely. Nonetheless, this controversial model (Dinh et al., 2019) is supported by studies (Pietro et al., 2018; Sarnthein and Jeanmonod, 2008; Sarnthein et al., 2003; Vanneste et al., 2018) showing a therapeutic lesion to the thalamus reduces excessive theta activity in neurogenic pain (Sarnthein et al., 2005; Stern et al., 2006). This is also supported by pain models in animal studies (LeBlanc et al., 2017; Leblanc et al., 2014).
We identified increased alpha power in the NP patients within multiple nodes of the DPC compared to HCs, and in the ANP when compared to the NNP patients. This suggests that increased alpha activity is unique to patients who also show signs of NP, at least AS-related NP. Additionally, the NP patients showed decreased gamma power. Alpha band activity is associated with attention (Palva and Palva, 2011), but alpha and gamma activity may have different roles in attention and executive function (Doesburg et al., 2008). A review paper on the role of alpha oscillation suggested that high alpha activity may point towards an ‘internal attentive state’ where the attention is oriented inward rather than outward. In this case, the appearance of an external stimulus is more likely to be missed (Hanslmayr et al., 2011; O'Connell et al., 2009). Notably, gamma activity is elevated when we attend to an external stimulus (Doesburg et al., 2008). Specifically, in pain, gamma oscillations were observed in response to tonic (Nickel et al., 2017; Schulz et al., 2015) and brief (Gross et al., 2007; Hauck et al., 2007) pain stimuli. Notably, increase in gamma oscillation was also reported in relation to ongoing chronic pain intensity, but this effect disappeared when the effect of time was considered (May et al., 2019). Together, we suggest that both increased alpha and decreased gamma power seem to support an inward attentive state in patients that are likely to have NP. As chronic pain patients show increased attention to pain-related stimuli (Schoth et al., 2012) and alpha activity is also associated to pain expectation and negative pain-related affect (Albu and Meagher, 2016), the increased alpha power might reflect pain vigilance as well as rumination and magnification. In line, alpha power in the NP subgroup was positively associated to higher trait-pain. This association was not observed in the inflammatory-NNP patients, in which alpha power was negatively associated with disease activity. Given the abnormal alpha activity in NP, increased alpha in this population may be associated with detrimental symptoms (e.g. increased pain). However, NNP show normal alpha activity and for these patients, increased alpha may be associated with protective qualities (e.g. reduced disease activity). Importantly, our measure of disease activity is based on the BASDAI score that reflects the inflammatory nature of AS pain. Nonetheless, some patients also suffer from a mix of inflammatory-NP. Our results suggest that the mechanisms for pain in the alpha band are different between these two subgroups.
NP patients showed temporal abnormalities in the DMN and SN. The DMN is active during rest and deactivated during a task while the SN shows the opposite pattern and is associated with the level of attention (Kucyi and Davis, 2015). These networks show abnormalities in chronic pain (Baliki et al., 2008; Bosma et al., 2018; Hemington et al., 2016; Kim et al., 2019; Kucyi and Davis, 2015) that might relate to the effect chronic pain has on attention and executive function (Berryman et al., 2014; Kucyi and Davis, 2015; Schoth et al., 2012). Temporal abnormalities were also observed in the ANP that include pain processing areas. Importantly, pain and disease activity were associated with the spectral power in various nodes of the DPC but this was more pronounced in the ANP. This supports other studies that showed associations between pain and the ANP (Pietro et al., 2018; Kim et al., 2019).
We do note a few considerations to interpret our findings conservatively: First, we previously demonstrated that chronic pain in AS is typically inflammatory or a mix of inflammatory and neuropathic pain (see our previous psychophysical study) (Wu et al., 2013). We divided the patients into those who are likely to have some NP (in addition to their inflammatory pain) and those with solely inflammatory-NNP based on painDETECT scores. We included in the NP subgroup patients with scores of 13–18 because these patients could have some NP in contrast, to the patients in the NNP subgroup which clearly had only inflammatory pain and was relatively homogenous. Second, we note that none of the patients were prescribed medication specifically for NP. Third, given the small n in the NP subgroup, the abnormalities identified should be interpreted with care (Button et al., 2013) and future studies are needed to determine if the findings replicate across other pain conditions.
Finally, some factors inherent to studies of chronic pain should be considered. Depression was unlikely to have contributed to the increased alpha power in the NP patients because it did not differ between the NP and NNP subgroups. We also considered age effects given that the NP group was older than the NNP group. Age effects have been reported to impact PAF and spectral power (Giaquinto and Nolfe, 1986; Hashemi et al., 2016), and accelerated gray matter aging has been shown in chronic pain patients (Kuchinad et al., 2007; Moayedi et al., 2012). However, our finding that the NP patients also showed temporal abnormalities when compared to age/sex-matched NNP patients provide evidence that our findings were not solely resulting from an age effect. A possible limitation of the study is that we were not able to completely rule out the effect of sex due to the smaller number of women in the study (AS is twice as prevalent in men than in women (Braun and Sieper, 2007)). Nonetheless, 50% of the mixed inflammatory-NP patients were women likely because NP is more prevalent in women (Bouhassira, 2019). Furthermore, given known sex differences in pain mechanisms (Hashmi and Davis, 2014; Jausovec and Jausovec, 2010; Mogil, 2012; Wang et al., 2014), the abnormalities that we found might be different between the sexes. Also, the results of the correlation analysis should be taken with care as it was not corrected for multiple comparisons. Finally, MEG findings from deep subcortical areas should be considered with care as signal extraction from deep sources is less reliable with MEG, although we employed a source reconstruction method (beamforming) that increases the signal from the ROI and supresses it from the surrounding areas (Hillebrand and Barnes, 2005). Nonetheless, the thalamus results only represent a minor aspect of our findings and methods such as beamforming were shown to locate signals in deep structures (Attal et al., 2012; Attal and Schwartz, 2013; Bardouille and Ross, 2008; Cornwell et al., 2007; Cornwell et al., 2008; Cornwell et al., 2008; Dumas et al., 2013; Hamada et al., 2004; Quraan et al., 2011; Roux et al., 2013; Tewarie et al., 2013).
In conclusion, we propose that high theta and low gamma activity in the dynamic pain connectome may be general markers of chronic inflammatory pain but high alpha-band activity may only be present when neuropathic pain is likely.
Funding
This work was supported by the Canadian Institute of Health Research (PJT 162347, and SCA-145102 Strategy for Patient-Oriented Research (SPOR) funding of the Canadian Chronic Pain Network), and The Mayday Fund. J.A. Kim was supported by an MS Society of Canada endMS Doctoral Studentship Award, K.S. Hemington, A. Rogachov and J.C. Cheng were supported by CIHR Doctoral Research Award and R.L. Bosma was a recipient of a CIHR Post-doctoral Research Award.
CRediT authorship contribution statement
Lee B. Kisler: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Junseok A. Kim: Conceptualization, Methodology, Software, Investigation, Writing - review & editing. Kasey S. Hemington: Investigation, Writing - review & editing. Anton Rogachov: Investigation, Writing - review & editing. Joshua C. Cheng: Investigation, Writing - review & editing. Rachael L. Bosma: Investigation, Writing - review & editing. Natalie R. Osborne: Investigation, Writing - review & editing. Benjamin T. Dunkley: Conceptualization, Methodology, Writing - review & editing. Robert D. Inman: Resources, Writing - review & editing. Karen D. Davis: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
All authors declare no conflict of interest.
Acknowledgments
The authors thank the late Eugen Hlasny, Keith Ta and Brian Li for expert technical assistance in MRI acquisition, Drs Luis Garcia Dominguez and Richard Wennberg for technical assistance in MEG acquisition. They also thank Renise Ayearst for clinical data retrieval, Daeria Lawson and Ammepa Anton for patient recruitment.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102241.
Appendix. Supplementary materials
References
- Albu S., Meagher M.W. Expectation of nocebo hyperalgesia affects EEG alpha-activity. Int. J. Psychophysiol. 2016;109:147–152. doi: 10.1016/j.ijpsycho.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Attal Y., Maess B., Friederici A., David O. Head models and dynamic causal modeling of subcortical activity using magnetoencephalographic/electroencephalographic data. Rev. Neurosci. 2012;23(1):85–95. doi: 10.1515/rns.2011.056. [DOI] [PubMed] [Google Scholar]
- Attal Y., Schwartz D. Assessment of subcortical source localization using deep brain activity imaging model with minimum norm operators: a MEG study. PLoS ONE. 2013;8(3):e59856. doi: 10.1371/journal.pone.0059856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardouille T., Ross B. MEG imaging of sensorimotor areas using inter-trial coherence in vibrotactile steady-state responses. Neuroimage. 2008;42(1):323–331. doi: 10.1016/j.neuroimage.2008.04.176. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995;57(1):289–300. [Google Scholar]
- Berryman C., Stanton T.R., Bowering K.J., Tabor A., McFarlane A., Moseley G.L. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin. Psychol. Rev. 2014;34(7):563–579. doi: 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Boord P., Siddall P., Tran Y., Herbert D., Middleton J., Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46(2):118. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- Bosma R.L., Kim J.A., Cheng J.C. Dynamic pain connectome functional connectivity and oscillations reflect multiple sclerosis pain. Pain. 2018;159(11):2267–2276. doi: 10.1097/j.pain.0000000000001332. [DOI] [PubMed] [Google Scholar]
- Bouhassira D. Neuropathic pain: definition, assessment and epidemiology. Rev. Neurol. (Paris) 2019;175(1–2):16–25. doi: 10.1016/j.neurol.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Bouhassira D., Lantéri-Minet M., Attal N., Laurent B., Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Braun J., Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Oxford University Press; 2006. Rhythms of the Brain. [Google Scholar]
- Cheng J.C., Rogachov A., Hemington K.S. Multivariate machine learning distinguishes cross-network dynamic functional connectivity patterns in state and trait neuropathic pain. Pain. 2018;159(9):1764–1776. doi: 10.1097/j.pain.0000000000001264. [DOI] [PubMed] [Google Scholar]
- Cornwell B.R., Baas J.M., Johnson L. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37(1):282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Carver F.W., Coppola R., Johnson L., Alvarez R., Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res. 2008;1244:103–112. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Johnson L.L., Holroyd T., Carver F.W., Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 2008;28(23):5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.D., Cheng J.C. Differentiating trait pain from state pain: a window into brain mechanisms underlying how we experience and cope with pain. Pain Rep. 2019;4(4):e735. doi: 10.1097/PR9.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.D., Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 2013;8(3):518–534. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- De Vries M., Wilder-Smith O.H., Jongsma M.L. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J. Pain Res. 2013;6:815. doi: 10.2147/JPR.S50919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro F., Macey P.M., Rae C.D. The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain. Hum. Brain Mapp. 2018;39(5):1945–1956. doi: 10.1002/hbm.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh S.T., Nickel M.M., Tiemann L. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain. 2019 doi: 10.1097/j.pain.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg S.M., Roggeveen A.B., Kitajo K., Ward L.M. Large-scale gamma-band phase synchronization and selective attention. Cereb. Cortex. 2008;18(2):386–396. doi: 10.1093/cercor/bhm073. [DOI] [PubMed] [Google Scholar]
- Dumas T., Dubal S., Attal Y. MEG evidence for dynamic amygdala modulations by gaze and facial emotions. PLoS ONE. 2013;8(9):e74145. doi: 10.1371/journal.pone.0074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S., Lawrence N.S., Dunkley B.T. Almost winning: induced MEG theta power in insula and orbitofrontal cortex increases during gambling near-misses and is associated with Bold signal and gambling severity. Neuroimage. 2014;91:210–219. doi: 10.1016/j.neuroimage.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Engels M., Hillebrand A., van der Flier W.M., Stam C.J., Scheltens P., van Straaten E.C. Slowing of hippocampal activity correlates with cognitive decline in early onset Alzheimer's disease. An MEG study with virtual electrodes. Front. Hum. Neurosci. 2016;10:238. doi: 10.3389/fnhum.2016.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayaz A., Croft P., Langford R., Donaldson L., Jones G. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6) doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freynhagen R., Baron R., Gockel U., Tölle T.R. opinion. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- Furman A.J., Meeker T.J., Rietschel J.C. Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage. 2018;167:203–210. doi: 10.1016/j.neuroimage.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Garrett S., Jenkinson T., Kennedy L.G., Whitelock H., Gaisford P., Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing spondylitis disease activity index. J. Rheumatol. 1994;21(12):2286–2291. [PubMed] [Google Scholar]
- Giaquinto S., Nolfe G. The EEG in the normal elderly: a contribution to the interpretation of aging and dementia. Electroencephalogr. Clin. Neurophysiol. 1986;63(6):540–546. doi: 10.1016/0013-4694(86)90141-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Moreno A., Aurtenetxe S., Lopez-Garcia M.-.E., del Pozo F., Maestu F., Nevado A. Signal-to-noise ratio of the MEG signal after preprocessing. J. Neurosci. Methods. 2014;222:56–61. doi: 10.1016/j.jneumeth.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Gross J., Schnitzler A., Timmermann L., Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5(5):e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y., Sugino K., Kado H., Suzuki R. Magnetic fields in the human hippocampal area evoked by a somatosensory oddball task. Hippocampus. 2004;14(4):426–433. doi: 10.1002/hipo.10196. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Gross J., Klimesch W., Shapiro K.L. The role of alpha oscillations in temporal attention. Brain Res. Rev. 2011;67(1–2):331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Harifi G., Amine M., Ait Ouazar M. Prevalence of chronic pain with neuropathic characteristics in the Moroccan general population: a national survey. Pain Med. 2013;14(2):287–292. doi: 10.1111/pme.12009. [DOI] [PubMed] [Google Scholar]
- Hashemi A., Pino L.J., Moffat G. Characterizing population EEG dynamics throughout adulthood. eNeuro. 2016;3(6) doi: 10.1523/ENEURO.0275-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi J.A., Davis K.D. Deconstructing sex differences in pain sensitivity. Pain. 2014;155(1):10–13. doi: 10.1016/j.pain.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Hauck M., Lorenz J., Engel A.K. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J. Neurosci. 2007;27(35):9270–9277. doi: 10.1523/JNEUROSCI.2283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemington K.S., Wu Q., Kucyi A., Inman R.D., Davis K.D. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 2016;221(8):4203–4219. doi: 10.1007/s00429-015-1161-1. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R. Beamformer analysis of MEG data. Int. Rev. Neurobiol. 2005;68:149–171. doi: 10.1016/S0074-7742(05)68006-3. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Singh K.D., Holliday I.E., Furlong P.L., Barnes G.R. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 2005;25(2):199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jausovec N., Jausovec K. Resting brain activity: differences between genders. Neuropsychologia. 2010;48(13):3918–3925. doi: 10.1016/j.neuropsychologia.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Kaplan C.M., Schrepf A., Vatansever D. Functional and neurochemical disruptions of brain hub topology in chronic pain. Pain. 2019;160(4):973. doi: 10.1097/j.pain.0000000000001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Mawla I., Kong J. Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain. 2019;160(7):1594–1605. doi: 10.1097/j.pain.0000000000001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.A., Bosma R.L., Hemington K.S. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. Pain. 2019;160(1):187–197. doi: 10.1097/j.pain.0000000000001391. [DOI] [PubMed] [Google Scholar]
- Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain. J. Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Davis K.D. The dynamic pain connectome. Trends Neurosci. 2015;38(2):86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Kucyi A., Davis K.D. The neural code for pain: from single-cell electrophysiology to the dynamic pain connectome. Neuroscientist. 2017;23(4):397–414. doi: 10.1177/1073858416667716. [DOI] [PubMed] [Google Scholar]
- Kucyi A., Salomons T.V., Davis K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl Acad. Sci. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc B.W., Cross B., Smith K.A. Thalamic bursts down-regulate cortical theta and nociceptive behavior. Sci. Rep. 2017;7(1):2482. doi: 10.1038/s41598-017-02753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc B.W., Lii T.R., Silverman A.E., Alleyne R.T., Saab C.Y. Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. Pain. 2014;155(4):773–782. doi: 10.1016/j.pain.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Lim M., Kim J.S., Kim D.J., Chung C.K. Increased low-and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front. Hum. Neurosci. 2016;10:111. doi: 10.3389/fnhum.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.R., Ribary U., Jeanmonod D., Kronberg E., Mitra P.P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E.S., Nickel M.M., Ta Dinh S. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019;40(1):293–305. doi: 10.1002/hbm.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaud L., Wu H., Barthelemy Q. Alpha-phase synchrony EEG training for multi-resistant chronic low back pain patients: an open-label pilot study. Eur. Spine J. 2019 doi: 10.1007/s00586-019-06051-9. [DOI] [PubMed] [Google Scholar]
- Meneses F.M., Queiros F.C., Montoya P. Patients with rheumatoid arthritis and chronic pain display enhanced alpha power density at rest. Front. Hum. Neurosci. 2016;10:395. doi: 10.3389/fnhum.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M., Weissman-Fogel I., Salomons T.V. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Nickel M.M., May E.S., Tiemann L. Brain oscillations differentially encode noxious stimulus intensity and pain intensity. Neuroimage. 2017;148:141–147. doi: 10.1016/j.neuroimage.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell R.G., Dockree P.M., Robertson I.H., Bellgrove M.A., Foxe J.J., Kelly S.P. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J. Neurosci. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S., Palva J.M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan E.M.I., Inman R.D. Pain in spondyloarthritis: a neuro-immune interaction. Best Pract. Res. Clin. Rheumatol. 2017;31(6):830–845. doi: 10.1016/j.berh.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Pinheiro E.S., de Queiros F.C., Montoya P. Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F., Ossipov M.H., Gebhart G. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Quraan M.A., Moses S.N., Hung Y., Mills T., Taylor M.J. Detection and localization of hippocampal activity using beamformers with MEG: a detailed investigation using simulations and empirical data. Hum. Brain Mapp. 2011;32(5):812–827. doi: 10.1002/hbm.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogachov A., Cheng J.C., Erpelding N., Hemington K.S., Crawley A.P., Davis K.D. Regional brain signal variability: a novel indicator of pain sensitivity and coping. Pain. 2016;157(11):2483–2492. doi: 10.1097/j.pain.0000000000000665. [DOI] [PubMed] [Google Scholar]
- Roux F., Wibral M., Singer W., Aru J., Uhlhaas P.J. The phase of thalamic alpha activity modulates cortical gamma-band activity: evidence from resting-state MEG recordings. J. Neurosci. 2013;33(45):17827–17835. doi: 10.1523/JNEUROSCI.5778-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnthein J., Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage. 2008;39(4):1910–1917. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Sarnthein J., Morel A., Von Stein A., Jeanmonod D. Thalamic theta field potentials and EEG: high thalamocortical coherence in patients with neurogenic pain, epilepsy and movement disorders. Thalamus Relat. Syst. 2003;2(3):231–238. [Google Scholar]
- Sarnthein J., Stern J., Aufenberg C., Rousson V., Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2005;129(1):55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Naranjo J.R., Brenneisen C. Pain ratings, psychological functioning and quantitative EEG in a controlled study of chronic back pain patients. PLoS ONE. 2012;7(3):e31138. doi: 10.1371/journal.pone.0031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoth D.E., Nunes V.D., Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin. Psychol. Rev. 2012;32(1):13–25. doi: 10.1016/j.cpr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Schulz E., May E.S., Postorino M. Prefrontal gamma oscillations encode tonic pain in humans. Cereb. Cortex. 2015;25(11):4407–4414. doi: 10.1093/cercor/bhv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J., Jeanmonod D., Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006;31(2):721–731. doi: 10.1016/j.neuroimage.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Biology. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51(7):1759. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Tewarie P., Schoonheim M.M., Stam C.J. Cognitive and clinical dysfunction, altered Meg resting-state networks and thalamic atrophy in multiple sclerosis. PLoS ONE. 2013;8(7):e69318. doi: 10.1371/journal.pone.0069318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C., Lander J., Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10(5):918–929. doi: 10.1111/j.1526-4637.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- van den Broeke E.N., Wilder-Smith O.H., van Goor H., Vissers K.C., van Rijn C.M. Patients with persistent pain after breast cancer treatment show enhanced alpha activity in spontaneous EEG. Pain Med. 2013;14(12):1893–1899. doi: 10.1111/pme.12216. [DOI] [PubMed] [Google Scholar]
- Van Der Linden S., Valkenburg H.A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., Van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vanneste S., Song J.J., De Ridder D. Thalamocortical dysrhythmia detected by machine learning. Nat. Commun. 2018;9(1):1103. doi: 10.1038/s41467-018-02820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan J., Sinha S., Satishchandra P. Magnetoencephalography recording and analysis. Ann. Indian Acad. Neurol. 2014;17(Suppl 1):S113. doi: 10.4103/0972-2327.128678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic A., Altaleb M.K.H., Fraser M., McGeady C., Purcell M. EEG correlates of self-managed neurofeedback treatment of central neuropathic pain in chronic spinal cord injury. Front. Hum. Neurosci. 2019;13:762. doi: 10.3389/fnins.2019.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic A., Hasan M.A., Fraser M., Conway B.A., Nasseroleslami B., Allan D.B. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J. Pain. 2014;15(6):645–655. doi: 10.1016/j.jpain.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K., Dubois M., Llinas R. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010;150(1):41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Wang G., Erpelding N., Davis K.D. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain. 2014;155(4):755–763. doi: 10.1016/j.pain.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Automat. Contr. 1967;15(2):70–73. [Google Scholar]
- Wu Q., Inman R.D., Davis K.D. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum. 2013;65(6):1494–1503. doi: 10.1002/art.37920. [DOI] [PubMed] [Google Scholar]
- Wydenkeller S., Maurizio S., Dietz V., Halder P. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. Eur. J. Neurosci. 2009;30(1):91–99. doi: 10.1111/j.1460-9568.2009.06801.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.