Abstract
Background
Insomnia symptoms are the most common parent-reported sleep complaints in children; however, little is known about the pathophysiology of childhood insomnia symptoms, including their association with hypothalamic-pituitary-adrenal (HPA) axis activation. The objective of this study is to examine the association between parent-reported insomnia symptoms, objective short sleep duration and cortisol levels in a population-based sample of school-aged children.
Design
A sample of 327 children from the Penn State Child Cohort (5–12 years old) underwent 9-h overnight polysomnography and provided evening and morning saliva samples to assay for cortisol. Objective short sleep duration was defined based on the median total sleep time (i.e., < 7·7 h). Parent-reported insomnia symptoms of difficulty initiating and/or maintaining sleep were ascertained with the Pediatric Behavior Scale.
Results
Children with parent-reported insomnia symptoms and objective short sleep duration showed significantly increased evening (0·33 ± 0·03 μg/dL) and morning (1·38 ± 0·08 μg/dL) cortisol levels. In contrast, children with parent-reported insomnia symptoms and ‘normal’ sleep duration showed similar evening and morning cortisol levels (0·23 ± 0·03 μg/dL and 1·13 ± 0·08 μg/dL) compared with controls with ‘normal’ (0·28 ± 0·02 μg/dL and 1·10 ± 0·04 μg/dL) or short (0·28 ± 0·02 μg/dL and 1·13 ± 0·04 μg/dL) sleep duration.
Conclusions
Our findings suggest that insomnia symptoms with short sleep duration in children may be related to 24-h basal or responsive physiological hyperarousal. Future studies should explore the association of insomnia symptoms with short sleep duration with physical and mental health morbidity.
Keywords: Children, cortisol, insomnia symptoms, objective sleep duration
Introduction
Insomnia symptoms are the most common parent-reported sleep complaints in children, with approximately 20–30% of parents describing problems with sleep initiation or maintenance in their child [1-7]. Whereas many studies report objective sleep data on adults with insomnia complaints, few studies have objectively explored them in children [8]. Our understanding of childhood insomnia symptoms, therefore, is primarily based on clinical observations and subjective parent reports.
In adults, insomnia has been associated with activation of both limbs of the stress system. Several studies have shown increased activity of the hypothalamic-pituitary-adrenal (HPA) and sympathetic-and sympatho-adreno-medullary axes in adults with chronic insomnia [9-25]. For example, 24-h urinary free cortisol, norepinephrine and catecholamine metabolite levels have been shown to be positively correlated with objective measures of sleep disturbance in adult insomniacs [9]. Moreover, among adult insomniacs, those with objective short sleep duration secrete significantly more cortisol than those with ‘normal’ sleep duration [10,14,15,26]. In contrast, few studies have examined the association between sleep duration or ‘poor sleep’ with impaired activity of the stress system in school-aged children [27-29] and none have examined the synergistic effect between insomnia symptoms and objective sleep duration observed in adults. Thus, although the relation between insomnia and the stress system has been well-described in adults, the extent to which this association exists in children with insomnia symptoms has not been established.
The aim of this study was to examine the association between parent-reported insomnia symptoms, objective sleep duration and salivary cortisol levels in children. We hypothesized that children with parent-reported insomnia symptoms and short sleep duration will show increased activity of the HPA axis, while controls with short sleep duration and those with parent-reported insomnia symptoms and ‘normal’ sleep duration will not. This is the first study to explore the synergistic effect of insomnia symptoms and objective sleep duration on HPA axis activity in children from the general population.
Materials and methods
Participants
Subjects for this study were participants in the Penn State Child Cohort (PSCC), a random general population sample of children aged 5–12 years. Detailed descriptions of the study design and data collection have been previously reported [6,7,30,31]. In brief, the PSCC was designed as a 2-phase study with the first phase designed for collecting general information from the parents about their child’s sleep and behavioural patterns (N = 5740). In the second phase, we collected more detailed data in our General Clinical Research Centre on a randomly selected stratified sample from the first phase (N = 700). As shown in Fig. 1, a total of 391 children in the second phase had complete data on sleep-related questions, polysomnography, and salivary cortisol. For the purpose of the present study, we excluded 61 children with a history of severe chronic medical conditions, genetic syndromes and/or use of medications known to affect the HPA axis as well as three children whose evening cortisol levels were inexplicably high (> 8 SDs above the mean). The final sample included in the present study consisted of 327 children that, as shown in Fig. 1, did not differ significantly from the original phase 2 sample of 700 subjects in terms of gender and race distribution (53·7% females and 21% minority), age (110 ± 21 months), or BMI percentile (61 ± 31). These data provide further support for the external validity of the sample. This study was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine. Informed consent from parents of all participants and assent from all children were obtained prior to participation.
Figure 1.
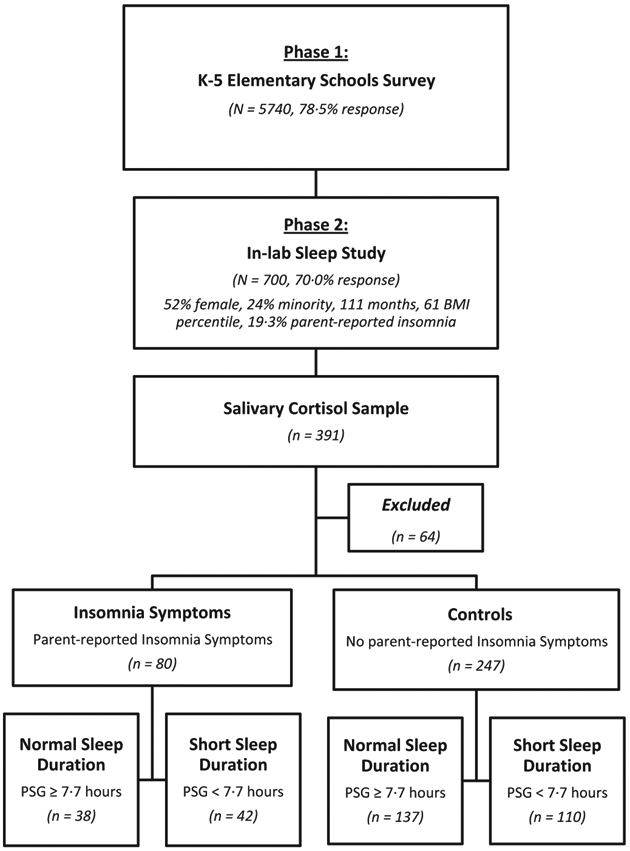
Penn State Child Cohort two-phase design and participants’ flow in the present study.
Saliva cortisol sampling technique and cortisol analysis
During the participants’ visit to the sleep laboratory in phase 2, an evening saliva sample (1800–1900) before dinner and a morning saliva sample (0600–0700) before breakfast were obtained for the assessment of cortisol. All samples were collected in salivary tubes and stored in a −20 °C freezer until assayed. Cortisol concentrations were assessed using commercially-available enzyme immunoassays (EIA; ALPCO Diagnostics, Salem, NH, USA).
Parent-reported insomnia symptoms
A parent completed the Pediatric Behavior Scale (PBS), a 165-item rating scale developed to evaluate behavioural problems in children, including sleep problems [32]. Each item is scored on a 0–3-point scale with 0 indicating no problems and 3 indicating that a behaviour is ‘very much’ or ‘very often’ a problem. Children were classified as having insomnia symptoms when the parent reported ‘often’ or ‘very often’ for either ‘has trouble falling asleep’ or ‘wakes up often in the night’. The prevalence of parent-reported insomnia symptoms in the sample included in the present study (19·5%) was similar to that previously reported in the 700 subjects and reported in Fig. 1 [6,7].
In-laboratory polysomnography
All children underwent a single overnight 9-h polysomnography (PSG) with a parent present in a sound-attenuated, light- and temperature-controlled room in the General Clinical Research Center at the Pennsylvania State University College of Medicine (Hershey, PA, USA). Each child’s bedtime and waketime approximated their typically sleep times (2100–2200 to 0600–0700). Children were monitored with an infrared video and computerized system (24-analogue channel and 10-dc channel TS amplifier using gamma software; Grass Telefactor, Inc., Warwick, RI, USA) including four channels of electroencephalogram (EEG), two-channel bilateral electrooculogram (EOG), a single-channel electrocardiogram (ECG), and chin and anterior tibialis electromyogram (EMG). Respiration was assessed throughout the night by use of a thermocouple at nose and mouth (model TCT R; Grass Telefactor, Inc.), nasal pressure (MP 45–871 ± 2 cm H2O; Validyne Engineering Corp., Northridge, CA, USA), and Piezo thoracic and abdominal respiratory effort electric belts (model 1312; Sleepmate Technologies, Glen Burnie, MD, USA). We obtained an objective estimate of snoring during the PSG by monitoring breathing sounds with a microphone attached to the throat (model 1250; Sleepmate Technologies), as well as a separate room microphone. All night haemoglobin oxygen saturation (SpO2) was obtained by pulse oximetry (model 8800; Nonin Medical, Inc., Plymouth, MN, USA). A hypopnoeic event was defined as a reduction of airflow of approximately 50% with an associated decrease in SpO2 of at least 3% or an associated breathing-related arousal. All PSG records were double-scored for breathing-related events in accordance with the American Thoracic Society standards for cardiopulmonary sleep studies in children [33] and an apnea/hypopnea index (AHI) was calculated ([apnea + hypopnea]/hours of sleep).
Results from the overnight PSG evaluation were used to classify the children’s total sleep time (TST) as short or ‘normal’ sleep duration. Short sleep duration was defined by a TST below the median of the overall sample (< 7·7 h), while ‘normal’ sleep duration was defined as a TST above the sample’s median (≥ 7·7–9·0 h).
Other key measures
As part of the initial questionnaire, parents also reported on children’s age and race. Furthermore, parents reported on children’s anxiety and depression in the PBS [32]. During the evening before the PSG, all children completed a physical examination, including height, weight and waist measurement. Height was measured in centimetres using a stadiometer (model 242, SECA Corp., Hanover, MD, USA) and weight was assessed in kilograms (model 758c, Cardinal Scale Manufacturing Co., Louisville, KY, USA). Age- and gender-adjusted body mass index (BMI) percentile was calculated based on CDC criteria. In the standing position, the waist circumference was measured in centimetres at the top of the iliac crest [34].
Statistical analyses
Means and proportions of the main variables were calculated for the entire study population, as well as stratified according to parent-reported insomnia status and objective sleep duration. We accounted for the sampling probability from Phase 1 to Phase 2 enrolments in all of the analyses to generate population level estimates and to make inference back to the population from which the Phase 2 study participants were selected [6,7,30,31]. anova was used to assess mean differences among groups on quantitative measures of descriptive data, while Chi-square tests were employed to assess gender and race differences. In order to control for experiment-wise error rate, a full factorial multivariate analysis of covariance (mancova) was used to examine the effects of parent-reported insomnia symptoms, objective sleep duration, and their interaction on morning and evening cortisol levels; we controlled for key potential confounders expected to affect cortisol levels as well as those variables significantly associated with parent-reported insomnia symptoms or objective sleep duration (i.e., gender, age, race, waist circumference, AHI and anxiety and depression). For posthoc analyses, all children were separated based on parent-reported insomnia symptoms status and objective sleep duration (short vs. ‘normal’), yielding four groups as depicted in Fig. 1: controls with ‘normal’ sleep duration (n = 137), controls with short sleep duration (n = 110), parent-reported insomnia symptoms with ‘normal’ sleep duration (n = 38), and parent-reported insomnia symptoms with short sleep duration (n = 42). To assess our a priori hypotheses that the joint effect of parent-reported insomnia symptoms and objective short sleep duration on cortisol levels is stronger than short sleep duration or parent-reported insomnia symptoms alone, least square difference planned tests, controlling for gender, age, race, waist circumference, AHI, and anxiety and depression, contrasted (i) insomnia symptoms with short duration versus. insomnia symptoms with ‘normal’ sleep duration, (ii) insomnia symptoms with short sleep duration versus. controls with short sleep duration, (iii) insomnia symptoms with ‘normal’ sleep duration versus. controls with ‘normal’ sleep duration, and (iv) controls with short sleep duration versus. controls with ‘normal’ sleep duration. The critical statistical confidence level for all analyses was P < 0·05 and two-tailed. All data analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Table 1 presents the characteristics of the overall sample as well as stratified by parent-reported insomnia symptoms and objective sleep duration. Parent-reported insomnia symptoms were significantly associated with female gender (P = 0·02) and higher anxiety and depression (P = 0·0001), while objective short sleep duration was associated with older age (P = 0·004), increased BMI percentile (P = 0·09) and waist circumference (P = 0·02), and, of course, shorter TST (P = 0·0001). No other comparisons between groups on any of the variables in Table 1 were marginally or fully statistically significant. Table 2 presents the characteristics of the study subgroups based on parent-reported insomnia symptoms and objective sleep duration.
Table 1.
Characteristics of the overall sample and stratified by parent-reported insomnia symptoms and objective sleep duration
| All | Controls | Insomnia symptoms | Normal sleep duration | Short sleep duration | |
|---|---|---|---|---|---|
| n | 327 | 247 | 80 | 175 | 152 |
| Males (%) | 46·3 | 49·0 | 34·0* | 47·0 | 45·0 |
| Age (months) | 110·0 ± 21·0 | 110·3 ± 20·0 | 107·9 ± 23·0 | 106·6 ± 21·4 | 113·0 ± 19·0*** |
| Minority (%) | 21·0 | 22·0 | 18·0 | 22·0 | 20·0 |
| BMI (%) | 61·0 ± 31·0 | 60·5 ± 30·1 | 60·7 ± 33·2 | 58·1 ± 29·8 | 63·8 ± 31·4† |
| Waist (cm) | 65·0 ± 10·0 | 64·5 ± 9·6 | 65·8 ± 11·9 | 63·5 ± 9·0 | 66·1 ± 11·2*** |
| TST (min) | 460·3 ± 46·6 | 460·7 ± 48·6 | 458·4 ± 37·1 | 492·8 ± 18·2 | 422·6 ± 40·6*** |
| AHI | 0·6 ± 0·9 | 0·6 ± 0·9 | 0·7 ± 1·2 | 0·6 ± 0·8 | 0·7 ± 1·1 |
| Anx/Dep (T) | 52·0 ± 12·9 | 50·3 ± 11·9 | 58·1 ± 14·7* | 52·6 ± 13·1 | 51·2 ± 13·0 |
Values are mean ± SD unless otherwise stated.
TST, total sleep time; AHI, apnea hypopnea index; Anx/Dep, anxiety and depression cluster T score.
P < 0·05 insomnia symptoms vs. controls.
P < 0·05 short vs. ‘normal’ sleep duration.
P < 0·10 short .vs. ‘normal’ sleep duration.
Table 2.
Characteristics of the subgroups based on parent-reported insomnia symptoms and objective sleep duration
| Controls with normal sleep duration |
Controls with short sleep duration |
Insomnia symptoms with normal sleep duration |
Insomnia symptoms with short sleep duration |
|
|---|---|---|---|---|
| n | 137 | 110 | 38 | 42 |
| Males (%) | 50·0 | 48·0 | 34·0* | 33·0* |
| Age (months) | 107·3 ± 20·8 | 113·8 ± 18·3** | 103·5 ± 24·1 | 111·9 ± 21·3** |
| Minority (%) | 23·0 | 21·0 | 20·0 | 16·0 |
| BMI (%) | 56·7 ± 29·7 | 65·1 ± 30·1*** | 63·3 ± 31·2 | 58·3 ± 35·2 |
| Waist (cm) | 63·3 ± 8·8 | 66·0 ± 10·4*** | 64·7 ± 9·9 | 66·9 ± 13·5† |
| TST (min) | 493·3 ± 18·3 | 419·8 ± 43·9 | 490·4 ± 18·7 | 426·6 ± 33·4 |
| AHI | 0·6 ± 0·9 | 0·6 ± 0·8 | 0·5 ± 0·6 | 1·1 ± 1·5‡ |
| Anx/Dep (T) | 50·8 ± 11·9 | 49·9 ± 11·9 | 58·5 ± 14·0* | 58·7 ± 15·1* |
Values are mean ± SD unless otherwise stated.
TST, total sleep time; AHI, apnea hypopnea index; Anx/Dep, anxiety and depression cluster T score.
P < 0·05 between insomnia symptoms with normal or short sleep duration and controls with normal or short sleep duration.
P < 0·05 between insomnia symptoms or controls with short sleep duration and insomnia symptoms or controls with normal sleep duration.
P < 0·05 between controls with normal sleep duration and controls with short sleep duration.
P < 0·05 between insomnia symptoms with short sleep duration and controls with normal sleep duration.
P < 0·05 between insomnia symptoms with short sleep duration and insomnia symptoms with normal sleep duration and controls.
Evening and morning cortisol levels by parent-reported insomnia symptoms and objective sleep duration adjusted for potential confounders are presented in Fig. 2. Factorial analyses showed nonsignificant and significant main effects of parent-reported insomnia symptoms on evening and morning cortisol levels (P = 0·97 and P = 0·04, respectively) as well as marginally significant and significant main effects of objective short sleep duration on evening and morning cortisol levels (P = 0·10 and P = 0·03, respectively). Importantly, these analyses showed marginally significant interaction effects between parent-reported insomnia symptoms and objective short sleep duration on evening and morning cortisol levels (P = 0·06 and P = 0·08, respectively). Specifically, children with parent-reported insomnia symptoms and short sleep duration had significantly higher evening cortisol levels (0·33 ± 0·03 μg/dL) compared with parent-reported insomnia symptoms with ‘normal’ sleep duration (0·23 ± 0·03 μg/dL, P = 0·048). Furthermore, children with parent-reported insomnia symptoms and short sleep duration had significantly higher morning cortisol levels (1·38 ± 0·08 μg/dL) than controls with short sleep duration (1·13 ± 0·04 μg/dL, P = 0·007), controls with ‘normal’ sleep duration (1·10 ± 0·04 μg/dL, P = 0·002), or parent-reported insomnia symptoms with ‘normal’ sleep duration (1·13 ± 0·08 μg/dL, P = 0·030). In contrast, evening and morning cortisol levels did not significantly differ between parent-reported insomnia symptoms and controls with ‘normal’ sleep duration (P = 0·200 and P = 0·781, respectively), nor did those between the two control subgroups (P = 0·825 and P = 0·614, respectively). Please see Table 3 for mean values for all study subgroups.
Figure 2.
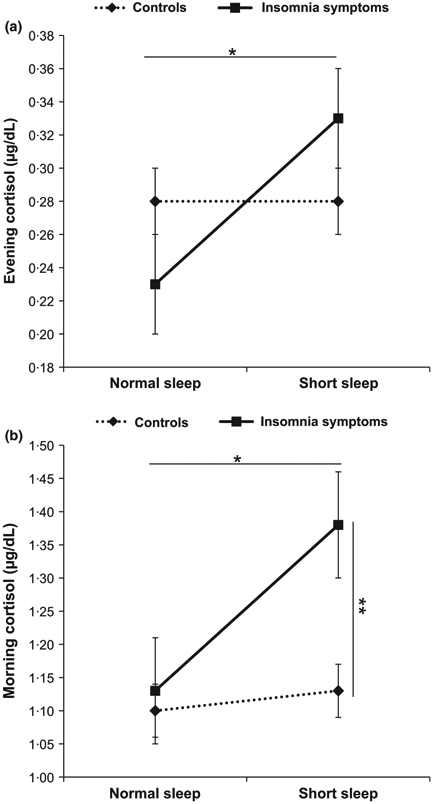
Salivary cortisol levels by insomnia symptoms and objective sleep duration. Error bars represent standard error of the mean (SEM). Data are adjusted for gender, age, race, waist circumference, AHI, anxiety and depression, and sampling weight. *P < 0·05 between insomnia symptoms with short sleep duration and insomnia symptoms with normal sleep duration (a, b); **P < 0·01 between insomnia symptoms with short sleep duration and controls with normal or short sleep duration (b).
Table 3.
Salivary cortisol levels across subgroups based on parent-reported insomnia symptoms and objective sleep duration
| Controls with normal sleep duration |
Controls with short sleep duration |
Insomnia symptoms with normal sleep duration |
Insomnia symptoms with short sleep duration |
|
|---|---|---|---|---|
| n | 137 | 110 | 38 | 42 |
| Evening, μg/dL | 0·28 ± 0·02 | 0·28 ± 0·02 | 0·23 ± 0·03 | 0·33 ± 0·03* |
| Morning, μg/dL | 1·10 ± 0·04 | 1·13 ± 0·04 | 1·13 ± 0·08 | 1·38 ± 0·08*, ** |
Values are mean ± standard error of the mean (SEM) adjusted for gender, age, race, waist circumference.
AHI, anxiety and depression, and sampling weight.
P < 0·05 between insomnia symptoms with short sleep duration and insomnia symptoms with normal sleep duration.
P < 0·01 between insomnia symptoms with short sleep duration and controls with normal or short sleep duration.
Discussion
This is the first study to assess the synergistic association between insomnia symptoms and objective short sleep duration on HPA axis activity in a population-based sample of young children. Our findings suggest that parent-reported insomnia symptoms with objective short sleep duration are associated with higher cortisol levels in the evening and morning, whereas insomnia symptoms with ‘normal’ sleep duration is not associated with significant activation of the HPA axis. Our novel findings on the synergistic effect of insomnia symptoms and objectively-measured sleep duration on cortisol levels, independent of sociodemographics, body weight, sleep-disordered breathing, anxiety and depression, may offer new insights into the pathophysiology of childhood insomnia symptoms.
In children, insomnia symptoms are typically conceptualized as behavioural problems of initiating or maintaining sleep resulting primarily from bedtime resistance and learned sleep-onset associations [8]. Our results add to the growing body of literature showing an association between disturbed sleep and activity of the HPA axis in school-aged children [27-29] and toddlers [35-38] and parallel those of previous findings in adults which characterize insomnia as a disorder of physiological hyperarousal [39,40]. Adult insomniacs with objective short sleep duration show 24-h elevations in cortisol levels as well as overall sympathetic activation, including enhanced catecholamine secretion, increased 24-h metabolic and heart rate and impaired heart rate variability [9-25], while those with ‘normal’ sleep duration do not [9,10,14,15,24,26,41-45]. In our study, elevations in both evening and morning cortisol levels suggest that insomnia symptoms with objective short sleep duration in children may be also a result of 24-h hyperarousal. Our study design, which included only one night in the laboratory without adaptation to the novel environment, cannot rule out the possibility that the elevated cortisol levels may reflect an increased response of the stress system to a stressful-unfamiliar environment [46]. In any event, it appears that children with insomnia symptoms and short sleep duration experience HPA axis alterations either in the form of basal hyperactivation or exaggerated response to novel, stressful stimuli. Future studies using multiple measures across different environments should examine these hypotheses.
In children and adolescents, several studies have shown an association between objective sleep and cortisol with behavioural or mental health problems [35-38,47] but not with physical health morbidity. In adults, recent studies have reported that insomnia with objective short sleep duration, in addition to its association with cortisol and mental health, is associated with increased risk of cardiometabolic morbidity – including hypertension and type 2 diabetes [48-50] – as well as neurocognitive impairment [51], depression [52,53], and increased mortality risk [54]. Future studies using objective and subjective measures of sleep should examine these associations in children.
Importantly, our findings should not lead to the conclusion that childhood insomnia is analogous to adult insomnia from a behavioural or pathophysiological stand point. For example, PSG differences in adult insomniacs compared with controls are significant and consistent across studies, whereas these differences in children are very subtle or not significant (e.g., TST in the present study), which may reflect stronger homeostatic sleep mechanisms in children than in adults. In adults, subjectively-defined insomnia has been associated with normal [9,10,14-16] or decreased morning awakening cortisol levels [55]. However, adult insomniacs with objective short sleep duration have increased plasma cortisol levels in the evening, during the sleep period, and upon awakening, that is, 6:30–8:00 am [10]. In the present and previous studies in children, objective sleep disturbances were associated with both increased evening and morning cortisol levels [27,28,35-37]. Together, these data suggest the possibility that childhood insomnia symptoms associated with HPA axis alterations (i.e., short sleep duration) may be in a continuum with adult insomnia, while childhood insomnia symptoms without HPA axis alterations (i.e., normal sleep duration) may include different behavioural phenotypes (e.g., bedtime resistance, sleep-onset associations) that are not in a continuum with adult insomnia [52]. Moreover, the question remains open whether pubertal maturity may play a role in the association of insomnia symptoms with activation of the HPA axis and its progression into adulthood. More data from other domains, for example, behavioural profiles, medical morbidity or treatment response, will help us clarify the nature of childhood insomnia symptoms and their natural course.
Some limitations should be taken into account when interpreting our results. First, the study is cross-sectional and does not allow causal conclusions; the possibility exists that HPA axis activation leads to insomnia and vice versa. Second, the objective sleep duration in this study was based on one night of fixed-time PSG, which may not be representative of the subjects’ ad libitum habitual sleep duration and may be affected by rebound and/or first-night effects. Nevertheless, our definitions of short (i.e., < 7·7 h) and ‘normal’ (i.e., 7·8–9·0 h) sleep duration are consistent with those of a previous study using week-long, at-home actigraphy, which may better represent children’s habitual sleep pattern of school-aged children [28]. Our cut-off for short sleep duration was based on a statistical approach (i.e., median sleep duration of the sample); future studies should explore the optimum cut-off of sleep duration based on clinical criteria. Third, we relied on parent-reported insomnia symptoms rather than self-reported data. Although self-reported sleep data may better reflect the child’s experience of insomnia symptoms, the use of parent-reports was deemed necessary given the young age of most children in this sample, which allowed comparison across children of all ages. Fourth, we did not assess daytime impairments or chronicity associated with insomnia symptoms, which would have delineated a more homogeneous phenotype of children (i.e., those with chronic insomnia). Future studies should examine the association of insomnia disorder, as defined by current diagnostic criteria, and HPA axis in children. Fifth, we only measured salivary cortisol levels at two time points. Future studies should examine the association of insomnia symptoms, objective sleep duration and activity of the HPA axis using 24-h blood or multiple time-points salivary sampling. Finally, the interactions between insomnia symptoms and objective sleep duration on cortisol levels were marginally significant, which may indicate lack of statistical power. However, given the data distribution across groups and the strength of the posthoc comparisons (Fig. 2), we expect future studies with a larger number of children with insomnia symptoms to replicate these observed interactions with sufficient statistical power.
In conclusion, young children with insomnia symptoms and objective short sleep duration have high evening and morning cortisol levels, while children with insomnia symptoms and ‘normal’ sleep duration do not show significant HPA axis activation. These findings were independent of sociodemographic factors, body weight, sleep-disordered breathing, anxiety and depression. These findings suggest that the pathophysiology of sleep disturbances may be related to basal or responsive hyperactivity of the stress system in a subgroup of children with insomnia symptoms. Future studies should examine the behavioural profiles, medical morbidity and subtypes of children with insomnia symptoms based on objective sleep measures.
Acknowledgements
The authors wish to thank the sleep technicians and staff of the General Clinical Research Center at the Pennsylvania State University College of Medicine for their dedication to this project, as well as the children and their families for their cooperation.
Footnotes
Conflict of interests
The authors declare no competing financial conflict of interests.
References
- 1.Smedje H, Broman JE, Hetta J. Parents’ reports of disturbed sleep in 5–7-year-old Swedish children. Acta Paediatr 1999;88:858–65. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Sun Z, Uchiyama M, Shibui K, Kim K, Okawa M. Prevalence and correlates of sleep problems in Chinese schoolchildren. Sleep 2000;23:1053–62. [PubMed] [Google Scholar]
- 3.Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics 2001;107:E60. [DOI] [PubMed] [Google Scholar]
- 4.Spruyt K, O’Brien LM, Cluydts R, Verleye GB, Ferri R. Odds, prevalence and predictors of sleep problems in school-age normal children. J Sleep Res 2005;14:163–76. [DOI] [PubMed] [Google Scholar]
- 5.Fricke-Oerkermann L, Pluck J, Schredl M, Heinz K, Mitschke A, Wiater A et al. Prevalence and course of sleep problems in childhood. Sleep 2007;30:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singareddy R, Moole S, Calhoun S, Vocalan P, Tsaoussoglou M, Vgontzas AN et al. Medical complaints are more common in young school-aged children with parent reported insomnia symptoms. J Clin Sleep Med 2009;5:549–53. [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med 2014;15:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual, 2nd Edn. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 9.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res 1998;45:21–31. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 2001;86:3787–94. [DOI] [PubMed] [Google Scholar]
- 11.Monroe LJ. Psychological and physiological differences between good and poor sleepers. J Abnorm Psychol 1967;72:255–64. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW, Gay TJA, Masterton JP, Bruce DW. Relationship between habits, adrenocortical activity and personality. Psychosom Med 1971;33:499–508. [DOI] [PubMed] [Google Scholar]
- 13.Adam K, Tomeny M, Oswald I. Physiological and psychological differences between good and poor sleepers. J Psychiatr Res 1986;20:301–16. [DOI] [PubMed] [Google Scholar]
- 14.Rodenbeck A, Huether G, Rüther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett 2002;324:159–63. [DOI] [PubMed] [Google Scholar]
- 15.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med 2002;64:793–802. [DOI] [PubMed] [Google Scholar]
- 16.Rodenbeck A, Cohrs S, Jordan W, Huether G, Ruther E, Hajak G. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. A placebo-controlled, double-blind, randomized, cross-over study followed by an open treatment over 3 weeks. Psychopharmacology 2003;170:423–8. [DOI] [PubMed] [Google Scholar]
- 17.Varkevisser M, Van Dongen HP, Kerkhof GA. Physiologic indexes in chronic insomnia during a constant routine: evidence for general hyperarousal? Sleep 2005;28:1588–96. [PubMed] [Google Scholar]
- 18.Bonnet MH. Hyperarousal as the basis for insomnia: effect size and significance. Sleep 2005;28:1500–1. [DOI] [PubMed] [Google Scholar]
- 19.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun 2003;17:365–72. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep 1995;18:581–8. [DOI] [PubMed] [Google Scholar]
- 21.Nofzinger EA, Nissen C, Germain A, Moul D, Hall M, Price JC et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med 2006;2:316–22. [PubMed] [Google Scholar]
- 22.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med 1994;10:261–6. [Google Scholar]
- 23.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med 1998;60:610–5. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Volderholzer U et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res 2010;20:137–45. [DOI] [PubMed] [Google Scholar]
- 25.Lichstein K, Johnson RS. Pupillometric discrimination of insomniacs. Behav Res Ther 1994;32:123–9. [DOI] [PubMed] [Google Scholar]
- 26.Riemann D, Klein T, Rodenbeck A, Feige B, Horny A, Hummel R et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res 2002;113:17–27. [DOI] [PubMed] [Google Scholar]
- 27.El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol 2008;27:26–33. [DOI] [PubMed] [Google Scholar]
- 28.Räikkönen K, Matthews KA, Pesonen AK, Pyhälä R, Paavonen EJ, Feldt K et al. Poor sleep and altered hypothalamic-pituitary-adrenocorticol and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab 2010;95:2254–61. [DOI] [PubMed] [Google Scholar]
- 29.Pesonen AK, Kajantie E, Heinonen K, Pyälä R, Lahti J, Jones A et al. Sex-specific associations between sleep problems and hypothalamic-pituitary-adrenocortical axis activity in children. Psychoneuroendocrinology 2012;37:238–48. [DOI] [PubMed] [Google Scholar]
- 30.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Fedok F et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension 2008;52:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep 2009;32:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindgren SD, Koeppl GK. Assessing child behavior problems in a medical setting: development of the Pediatric Behavior Scale In: Prinz RJ, editor. Advances in Behavioral Assessment of Children and Families. Greenwich: JAI; 1987: pp 57–90. [Google Scholar]
- 33.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–78. [DOI] [PubMed] [Google Scholar]
- 34.National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel. The practical guide: Identification, evaluation and treatment of overweight and obesity in adults. 2000; NIH publication 00-4084. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed on 1 November 2013. [Google Scholar]
- 35.Hatzinger M, Brand S, Perren S, Stadelmann S, von Wyl A, von Klitzing K et al. Electroencephalographic sleep profiles and hypothalamic-pituitary-adrenocortical (HPA)-activity in kindergarten children: early indication of poor sleep quality associated with increased cortisol secretion. J Psychiatr Res 2008;42:532–43. [DOI] [PubMed] [Google Scholar]
- 36.Hatzinger M, Brand S, Perren S, Stadelmann S, von Wyl A, von Klitzing K et al. Sleep actigraphy pattern and behavioral/emotional difficulties in kindergarten children: association with hypothalamic-pituitary-adrenal (HPA) activity. J Psychiatric Res 2009;44:253–61. [DOI] [PubMed] [Google Scholar]
- 37.Scher A, Hall WA, Zaidman-Zait A, Weinberg J. Sleep quality, cortisol levels, and behavioral regulation in toddlers. Dev Psychobiol 2010;52:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gribbin CE, Watamura SE, Cairns A, Harsh JR, Lebourgeois MK. The cortisol awakening response (CAR) in 2- to 4-year-old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol 2012;54:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev 2010;14:9–15. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel BL, Buchbinder R, Coursey R, Snyder F. Sleep patterns and psychological test characteristics of chronic primary insomniacs. Sleep Res 1973;2:149. [Google Scholar]
- 42.Freedman RR, Sattler HL. Physiological and psychological factors in sleep onset insomnia. J Abnorm Psychol 1982;91:380–9. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet MH, Arand DL. Physiological activation in patients with sleep state misperception. Psychosom Med 1997;59:533–40. [DOI] [PubMed] [Google Scholar]
- 44.Lichstein KL, Johnson RS, Sen Gupta S, O’Laughlin DL, Dykstra TA. Are insomniacs sleepy during the day? A pupillometric assessment Behav Res Ther 1992;30:283–92. [DOI] [PubMed] [Google Scholar]
- 45.Lichstein KL, Wilson NM, Noe SL, Aguillard RN, Bellur SN. Daytime sleepiness in insomnia: behavioral, biological and subjective indices. Sleep 1994;17:693–702. [DOI] [PubMed] [Google Scholar]
- 46.Drake CL, Roth T. Predisposition in the evolution of insomnia: evidence, potential mechanisms and future direction. Sleep Med Clin 2006;1:333–49. [Google Scholar]
- 47.Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry 2006;59:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension 2012;60:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009;32:1980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep 2010;33:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Mendoza J, Calhoun S, Bixler EO, Karataraki M, Liao D, Vela-Bueno A et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med 2011;73:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Troxel WM, Kupfer DJ, Reynolds CF 3rd, Frank E, Thase ME, Miewald JM et al. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry 2012;73:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep 2010;33:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 2004;29:1184–91. [DOI] [PubMed] [Google Scholar]


