Abstract
Based on previous studies reporting on the association of objective sleep duration and physiologic changes (i.e., increased cortisol) in children, we examined the role of objective sleep duration on differentiating behavioral profiles in children with insomnia symptoms. Seven hundred children (ages 5–12, 47.8% male) from the Penn State Child Cohort underwent a nine-hour polysomnography and parent completed Pediatric Behavior Scale. Insomnia symptoms were defined as parent report of difficulty falling and/or staying asleep, sleep disordered breathing as an AHI of ≥1, and objective short sleep duration as a total sleep time < 7.7 h. Children with insomnia symptoms demonstrated more overall behavioral problems than controls. Significant interactions between insomnia symptoms and objective sleep duration on scores of externalizing behaviors, mood variability and school problems were found. Profile analyses showed that children with insomnia symptoms and normal sleep duration were associated with clinically elevated externalizing behaviors, inattention, mood variability, and school problems, while children with insomnia and short sleep duration were associated with an overall elevated profile in which internalizing behaviors were more prominent. Childhood insomnia symptoms are associated with a wide array of behavioral problems, for which objective sleep duration is useful in differentiating behavioral profiles. Children with insomnia symptoms and normal sleep duration had a behavioral profile consistent with limit-setting and rule-breaking behaviors, while children with insomnia symptoms and short sleep duration had a behavioral profile more consistent with internalizing behaviors resembling that of psychophysiological disorders.
Keywords: Childhood insomnia, Behavior problems, Anxiety and depression, Objective sleep duration
There is increasing recognition that sleep disturbances are important factors in child development. When children do not get enough sleep, aspects of their physical, emotional, cognitive and social development are negatively affected and can impair both the parent’s and child’s daytime functioning. Insomnia is the most common parent-reported sleep complaint in children, with approximately 15-30% of children having problems with difficulty falling asleep and/or difficulty staying asleep (Calhoun et al. 2014; Camhi et al. 2000; Singareddy et al. 2009; Sprugt et al. 2005). These high prevalence rates are particularly alarming given that a great deal of evidence suggests that untreated sleep problems may persist across a range of developmental stages (Roberts et al. 2008). From a clinical perspective, these sleep difficulties are commonly conceptualized as behavioral problems of initiating or maintaining sleep resulting primarily from bedtime resistance and learned sleep-onset associations (American Academy of Sleep Medicine 2005). Although there is substantial published literature examining behavioral and emotional problems in adolescents (Liu and Zhou 2002; Roberts et al. 2008) and adults with insomnia (Fernandez-Mendoza et al. 2009; Fernandez-Mendoza et al. 2010), and in children with sleep disordered breathing (SDB) (Lewin et al. 2002; O’Brien et al. 2004; Owens et al. 2008; Zhao et al. 2008);only two epidemiological studies have reported on the association of sleeping difficulties and emotional problems and externalizing behaviors in children (Paavonen et al. 2000; Smedje et al. 2000). Therefore, the significance and potential associations of childhood insomnia symptoms per se with behavioral problems are very limited, particularly in young children from the general population.
Smedje et al. (2000) reported an association between parent-reported disturbed sleep, and a broad index of emotional symptoms in 635 children ages 6 to 8 from the general population. They reported specific associations between particular sleep related disturbances and particular dimensions of behavior (e.g., conduct problems were associated with bedtime resistance, and emotional symptoms were associated with night terrors and difficulty falling asleep). Another study in younger children from a community sample (Paavonen et al. 2000) found an association between parent-reported difficulty falling and staying asleep and a total problem behavior score on a parent completed rating scale. Others have suggested that subjectively reported insomnia symptoms may be more closely associated with anxiety in childhood and depression in adolescence (Alfano et al. 2009). Also, a recent clinical study suggested that insomnia symptoms are more frequent in children age 7–11 with attention deficit hyperactivity disorder (ADHD) than in controls (Wiebe et al. 2013).
Furthermore, emerging evidence suggests that sleep problems in children predict the onset of externalizing problems as well as internalizing problems such as depression and anxiety (Gregory and O’Connor 2002; Gregory et al. 2004). For example, sleep problems in very young children ages 3–4 have been shown to predict anxiety, conduct disorder, and hyperactivity at age 7 (Gregory et al. 2004). Also, sleep problems in childhood appear to be a risk factor for anxiety and depression in adulthood (Gregory et al. 2005). In contrast, some studies have shown a possible connection from anxiety to sleep problems and subsequent depression (Johnson et al. 2006).
Many studies have reported objective sleep data on adults with insomnia, compared to only two studies that have objectively explored them in children from the general population (Calhoun et al. 2014; Singareddy et al. 2009). Therefore, our understanding of childhood insomnia has primarily been based on clinical observations and subjective parent reports. In adults, objective sleep measures are a useful marker in distinguishing two insomnia subtypes;one associated with short sleep duration, increased hypothalamic-pituitary-adrenal axis and central and sympathetic activity and medical morbidity (e.g., hypertension, cognitive impairment), and the other associated with normal sleep duration, lack of hypothalamic-pituitary-adrenal axis and sympathetic activation or medical morbidity (Fernandez-Mendoza et al. 2010; Fernandez-Mendoza et al. 2012; Vgontzas et al. 2001; Vgontzas et al. 2009; Vgontzas et al. 2013). A recent general population study of young children showed that insomnia with short sleep duration is related to hypothalamic-pituitary-adrenal axis activation when compared to children with insomnia with “normal” sleep duration or controls (Fernandez-Mendoza et al. 2014). This study hypothesized that objective sleep duration may be a useful marker in ascertaining different behavioral profiles (e.g., oppositional behaviors when going to bed), which would explain why parents report insomnia symptoms in some children despite their objective normal sleep duration. In other words, insomnia symptoms with short sleep duration may be associated with emotional/physiological hyperarousal and, therefore, primarily internalizing behaviors, while insomnia symptoms with “normal” sleep duration may be associated with externalizing/oppositional behaviors in young children.
Thus, the aims of this study were to examine 1) the association of parent-reported insomnia symptoms with internalizing and externalizing behavior problems, 2) the role of objective sleep duration on the association of parent-reported insomnia symptoms with internalizing and externalizing behavioral ratings, and 3) the behavioral profiles of children with insomnia symptoms based on objective sleep duration in a general population sample of young children.
Materials and Methods
Participants
This study was designed in two-phases, with the first phase designed for collecting general information from the parents about their child’s sleep and behavioral patterns. In the first phase, elementary schools (kindergarten through grade 5) with approximately 1500 students were selected over the course of five years. We sent home with every child in the selected schools a questionnaire with consent forms to be completed by the parent. The questionnaire was based on the validated survey published by Ali et al. (1993) and assessed breathing disorders in children. We added additional questions regarding height, weight, age, gender, race and ethnicity. We assessed 18 public elementary schools within three school districts of Dauphin County, Pennsylvania. Overall, we sent home 7312 questionnaires; 5740 were returned for a 78.5% response rate.
The second phase involved collection of more detailed data in our General Clinical Research Center (GCRC) targeting a randomly selected subset of 1000 children from the first phase. The second phase of this study was initiated each year by randomly selecting 200 children based on a stratification of grade, sex and risk for sleep disordered breathing from the current year’s returned questionnaires. The average time between Phase I and II assessment was seven months. The distribution of gender and age were similar across assessment years. In total, we studied 704 children in this phase. Four children did not complete the PSG recording; thus, 700 children were included in this study for a final response rate of 70%. We contrasted the 700 subjects who completed the PSG recordings with those who were selected and did not complete phase II (n = 300). There were no significant differences between the two groups. We established compensatory weights to obtain estimates of the original target population as well as to adjust for the age and gender distribution of the nation as a whole. More details regarding the sampling methods used in the Penn State Child Cohort are reported in Bixler et al. (2009). This study was approved by the Institutional Review Board of Penn State College of Medicine. Informed consent was obtained from parents of all participants and assent was obtained from all children prior to participation.
Sleep Laboratory Evaluation
During their visit in the laboratory, all subjects underwent a series of subjective and objective measurements including parent completed questionnaires and rating scales (e.g., behavior, sleep and child development), and measurement of height and weight were recorded for each child. Body mass index was calculated and expressed as body mass index percentiles adjusted for age and gender, using the formula and data of the NHANES CDC growth charts (Kuczmarski et al. 2000). Parents reported on the race and ethnicity of each child. ADHD diagnosis was based on parent-report of physician diagnosis of ADHD and/or treatment with a stimulant collected during the medical history and physical examination.
All children underwent a nine-hour PSG with a parent present in a sound-attenuated, light and temperature controlled room in our General Clinical Research Center. Children’s bedtime and wakeup time approximated their typical sleep times (average 21:00–22:00 to 06:00–07:00). Each child was monitored with an infrared video and a computerized system (24 analog channel and 10 dc channel TS amplifier using Gamma software, Grass Telefactor, Inc.) including four channels of electroencephalogram two channel bilateral electroculogram, and chin and anterior tibial electromyogram. Respiration was assessed throughout the night by use of a thermocouple at nose and mouth (model TCT R, Grass Telefactor, Inc), nasal pressure (MP 45–871 ± 2 cm H2O, Validyne Engineering Corp), and Piezo thoracic and abdominal respiratory effort electric belts (model 1312, Sleepmate). We obtained an objective estimate of snoring during the PSG by monitoring breathing sounds with a microphone attached to the throat (model 1250, Sleepmate Technologies) as well as a separate room microphone. All night hemoglobin oxygen saturation was obtained by pulse oximeter (model 8800, Nonin Medical) attached to the finger. A single channel electrocardiogram was also recorded. All of the polysomnography records were double-scored in accordance with a modified version of the American Thoracic Society standards for cardiopulmonary sleep studies in children (American Academy of Pediatrics 2002; American Thoracic Society 1996). These modifications included the addition of breathing-related arousals and elimination of central apneas in the calculation of the apnea/hyponea index. Obstructive apnea was defined as a cessation of airflow with a minimum duration of five seconds and an out of phase strain gauge movement. A hypopneic event was defined as a reduction of airflow of approximately 50% with an associated decrease in oxygen saturation (SpO2) of at least 3% or an associated breathing related arousal. Based on these data, the AHI was calculated as [(apnea + hypopnea)/hours of sleep]. Results from the overnight polysomnography evaluation were used to classify the children as having or not having sleep disordered breathing, defined as an apnea/hyponea index ≥1. Results from the overnight PSG evaluation were also used to classify the children’s total sleep time as short or normal sleep duration. Short sleep duration was defined by a total sleep time below the median of the overall sample (< 7.7 h), while normal sleep duration was defined as a total sleep time above the sample’s median (7.7–9.0 h). This definition of objective short sleep duration is commensurate with that used in our previous study (Fernandez-Mendoza et al. 2014) and other studies using actigraphy data, a more typical representation of habitual sleep duration (Pesonen et al. 2010; Raikkonen et al. 2010).
Pediatric Behavior Scale
A parent-completed the Pediatric Behavior Scale (Lindgren and Koeppl 1987) a 165-item rating scale which has normreferenced t scores for subscales including Opposition (e.g., defies authority, disobedient), Aggression (e.g., starts fights, destructive), Explosiveness (e.g., loses temper, irritable), Inattention (e.g., does not listen to instructions, easily distracted), Impulsivity (e.g., wants things right away, acts without stopping to think), Hyperactivity (e.g., cannot sit still, fidgets), Anxiety/Depression (e.g., worried, anxious, sad, low self-esteem), Inappropriate Social Behavior (e.g., poor social judgment, emotional immaturity), Mood Variability (e.g., inconsistent behavior, changes in personality), and School Problems (e.g., difficulty learning, low grades). A parent was asked to rate these items over the past 2 months, on a 4-point Likert scale from 0 to 3, with 0 (Almost never or not at all), 1 (Sometimes or just a little), 2 (Often or pretty much), and 3 (Very often or very much). A clinically meaningful cut-off for the Pediatric Behavior Scale subscales of a t score ≥ 65 was used. None of the subscales mentioned above included any sleep-related items. In the present and previous studies (Calhoun et al. 2014; Fernandez-Mendoza et al. 2014; Singareddy et al. 2009), children were classified as having insomnia symptoms within the past two months when the parent reported 2 (“Often or pretty much”) or 3 (“Very often or very much”) for either “Has trouble falling asleep” or “Wakes up often in the night” items of the Pediatric Behavior Scale sleep subscale. The Pediatric Behavior Scale has been used in several studies to assess sleep problems in children with autism and ADHD and the general population (Mayes et al. 2008, 2009a, b; Mayes and Calhoun 2009).
Statistical Analyses
The sample was divided into two groups based on presence or absence of parent-reported insomnia symptoms. Comparisons of the distribution of demographics according to group membership were made with independent t or χ2 tests. A two-way multivariate analysis of covariance (MANCOVA) was used to examine the association of insomnia symptoms (no versus yes), objective sleep duration (short vs. normal), and their interaction on Pediatric Behavior Scale scaled scores while controlling for age, race, gender, body mass index%, ADHD, and sleep disordered breathing. For post-hoc analyses, all children were separated based on parent-reported insomnia symptoms status and objective sleep duration (short vs. normal), yielding four groups: controls with normal sleep duration (n = 254), controls with short sleep duration (n = 311), parent-reported insomnia symptoms with normal sleep duration (n = 59), and parent-reported insomnia symptoms with short sleep duration (n = 76). In order to assess our a priori hypotheses that the joint effect of parent-reported insomnia symptoms and objective short sleep duration on behavior, mood and learning ratings is stronger than short sleep duration or parent-reported insomnia symptoms alone, least square difference planned tests, contrasted 1) insomnia symptoms with short duration vs. insomnia symptoms with normal sleep duration, 2) insomnia symptoms with short sleep duration vs. controls with short sleep duration, 3) insomnia symptoms with normal sleep duration vs. controls with normal sleep duration, and 4) controls with short sleep duration vs. controls with normal sleep duration. Third, in order to study clinically meaningful behavioral profiles we examined the proportion of each Pediatric Behavior subscale that was rated above the clinical cutoff (t score ≥ 65) across controls and insomnia symptoms subgroups based on objective sleep duration. Parametric values are expressed as mean ± standard deviation. Effect sizes (d) were calculated for standardized mean differences or binary proportions using standard methods (i.e., Cohen’s and Logit, respectively). The critical statistical confidence level for all analyses was p < 0.05 and two-tailed. Analyses were performed using PASW software, version 20.
Results
Descriptive characteristics by parent-reported insomnia symptoms for age, gender, ADHD, body mass index percentile, sleep disordered breathing, race, and apnea/hypopnea index are presented in Table 1. The percent of white, black, Asian, and other race was not significantly different (p = .73) between controls (79.7%, 11.3%, 2.7%, and 6.3%, respectively) and insomniacs (80.5%, 10.7%, 1.8%, and 7.0%, respectively). Also, the percent of Hispanic and non-Hispanic ethnicity was not significantly different (p = .40) between controls (5.8% and 94.2%, respectively) and insomniacs (7.6% and 92.4%, respectively). Given the lack of significant differences in terms of race and ethnicity, we collapsed them into Caucasian and Minority (Table 1). No significant differences were found on any of these demographic variables between controls and children with insomnia symptoms with the exception of ADHD, which was more frequent in children with insomnia symptoms.
Table 1.
Descriptive characteristics of population sample
| Controls | Insomnia | p-value | |
|---|---|---|---|
| (n = 565) | (n = 135) | ||
| Age | 8.8 ± 1.8 | 8.8 ± 1.7 | .94 |
| BMI percentile | 60.9 ± 30.6 | 61.2 ± 28.7 | .84 |
| AHI | 0.85 ± 1.7 | 0.72 ± 1.1 | .52 |
| % SDB | 27.1 | 23.0 | .33 |
| % Male | 45.5 | 48.3 | .62 |
| % Minority | 19.6 | 17.9 | .98 |
| % ADHD diagnosis | 6.8 | 19.4 | <.001 |
BMI Body Mass Index. AHI = Apnea/Hypopnea Index. ADHD = attention deficit hyperactivity disorder. SDB = sleep disordered breathing
As shown in Table 2, the overall two-way MANCOVA revealed significant associations between insomnia symptoms (Wilk’s λ = 0.85, F = 12.2, p < 0.001), objective sleep duration (Wilk’s λ = 0.96, F = 3.0, p = 0.001), and their interaction (Wilk’s λ = 0.96, F = 2.5, p = .01) with Pediatric Behavior Scale t scores. Specifically, children with insomnia symptoms demonstrated more problems on all behavioral indices as compared to controls independent of age, gender, race, body mass index %, ADHD, and sleep disordered breathing. Significant interactions between insomnia symptoms and objective sleep duration were found on specific Pediatric Behavior scales of opposition, hyperactivity, inappropriate social behavior, mood variability and school problems (Table 2). Post hoc comparisons showed that children with insomnia symptoms and normal sleep duration scored significantly higher than children with insomnia and short sleep duration on scales of externalizing behaviors such as opposition (p = 0.05, d = .41), hyperactivity (p = .03, d = .42), inappropriate social behavior (p = .02, d = .47), mood variability (p = .03, d = .37), and school problems (p = 0.001, d = .40). No significant differences were found between control subgroups based on objective sleep duration.
Table 2.
Behavioral scores by control and insomnia symptom subgroups
| Controls ≥7.7h | Controls <7.7h | Insomnia ≥7.7h | Insomnia <7.7h | Insomnia | Sleep Duration | Interaction | |
|---|---|---|---|---|---|---|---|
| n | 254 | 311 | 59 | 76 | |||
| Opposition | 47.6 ± 10.4 | 45.9 ± 9.2 | 58.7 ± 13.3 | 53.3 ± 13.3 | <.001 | <.001 | .05 |
| Aggression | 50.1 ± 12.4 | 47.6 ± 7.9 | 59.5 ± 21.4 | 53.1 ± 13.6 | <.001 | <.001 | .08 |
| Explosiveness | 50.0 ± 11.8 | 47.4 ± 9.2 | 61.4 ± 17.5 | 55.4 ± 15.5 | <.001 | <.001 | .12 |
| Inattention | 53.2 ± 14.1 | 50.7 ± 12.4 | 65.4 ± 18.8 | 60.3 ± 15.7 | <.001 | .004 | .32 |
| Impulsivity | 49.8 ± 13.1 | 48.9 ± 10.9 | 61.8 ± 16.9 | 57.1 ± 16.9 | <.001 | .013 | .30 |
| Hyperactivity | 52.9 ± 14.1 | 51.6 ± 12.4 | 66.5 ± 20.0 | 58.9 ± 16.5 | <.001 | .005 | .03 |
| Anxiety/Depression | 51.4 ± 11.2 | 49.3 ± 8.2 | 55.0 ± 12.5 | 57.5 ± 13.7 | <.001 | .005 | .71 |
| Inappropriate Behavior | 50.9 ± 12.7 | 48.5 ± 10.2 | 66.6 ± 20.2 | 58.0 ± 16.9 | <.001 | <.001 | .02 |
| Mood Variability | 52.2 ± 14.6 | 49.7 ± 11.0 | 67.9 ± 27.0 | 59.1 ± 20.6 | <.001 | <.001 | .03 |
| School Problems | 54.7 ± 17.0 | 54.5 ± 15.9 | 74.7 ± 30.7 | 63.9 ± 24.5 | <.001 | <.001 | .001 |
Data are mean ± standard deviation adjusted for age, race, gender, BMI percentile, ADHD, and Sleep Disordered Breathing
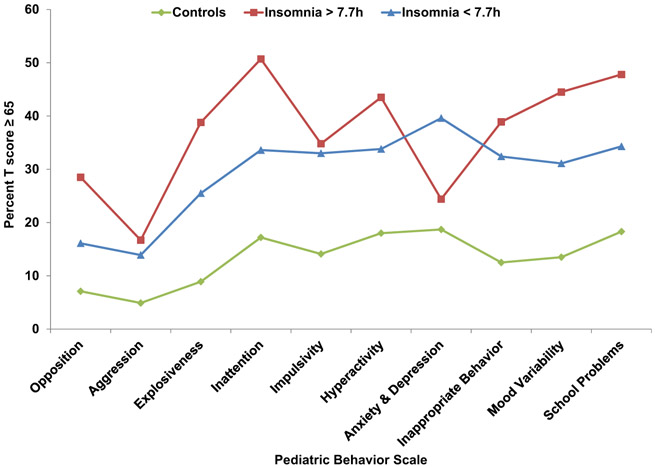
Finally, we examined the behavioral profiles across insomnia subgroups based on clinically elevated scores on the Pediatric Behavior Scale while excluding children with sleep disordered breathing considering its potential confounding effect (Fig 1). Controls were used as the reference group given the previous lack of association of objective sleep duration with Pediatric Behavior Scale t scores within this group. As shown in Fig 1, children with insomnia symptoms had significantly elevated profiles in all behavioral indices as compared to controls (d = .31 to .90), except children with insomnia symptoms and normal sleep duration who did not differ from controls on internalizing behaviors, i.e., anxiety and depression (p = .34, d = .15). Specifically, children with insomnia symptoms and normal sleep duration were associated with clinically meaningful elevations on externalizing behaviors, i.e., opposition (p = .02, d = .42) and explosiveness (p = .03, d = .39), as well as inattention (p = .02, d = .42), mood variability (p = 0.05, d = .35), and school problems (p = .07, d = .33) as compared to children with insomnia symptoms and short sleep duration. Consistently, children with insomnia symptoms and normal sleep duration had a significantly higher frequency of ADHD diagnosis as compared to those with short sleep duration (26.7% vs. 13.2%, p = .04), while the frequency of ADHD diagnosis in controls was similar regardless of sleep duration (5.5% vs. 4.5% for normal and short sleep duration, respectively). In contrast, children with insomnia symptoms and short sleep duration were associated with an overall elevated profile as compared to controls, on which internalizing behaviors, i.e., anxiety and depression, were more prominent as compared to controls (p < 0.001, d = .53) and children with insomnia symptoms and normal sleep duration (p = .04, d = .37). In terms of absolute effect sizes in the proportion of clinically elevated subscales between the two insomnia subgroups, the significant differences were of at least 10% (e.g., 28.5% vs. 16.1% in opposition and 24.4% vs. 39.6% in anxiety and depression), which is considered clinically meaningful.
Fig 1.
Behavioral profiles of subgroups of insomnia symptoms based on objective sleep duration. Data are percent of clinically elevated scores (T ≥ 65) across insomnia symptoms subgroups and controls adjusted for age, race, gender, BMI%, and ADHD diagnosis and excluding those with SDB
Discussion
This general population study demonstrated that young children with parent-reported insomnia symptoms have more problems on all behavioral indices than controls that are not explained by differences in sociodemographic factors or other clinical conditions. Importantly, this is the first study to examine the joint effect of parent-reported insomnia symptoms and objectively-measured sleep duration on behavioral profiles in young children. In fact, children with insomnia symptoms and normal sleep duration had a behavioral profile consistent with limit-setting, oppositional, and explosive behaviors, while children with insomnia symptoms and short sleep duration had a behavioral profile characterized by internalizing behaviors, which is consistent with previous findings of increased cortisol levels in this insomnia subtype.
The findings in the present study are consistent with a previous report (Fernandez-Mendoza et al. 2014) of a synergistic effect of insomnia symptoms and objectively-measured sleep duration on cortisol levels. Children with insomnia symptoms and short sleep duration were found to have increased evening and morning cortisol levels, while those with normal sleep duration had normal, or even low, cortisol levels as compared to controls. Together, these data suggest that insomnia symptoms with objective short duration in children are associated with internalizing behaviors (with specific agerelated mild elevations in externalizing behaviors), and may be in a continuum with adult insomnia associated with emotional and physiological hyperarousal (Bonnet and Arand 2010; Vgontzas et al. 2013), whereas children with insomnia symptoms and normal sleep duration are more likely to suffer from behavioral insomnia of childhood such as bedtime resistance, sleep-onset associations.
Until recently, the International Classification of Sleep Disorders (ICSD-2) defined behavioral insomnia of childhood as a disorder characterized by either a problem with parental limit-setting leading to bedtime resistance (e.g., struggle enforcing limits such as bedtime) and/or learned sleep-onset associations (e.g., need for a specific item or person to fall asleep) (American Academy of Sleep Medicine 2014). It is also commonly inferred that the degree of insufficient sleep negatively impacts children’s daytime functioning in the areas of learning, attention, and memory, behavior, academic performance, mood and emotional control, and parent-child relationships (American Academy of Sleep Medicine 2005). This nosology has been revised and the ICSD-3 no longer separates insomnia disorders into discrete diagnostic categories such as behavioral insomnia of childhood, so that the same basic diagnostic criteria used to diagnose insomnia disorder in adolescents or adults is used in young children as per parent report (i.e., difficulty initiating sleep or returning to sleep without caregiver intervention) (American Academy of Sleep Medicine 2014).
In contrast to this recent shift towards simplifying insomnia nosology, our data appears to support that behavioral insomnia of childhood associated with limit-setting, rule-breaking and explosive behaviors may indeed require a separate diagnosis, and challenges the commonly held belief that the degree of insufficient sleep (e.g., as a result of bedtime resistance and/or sleep-onset associations) is related to the severity of behavioral dysfunction in children with insomnia symptoms. In fact, based on the behavioral profiles found, one might hypothesize that the elevated inattention and school problems observed in children with insomnia symptoms and normal sleep duration may be driven by their prominent externalizing behaviors. In contrast, the inattention and school problems observed in children with insomnia symptoms and short sleep duration, while also potentially driven by other behavioral problems (particularly internalizing behaviors), could be the result of their sleep disorder per se. Future studies collecting specific data on bedtime resistance, sleep-onset association, behavior and objective sleep should further test this hypothesis.
The proposed subtyping of childhood insomnia based on physiological data, (e.g., cortisol), and behavioral profiles, may have an effect toward more individualized treatment that could result in improved outcomes. For example, it may be more likely that children with insomnia symptoms and normal sleep duration respond best to behavioral interventions targeting externalizing behaviors and sleep-onset associations, while children with parent-report of insomnia symptoms whose sleep is quantitatively shorter and present with more internalizing behaviors may respond best to interventions targeting their emotional distress and physiological hyperarousal. Future clinical trials should test this hypothesis.
Several limitations should be taken into account when interpreting the results of our study. First, the study is crosssectional and does not allow causal conclusions. It is likely that the relationship between sleep disturbance and behavior problems in young children is complex and bidirectional. It remains unknown whether it is the behavioral problems that lead to parent reports of insomnia symptoms and associated objective short sleep duration, or whether it is the insomnia symptoms that lead to behavioral problems through shorter sleep duration, or both Furthermore, the underlying assumption that behavioral problems are a consequence of short sleep in the context of parent reports of insomnia may not hold true and, thus, behavior problems may occur even with normal amounts of objective sleep, suggesting the existence of insomnia subtypes among children. Second, the objective sleep duration in this study was based on one night of fixed-time PSG, which may not be representative of the subjects’ habitual sleep duration and may be affected by first-night effect (the effect of the environment and polysomnographic recording equipment on the quality of the subject’s sleep the first night of recording). Our cut-off for short sleep duration was based on an empirical approach (i.e., median sleep duration of the sample); future studies should explore the optimum cut-off of sleep duration based on clinical criteria and explore the use of multiple PSG nights or less expensive objective measures of habitual sleep, (e.g., actigraphy). Nevertheless, our definitions of short (i.e., < 7.7 h) and normal (i.e., 7.8–9.0 h) sleep duration are consistent with those of a recently published study by our group (Fernandez-Mendoza et al. 2014), and in two previous studies using week-long, at-home actigraphy, which may better represent children’s habitual sleep pattern of school-aged children (Pesonen etal. 2010; Raikkonen etal. 2010). In terms of concerns with ecological validity of the PSG, our in-lab, 9-h (21:00–22:00 to 6:00–7:00), fixed-time PSG recording is consistent with that used in previous large normative PSG studies in young children (Montgomery-Downs et al. 2006) and a meta-analysis (Ohayon et al. 2004), which showed that the vast majority of studies in children aged 5–12 years old found average PSG total sleep times ranging from 450 to 550 min. Together, these data indicate that a nine-hour PSG protocol at the typical bedtime and wakeup time does not curtail the sleep duration of children 5–12 years old, and may provide a reliable index of in-lab physiological sleep duration.
Third, the definition of insomnia was that of symptoms rather than a disorder or syndrome, as it did not include general diagnostic criteria (ICSD-3) or specific behavioral insomnia of childhood criteria (ICSD-2). Future large, epidemiological studies should use current diagnostic criteria for childhood insomnia and its subgroups. Fourth, we relied on parent-reported insomnia symptoms rather than self-reported data. Although self-reported sleep data may better reflect the child’s experience of insomnia symptoms, the use of parent-report was deemed necessary given the young age of most children in this sample, which allowed comparison across children of all ages. Finally, although we adjusted for gender and other potential confounders in all of our analyses, future studies may prove helpful in understanding the potential modifying effect of gender on behavior in children with insomnia. Despite these limitations, this study extends the limited previous knowledge on insomnia symptoms in young children using a random general population sample and objective sleep measures.
In summary, children with parent-reported insomnia symptoms have increased ratings on measures of externalizing and internalizing behaviors as well as school problems. Importantly, children with insomnia symptoms and normal sleep duration have more externalizing behaviors as well as inattention, mood variability, and school problems, while children with insomnia symptoms and short sleep duration have significant behavioral problems, particularly in internalizing behaviors. These differential behavioral profiles suggest that subtyping of insomnia could be introduced as early as childhood. Such a nosological approach may provide clinicians with the ability to differentiate insomnia subtypes based on behavioral measures without having to rely on expensive, time-consuming sleep studies and better guide clinical evaluation and application of specific interventions.
Acknowledgments
Funding The authors report no financial or other relationship relevant to the subject of this article.
Sources of Supports This work was supported by National Institutes of Health grants R01 HL063772, M01 RR010732, and C06 RR016499.
Footnotes
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Alfano CA, Zakem AH, Costa NM, Taylor LK, & Weems CF (2009). Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents. Depression and Anxiety, 26, 503–512. [DOI] [PubMed] [Google Scholar]
- Ali NJ, Pitson D, & Stradling JR (1993). Snoring, sleep disturbance, and behaviour in 4-5 year olds. Archives of Disease in Childhood, 68, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics (2002). Clinical practice guidelines: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics, 109, 704–712. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine. (2005). The international classification of sleep disorders: Diagnostic and coding manual. In 2nd ed rev. Westchester: American Academy of Sleep Medicine. [Google Scholar]
- American Academy of Sleep Medicine. (2014). The international classification of sleep disorders: Diagnostic and coding manual. In 3rd ed rev. Westchester: American Academy of Sleep Medicine. [Google Scholar]
- American Thoracic Society (1996). Standards and indications for cardiopulmonary sleep studies in children. American Journal of Respiratory and Critical Care Medicine, 153, 866–878. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Liao DP, Calhoun SL, Vela-Bueno A, et al. (2009). Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep, 32, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, & Arand DL (2010). Hyperarousal and insomnia: state of the science. Sleep Medicine Review, 14, 9–15. [DOI] [PubMed] [Google Scholar]
- Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao DP, & Bixler EO (2014). Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Medicine, 15, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SL, Morgan WJ, Pernisco N, & Quan SF (2000). Factors affecting sleep disturbances in children and adolescents. Sleep Medicine, 1, 117–123. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, Olavarrieta-Bernardino S, Ramos-Platon MJ, & Bixler EO (2009). Nighttime sleep and daytime functioning correlates of the insomnia complaint in young adults. Journal of Adolescence, 32, 1059–1074. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao DP, et al. (2010). Insomnia with objective sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep, 33, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. (2012). Insomnia with objective short sleep duration and incident hypertension: the Penn State cohort. Hypertension, 60, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Calhoun SL, Vgontzas A, Tsaoussoglou M, Gaines J, et al. (2014). Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. European Journal of Clinical Investigation, 44, 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, & O’Connor TG (2002). Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 964–971. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC, O’Connor TG, & Plomin R (2004). Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 744–751. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, & Poulton R (2005). Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of Abnormal Child Psychology, 33, 157–163. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, & Breslau N (2006). The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. Journal of Psychiatric Research, 40, 700–708. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. (2000). CDC growth charts: United States. Adv Data, 8, 1–27. [PubMed] [Google Scholar]
- Lewin DS, Rosen RC, England SJ, & Dahl R (2002). Preliminary evidence of behavioral and cognitive sequelae obstructive sleep apnea in children. Sleep Medicine, 3, 5–13. [DOI] [PubMed] [Google Scholar]
- Lindgren SD, & Koeppl GK (1987). Assessing child behavior problems in a medical setting: development of the pediatric behavior scale In Prinz RJ (Ed.), Advances in behavioral assessment of children and families: Vol 3(57–90). JAI: Greenwich, CT. [Google Scholar]
- Liu X, & Zhou H (2002). Sleep duration, insomnia and behavioral problems in Chinese adolescents. Psychiatry Research, 111, 75–85. [DOI] [PubMed] [Google Scholar]
- Mayes SD, & Calhoun SL (2009). Variables related to sleep problems in children with autism. Research on Autism Spectrum Disorder, 3, 931–941. [Google Scholar]
- Mayes SD, Calhoun SL, Bixler EO, & Vgontzas AN (2008). Nonsignificance of sleep relative to IQ and neuropsychological scores in predicting academic achievement. Journal of Developmental and Behavioral Pediatrics, 29, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Bixler EO, Vgontzas AN, Mahr F, Hillwig-Garcia J, et al. (2009a). ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: differences in sleep problems. Journal of Pediatric Psychology, 34, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Bixler EO, & Vgontzas AN (2009b). Sleep problems in children with autism, ADHD, anxiety, depression, acquired brain injury, and typical development. Sleep Medicine Clinics, 4, 19–25. [Google Scholar]
- Montgomery-Downs HE, O’Brien LM, Gulliver TE, & Gozal D (2006). Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics, 117, 741–753. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Mervis CB, Holbrook CR, Bruner J, Smith N, McNally N, et al. (2004). Neurobehavioral correlates of sleep disordered breathing. Journal of Sleep Research, 13, 165–172. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, & Vitiello MV (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals; developing normative sleep values across the human lifespan. Sleep, 27, 1255–1273. [DOI] [PubMed] [Google Scholar]
- Owens JA, Mehlenbeck R, Lee J, & King MM (2008). Effect of weight, sleep duration, and comorbid sleep disorders on behavioral outcomes in children with sleep disordered breathing. Archives of Pediatric Adolescent Medicine, 162, 313–321. [DOI] [PubMed] [Google Scholar]
- Paavonen EJ, Aronen ET, Moilanen I, Piha J, Rasanen E, Tamminen T, et al. (2000). Sleep problems of school aged children: a complementary view. Acta Paediatrica, 89, 223–228. [DOI] [PubMed] [Google Scholar]
- Pesonen A-K, Raikkonen K, Paavonen EJ, Heinonen K, Komsi N, Lahti J, et al. (2010). Sleep duration and regularity are associated with behavioral problems in 8-year old children. International Journal of Behavioral Medicine, 17, 298–305. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Matthews KA, Pesonen A-K, Pyhälä R, Paavonen EJ, Feldt K, et al. (2010). Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. Journal of Clinical Endocrinology and Metabolism, 95, 2254–2261. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, & Duong HT (2008). Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12 month prospective study. Journal of Adolescent Health, 42, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singareddy R, Moole S, Calhoun SL, Vocalan P, Tsaoussoglou M, Vgontzas AN, et al. (2009). Medical complaints are more common in young school aged children with parent reported insomnia symptoms. Journal of Clinical Sleep Medicine, 5, 549–553. [PMC free article] [PubMed] [Google Scholar]
- Smedje H, Broman JE, & Hetta J (2000). Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight: a study based on parents’ perceptions. European Child & Adolescent Psychiatry, 10, 1–9. [DOI] [PubMed] [Google Scholar]
- Sprugt K, O’Brien LM, Cluydts R, Verleye GB, & Ferri R (2005). Odds, prevalence and predictors of sleep problems in school age normal children. Journal of Sleep Research, 14, 163–176. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. (2001). Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. Journal of Clinical Endocrinology and Metabolism, 86, 3787–3794. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, & Bixler EO (2009). Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care, 32, 1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, & Bixler EO (2013). Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Medicine Review, 17, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Carrier J, Frenette S, & Gruber R (2013). Sleep and sleepiness in children with attention deficit/hyperactivity disorder and controls. Journal of Sleep Research, 22, 24–29. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Sherrill DL, Goodwin JL, & Quan SF (2008). Association of sleep disordered breathing and behavior in school-aged children: the Tucson Children’s assessment of sleep apnea study. Open Epidemiology Journal, 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]